Abstract
Background
It is well known that the incidence of developing hepatocelluler carcinoma (HCC) is increased in liver cirrhosis of different etiologies. However, comparison of HCC incidence in various liver diseases has not yet been estimated. We surveyed this comparison.
Methods
The PubMed database was examined (1989‐2017) for studies published in English language regarding the prospective follow‐up results for the development of HCC in various liver diseases. A meta‐analysis was performed for each liver disease.
Results
The annual incidence (%) of HCC in the non‐cirrhotic stage and cirrhotic stage, and the ratio of HCC incidence in the cirrhotic stage/non‐cirrhotic stage were as follows. (a) hepatitis B virus liver disease: 0.37%→3.23% (8.73‐fold), (b) hepatitis C virus liver diseases: 0.68%→4.81% (7.07‐fold), (c) primary biliary cholangitis (0.26%→1.79%, 6.88‐fold), (d) autoimmune hepatitis (0.19%→0.53%, 2.79‐fold), and (e) NASH (0.03%→1.35%, 45.00‐fold). Regarding primary hemochromatosis and alcoholic liver diseases, only follow‐up studies in the cirrhotic stage were presented, 1.20% and 2.06%, respectively.
Conclusions
When the liver diseases advance to cirrhosis, the incidence of HCC is markedly increased. The development of HCC must be closely monitored by ultrasonography, magnetic resonance imaging, and computed tomography, irrespective of the different kinds of liver diseases.
Keywords: hepatocellular carcinoma, liver cirrhosis, liver diseases, meta‐analysis, risk of HCC
1. INTRODUCTION
It is well known that cirrhosis is the most potent risk factor for the development of hepatocellular carcinoma (HCC), irrespective of the etiology of liver disease. However, precise comparison of the incidence of HCC in various liver diseases especially in liver cirrhosis (LC) has not yet been elucidated. Moreover, the degree of increase in cirrhotic state in various liver diseases has not also yet been estimated. In this study, we compared the incidence of HCC in LC in various liver diseases by meta‐analysis, and also surveyed how the incidence of developing HCC is increasing in the cirrhotic state as compared with that in the non‐cirrhotic state in various liver diseases. Furthermore, we discuss the possible mechanisms of HCC development in various liver diseases.
2. MATERIAL AND METHOD
2.1. Search strategy
The PubMed database was searched (1989‐2017) for studies published in English regarding the follow‐up results for the development of HCC in various liver diseases to perform meta‐analyses. Only prospective studies for the development of HCC were used, and retrospective studies were omitted. In this search, review articles were omitted. Among studies on hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, those associated with therapeutic intervention were excluded, and studies that followed the natural course were included. Among reports on primary biliary cholangitis, the major pathological classification was Sheuer's Ⅰ/Ⅱ and Ⅲ (pre‐cirrhosis)/Ⅳ (cirrhosis) in almost all papers demonstrated. Thus, the ratio of HCC incidence represents Sheuer's Ⅲ/Ⅳ compared with Ⅰ/Ⅱ.
2.2. Statistical analysis
For the seven diseases, we calculated the weighted mean of the HCC incidence rate for LC and non‐LC using the random effects model (ref: Dersimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials. 1986; 7:177‐188). To assess whether the incidence rate among LC patients was higher than that among non‐LC patients, we calculated the incidence rate ratio and P‐value using the Z‐test. All reported P‐values correspond to two‐sided tests, and those <0.05 were considered significant. Analyses were performed with JMP version 12 (SAS Institute, Cary, NC).
3. RESULTS
3.1. Incidence of HCC according to the etiology of cirrhosis
3.1.1. Incidence of HCC in HBV infection
The annual incidence (%) of HCC in the non‐cirrhotic state was 0.37% and that in the cirrhotic state was 3.23% (LC vs non‐LC P < 0.001).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 The ratio of HCC incidence for the cirrhotic state/non‐cirrhotic state was 8.73‐fold. In this group, cases with interventional therapy were excluded. The majority of reports describing the follow‐up results of non‐cirrhotic HB patients included follow‐up results of patients with chronic hepatitis B, and not inactive HBV healthy carriers (Figure 1).
Figure 1.

Incidence of hepatocelluler carcinoma in hepatitis B virus infection
3.1.2. Incidence of HCC in HCV infection
The annual incidence (%) of HCC in the non‐cirrhotic state was 0.68% and that in the cirrhotic state was 4.81% (LC vs non‐LC P < 0.001).26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 The ratio of HCC incidence for cirrhotic state/non‐cirrhotic state was 7.07‐fold. In this group, patients with interventional therapy were excluded. The majority of the non‐cirrhotic patients had chronic hepatitis C (Figure 2).
Figure 2.

Incidence of hepatocelluler carcinoma in hepatitis C virus infection
3.1.3. Incidence of HCC in primary biliary cholangitis (PBC)
The annual incidence (%) of HCC in the non‐cirrhotic state (Scheuer's stage Ⅰ~Ⅱ) was 0.26% and that in the pre‐cirrhotic ~cirrhotic state (Scheuer's stage Ⅲ~Ⅳ) was 1.79% (LC vs non‐LC P < 0.001).54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74 The ratio of HCC incidence for stage Ⅲ~Ⅳ/stageⅠ~Ⅱ was 6.88‐fold. In the majority of patients with PBC, the background of the liver was in cirrhotic state when HCC developed. The majority of patients with PBC was treated with ursodeoxycholic acid in both cirrhotic and non‐cirrhotic patients (Figure 3).
Figure 3.

Incidence of hepatocelluler carcinoma in primary biliary cholangitis
3.1.4. Incidence of HCC in autoimmune hepatitis
The annual incidence (%) of HCC in the non‐cirrhotic state was 0.19% and that in the cirrhotic state was 0.53% (LC vs non‐LC P = 0.030).75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90 The ratio of HCC incidence for the cirrhotic state/non‐cirrhotic state was 2.79‐fold (Figure 4).
Figure 4.

Incidence of hepatocelluler carcinoma in autoimmune hepatitis
3.1.5. Incidence of HCC in nonalcoholic steatohepatitis (NASH)
The annual incidence (%) of HCC in the non‐cirrhotic state was 0.03% and that in the cirrhotic state was 1.35% (LC vs non‐LC P < 0.001).91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101 The ratio of HCC incidence for the cirrhotic state/non‐cirrhotic state was 45.00‐fold. Of the NASH patients, 52% had diabetes mellitus and 81% were obese (Figure 5).
Figure 5.

Incidence of hepatocelluler carcinoma in nonalcoholic steatohepatitis
3.1.6. Incidence of HCC in genetic hemochromatosis
Only follow‐up studies for the cirrhotic state were reported and the incidence was 1.20%/year (Figure 6).102, 103, 104, 105, 106, 107, 108, 109
Figure 6.

Incidence of hepatocelluler carcinoma in primary hemochromatosis
3.1.7. Incidence of HCC in alcoholic liver disease
Only follow‐up studies for alcohol‐related cirrhosis were reported and the incidence was 2.06% (Figure 7).110, 111, 112, 113, 114, 115, 116, 117, 118
Figure 7.
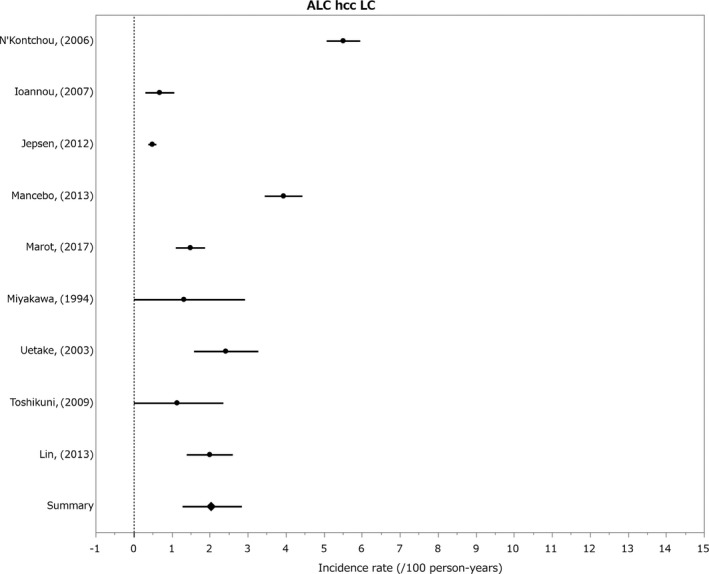
Incidence of hepatocelluler carcinoma in alcoholic liver diseases
The above results are summarized in the Table 1.
Table 1.
Incidence of HCC (%/year) and rate ratio (LC/non LC) in liver diseases
| Incidence rate (%/year) | Rate/ratio (LC/non LC) | ||
|---|---|---|---|
| LC | Non LC | ||
| HBV infection | 3.23 | 0.37 | 8.73 |
| HCV infection | 4.81 | 0.68 | 7.07 |
| PBC | 1.79 | 0.26 | 6.88 |
| AIH | 0.53 | 0.19 | 2.79 |
| NASH | 1.35 | 0.03 | 45.00 |
| Primary hemochoromatosis | 1.20 | — | — |
| Alcholic liver diseases | 2.06 | — | — |
HCC, hepatocelluler carcinoma; LC, liver cirrhosis.
4. DISCUSSION
It is known that cirrhosis is present in 80~90% of HCC patients with any underlying liver disease,119 and it is the most important risk factor for HCC. However, comparison of the incidence of HCC in various liver diseases was not accurately and precisely evaluated in previous studies. In this study, we found that the incidence of HCC is highest in HCV LC (4.81%/year) and second highest in HBV LC (3.23%), followed by alcoholic LC (2.06%), PBC LC (1.79%), NASH LC (1.35%), primary hemochromatosis (1.20%), and AIH (0.53%).
The incidence of HCC has been wildly studied in patients with HBV LC and HCV LC, and was reported to be 3% and 5%,29, 120 which was almost the same as that in our study.
In this study, we also demonstrated that the incidence of HCC is markedly increased (2.79‐ to 45.00‐fold) in the cirrhotic state compared with non‐cirrhotic state, irrespective of the etiology of liver disease. Why this increase in HCC development occurs in the cirrhotic state must be considered.
First, chronic inflammation may be a key mechanism for HCC development in the cirrhotic state. In this respect, we made clinical observation in the HCV LC patients (Child A) in the past. The LC patients were divided into three groups: Group A: 28 patients whose annual average serum alanine aminotransferase (ALT) level was persistently high (≧80 IU; high‐ALT group); Group B: 28 patients whose annual average serum ALT levels was persistently low (<80 IU; low‐ALT group), and Group C: 13 unclassified patients. The patients had been followed up prospectively. The 5‐year incidence rate of HCC in the high‐ALT group was as high as 53.6% compared with only 7.1% in the low‐ALT group (P < 0.001).120 Thus, this clinical observation demonstrated the importance of chronic inflammation in the development of HCC in HCV LC.
The same tendency was demonstrated in HBV LC. Chen et al121 reported that high serum levels of HBV DNA and ALT were independent risk factors for HCC development in HBV infection. Ishikawa et al20 also reported that serum aminotransferase are one of predictive factor for the development of HCC. One important mechanism for high incidence of HCC in HCV LC and HBV LC as compared with the incidence of HCC in LC caused by other etiologies may be the sustained high‐grade inflammation.
Furthermore, in autoimmune hepatitis, persistent elevation of serum aminotransaminase was reported to lead to the development of HCC.78 This suggests a role for inflammation in the development of HCC, but this hypothesis has not been confirmed solidly.
Second, increases in DNA synthesis in the hepatocytes of cirrhotic patients is suspected as a possible mechanism of HCC development. In our previous study, we demonstrated that in the high DNA synthetic group [BrdU (thymidine analog) labeling index ≧1.5%] 64.3% of patients developed HCC in 5 years, in contrast to 14.3% in the low DNA synthesis (Brd U LI <1.5%) group in HCV LC patients (P < 0.05).122 We further demonstrated that in HCV‐associated cirrhosis patients who showed nodular formation on ultrasound, the cell cycle of hepatocytes was markedly accelerated, as estimated by the bromodeoxyuridine (thymidine analog) uptake into hepatocytes in vitro, and the incidence of HCC was greatly increased.123
Third, accumulated genomic change was also important factor for HCC development. In this respect, Hiatt et al124 reported that C282Y mutation itself may increase the risk of HCC development in hereditary hemochromatosis. In addition, in alcoholic LC, the genetic effect of long‐term alcoholic intake may influence the development of HCC.125
Fourth, obesity, and diabetes are suspected to increase the incidence of developing HCC. In a large epidemiologic study, patients with BMI >35 had an increased risk of cancer, especially HCC, with hazard ratio (HR) of 4.52.126 In addition, diabetes is associated with HCC occurrence, with a HR of 2.39 in a US cohort.127 It is generally accepted that NASH is associated with obesity and diabetes in high percentages.
Notably, in 2014, Flemming et al128 predicted the risk of HCC in patients with cirrhosis, using the ADRESS‐HCC risk model. They examined the l‐year probability of HCC in various liver diseases in 34 932 patients in the national transplantation waitlist database. HCC developed in 1960 patients (5.6%) during a median follow‐up of 1.3 years. Six baseline clinical variables including age, diabetes, race, etiology of cirrhosis, sex, and severity (ADRESS) of liver dysfunction were independently associated with HCC. They settled ADRESS‐HCC risk model from these data and the risk model well‐coincided with the clinical observation. They concluded that the risk model was clinically useful tool for predicting the risk of HCC in patients with diverse etiologies.
In addition, the aging of the patients must be taken into the consideration, as the cirrhotic patients were considered to be older than the non‐cirrhotic patients in almost all liver diseases. In this regard, Asahina et al129 investigated the difference of HCC incidence in aging in HCV‐associated liver disease, and found that the incidence of HCC was higher in the older patients (>65 years old) than the younger patients (<65 years old). The same tendency was observed by Taura et al130 However, the difference in incidence was approximately twofold. So, it is difficult to explain the marked difference in HCC incidence between the cirrhotic state and non‐cirrhotic state found in this meta‐analysis. Regarding other liver diseases, there were very few reports which demonstrated a difference between patients in the non‐cirrhotic and cirrhotic states concerning age.
The next consideration is the prevention of patients not to become cirrhosis state. We demonstrated that the incidence of HCC in the cirrhotic state and that in the non‐cirrhotic state were markedly different in many liver diseases; therefore, efforts to prevent patients with liver diseases from progressing to the cirrhotic state by all means is very important.
In HBV infection, the effort to diminish HBV‐DNA by pegylated interferon (Peg IFN)19 or oral antiviral agents, such as entecavir (ETV), tenofovir, and tenofovir anafenamide, is important. Indeed, ETV and tenofovir were reported to prevent the occurrence of HCC.131, 132
In HCV infection, Peg IFN plus ribavirin or direct‐acting antivirals (DAAs) are effective to eliminate HCV, and may be prevent the progression of the disease, resulting in prevention of HCC development.
In primary biliary cholangitis, administration of ursodeoxycholic acid is important. Indeed, the risk of HCC in UDCA‐treated PBC patients was reported to be relatively low.59, 63
In autoimmune hepatitis, well‐designed corticosteroid therapy is important to prevent HCC development, and persistent elevation of serum aminotransaminase is reported to lead the development of HCC.78 It seems that alleviation of serum ALT levels might prevent HCC development, but this hypothesis is not confirmed solidly.
In NASH patients, improvement in metabolic syndrome, especially improvement in diabetes mellitus and obesity, is important.
Next, we mentioned whether the treatment of underlying liver diseases substantially modulates the HCC risk or not. We restricted the survey chiefly to the cirrhotic patients because they are at high risk of HCC development.
4.1. HBV‐related cirrhosis
4.1.1. Interferon (IFN)
Ikeda et al19 investigated influence of long‐term IFN administration on the rate of occurrence of HCC in patients with HBV‐related cirrhosis and found that cumulative occurrence rates of HCC in the group treated with IFN and the untreated group were 4.5% and 13.3%, respectively, at the end of 3 years; 7.0% and 19.6%, respectively, at the end of 5 years; and 17.0% and 30.8%, respectively, at the end of 10 years. The rate of HCC development in the treated group was significantly lower than that of the untreated group (P = 0.0124). Interferon treatment was an independent contributing factor in lowering the rate of carcinogenesis (odds ratio = 0.39; P = 0.031). The same tendency was observed by Lin et al14
4.1.2. Oral nucleos(t)ide analogues
Wong et al17 compared the incidence of HCC between the 482 ETV‐treated HBV cirrhotic patients and 69 untreated patients, and found that the 5‐year cumulative incidence of HCC was 13.8% in the ETV‐treated and 26.4% in the untreated patients (P = 0.036) and concluded that ETV treatment reduces HCC development in chronic hepatitis B patients with liver cirrhosis. The same tendency was observed by Su et al25
Furthermore, Hosaka et al18 compared the incidence of HCC between lamivudine (LAM)‐treated, ETV‐treated HBV‐cirrhotic patients, and control HBV cirrhotic patients, and found that the cumulative incidence of HCC in 5 years were 22.2% in the LAM‐treated, 7% in the ETV‐treated, and 38.9% in the control groups, and HCC suppression effect was greater in the ETV‐treated group (P < 0.001) than in the LAM‐treated group (P = 0.019) and the control group.
Additionally, Kim et al131 examined the impact of tenofovir disoproxil fumarate (TDF) on the incidence of HCC, and found that the long‐term (384 weeks) therapy with TDF was associated with the reduced incidence of HCC among patients without cirrhosis.
4.2. HCV‐related cirrhosis
4.2.1. Interferon
Nishiguchi et al27 investigated the effects of IFN‐α on the development of HCC in the HCV‐related cirrhotic patients. IFN‐α (6 MU three times weekly) was administered for 12‐24 weeks in 45 Child A cirrhotic patients and compared with the 45 patients with symptomatic treatment. HCC was detected in 4% of IFN patients and 38% in controls (P = 0.002), and the risk ratio of IFN‐α treatment vs symptomatic treatment was 0.067.
Ikeda et al47 also investigated the effect of IFN on HCC development in the HCV‐related cirrhosis. HCC developing rates in the untreated and IFN groups were 14.8 and 9.1% at the end of 3rd year, 28.4 and 14.1% at the end of the 5th year, and 52.5 and 36.7% at the 10th year, respectively. The carcinogenesis rate of the IFN‐treated group was significantly lower than that of the untreated group (P = 0.0028). The same tendency was observed by Benvegnú et al33 and Yoshida et al42
4.2.2. Direct‐acting antiviral agents
Kanwal et al133 compared the risk of HCC in patients with vs those without sofosbuvir (SVR) (chiefly sofosbuvir + ledipasvir).
Nineteen thousand five hundred and eighteen patients with sustained virological response (SVR) and 2,982 patients without SVR (39.0% had cirrhosis) were included. Compared with patients without SVR, those with SVR had a significantly reduced risk of HCC (0.90 vs 3.45 HCC/100 person‐years, HR 0.28). Moreover, the magnitude of SVR protective effect was similar in patients with and without cirrhosis (HR = 0.32 and HR = 0.18).
Furthermore, Ioannou et al134 investigated the impact of DAA‐induced SVR on HCC risk. They included 35,871 (58%) IFN (chiefly Peg IFN)‐only regimens, 4,535 (7.2%) DAA + IFN regimens, and 21,948 (35%) DAA (chiefly sofosbuvir/ledipasvir)‐only regimens. They retrospectively followed up patients for a mean follow‐up period of 6.1 years. SVR was associated with a significantly decreased risk of HCC irrespective of whether antiviral treatment was DAA‐only (adjusted hazard ratio [AHR] 0.29), DAA + IFN (AHR 0.48), or IFN‐only (AHR 0.32). DAA‐induced SVR is associated with a 71% reduction in HCC risk. Treatment with DAAs is not associated with increased HCC risk compared with treatment with IFN. Patients who achieved SVR had a lower incidence of HCC compared with those who did not achieve SVR among both patients with cirrhosis and those without.
4.3. Primary biliary cirrhosis
Jackson58 investigated whether the risk of developing HCC is reduced by use of ursodeoxycholic acid. They categorized the patients into regular ursodeoxycholic acid–treated patients (with six or more prescriptions) and nonregular‐treated patients (with less than six), and compared the incidence of HCC. The increased risk of HCC in regular ursodeoxycholic acid–treated patients as compared with control subjects was threefold (HR, 3.17) in contrast to an eightfold increase (HR, 7.77) in those nonregular treated.
Furthermore, Kuiper et al62 investigated the risk factor for HCC development in PBC patients, and found that the strongest risk factor for HCC was the absence of biochemical response after 1‐year treatment with UDCA (P < 0.001). (The response to UDCA was defined as normalization of bilirubin and/or albumin levels after administration of UDCA for one year.)
4.4. Autoimmune cirrhosis
There are no reports which deals with the incidence of HCC in the untreated autoimmune patients. However, there are some studies which showed increased incidence of HCC in patients who showed no effectiveness to immune suppressive agent and had continued active hepatitis.
Yoshizawa et al88 followed up 203 well‐defined AIH patients for a mean follow‐up period of 131 months. All patients were treated with corticosteroid with or without azathioprine. They found that the prognosis of two or more relapses were identified as the only risk factor for development of hepatic malignancy (hazard ratio 9.1, P = 0.007). Also, Montano‐Loza et al78 demonstrated that worsening laboratory tests during corticosteroid therapy was associated with a higher risk of HCC. Moreover, Miyake86 et al demonstrated elevation of serum ALT levels leads to HCC development. Furthermore, Hino‐Arinaga et al90 surveyed the risk factors for HCC in autoimmune patients and found that cirrhosis at diagnosis of AIH and abnormal ALT at final observation were independently associated with HCC development.
From the above‐mentioned studies, lowering the serum ALT levels, and preventing relapses seemed to be effective to decrease the risk of developing HCC.
4.5. NASH cirrhosis
The standard therapy of NASH cirrhosis has not been established completely. However, there is some possibility that improvement in metabolic syndrome, especially improvement in diabetes mellitus and obesity may diminish the incidence of HCC in NASH cirrhosis.
4.6. Hereditary hemochromatosis
Niederau et al103 followed up 251 patients with hemochromatosis for up to 33 years (mean, 14.1 years). They demonstrated iron removal by phlebotomy therapy markedly improved survival, including liver cirrhosis. They mentioned that all 21 cases of liver cancer developed in cirrhotic livers, and that the development of liver cancer may depend on the amount and duration of iron overload because patients who died from liver cancer had significantly greater iron stores than patients who died from other cancers. From these statements, iron removal by phlebotomy in cirrhotic patients may supposed to decrease the incidence of HCC, although the beneficial effect of iron removal on HCC development has never been proven by controlled trial.
4.7. Alcoholic cirrhosis
The published paper dealing with the effect of alcoholic abstinence or diminished alcohol intake on the ratio of HCC development was not confirmed. However, Chuang et al135 surveyed by a meta‐analysis the meta‐relative risk (mRR) and dose‐response trend and found that the dose‐response relation between alcohol and development of HCC; the relative risk (RR) was 1.08 for 12 g/day (~1 drink), 1.54 for 50 g/day, 2.14 for 75 g/day, 3.21 for 100 g/day, and 5.20 for 125 g/day of alcohol consumption. It is presumed from these results that the abstinence or diminished alcohol intake would reduce the risk of HCC development.
Another important aspect is surveillance of HCC by imaging modalities, such as ultrasonography (US), magnetic resonance imaging (MRI), or computed tomography (CT) at appropriate intervals (4~6 months) irrespective of the etiology of liver disease when cirrhosis is noted to detect HCC in the early stage.
We found that the incidence of HCC is markedly increased in the cirrhotic state as compared with in the non‐cirrhotic state irrespective of the etiology of liver disease. Therefore, the patients need to be closely monitored for the development of HCC when their liver diseases progress to the cirrhotic state by US, MRI, or CT at appropriate intervals (every 4 months is ideal) to find HCC in the early stage.
CONFLICT OF INTEREST
Tanaka K has received research funding from Bristol‐Myers Squibb and Abb Vie. Nozaki A has received research funding from Gilead Sciences. Tarao K, Ikeda T, Sato A, Komatsu H, Komatsu T, and Taguri M declare that they have no conflict of interest.
Tarao K, Nozaki A, Ikeda T, et al. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases—meta‐analytic assessment. Cancer Med. 2019;8:1054–1065. 10.1002/cam4.1998
Funding information
This work was supported by the Kanagawa Association of Medical and Dental Practitioners.
REFERENCES
- 1. Davidsdôttir L, Duberg AS, Törner A, et al. Hepatocellular carcinoma in individuals with HBV infection or HBV–HCV co‐infection in a low endemic country. Scand J Gastroenterol. 2010;45:944‐952. [DOI] [PubMed] [Google Scholar]
- 2. Gordon SC, Lamerato LE, Rupp LB, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12(5):885‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carries of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22(2):432‐438. [PubMed] [Google Scholar]
- 4. Beasley RP. Hepatitis . B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61(10):1942‐1956. [DOI] [PubMed] [Google Scholar]
- 5. Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow‐up study in Taiwan. Gastroenterology. 2008;135:111‐121. [DOI] [PubMed] [Google Scholar]
- 6. Fang ZL, Sabin CA, Dong BQ, et al. HBV A1762T, G1764A mutations are a valuable biomarker for identifying a subset of male HBs Ag carriers at extremely high risk of hepatocellular carcinoma: a prospective study. Am J Gastroenterol. 2008;103:2254‐2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fwu CW, Chien YC, Kirk GD, et al. Hepatitis B virus infection and hepatocellular carcinoma among parous Taiwanese women: nationwide cohort study. J Natl Cancer Institute. 2009;101:1019‐1027. [DOI] [PubMed] [Google Scholar]
- 8. Kusakabe A, Tanaka Y, Inoue M, et al. A population based cohort study for the risk factors of HCC among hepatitis B virus mono‐infected subjects in Japan. J Gastroenterol. 2011;46:117‐124. [DOI] [PubMed] [Google Scholar]
- 9. Sung FY, Jung CM, Wu CF, et al. Hepatitis B virus core variants modify natural course of viral infection and hepatocellular carcinoma progression. Gastroenterology. 2009;137:1687‐1697. [DOI] [PubMed] [Google Scholar]
- 10. Tong MJ, Trieu J. Hepatitis B inactive carriers: clinical course and outcomes. J Dig Dis. 2013;14(6):311‐317. [DOI] [PubMed] [Google Scholar]
- 11. Di Marco V, Lo Iacono O, Cammà C, et al. The long‐term course of chronic hepatitis B. Hepatology. 1999;30:257‐264. [DOI] [PubMed] [Google Scholar]
- 12. Papatheodoridis GV, Dimou E, Dimakopoulos K, et al. Outcome of hepatitis B e antigen‐negative chronic hepatitis B on long‐term nucleos(t)ide analog therapy starting with lamivudine. Hepatology. 2005;42:121‐129. [DOI] [PubMed] [Google Scholar]
- 13. Tong MJ, Blatt LM, Kao JH, Cheng JT, Corey WG. Precore/ basal core promoter mutants and hepatitis B viral DNA levels as predictors for liver deaths and hepatocellular carcinoma. World J Gastroenterol. 2006;12:6620‐6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin SM, Yu ML, Lee CM, et al. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45‐52. [DOI] [PubMed] [Google Scholar]
- 15. Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650‐655. [PubMed] [Google Scholar]
- 16. Tseng TC, Liu CJ, Yang HC, et al. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142(1140–1149):e3. [DOI] [PubMed] [Google Scholar]
- 17. Wong GL, Chan HL, Mak CW, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537‐1547. [DOI] [PubMed] [Google Scholar]
- 18. Hosaka T, Suzuki F, Kobayashi M, et al. Long‐term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98‐107. [DOI] [PubMed] [Google Scholar]
- 19. Ikeda K, Saitoh S, Suzuki Y, et al. Interferon decreases hepatocellular carcinogenesis in patients with cirrhosis caused by the hepatitis B virus: a pilot study. Cancer. 1998;82:827‐835. [DOI] [PubMed] [Google Scholar]
- 20. Ishikawa T, Ichida T, Yamagiwa S, et al. High viral loads, serum alanine aminotransferase and gender are predictive factors for the development of hepatocellular carcinoma from viral compensated liver cirrhosis. J Gastroenterol Hepatol. 2001;16:1274‐1281. [DOI] [PubMed] [Google Scholar]
- 21. Kato Y, Nakata K, Omagari K, et al. Risk of hepatocellular carcinoma in patients with cirrhosis in Japan. Analysis of infectious hepatitis viruses. Cancer. 1994;74:2234‐2238. [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi M, Ikeda K, Hosaka T, et al. Natural history of compensated cirrhosis in the Child‐Pugh class A compared between 490 patients with hepatitis C and 167 with B virus infections. J Med Virol. 2006;78:459‐465. [DOI] [PubMed] [Google Scholar]
- 23. Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521‐1531. [DOI] [PubMed] [Google Scholar]
- 24. Sinn DH, Lee J, Goo J, et al. Hepatocellular carcinoma risk in chronic hepatitis B virus‐infected compensated cirrhosis patients with low viral load. Hepatology. 2015;62(3):694‐701. [DOI] [PubMed] [Google Scholar]
- 25. Su TH, Hu TH, Chen CY, et al. Four‐year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36(12):1755‐1764. [DOI] [PubMed] [Google Scholar]
- 26. Kato Y, Nakata K, Omagari K, et al. Risk of hepatocellular carcinoma in patients with cirrhosis in Japan. Cancer. 1994;74:2234‐2238. [DOI] [PubMed] [Google Scholar]
- 27. Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon‐alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346(8982):1051‐1055. [DOI] [PubMed] [Google Scholar]
- 28. Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatolog. 1995;21:650‐655. [PubMed] [Google Scholar]
- 29. Mazzella G, Accogli E, Sottili S, et al. Alpha interferon treatment may prevent hepatocellular carcinoma in HCV‐related liver cirrhosis. J Hepatol. 1996;24(2):141‐147. [DOI] [PubMed] [Google Scholar]
- 30. Chiba T, Matsuzaki Y, Abei M, et al. The role of previous hepatitis B virus infection and heavy smoking in hepatitis C virus‐related hepatocellular carcinoma. Am J Gastroenterol. 1996;91(6):1195‐1203. [PubMed] [Google Scholar]
- 31. Bruno S, Silini E, Crosignani A, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a prospective study. Hepatology. 1997;25(3):754‐758. [DOI] [PubMed] [Google Scholar]
- 32. Fattovich G, Giustina G, Degos F, et al. Effectiveness of interferon alfa on incidence of hepatocellular carcinoma and decompensation in cirrhosis type C. European Concerted Action on Viral Hepatitis (EUROHEP). J Hepatol. 1997;27(1):201‐205. [DOI] [PubMed] [Google Scholar]
- 33. Benvegnú L, Chemello L, Noventa F, Fattovich G, Pontisso P, Alberti A. Retrospective analysis of the effect of interferon therapy on the clinical outcome of patients with viral cirrhosis. Cancer. 1998;83(5):901‐909. [DOI] [PubMed] [Google Scholar]
- 34. Imai Y, Kawata S, Tamura S, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Ann Intern Med. 1998;129(2):94‐99. [DOI] [PubMed] [Google Scholar]
- 35. Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28(6):1687‐1695. [DOI] [PubMed] [Google Scholar]
- 36. International Interferon Hepatocellular Carcinoma Study Group . Effect of interferon‐alpha on progression of cirrhosis to hepatocellular carcinoma: a retrospective cohort study. Lancet. 1998;351(9115):1535‐1539. [PubMed] [Google Scholar]
- 37. Serfaty L, Aumaître H, Chazouillères O, et al. Determinants of outcome of compensated hepatitis C virus‐related cirrhosis. Hepatology. 1998;27(5):1435‐1440. [DOI] [PubMed] [Google Scholar]
- 38. Gordon SC, Bayati N, Silverman AL. Clinical Outcome of Hepatitis C as a Function of Mode of Transmission. Hepatology. 1998;28:562‐567. [DOI] [PubMed] [Google Scholar]
- 39. Dutta U, Byth K, Kench J, et al. Risk factors for development of hepatocellular carcinoma among Australians with hepatitis C: a case‐control study. Aust N Z J Med. 1999;29(3):300‐307. [DOI] [PubMed] [Google Scholar]
- 40. Chiaramonte M, Stroffolini T, Vian A, et al. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer. 1999;85:2132‐2137. [PubMed] [Google Scholar]
- 41. Okanoue T, Itoh Y, Minami M, et al. Interferon therapy lowers the rate of progression to hepatocellular carcinoma in chronic hepatitis C but not significantly in an advanced stage: a retrospective study in 1148 patients. J of Hepatology. 1999;30:653‐659. [DOI] [PubMed] [Google Scholar]
- 42. Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. Ann Intern Med. 1999;131(3):174‐181. [DOI] [PubMed] [Google Scholar]
- 43. Valla DC, Chevallier M, Marcellin P, et al. Treatment of hepatitis C virus‐related cirrhosis: a randomized, controlled trial of interferon alfa‐2b versus no treatment. Hepatology. 1999;29(6):1870‐1875. [DOI] [PubMed] [Google Scholar]
- 44. Degos F, Christidis C, Ganne‐Carrie N, Farmachidi J‐P, Degott C, Guettier C. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gramenzi A, Andreone P, Fiorino S, et al. Impact of interferon therapy on the natural history of hepatitis C virus related cirrhosis. Gut. 2001;48(6):843‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nishiguchi S, Shiomi S, Nakatani S, et al. Prevention of hepatocellular carcinoma in patients with chronic active hepatitis C and cirrhosis. Lancet. 2001;357:196‐197. [DOI] [PubMed] [Google Scholar]
- 47. Ikeda K, Saitoh S, Kobayashi M, et al. Long‐term interferon therapy for 1 year or longer reduces the hepatocellular carcinogenesis rate in patients with liver cirrhosis caused by hepatitis C virus: a pilot study. J Gastroenterol Hepatol. 2001;16(4):406‐415. [DOI] [PubMed] [Google Scholar]
- 48. Tarao K, Rino Y, Ohkawa S, et al. Close association between high serum alanine aminotransferase levels and multicentric hepatocarcinogenesis in patients with hepatitis C virus‐associated cirrhosis. Cancer. 2002;94:1787‐1795. [DOI] [PubMed] [Google Scholar]
- 49. Mazziotti G, Sorvillo F, Morisco F, et al. Serum insulin‐like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C Virus‐related cirrhosis. Cancer. 2002;95:2539‐2545. [DOI] [PubMed] [Google Scholar]
- 50. Fattovich G, Pantalena M, Zagni I, et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97:2886‐2895. [DOI] [PubMed] [Google Scholar]
- 51. Velazquez RF, Rodriguez M, Navascues CA, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520‐527. [DOI] [PubMed] [Google Scholar]
- 52. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: Incidence and Risk Factors. Gastroenterology. 2004;127:S35‐S50. [DOI] [PubMed] [Google Scholar]
- 53. Bedogni G, Miglioli L, Masutti F, et al. Natural course of chronic HCV and HBV infection and role of alcohol in the general population: the Dionysos Study. Am J Gastroenterol. 2008;103(9):2248‐2253. [DOI] [PubMed] [Google Scholar]
- 54. Jones DE, Metcalf JV, Collier JD, Bassendine MF, James OF. Hepatocellular carcinoma in primary biliary cirrhosis and its impact on outcomes. Hepatology. 1997;26:1138‐1142. [DOI] [PubMed] [Google Scholar]
- 55. Floreani A, Baragiotta A, Baldo V, Menegon T, Farinati F, Naccarato R. Hepatic and extrahepatic malignancies in primary biliary cirrhosis. Hepatology. 1999;29:1425‐1428. [DOI] [PubMed] [Google Scholar]
- 56. Nijhawan PK, Therneau TM, Dickson ER, Boynton J, Lindor KD. Incidence of cancer in primary biliary cirrhosis: the Mayo experience. Hepatology. 1999;29:1896‐1898. [DOI] [PubMed] [Google Scholar]
- 57. Caballería L, Parés A, Castella A, Ginés A, Bru C, Rodés J. Hepatocellular carcinoma in primary biliary cirrhosis: similar incidence to that in hepatitis C virus related cirrhosis. Am J Gastroenterol. 2001;96:1160‐1163. [DOI] [PubMed] [Google Scholar]
- 58. Jackson H, Solaymani‐Dodaran M, Card TR, Aithal GP, Logan R, West J. Influence of ursodeoxycholic acid on the mortality and malignancy associated with primary biliary cirrhosis: a population‐based cohort study. Hepatology. 2007;46:1131‐1137. [DOI] [PubMed] [Google Scholar]
- 59. Silveira MG, Suzuki A, Lindor KD. Surveillance for hepatocellular carcinoma in patients with primary biliary cirrhosis. Hepatology. 2008;48:1149‐1156. [DOI] [PubMed] [Google Scholar]
- 60. Deutsch M, Papatheodoridis GV, Tzakou A, Hadziyannis SJ. Risk of hepatocellular carcinoma and extrahepatic malignancies in primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2008;20:5‐9. [DOI] [PubMed] [Google Scholar]
- 61. Cavazza A, Caballeria L, Floreani A, et al. Incidence, risk factors, and survival of hepatocellular carcinoma in primary biliary cirrhosis: comparative analysis from two centers. Hepatology. 2009;50:1162‐1168. [DOI] [PubMed] [Google Scholar]
- 62. Kuiper EM, Hansen BE, Adang RP, et al. Relatively high risk for hepatocellular carcinoma in patients with primary biliary cirrhosis not responding to ursodeoxycholic acid. Eur J Gastroenterol Hepatol. 2010;22:1495‐1502. [DOI] [PubMed] [Google Scholar]
- 63. Boonstra K, Bokelear R, Stadhouders PH, et al. Increased cancer risk in a large population‐based cohort of patients with primary biliary cirrhosis: follow up for up to 86 years. Hepatol Int. 2014;8(2):266‐274. [DOI] [PubMed] [Google Scholar]
- 64. Trivedi PJ, Lammers WJ, van Buuren HR, et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut. 2016;65(2):321‐329. [DOI] [PubMed] [Google Scholar]
- 65. Shibuya A, Tanaka K, Miyakawa H, et al. Hepatocellular carcinoma and survival in patients with primary biliary cirrhosis. Hepatology. 2002;35(5):1172‐1178. [DOI] [PubMed] [Google Scholar]
- 66. Watanabe T, Soga K, Hirono H, et al. Features of hepatocellular carcinoma in cases with autoimmune hepatitis and primary biliary cirrhosis. World J Gastroenterol. 2009;15(2):231‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harada K, Hirohara J, Ueno Y, et al. Incidence of and risk factors for hepatocellular carcinoma in primary biliary cirrhosis: national data from Japan. Hepatology. 2013;57:1942‐1949. [DOI] [PubMed] [Google Scholar]
- 68. Tomiyama Y, Takenaka K, Kodama T, et al. Risk factors for survival and the development of hepatocellular carcinoma in patients with primary biliary cirrhosis. Intern Med. 2013;52(14):1553‐1559. [DOI] [PubMed] [Google Scholar]
- 69. Hosonuma K, Sato K, Yanagisawa M, et al. Incidence, mortality, and predictive factors of hepatocellular carcinoma in primary biliary cirrhosis. Gastroenterol Res Pract. 2013;2013:168012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang XX, Wang LF, Jin L, et al. Primary biliary cirrhosis‐associated hepatocellular carcinoma in Chinese patients: incidence and risk factors. World J Gastroenterol. 2015;21(12):3554‐3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rong G, Wang H, Bowlus CL, et al. Incidence and risk factors for hepatocellular carcinoma in primary biliary cirrhosis. Clin Rev Allergy Immunol. 2015;48(2–3):132‐141. [DOI] [PubMed] [Google Scholar]
- 72. Kim KA, Ki M, Choi HY, Kim BH, Jang ES, Jeong SH. Population‐based epidemiology of primary biliary cirrhosis in South Korea. Aliment Pharmacol Ther. 2016;43(1):154‐162. [DOI] [PubMed] [Google Scholar]
- 73. Cheung KS, Seto WK, Fung J, Lai CL, Yuen MF. Epidemiology and natural history of primary biliary cholangitis in the Chinese: a territory‐based study in Hong Kong between 2000 and 2015. Clin Tranel Gastroenterol. 2017;8(8):e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cheung KS, Seto WK, Fung J, Mak LY, Lai CL, Yuen MF. Prediction of hepatocellular carcinoma development by aminotransferase to platelet ratio index in primary biliary cholangitis. World J Gastroenterol. 2017;23(44):7863‐7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Park SZ, Nagorney DM, Czaja AJ. Hepatocellular carcinoma in autoimmune hepatitis. Dig Dis Sci. 2000;45(10):1944‐1948. [DOI] [PubMed] [Google Scholar]
- 76. Seela S, Sheela H, Boyer JL. Autoimmune hepatitis type 1: safety and efficacy of prolonged medical therapy. Liver Int. 2005;25(4):734‐739. [DOI] [PubMed] [Google Scholar]
- 77. Floreani A, Niro G, Rosa Rizzotto E, et al. Type 1 autoimmune hepatitis: clinical course and outcome in an Italian multicentre study. Aliment pharmacol Ther. 2006;24:1051‐1057. [DOI] [PubMed] [Google Scholar]
- 78. Montano‐Loza AJ, Carpenter HA, Czaja AJ. Predictive factors for hepatocellular carcinoma in type 1 autoimmune hepatitis. Am J Gastroenterol. 2008;103(8):1944‐1951. [DOI] [PubMed] [Google Scholar]
- 79. Seo S, Toutounjian R, Conrad A, Blatt L, Tong MJ. Favorable outcome of autoimmune hepatitis in a community clinic setting. J Gastroenterol Hepatol. 2008;23(9):1410‐1414. [DOI] [PubMed] [Google Scholar]
- 80. Yeoman AD, Al‐Chalabi T, Karani JB, et al. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: implications for follow‐up and screening. Hepatology. 2008;48(3):863‐870. [DOI] [PubMed] [Google Scholar]
- 81. Teufel A, Weinmann A, Centner C, et al. Hepatocellular carcinoma in patients with autoimmune hepatitis. World J Gastroenterol. 2009;15(5):578‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wong RJ, Gish R, Frederick T, Bzowej N, Frenette C. Development of hepatocellular carcinoma in autoimmune hepatitis patients: a cace series. Dig Dis Sci. 2011;56:578‐585. [DOI] [PubMed] [Google Scholar]
- 83. Danielsson Borssen D, Almer S, Prytz H, et al. Hepatocellular and extrahepatic cancer in patients with autoimmune hepatitis – a long‐term follow‐up study in 634 Swedish patients. Scand J Gastroenterol. 2015;50(2):217‐223. [DOI] [PubMed] [Google Scholar]
- 84. Werner M, Almer S, Prytz H, et al. Hepatic and extrahepatic malignancies in autoimmune hepatitis. A long‐term follow‐up in 473 Swedish patients. J of Hepatol. 2009;50:388‐393. [DOI] [PubMed] [Google Scholar]
- 85. Wang KK, Czaja AJ. Hepatocellular carcinoma in corticosteroid‐treated severe autoimmune chronic active hepatitis. Hepatology. 1988;8(6):1679‐1683. [DOI] [PubMed] [Google Scholar]
- 86. Miyake Y, Iwasaki Y, Terada R, et al. Persistent elevation of serum alanine aminotransferase levels leads to poor survival and hepatocellular carcinoma development in type 1 autoimmune hepatitis. Aliment Pharmacol Ther. 2006;24(8):1197‐1205. [DOI] [PubMed] [Google Scholar]
- 87. Kil JS, Lee, et al. Long term treatment outcome for autoimmune hepatitis in Korea. J Korean Med Sci. 2010;25:54‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yoshizawa K, Matsumoto A, Ichijo T, et al. Long‐term outcome of Japanese patients with type 1 autoimmune hepatitis. Hepatology. 2012;56:668‐676. [DOI] [PubMed] [Google Scholar]
- 89. Migita K, Watanabe Y, Jiuchi Y, et al. Hepatocellular carcinoma and survival in patients with autoimmune hepatitis (Japanese national hospital organization‐ autoimmune hepatitis prospective study). Liver Int. 2012;32(5):837‐844. [DOI] [PubMed] [Google Scholar]
- 90. Hino‐Arinaga T, Ide T, Kuromatsu R, et al. Risk factors for hepatocellular carcinoma in Japanese patients with autoimmune hepatitis type 1. J Gastroenterol. 2012;47:569‐576. [DOI] [PubMed] [Google Scholar]
- 91. Powell EE, Cooksley W, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow‐up study of fourty‐two patients for up to 21 years. Hepatology. 1990;11:74‐80. [DOI] [PubMed] [Google Scholar]
- 92. Hui JM, Kench JG, Chitturi S, et al. Long‐term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420‐427. [DOI] [PubMed] [Google Scholar]
- 93. Adams LA, Lymp JF, Sauver J, et al. The natural history of nonalcoholic fatty liver diseases: A population‐based cohort study. Gastroenterology. 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 94. Sanyal A, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682‐689. [DOI] [PubMed] [Google Scholar]
- 95. Ekstedt M, Franźen LE, Mathiesen UK, et al. Long‐term follow‐up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865‐873. [DOI] [PubMed] [Google Scholar]
- 96. Ascha M, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972‐1978. [DOI] [PubMed] [Google Scholar]
- 97. Bhala N, Angulo P, Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaboratative study. Hepatology. 2011;54(4):1208‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non‐alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248‐254. [DOI] [PubMed] [Google Scholar]
- 99. Hashimoto E, Yatsuji S, Tobari M, et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(Suppl 19):89‐95. [DOI] [PubMed] [Google Scholar]
- 100. Kawamura Y, Arase Y, Ikeda K, et al. Large‐scale long‐term follow‐up study of Japanese patients with non‐alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253‐261. [DOI] [PubMed] [Google Scholar]
- 101. Tobari M, Taniai M, Hashimoto E. NAFLD/NASH and hepatocellular carcinoma: Difference with viral hepatocellular carcinoma. Med J. 2012;48(12):2847‐2852. (in Japanese). [Google Scholar]
- 102. Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313(20):1256‐1262. [DOI] [PubMed] [Google Scholar]
- 103. Niederau C, Fischer R, Purschel A, Stremmel W, Haussinger D, Strohmeyer G. Long‐term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110(4):1107‐1119. [DOI] [PubMed] [Google Scholar]
- 104. Fargion A, Mandelli C, Piperno A, et al. Survival and Prognostic factors in 212 Italian patients with genetic hemochromatosis. Hepatology. 1992;15(4):655‐659. [DOI] [PubMed] [Google Scholar]
- 105. Deugnier YM, Guyader D, Crantock L, et al. Primary liver cancer in genetic hemochromatosis: A clinical, pathological, and pathogenetic study of 54 cases. Gastroenterology. 1993;104(1):228‐234. [DOI] [PubMed] [Google Scholar]
- 106. Pleass H, Garden OJ. Early diagnosis of hepatocellular carcinoma in haemochromatosis influences surgical management. Scot Med J. 1998;43:114‐115. [DOI] [PubMed] [Google Scholar]
- 107. Fracanzani AL, Conte D, Fraquelli M, et al. Increased cancer risk in a Cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with non‐iron‐related chronic liver disease. Hepatology. 2001;33(3):647‐651. [DOI] [PubMed] [Google Scholar]
- 108. Morcos M, Dubois S, Bralet MP, Belghiti J, Degott C, Terris B. Primary liver carcinoma in genetic hemochromatosis reveals a broad histologic spectrum. Am J Clin Pathol. 2001;116:738‐743. [DOI] [PubMed] [Google Scholar]
- 109. Deugnier YM, Charalambous P, Quilleuc DL, et al. Preneoplastic significance of hepatic iron‐free foci in genetic hemochromatosis: a study of 185 patients. Heptology. 1993;18(6):1363‐1369. [PubMed] [Google Scholar]
- 110. N’Kontchou G, Pries J, Htar MT, et al. Risk factors for hepatocellular carcinoma in patients with alcohol or viral C cirhosis. Clin Gastroenterol Hepatol. 2006;4(8):1062‐1068. [DOI] [PubMed] [Google Scholar]
- 111. Ioannou GN, Splan MF, Weiss NS, et al. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5(8):935‐945. [DOI] [PubMed] [Google Scholar]
- 112. Jepsen P, Ott P, Andersen PK, et al. Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis; a Danish nationwide cohort study. Ann Intern Med. 2012;156(2):841‐847. [DOI] [PubMed] [Google Scholar]
- 113. Mancebo A, Gonzalez‐Dieguez ML, Cadahia V, et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clon Gastroenterol Hepatol. 2013;11(1):95‐101. [DOI] [PubMed] [Google Scholar]
- 114. Marot A, Henrion J, Knebel JF, et al. Alcoholic liver disease confers a worse prognosis than HCV infection and non‐alcoholic fatty liver disease among patients with cirrhosis: an obsevational study. PLoS ONE. 2017;12(10):e0186715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Miyakawa H, Sato C, Tazawa J, et al. A prospective study on hepatocellular carcinoma in liver cirrhosis: respective roles of alcohol and hepatitis C virus infection. Alcohl Alcohol Suppl. 1994;29:75‐79. [PubMed] [Google Scholar]
- 116. Uetake S, Yamauchi M, Itoh S, et al. Analysis of risk factors for hepatocellular carcinoma in patients with HBs antigen‐and anti‐HCV antibody‐negative alcoholic cirrhosis: clinical significance of prior hepatitis B virus infection. Alcohol Clin Exp Res. 2003;27(8 Suppl):47S–51S. [DOI] [PubMed] [Google Scholar]
- 117. Toshikuni N, Izumi A, Nishino K, et al. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus‐related cirrhosis. J Gastroenterol Hepatol. 2009;24(7):1276‐1283. [DOI] [PubMed] [Google Scholar]
- 118. Lin CW, Lin CC, Mo LR, et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus‐related cirrhosis. J Hepatol. 2013;58(4):730‐735. [DOI] [PubMed] [Google Scholar]
- 119. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245‐1255. [DOI] [PubMed] [Google Scholar]
- 120. Tarao K, Rino Y, Ohkawa S, et al. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus‐associated cirrhosis. Cancer. 1999;86:589‐595. [DOI] [PubMed] [Google Scholar]
- 121. Chen C‐F, Lee W‐C, Yang H, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141:1240‐1248. [DOI] [PubMed] [Google Scholar]
- 122. Tarao K, Ohkawa S, Shimizu A, et al. Significance of hepatocellular proliferation in the development of hepatocellular carcinoma from anti‐hepatitis C virus‐positive cirrhotic patients. Cancer. 1994;73:1149‐1154. [DOI] [PubMed] [Google Scholar]
- 123. Tarao K, Hoshino H, Shimizu A, et al. Patients with ultrasonic coarse‐nodular cirrhosis who are anti‐hepatitis C virus‐positive are at high risk for hepatocellular carcinoma. Cancer. 1995;75:1255‐1262. [DOI] [PubMed] [Google Scholar]
- 124. Hiatt T, Trotter JF, Kam I. Hepatocellular carcinoma in a noncirrhotic patient with hereditary hemochromatosis. Am J Med Sci. 2007;334(3):228‐230. [DOI] [PubMed] [Google Scholar]
- 125. Testino G, Leone S, Borro P. Alcohol and hepatocellular carcinoma: a review and point of view. World J Gastroenterology. 2014;20(43):15943‐15954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625‐1638. [DOI] [PubMed] [Google Scholar]
- 127. El‐Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460‐468. [DOI] [PubMed] [Google Scholar]
- 128. Flemming JA, Mas F, Yang JD, et al. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS‐HCC risk model. Cancer. 2014;120:3485‐3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52:518‐527. [DOI] [PubMed] [Google Scholar]
- 130. Taura N, Hamasaki K, Nakao K, et al. Aging of patients with hepatitis C virus‐associated hepatocellular carcinoma: Long‐term trends in Japan. Oncol Rep. 2006;16:837‐843. [PubMed] [Google Scholar]
- 131. Kim WR, Loomba R, Berg T, et al. Impact of Long‐Term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer. 2015;121:3631‐3638. [DOI] [PubMed] [Google Scholar]
- 132. Singal AK, Salameh H, Kuo YF, Fontane RJ. Meta‐analysis: the impact of oral anti‐viral agents on incidence of hepatocellular carcinoma in chronic hepatitis B. Alimert Pharmacol Ther. 2013;38:98‐106. [DOI] [PubMed] [Google Scholar]
- 133. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El‐Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct‐acting antiviral agents. Gastroenterology. 2017;153:996‐1005. [DOI] [PubMed] [Google Scholar]
- 134. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct‐acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatology. 2018;68:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chuang SC, Lee Y‐CA, Wu GJ, Straif K, Hashibe M. Alcohol consumption and liver cancer risk: a meta‐analysis. Cancer Causes Control. 2015;26:1205‐1231. [DOI] [PubMed] [Google Scholar]


