Abstract
Moringa oleifera has potential anti-hyperglycaemic effects that have been reported earlier by different scientific groups using animal models of diabetes. We aimed to explore the possible mechanisms of action of M. oleifera extract through different methods. Primarily, we measured fasting blood glucose and performed glucose tolerance test, in Type 2 diabetic rats. Further, we studied the effects of extracts on pancreatic insulin concentration. Extracts’ effect on carbohydrate breakdown was assayed using α-amylase inhibition assays and assay of six different segments of gastrointestinal (GI) tracts. An in situ intestinal perfusion model and a glucose fibre assay were performed to see the potentiality of M. oleifera on glucose absorption. M. oleifera showed no significant change in insulin secretion in vivo. Additionally, substantial effect of the extract was seen on retarded glucose absorption and in the in situ perfusion study of rat intestinal model. α-amylase action was inhibited by the extract, yet again, these findings were further confirmed via the Six Segment assay, where sucrose digestion was found to be inhibited throughout the length of the GI tract. A combined in vitro, in vivo and in situ tests justified the potential of anti-hyperglycaemic activity of M. oleifera and its tissue level mechanism is also justified.
Keywords: glucose tolerance, ELISA, hypoglycemia, type 2 diabetes
Introduction
For the last few decades, diabetes has held its position as one of the world’s predominant endocrine disorder [1]. By nature, it cannot be completely cured but it should be kept under tight control. Usually modified lifestyle, medications, diet or a combination of all these are prescribed to diabetic patients to control diabetes. Complementary and alternative medicines (CAMs) have become popular among people from developing countries for managing a multitude of disorders including diabetes. However, unclear mechanism of action and lack of scientific evidence of efficacy of these therapies keeps them far behind from important use. Another drawback of these plants used in various ailments is that they have no established safety profile; although it is thought that they are safe besides being economical, effective and their easy availability. Traditional practitioners globally emphasize these advantages of medicinal plants in their day-to-day practice. Traditional use of medicines is recognized as a way to learn about potential future medicines. In 2001, researchers identified 122 compounds used in mainstream medicine that were derived from ‘ethnomedical’ plant sources; 80% of these compounds were used in the same or related manner as the traditional ethnomedical use [2].
Moringa oleifera is also known as Moringa pterygosperma Gaertn, is a member of the Moringaceae family of perennial angiosperm plants, which includes 12 other species. This plant is commonly named in English as Moringa and drumstick tree [3]. M. oleifera is one of the most useful tropical trees [4], it has the following characteristics: high protein, vitamins, minerals and carbohydrate content. This plant provides high value of nutrition for both humans and domestic animals; the seeds contain high oil content (42%), which is edible and has medicinal uses. Previous studies by Amaglo et al. [5], Limon-Pacheco and Gonsebatt [6] and Mahajan and Mehta [7] have reported different pharmacological potentialities including antioxidant and anti-inflammatory properties of M. oleifera. Moreover, Awodele et al. [8] have studied to evaluate toxicological property of the aqueous extract of M. oleifera Lam. (Moringaceae). Oyedepo et al. [9] evaluated the anti-hyperlipidaemic effect of aqueous leaves extract of M. oleifera while Gupta et al. [10] worked on the evaluation of anti-diabetic and antioxidant activity of M. oleifera in experimental diabetes model. Again, Jaiswal et al. [11] investigated the role of M. oleifera in regulation of diabetes-induced oxidative stress, while Choudhary et al. [12] assessed the anti-ulcer potential of M. oleifera root bark extract in rats. Although there have been several reports on the cholesterol and blood glucose reducing effect of different fractions of leaf extracts of M. oleifera in rats [13–17], there is still paucity of information on the hypoglycaemic activity of the extract at the doses investigated in the present study.
However, basic mechanism of action of M. oleifera still remains unclear till today as an anti-hyperglycaemic herb. The aim of the present study is to paint a comprehensive picture of effects of M. oleifera on sucrose breakdown and glucose absorption, insulin release and intestinal enzyme functions. Our study will help to identify the particular organ or organ system responsible for the previously seen hypoglycaemic activity of this plant.
Materials and methods
Plant collection and processing
M. oleifera leaves were collected from Jahangirnagar University, University Ayurvedic Research Centre (UARC), Dhaka, Bangladesh. The plant was identified by a taxonomist prior to further processing and a voucher specimen was deposited at the National Herbarium at Mirpur, Dhaka, Bangladesh with accession number DACB-12290. Then, the leaves were cleaned off of dirt and other debris and thoroughly washed under running tap water followed by air drying for granules preparation.
The solvent (ethanol 80%) was collected from organic chemistry laboratory of North South University, Dhaka. Approximately 400 g of ground powder was taken in flat-bottomed container, in which 2000 ml of solvent was added gradually with regular stirring. The container was sealed and left in the mechanical shaker for 6 days, on an average 2–3 h daily. The mixture was filtrated in stepwise processes. A clean, white cotton cloth was used which was double folded and the mixture was passed through it. It was the primary filtration where larger fibres were separated from the mixture. Then cotton was used later to filter the mixture to separate larger materials. Finally, filter paper was used for the separation of clear filtrate from the mixture. The amount of filtrate obtained was 150 ml. The filtrate (ethanol extract) obtained was evaporated by Rotary evaporator (Bibby RE-200, Sterilin Ltd., U.K.) at 5–6 rpm and at 57°C. It rendered a gummy concentrate of greenish colour that was designated as crude extract or ethanolic extract. This crude ethanolic extract was then dried by freeze drier and preserved at 4°C until further use.
Animal handling
Long Evan type normal healthy and Type 2 diabetic rats of both sexes were farmed in the animal house of the Department of Pharmaceutical Sciences, North South University, Dhaka and the weights of rats were approximately 180–220 g. All test animals were kept at an ambient temperature of 22 ± 5°C and humidity was maintained at 50–70%. Twelve hours day-night cycle was maintained to avoid fluctuations in the circadian rhythm and the rats were kept in translucent plastic cages with wood shavings provided as bedding; while grilled cages were replaced with bedding prior to fasted rat testing, to prevent corpophagy. Standard rat pellets and filtered drinking water were provided to the test animals ad libitum throughout the experiment apart from certain tests that required fasting and during that time only water was given to them. The experimental protocol was designed and subsequently approved by the Ethics Committee on Animal Research, North South University, following the ‘Revised guide for the care and use of laboratory animals by American Physiological Society’ [18].
Diabetes induction
Intraperitoneal streptozotocin (STZ) was given to normal and healthy rats to induce Type 2 diabetes in citrate buffer solution at a dose of 90 mg/kg. Newly born rats aged less than 48 h and weighing 7 g were chosen for this procedure. Three months later, fasting blood glucose levels of 8–12 mmol/l were selected for the experiments and an oral glucose tolerance test (OGTT) was performed for further confirmation of Type 2 diabetes [19].
Acute effects of ethanolic extracts of M. oleifera on glucose homoeostasis
M. oleifera extract was suspended in distilled water and orally administered to 12-h fasted rats and control group animals received only an equal volume of distilled water; to test blood glucose change in fasting condition. Effects on glucose tolerance were similarly evaluated by administration of M. oleifera extracts together with glucose (2.5 g/10 ml per kg body weight) after a fasting period of 12 h and control group received only glucose solution. In either cases, blood sample was collected from the tail vein of rats, serum separated by centrifugation and stored at –22°C until further analysis. Blood glucose was analysed by GOD-PAP method [20] (Glucose kit, Randox™, U.K.).
Effects of M. oleifera on plasma insulin
Blood was drawn out from Type 2 diabetic rats, 1 h after administration of M. oleifera. The amount of insulin released from the pancreas in vivo, was determined using Rat Insulin ELISA Kit (Crystal Chem™, U.S.A.).
Effects of M. oleifera on intestinal glucose absorption
An in situ intestinal perfusion technique [21] was used to determine the effect of M. oleifera intestinal absorption of glucose in 36-h fasted non-diabetic rats anaesthetized using Ketamine (80 mg/kg). Ethanol extract of M. oleifera (10 mg/ml equivalent to 0.5 g/kg was suspended in Krebs Ringer buffer, along with glucose (54 g/l). These were passed through rat pyloris via a butterfly cannula and the perfusate was collected by means of a tube inserted at the end of ileum. The control group was perfused with Krebs Ringer buffer along with glucose only. Perfusion was carried out at a rate of 0.5 ml/min for 30 min at 37°C. The results were presented as percentage of absorbed glucose, calculated from the percentage change in the amount of glucose in solution before and after the perfusion.
Effects of M. oleifera on sucrose absorption from the gut
The effect of M. oleifera on sucrose absorption from gastrointestinal tract (GIT) was assayed by determining the unabsorbed sucrose content following oral sucrose load by sucrose malabsorption study as described by Hannan et al. [22].Twelve hours fasted, Type 2 diabetic rats were administered 50% sucrose solution per oral (2.5 g/kg body mass) along with 500 mg/kg dose of M. oleifera and equal volume of water for control. Blood was sampled at the following time intervals: 30, 60, 120 and 240 min, after sucrose load for the quantification of blood glucose. At these time intervals, some of the rats were killed for determining unabsorbed sucrose contents of the GI tract. The GI tract was excised and separated into six segments: the stomach, the upper 20 cm, middle and lower 20 cm of the small intestine, the caecum and the large intestine. Each segment was rinsed with acidified, ice-cold, saline followed by centrifugation at 3000 rpm (1000 g) for 10 min. The supernatant was pipetted out and boiled for 2 h in sulfuric acid to hydrolyse the sucrose. The sulfuric acid was later neutralized by NaOH solution. Both plasma glucose concentration and the amount of glucose released from residual sucrose in the GI tract was determined. The GI sucrose content was calculated from the amount of liberated glucose [23].
Effects of M. oleifera on gut motility
GI motility was determined by means of BaSO4 milk following the previously described method of Chattarjee [24]. BaSO4 milk was prepared by mixing BaSO4 as 10% (w/v) in 0.5% CM-cellulose to form a suspension. The ethanol extract was administered orally, 1 h before the oral administration of BaSO4 milk. Control group was administered distilled water only (10 ml/kg). Rats belonging to all groups were killed 15 min after BaSO4 administration. The distance travelled by BaSO4 milk was measured and represented as a percentage of total length of the small intestine (from pylorus to ileocaecal junction).
Effect of M. oleifera on jejunal nutrient absorption by glucose dialysis tube retardation assay
Dry, precut dialysis sacs (inflated diameter approximately 16 mm, length =30 cm, Sigma–Aldrich™, U.S.A.) were soaked in 1 g sodium azide/l. The bag was loaded with 6 ml sodium azide (1 gm/l) and 36 mg glucose alone (the control sac) or after addition of fine powder of M. oleifera. The dry fibrous powder was wetted by an aqueous solution of sodium azide (1 g/l) for 14 h prior to the experiment. The sacs were closed at the ends and hung in a solution of 100 ml of sodium azide (1 g/l) and then placed in a stirred bath at 37°C for 1 h. At 30 and 60 min time interval, 2 ml of the dialysate was analysed for glucose by the GOD-PAP method as previously described.
The effect of fibre on nutrient absorption was indicated by the glucose dialysis retardation index (GDRI):
Effect of M. oleifera on α-amylase activity
The effects of M. oleifera powder on starch digestibility was determined as a function of time in a fibre-enzyme-starch mixture system using a dialysis membrane with a cut-off molecular weight of 12000 Da (inflated diameter approximately 16 mm, length =30 cm, Sigma–Aldrich™, U.S.A.) as previously described with minor modifications [25 ]. A solution was prepared by mixing 0.2 g powdered M. oleifera and 0.04 g α-amylase (obtained from human saliva, Sigma–Aldrich™, U.S.A.) in 10 ml of potato starch solution (4 g/100 ml) was dialysed in 200 ml deionised water at 37°C. Following the incubation period: 10, 30, 60, and 120 min, glucose concentration in the dialysate solution was assayed using the GOD-PAP method as described previously. The control was run without the addition of powder.
Determination of glucose-adsorption capacity
The assay was conducted following the procedure by Ou et al. [26], where the glucose-adsorption ability (mM/mol g–1) was measured by mixing 1 g of insoluble plant powder or CM-cellulose with 100 ml of glucose solution at a constant temperature of 37°C for 6 h. This was then followed by centrifugation at 3500 rpm for 15 min. Glucose concentration in the supernatant was assayed using GOD-PAP method as previously described.
Statistical analysis
Statistical tests were conducted using GraphPad Prism 6. Results are presented as means ± S.E.M. Experiments with data being collected at several time intervals, were analysed using repeated-measures ANOVA followed by Bonferroni adjustment ensuring an error margin within ≤5%. One-way ANOVA was carried out and pairwise comparisons were made with the control group using Dunnett’s test to maintain an acceptable error margin of 5%. A two-tailed P value of <0.05 was considered statistically significant.
Results
Acute effects of M. oleifera on glucose homoeostasis
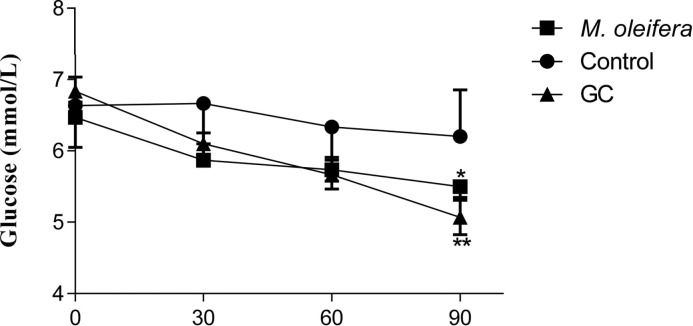
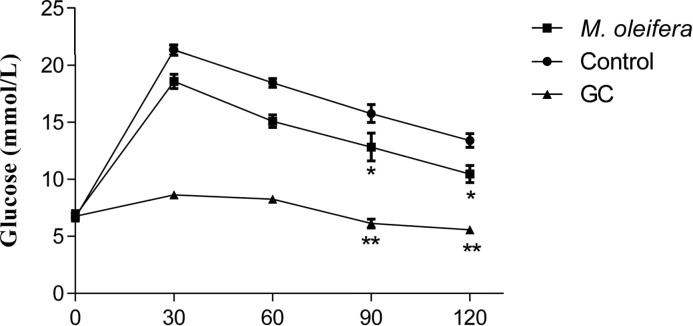
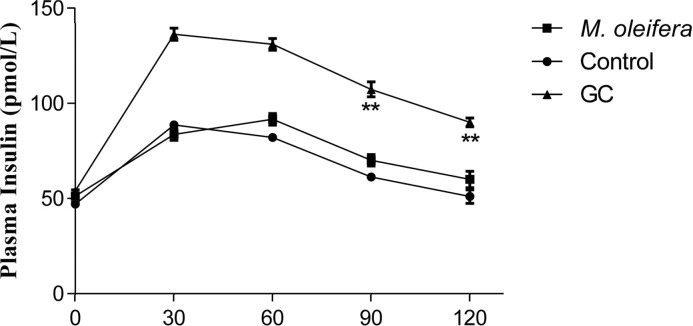
Oral administration of M. oleifera, at 500 mg/kg, altered the hyperglycaemic condition of fasted, Type 2 diabetic rats after 30 min (Figure 1). The extract also showed significant effect on glucose tolerance at 90 min and 120 min but there was no significant increase in plasma insulin level after an acute glucose insult (Figures 2 and 3).
Figure 1. Effects of ethanol extract of M. oleifera on fasting blood glucose level in Type 2 diabetic rats.
Values are means ± S.E.M represented by vertical bars (n=10). Fasted rats were given ethanol extract of M. oleifera (500 mg/kg) or Glibenclamide (GC) (0.5 mg/kg) or only water (control) by oral administration. Mean values marked with an asterisk (*) were significantly different from those of respective control rats (*P <0.05 and ,**P<0.001) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
Figure 2. Effects of ethanol extract of M. oleifera on glucose tolerance in Type 2 diabetic rats.
Values are means ± S.E.M represented by vertical bars (n=10). Fasted rats were given ethanol extract of M. oleifera (500 mg/kg body weight) or Glibenclamide (GC) (0.5 mg/kg) or only water (control) by oral administration with glucose (2.5 g/kg body weight). Mean values marked with an asterisk (*) were significantly different from those of respective control rats (*P <0.05, **P<0.001) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
Figure 3. Effects of ethanol extract of M. oleifera on plasma insulin level in Type 2 diabetic rats.
Values are means ± S.E.M represented by vertical bars (n=8). Rats were given ethanol extract of M. oleifera (500 mg/kg) or Glibenclamide (GC) (0.5 mg/kg) or only water (control) by oral administration. Mean values marked with an asterisk (*) were significantly different from those of respective control rats (**P <0.001) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
Effect of M. oleifera on serum glucose after sucrose load
M. oleifera showed a significant (P<0.05) suppression of serum glucose level after 60 min compared with control, where peak serum glucose was observed after administration of sucrose load and 500 mg/kg dose of M. oleifera maintained this trend of suppression of glucose level at 120 min too (Figure 4).
Figure 4. Effects of ethanol extract of M. oleifera on serum glucose after the sucrose load in Type 2 diabetic rats.
Rats were fasted for 20 h and administered orally with a sucrose solution (2.5 g/kg body weight) with or without ethanol extract of M. oleifera (500 mg/kg body weight) or Glibenclamide (GC) (0.5 mg/kg) or only water (control). Means ± S.E.M. represented by vertical bars (n=8). Mean values marked with an asterisk (*) were significantly different from those of respective control rats (*P <0.05, **P<0.001) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
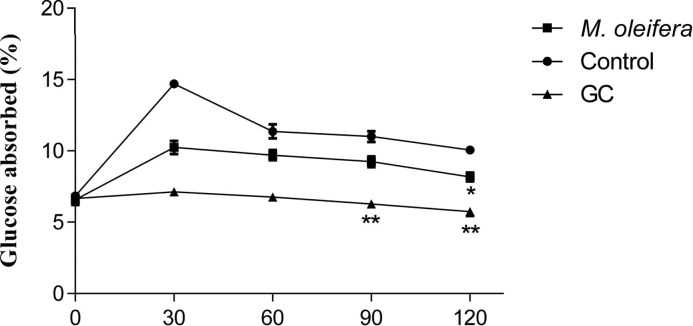
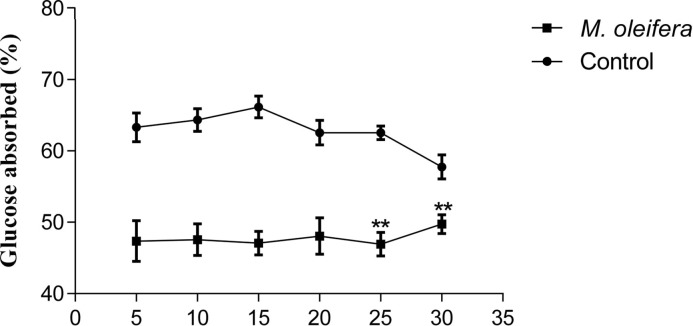
Effect of M. oleifera on intestinal glucose absorption
Five hundred milligrams per kilogram doses of M. oleifera extract, when perfusated with glucose, showed significant (P<0.05) reduction in the percentage of glucose absorption during most of the perfusion period (Figure 5).
Figure 5. Effects of ethanol extract of M. oleifera on intestinal glucose absorption in Type 2 diabetic rats.
Rats were fasted for 36 h and the intestine was perfused with glucose (54 g/l) with (treated group) or without (control group) ethanol extract of M. oleifera (10 mg/ml each subject received 15 ml of perfusion). Values are means ± S.E.M represented by vertical bars (n=8). Mean values marked with an asterisk (*) were significantly different from those of respective control rats (**P <0.001) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
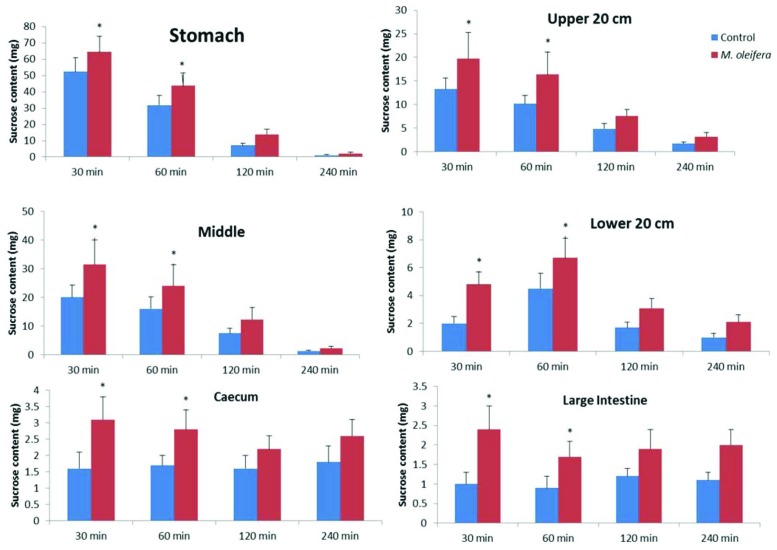
Effect of M. oleifera on unabsorbed sucrose content in the GI tract
Upon oral administration of sucrose along with M. oleifera (500 mg/kg), significant amount of unabsorbed sucrose remained in the stomach, upper, middle and lower intestines at 30 min and 60 min. This amount of residual sucrose remained significant in caecum and large intestine till 4 h (P<0.05; Figure 6).
Figure 6. Effects of ethanol extract of M. oleifera on GI tract sucrose content after oral sucrose loading in Type 2 diabetic rats.
Rats were fasted for 20 h before the oral administration of a sucrose solution (2.5 g/kg body weight) with (treated group) or without (control group) ethanol extract of M. oleifera (500 mg/kg body weight). Values are means ± S.E.M represented by vertical bars (n= 8). Mean values marked with an asterisk (*) were significantly different from those of respective control rats (*P<0.05) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
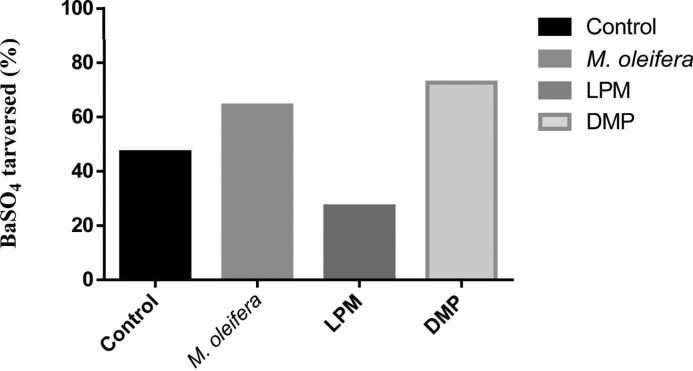
Effect of M. oleifera on gut motility
M. oleifera extract increased the GI motility significantly (P<0.05) at 500 mg/kg dose in Figure 7.
Figure 7. Effects of ethanol extract of M. oleifera on GI tract motility (by BaSO4 traversed).
Rats were fasted for 20 h before the oral administration of ethanol extract of M. oleifera or water (control). BaSO4 was administered at 60 min. Motility was measured over the following 15 min. Loperamide (LPM) (5 mg/kg) and domperidone (DMP) (10 mg/kg) were used as reference controls for GI motility test (n=12). Mean values and significance (P<0.05) were derived from repeated-measures ANOVA and adjusted using Bonferroni correction.
Effect of M. oleifera powder on in vitro GDRI
M. oleifera powder reduced the amount of glucose present in the dialysate. GDRI was 36.97% and 46.20% at 30 and 60 min respectively (P<0.05; Table 1).
Table 1. Retarding effect of insoluble fibre of M. oleifera on the glucose movement (GDRI).
| Treatment | Dialysis for 30 min | Dialysis for 60 min | ||
|---|---|---|---|---|
| Glucose in dialysate (mmol/l) | GDRI (%) | Glucose in dialysate (mmol/l) | GDRI (%) | |
| M. oleifera | 0.75 ± 0.18* | 36.97 | 0.92 ± 0.12* | 46.20 |
| CM-cellulose 1000 mg | 0.62 ± 0.11* | 42.86 | 0.83 ± 0.07* | 51.46 |
| Control | 1.19 ± 0.21 | 0 | 1.71 ± 0.14 | 0 |
Data are presented as means ± SD (n=3). Glucose dialysis retardation index = control (100%) – fiber (% of control value). Mean values marked with an asterisk (*) were significantly different from those of respective control groups (*P<0.05) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
Data are presented as means ± S.E.M (n=4). GDRI = control (100%) – fibre (% of control value). Mean values marked with an asterisk (*) were significantly different from those of respective control groups (*P<0.05) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
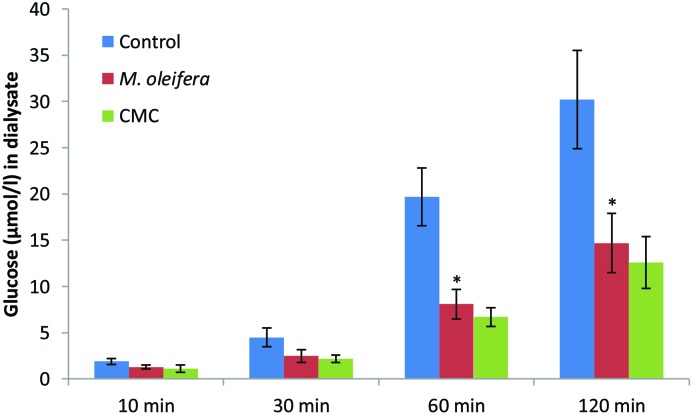
Effect of M. oleifera powder on α-amylase activity
The effect of M. oleifera powder on starch digestibility was determined by the alteration in the glucose concentration in the dialysate with time. There was significant change, compared with control, in the glucose content at 60 min and 120 min (P<0.05; Figure 8).
Figure 8. Effect of M. oleifera powder on α-amylase activity.
Data are presented as means ± S.E.M ( n= 4). Values are represent the glucose concentration (μmol) in dialysate. Mean values marked with an asterisk (*) were significantly different from those of respective control rats (*P<0.05) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
Effect of M. oleifera powder on in vitro glucose-adsorption capacity
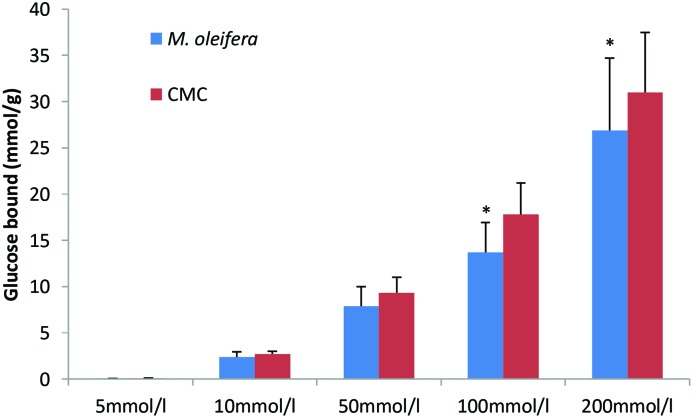
M. oleifera powder depicted the capacity of glucose adsorption in the presence of different levels of glucose in the solution. This activity of glucose adsorption was found to continue from higher level of glucose to low level of glucose present in the solution (Figure 9).
Figure 9. Effect of M. oleifera powder on in vitro glucose-adsorption capacity.
Data are presented as means ± S.E.M (n= 4). Values are represent the millimoles of glucose bound by each gram of M. oleifera powder at different glucose concentration (5–200 mmol/l). Glucose bound = (glucose concentration of original solution - concentration when the adsorption reached equilibrium) × volume of solution ÷ weight of dietary fiber. Mean values marked with an asterisk (*) were significantly different from those of respective control rats (*P<0.05) (derived from repeated-measures ANOVA and adjusted using Bonferroni correction).
Discussion
Streptozotocin used in the present study to induce Type 2 diabetes in adult animals that causes DNA damage and generates superoxide radicals to destroy the β cells [26]. We have used Glibenclamide (GC) in this experiment as a reference drug, which is a synthetic hypoglycaemic agent and has been used as an anti-diabetic drug in Type 2 diabetic patients since 1973 and is still being used [27]. This drug acts by stimulation of insulin release [28]. Only one oral dose of 500 mg/kg BW of M. oleifera ethanolic leaf extract was administered for evaluation of anti-hyperglycaemic properties by increasing blood glucose tolerance in the normal rat, which was less potent than those of STZ-induced diabetic rat. M. oleifera leaves contain many powerful antioxidant phytochemicals, especially quercetin and kaempferol [29]. There are many reports about hypoglycaemic activities of kaempferol derivatives from many medicinal plants such as Sterculia rupestris [30] and Equisetum myriochaetum [31]. Furthermore, they also improved chronic hyperglycaemia impaired pancreatic β cells viability and insulin secretion in vitro [32]. Quercetin, a strong antioxidant flavonoid revealed a protective effect against STZ-induced diabetes in rats by intraperitoneal injection of quercetin 15 mg/kg BW for 3 days prior to STZ administration [33] and protected an insulin secreting cell line (INS-1) against oxidative damage [34,35]. It also exhibited hypoglycaemic properties in diabetic rats [36]. Vessal et al. [37] reported that quercetin significantly increased hepatic glucokinase activities as an insulin-like effect.
Hyperglycaemia causes cellular damage that hinders homoeostasis of internal glucose concentration, which results in acutely altered cellular metabolism and long-term changes in cellular macromolecular content [38–40]. Postprandial glucose spike causes perturbation in endothelial cell function, [41,42] and increases the risk of blood coagulation [42]. Hyperglycaemic states also increase products of glycosylation, which has a significant influence on the development of diabetes-induced vascular disease [43]. Therefore, management of hyperglycaemic states is an important method of diabetes control. There are some basic pathways used in anti-diabetic drugs that include enhanced insulin secretion, enhanced sensitivity to insulin, improved peripheral glucose utilization, inhibition of glucose absorption and inhibition of carbohydrate digestion [44]. M. oleifera leaves have shown promising glucose-lowering effect in the present study with chemically induced hyperglycaemic rats. However, there are no reports that have been issued to reveal the tissue level mechanism of action of M. oleifera. In our present study, we have employed different techniques, which suggest one or more of the aforementioned modes of action.
Different studies have proved that blood glucose level in the upper normal range is a probable risk factor for cardiovascular disease, a common condition in case of chronic Type 2 diabetic patients [45]. In our study, fasting blood glucose in Type 2 diabetic rats in treatment group was changed significantly (p<0.05) and it was highly significant when compared to standard (Glibenclamide). In glucose tolerance test, the peak glucose concentration after glucose induction whereas M. oleifera treated group (500 mg/kg) significantly (p<0.05) declined the glucose absorption after 60 min compared to control group.
To further ascertain this, we measured the plasma insulin level of the test animals and found no significant increase in insulin secretion on M. oleifera administration. Therefore, increased insulin secretion from pancreatic β cell can be ruled out as possible mechanism of action.
An in situ intestinal perfusion of the GI tract shows marked reduction in glucose absorption. In BaSO4 GI motility assay, intestinal motility was found to be significantly higher. Six Segment test showed significantly higher amount of unabsorbed sucrose in stomach, upper, middle and lower intestines in M. oleifera-administered groups. The last three parts of GI tract are most important for absorption of nutrients including sugars [46]. Highly unabsorbed sucrose content in the GI tract indicates that sucrose digestion has been reduced. Thus, a significantly higher concentration of sucrose has reached to the large intestine and caecum, which eventually remains unabsorbed and egested with faeces. An in vitro study was conducted to evaluate fibre-binding capacity of the crude extract, no substantial effect was found that clearly demonstrates no dietary fibres are available in M. oleifera. Glucose is carried by specific transporter proteins [47]. Bound glucose is probably incapable of fitting the active site of these transporter proteins. This finding validates our initial findings in the gut perfusion experiments, which too showed no hindrance in glucose absorption. This is now fully understood that there is no glucose-fibre binding capacity of the crude extract.
M. oleifera in the α-amylase activity assay showed promising decrease in the catabolism of starch. As complex carbohydrates and disaccharides need to be broken down into simpler monosaccharides [48] prior to absorption, any inhibition of this catabolic process would retard sugar absorption, which in turn lowers glycaemic peak. However, the precise mechanism of this inhibitory action remains to be studied.
Conclusions
The present study showed that ethanolic leaves extract of M. oleifera possessed hypoglycaemic and anti-hyperglycaemic properties in chemically induced Type 2 diabetic rats, which suggest the presence of biologically active components that may be worth further investigation and elucidation. Studies confirm the previous claims regarding anti-hyperglycaemic action of M. oleifera. Additionally, we have elucidated that M. oleifera is capable of inhibiting absorption of glucose by inhibition of α-amylase. Therefore, its traditional use, as mentioned above, is justified and a primary mechanism of action is now known that was unrevealed until the present study.
Acknowledgments
We thank the staff members of Pharmacology Lab, Department of Pharmaceutical Sciences, North South University for allowing us to continue our project in their laboratory and providing us with their utmost research facility.
Abbreviations
- ACB
acarbose
- GC
glibenclamide
- GDRI
glucose dialysis retardation index
- GI
gastrointestinal
- STZ
streptozotocin
- BW
body weight
- GOD-PAP
enzymatic colorimetric method for glucose determination
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author contribution
P.A. and J.M.A.H. designed the whole project. S.B.A., S.M.H. and M.I.B.S. were involved in the collection and identification of the plant material. S.A. and M.H. collected and arranged all the chemicals used in this project. P.A., S.B.A. and M.I.B.S. executed the designed project, while S.M.H assisted them in data collection, processing and interpretation. P.A. and S.A. wrote the manuscript. All the authors scrutinized the paper and approved it.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.World Health Organization (2003) Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. WHO Technical Report Series 916, WHO, Geneva: [PubMed] [Google Scholar]
- 2.Mishra D. (2014) In vitro rapid clonal propagation of an important medicinal plant of herbal origin Ocimum americanum for field plantation. Online Int. Interdis. Res. J. 4, 192–196 [Google Scholar]
- 3.Verzosa B.R. and Caryssa L.H. (2012) Malunggay and spinach powder (investigatory project sample). Scribd.com.http://www.scribd.com/doc/29164852/Malunggay-and-Spinach-Powder-Investigatory-ProjectSample [Google Scholar]
- 4.Foidl N., Makkar H.P.S. and Becker K. (2001) The potential of Moringa oleifera for agricultural and industrial uses. In The Miracle Tree: The Multiple Attributes of Moringa (Fuglie L.J., ed.), CTA, U.S.A. [Google Scholar]
- 5.Amaglo N.K., Bennett R.N., Lo Curto R.B., Rosa E.A.S., Lo Turco V., Giuffrid A. et al. (2010) Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 122, 1047–1054 [Google Scholar]
- 6.Limon-Pacheco J. and Gonsebatt M.E. (2009) The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. 674, 137–147 [DOI] [PubMed] [Google Scholar]
- 7.Mahajan S.G. and Mehta A.A. (2009) Curative effect of hydroalcoholic extract of leaves of Moringa oleifera lam. against adjuvant induced established arthritis in rats. Niger. J. Nat. Prod. Med. 13, 13–22 [Google Scholar]
- 8.Awodele O., Oreagba I.A., Odoma S., da Silva J.A.T. and Osunkalu V.O. (2012) Toxicological evaluation of the aqueous extract of Moringa oleifera Lam. (Moringaceae). J. Ethnopharmacol. 139, 330–336 [DOI] [PubMed] [Google Scholar]
- 9.Oyedepo T.A., Babarinde S.O. and Ajayeoba T.A. (2013) Evaluation of anti-hyperlipidemic effect of aqueous leaves extract of Moringa oleifera in alloxan induced diabetic rats. Int. J. Biochem. Res. Rev. 3, 162–170 [Google Scholar]
- 10.Gupta R., Mathur M., Bajaj V.K., Katariya P., Yadav S., Kamal R. et al. (2012) Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J. Diabetes 4, 164–171 [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal D., Rai P.K., Mehta S., Chatterji S., Shukla S., Rai D.K. et al. (2013) Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac. J. Trop. Med. 6, 426–432 [DOI] [PubMed] [Google Scholar]
- 12.Choudhary M.K., Bodakhe S.K. and Gupta S.K. (2013) Assessment of the antiulcer potential of Moringa oleifera root-bark extract in rats. J. Acupunct. Meridian Stud. 6, 214–220 [DOI] [PubMed] [Google Scholar]
- 13.Ara N., Rashid M. and Amran M.S. (2008) Comparison of Moringa oleifera leaves extract with atenolol on serum triglyceride, serum cholesterol, blood glucose, heart weight, body weight in adrenaline induced rats. Saudi J. Biol. Sci. 15, 253–258 [Google Scholar]
- 14.Atsukwei D., Eze D.E., Adams M.D., Adinoyi S.S. and Ukpabi C.N. (2014) Hypolipidaemic effect of ethanol leaf extract of Moringa oleifera Lam. in experimentally induced hypercholesterolemic Wistar rats. Int. J. Nutr. Food Sci. 3, 355–360 [Google Scholar]
- 15.Fombang E.N. and Saa R.W. (2016) Antihyperglycemic activity of Moringa oleifera Lam leaf functional tea in rat models and human subjects. Food Nutr. Sci. 7, 1021–1032 [Google Scholar]
- 16.Luka C.D., Tijjani H., Joel E.B., Ezejiofor U.L. and Onwukike P. (2013) Hypoglycaemic properties of aqueous extracts of Anacardium occidentale, Moringa oleifera, Vernonia amygdalina and Helianthus annuus: a comparative study on some biochemical parameters in diabetic rats. Int. J. Pharm. Sci. Invent. 2, 16–22 [Google Scholar]
- 17.Luangpiom A., Kourjampa W. and Junaimaung T. (2013) Anti-hyperglycemic properties of Moringa oleifera Lam. aqueous leaf extract in normal and mildly diabetic mice. Br. J. Pharmacol. Toxicol. 4, 106–109 [Google Scholar]
- 18.Bayne K. (1996) Revised guide for the care and use of laboratory animals available. American Physiological Society. Physiologist 39, 208–211 [PubMed] [Google Scholar]
- 19.Lenzen S. (2008) The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 51, 216–226 [DOI] [PubMed] [Google Scholar]
- 20.Trinder P. (1969) Enzymatic colorimetric method for estimation of glucose test (GOD-PAP method), uric acid and phospholipids. Ann. Clin. Biochem. 6, 25 [Google Scholar]
- 21.Moskalewski S. (1969) Studies on the culture and transplantation of isolated islets of langerhans of the guinea pig. Proc. Kon. Ned. Akad. Wetensch., C 72, 157. [PubMed] [Google Scholar]
- 22.Hannan J.M.A., Ali L., Rokeya B., Khaleque J., Akhter M., Flatt P. et al. (2007) Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 97, 514–521 [DOI] [PubMed] [Google Scholar]
- 23.Swintosky J. and Pogonowska-Wala E. (1982) The in situ rat gut technique. Pharmacy Int. 3, 163–167 [Google Scholar]
- 24.Chatterjee T. (1993) Handbook of Laboratory Mice and Rats, pp. 133–139, Jadavpur University, India [Google Scholar]
- 25.Goto Y., Yamada K., Ohyama T., Matsuo T., Odaka H. and Ikeda H. (1995) An α-glucosidase inhibitor, AO-128, retards carbohydrate absorption in rats and humans. Diabetes Res. Clin. Pract. 28, 81–87 [DOI] [PubMed] [Google Scholar]
- 26.Ou S., Kwok K-c, Li Y. and Fu L. (2001) In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 49, 1026–1029 [DOI] [PubMed] [Google Scholar]
- 27.Szkudelski T. (2001) The mechanism of alloxan and streptozotocin action on beta cell of the rat pancreas. Physiol. Res. 50, 537–546 [PubMed] [Google Scholar]
- 28.World Health Organization (WHO) (2007) WHO Model List of Essential Medicines, 15th edn. p. 21, World Health Organization [Google Scholar]
- 29.Serranto-Martin X., Payares G. and Mendoza-Leon A. (2006) Glibenclamide, a blocker of K+ATP channels, shows antileishmannial activity in experimental murine cutaneous leishmaniasis. Antimicrob. Agents Chemother. 50, 14–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuglie L.J. (1999) The Miracle Tree: Moringa oleifera Natural Nutrition for the Tropics, p. 63, Church World Service, New York [Google Scholar]
- 31.Desoky E.K. and Youssef S.A. (1997) Hypoglycemic effect of Sterculia rupestris and a comparative study of its flavonoids with Sterculia diversifolia. Bull. Faculty Pharm. Cairo Univ. 35, 257–261 [Google Scholar]
- 32.Andrade-Cetto A.A., Wiedenfeld H., Revilla M.C. and Sergio I.A. (2000) Hypoglycemic effect of Equisetum myriochaetum aerial parts on streptozotocin diabetic rats. J. Ethnopharmacol. 72, 129–133 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y. and Liu D. (2001) Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 670, 325–332 [DOI] [PubMed] [Google Scholar]
- 34.Coskun O., Kanter M., Kormaz A. and Oter S. (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol. Res. 51, 117–123 [DOI] [PubMed] [Google Scholar]
- 35.Youl E., Bardy G., Magous R., Cros G., Sejalon F., Virsolvy A., Richard S., Quignard J.F., Gross R., Petit P., Bataille D. and Oiry C. (2010) Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br. J. Pharmacol. 161, 799–814 10.1111/j.1476-5381.2010.00910.x 20860660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shetty A.K., Rashmi R., Rajan M.G.R., Sambaiah K. and Alimath P.V. (2004) Antidiabetic influence of quercetin in streptozotocin-induced diabetic rats. Nutr. Res. 24, 373–381 [Google Scholar]
- 37.Vessal M., Hemmati M. and Vasei M. (2003) Antidiabetic effects of quercetin in streptozocin induced diabetic rats. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 135C, 357–364 [DOI] [PubMed] [Google Scholar]
- 38.UK Prospective Diabetes Study (UKPDS) Group, (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 [PubMed] [Google Scholar]
- 39.Heilig C.W., Concepcion L.A., Riser B.L, Freytag S.O, Zhu M. and Cortes P. (1995) Overexpression of glucose transporters in rat mesangial cells cultured in a normal glucose milieu mimics the diabetic phenotype. J. Clin. Invest. 96, 1802–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiser N., Sasson S., Feener E.P., Boukobza-Vardi N., Higashi S., Moller D.E. et al. (1993) Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 42, 80–89 [DOI] [PubMed] [Google Scholar]
- 41.Haller H. (1997) Postprandial glucose and vascular disease. Diabet. Med. 14, S50–S56 [DOI] [PubMed] [Google Scholar]
- 42.Koya D. and King G.L. (1998) Protein kinase C activation and the development of diabetic complications. Diabetes 47, 859–866 [DOI] [PubMed] [Google Scholar]
- 43.Ceriello A., Taboga C., Tonutti L., Giacomello R., Stel L., Motz E. et al. (1996) Postmeal coagulation activation in diabetes mellitus: the effect of acarbose. Diabetologia 39, 469–473 [DOI] [PubMed] [Google Scholar]
- 44.Thornalley P.J. (1996) Advanced glycation and the development of diabetic complications. Unifying the involvement of glucose, methylglyoxal and oxidative stress. Endocrinol. Metab. London 3, 149–166 [Google Scholar]
- 45.UK Prospective Diabetes Study Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317, 703–713 [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson P.W., Cupples L.A. and Kannel W.B. (1991) Is hyperglycemia associated with cardiovascular disease? The Framingham Study. Am. Heart. J. 121, 586–590 [DOI] [PubMed] [Google Scholar]
- 47.Crane R.K. (1975) The physiology of the intestinal absorption of sugars. In Physiological Effects of Food Carbohydrates vol. 15, (Jeanes A. and Hodge J., eds.), pp. 2–19, American Chemical Society, U.S.A. [Google Scholar]
- 48.Reynell P. and Spray G. (1956) The simultaneous measurement of absorption and transit in the gastro-intestinal tract of the rat. J. Physiol. 131, 452–462 [DOI] [PMC free article] [PubMed] [Google Scholar]