Abstract
Hypoxia-inducible factor (HIF)-1α protein, which is upregulated by hypoxia, serves an important role in angiogenesis during osteogenesis. The aim of the present study was to investigate the effect of HIF-1α on alveolar ridge preservation in a dog tooth extraction model. Six beagle dogs were used in the present study. The second and fourth premolar teeth of the lower jaws on both sides were extracted. Two unilateral extraction sockets were randomly selected and filled with Bio-Oss and Bio-Oss + HIF-1α. The contralateral sockets remained unfilled and served as the negative control. Micro-computed tomography examination and histological staining were performed to examine the difference of new bone formation among the three groups. Western blotting and reverse transcription-quantitative polymerase chain reaction analysis were used to detect the expression levels of osteogenesis- and angiogenesis-associated genes in the bone tissues of the three groups. Twelve weeks post-surgery, trabecular bone formation in the Bio-Oss + HIF-1α group was significantly increased compared with the other groups. The expression levels of osteogenesis-associated genes (runt-related transcription factor 2, osteoblast-specific transcription factor osterix and osteocalcin) and angiogenesis-associated genes (HIF-1α and vascular endothelial growth factor) were all significantly increased in the Bio-Oss + HIF-1α group compared with the other two groups (P<0.05). The present results indicated that Bio-Oss with HIF-1α can promote osteogenesis and angiogenesis in vivo and may be used as an effective treatment for the preservation of the alveolar ridge.
Keywords: alveolar ridge preservation, hypoxia-inducible factor-1α protein, osteogenesis, angiogenesis
Introduction
An ideal dental implant requires adequate alveolar ridge dimensions following tooth extraction. In recent years, alveolar bone regeneration has been widely investigated by filling the extraction socket with bone substitutes or growth factors to protect alveolar bone against resorption. These substitutes include deproteinized bovine bone (Bio-Oss) particles (1), deproteinized bovine bone and collagen matrix Bio-Oss Collagen (2) and demineralized freeze-dried bone allograft (3). The growth factors include recombinant human bone morphogenetic protein-2 (4), recombinant human platelet-derived growth factor (5) and plasma-rich growth factor (6), among others. These growth factors exert different effects during the bone formation, such as inflammatory reactions, soft and hard callus formation, and bone remodelling. Despite the osteoinductive agents of these growth factors, their use in bone regeneration are limited due to the drawbacks, including osteolysis, heterotopic ossification, easy degradation, safety and efficiency (7,8). As a result, they are far from being ready for extensive clinical use. A new treatment aim for the preservation of the alveolar ridge includes how to achieve optimal osteoinduction effects and provide sufficient nutrition and oxygen to the osteoblasts during the process of new bone formation.
Molecular oxygen is essential for metabolic processes, including oxidative phosphorylation, in which oxygen serves as an electron acceptor during ATP formation (9). Lack of oxygen may result in failure to generate sufficient ATP to maintain essential cellular functions. Hypoxia-inducible factor 1 (HIF-1), which is composed of HIF-1α and HIF-1β, is a heterodimeric basic helix-loop-helix transcription factor that regulates hypoxia-inducible genes, including the human erythropoietin (EPO) gene (10). HIF-1α is not expressed in normal cells, but can be induced under hypoxic conditions; it acts as one of the key transcription factors that regulate numerous downstream responses to hypoxia, including the expression of several angiogenic factors (11). Previous findings have demonstrated that hypoxia transcriptionally upregulates the expression of HIF-1α, vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-β and insulin-like growth factor-2, which may serve an important role in angiogenesis during osteogenesis (12). It has long been recognized that angiogenesis is essential for osteogenesis. Under hypoxic conditions, the HIF-1α protein is stabilized as it dimerizes with HIF-1β and binds to the hypoxia response elements in target gene promoter sequences (13). The oxygen-dependent prolyl hydroxylases, which act upon HIF-1α, are members of the same protein family that contains the procollagenprolyl 4-hydroxylase, an enzyme essential for collagen formation (14). However, the application of HIF-1α for bone regeneration remains controversial due to its seemingly contradictory dual role in osteoblast differentiation.
Endochondral bone formation and fracture repair are dependent upon blood vessel invasion (1) and exercise-induced bone formation, which is associated with increased blood supply. However, the effect of oxygenation on the function of osteoblasts has not been extensively investigated to date. Previous results have demonstrated that VEGF, a potent angiogenic peptide with specific mitogenic and chemotactic properties, has a key role in angiogenesis in cartilage and temporomandibular joint osteoarthritis (2). As an important transcription factor in angiogenesis, HIF-1α is one of the most important regulators of VEGF expression. Therefore, it was hypothesized that the application of HIF-1α in tooth extraction sites may exert osteoinductive and angiogenic effects during the bone healing process. Bio-Oss has been extensively examined in vitro and in vivo in a variety of clinical and preclinical studies (15–17). To examine the function of HIF-1α in the preservation of the alveolar ridge in a dog model, HIF-1α was applied to the tooth extraction sites by using Bio-Oss as the protein carrier.
Materials and methods
Preparation of HIF-1α protein carrier
Bio-Oss (Geistlich Pharma AG, Wolhusen, Switzerland) was used as a carrier for the HIF-1α protein (AnaSpec Inc., Fremont, CA, USA). Pieces of Bio-Oss, 1 mm in diameter, were submerged in a concentration of 100 mg/ml HIF-1α protein prior to use.
Animal protocol
Six beagle dogs (age, 1.5 years; weight, 15–20 kg) were obtained from the Animal Centre of the Ninth People's Hospital, Shanghai Jiao Tong University. The Institutional Animal Care and Use Committee of the Ninth People's Hospital, Shanghai Jiao Tong University, approved the protocol used in the present study (ethics approval number, 2013-304). All animals were handled according to the guidelines established by the Institutional Animal Care Committee.
Surgical procedures
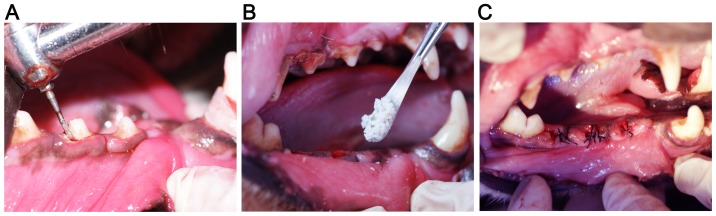
Each animal was anesthetized with an intramuscular injection of ketamine (10 mg/kg; Fujian Gutian Pharma Co., Ltd., Fujian, China) and xylazine (1 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). According to a previous study (18), the second and fourth premolar teeth of the lower jaws were carefully extracted following vertical tooth separation. Two randomly selected extraction sockets on one side were filled with Bio-Oss alone or Bio-Oss + HIF-1α, while the contralateral sockets remained unfilled. There were three groups of extraction sites in the present study: Blank group (no fillings in the sockets), Bio-Oss group (Bio-Oss filling), and Bio-Oss + HIF-1α group (Bio-Oss + HIF-1α filling). The wounds were closed following vertical flap elevation. All the operative procedures were performed under sterile conditions (Fig. 1A-C).
Figure 1.
Images captured intraoperatively. (A) Minimally invasive surgical extraction of the dog teeth was performed by using a dental handpiece. (B) Filling the extraction socket with Bio-Oss or hypoxia-inducible factor-1α + Bio-Oss. (C) Stitching the wounds post-surgery.
Sample harvest
At 12 weeks post-surgery, all the animals were euthanized with intravenous injection of pentobarbital (100 mg/kg; Sigma-Aldrich; Merck KGaA). The alveolar bone surrounding the extraction socket was dissected and cut into blocks. Protein and RNA were isolated from the tissues around the extraction sites. The bone samples from the extraction sites were fixed in 4% paraformaldehyde overnight at 4°C and were then dehydrated in ethanol solution.
Micro-computed tomography (CT) measurement
Specimens were harvested and assessed with the microtomographic imaging system (MicroCT-80, Siemens Healthineers, Erlangen, Germany), as previously reported (19). All micro-CT scans were performed with the following acquisition parameters: Tube voltage: 80 kV; tube current: 0.50 mA; scan time: 1,500 msec; tomographic rotation: 360°; a rotation step of 360; and a cool-down time of 2,000 msec. The setting of the charge-coupled Device (CCD) readout was as follows: Transaxial, 2048; axial, 2048; and binning, 1. The settings of the effective scope CCD-FOV were as follows: Transaxial, 19.03 mm; axial, 19.03 mm; and effective pixel size, 9.29 µm. The morphology of reconstructed mandible was assessed using an animal micro-CT scanner (Inveon Research Workplace, Siemens Healthineers). Micro-CT measurements included bone volume to tissue volume (BV/TV), bone mineral density and trabecular number of the extraction sockets.
Histological and histomorphometric observations
Following micro-CT examination, all samples were decalcified in 10% EDTA for 3 weeks and were then dehydrated in 50 and 70% alcohol for 30 min each. Following paraffin embedding, the samples were cut into 6-µm sections with a Leica microtome and mounted on slides (Thermo Fisher Scientific, Inc., Waltham, MA, USA). For hematoxylin and eosin (H&E) staining, the sections were deparaffinized and hydrated through a xylene and graded ethanol series (100, 90, 70 and 50%) for 5 min each, then stained with hematoxylin for 5 min and eosin for 3 min at room temperature. The samples were observed under a Leica microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Immunohistochemical (IHC) staining
IHC staining was performed to analyze osteoblast-associated factors. First, bone sections were processed for antigen retrieval by digestion with 0.05% trypsin at 37°C for 15 min, and then incubated with primary antibodies against osteocalcin (OCN; 1:200; ab93876, Abcam, Cambridge, UK) and runt-related transcription factor 2 (Runx2; 1:200; ab23981, Abcam) overnight at 4°C. Subsequently, the sections were incubated with the horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibodies (1:1,000; ab150077; Abcam, Cambridge, UK) for 1 h at room temperature, and developed in 3,3′-diaminobenzidine chromogen (8801-4965-72; Invitrogen; Thermo Fisher Scientific, Inc.). The sections were counterstained with hematoxylin for 5 min at room temperature and observed under a light microscope (magnification, ×20; Leica Microsystems GmbH).
Western blotting
For protein extraction, the frozen samples were transferred to a tube prefilled with beads (Nextadvance, Inc., Troy, NY, USA) and immersed with 100 µl 2X SDS lysis buffer (100 mM Tris-HCl, 4% SDS, 0.1% bromophenol blue, 20% glycerol, supplemented with DTT and protease inhibitors), lysed via centrifugation at 15,000 × g for 15 min at 4°C using an electronic homogenizer (Nextadvance, Inc.). The supernatants were then collected and protein concentrations were measured using a bicinchoninic acid assay protein assay kit (23225; Thermo Fisher Scientific, Inc.). Protein was denatured at 95°C for 10 min, then cooled to room temperature. Samples (10 µl) were resolved on a 10% SDS-PAGE gel and transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline with Tween 20 for 1 h at room temperature, and incubated overnight at 4°C with primary antibodies against HIF-1α (1:800; ab228649, Abcam), OCN (1:800, ab93876, Abcam), Runx2 (1:1,000; ab23981, Abcam), βactin (1:3,000; ab8226; Acbam) and osteoblast-specific transcription factor osterix (OSX; 1:500; SC22538, Santa Cruz Biotechnology, Inc., Dallas, TX, USA); respectively. Membranes were subsequently washed 3 times using phosphate buffered saline with Tween 20 and incubated with the following secondary antibodies for 1 h at room temperature: HRP conjugated- Goat anti-Rabbit Immunoglobulin G (IgG; 1:8,000; ab6721; Abcam) and HRP conjugated Goat anti-Mouse IgG (1:5,000; ab6789; Abcam). Antibody-antigen complexes were detected using the Luminata Forte HRP substrate (EMD Millipore). Membranes were visualized and quantified using a blot scanner (LI-COR Biosciences, Lincoln, NE, USA) and Image Studio™ Lite software (Li-COR Biosciences).
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
RNA samples from bone tissues were transferred to the aforementioned tube prefilled with beads (Nextadvance, Inc.) and homogenized using a Bullet blender (Nextadvance, Inc.). RNA extraction was performed using the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturers' protocol. Isolated RNA was dissolved in RNAse-free water and quantified by measuring the absorbance at 260 and 280 nm with a spectrophotometer. The RNA samples were then treated with DNAse I, and cDNA was prepared for each sample using RevertAid H Minus Reverse Transcriptase (EP0452, Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The primers of the target genes were indicated in (Table I). To evaluate the gene expression levels, qPCR was performed with SYBR Green PCR Kit using iCycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). PCR reactions were performed at 0.5 µM for each primer in a 25-µl volume containing 1 µl cDNA sample. The reaction was initiated by activating the polymerase with a 5-min pre-incubation at 95°C. Amplification was achieved with 45 cycles of 15 sec denaturation at 94°C, 20 sec annealing at 65°C and 10 sec extension at 72°C. The program was concluded by a melting curve analysis. All experiments were performed in triplicate. The copy numbers of each gene were determined with the quantification cycle (2−ΔΔCq) method (20). The means of the copy numbers of glyceraldehyde 3-phosphate dehydrogenase GAPDH were used as internal controls. Standard curves of all primers were prepared from total normal cDNA, amplified by semi-quantitative PCR and cloned using the TOPO II TA Cloning Kit (Thermo Fisher Scientific, Inc.) following the manufacturer's recommendations.
Table I.
Primer sequences utilized for reverse transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequences (5′-3′) |
|---|---|
| Runx2 | F: TCCAGACCAGCAGCACTCCATA |
| R: TTCCATCAGCGTCAACACCATC | |
| ALP | F: CTGCGGGACTCAACGACACT |
| R: AGGAGGGTACTCATTGGC ATAGC | |
| OCN | F: TCACAGACCCAGACAGAACCG |
| R: AGCCCAGAGTCCAGGTAGCG | |
| HIF-1α | F: GTGTACCCTAACTAGCCGAGGA |
| R: GTTCACAAATCAGCACCAAGC | |
| VEGF | F: AAGAGCGATCCCCACGTCAA |
| R: TTCGTTTCAGTGCCACATACCA | |
| GAPDH | F: ATGTTTGTGATGGGCGTGAAGGTC |
| R: TTCTGGGTGGCAGTGAT |
F, forward; R, reverse; Runx2, runt-related transcription factor 2; ALP, alkaline phosphatase; OCN, osteocalcin; HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
Data are presented as mean ± standard deviation (n>3). Individual pairwise comparisons were analyzed by 2-sample, 2-tailed Student's t-tests. The statistical significance of differences among more than 2 groups was analyzed using one-way analysis of variance. All statistical analyses were performed using SPSS 18.0 (SPSS, Inc., Chicago. IL, USA) and P<0.05 was considered to indicate a statistically significant difference.
Results
General observations
Following surgery, all 6 dogs remained in good health and had no complications. All extraction sockets healed uneventfully and there were no signs of inflammation or other adverse reactions.
Micro-CT measurement
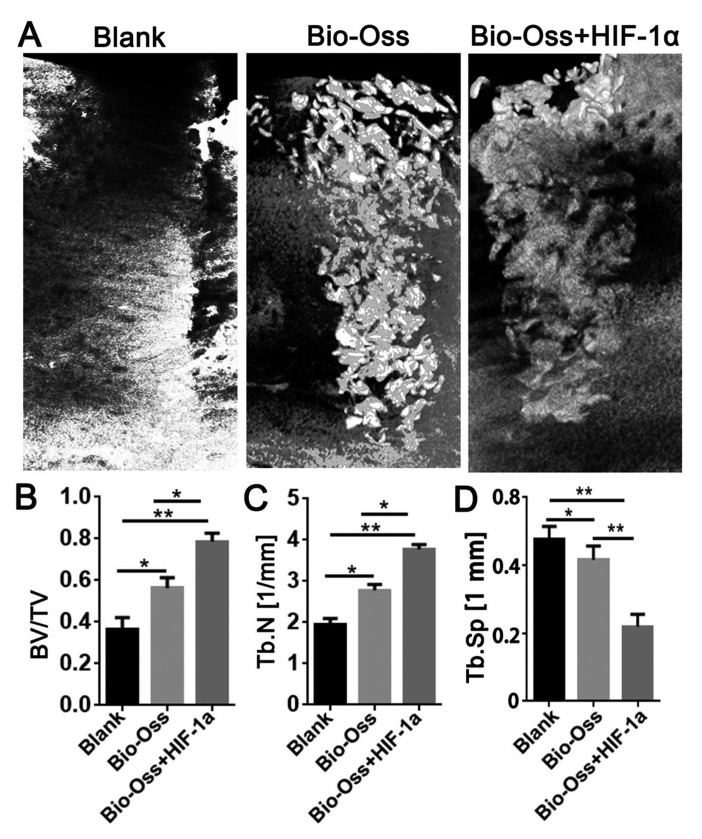
Micro-CT software was used for the three-dimensional reconstruction of the mandibular alveolar sockets, and the alveolar bone regeneration in the hollow of the extraction site was further evaluated. The results of micro-CT examination revealed that Bio-Oss combined with HIF-1α resulted in a significantly higher BV/TV (P<0.05 and P<0.01; Fig. 2B) and Tb.N (P<0.05 and P<0.01; Fig. 2C), and a significantly lower trabecular separation (Tb.Sp; each, P<0.01; Fig. 2D) compared with the other two groups.
Figure 2.
Micro-CT examination of the extraction sockets in the three groups 12 weeks post-surgery. (A) Three-dimensional reconstruction of the extraction sockets in Blank group, Bio-Oss group and Bio-Oss + HIF-1α group. (B) BV/TV. (C) Tb.N. (D) Tb.Sp. Data were presented as mean ± standard deviation, n>3. *P<0.05 and **P<0.01 as indicated. BV/TV, bone volume/tissue volume; Tb.N, trabecular number; Tb.Sp, trabecular separation; HIF-1α, hypoxia-inducible factor-1α.
Histological and histomorphometric analysis
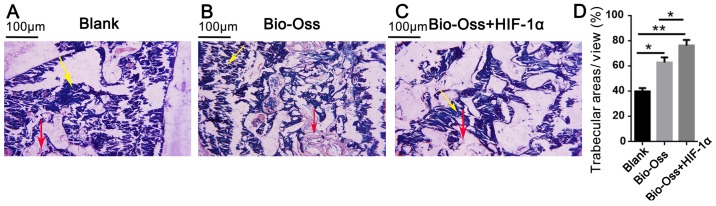
A total of 12 weeks post-surgery, all the extraction sockets were filled with new bone, and there were clear boundaries between old and new bone in each group. New bone consisted primarily of mature woven bone, a part of which was transformed into lamellar bone (Fig. 3A-C). In the Bio-Oss and Bio-Oss + HIF-1α groups, there was more active new bone formation, trabecular bone maturation and calcification compared with the blank group (Fig. 3). Furthermore, the trabecular bone areas were significantly more extensive in the Bio-Oss + HIF-1α group as compared with those in the blank and Bio-Oss groups (P<0.05 and P<0.01; Fig. 3D).
Figure 3.
Hematoxylin & eosin staining of the extraction sockets in each group at 12 weeks after surgery. (A) Blank group. (B) Bio-Oss group. (C) Bio-Oss + HIF-1α group. Yellow arrows indicate the bone marrow; red arrows indicate the trabecular bone. (D) Quantification of the trabecular bone areas in the three groups. Data were presented as mean ± standard deviation, n>3. *P<0.05 and **P<0.01 as indicated. HIF-1α, hypoxia-inducible factor-1α.
HIF-1α combined with Bio-Oss improves the expression of Runx2 and OCN in vivo
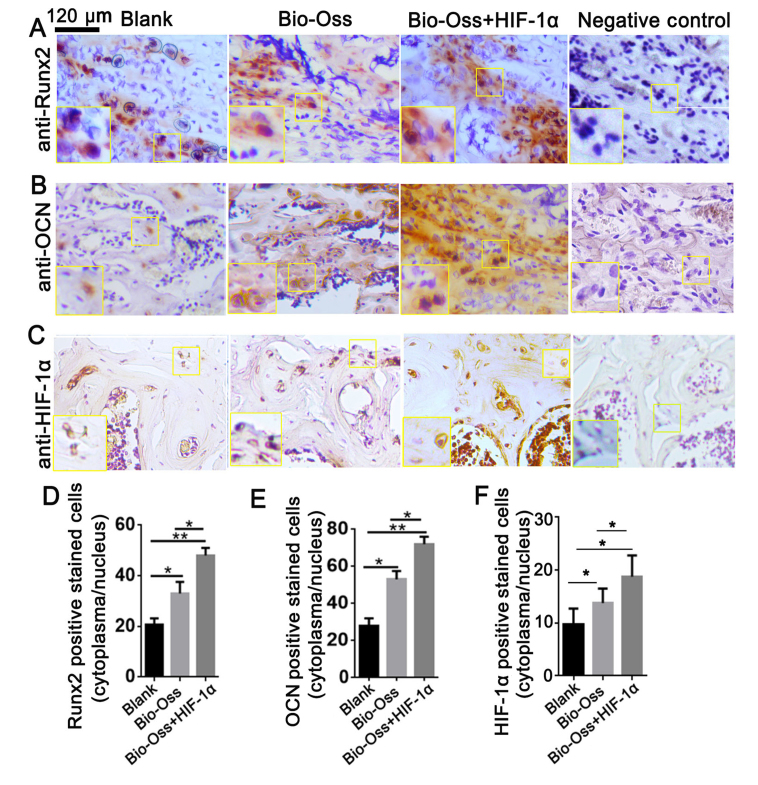
To further investigate the role of HIF-1α in osteoblast differentiation in the bone marrow, IHC staining was performed to analyze the protein expression level of Runx2, OCN and HIF-1α in bone sections (Fig. 4). Runx2 is the first transcription factor required for the determination of osteoblast commitment, and is predominantly expressed in preosteoblasts and immature osteoblasts (21). The present results demonstrated that the Runx2 expression level was significantly upregulated in the Bio-Oss + HIF-1α group compared with the other two groups (P<0.05 and P<0.01; Fig. 4A and D). The results also indicated that the number of HIF-1α-positive stained cells was significantly increased in the Bio-Oss + HIF-1α group compared with the other groups (both P<0.01; Fig. 4C and F). Notably, the expression level of OCN, which is primarily expressed in mature osteoblasts (22), was also significantly increased in the Bio-Oss + HIF-1α group compared with the other two groups (P<0.05 and P<0.01; Fig. 4B and E). These results indicated that HIF-1α treatment in the extraction sites promoted osteoblast differentiation and mineralization in bone tissues.
Figure 4.
HIF-1α combined with Bio-Oss upregulates the expression of Runx2 and OCN in vivo. Immunohistochemistry staining with (A) anti-Runx2 antibody, (B) anti-OCN antibody and (C) anti-HIF-1α antibody of paraffin sections from bones in the extraction sites. (D) Quantification of Runx2-positive stained cells in (A). (E) Quantification of OCN-positive stained cells in (B). (F) Quantification of HIF-1α-positive stained cells in (C). Data were presented as mean ± standard deviation, n>3. *P<0.05, **P<0.01 as indicated. HIF-1α, hypoxia-inducible factor-1α; Runx2, runt-related transcription factor 2; OCN, osteocalcin.
HIF-1α combined with Bio-Oss activates the mRNA expression levels of angiogenesis- and osteogenesis-associated genes in extraction sites
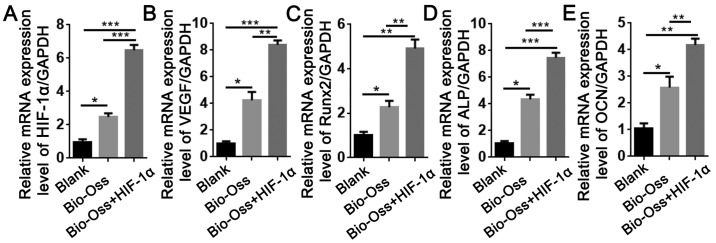
To examine the effects of HIF-1α treatment on the expression of angiogenesis- and osteogenesis-associated genes, RNA was isolated from the bone tissues around the extraction sites. The results of RT-qPCR revealed that the mRNA expression levels of HIF-1α and VEGF in the Bio-Oss + HIF-1α group were 7-fold and 9-fold higher, respectively, compared with the blank group, and 2-fold and 1.5-fold higher, respectively, compared with the Bio-Oss group (Fig. 5A and B). In addition, the expression of Runx2, alkaline phosphatase (ALP) and OCN in the Bio-Oss + HIF-1α group was significantly upregulated compared with the other two groups (P<0.05; Fig. 5C-E), which was consistent with the results of IHC staining. These results demonstrated that HIF-1α enhanced the function of Bio-Oss in promoting angiogenesis and osteogenesis in the extraction sites.
Figure 5.
HIF-1α combined with Bio-Oss upregulates the mRNA expression levels of the angiogenesis-associated genes. (A) HIF-1α and (B) VEGF, and the osteogenesis-associated genes (C) Runx2, (D) ALP and (E) OCN were indicated. Data are presented as mean ± standard deviation, n>3. *P<0.05, **P<0.01 and ***P<0.005 as indicated. HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; Runx2, runt-related transcription factor 2; ALP, alkaline phosphatase; OCN, osteocalcin.
HIF-1α combined with Bio-Oss upregulates the protein expression levels of osteogenesis- and angiogenesis-associated factors in extraction sites
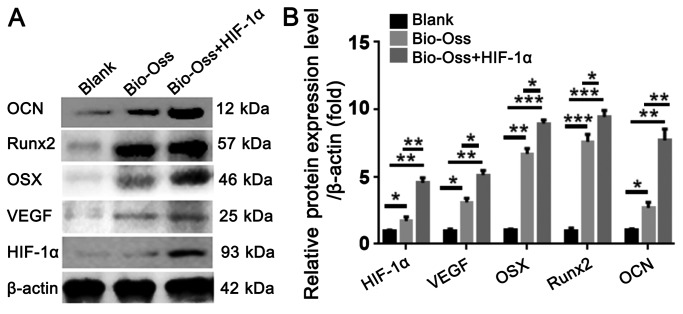
To further examine the effect of HIF-1α on osteogenesis and angiogenesis in the extraction sites at the protein level, protein was isolated from the bone tissues around the extraction sites. The results of western blotting indicated that the protein level of VEGF, OSX and Runx2 were significantly increased in the Bio-Oss + HIF-1α group compared with those in the blank group and Bio-Oss group. Furthermore, the protein level of OCN in the Bio-Oss + HIF-1α group was ~3-fold and 7-fold higher than those in the Bio-Oss group and Blank group (Fig. 6A and B). These results confirmed that application of HIF-1α in the extraction sites increased the protein expression levels of angiogenesis- and osteogenesis-associated factors.
Figure 6.
HIF-1α combined with Bio-Oss upregulates the protein expression levels of osteogenesis- and angiogenesis-associated factors in extraction sites. (A) Western blotting was used to detect the protein levels of OCN, Runx2, OSX, VEGF and HIF-1α in the bone tissues around the extraction sites. (B) Quantification of the protein levels in (A) by normalizing to β-actin. The results are presented as mean ± standard deviation, n>3. *P<0.05, **P<0.01 and ***P<0.005 as indicated. HIF-1α, hypoxia-inducible factor-1α; VEGF, vascular endothelial growth factor; Runx2, runt-related transcription factor 2; OCN, osteocalcin; OSX, osteoblast-specific transcription factor osterix.
Discussion
A variety of studies have demonstrated that HIF-1α is subjected to proteasomal degradation under non-hypoxic conditions (23,24). The present study investigated the effect of HIF-1α on alveolar ridge preservation. The present results demonstrated that the HIF-1α protein provided a superior micro-environment for alveolar ridge preservation compared with the Bio-Oss alone and blank groups. Therefore, Bio-Oss + HIF-1α appears to be an effective treatment for enhancing new bone formation in tooth extraction sites, as it promotes osteogenesis and angiogenesis.
A good substitute for alveolar ridge preservation should not only repair the bone defect, but must also be able to preserve the alveolar shape (15). The most frequently used method is the alveolar fossa plug, which, however, only fills the space rather than promoting the formation of new bone. In the present study, Bio-Oss in combination with HIF-1α was used to fill the extraction socket, which is a hypoxic environment following tooth extraction, similar to humans (25). The activation of HIF-1α is a specific and sensitive response to hypoxia, which may induce the expression of several pro-angiogenic factors, such as VEGF, inducible nitric oxidase, heme oxygenase-1, angiotensin-2 and EPO (26). Furthermore, HIF-1α can increase the number of osteoblasts, improve their ability to survive under hypoxic and ischemic conditions, and increase angiogenesis in hypoxic tissues (27). In addition, as an ideal support, Bio-Oss is similar to the human bone structure and has a large internal surface area, which makes it suitable for the adsorption of growth factors and biological proteins (28). The different pore sizes of the internal structure of Bio-Oss can promote the regeneration of blood vessels and maintain the stability of blood clots (29). Bio-Oss consists of small granules, with a diameter of 10–60 nm. In addition, its physical structure is similar to that of human bone, and can be absorbed during bone regeneration (30). As angiogenesis and osteogenesis are tightly coupled during bone regeneration, the combination of HIF-1α and Bio-Oss appears to be an optimal choice for alveolar ridge preservation following tooth extraction.
However, the application of HIF-1α in bone regeneration remains controversial. According to a previous report, HIF-1α is a pro-osteogenic factor in woven bone formation following damaging loading, but is considered an anti-osteogenic factor in lamellar bone formation following non-damaging mechanical loading (31). In the present study, the HIF-1α protein combined with Bio-Oss significantly increased trabecular bone formation in the extraction sockets compared with the other two groups, as indicated by micro-CT examination and H&E staining. Furthermore, the arrangement of the vascular network was more concentrated in the HIF-1α + Bio-Oss group compared with the other two groups. These results demonstrated that HIF-1a combined with Bio-Oss not only promoted osteogenesis in the extraction socket, but also stimulated the formation of new blood vessels around the bone. The results can be explained by the fact that tooth extraction may represent damaging mechanical loading that activates the supportive function of HIF-1α in trabecular bone formation. In addition, Tomlinson and Silva (32) also revealed that the osteogenesis-promoting function of HIF-1α in sites with damaging mechanical loading may be associated with its role as a transcription factor during angiogenesis. VEGF is the primary target of HIF-1α; it serves an important role in the vascularization of bone and it is upregulated after damaging bone loading (33). Additionally, inhibition of angiogenesis results in reduced woven bone formation following damaging mechanical loading (34). Consistently, the present results demonstrated that the expression levels of osteogenesis-associated genes (Runx2, OSX and OCN) and an angiogenesis-associated gene (VEGF) were significantly upregulated at the mRNA and protein levels in the Bio-Oss + HIF-1α group compared with the other two groups. The results indicate that HIF-1α may be used as an enhancer for trabecular bone formation in tooth extraction sites.
As previous reports have indicated, HIF-1α promotes the expression of osteogenic and angiogenic related genes in hypoxia, which indicates HIF-1α is one of the major regulators of angiogenic-osteogenic coupling in bone formation (35,36). Based on the results of the present study, the increased bone formation in the HIF-1α + Bio-Oss group may have occurred via the several mechanisms. First, HIF-1α may transactivates the expression of VEGF, which stimulates blood vessel formation in the extraction sites. The increased blood supply not only delivers the oxygen and nutrients to the bone, but also introduces more numbers of mesenchymal stem cells in the extraction sites that then commit to osteoblasts. The other is that, HIF-1α may act directly on the osteoblast-associated genes to promote osteoblast differentiation and mineralization. It has been demonstrated that in some experimental settings, HIF-1α and VEGF can regulate bone cell function without stimulating angiogenesis (37). The results from the present IHC staining and qPCR analysis also revealed increased expression levels of Runx2 and OCN in the HIF-1α+Bio-Oss group.
Taken together, the findings of the present study demonstrated that HIF-1α combined with Bio-Oss promotes trabecular bone formation in the extraction sockets by activating the expression of osteogenesis- and angiogenesis-associated genes. This study provides important insights into the role of HIF-1α under conditions of damaging mechanical loading during bone development. Further elucidation of the mechanisms underlying the regulatory effect of HIF-1α on the expression of Runx2, OSX and OCN may provide a useful tool for its application in bone regeneration.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (NSFC) (grant no. 81470768). and by grants from the Leading Academic Discipline Project of Pudong New Area Health System Discipline (grant no. PWRd2016-09) and Jiangxi Natural Science Foundation (grant no. 20181BAB2050380).
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YL, YHL and LT conceived and designed the experiments of the current study. LT, YZ and YL performed the experiments. YH and YHL analyzed the data. YL and YHL wrote and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee of the Ninth People's Hospital, Shanghai Jiao Tong University, approved the protocol used in this study. All animals were handled according to the guidelines established by the Institutional Animal Care Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests to disclose.
References
- 1.Mardas N, Chadha V, Donos N. Alveolar ridge preservation with guided bone regeneration and a synthetic bone substitute or a bovine-derived xenograft: A randomized, controlled clinical trial. Clin Oral Implants Res. 2010;21:688–698. doi: 10.1111/j.1600-0501.2010.01918.x. [DOI] [PubMed] [Google Scholar]
- 2.Araújo MG, Liljenberg B, Lindhe J. Dynamics of Bio-Oss Collagen incorporation in fresh extraction wounds: An experimental study in the dog. Clin Oral Implants Res. 2010;21:55–64. doi: 10.1111/j.1600-0501.2009.01854.x. [DOI] [PubMed] [Google Scholar]
- 3.Becker W, Clokie C, Sennerby L, Urist MR, Becker BE. Histologic findings after implantation and evaluation of different grafting materials and titanium micro screws into extraction sockets: Case reports. J Periodontol. 1998;69:414–421. doi: 10.1902/jop.1998.69.4.414. [DOI] [PubMed] [Google Scholar]
- 4.Jung RE, Glauser R, Schärer P, Hämmerle CH, Sailer HF, Weber FE. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res. 2003;14:556–568. doi: 10.1034/j.1600-0501.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 5.Simion M, Rocchietta I, Monforte M, Maschera E. Three-dimensional alveolar bone reconstruction with a combination of recombinant human platelet-derived growth factor BB and guided bone regeneration: A case report. Int J Periodontics Restorative Dent. 2008;28:239–243. [PubMed] [Google Scholar]
- 6.Anitua E. The use of plasma-rich growth factors (PRGF) in oral surgery. Pract Proced Aesthet Dent. 2001;13:487–493. [PubMed] [Google Scholar]
- 7.Cao J, Wang L, Lei DL, Liu YP, Du ZJ, Cui FZ. Local injection of nerve growth factor via a hydrogel enhances bone formation during mandibular distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:48–53. doi: 10.1016/j.tripleo.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Hu K, Olsen BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–38. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 11.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 12.Warren SM, Steinbrech DS, Mehrara BJ, Saadeh PB, Greenwald JA, Spector JA, Bouletreau PJ, Longaker MT. Hypoxia regulates osteoblast gene expression. J Surg Res. 2001;99:147–155. doi: 10.1006/jsre.2001.6128. [DOI] [PubMed] [Google Scholar]
- 13.Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor-HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19:90–97. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- 14.Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res. 2006;312:1693–1702. doi: 10.1016/j.yexcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Aludden HC, Mordenfeld A, Hallman M, Dahlin C, Jensen T. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: A systematic review. Int J Oral Maxillofac Surg. 2017;46:1030–1038. doi: 10.1016/j.ijom.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Paolantonio M, Scarano A, Di Placido G, Tumini V, D'Archivio D, Piattelli A. Periodontal healing in humans using anorganic bovine bone and bovine peritoneum-derived collagen membrane: A clinical and histologic case report. Int J Periodontics Restorative Dent. 2001;21:505–515. [PubMed] [Google Scholar]
- 17.Shabanovich A. P.067 application of BIO-OSS® and BIO-GIDE® at carrying out immediate dental implantology. J Cranio-Maxillofac Surg. 2006;34(Suppl 1):S148–S149. doi: 10.1016/S1010-5182(06)60574-X. [DOI] [Google Scholar]
- 18.He F, Ren W, Tian X, Liu W, Wu S, Chen X. Comparative study on in vivo response of porous calcium carbonate composite ceramic and biphasic calcium phosphate ceramic. Mater Sci Eng C Mater Biol Appl. 2016;64:117–123. doi: 10.1016/j.msec.2016.03.085. [DOI] [PubMed] [Google Scholar]
- 19.Roldán JC, Jepsen S, Miller J, Freitag S, Rueger DC, Açil Y, Terheyden H. Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone. 2004;34:80–90. doi: 10.1016/j.bone.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Komori T. Roles of Runx2 in skeletal development. Adv Exp Med Biol. 2017;962:83–93. doi: 10.1007/978-981-10-3233-2_6. [DOI] [PubMed] [Google Scholar]
- 22.Komori T. Regulation of skeletal development by the Runx family of transcription factors. J Cell Biochem. 2005;95:445–453. doi: 10.1002/jcb.20420. [DOI] [PubMed] [Google Scholar]
- 23.Zou D, Zhang Z, He J, Zhu S, Wang S, Zhang W, Zhou J, Xu Y, Huang Y, Wang Y, et al. Repairing critical-sized calvarial defects with BMSCs modified by a constitutively active form of hypoxia-inducible factor-1α and a phosphate cement scaffold. Biomaterials. 2011;32:9707–9718. doi: 10.1016/j.biomaterials.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daly B, Sharif MO, Newton T, Jones K, Worthington HV. Local interventions for the management of alveolar osteitis (dry socket) Cochrane Database Syst Rev. 2012;12:CD006968. doi: 10.1002/14651858.CD006968.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Eskandani M, Vandghanooni S, Barar J, Nazemiyeh H, Omidi Y. Cell physiology regulation by hypoxia inducible factor-1: Targeting oxygen-related nanomachineries of hypoxic cells. Int J Biol Macromol. 2017;99:46–62. doi: 10.1016/j.ijbiomac.2016.10.113. [DOI] [PubMed] [Google Scholar]
- 27.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeo EJ, Chun YS, Park JW. New anticancer strategies targeting HIF-1. Biochem Pharmacol. 2004;68:1061–1069. doi: 10.1016/j.bcp.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 29.Luo G, Huang Y, Gu F. rhBMP2-loaded calcium phosphate cements combined with allogenic bone marrow mesenchymal stem cells for bone formation. Biomed Pharmacother. 2017;92:536–543. doi: 10.1016/j.biopha.2017.05.083. [DOI] [PubMed] [Google Scholar]
- 30.Kellner J, Sivajothi S, McNiece I. Differential properties of human stromal cells from bone marrow, adipose, liver and cardiac tissues. Cytotherapy. 2015;17:1514–1523. doi: 10.1016/j.jcyt.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal S, Loder S, Brownley C, Cholok D, Mangiavini L, Li J, Breuler C, Sung HH, Li S, Ranganathan K, et al. Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proc Natl Acad Sci USA. 2016;113:E338–E347. doi: 10.1073/pnas.1515397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomlinson RE, Silva MJ. HIF-1α regulates bone formation after osteogenic mechanical loading. Bone. 2015;73:98–104. doi: 10.1016/j.bone.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hankenson KD, Gagne K, Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv Drug Deliv Rev. 2015;94:3–12. doi: 10.1016/j.addr.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Segar CE, Ogle ME, Botchwey EA. Regulation of angiogenesis and bone regeneration with natural and synthetic small molecules. Curr Pharm Des. 2013;19:3403–3419. doi: 10.2174/1381612811319190007. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki N, Gradin K, Poellinger L, Yamamoto M. Regulation of hypoxia-inducible gene expression after HIF activation. Exp Cell Res. 2017;356:182–186. doi: 10.1016/j.yexcr.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Riddle RC, Khatri R, Schipani E, Clemens TL. Role of hypoxia-inducible factor-1alpha in angiogenic-osteogenic coupling. J Mol Med (Berl) 2009;87:583–590. doi: 10.1007/s00109-009-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.