Abstract
Peroxisomes are ubiquitous cellular organelles required for specific pathways of fatty acid oxidation and lipid synthesis, and until recently their functions in adipocytes have not been well appreciated. Importantly, peroxisomes host many oxygen-consumption reactions and play a major role in generation and detoxification of reactive oxygen species (ROS) and reactive nitrogen species (RNS), influencing whole cell redox status. Here, we review recent progress in peroxisomal functions in lipid metabolism as related to ROS/RNS metabolism and discuss the roles of peroxisomal redox homeostasis in adipogenesis and adipocyte metabolism. We provide a framework for understanding redox regulation of peroxisomal functions in adipocytes together with testable hypotheses for developing therapies for obesity and the related metabolic diseases.
Keywords: Peroxisomes, Redox, Lipid metabolism, Adipocytes, Mitochondria
Abbreviations: Akt, protein kinase B; ACOX, acyl-CoA oxidase; BCFA, branched chain fatty acid; BAT, brown adipose tissue; C/EBPα, CCAAT-enhancer binding protein α; DHAP, dihydroxy acetone phosphate; EET, epoxyeicosatrienoic acid; FA, fatty acid; FAS, fatty acid synthase; H2O2, hydrogen peroxide; HACL1, 2-hydroxyacyl-CoA lyase 1; HAOX, l-hydroxy acid oxidase; HFD, high fat diet; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinase; LDs, lipid droplets; 2-OHFA, 2-hydroxy fatty acid; PPARγ, peroxisome proliferator-activated receptor γ; PRDX5, peroxiredoxin 5; PUFA, polyunsaturated fatty acid; RNS, reactive nitrogen species; ROS, reactive oxygen species; RXR, retinoid X receptor; sEH, soluble epoxide hydrolase; SOD, superoxide dismutase; TAG, triacylglycerol; VLCFA, very long chain fatty acid; WAT, white adipose tissue
Graphical abstract
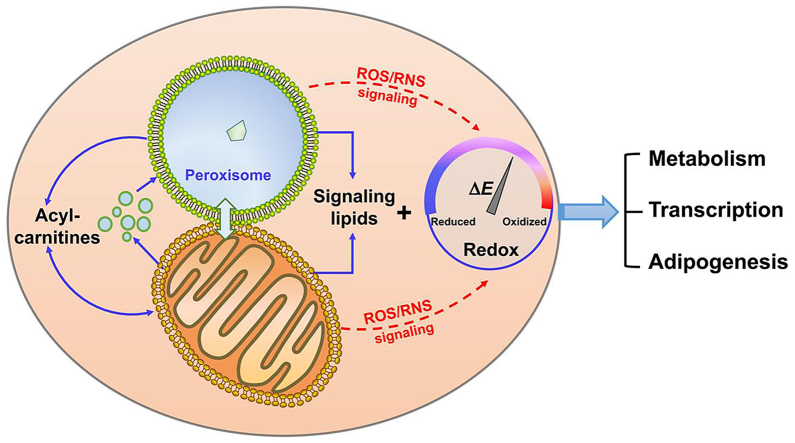
Peroxisomes and mitochondria coordinate lipid metabolism and ROS production to establish a redox tone to control adipogenesis and lipid metabolism.
Highlights
-
•
Peroxisomal proliferation is critical to promote adipogenesis and regulate lipogenesis.
-
•
Peroxisomes host many oxygen-consumption reactions and influence whole cell redox status
-
•
Both bioactive lipids and ROS/RNS contribute to peroxisomal regulation of metabolic functions in adipocytes.
-
•
Peroxisomal functions and redox regulation in adipose are depot-dependent.
-
•
Peroxisomes and mitochondria cooperate to control generation of signaling lipid and regulation of redox hemostasis.
1. Introduction
The ability of adipose tissues to dispose of circulating fatty acids (FA) by storage as triacylglycerols (TAG) or by oxidation has important metabolic and clinical implications [1]. Increased and dysregulated FA disposal contributes to the accumulation of potentially harmful FA metabolites in peripheral organs, which can cause insulin resistance and cardiometabolic diseases [2]. In addition, adverse remodeling and expansion of white adipose tissue (WAT) is associated with alterations in metabolic function and adipokine secretion, and accelerated development of insulin resistance [3]. The expansion of adipose depots can be driven either by the increase in adipocyte size (hypertrophy) or by the formation of new adipocytes differentiated from their precursors (hyperplasia). Based on different adipogenic sites, WAT is usually divided into subcutaneous and visceral depots, which have intrinsically different metabolic functions in maintaining health. The propensity to generate new adipocytes varies between the different adipose depots, and these processes are differentially regulated and have different metabolic consequences [4].
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are signaling messengers that mediate various biological responses. The primary ROS/RNS species generated in cells are superoxide radical (O2•-), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and nitric oxide radical (•NO) and its derivative peroxynitrite (ONOO−) [5]. Obesity is often associated with an imbalance in oxidation-reduction (redox) in WAT, which contributes to adipocyte dysfunction and can inhibit the insulin signaling cascade [6,7]. Mitochondria and peroxisomes are involved in major cellular catabolic pathways that are the major sources of ROS generation. Mitochondrial ROS signaling in supporting adipocyte thermogenic identity and function has been demonstrated in numerous animal and cellular models [8]. In contrast, much less is known about peroxisomes, where FA oxidation is coupled with the generation of H2O2 and minimal ATP production.
Peroxisomes are dynamic cellular organelles with diverse metabolic functions. Increase of peroxisomal gene expression and peroxisomal proliferation were observed during both brown and white adipogenesis [9]. In adipocytes, peroxisomes generate a variety of signaling molecules that are involved in regulating adipogenesis and adipocyte FA metabolism. Peroxisomes are named based on their function in producing and detoxifying H2O2 that serves as a cellular redox buffering system [10]. Despite the widespread perception that ROS are toxic by-products of aerobic metabolism, there is increasing evidence that ROS species can act as signaling molecules involved in cellular metabolic functions, including cell proliferation, differentiation, migration, and apoptosis [11]. Moreover, accumulating evidence supports that peroxisomal functions as related to ROS metabolism undergo differential dynamic regulation in different adipose depots. In this paper, we review the importance of peroxisomal redox signaling in adipogenesis and adipocyte metabolism.
2. Overview of the metabolic pathways in peroxisomes
Lipid storage and utilization rely on the interplay among the endoplasmic reticulum (ER), mitochondria, peroxisomes and lipid droplets (LD) [12]. Peroxisomes uniquely integrate cellular lipid storage and removal where FAs can be conjugated with dihydroxyacetone phosphate (DHAP) to synthesize glycerolipids or be shuttled into oxidative pathways [13]. Peroxisomes are involved in a broad range of metabolic pathways in humans that include: 1) FA β-oxidation; 2) FA α-oxidation; 3) ether lipid synthesis; 4) bile acid synthesis, 5) amino acid catabolism and 6) glyoxylate detoxification [13]. The importance of peroxisome lipid metabolism in human health is highlighted by the observations made in patients with Zellweger syndrome, who have a reduction or absence of functional peroxisomes that cause an accumulation of plasma very-long-chain FA (VLCFA) and branched chain FA (BCFA) and impaired function in multiple organs [14], including brain and lung functions [13].
3. ROS-generating pathways in peroxisomes
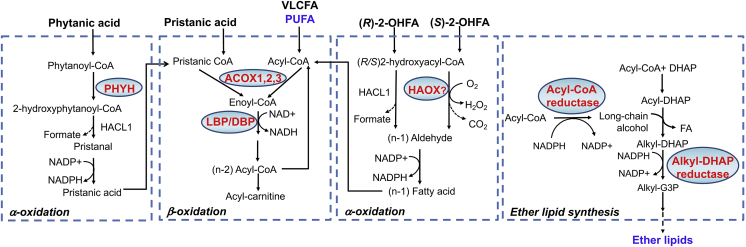
Peroxisomal FA oxidation shows substrate preference for VLCFA, 2-methyl-BCFA (e.g. pristanic acid), 2-hydroxy FA (2-OHFA), long chain dicarboxylic acid, polyunsaturated FA (PUFA) and precursors of bile acids. In contrast with mitochondrial FA β-oxidation initiated by acyl-CoA dehydrogenases and coupled to generation of FADH2, peroxisomal FA β-oxidation is initiated by fatty acyl-CoA oxidases (ACOXs) with generation of H2O2. The resulting acetyl-CoA and acyl-CoAs with shorter chain length are converted to their carnitine conjugates, exported out of peroxisomes and taken up by mitochondria for complete oxidation (Fig. 1).
Fig. 1.
Metabolic pathways in peroxisomes and redox regulation. Enzymes in red are known to generate ROS or act as antioxidants. Metabolites in blue act as non-enzymatic antioxidants. Refer to the text for details. ACOX, acyl-CoA oxidase; Alkyl-G3P, 1-O-alkyl glycerol-3-phosphate; DBP, D-bifunctional protein; DHAP, dihydroxy acetone phosphate; HAOX, l-hydroxy acid oxidase; HACL1, 2-hydroxyacyl-CoA lyase 1; LBP, L-bifunctional protein; 2-OHFA, 2-hydroxy fatty acid; PHYH, phytanoyl-CoA 2-hydroxylase; PUFA, poly unsaturated fatty acid; VLCFA, very long chain fatty acid.
Peroxisomal FA α-oxidation is another pathway influencing cellular ROS generation. Phytanic acid with a methyl group at the C3 position prevents its degradation by β−oxidation, but can be converted into pristanic acid by α oxidation [13]. This reaction is initiated by phytanoyl CoA hydroxylase (PHYH) using O2 as electron acceptor. The resulting 2-hydroxyphytanoyl-CoA is processed sequentially by 2-hydroxyacyl-CoA lyase 1 (HACL1) and by an unknown peroxisomal aldehyde dehydrogenase. Treatment of SH-SY5Y cells and astrocytes with phytanic acid results in higher ROS generation, suggesting that the overall metabolism of pristanic acid contributed to higher cellular oxidative stress [15]. Another group of FA substrates that undergo α-oxidation is straight chain 2-hydroxy FA (2-OHFA), (R)-enantiomers of which can be oxidized by HACL1 [16] and (S)-enantiomers by α-hydroxy acid oxidases (HAOX1 and HAOX2) leading to H2O2 release [17] (Fig. 1). Moreover, formate generated by FA α-oxidation can influence cellular ROS generation by stimulating mitochondrial superoxide/hydrogen peroxide release [18].
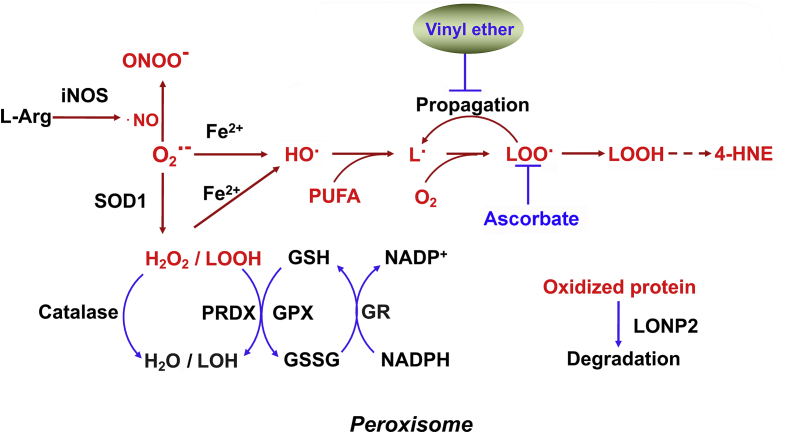
Additional peroxisomal ROS-producing oxidative enzymes are d-amino acid oxidase, d-aspartate oxidase, l-pipecolic acid oxidase, polyamine oxidase, xanthine oxidase (XDH) and many heme-containing enzymes [19]. H2O2 and the superoxide anion O2•- are usually generated as byproducts of their catalytic cycle. O2•- reacts rapidly with H2O2 and nitrates to generate hydroperoxide species and the highly oxidative ONOO−. O2•- can also be dismutated to form H2O2, which is decomposed through the Fenton reaction to •OH. The peroxisomal ROS species react rapidly with lipids to form lipid radicals and lipid peroxidation derivatives and with proteins to instigate damage [20]. Moreover, peroxisomes contain inducible nitric oxide synthase (iNOS), which catalyzes the oxidation of l-arginine to generate •NO; targeted disruption of the iNOS gene decreases adipose tissue inflammation [21] (Fig. 2).
Fig. 2.
Peroxisomal ROS scavenge system. Red characters denote ROS/RNS and macromolecular damaged by ROS. Blue characters denote nonenzymatic antioxidants. Refer to the text for details. GPX, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; H2O2, hydrogen peroxide; iNOS, inducible nitric oxide synthase; L•, lipid radical; LOO•, lipid peroxyl radical; LOOH, lipid peroxide; LOH, lipid alcohol; LONP2, Lon protease 2; •NO, nitric oxide radical; O2•-, superoxide radical; OH•, hydroxyl radical; ONOO−, peroxynitrite; PRDX, peroxiredoxin; PUFA, polyunsaturated fatty acid; SOD, superoxide mutase.
4. Peroxisomal ROS/RNS buffering systems
Multiple antioxidant systems have evolved within peroxisomes to detoxify ROS/RNS and maintain redox homeostasis. The identified peroxisomal antioxidant enzymes include catalase, superoxide dismutases (SODs), peroxidases, peroxiredoxin I and V, and soluble epoxide hydrolase (sEH). The copper-zinc SOD (SOD1) is the cytoplasmic isoform without an endogenous peroxisomal targeting sequence, but can be targeted to peroxisomes by using its physiological interaction partner, copper chaperone of SOD1, as a shuttle [22]. SOD catalyzes the dismutation of O2•- radical into O2 and H2O2, which are then removed by catalase and glutathione peroxidase within peroxisomes [23]. Peroxisomal peroxidases remove superoxide anion-induced lipid radicals and organic hydroperoxide species, and peroxiredoxin I and peroxiredoxin V control peroxide levels [24]. The epoxy FA neutralizing enzyme sEH has been localized in both the cytosol and peroxisomes, and the monomeric form with disrupting dimerization is preferentially targeted to peroxisomes [25]. Finally, peroxisomes also contain several proteases whose functions are linked to ROS-production. Peroxisomal lon peptidase 2 (LonP2), an ATP-dependent serine protease, is implicated in the degradation of damaged proteins by oxidation due to increased ROS levels, leading to regulation of FA β-oxidation in peroxisomal matrix [26] (Fig. 2).
In addition to the catabolism of various FAs, peroxisomes harbor metabolic enzymes initiating the synthesis of plasmalogens with vinyl ether linkage, which can function as non-enzymatic antioxidants [27]. Vinyl ether lipids prevent the propagation of lipid peroxidation [28] and their deficiency makes cells more vulnerable to oxidative stress [29,30]. Other small molecule antioxidants in peroxisomes include glutathione and ascorbic acid (vitamin C). Removal of H2O2 is coupled to the oxidation of GSH to GSSG and the subsequent regeneration of GSH requires glutathione reductase as well as NADPH (Fig. 2). The oxidized GSSG may be exported out of peroxisomes for GSH regeneration in cytosol and be re-uptaken to eliminate ROS.
NAD(P)H plays an important role in both peroxisomal ROS generation and elimination. Peroxisomes contain two different systems to regenerate NADPH: the pentose phosphate pathway (PPP) and the isocitrate dehydrogenase (IDH) pathway. Since NADPH is more abundant than NADH, peroxisomal redox status is often evaluated by assessing the NADPH/NADP+ ratio. NADPH can slowly diffuse out of the peroxisome in vitro, suggesting that peroxisomal NADPH/NADP+ is able to influence redox potential in cytosol [31]. In contrast, regeneration of NADH, which is required for continuous β-oxidation in peroxisomes, is accomplished by a pyruvate-lactate redox shuttle localized on the peroxisomal membrane [32].
5. Peroxisomal regulation of adipogenesis
Accumulating evidence supports the importance of peroxisomes in the regulation of white and brown adipocyte differentiation (adipogenesis). Adipogenesis relies on a delicate transcriptional program including CCAAT-enhancer binding protein α (C/EBPα) and peroxisome proliferator-activated receptor (PPARγ) that are also involved in the regulation of peroxisome proliferation in adipocytes [33]. Peroxisomes are essential for the metabolism of PUFA, providing natural ligands for PPARγ [34]. Additionally, 1-O-octadecenyl-2-palmitoyl-3-glycerophosphocholine (18:1e/16:0-GPC) has been identified as a potential endogenous ligand for PPARγ [35] and monoalkylglycerol ether lipids as a new class of adipogenic promoters [36]. The peroxisomal ether lipid synthesis may thus represent a positive feedback loop to sustain the adipogenic program. In addition to its signaling functions in promoting adipogenesis, peroxisomes are also significantly involved in TAG synthesis (lipogenesis). In cultured adipocytes, peroxisomes initiated the synthesis of almost half of the TAG [37] and were closely associated with LD [12]. Approximately 10–20% of the neutral lipids in LD were the ether lipid, monoalk(en)yl diacylglycerol, whose synthesis was exclusively initiated in peroxisomes [38]. Silencing of Pex16 in 3T3-L1 cells impaired adipocyte differentiation and cellular TAG stores, which are rescued by addition of PPARγ agonist rosiglitazone and peroxisome-related lipid species, highlighting the relevance of peroxisomes for adipogenesis [39]. Pex7 knockout mice, which are characterized by the absence of plasmalogens, have markedly reduced WAT and abnormally small LD within the cytoplasm of individual brown adipocytes [40]. In contrast, aP2-Pex5 conditional knockout mice showed dysfunctional WAT with increased fat mass and reduced lipolysis, which may be due to nonselective inhibition of peroxisomal functions in nervous system by the aP2 promotor, leading to reduced levels of catecholamine [41]. However, based on normal white adipose and muscle function in Nestin-Pex5 and Wnt-Pex5 knockout mice respectively, it is unlikely that peroxisome absence from the central and peripheral nervous system caused the observed adipose phenotype. Understanding PEX5 regulation of adipose expansion in vivo requires additional models of Pex5 deficiency specific in adipose. Collectively, these data indicate that biogenesis of peroxisomes provides signaling lipids to promote adipogenesis and regulate lipogenesis.
6. Redox regulation of adipogenesis
Chronic stimulation of ROS production in adipose during unhealthy obesity is associated with inhibited insulin signaling and thus impaired adipogenesis [42]. Peroxisomes may influence adipogenesis by regulating ROS homeostasis and multiple mechanisms have been proposed for ROS regulation of adipogenesis. Inhibition of complex I and ATP synthase increases cellular H2O2 and may act as anti-growth signal on 3T3-L1 preadipocytes to impair their potential to differentiate [43]. The preadipocytes acquire resistance against oxidative stress via FoxO-regulated expression of ROS-scavenging enzymes during differentiation [44] and in vitro study reveals that oxidative stress decreased GLUT4 expression and lipogenesis by diminished binding of C/EBP-dimer to GLUT4 promoter [45]. This regulation is supported by recent study that 4-hydroxynonenal (4-HNE)-induced oxidative stress impairs the adipogenic capacity of preadipocytes obtained from human subjects [46]. Moreover, cellular redox homeostasis may function through multiple crucial adipogenic transcriptional factors including C/EBPs, PPARγ, retinoid X receptor (RXR) as well as PPARγ coactivator 1α (PGC1α), whose activities are sensitive to their phosphorylation by a myriad of redox sensitive kinase pathways including c-Jun N-terminal kinase (JNK), p38-mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK) and protein kinase B (Akt) pathways [47]. Specifically, an increase in ROS by treatment with octanoate in adipocytes was linked to a decrease in TAG synthesis, and reduction of lipogenic gene expression by inactivation of PPARγ [48].
On the other hand, accumulating evidence supports that ROS generation seems important for adipocyte differentiation. Adipogenic differentiation in the 3T3-L1 preadipocytes were stimulated by addition of H2O2 [49]. Moreover, increased endogenous ROS production is observed even with enhanced expression of ROS-scavenging enzymes [44] in differentiating 3T3-L1 cells and in primary human mesenchymal stem cells undergoing differentiation into adipocytes [50]. This stimulated ROS generation facilitates adipocyte differentiation by enhancing C/EBPβ DNA binding activity and accelerating mitotic clonal expansion during early stage of differentiation [51]. Conversely, decrease of intracellular ROS or addition of antioxidants inhibits adipocyte differentiation [52,53]. Mutual interaction between ROS and insulin signaling has significant impact on adipogenesis. ROS generation through activation of NADPH oxidase homolog -NOX4 induces insulin signaling and adipogenesis by inhibiting protein phosphatase PIP1B [54]. Alternatively, oxidative stress inhibits expression of the adipogenic suppressor Sirt1, inducing adipogenesis along with adipocyte hypertrophy in mesenchymal stem cells and mouse pre-adipocytes [55]. Moreover, Sirt1 activity is sensitive to [NAD+]/[NADH] ratio which impact the acetylation status and metabolic functions of the adipogenic transcriptional factors [56,57].
The involvement of •NO in regulating adipogenesis and adipocyte biology has also been studied. •NO is essential for maintaining the balance between osteoblast and adipocyte lineage differentiation in periodontal ligament stem cells via the JNK/MARK signaling pathway. Increasing the physiological level of •NO with sodium nitroprusside significantly promoted the osteogenic differentiation capacity, but reduced its adipogenic differentiation capacity [58]. In contrast, a positive role of •NO in modulating adipogenesis is evidenced in cultured preadipocytes. •NO level in preadipocytes derived from rat WAT is increased during the early phase of differentiation and its deprivation partially abrogated the differentiation process [59]. S-nitrosylation of fatty acid synthase induced by •NO increases its activity through dimerization and may contribute to •NO regulation of adipogenesis [60]. Collectively, different cellular ROS and RNS may play distinct roles in different stages of adipogenesis [6], whose effects are determined by the levels and duration of specific oxidative signaling.
7. Depot-dependent redox regulation in adipose
Although brown adipose tissue (BAT) and WAT have comparable levels of ROS in mice under chow diet, BAT is more sensitive to HFD-induced ROS production [61]. Meanwhile, high fat feeding also increases expression of peroxisomal genes in BAT [9], suggesting potential involvement of peroxisomes in BAT ROS metabolism. Regulation of ROS metabolism in WAT also shows depot dependency. Mouse visceral WAT exhibits higher ROS as compared with the subcutaneous depot and aging induces ROS in visceral, but not in subcutaneous depot [62]. In humans, obesity is associated with lower GSH levels in both WAT depots, but additional pro-oxidative changes exist only in visceral WAT, which may be signs of visceral WAT metabolic dysfunction [63]. Cholesterol epoxide increases the expression of α-oxidation related genes in visceral adipocytes of obese subjects, but not in the subcutaneous adipocytes [64]. Collectively, these observations point out the complex regulation of oxidative stress in WAT depots and ROS in visceral WAT seems more sensitive to regulation by aging and obesity. However, contribution of peroxisomes in the depot-specific regulation of ROS metabolism remains to be determined.
8. Peroxisomal redox signaling in adipogenesis
Peroxisome biogenesis and its capacity for lipid metabolism dramatically increase during adipogenesis [65,66], suggesting that peroxisome proliferation can act as a positive feedback circuit to support adipocyte lipid metabolism and differentiation. Accumulating evidence supports that peroxisomal ROS generation promotes adipogenesis and adipocyte hypertrophy. Treatment with a SOD mimetics reduces visceral adiposity in mice through a reduction in adipocyte hypertrophy and adipogenesis, suggesting a causal role for ROS in the response to increased energy intake and diet-induced obesity [67]. Epoxyeicosatrienoic acid (EET), a type of PUFA that could act as an endogenous antioxidant, reduces adipogenesis in human mesenchymal stem cells and mouse 3T3-L1 preadipocytes, and injection of EET leads to reduced weight gain in obese mice [68]. Deletion of sEH that removes EET results in a significant decrease in adipocyte size and a decrease in visceral fat by reprograming of WAT to express mitochondrial and thermogenic genes, a characteristic phenotype of beige fat [69]. Conversely, silencing peroxiredoxin 5 (PRDX5) promotes preadipocytes to differentiate into adipocytes and Prdx5 knockout mice exhibit significant increase in body weight and enormous fat pads, suggesting ROS regulation of adipogenic gene expression and adipogenesis [70]. Similarly, catalase knockout mice exhibit an obese phenotype, manifested as an increase in body weight that becomes more pronounced with age and develop a pre-diabetic phenotype [71].
In contrast, a recent study using mouse models with peroxisomal ROS metabolism manipulated specifically in adipocytes suggests that oxidative stress inhibits healthy adipose expansion [72]. SOD1 and catalase over-expression in adipocytes exhibit adipose expansion with decreased ectopic lipid accumulation and improved insulin sensitivity, potentially by attenuation of SREBF1-mediated lipogenic transcriptional activities [72]. These data support the idea that peroxisomal functions in ROS metabolism and adipogenic transcriptional activation synergistically regulate lipid synthesis and expansion of adipose tissue. More importantly, it seems that ROS regulation of adipogenesis might contribute to WAT remodeling and determine its impact on peripheral tissues. The overall effect of peroxisomal ROS in adipogenesis and adipocyte functions is determined by temporal regulation of specific metabolic pathways of ROS.
9. Peroxisomal regulation of lipid mobilization
During adipogenesis, peroxisomes increase in number and form close contact with LD. Moreover, the biogenesis of peroxisomes and LD occur at the same ER subdomains [73], suggesting their intimate connection and potential functional coupling. Potential peroxisomal regulation of lipolysis has been suggested in peroxisome deficient aP2-Pex5 knockout mice, which have reduced lipolytic rates [41]. In Saccharomyces cerevisiae, peroxisomes can even extend into lipid body cores and promote the coupling of lipolysis in lipid bodies with peroxisomal FA oxidation, without passing through the cytosolic compartment [74]. Moreover, the coordination among LD, peroxisomes, and mitochondria regulated by ATGL-PPARα is important for lipolysis and FA oxidation in ob/ob mice deficient in Cidea and Cidec [75]. Lipidomics analysis of differentiating adipocytes revealed continuous accumulation of odd chain FAs [76], suggesting a concurrent increase in lipid turnover as well as the peroxisomal α-oxidation activity.
Level of lipolysis in visceral adipose is higher and declines with age. In contrast, subcutaneous adipose keeps relatively constant low level. These depot differences are not due to different oxidative damage or enzymatic anti-oxidant defense, but may be explained by site variations in insulin and adrenergic signaling [77]. In humans, both basal and adrenergic stimulated lipolysis is higher in adipocytes derived from visceral adipose as compared to cells from subcutaneous depot [78]. In line with depot specific regulation of lipolysis, adipocytes from visceral fat are more insulin-resistant and more sensitive to adrenergic stimulation than subcutaneous WAT.
Cellular redox state influences lipolysis in adipocytes and peroxisomal redox signaling may contribute to its regulation of lipolysis. Lactate inhibits lipolysis by blocking the cAMP/PKA signaling cascade [79] and the NAD+-dependent Sirt1 deacetylase activity promotes fat mobilization in white adipocytes by repressing PPARγ [80]. Physiological levels of •NO produced in adipose tissue stimulates lipolysis and FA oxidation through both AMPK-dependent and independent mechanisms, whereas high •NO levels inhibit basal as well as adrenergic stimulated lipolysis [81]. Moreover, iNOS impairs the anti-lipolytic effect of insulin in postprandial adipocytes, possibly via increased S-nitrosylation and inactivation of Akt/phosphodiesterase 3B axis [82,83].
10. Peroxisomal regulation of WAT browning and metabolic remodeling
Intake of HFD or cold exposure induces the acquisition of thermogenic features in subcutaneous WAT [84]. Characterization of the transcriptional network responsible for thermogenic fat activation revealed the involvement of PPARγ and PGC1α, which are key regulators of peroxisomal proliferation [85]. HFD did not increase expression of peroxisomal genes in WAT [9], arguing against the involvement of peroxisomes in HFD-induced WAT browning. In contrast, cold exposure increased the expression of genes involved in peroxisomal biogenesis in BAT, as well as in subcutaneous WAT, which is highly susceptible to cold-induced browning [86]. Collectively, these results suggest depot-specific regulation of peroxisomal signaling may be involved in WAT browning in response to cold exposure. Although higher ROS level and more sensitive regulation are observed in visceral fat, which plays minimal roles in thermogenic activation in response to cold exposure, whether peroxisomal ROS signaling represents an intrinsic inhibitory regulator of WAT browning remains elusive.
Depot specific regulation of adipokine secretion has also been observed. Subcutaneous WAT is the major source of leptin, while visceral WAT is more capable of generating adiponectin, angiotensinogen, plasminogen activator inhibitors-1 and pro-inflammatory cytokines [87]. Depot specific peroxisomal regulation of lipolysis, insulin signaling, glucose and lipid metabolism and adipokine secretion are mainly unknown due to unavailable depot specific manipulation of peroxisomal signaling. However, studies using models of global or adipose specific peroxisome deficiencies reveal important metabolic regulation in adipose by peroxisomal signaling. Pex11a deficiency impairs physical activity and energy expenditure, decreases fatty acid oxidation, increases de novo lipogenesis and results in dyslipidaemia and obesity [88]. aP2-Pex5 conditional knockout mice showed dysfunctional white adipose tissue with reduced lipolysis and increased insulin resistance, suggesting that peroxisomal signaling is necessary to maintain the adrenergic tone in mice, which in turn determines metabolic homeostasis [41]. Mice with adipose-specific knockout of Pex16 have increased diet-induced obesity and impaired thermogenesis due to inhibited plasmalogen synthesis, leading to decreased insulin sensitivity and glucose tolerance [9].
11. Obesity-induced peroxisomal regulation in adipose tissues
In adipose biology, diabetic obesity is correlated with increased ROS in depot-specific manner and is mechanistically linked to obesity-related metabolic diseases. Subcutaneous WAT from obese diabetic subjects exhibit increased H2O2 production compared with that from lean controls. Moreover, oxidized lipids and proteins accumulate to a greater extent in visceral depots compared with subcutaneous depots [89]. Obesity is associated with lower GSH levels in both WAT depots [63], but whether this redox regulation in WAT is related to peroxisomal signaling is not clear. Levels of PGC-1α and peroxisomal markers in BAT are lower in ob/ob mice than in lean control [75]. Moreover, as compared to obesity-prone animal, obesity-resistant animals show enhanced peroxisomal β-oxidation metabolism and reduced fat accumulation in visceral adipose tissues by up-regulating the expression of peroxisomal β-oxidation marker genes, which may be driven or enhanced by the up-regulation of the expression of PPARα [90]. In contrast, HFD-induced obesity shows increased expression of peroxisomal genes in BAT, but not in WAT [9]. These results indicate that peroxisomal functions in adipose are not determined by obesity per se, but may be regulated by specific pathway leading to adipose expansion in depot-specific manner.
12. Interplay between peroxisomes and mitochondria in the control of cellular redox signaling
Mitochondria are important in redox regulation and signaling where spontaneous leakage of electrons to oxygen from various dehydrogenases and respiratory chain complexes contributes to the generation of ROS. Previous studies suggest that ROS derived from mitochondria are important in the regulation of adipose expansion. Deletion of p66(Shc), a redox enzyme that generates mitochondrial ROS, also reduced triglyceride accumulation in adipocytes and in vivo decreased fat mass and resistance to diet-induced obesity [91]. Prx3 KO mice had increased fat mass with increased adipogenic transcription factors and lipogenic gene expression, possibly due to increased oxidative stress as evidenced by mitochondrial protein carbonylation [92]. Moreover, mitochondrial complex III ROS was demonstrated to be a causal factor in promoting adipocyte differentiation [50]. In contrast, adipose specific deletion of SOD2 increased mitochondrial superoxide and triggered an adaptive stress response, thereby preventing diet-induced obesity and insulin resistance [93], suggesting differential regulation of adipose expansion by different ROS metabolic pathways.
Peroxisomes cooperate with mitochondria in multiple metabolic pathways that affect redox-linked physiological processes and dysfunction of one organelle may affect the redox balance and function of the other [94]. Mitochondrial dysfunction is observed in patients with X-linked adrenoleukodystrophy (X-ALD) and peroxisome deficiency. Pex5 knockout mice with defective peroxisome biogenesis have altered mitochondrial ultrastructure with increased mitochondrial fission and changes in respiratory chain activity, and Pex13 null MEFs show mitochondrial oxidative stress and altered mitochondrial dynamics [41]. Mitochondrial dysfunction is also observed in ABCD1 deficiency as well as in Zellweger syndrome patients with Pex16 mutation [95]. Increasing peroxisomal FA oxidation boosts mitochondrial FA oxidation and ROS generation, indicating an intimate relationship between peroxisomes and mitochondria in regulating substrate oxidation and cellular redox status. On the other hand, inhibition of FA transport into mitochondria by Cpt1 knockout increases adaptive expression of peroxisomal FA oxidation genes, suggesting the presence of a mitochondrial retrograde signaling to regulate peroxisomal FA oxidation [96]. A recent publication supports a surprising interaction between peroxisomes and mitochondria. Peroxisomes regulate mitochondrial dynamics and thermogenesis, by directing plasmalogens to mitochondria to mediate mitochondrial fission [9]. Moreover, mitochondria may act as dynamic receivers, integrators, and transmitters of peroxisome-derived mediators of oxidative stress, suggesting a potential broader role for the peroxisome in regulating oxidative stress and adipogenesis [97,98]. The intimate redox interplay between mitochondria and peroxisomes in regulation of signaling and metabolism makes it difficult to estimate the relative contribution of peroxisomal ROS as compared to mitochondrial ROS to these processes.
Peroxisomes oxidize a variety of FAs and generate peroxisomal H2O2 by ACOX-mediated desaturation, while mitochondria are important for further metabolism of the end-products of peroxisomal β oxidation which include chain-shortened acyl-CoAs, acetyl-CoA, propionyl-CoA as well as NADH. The transport of acyl-CoAs is achieved by interconversion with their acyl carnitine counterparts catalyzed by multiple carnitine acyltransferases and translocase. A recent study identifies the peroxisome-mitochondrial contact by a proximity detection method based on split fluorophores, and demonstrates its contribution to complete β-oxidation of FAs [99]. Moreover, cargo transport from the mitochondria to peroxisomes can be achieved by vesicular carriers [100], providing additional mechanism for active substrate exchange between these two organelles.
13. Conclusions and future perspectives
Adipocyte peroxisomes not only contribute significantly to the synthesis of TAG and ether lipids via the DHAP pathway, but also generate important lipid molecules and redox signals that influence adipogenesis and adipocyte metabolism. Peroxisomes form close contacts with various subcellular organelles, including the ER, LD and mitochondria to coordinate key adipocyte functions of lipid synthesis, lipolysis and FA oxidation. Importantly, peroxisomes are major site of ROS/RNS formation and detoxification and are therefore important in maintaining cellular redox homeostasis and regulating various signaling cascades which are important in adipogenesis and adipocyte functions.
Although convincing evidences have been provided to support that peroxisomal redox signaling plays critical roles in regulating adipocyte functions, the underlying mechanisms are still poorly understood. Additional studies are necessary to answer how ROS signaling may influence bioactive signaling lipid generation in adipocyte peroxisomes. It is also not clear how ROS signaling influences remodeling of WAT and regulation of whole body insulin sensitivity. A better understanding of the specific roles of peroxisomes in different metabolic pathways and their contribution to the pathogenesis of metabolic diseases could identify novel directions and targets for treating patients with peroxisomal disorders, oxidative stress, and metabolic diseases.
Declarations of interest
None.
Acknowledgement
This work was supported by the National Institutes of Health Grant DK097608 and Nutrition and Obesity Research Center Grant P30-DK056341, the National Natural Science Foundation of China (31570806, 31701251 and 31620103906); Natural Science Foundation of Jiangsu Province Grant BK20150006; a project funded by Priority Academic Programme Development of Jiangsu Higher Education Institutions; College Research Program of Science and Technology Commission Foundation of Jiangsu Province (17KJB310013) and Suzhou Basic and Applied Medical Research Plan (SYS201673).
References
- 1.Kusminski C.M., Bickel P.E., Scherer P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016;15:639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 2.Czech M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017;23:804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka M., Itoh M., Ogawa Y., Suganami T. Molecular mechanism of obesity-induced ʻmetabolicʼ tissue remodeling. J. Diabetes Investig. 2018;9:256–261. doi: 10.1111/jdi.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghaben A.L., Scherer P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019 Jan 4 doi: 10.1038/s41580-018-0093-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell Longev. 2016;2016 doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jankovic A., Korac A., Buzadzic B., Otasevic V., Stancic A., Daiber A., Korac B. Redox implications in adipose tissue (dys)function--A new look at old acquaintances. Redox Biol. 2015;6:19–32. doi: 10.1016/j.redox.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newsholme P., Cruzat V.F., Keane K.N., Carlessi R., de Bittencourt P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016;473:4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- 8.Chouchani E.T., Kazak L., Spiegelman B.M. Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. J. Biol. Chem. 2017;292:16810–16816. doi: 10.1074/jbc.R117.789628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park H., He A., Tan M., Johnson J.M., Dean J.M., Pietka T.A., Chen Y., Zhang X., Hsu F.F., Razani B. Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J. Clin. Investig. 2019;129:694–711. doi: 10.1172/JCI120606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker C.L., Pomatto L.C.D., Tripathi D.N., Davies K.J.A. Redox regulation of homeostasis and proteostasis in peroxisomes. Physiol. Rev. 2018;98:89–115. doi: 10.1152/physrev.00033.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripathi D.N., Walker C.L. The peroxisome as a cell signaling organelle. Curr. Opin. Cell Biol. 2016;39:109–112. doi: 10.1016/j.ceb.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuldiner M., Bohnert M. A different kind of love - lipid droplet contact sites. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1188–1196. doi: 10.1016/j.bbalip.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Wanders R.J. Metabolic functions of peroxisomes in health and disease. Biochimie. 2014;98:36–44. doi: 10.1016/j.biochi.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Klouwer F.C.C., Berendse K., Ferdinandusse S., Wanders R.J.A., Engelen M., Poll-The B.T. Zellweger spectrum disorders: clinical overview and management approach. Orphanet J. Rare Dis. 2015;10 doi: 10.1186/s13023-015-0368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhary S., Sahu U., Kar S., Parvez S. Phytanic acid-induced neurotoxicological manifestations and apoptosis ameliorated by mitochondria-mediated actions of melatonin. Mol. Neurobiol. 2017;54:6960–6969. doi: 10.1007/s12035-016-0209-4. [DOI] [PubMed] [Google Scholar]
- 16.Foulon V., Sniekers M., Huysmans E., Asselberghs S., Mahieu V., Mannaerts G.P., Van Veldhoven P.P., Casteels M. Breakdown of 2-hydroxylated straight chain fatty acids via peroxisomal 2-hydroxyphytanoyl-CoA lyase. J. Biol. Chem. 2005;280:9802–9812. doi: 10.1074/jbc.M413362200. [DOI] [PubMed] [Google Scholar]
- 17.Jones J.M., Morrell J.C., Gould S.J. Identification and characterization of HAOX1, HAOX2, and HAOX3, three human peroxisomal 2-hydroxy acid oxidases. J. Biol. Chem. 2000;275:12590–12597. doi: 10.1074/jbc.275.17.12590. [DOI] [PubMed] [Google Scholar]
- 18.Young A., Gardiner D., Brosnan M.E., Brosnan J.T., Mailloux R.J. Physiological levels of formate activate mitochondrial superoxide/hydrogen peroxide release from mouse liver mitochondria. FEBS Lett. 2017;591:2426–2438. doi: 10.1002/1873-3468.12777. [DOI] [PubMed] [Google Scholar]
- 19.Fransen M., Nordgren M., Wang B., Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim. Et Biophys. Acta Mol. Basis Dis. 2012;1822:1363–1373. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Moldogazieva N.T., Mokhosoev I.M., Feldman N.B., Lutsenko S.V. ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic. Res. 2018;52:507–543. doi: 10.1080/10715762.2018.1457217. [DOI] [PubMed] [Google Scholar]
- 21.Becerril S., Rodriguez A., Catalan V., Mendez-Gimenez L., Ramirez B., Sainz N., Llorente M., Unamuno X., Gomez-Ambrosi J., Fruhbeck G. Targeted disruption of the iNOS gene improves adipose tissue inflammation and fibrosis in leptin-deficient ob/ob mice: role of tenascin C. Int. J. Obes. (Lond) 2018;42:1458–1470. doi: 10.1038/s41366-018-0005-5. [DOI] [PubMed] [Google Scholar]
- 22.Islinger M., Li K.W., Seitz J., Volkl A., Luers G.H. Hitchhiking of Cu/Zn superoxide dismutase to peroxisomes--evidence for a natural piggyback import mechanism in mammals. Traffic. 2009;10:1711–1721. doi: 10.1111/j.1600-0854.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Branicky R., Noe A., Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walbrecq G., Wang B., Becker S., Hannotiau A., Fransen M., Knoops B. Antioxidant cytoprotection by peroxisomal peroxiredoxin-5. Free Radic. Biol. Med. 2015;84:215–226. doi: 10.1016/j.freeradbiomed.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 25.Nelson J.W., Das A.J., Barnes A.P., Alkayed N.J. Disrupting dimerization translocates soluble epoxide hydrolase to peroxisomes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okumoto K., Kametani Y., Fujiki Y. Two proteases, trypsin domain-containing 1 (Tysnd1) and peroxisomal lon protease (PsLon), cooperatively regulate fatty acid beta-oxidation in peroxisomal matrix. J. Biol. Chem. 2011;286:44367–44379. doi: 10.1074/jbc.M111.285197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broniec A., Zadlo A., Pawlak A., Fuchs B., Klosinski R., Thompson D., Sarna T. Interaction of plasmenylcholine with free radicals in selected model systems. Free Radic. Biol. Med. 2017;106:368–378. doi: 10.1016/j.freeradbiomed.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Engelmann B. Plasmalogens: targets for oxidants and major lipophilic antioxidants. Biochem. Soc. Trans. 2004;32:147–150. doi: 10.1042/bst0320147. [DOI] [PubMed] [Google Scholar]
- 29.Broniec A., Klosinski R., Pawlak A., Wrona-Krol M., Thompson D., Sarna T. Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic. Biol. Med. 2011;50:892–898. doi: 10.1016/j.freeradbiomed.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luoma A.M., Kuo F., Cakici O., Crowther M.N., Denninger A.R., Avila R.L., Brites P., Kirschner D.A. Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic. Biol. Med. 2015;84:296–310. doi: 10.1016/j.freeradbiomed.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Antonenkov V.D., Hiltunen J.K. Transfer of metabolites across the peroxisomal membrane. Biochim. Et Biophys. Acta Mol. Basis Dis. 2012;1822:1374–1386. doi: 10.1016/j.bbadis.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Schueren F., Lingner T., George R., Hofhuis J., Dickel C., Gartner J., Thoms S. Peroxisomal lactate dehydrogenase is generated by translational read through in mammals. Elife. 2014;3 doi: 10.7554/eLife.03640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuri-Harcuch W., Velez-delValle C., Vazquez-Sandoval A., Hernandez-Mosqueira C., Fernandez-Sanchez V. A cellular perspective of adipogenesis transcriptional regulation. J. Cell. Physiol. 2019;234(2):1111–1129. doi: 10.1002/jcp.27060. [DOI] [PubMed] [Google Scholar]
- 34.Muralikumar S., Vetrivel U., Narayanasamy A., U N.D. Probing the intermolecular interactions of PPARgamma-LBD with polyunsaturated fatty acids and their anti-inflammatory metabolites to infer most potential binding moieties. Lipids Health Dis. 2017;16:17. doi: 10.1186/s12944-016-0404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodhi I.J., Yin L., Jensen-Urstad A.P., Funai K., Coleman T., Baird J.H., El Ramahi M.K., Razani B., Song H., Fu-Hsu F. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metabol. 2012;16:189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homan E.A., Kim Y.G., Cardia J.P., Saghatelian A. Monoalkylglycerol ether lipids promote adipogenesis. J. Am. Chem. Soc. 2011;133:5178–5181. doi: 10.1021/ja111173c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajra A.K., Larkins L.K., Das A.K., Hemati N., Erickson R.L., MacDougald O.A. Induction of the peroxisomal glycerolipid-synthesizing enzymes during differentiation of 3T3-L1 adipocytes. Role in triacylglycerol synthesis. J. Biol. Chem. 2000;275:9441–9446. doi: 10.1074/jbc.275.13.9441. [DOI] [PubMed] [Google Scholar]
- 38.Bartz R., Li W.H., Venables B., Zehmer J.K., Roth M.R., Welti R., Anderson R.G., Liu P., Chapman K.D. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Hofer D.C., Pessentheiner A.R., Pelzmann H.J., Schlager S., Madreiter-Sokolowski C.T., Kolb D., Eichmann T.O., Rechberger G., Bilban M., Graier W.F. Critical role of the peroxisomal protein PEX16 in white adipocyte development and lipid homeostasis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:358–368. doi: 10.1016/j.bbalip.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brites P., Ferreira A.S., da Silva T.F., Sousa V.F., Malheiro A.R., Duran M., Waterham H.R., Baes M., Wanders R.J. Alkyl-glycerol rescues plasmalogen levels and pathology of ether-phospholipid deficient mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martens K., Bottelbergs A., Peeters A., Jacobs F., Espeel M., Carmeliet P., Van Veldhoven P.P., Baes M. Peroxisome deficient aP2-Pex5 knockout mice display impaired white adipocyte and muscle function concomitant with reduced adrenergic tone. Mol. Genet. Metabol. 2012;107:735–747. doi: 10.1016/j.ymgme.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carriere A., Fernandez Y., Rigoulet M., Penicaud L., Casteilla L. Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Lett. 2003;550:163–167. doi: 10.1016/s0014-5793(03)00862-7. [DOI] [PubMed] [Google Scholar]
- 44.Kojima T., Norose T., Tsuchiya K., Sakamoto K. Mouse 3T3-L1 cells acquire resistance against oxidative stress as the adipocytes differentiate via the transcription factor FoxO. Apoptosis. 2010;15:83–93. doi: 10.1007/s10495-009-0415-x. [DOI] [PubMed] [Google Scholar]
- 45.Pessler-Cohen D., Pekala P.H., Kovsan J., Bloch-Damti A., Rudich A., Bashan N. GLUT4 repression in response to oxidative stress is associated with reciprocal alterations in C/EBP alpha and delta isoforms in 3T3-L1 adipocytes. Arch. Physiol. Biochem. 2006;112:3–12. doi: 10.1080/13813450500500399. [DOI] [PubMed] [Google Scholar]
- 46.Elrayess M.A., Almuraikhy S., Kafienah W., Al-Menhali A., Al-Khelaifi F., Bashah M., Zarkovic K., Zarkovic N., Waeg G., Alsayrafi M. 4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic. Biol. Med. 2017;104:129–137. doi: 10.1016/j.freeradbiomed.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Brunmeir R., Xu F. Functional regulation of PPARs through post-translational modifications. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo W., Xie W., Han J. Modulation of adipocyte lipogenesis by octanoate: involvement of reactive oxygen species. Nutr Metab. (Lond) 2006;3:30. doi: 10.1186/1743-7075-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H., Lee Y.J., Choi H., Ko E.H., Kim J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009;284:10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tormos K.V., Anso E., Hamanaka R.B., Eisenbart J., Joseph J., Kalyanaraman B., Chandel N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metabol. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murakami N., Ohtsubo T., Kansui Y., Goto K., Noguchi H., Haga Y., Nakabeppu Y., Matsumura K., Kitazono T. Mice heterozygous for the xanthine oxidoreductase gene facilitate lipid accumulation in adipocytes. Arterioscler. Thromb. Vasc. Biol. 2014;34:44. doi: 10.1161/ATVBAHA.113.302214. [DOI] [PubMed] [Google Scholar]
- 52.Calzadilla P., Sapochnik D., Cosentino S., Diz V., Dicelio L., Calvo J.C., Guerra L.N. N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2011;12:6936–6951. doi: 10.3390/ijms12106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vigilanza P., Aquilano K., Baldelli S., Rotilio G., Ciriolo M.R. Modulation of intracellular glutathione affects adipogenesis in 3T3-L1 cells. J. Cell. Physiol. 2011;226:2016–2024. doi: 10.1002/jcp.22542. [DOI] [PubMed] [Google Scholar]
- 54.Mahadev K., Motoshima H., Wu X., Ruddy J.M., Arnold R.S., Cheng G., Lambeth J.D., Goldstein B.J. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puri N., Sodhi K., Haarstad M., Kim D.H., Bohinc S., Foglio E., Favero G., Abraham N.G. Heme induced oxidative stress attenuates sirtuin1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes. J. Cell. Biochem. 2012;113:1926–1935. doi: 10.1002/jcb.24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kraakman M.J., Liu Q., Postigo-Fernandez J., Ji R., Kon N., Larrea D., Namwanje M., Fan L., Chan M., Area-Gomez E. PPARgamma deacetylation dissociates thiazolidinedione's metabolic benefits from its adverse effects. J. Clin. Investig. 2018;128:2600–2612. doi: 10.1172/JCI98709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryu K.W., Nandu T., Kim J., Challa S., DeBerardinis R.J., Kraus W.L. Metabolic regulation of transcription through compartmentalized NAD(+) biosynthesis. Science. 2018;360 doi: 10.1126/science.aan5780. 618-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang S., Guo L., Su Y., Wen J., Du J., Li X., Liu Y., Feng J., Xie Y., Bai Y. Nitric oxide balances osteoblast and adipocyte lineage differentiation via the JNK/MAPK signaling pathway in periodontal ligament stem cells. Stem Cell Res. Ther. 2018;9:118. doi: 10.1186/s13287-018-0869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan H., Aziz E., Shillabeer G., Wong A., Shanghavi D., Kermouni A., Abdel-Hafez M., Lau D.C. Nitric oxide promotes differentiation of rat white preadipocytes in culture. J. Lipid Res. 2002;43:2123–2129. doi: 10.1194/jlr.m200305-jlr200. [DOI] [PubMed] [Google Scholar]
- 60.Choi M.S., Jung J.Y., Kim H.J., Ham M.R., Lee T.R., Shin D.W. S-nitrosylation of fatty acid synthase regulates its activity through dimerization. J. Lipid Res. 2016;57:607–615. doi: 10.1194/jlr.M065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alcala M., Calderon-Dominguez M., Bustos E., Ramos P., Casals N., Serra D., Viana M., Herrero L. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci. Rep. 2017;7:16082. doi: 10.1038/s41598-017-16463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L., Ebenezer P.J., Dasuri K., Fernandez-Kim S.O., Francis J., Mariappan N., Gao Z.G., Ye J.P., Bruce-Keller A.J., Keller J.N. Aging is associated with hypoxia and oxidative stress in adipose tissue: implications for adipose function. Am. J. Physiol. Endocrinol. Metab. 2011;301:E599–E607. doi: 10.1152/ajpendo.00059.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jankovic A., Korac A., Srdic-Galic B., Buzadzic B., Otasevic V., Stancic A., Vucetic M., Markelic M., Velickovic K., Golic I. Differences in the redox status of human visceral and subcutaneous adipose tissues--relationships to obesity and metabolic risk. Metabolism. 2014;63:661–671. doi: 10.1016/j.metabol.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Jove M., Moreno-Navarrete J.M., Pamplona R., Ricart W., Portero-Otin M., Fernandez-Real J.M. Human omental and subcutaneous adipose tissue exhibit specific lipidomic signatures. FASEB J. 2014;28:1071–1081. doi: 10.1096/fj.13-234419. [DOI] [PubMed] [Google Scholar]
- 65.Roberts L.D., Virtue S., Vidal-Puig A., Nicholls A.W., Griffin J.L. Metabolic phenotyping of a model of adipocyte differentiation. Physiol. Genom. 2009;39:109–119. doi: 10.1152/physiolgenomics.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bagattin A., Hugendubler L., Mueller E. Transcriptional coactivator PGC-1alpha promotes peroxisomal remodeling and biogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20376–20381. doi: 10.1073/pnas.1009176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pires K.M., Ilkun O., Valente M., Boudina S. Treatment with a SOD mimetic reduces visceral adiposity, adipocyte death, and adipose tissue inflammation in high fat-fed mice. Obesity (Silver Spring) 2014;22:178–187. doi: 10.1002/oby.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cao J., Singh S.P., McClung J.A., Joseph G., Vanella L., Barbagallo I., Jiang H., Falck J.R., Arad M., Shapiro J.I. EET intervention on Wnt1, NOV, and HO-1 signaling prevents obesity-induced cardiomyopathy in obese mice. Am. J. Physiol. Heart Circ. Physiol. 2017;313:H368–H380. doi: 10.1152/ajpheart.00093.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu L., Puri N., Raffaele M., Schragenheim J., Singh S.P., Bradbury J.A., Bellner L., Vanella L., Zeldin D.C., Cao J. Ablation of soluble epoxide hydrolase reprogram white fat to beige-like fat through an increase in mitochondrial integrity, HO-1-adiponectin in vitro and in vivo. Prostag. Other Lipid Mediat. 2018;138:1–8. doi: 10.1016/j.prostaglandins.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim M.H., Park S.J., Kim J.H., Seong J.B., Kim K.M., Woo H.A., Lee D.S. Peroxiredoxin 5 regulates adipogenesis-attenuating oxidative stress in obese mouse models induced by a high-fat diet. Free Radic. Biol. Med. 2018;123:27–38. doi: 10.1016/j.freeradbiomed.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 71.Heit C., Marshall S., Singh S., Yu X.Q., Charkoftaki G., Zhao H.Y., Orlicky D.J., Fritz K.S., Thompson D.C., Vasiliou V. Catalase deletion promotes prediabetic phenotype in mice. Free Radic. Biol. Med. 2017;103:48–56. doi: 10.1016/j.freeradbiomed.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okuno Y., Fukuhara A., Hashimoto E., Kobayashi H., Kobayashi S., Otsuki M., Shimomura I. Oxidative stress inhibits healthy adipose expansion through suppression of SREBF1-mediated lipogenic pathway. Diabetes. 2018;67:1113–1127. doi: 10.2337/db17-1032. [DOI] [PubMed] [Google Scholar]
- 73.Joshi A.S., Nebenfuehr B., Choudhary V., Satpute-Krishnan P., Levine T.P., Golden A., Prinz W.A. Lipid droplet and peroxisome biogenesis occur at the same ER subdomains. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-05277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Binns D., Januszewski T., Chen Y., Hill J., Markin V.S., Zhao Y., Gilpin C., Chapman K.D., Anderson R.G., Goodman J.M. An intimate collaboration between peroxisomes and lipid bodies. J. Cell Biol. 2006;173:719–731. doi: 10.1083/jcb.200511125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou L.K., Yu M., Arshad M., Wang W.M., Lu Y.Y., Gong J.Y., Gu Y.N., Li P., Xu L. Coordination among lipid droplets, peroxisomes, and mitochondria regulates energy expenditure through the CIDE-ATGL-PPAR alpha pathway in adipocytes. Diabetes. 2018;67:1935–1948. doi: 10.2337/db17-1452. [DOI] [PubMed] [Google Scholar]
- 76.Su X., Han X., Yang J., Mancuso D.J., Chen J., Bickel P.E., Gross R.W. Sequential ordered fatty acid alpha oxidation and Delta9 desaturation are major determinants of lipid storage and utilization in differentiating adipocytes. Biochemistry. 2004;43:5033–5044. doi: 10.1021/bi035867z. [DOI] [PubMed] [Google Scholar]
- 77.Liu R., Pulliam D.A., Liu Y., Salmon A.B. Dynamic differences in oxidative stress and the regulation of metabolism with age in visceral versus subcutaneous adipose. Redox Biol. 2015;6:401–408. doi: 10.1016/j.redox.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verboven K., Hansen D., Moro C., Eijnde B.O., Hoebers N., Knol J., Bouckaert W., Dams A., Blaak E.E., Jocken J.W. Attenuated atrial natriuretic peptide-mediated lipolysis in subcutaneous adipocytes of obese type 2 diabetic men. Clin. Sci. (Lond.) 2016;130:1105–1114. doi: 10.1042/CS20160220. [DOI] [PubMed] [Google Scholar]
- 79.Offermanns S. Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol. Metabol. 2017;28:227–236. doi: 10.1016/j.tem.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Chakrabarti P., English T., Karki S., Qiang L., Tao R., Kim J., Luo Z.J., Farmer S.R., Kandror K.V. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J. Lipid Res. 2011;52:1693–1701. doi: 10.1194/jlr.M014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jankovic A., Korac A., Buzadzic B., Stancic A., Otasevic V., Ferdinandy P., Daiber A., Korac B. Targeting the NO/superoxide ratio in adipose tissue: relevance to obesity and diabetes management. Br. J. Pharmacol. 2017;174:1570–1590. doi: 10.1111/bph.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loughran P.A., Stolz D.B., Barrick S.R., Wheeler D.S., Friedman P.A., Rachubinski R.A., Watkins S.C., Billiar T.R. PEX7 and EBP50 target iNOS to the peroxisome in hepatocytes. Nitric Oxide. 2013;31:9–19. doi: 10.1016/j.niox.2013.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ovadia H., Haim Y., Nov O., Almog O., Kovsan J., Bashan N., Benhar M., Rudich A. Increased adipocyte S-nitrosylation targets anti-lipolytic action of insulin: relevance to adipose tissue dysfunction in obesity. J. Biol. Chem. 2011;286:30433–30443. doi: 10.1074/jbc.M111.235945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garcia-Ruiz E., Reynes B., Diaz-Rua R., Ceresi E., Oliver P., Palou A. The intake of high-fat diets induces the acquisition of brown adipocyte gene expression features in white adipose tissue. Int. J. Obes. (Lond) 2015;39:1619–1629. doi: 10.1038/ijo.2015.112. [DOI] [PubMed] [Google Scholar]
- 85.Emont M.P., Kim D.I., Wu J. Development, activation, and therapeutic potential of thermogenic adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864:13–19. doi: 10.1016/j.bbalip.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang W., Seale P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016;17:691–702. doi: 10.1038/nrm.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ibrahim M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes. Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 88.Chen C., Wang H., Chen B., Chen D., Lu C., Li H., Qian Y., Tan Y., Weng H., Cai L. Pex11a deficiency causes dyslipidaemia and obesity in mice. J. Cell Mol. Med. 2019;23:2020–2031. doi: 10.1111/jcmm.14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hauck A.K., Huang Y., Hertzel A.V., Bernlohr D.A. Adipose oxidative stress and protein carbonylation. J. Biol. Chem. 2019;294:1083–1088. doi: 10.1074/jbc.R118.003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie W.D., Wang H., Zhang J.F., Li J.N., Can Y., Qing L., Kung H.F., Zhang Y.O. Enhanced peroxisomal beta-oxidation metabolism in visceral adipose tissues of high-fat diet-fed obesity-resistant C57BL/6 mice. Exp. Ther. Med. 2011;2:309–315. doi: 10.3892/etm.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berniakovich I., Trinei M., Stendardo M., Migliaccio E., Minucci S., Bernardi P., Pelicci P.G., Giorgio M. p66Shc-generated oxidative signal promotes fat accumulation. J. Biol. Chem. 2008;283:34283–34293. doi: 10.1074/jbc.M804362200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huh J.Y., Kim Y., Jeong J., Park J., Kim I., Huh K.H., Kim Y.S., Woo H.A., Rhee S.G., Lee K.J. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxidants Redox Signal. 2012;16:229–243. doi: 10.1089/ars.2010.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han Y.H., Buffolo M., Pires K.M., Pei S., Scherer P.E., Boudina S. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Diabetes. 2016;65:2639–2651. doi: 10.2337/db16-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lismont C., Nordgren M., Van Veldhoven P.P., Fransen M. Redox interplay between mitochondria and peroxisomes. Front. Cell Dev. Biol. 2015;3:35. doi: 10.3389/fcell.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salpietro V., Phadke R., Saggar A., Hargreaves I.P., Yates R., Fokoloros C., Mankad K., Hertecant J., Ruggieri M., McCormick D. Zellweger syndrome and secondary mitochondrial myopathy. Eur. J. Pediatr. 2015;174:557–563. doi: 10.1007/s00431-014-2431-2. [DOI] [PubMed] [Google Scholar]
- 96.Wicks S.E., Vandanmagsar B., Haynie K.R., Fuller S.E., Warfel J.D., Stephens J.M., Wang M., Han X., Zhang J., Noland R.C. Impaired mitochondrial fat oxidation induces adaptive remodeling of muscle metabolism. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E3300–E3309. doi: 10.1073/pnas.1418560112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ivashchenko O., Van Veldhoven P.P., Brees C., Ho Y.S., Terlecky S.R., Fransen M. Intraperoxisomal redox balance in mammalian cells: oxidative stress and interorganellar cross-talk. Mol. Biol. Cell. 2011;22:1440–1451. doi: 10.1091/mbc.E10-11-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang B., Van Veldhoven P.P., Brees C., Rubio N., Nordgren M., Apanasets O., Kunze M., Baes M., Agostinis P., Fransen M. Mitochondria are targets for peroxisome-derived oxidative stress in cultured mammalian cells. Free Radic. Biol. Med. 2013;65:882–894. doi: 10.1016/j.freeradbiomed.2013.08.173. [DOI] [PubMed] [Google Scholar]
- 99.Shai N., Yifrach E., van Roermund C.W.T., Cohen N., Bibi C., L I.J., Cavellini L., Meurisse J., Schuster R., Zada L. Systematic mapping of contact sites reveals tethers and a function for the peroxisome-mitochondria contact. Nat. Commun. 2018;9:1761. doi: 10.1038/s41467-018-03957-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Neuspiel M., Schauss A.C., Braschi E., Zunino R., Rippstein P., Rachubinski R.A., Andrade-Navarro M.A., McBride H.M. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr. Biol. 2008;18:102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]