Abstract
Background
Rotavirus results in more diarrhoea‐related deaths in children under five years than any other single agent in countries with high childhood mortality. It is also a common cause of diarrhoea‐related hospital admissions in countries with low childhood mortality. Rotavirus vaccines that have been prequalified by the World Health Organization (WHO) include a monovalent vaccine (RV1; Rotarix, GlaxoSmithKline), a pentavalent vaccine (RV5; RotaTeq, Merck), and, more recently, another monovalent vaccine (Rotavac, Bharat Biotech).
Objectives
To evaluate rotavirus vaccines prequalified by the WHO (RV1, RV5, and Rotavac) for their efficacy and safety in children.
Search methods
On 4 April 2018 we searched MEDLINE (via PubMed), the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (published in the Cochrane Library), Embase, LILACS, and BIOSIS. We also searched the WHO ICTRP, ClinicalTrials.gov, clinical trial reports from manufacturers' websites, and reference lists of included studies and relevant systematic reviews.
Selection criteria
We selected randomized controlled trials (RCTs) in children comparing rotavirus vaccines prequalified for use by the WHO versus placebo or no intervention.
Data collection and analysis
Two review authors independently assessed trial eligibility and assessed risks of bias. One review author extracted data and a second author cross‐checked them. We combined dichotomous data using the risk ratio (RR) and 95% confidence interval (CI). We stratified the analysis by country mortality rate and used GRADE to evaluate evidence certainty.
Main results
Fifty‐five trials met the inclusion criteria and enrolled a total of 216,480 participants. Thirty‐six trials (119,114 participants) assessed RV1, 15 trials (88,934 participants) RV5, and four trials (8432 participants) Rotavac.
RV1
Children vaccinated and followed up the first year of life
In low‐mortality countries, RV1 prevents 84% of severe rotavirus diarrhoea cases (RR 0.16, 95% CI 0.09 to 0.26; 43,779 participants, 7 trials; high‐certainty evidence), and probably prevents 41% of cases of severe all‐cause diarrhoea (RR 0.59, 95% CI 0.47 to 0.74; 28,051 participants, 3 trials; moderate‐certainty evidence). In high‐mortality countries, RV1 prevents 63% of severe rotavirus diarrhoea cases (RR 0.37, 95% CI 0.23 to 0.60; 6114 participants, 3 trials; high‐certainty evidence), and 27% of severe all‐cause diarrhoea cases (RR 0.73, 95% CI 0.56 to 0.95; 5639 participants, 2 trials; high‐certainty evidence).
Children vaccinated and followed up for two years
In low‐mortality countries, RV1 prevents 82% of severe rotavirus diarrhoea cases (RR 0.18, 95% CI 0.14 to 0.23; 36,002 participants, 9 trials; high‐certainty evidence), and probably prevents 37% of severe all‐cause diarrhoea episodes (rate ratio 0.63, 95% CI 0.56 to 0.71; 39,091 participants, 2 trials; moderate‐certainty evidence). In high‐mortality countries RV1 probably prevents 35% of severe rotavirus diarrhoea cases (RR 0.65, 95% CI 0.51 to 0.83; 13,768 participants, 2 trials; high‐certainty evidence), and 17% of severe all‐cause diarrhoea cases (RR 0.83, 95% CI 0.72 to 0.96; 2764 participants, 1 trial; moderate‐certainty evidence).
No increased risk of serious adverse events (SAE) was detected (RR 0.88 95% CI 0.83 to 0.93; high‐certainty evidence). There were 30 cases of intussusception reported in 53,032 children after RV1 vaccination and 28 cases in 44,214 children after placebo or no intervention (RR 0.70, 95% CI 0.46 to 1.05; low‐certainty evidence).
RV5
Children vaccinated and followed up the first year of life
In low‐mortality countries, RV5 probably prevents 92% of severe rotavirus diarrhoea cases (RR 0.08, 95% CI 0.03 to 0.22; 4132 participants, 5 trials; moderate‐certainty evidence). We did not identify studies reporting on severe all‐cause diarrhoea in low‐mortality countries. In high‐mortality countries, RV5 prevents 57% of severe rotavirus diarrhoea (RR 0.43, 95% CI 0.29 to 0.62; 5916 participants, 2 trials; high‐certainty evidence), but there is probably little or no difference between vaccine and placebo for severe all‐cause diarrhoea (RR 0.80, 95% CI 0.58 to 1.11; 1 trial, 4085 participants; moderate‐certainty evidence).
Children vaccinated and followed up for two years
In low‐mortality countries, RV5 prevents 82% of severe rotavirus diarrhoea cases (RR 0.18, 95% CI 0.08 to 0.39; 7318 participants, 4 trials; moderate‐certainty evidence). We did not identify studies reporting on severe all‐cause diarrhoea in low‐mortality countries. In high‐mortality countries, RV5 prevents 41% of severe rotavirus diarrhoea cases (RR 0.59, 95% CI 0.43 to 0.82; 5885 participants, 2 trials; high‐certainty evidence), and 15% of severe all‐cause diarrhoea cases (RR 0.85, 95% CI 0.75 to 0.98; 5977 participants, 2 trials; high‐certainty evidence).
No increased risk of serious adverse events (SAE) was detected (RR 0.93 95% CI 0.86 to 1.01; moderate to high‐certainty evidence). There were 16 cases of intussusception in 43,629 children after RV5 vaccination and 20 cases in 41,866 children after placebo (RR 0.77, 95% CI 0.41 to 1.45; low‐certainty evidence).
Rotavac
Children vaccinated and followed up the first year of life
Rotavac has not been assessed in any RCT in countries with low child mortality. In India, a high‐mortality country, Rotavac probably prevents 57% of severe rotavirus diarrhoea cases (RR 0.43, 95% CI 0.30 to 0.60; 6799 participants, moderate‐certainty evidence); the trial did not report on severe all‐cause diarrhoea at one‐year follow‐up.
Children vaccinated and followed up for two years
Rotavac probably prevents 54% of severe rotavirus diarrhoea cases in India (RR 0.46, 95% CI 0.35 to 0.60; 6541 participants, 1 trial; moderate‐certainty evidence), and 16% of severe all‐cause diarrhoea cases (RR 0.84, 95% CI 0.71 to 0.98; 6799 participants, 1 trial; moderate‐certainty evidence).
No increased risk of serious adverse events (SAE) was detected (RR 0.93 95% CI 0.85 to 1.02; moderate‐certainty evidence). There were eight cases of intussusception in 5764 children after Rotavac vaccination and three cases in 2818 children after placebo (RR 1.33, 95% CI 0.35 to 5.02; very low‐certainty evidence).
There was insufficient evidence of an effect on mortality from any rotavirus vaccine (198,381 participants, 44 trials; low‐ to very low‐certainty evidence), as the trials were not powered to detect an effect at this endpoint.
Authors' conclusions
RV1, RV5, and Rotavac prevent episodes of rotavirus diarrhoea. Whilst the relative effect estimate is smaller in high‐mortality than in low‐mortality countries, there is a greater number of episodes prevented in these settings as the baseline risk is much higher. We found no increased risk of serious adverse events.
Keywords: Adult; Child; Child, Preschool; Humans; Infant; Infant, Newborn; Young Adult; Diarrhea; Diarrhea/prevention & control; Diarrhea/virology; Diarrhea, Infantile; Diarrhea, Infantile/prevention & control; Diarrhea, Infantile/virology; Randomized Controlled Trials as Topic; Rotavirus Infections; Rotavirus Infections/prevention & control; Rotavirus Vaccines; Rotavirus Vaccines/classification; Rotavirus Vaccines/therapeutic use; Vaccines, Attenuated; Vaccines, Attenuated/therapeutic use
Vaccines for preventing rotavirus diarrhoea: vaccines in use
What is the aim of this review?
The aim of this Cochrane Review was to find out if rotavirus vaccines are effective in preventing diarrhoea and deaths in infants and young children. We also aimed to find out if the rotavirus vaccines are safe. We collected and analyzed all relevant studies to answer these questions, and found 55 studies.
Key messages
RV1, RV5, and Rotavac prevent episodes of rotavirus diarrhoea (moderate‐ to high‐certainty evidence). We found no increased risk of serious adverse events (moderate‐ to high‐certainty evidence) including intussusception (where the bowel telescopes on itself, and can cause obstruction) (very low to low‐certainty evidence).
What was studied in the review?
Rotavirus infection is a common cause of diarrhoea in infants and young children, and can cause mild illness, hospitalization, and death. Since 2009, the World Health Organization (WHO) has recommended that a rotavirus vaccine be included in all national infant and child immunization programmes, and 95 countries have so far followed this recommendation. In the years before infants and children started receiving rotavirus vaccine, rotavirus infection resulted in about half a million deaths a year in children aged under five years, mainly in low‐ and middle‐income countries.
In this review we included randomized controlled trials in infants and young children that evaluated a monovalent rotavirus vaccine (RV1; Rotarix, GlaxoSmithKline) or a pentavalent rotavirus vaccine (RV5; RotaTeq, Merck). These vaccines have been evaluated in several large trials and are approved for use in many countries. We also included trials that evaluated another monovalent rotavirus vaccine (Rotavac; Bharat Biotech), which is used in India only. The rotavirus vaccines were compared with placebo or with no vaccine. The included studies did not allow comparisons between the vaccines.
What are the main results of the review?
We found 55 relevant studies with 216,480 participants. The trials took place in several locations worldwide. These studies compared a rotavirus vaccine versus placebo or versus no vaccine for infants and young children. The vaccines tested were RV1 (36 trials with 119,114 participants), RV5 (15 trials with 88,934 participants), and Rotavac (four trials with 8432 participants). Fifty‐one studies were funded or co‐funded by vaccine manufacturers, while four were independent of manufacturer funding.
In the first two years of life, RV1:
●prevents more than 80% of severe cases of rotavirus diarrhoea in countries with low death rates (high‐certainty evidence) ●prevents 35% to 63% of severe rotavirus diarrhoea in countries with high death rates (high‐certainty evidence) ●probably prevents 37% to 41% of severe cases of diarrhoea from all causes (such as any viral infection, bacterial infection, or parasitic infection) in countries with low death rates (moderate‐certainty evidence) ●probably prevents 18% to 27% of severe cases of diarrhoea from all causes in countries with high death rates (moderate‐ to high‐certainty evidence).
In the first two years of life, RV5:
●probably prevents 82% to 92% of severe cases of rotavirus diarrhoea in countries with low death rates (moderate‐certainty evidence) ●prevents 41% to 57% of severe cases of rotavirus diarrhoea in countries with high death rates (high‐certainty evidence) ●probably prevents 15% of severe cases of diarrhoea from all causes in countries with high death rates (moderate‐ to high‐certainty evidence); we did not identify any studies that reported on diarrhoea from all causes in countries with low death rates.
In the first two years of life, Rotavac:
●probably prevents more than 50% of severe cases of rotavirus diarrhoea in India, a country with high death rates (moderate‐certainty evidence) ●probably prevents 18% of severe cases of diarrhoea from all causes in India (moderate‐certainty evidence). Rotavac has not been evaluated in a randomized controlled trial in a country with low death rates.
We found little or no difference in the number of serious adverse events (moderate‐ to high‐certainty evidence), or intussusception cases (low‐ to very low‐certainty evidence), between those receiving RV1, RV5, or Rotavac compared with placebo or no intervention.
How up‐to‐date is this review?
We searched for studies that had been published up to 4 April 2018.
Summary of findings
Summary of findings for the main comparison.
RV1 compared to placebo for preventing rotavirus diarrhoea in low‐mortality countries
| Patient or population: children Setting: low‐mortality countries (WHO strata A and B) Intervention: RV1 Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | RV1 | |||||
| Severe cases of rotavirus diarrhoea Follow‐up: up to 1 year | 13 per 1000 | 2 per 1000 (1 to 3) | RR 0.16 (0.09 to 0.26) | 43,779 (7 studies) | ⊕⊕⊕⊕ higha | RV1 reduces severe rotavirus diarrhoea compared to placebo at up to one year follow‐up. One study (RV1 Vesikari 2007a‐EU) reported higher efficacy compared to the pooled data. When we excluded this study from the analysis, there was no heterogeneity observed in the pooled data |
| Severe cases of rotavirus diarrhoea Follow‐up: up to 2 years | 24 per 1000 | 4 per 1000 (3 to 5) | RR 0.18 (0.14 to 0.23) | 36,002 (9 studies) | ⊕⊕⊕⊕ high | RV1 reduces severe rotavirus diarrhoea compared to placebo at up to two years follow‐up. |
| Severe cases of all‐cause diarrhoea Follow‐up: up to 1 year | 41 per 1000 | 24 per 1000 (19 to 30) | RR 0.59 (0.47 to 0.74) | 28,051 (3 studies) | ⊕⊕⊕⊝ moderateb due to reporting bias |
RV1 probably reduces severe all‐cause diarrhoea compared to placebo at up to one year follow‐up. |
| Severe episodes of all‐cause diarrhoea Follow‐up: up to 2 years | 39 per 1000 | 24 per 1000 (22 to 28) | Rate Ratio 0.63 (0.56 to 0.71) | 39,091 (2 studies) | ⊕⊕⊕⊝ moderatec due to reporting bias |
RV1 probably reduces severe all‐cause diarrhoea compared to placebo at up to two years follow‐up. Three additional studies reported on cases of children with severe all‐cause diarrhoea (RR 0.60, 95% CI 0.36 to 1.02; 9417 participants); these data could not be pooled with the studies reporting on number of episodes |
| All‐cause death Follow‐up: 2 months to 2 years | 1 per 1000 | 2 per 1000 (1 to 2) | RR 1.22 (0.87 to 1.71) | 97,597 (22 studies) | ⊕⊕⊝⊝ lowd due to imprecision |
RV1 may make little or no difference to all‐cause death compared to placebo. |
| All serious adverse events Follow‐up: 2 months to 2 years | 45 per 1000 | 40 per 1000 (37 to 42) | RR 0.88 (0.83 to 0.93) | 96,233 (24 studies) | ⊕⊕⊕⊕ high | RV1 slightly reduces serious adverse events compared to placebo. |
| Serious adverse events: intussusception Follow‐up: 2 months to 2 years | 1 per 1000 | 1 per 1000 (0 to 1) | RR 0.69 (0.45 to 1.04) | 96,513 (17 studies) | ⊕⊕⊝⊝ lowe due to imprecision |
RV1 may make little or no difference to intussusception compared to placebo. |
| *The basis for the assumed risk is the control group risk across studies included in the meta‐analysis. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aWe observed heterogeneity (I2 statistic = 61%) in the pooled data, but given the strength of the evidence, and that estimates were all in the same direction, we did not downgrade the outcome. bDowngraded by one for risk of selective reporting bias. Only three of the seven studies reporting on severe rotavirus diarrhoea provided data for this outcome. cDowngraded by one for risk of selective reporting bias. Only five of the nine studies reporting on severe rotavirus diarrhoea provided data for this outcome. dDowngraded by two for imprecision. These trials were not powered to detect an effect on mortality. eDowngraded by two for imprecision. There was a 1:10,000 to 1:32,000 increased risk of intussusception with a previous rotavirus vaccine (Bines 2005), so these trials were not powered to detect an association between RV1 and intussusception.
Summary of findings 2.
RV1 compared to placebo for preventing rotavirus diarrhoea in high‐mortality countries
| Patient or population: children Settings: high‐mortality countries (WHO strata D and E) Intervention: RV1 Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | RV1 | |||||
| Severe cases of rotavirus diarrhoea Follow‐up: up to 1 year | 60 per 1000 | 22 per 1000 (14 to 36) | RR 0.37 (0.23 to 0.60) | 6114 (3 studies) | ⊕⊕⊕⊕ high | RV1 reduces severe rotavirus diarrhoea compared to placebo or no intervention at up to one year follow‐up. We did not downgrade for inconsistency as the heterogeneity observed in the pooled data (I2 statistic = 57%) was due to within‐study heterogeneity (RV1 Madhi 2010‐AF results split by country) |
| Severe cases of rotavirus diarrhoea Follow‐up: up to 2 years | 43 per 1000 | 28 per 1000 (22 to 35) | RR 0.65 (0.51 to 0.83) | 13,768** (2 studies) | ⊕⊕⊕⊕ high | RV1 reduces severe rotavirus diarrhoea compared to placebo or no intervention at up to two years follow‐up. Sensitivity analysis excluding the cluster‐RCT (RV1 Zaman 2017‐BGD) that contributed data to this outcome showed no significant change in effect estimate or 95% CI (RR 0.58, 95% CI 0.42 to 0.79, n = 2764, 1 RCT) |
| Severe cases of all‐cause diarrhoea Follow‐up: up to 1 year | 176 per 1000 | 129 per 1000 (99 to 167) | RR 0.73 (0.56 to 0.95) | 5639 (2 studies) | ⊕⊕⊕⊕ high | RV1 reduces severe all‐cause diarrhoea compared to placebo or no intervention at up to one year follow‐up. We did not downgrade for inconsistency as the heterogeneity observed in the pooled data (I2 statistic = 75%) was due to within‐study heterogeneity (RV1 Madhi 2010‐AF results split by country) |
| Severe cases of all‐cause diarrhoea Follow‐up: up to 2 years | 233 per 1000 | 191 per 1000 (166 to 222) | RR 0.82 (0.71 to 0.95) | 2764 (1 study) | ⊕⊕⊕⊝ moderatea due to indirectness |
RV1 probably slightly reduces severe all‐cause diarrhoea compared to placebo or no intervention at up to two years follow‐up. |
| All‐cause death Follow‐up: 2 months to 2 years | 24 per 1000 | 21 per 1000 (16 to 30) | RR 0.88 (0.64 to 1.22) | 8181 (8 studies) | ⊕⊕⊝⊝ lowb due to imprecision |
RV1 may make little or no difference to all‐cause death compared to placebo or no intervention. |
| All serious adverse events Follow‐up: 2 months to 2 years | 95 per 1000 | 84 per 1000 (72 to 99) | RR 0.89 (0.76 to 1.04) | 7481 (7 studies) | ⊕⊕⊕⊕ high | RV1 makes little or no difference to serious adverse events compared to placebo or no intervention. |
| Serious adverse events: intussusception Follow‐up: 2 months to 2 years | 0 per 100,000 | 0 per 100,000 (0 to 0) | RR 1.49 (0.06 to 36.63) | 17,492** (4 studies) | ⊕⊕⊝⊝ lowc due to imprecision |
RV1 may make little or no difference to intussusception compared to placebo or no intervention. Sensitivity analysis excluding the cluster‐RCT (RV1 Zaman 2017‐BGD) that contributed data to this outcome showed no change in effect estimate or 95% CI |
| *The basis for the assumed risk is the control group risk across studies included in the meta‐analysis. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **Number of participants in this table shows the true number of participants for this outcome; the number of events and the number of participants in the analysis has been adjusted for the included cluster trial RV1 Zaman 2017‐BGD using a design effect of 2.53. CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by one for indirectness. Trials were conducted in Malawi and South Africa, so generalization to any high‐mortality country is difficult. bDowngraded by two for imprecision. These trials were not powered to detect an effect on mortality. cDowngraded by two for imprecision. There was a 1:10,000 to 1:32,000 increased risk of intussusception with a previous rotavirus vaccine (Bines 2005), so these trials were not powered to detect an association between RV1 and intussusception.
Summary of findings 3.
RV5 compared to placebo for preventing rotavirus diarrhoea in low‐mortality countries
| Patient or population: children Settings: low‐mortality countries (WHO strata A and B) Intervention: RV5 Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | RV5 | |||||
| Severe cases of rotavirus diarrhoea Follow‐up: up to 1 year | 17 per 1000 | 1 per 1000 (1 to 5) | RR 0.08 (0.03 to 0.22) | 4132 (5 studies) | ⊕⊕⊕⊝ moderatea due to imprecision |
RV5 probably reduces severe rotavirus diarrhoea compared to placebo at up to one year follow‐up. |
| Severe cases of rotavirus diarrhoea Follow‐up: up to 2 years | 25 per 1000 | 4 per 1000 (2 to 10) | RR 0.18 (0.08 to 0.39) | 7318 (4 studies) | ⊕⊕⊕⊝ moderateb due to inconsistency |
RV5 probably reduces severe rotavirus diarrhoea compared to placebo at up to two years follow‐up. |
| Severe all‐cause diarrhoea Follow‐up: up to 1 year | ‐ | ‐ | ‐ | ‐ | ‐ | We found no studies that reported on this outcome in this setting |
| Severe all‐cause diarrhoea Follow‐up: up to 2 years | ‐ | ‐ | ‐ | ‐ | ‐ | We found no studies that reported on this outcome in this setting |
| All‐cause death Follow‐up: 2 months to 2 years | 1 per 1000 | 1 per 1000 (0 to 1) | RR 1.13 (0.65 to 1.96) | 77,642 (9 studies) | ⊕⊕⊝⊝ lowc due to imprecision |
RV5 may make little or no difference to all‐cause death compared to placebo. |
| All serious adverse events Follow‐up: 2 months to 2 years | 27 per 1000 | 25 per 1000 (23 to 28) | RR 0.93 (0.86 to 1.02) | 75,672 (8 studies) | ⊕⊕⊕⊕ high | RV5 makes little or no difference to serious adverse events compared to placebo. |
| Serious adverse events: intussusception Follow‐up: 2 months to 2 years | 1 per 1000 | 0 per 1000 (0 to 1) | RR 0.77 (0.41 to 1.45) | 78,907 (12 studies) | ⊕⊕⊝⊝ lowd due to imprecision |
RV5 may make little or no difference to intussusception compared to placebo. |
| *The basis for the assumed risk is the control group risk across studies included in the meta‐analysis. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by one for imprecision. The total number of events was very low. bDowngraded by one for inconsistency. We found substantial heterogeneity (I2 statistic = 44%). Consistency was restored when removing the one study carried out only in a very low‐mortality (stratum A) country, with results then showing a slightly smaller effect (RR 0.22, 95% CI 0.13 to 0.36, 6291 participants, 3 studies). cDowngraded by two for imprecision. These trials were not powered to detect an effect on mortality. dDowngraded by two for imprecision. There was a 1:10,000 to 1:32,000 increased risk of intussusception with a previous rotavirus vaccine (Bines 2005), so these trials were not powered to detect an association between RV1 and intussusception.
Summary of findings 4.
RV5 compared to placebo for preventing rotavirus diarrhoea in high‐mortality countries
| Patient or population: children Settings: high‐mortality countries (WHO strata D and E) Intervention: RV5 Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | RV5 | |||||
| Severe cases of rotavirus diarrhoea Follow‐up: up to 1 year | 30 per 1000 | 13 per 1000 (9 to 19) | RR 0.43 (0.29 to 0.62) | 5916 (2 studies) | ⊕⊕⊕⊕ high | RV5 reduces severe rotavirus diarrhoea compared to placebo at up to one year follow‐up. |
| Severe cases of rotavirus diarrhoea Follow‐up: up to 2 years | 63 per 1000 | 37 per 1000 (27 to 51) | RR 0.59 (0.43 to 0.82) | 5885 (2 studies) | ⊕⊕⊕⊕ high | RV5 reduces severe rotavirus diarrhoea compared to placebo at up to two years follow‐up. |
| Severe cases of all‐cause diarrhoea Follow‐up: up to 1 year | 77 per 1000 | 62 per 1000 (45 to 85) | RR 0.8 (0.58 to 1.11) | 4085 (1 study) | ⊕⊕⊕⊝ moderatea due to indirectness |
RV5 probably makes little or no difference to severe all‐cause diarrhoea compared to placebo at up to one year follow‐up. |
| Severe cases of all‐cause diarrhoea Follow‐up: up to 2 years | 130 per 1000 | 110 per 1000 (97 to 127) | RR 0.85 (0.75 to 0.98) | 5977 (2 studies) | ⊕⊕⊕⊕ high | RV5 slightly reduces severe all‐cause diarrhoea compared to placebo at up to two years follow‐up. |
| All‐cause death Follow‐up: 2 months to 2 years | 26 per 1000 | 23 per 1000 (17 to 32) | RR 0.92 (0.68 to 1.24) | 6806 (3 studies) | ⊕⊕⊝⊝ lowb due to imprecision |
RV5 may make little or no difference to all‐cause death compared to placebo. |
| All serious adverse events Follow‐up: 2 months to 2 years | 21 per 1000 | 19 per 1000 (14 to 27) | RR 0.92 (0.66 to 1.28) | 6830 (4 studies) | ⊕⊕⊕⊝ moderatec due to imprecision |
RV5 probably makes little or no difference to serious adverse events compared to placebo. |
| Serious adverse events: intussusception Follow‐up: 2 months to 2 years | See comment | See comment | Not estimable | 6588 (2 studies) | ⊕⊕⊝⊝ lowd due to imprecision |
No events were reported. RV5 may make little or no difference to intussusception compared to placebo. |
| *The basis for the assumed risk is the control group risk across studies included in the meta‐analysis. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by one for indirectness. Single trial conducted in three African countries (Mali, Ghana, and Kenya), so generalization to any high‐mortality country is difficult. bDowngraded by two for imprecision. These trials were not powered to detect an effect on mortality. cDowngraded by one for imprecision. The 95% CI includes both no effect and appreciable harm. dDowngraded by two for imprecision. There was a 1:10,000 to 1:32,000 increased risk of intussusception with a previous rotavirus vaccine (Bines 2005), so these trials were not powered to detect an association between RV1 and intussusception.
Summary of findings 5.
Rotavac compared to placebo for preventing rotavirus diarrhoea in high‐mortality countries
|
Patient or population: children Settings: one high‐mortality country (India) (WHO stratum D) Intervention: Rotavac Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Rotavac | |||||
|
Severe cases of rotavirus diarrhoea follow‐up: up to 1 year |
31 per 1000 | 13 per 1000 (9 to 19) | RR 0.43 (0.30 to 0.60) | 6799 (1 study) | ⊕⊕⊕⊝ moderatea due to indirectness |
Rotavac probably reduces severe rotavirus diarrhoea compared to placebo at up to one year follow‐up. |
| Severe cases of rotavirus diarrhoea follow‐up: up to 2 years | 47 per 1000 | 21 per 1000 (16 to 28) | RR 0.46 (0.35 to 0.60) | 6541 (1 study) | ⊕⊕⊕⊝ moderatea due to indirectness |
Rotavac probably reduces severe rotavirus diarrhoea compared to placebo at up to two years follow‐up. |
|
Severe cases of all‐cause diarrhoea follow‐up: up to 2 years |
93 per 1000 | 78 per 1000 (66 to 91) | RR 0.84 (0.71 to 0.98) | 6799 (1 study) | ⊕⊕⊕⊝ moderatea due to indirectness |
Rotavac probably slightly reduces severe all‐cause diarrhoea compared to placebo at up to one year follow‐up. |
|
All‐cause death follow‐up: up to 2 years |
7 per 1000 | 6 per 1000 (4 to 11) | RR 0.92 (0.52 to 1.62) | 8155 (2 studies) | ⊕⊝⊝⊝ very lowb,c due to indirectness and imprecision |
We are uncertain whether Rotavac reduced all‐cause death as the certainty of the evidence is very low. |
|
All serious adverse events follow‐up: up to 2 years |
204 per 1000 | 189 per 1000 (173 to 208) | RR 0.93 (0.85 to 1.02) | 8210 (3 studies) | ⊕⊕⊕⊝ moderateb due to indirectness |
Rotavac probably makes little or no difference to serious adverse events compared to placebo. |
|
Serious adverse events: intussusception follow‐up: up to 2 years |
1 per 1000 | 1 per 1000 (0 to 5) | RR 1.33 (0.35 to 5.02) | 8582 (4 studies) | ⊕⊝⊝⊝ very lowb,d due to indirectness and imprecision |
No events were reported in three of the four studies. We are uncertain whether Rotavac has an effect on intussusception as the certainty of the evidence is very low. |
| *The basis for the assumed risk is the control group risk across studies included in the meta‐analysis. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
aDowngraded by one for indirectness. Single trial conducted in India, so generalization to any high‐mortality country is difficult. bDowngraded by one for indirectness. All trials were conducted in India, so generalization to any high‐mortality country is difficult. cDowngraded by two for imprecision. These trials were not powered to detect an effect on mortality. dDowngraded by two for imprecision. There was a 1:10,000 to 1:32,000 increased risk of intussusception with a previous rotavirus vaccine (Bines 2005), therefore, these trials were not powered to detect an association between Rotavac and intussusception.
Background
Description of the condition
The global impact of rotavirus infection
Rotavirus is the leading known cause of severe gastroenteritis in infants and young children worldwide (Parashar 2006a; Vesikari 1997; WHO 2013). While nearly every child experiences at least one rotavirus infection in early childhood regardless of setting, the vast majority of rotavirus‐associated deaths occur in children in low‐ and middle‐income countries, particularly in sub‐Saharan Africa and in the Indian subcontinent. Prior to the rollout of rotavirus vaccination, rotavirus caused 37% of diarrhoeal deaths (˜ 450,000 deaths worldwide in 2008) in children younger than five years. Five countries accounted for more than half of all deaths, and 22% of deaths attributable to rotavirus infection occurred in India (Tate 2012). In high‐income countries, where deaths due to rotavirus are rare, rotavirus accounted for 40% to 50% of hospital admissions due to diarrhoeal disease in the pre‐rotavirus vaccine period (Linhares 2008; Parashar 2006a; Tate 2012).
Epidemiology of rotavirus infection
Rotavirus is transmitted primarily via the faecal‐oral route, with symptoms typically developing one to two days following infection. Rotavirus infection occurs throughout life, and successive rotavirus infections occur during infancy and early childhood. The first rotavirus infection typically results in the most severe disease outcome; subsequent rotavirus infections are associated with milder disease or may be asymptomatic. However, differences in the age of first infection and number of infections required to acquire protection from symptomatic disease vary from one population to another. Rotavirus diarrhoea is particularly associated with severe outcomes between the ages of three and 35 months (Parashar 2006b), with a peak incidence of all episodes occurring between six and 24 months (CDC‐ASIP 1999; Linhares 2008). The peak incidence of severe rotavirus disease occurs earlier in high‐mortality countries than in low‐mortality countries; an estimated 43% of all rotavirus hospitalizations in children aged under five occur by eight months of age in Africa compared with 27% in Europe (Crawford 2017; Sanderson 2011). Typically, infants in low‐income countries experience a greater number of symptomatic episodes (Gladstone 2011; Velázquez 1996). In temperate countries rotavirus infections display marked seasonality, with distinct peaks during the winter months and few infections identified outside this period, whereas rotavirus infections occur year‐round in most tropical countries.
Rotavirus classification
Rotaviruses are double‐stranded (ds) RNA viruses: genus Rotavirus, family Reoviridae. Each of the 11 dsRNA segments, contained within the core of a triple‐layered viral particle, encodes one or more viral proteins. Rotavirus A, which causes most human disease, is genetically diverse in each of its 11 genome segments (called genotypes), and a nucleotide sequence‐based, complete genome classification system is used. Because of their importance in protective immunity, the outer capsid proteins VP7 and VP4 have been most extensively investigated. Species A rotaviruses are classified into G and P genotypes, based on the sequence diversity of the RNA segments encoding VP7 and VP4, respectively; 32 G genotypes and 47 P genotypes have been described (Crawford 2017) (see Figure 1 for details). Rotavirus vaccines are designed to protect against disease caused by the most prevalent strain types; globally, G1P[8], G2P[4], G3P[8], G4P[8], G9P[8] and G12 in combination with P[6] or P[8] account for over 90% of the genotypes that infect humans (Bányai 2012).
Figure 1.
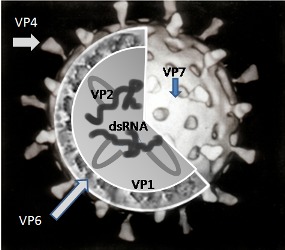
A simplified diagram of the location of rotavirus structural proteins (source: Graham Cohn, Wikipedia (public domain image)): Rotaviruses are segmented, double‐stranded RNA viruses. The mature, triple‐layered virus particle comprises a core (which contains the viral genome), a middle layer (comprised of viral protein (VP)6, and an outer layer (comprised of VP7 and VP4) as shown in the figure. VP6 defines rotavirus group, and most rotaviruses that infect humans are of group A. The two outer capsid proteins independently induce neutralizing antibodies: VP7, a glycoprotein, defines G‐serotype; and the protease‐sensitive VP4 protein defines P‐serotype. G‐serotype determined by serological methods correlates precisely with G‐genotype obtained through molecular assays, whereas there is an imperfect correlation of P‐serotype and P‐genotype; P‐genotype is thus included in square brackets.
Description of the intervention
Vaccines approved for use
This review evaluates three vaccines, including a monovalent rotavirus vaccine (RV1; Rotarix, GlaxoSmithKline Biologicals) and a pentavalent rotavirus vaccine (RV5; RotaTeq, Merck & Co., Inc.), which have been evaluated in several large trials and are in routine use in many countries; and a further monovalent vaccine (Rotavac, Bharat Biotech Ltd.), which is currently licensed in India only. All three vaccines are listed as prequalified vaccines by the WHO (Dellepiane 2015; WHO 2018). As of April 2018, 95 countries have introduced rotavirus vaccines into their immunization programmes (ROTA council 2018).
RV1 is an oral, live‐attenuated, human rotavirus vaccine derived from the most common circulating wild‐type strain G1P[8]. RV1 is based on a rotavirus of entirely human origin and is administered to infants in two oral doses with an interval of at least four weeks between doses. The manufacturer states that the "vaccination course should preferably be given before 16 weeks of age, but must be completed by the age of 24 weeks" (EMA 2011). As of May 2016, RV1 had been introduced in national immunization programmes in 63 countries around the world (PATH 2016).
RV5 is an oral, live, human‐bovine, reassortant, multivalent rotavirus vaccine developed from an original Wistar calf 3 (WC3) strain of bovine rotavirus. The vaccine contains five live, human‐bovine reassortant rotavirus strains. Four reassortant rotavirus strains each express one of the common human VP7 (G) types including G1, G2, G3, and G4, and the fifth reassortant expresses the common human VP4 (P) type P[8]. The three‐dose liquid vaccine is intended for infants aged between six and 32 weeks, with the first dose given at six to 12 weeks and subsequent doses administered at four‐ to 10‐week intervals; however, the third dose should not be given after 32 weeks of age (Merck 2008). As of May 2016, RV5 had been introduced in national immunization programmes in 22 countries around the world (PATH 2016).
Rotavac is a live‐attenuated, monovalent vaccine derived from a naturally‐occurring reassortant G9P[11] strain [116E] isolated from a newborn child in India (Yen 2014). This oral vaccine was developed by Bharat Biotech Ltd. in India and was licensed in India in 2014 (VAC Chandola 2017‐IND). Three doses are recommended, to be administered at 6, 10, and 14 weeks of age.
There are a further three rotavirus vaccines that have been licensed and approved for use in individual countries, but are not yet prequalified by the WHO. Lanzhou lamb rotavirus vaccine (LLR; Lanzhou Institute of Biomedical Products) which is licensed and used in China; a bovine rotavirus pentavalent vaccine (BRV‐PV, Rotasiil, Serum Institute of India Ltd.) which is licensed and used in India; and a monovalent vaccine (Rotavin‐M1, POLYVAC) which is licensed and used in Vietnam.
Vaccines no longer in use
Several vaccines, including the first licensed rotavirus vaccine (RRV‐TV; RotaShield, Wyeth Laboratories) were developed, tested in trials, and later abandoned or withdrawn from use. These vaccines are covered in a separate Cochrane Review (Soares‐Weiser 2004). RRV‐TV, a tetravalent rhesus‐human reassortant vaccine, was withdrawn from use in 1999 following reports of intussusception (bowel obstruction which occurs when one segment of bowel becomes enfolded within another segment). Evaluations have since suggested that the risk of intussusception was age‐related, with 80% of intussusception cases occurring in infants who were more than 90 days old when the first vaccine dose was administered (Simonsen 2005). Although it is still currently licensed, this vaccine is no longer in clinical use (Dennehy 2008).
How the intervention might work
Recommendations for rotavirus vaccine use
Vaccination with RV1 and RV5 was first recommended in 2006 in Europe and the Americas, where clinical trials had demonstrated vaccine efficacy of 85% to 100% (RV1 Ruiz‐Palac 06‐LA/EU; RV5 Vesikari 2006b‐INT). In April 2009, following clinical trials of RV1 and RV5 in low‐ and middle‐income countries in Africa and Asia, the WHO Strategic Advisory Group of Experts (SAGE) on Immunization recommended "the inclusion of rotavirus vaccination of infants into all national immunization programmes", with a stronger recommendation for countries where "diarrhoeal deaths account for ≥10% of mortality among children aged <5 years" (SAGE 2009). Due to an age‐related risk of intussusception identified with RRV‐TV (Murphy 2001), SAGE recommended administering the first dose of RV1 or RV5 to infants of six to 15 weeks of age, with the last dose administered before 32 weeks of age (SAGE 2009). In April 2012, SAGE relaxed the age restricted recommendation and advised to vaccinate "as soon as possible after the age of six weeks" because "the current age restrictions for the first dose (< 15 weeks) and last dose (< 32 weeks) are preventing vaccination of many vulnerable children" (Patel 2012; SAGE 2012).
Performance of oral rotavirus vaccines by setting
Many oral vaccines, including rotavirus vaccines, have demonstrated lower immunogenicity and efficacy in low‐ and middle‐income countries in Africa and Asia compared to high‐income countries in North America, South America, and Europe (Levine 2010). A systematic review demonstrated a correlation between lower vaccine efficacy against severe rotavirus diarrhoea and high child mortality rates (Fischer Walker 2011). The reasons for reduced oral vaccine efficacy in countries with higher child mortality rates are unknown; factors may include interference by maternal antibody, co‐administration with oral poliovirus vaccine, histoblood group antigen, diverse rotavirus strain types, micronutrient deficiencies, endemic infections such as malaria, tuberculosis, or HIV, concomitant enteric infections, gut inflammation, and altered gut microbiota (Czerkinsky 2015).
Outcomes of interest
The safety and efficacy of the licensed vaccines for the prevention of rotavirus gastroenteritis in infants have been assessed in several randomized controlled trials (RCTs) worldwide. The goal of this review is to systematically assess these trials and evaluate vaccine efficacy against rotavirus diarrhoea, all‐cause diarrhoea, and diarrhoea‐related medical visits and hospitalization. We also examine the occurrence of deaths and serious adverse events, including intussusception, to provide decision‐makers, clinicians, and caregivers with the relevant information to aid decisions about vaccine use.
Why it is important to do this review
Development of Cochrane systematic rotavirus vaccine reviews
The original Cochrane Review of rotavirus vaccines (Soares‐Weiser 2004) examined vaccines in use and other vaccines, including those that were no longer in use or were in development. Soares‐Weiser 2004 concluded that more trials were needed before routine vaccine use could be recommended. An update in 2009 included a new search, revised inclusion criteria (only vaccines in use in children), updated review methods and new authors. The review was updated again in 2010 with nine new studies (Soares‐Weiser 2010). The 2010 version of the review concluded that RV1 and RV5 are both effective vaccines for the prevention of rotavirus diarrhoea. Another update in February 2012 added a further nine new studies, GRADE ‘Summary of findings' tables and, again, new authors joined the team (Soares‐Weiser 2012a). The November 2012 update included a new search, major restructuring of analyses, including re‐evaluating primary outcomes in consultation with the WHO to reflect the observation that vaccine efficacy profiles are different in countries with different mortality rates (Soares‐Weiser 2012b). This current update adds a further 10 RV1 and RV5 studies to the review and four studies of a new vaccine, Rotavac, that has been prequalified by the WHO since the previous version of the review.
Objectives
To evaluate rotavirus vaccines prequalified by the WHO (RV1, RV5, and Rotavac) for their efficacy and safety in children.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Children (age as defined in the trials).
Types of interventions
Intervention
Rotavirus vaccines approved by the WHO vaccine prequalification programme (Dellepiane 2015; WHO 2018).
Control
Placebo, no vaccination, or other vaccine.
Types of outcome measures
Primary
We selected our primary outcome measures in consultation with the WHO, and stratified them according to high‐ or low‐mortality rate, based on WHO mortality strata (WHO 1999), and up to one and up to two years follow‐up.
Rotavirus diarrhoea: severe (as defined in trial report)
All‐cause diarrhoea: severe
All‐cause death
Serious adverse events (that are fatal, life‐threatening, or result in hospitalization); e.g. Kawasaki disease
Intussusception
Secondary
Rotavirus diarrhoea: of any severity
All‐cause diarrhoea (as defined in trial report)
Rotavirus diarrhoea: requiring hospitalization
All‐cause diarrhoea: requiring hospitalization
Emergency department visit
Hospital admission: all‐cause
Reactogenicity (capacity to produce an adverse reaction, such as fever, diarrhoea, and vomiting)
Adverse events that require discontinuation of vaccination schedule
Other
-
Immunogenicity
Vaccine virus shedding in stool
Seroconversion: conversion from seronegative to seropositive for anti‐rotavirus IgA antibodies
Dropouts
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and ongoing).
For this review update, Dr Vittoria Lutje (Information Specialist, Cochrane Infectious Diseases Group) searched the following databases using the search terms and strategy described in Appendix 1.
Cochrane Infectious Diseases Group Specialized Register (4 April 2018)
Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library (2018, Issue 4)
MEDLINE (via PubMed; 1966 to April 2018)
Embase (1974 to 4 April 2018)
LILACS (1982 to 4 April 2018)
BIOSIS (1926 to 4 April 2018)
We also searched the WHO International Clinical Trials Registry Platform (ICTRP) and Clinicaltrials.gov Clinical Study Register (www.clinicaltrials.gov) on 4 April 2018, using ‘rotavirus' as the search term.
We searched manufacturers' websites for clinical trial reports. We also checked the reference lists of relevant systematic reviews and included studies.
Data collection and analysis
Selection of studies
For this review update, we uploaded and screened references in DistillerSR online. Two review authors independently screened each title and abstract identified in the search. We retrieved full texts for potentially relevant references and two review authors again screened them independently, resolving disagreements by recourse to a third review author. We tabulated the excluded studies along with the reason for excluding them in the Characteristics of excluded studies tables. We ensured that data from each trial were entered only once in our review. In previous versions of this review we had screened references in an EndNote database.
Data extraction and management
For this review update, we extracted data in DistillerSR online. We created forms for data collection, which were piloted and then revised after the review author team's discussion. For previous versions of this review we had used Microsoft Word or Excel data collection forms.
One review author extracted data and another review author cross‐checked them. All outcomes were dichotomous, and we extracted the total number of participants and the number of participants who experienced the event. We cross‐checked the extracted data to identify errors, resolving disagreements by referring to the trial report or by consulting a third review author. One review author entered data into Review Manager 5 (RevMan 5) (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors independently assessed the risks of bias of each trial, using the Cochrane ‘Risk of bias' tool (Higgins 2017). Based on the guidance of the Cochrane ‘Risk of bias' tool (Higgins 2017), we created a form to make judgements on the risk of bias for the rotavirus diarrhoea outcome measure in six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other potential sources of bias. We categorized these judgements as ‘low', ‘high', or ‘unclear' risk of bias. We resolved disagreements through discussion with a third review author.
For the 2012 published version of this review, we asked for help from Dr Ana Maria Restrepo at the WHO Initiative for Vaccine Research, who contacted the vaccine manufacturers GlaxoSmithKline (RV1) and Merck (RV5), who were involved in designing and funding most of the included trials. We provided them with an Excel spreadsheet with specific details of each trial that would impact on the assessment of risk of bias. We received details from Merck (RV5), (see Characteristics of included studies for details). For this review update, we matched most of the previously‐included RV1 studies to the full clinical trial reports available on the manufacturer's website (www.gsk‐clinicalstudyregister.com). More details were available in these trial reports than in the published studies, that were helpful in assessing the risks of bias for these studies.
Measures of treatment effect
We analyzed dichotomous data of cases by calculating the risk ratio (RR) for each trial (expressed using blue squares in forest plots) with the uncertainty in each result expressed using 95% confidence intervals (CIs). For dichotomous data of events that could occur more than once in one participant, we calculated the rate ratio (expressed using red squares in forest plots) on the logarithmic scale using the generic inverse variance method (see Data synthesis for more details). For outcomes that included cluster‐RCTs we calculated risk ratios (expressed using red squares in forest plots) using the generic inverse variance method (see Unit of analysis issues for more details).
Unit of analysis issues
When trials had multiple treatment arms and we considered it suitable, we grouped the trial arms. We excluded irrelevant trial arms.
We pooled cluster‐RCT data that had been adjusted for clustering with data from trials that randomly assigned individuals (individual‐RCTs). For outcomes that included cluster‐RCTs, we pooled risk ratios on the logarithmic scale with their standard errors using the generic inverse variance method (16.3.3. in Higgins 2011). When the results of a cluster‐RCT had not been adjusted for clustering, we imputed the clustering effect (intracluster correlation coefficient (ICC)) from another study, and performed sensitivity analyses excluding these studies.
Dealing with missing data
We undertook a complete‐case analysis (the number analyzed) and an intention‐to‐treat analysis when data were available.
Assessment of heterogeneity
We initially assessed heterogeneity in the results of the trials by inspecting the graphical presentations and by calculating the Chi2 test of heterogeneity. However, we were aware of the fact that the Chi2 test has a poor ability to detect statistically significant heterogeneity among studies. We therefore also quantified the impact of heterogeneity in the meta‐analysis using a measure of the degree of inconsistency in the studies' results (Higgins 2003). This measure (the I2 statistic) describes the percentage of total variation across studies that are due to heterogeneity rather than to the play of chance (Higgins 2003). The I2 statistic values lie between 0% and 100%, and a simplified categorization of heterogeneity could be low, moderate, and high for I2 statistic values of 25%, 50%, and 75% respectively (Higgins 2003).
Assessment of reporting biases
If 10 or more studies were included in an outcome, we examined a funnel plot for the primary outcome (severe rotavirus diarrhoea), estimating the precision of trials (plotting the RR against the standard error (SE) of the log of RR) to estimate potential asymmetry.
Data synthesis
We stratified all analyses by the type of vaccine, RV1, RV5 or Rotavac. Subsequently, we grouped all outcomes in the meta‐analyses according to the time point when the outcome was measured or the number of rotavirus seasons, or both, as follows: less than two months; up to one year (one rotavirus season); up to two years (up to two rotavirus seasons); and up to three years (three rotavirus seasons). If data were available for more than one time point, we used the number of completers for each time point in the trial.
For the current update, we stratified each primary outcome (rotavirus diarrhoea, all‐cause diarrhoea, all‐cause death, all serious adverse events, and intussusception) and selected secondary outcomes (rotavirus diarrhoea and all‐cause diarrhoea of any severity, and all‐cause hospitalization) by country mortality rate according to WHO mortality strata (WHO 1999), as follows:
Low‐mortality: countries in WHO strata A and B (very low/low child mortality and low adult mortality)
High‐mortality: countries in WHO strata D and E (high child mortality and high/very high adult mortality)
We used a fixed‐effect model, unless we found statistically significant heterogeneity (P < 0.10) for a specific outcome, in which case we used the random‐effects model.
We included separate analyses for cases of diarrhoea (e.g. a child who has diarrhoea regardless of the number of episodes) and episodes (i.e. one child can experience more than one episode), where data permitted. We combined episodes using the rate ratio in the logarithmic scale and SE, with the uncertainty in each result being expressed using a 95% CI (9.4.8. in Higgins 2011).
Certainty of the evidence
We interpreted the findings of this review using the GRADE approach (Schünemann 2017), and we used GRADE profiler (GRADE 2004) to import data from RevMan 5 (RevMan 2014) to create ‘Summary of findings' tables. These tables provide outcome‐specific information concerning the overall certainty of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient care and decision‐making, and is reflected as follows: high certainty ("vaccine prevents...."); moderate certainty ("vaccine probably prevents..."); low certainty ("vaccine may prevent...."); and very low certainty ("we do not know whether or not the vaccine prevents....").
We selected primary outcomes, all stratified by vaccine and high or low country mortality, for inclusion in the ‘Summary of findings' tables: severe rotavirus diarrhoea; severe all‐cause diarrhoea; all‐cause death; serious adverse events; and intussusception.
Subgroup analysis and investigation of heterogeneity
In addition to stratifying the results by country‐based high‐mortality and low‐mortality rates using WHO mortality country strata (WHO 1999), we planned to perform subgroup analyses to assess the impact of the following possible sources of heterogeneity for any of the included vaccines: vaccine protection against specific rotavirus G types; and vaccination of special groups, including immunocompromised (including HIV‐infected) children and children with malnutrition. In previous versions of this review (Soares‐Weiser 2010; Soares‐Weiser 2012a), we also analyzed vaccine effect according to each study's country income, use of other childhood vaccines, number of doses administered, source of funding, and whether infants were born prematurely or were breast‐ or formula‐fed. These subgroup analyses did not show any differences, and are not presented in this updated version; they can be found in Soares‐Weiser 2010 and Soares‐Weiser 2012a.
Sensitivity analysis
We also planned to conduct sensitivity analyses for the primary outcomes according to allocation concealment (high, low, and unclear risk of bias) for outcomes in which data could not be pooled because of significant heterogeneity (I2 statistic > 75%).
Results
Description of studies
Results of the search
The update search in 2017 identified 1247 records and the update search in 2018 identified a further 488 records. After de‐duplication, we screened 1614 records and considered 1500 to be irrelevant. We reviewed the full texts of 114 records. In the previously published version of this review there were 41 included studies. The review now includes 55 independent trials (see Characteristics of included studies), 14 of which are new to this update (RV1 Colgate 2016‐BGD; RV1 Kim 2012‐KOR; RV1 Li 2013a‐CHN; RV1 Li 2013b‐CHN; RV1 Li 2014‐CHN; RV1 NCT00158756‐RUS; RV1 Zaman 2017‐BGD; RV5 Dhingra 2014‐IND; RV5 Levin 2017‐AF; RV5 Mo 2017‐CHN; VAC Bhandari 2006‐IND; VAC Bhandari 2009‐IND; VAC Bhandari 2014‐IND; VAC Chandola 2017‐IND) and we also added another 23 new companion papers to previously included trials with this update. The review also includes 15 ongoing studies (see Characteristics of ongoing studies). We excluded 78 studies for the reasons given in the Characteristics of excluded studies section.
Included studies
The 55 included trials enrolled about 216,480 participants (approximate number, as some trials provided only the number evaluable), and each trial compared a rotavirus vaccine with a placebo. The vaccines tested were RV1 (36 trials reported in 171 publications or reports; 119,114 participants), RV5 (15 trials reported in 60 publications or reports; 88,934 participants), and Rotavac (4 trials reported in 13 publications or reports; 8432 participants).
The trials were conducted in Africa, Asia, Europe, and the Americas, and the location can be identified in the study reference: AF, Africa; AS, Asia; EU, Europe; INT, several international locations; LA, Latin America; NA, North America; or country three‐letter acronym according to ISO 3166‐1 Alpha‐3 (e.g. BGD for Bangladesh) from www.all‐acronyms.com/special/countries_acronyms_and_abbreviations, if the study was conducted in a single country.
1. RV1
The 36 RV1 trials were published between 1998 and 2017. Five of the trials are unpublished and were located on the GlaxoSmithKline website through clinicalstudyresults.org or clinicaltrials.gov. One trial (RV1 Madhi 2010‐AF) provided country‐specific data for efficacy outcomes but not for safety outcomes, and was consequently split into RV1 Madhi 2010‐MWI and RV1 Madhi 2010‐ZAF for the Malawi‐ and South Africa‐specific data. Twenty‐five trials enrolled around 500 participants or fewer, three trials enrolled around 1000 participants, seven trials enrolled between 2155 and 12,318 participants, and one large trial enrolled 63,225 participants. Most children were aged between one and three months at the time of the first vaccination.
Population
Most trials included healthy infants. Two trials included HIV‐infected or ‐exposed infants (RV1 Madhi 2010‐AF; RV1 Steele 2010a‐ZAF), one trial included premature infants (RV1 Omenaca 2012‐EU), and one trial included children aged two to six years (RV1 Li 2013a‐CHN).
Outcome measures
Each trial reported on one or more of the outcome measures specified for this review (see Appendix 2). We included data on participants requiring medical visits, as this was reported in some trials and is a similar outcome measure to participants requiring hospitalization.
Twenty‐three trials were safety studies, reporting mainly safety outcomes (e.g. serious adverse events and reactogenicity), immunogenicity outcomes, or both. Eleven of these trials also reported efficacy outcomes with a follow‐up of up to two months. Eleven trials reported one or more efficacy outcomes (e.g. rotavirus diarrhoea) in addition to safety outcomes; most reported one or more immunogenicity outcomes. Two trials reported on efficacy or effectiveness but not safety or immunogenicity (RV1 Colgate 2016‐BGD; RV1 Zaman 2017‐BGD). The trials varied in the length of follow‐up, but in general the trials that specified efficacy outcome measures had longer follow‐up times (Appendix 2).
As shown in Appendix 3, rotavirus diarrhoea (of any severity) was the most common efficacy outcome reported (by 23 trials); 14 trials reported on severe rotavirus diarrhoea, and 10 reported on rotavirus diarrhoea requiring hospitalization. Data on all‐cause diarrhoea were provided by 17 trials, and severe all‐cause diarrhoea by nine trials. Most reported all‐cause death and dropouts, but other efficacy outcomes were reported by few trials.
For safety outcomes (Appendix 4), 29 trials reported on reactogenicity, all but four trials reported on serious adverse events, and 24 reported on adverse events leading to discontinuation of the intervention.
Most trials reported on one or more immunogenicity outcomes; see Appendix 4.
Location
Early trials were conducted in North America and Europe, but since 2005 trials have also been conducted in Asia (Bangladesh, China, India, Japan, Philippines, South Korea, Singapore, Thailand, Vietnam; 17 trials), Latin America (Argentina, Brazil, Chile, Colombia, Dominican Republic, Honduras, Mexico, Nicaragua, Panama, Peru, Venezuela; six trials), and Africa (South Africa, Malawi; four trials); see Appendix 5. Most trials had multiple sites, often in several countries; RV1 Vesikari 2007a‐EU included 98 sites in six European countries.
Country mortality rate
Most trials were conducted in countries with low mortality rates, corresponding to WHO mortality strata A and B. Eight trials were conducted in countries with high mortality rates (RV1 Colgate 2016‐BGD; RV1 Madhi 2010‐AF; RV1 Narang 2009‐IND; RV1 Steele 2008‐ZAF; RV1 Steele 2010a‐ZAF; RV1 Steele 2010b‐ZAF; RV1 Zaman 2009‐BGD; RV1 Zaman 2017‐BGD), corresponding to WHO mortality strata D and E; see Appendix 5. For RV1 Madhi 2010‐AF, available data were split between countries into RV1 Madhi 2010‐MWI and RV1 Madhi 2010‐ZAF. Two trials were conducted in several countries with both low and high mortality: RV1 GSK[033] 2007‐LA was conducted in four study centres in a high‐mortality country (Peru), but also in three study centres in two low‐mortality countries (Colombia and Mexico), and was placed in the high‐mortality group; and RV1 Ruiz‐Palac 06‐LA/EU was conducted mainly in low‐mortality countries in Latin America and in Finland, but also in two high‐mortality countries (Nicaragua and Peru), and was placed in the low‐mortality group.
Vaccine schedule
The trials varied in the vaccine dose and schedule (see Appendix 6). Most trials gave two doses of the vaccine with virus concentration of more than 106 plaque‐forming units (PFUs). Older trials, conducted between 1998 and 2005, tended to include slightly lower PFUs or a range of PFUs for comparison.
RV1 was given as two doses in all but five trials: one trial conducted in partnership with GlaxoSmithKline and PATH Rotavirus Vaccine Program tested two and three doses of the vaccine (RV1 Madhi 2010‐AF); another trial conducted by GlaxoSmithKline in which the poliovirus vaccine was co‐administered with RV1, tested two or three vaccine doses to investigate differences in immune response (RV1 Steele 2010b‐ZAF); a third study tested three vaccine doses in HIV‐positive infants (RV1 Steele 2010a‐ZAF); a fourth study tested three vaccine doses in healthy infants (RV1 GSK[021] 2007‐PAN); a fifth study that included children aged two to six years administered one dose only (RV1 Li 2013a‐CHN).
Some trials compared more than one arm: different PFU virus concentrations (RV1 Vesikari 2004a‐FIN; RV1 Dennehy 2005‐NA; RV1 Phua 2005‐SGP; RV1 Salinas 2005‐LA; RV1 Ward 2006‐USA); different formulations (RV1 GSK[021] 2007‐PAN; RV1 GSK[033] 2007‐LA; RV1 GSK[101555] 2008‐PHL; RV1 Kerdpanich 2010‐THA; RV1 Vesikari 2011‐FIN); co‐administration of other vaccine (RV1 Steele 2008‐ZAF; RV1 Zaman 2009‐BGD; RV1 NCT00158756‐RUS; RV1 Li 2014‐CHN); and different intervals between doses (RV1 Anh 2011‐PHL; RV1 Anh 2011‐VNM).
Infant vaccination status
All but four trial reports referred to vaccination with other infant vaccines (see Appendix 6). Most trials co‐administered other routine infant vaccines, such as diphtheria‐tetanus‐acellular pertussis, Haemophilus influenzae type b (HiB), inactivated polio vaccine, and hepatitis B vaccine (HBV). Some trials also co‐administered oral polio vaccine. Other trials imposed a two‐week separation between other infant vaccines and rotavirus vaccine or placebo, or specified other vaccines as not allowed.
Methods for collecting adverse event data
Fifteen of the 36 trials did not provide details of how adverse event data were collected. Out of the trials that did report the method of collecting adverse event data, 13 trials used passive methods (e.g. diary cards), two used an active method ("active surveillance system"), and five used both passive and active methods (e.g. diary card plus regular telephone calls to parents); see Appendix 7.
Source of funding
Most trials were supported by GlaxoSmithKline Biologicals, three of which were in partnership with PATH Rotavirus Vaccine Program (RV1 Li 2014‐CHN; RV1 Madhi 2010‐AF; RV1 Zaman 2009‐BGD), and another two in partnership with RAPID trials and the WHO (RV1 Steele 2008‐ZAF; RV1 Steele 2010a‐ZAF). One trial was funded by The Bill and Melinda Gates Foundation (RV1 Colgate 2016‐BGD) and one by GAVI and PATH (RV1 Zaman 2017‐BGD). Three trials were sponsored by Avant Immunotherapeutics (formerly Virus Research Institute, Inc.) (RV1 Bernstein 1998‐USA; RV1 Bernstein 1999‐USA; RV1 Ward 2006‐USA).
2. RV5
We identified 15 trials of RV5 vaccine. The earliest was reported in 2003 and the most recent in 2017. One of the trials is unpublished and was accessed via clinicalstudyresults.org. Two trials (RV5 Armah 2010‐AF and RV5 Zaman 2010‐AS) provided country‐specific data for some outcomes but not for all outcomes, and were consequently split into RV5 Armah 2010‐GHA; RV5 Armah 2010‐KEN; and RV5 Armah 2010‐MLI for the Ghana‐, Kenya, and Mali‐specific data, and RV5 Zaman 2010‐BGD and RV5 Zaman 2010‐VNM for the Bangladesh‐ and Vietnam‐specific data. Overall, 88,934 participants were included in the trials; the largest trial included 70,301 participants (RV5 Vesikari 2006b‐INT) and the smallest included 48 participants (RV5 Lawrence 2012‐CHN). For the 2012 update of this review, we received new information from Merck (Merck 2012) for some of the trials on the outcomes serious adverse events, intussusception, and deaths. We have incorporated the new information into the analyses and have indicated this in the Characteristics of included studies section.
Population
Most trials included healthy infants. One trial included both healthy and HIV‐infected infants (RV5 Armah 2010‐KEN), another trial included HIV‐exposed but uninfected and HIV‐infected infants (RV5 Levin 2017‐AF), and one trial included prematurely‐born infants as well as those born at normal gestation (RV5 Vesikari 2006b‐INT). All but two trials enrolled children aged between one month and three months; the children in RV5 Vesikari 2006a‐FIN were aged between three months and six months, and there was a child cohort (2‐ to 6‐year‐old children) in addition to an infant cohort in RV5 Lawrence 2012‐CHN.
Outcome measures
Six trials were safety studies (Appendix 2), reporting safety outcomes (e.g. serious adverse events and reactogenicity) and generally immunogenicity outcomes as well. The other nine trials reported one or more efficacy and safety outcomes, and seven out of those nine also reported immunogenicity outcomes (Appendix 2). The trials varied in the length of follow‐up (Appendix 2), but in general the trials that specified efficacy outcome measures had longer follow‐up times (up to three years). Similar to the RV1 trials, we included data on participants requiring medical visits, as this was reported in some trials and is a similar outcome measure to participants requiring hospitalization.
As shown in Appendix 3, rotavirus diarrhoea, severe cases and cases of any severity, were the most common efficacy outcomes reported (by eight trials); only one of these reported rotavirus diarrhoea requiring hospitalization. Three trials provided data on severe cases of all‐cause diarrhoea; two also presented data on cases with any severity. Eleven trials reported all‐cause death, and 13 of the 15 trials reported dropouts.
For safety outcomes, all trials reported on serious adverse events and reactogenicity, and 13 trials reported on adverse events leading to discontinuation of the intervention; see Appendix 4.
Twelve trials reported on an immunogenicity outcome (Appendix 4).
Location
Half of the trials were conducted in low‐mortality countries in North America and Europe. Six trials, including the smallest and the largest trials, were conducted in other regions: RV5 Armah 2010‐AF was conducted in Ghana, Kenya and Mali; RV5 Levin 2017‐AF was conducted in Botswana, Tanzania, Zambia and Zimbabwe, RV5 Dhingra 2014‐IND was conducted in India, RV5 Kim 2008‐KOR was conducted in South Korea; RV5 Iwata 2013‐JPN was conducted in Japan; RV5 Lawrence 2012‐CHN and RV5 Mo 2017‐CHN were conducted in China; RV5 Vesikari 2006b‐INT was conducted in 12 countries in Asia, the Caribbean, Europe, Latin America, North America; and RV5 Zaman 2010‐AS was conducted in Bangladesh and Vietnam. Each trial had multiple sites, ranging from three (RV5 Vesikari 2006a‐FIN) to 356 sites (RV5 Vesikari 2006b‐INT); see Appendix 5.
Country mortality rate
Most trials were conducted in countries with low mortality rates, corresponding to WHO mortality strata A and B; see Appendix 5. One trial was conducted in high‐mortality India (RV5 Dhingra 2014‐IND). Four trials were conducted in several low‐ and high‐mortality countries. RV5 Armah 2010‐AF was conducted in three high‐mortality countries, Ghana, Kenya, and Mali, and when available the data were split into RV5 Armah 2010‐GHA, RV5 Armah 2010‐KEN and RV5 Armah 2010‐MLI. RV5 Levin 2017‐AF was conducted in four high‐mortality countries (Botswana, Tanzania, Zambia and Zimbabwe). RV5 Vesikari 2006b‐INT was conducted mainly in European and Latin American low‐mortality countries, but also in Guatemala, a high‐mortality country, and was placed in the low‐mortality group. RV5 Zaman 2010‐AS was conducted in one high‐mortality country (Bangladesh) with 1136 participants, and in one low‐mortality country (Vietnam) with 900 participants, and was placed in the high‐mortality group, except when data could be split into RV5 Zaman 2010‐BGD and RV5 Zaman 2010‐VNM.
Vaccine schedule
Each trial used three doses of RV5 vaccine, with intervals between doses of four and 10 weeks (see Appendix 6). All but two trials had one vaccine and one placebo arm; RV5 Vesikari 2006a‐FIN included three vaccine arms in which there were different RV5 components (G1‐4, P1A, G1‐4, and P1A), and RV5 Dhingra 2014‐IND included a RV5 arm, a placebo arm, and three arms with different concentrations of BRV‐TV vaccine.
Infant vaccination status
Most trials did not restrict the use of other childhood vaccines (see Appendix 6). Two trials co‐administered hepatitis B, diphtheria‐tetanus‐pertussis, poliovirus, and H influenzae type b vaccines with RV5 (RV5 Ciarlet 2009‐EU; RV5 Dhingra 2014‐IND). One trial randomized participants to either concomitant or staggered administration of other childhood vaccines (OPV, DTaP) with RV5 or placebo (RV5 Mo 2017‐CHN). Three trials allowed the use of oral polio vaccine, in addition to other licensed childhood vaccines (RV5 Armah 2010‐AF; RV5 Mo 2017‐CHN; RV5 Zaman 2010‐AS). Three trials did not allow the use of other vaccines (RV5 Clark 2003‐USA; RV5 Clark 2004‐USA; RV5 Lawrence 2012‐CHN), and one trial did not mention their use (RV5 Iwata 2013‐JPN).
Methods for collecting adverse event data
As shown in Appendix 7, seven trials used a combination of passive methods (e.g. diary cards for parents) and active methods (directly contacting parents) to collect adverse event data. The other trials used passive methods only (diary cards, three trials), active methods only ("active surveillance", three trials), or the information was not provided (two trials).
Source of funding
All but one trial was funded by Merck & Co., Inc. Two of those trials also received funding and were run by PATH (GAVI Alliance grant) (RV5 Armah 2010‐AF; RV5 Zaman 2010‐AS), and one trial also received funding from the International Maternal, Pediatric, and Adolescent AIDS Clinical Trial Network (IMPAACT) through the National Institute of Health (RV5 Levin 2017‐AF). One trial was funded by Shantha Biotechnics Ltd (RV5 Dhingra 2014‐IND).
3. Rotavac
We identified four trials of Rotavac vaccine. The earliest was reported in 2006 and the most recent in 2017. Overall, 8432 participants were included in the trials; the largest trial included 6799 participants (VAC Bhandari 2014‐IND) and the smallest included 90 participants (VAC Bhandari 2006‐IND).
Population
All trials included healthy infants. Trials enrolled infants aged between six weeks and nine weeks.
Outcome measures
Three trials were safety studies (Appendix 2) reporting safety outcomes and immunogenicity outcomes. They reported on follow‐up results for one to 12 months after the last vaccine dose. The other trial (VAC Bhandari 2014‐IND) reported on efficacy, safety, and immunogenicity outcomes until the infants were two years of age.
As shown in Appendix 3, VAC Bhandari 2014‐IND reported on rotavirus diarrhoea (severe cases, cases of any severity, and cases requiring medical attention). The same trial also provided data on severe cases of all‐cause diarrhoea. Two trials reported all‐cause death, and three of the four trials reported dropouts.
For safety outcomes, all trials reported on serious adverse events and two reported on reactogenicity. All trials reported on an immunogenicity outcome (Appendix 4).
Location
All trials were conducted in India, one at three sites in the cities of Delhi, Pune, and Vellore (VAC Bhandari 2014‐IND), and the remaining three studies at one site in Delhi.
Country mortality rate
All trials were conducted in India, a high‐mortality country (WHO mortality stratum D).
Vaccine schedule
Most trials used three doses of Rotavac vaccine, with intervals between doses of four to eight weeks (see Appendix 6). One trial (VAC Bhandari 2006‐IND) administered one dose. One trial had one vaccine and one placebo arm (VAC Bhandari 2014‐IND). VAC Bhandari 2006‐IND included an additional vaccine arm for a rotavirus vaccine candidate (I321) that we did not include for analysis in this review. VAC Bhandari 2009‐IND randomized participants to high‐ (1 x 105 ffu) and low‐dose (1 x 104 ffu) vaccine arms which we combined in this review. VAC Chandola 2017‐IND randomized participants to three vaccine production lots as well as to placebo. We combined the different production lot arms in our analyses.
Infant vaccination status
Two trials separated the use of other routine childhood vaccines from Rotavac administration by at least two weeks (VAC Bhandari 2006‐IND; VAC Bhandari 2009‐IND). Two trials co‐administered other routine childhood vaccines (OPV, DPT, Hep B and Hib) with Rotavac (VAC Bhandari 2014‐IND; VAC Chandola 2017‐IND).
Methods for collecting adverse event data
As shown in Appendix 7, three trials used a combination of passive methods (e.g. diary cards for parents) and active methods (directly contacting parents) to collect adverse event data. The other trial (VAC Chandola 2017‐IND) used active methods only (directly contacting parents).
Source of funding
One trial was funded by Bharat Biotech (VAC Bhandari 2006‐IND), one trial was co‐funded by Bharat Biotech (VAC Bhandari 2009‐IND) and the other two trials were funded by PATH, the Government of India, and other not‐for‐profit organizations (VAC Bhandari 2014‐IND; VAC Chandola 2017‐IND).
Ongoing studies
We identified 15 ongoing trials, three of RV1, one of RV5 and 11 others (RV1 together with RV5; RV3‐BB; Rotasiil; Rotavac; BRV‐TV; Trivalent P2VP8; Bio Farma's rotavirus vaccine) (see Characteristics of ongoing studies). As shown in Appendix 8, the RV1 trials are being conducted in South Africa and Bangladesh. The ongoing RV5 trial is in Bangladesh, and the studies testing other vaccines are located in Australia, Bangladesh, China, India, Indonesia, Malawi, Mexico, South Africa, and the USA.
Excluded studies
There are 78 excluded studies with 100 references (Figure 2). We excluded most studies because they were not RCTs (34 studies). We excluded 27 studies because they reported on comparisons not relevant to this review, three studies because they did not report on RV vaccines, three because they included adult populations, 10 because they reported on unlicensed vaccines in development (OTHER Bines 2015; OTHER Bines 2018; OTHER Cowley 2017; OTHER Groome 2017) or licensed vaccines that have not been prequalified by the WHO (OTHER CTRI/2009/091/000821; OTHER Dang 2012; OTHER Isanaka 2017‐NER; OTHER Kulkarni 2017; OTHER Zade 2014a‐IND; OTHER Zade 2014b‐IND), and one because it reported on a withdrawn vaccine (OTHER Armah 2013).
Figure 2.
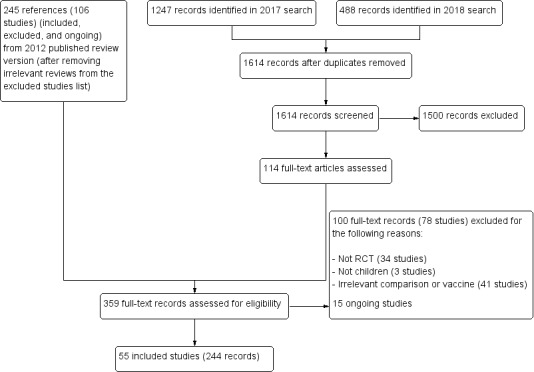
PRISMA diagram.
Risk of bias in included studies
We prepared a ‘Risk of bias' assessment for each trial, with a focus on the rotavirus diarrhoea outcome measure. Of the 55 RCTs analyzed in this review, 48 (87%) reported an adequate generation of allocation sequence, while the method of assignment was unclear in the remaining studies. We considered the methods used to conceal allocation to be adequate in 46 trials (84%), and unclear in the remaining studies. Information about blinding of participants, care providers, or outcome assessors was provided and we considered it to be adequate in 42 studies (76%), unclear for nine studies, and at high risk of bias for four studies (RV1 Colgate 2016‐BGD; RV1 Kerdpanich 2010‐THA; RV1 Zaman 2017‐BGD; RV5 Dhingra 2014‐IND). Incomplete outcome data were adequately addressed in 46 studies (84%), unclear in eight studies, and was not addressed adequately in one study. Thirty‐eight (69%) trials were free from selective reporting bias, nine were not, and the remaining eight trials were unclear. No other bias was apparent for 31 trials (56%). An overall pictorial summary of the ‘Risk of bias' assessment is shown in Figure 3 and Figure 4.
Figure 3.
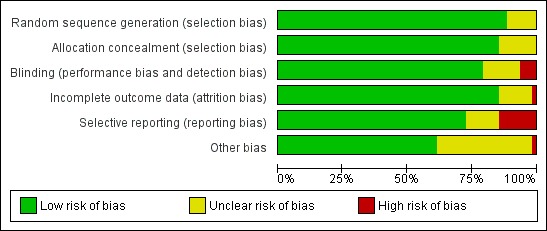
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 4.
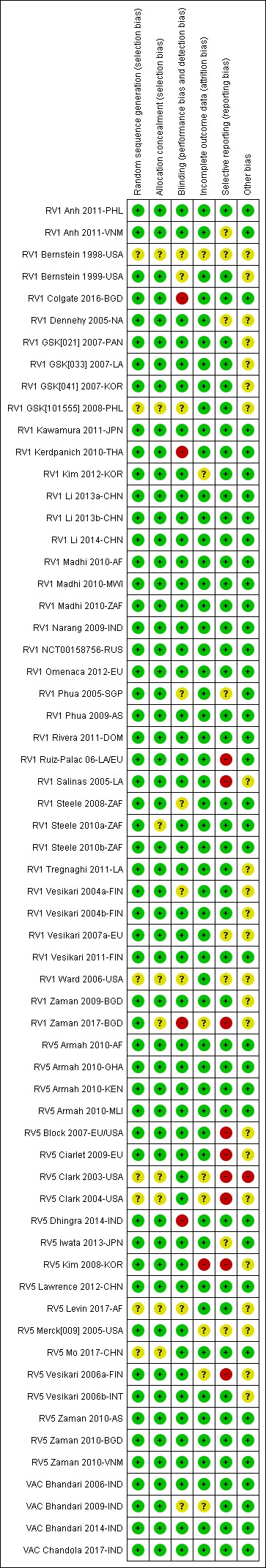
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
RV1
Since the previous update of this review, detailed clinical study reports of most of the GlaxoSmithKline‐sponsored studies (another five, totaling 27 of the 36 trials) have been published online (gsk‐clinicalstudyregister.com). Full details of blinding, participant selection, and attrition are available from these reports, and we could subsequently update risks of bias for these studies, where previously there was no information available. We rated five trials as at high risk of bias for at least one domain; three trials for blinding (RV1 Colgate 2016‐BGD; RV1 Kerdpanich 2010‐THA; RV1 Zaman 2017‐BGD), and three trials for selective reporting bias (RV1 Ruiz‐Palac 06‐LA/EU; RV1 Salinas 2005‐LA; RV1 Zaman 2017‐BGD).
RV5
Based on unpublished information provided by Merck, many of the trials' risks of bias were upgraded for the previous 2012 version of this review. Details of the new information are indicated in the ‘Risk of bias' tables in the Characteristics of included studies section. We judged 10 of the 15 RV5 trials as having a low risk of bias for sequence generation, allocation concealment, and blinding, and varying risks of bias for attrition, selective reporting and other bias. We rated two of these trials (RV5 Armah 2010‐AF; RV5 Zaman 2010‐AS) at an overall low risk of bias. Seven of the 15 RV5 trials had a high risk of bias for one or more domains, most commonly a high risk of selective reporting.
Rotavac
Peer‐reviewed articles for most Rotavac studies reported clearly on how the trials were conducted. Full details about blinding, participant selection, attrition, and outcome reporting could be obtained from most of these reports. We rated only one of the trials at unclear risk of performance and detection bias, since no details about blinding were provided and unclear risk of attrition bias since not all outcomes were assessed with the full study population and the reason for this was not clear (VAC Bhandari 2009‐IND).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
1. RV1
1.1. Primary outcomes
1.1.1. Rotavirus diarrhoea: severe
Eleven trials provided data on the efficacy of RV1 to prevent severe rotavirus diarrhoea in children; see Analysis 1.1 for up to one‐year follow‐up and Analysis 1.2 for two years follow‐up. Trials were performed in low‐mortality countries (RV1 Bernstein 1999‐USA; RV1 Kawamura 2011‐JPN; RV1 Li 2014‐CHN; RV1 Phua 2005‐SGP; RV1 Phua 2009‐AS; RV1 Ruiz‐Palac 06‐LA/EU; RV1 Salinas 2005‐LA; RV1 Tregnaghi 2011‐LA; RV1 Vesikari 2004b‐FIN; RV1 Vesikari 2007a‐EU), and high‐mortality countries (RV1 Colgate 2016‐BGD; RV1 Madhi 2010‐MWI; RV1 Madhi 2010‐ZAF; RV1 Steele 2010b‐ZAF; RV1 Zaman 2017‐BGD). Data below are grouped accordingly.
Analysis 1.1.
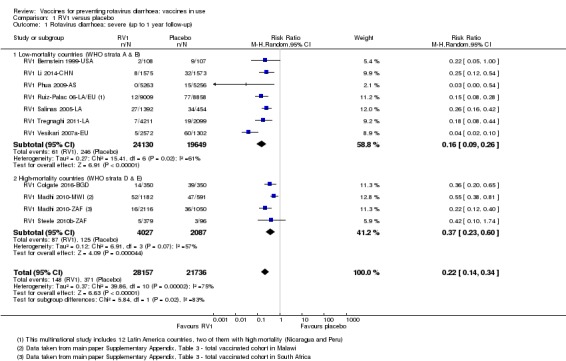
Comparison 1 RV1 versus placebo, Outcome 1 Rotavirus diarrhoea: severe (up to 1 year follow‐up).
Analysis 1.2.
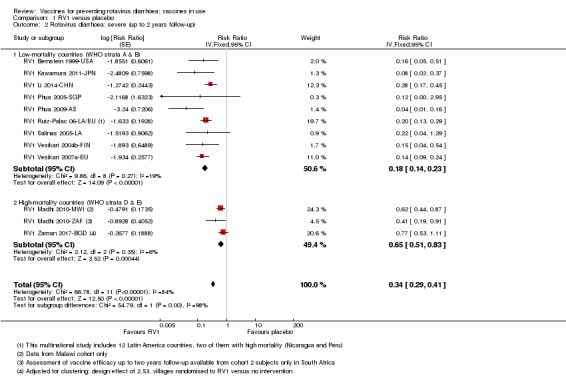
Comparison 1 RV1 versus placebo, Outcome 2 Rotavirus diarrhoea: severe (up to 2 years follow‐up).
Low‐mortality countries (WHO strata A and B)
RV1 reduced severe rotavirus diarrhoea cases by 84% after one year (RR 0.16, 95% CI 0.09 to 0.26; 43,779 participants, 7 trials) and by 82% after two years (RR 0.18, 95% CI 0.14 to 0.23; 36,002 participants, 9 trials; Analysis 1.2). After three years there was no statistically significant difference between RV1 and placebo (RR 0.10, 95% CI 0.01 to 1.52; 12,109 participants, two trials (RV1 Phua 2009‐AS and RV1 Vesikari 2007a‐EU; data not shown)). Pooled results showed statistical heterogeneity at one‐year (I2 statistic = 61%, Analysis 1.1) and three years (I2 statistic = 69%, data not shown) follow‐up.
High‐mortality countries (WHO strata D and E)
RV1 reduced severe rotavirus diarrhoea cases by 63% during the first year of follow‐up (RR 0.37, 95% CI 0.23 to 0.60; 6114 participants, 4 comparisons from 3 trials) and by 35% after two years (RR 0.65, 95% CI 0.51 to 0.83; 7113 participants, 3 comparisons from 2 trials; Analysis 1.2). Pooled results showed statistical heterogeneity at one‐year follow‐up (I2 statistic = 57%, Analysis 1.1).
We noted a funnel plot asymmetry for trials reporting results up to one year (Figure 5).
Figure 5.
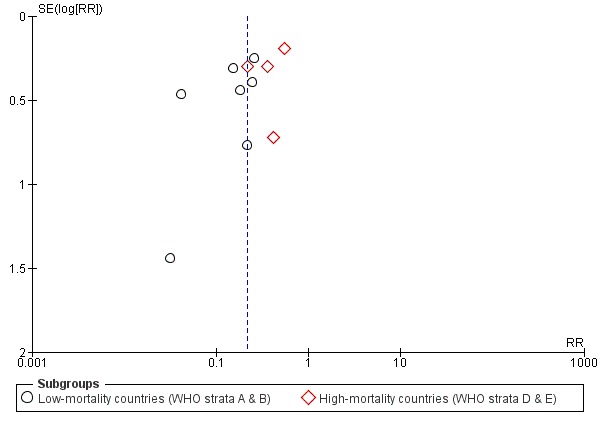
Funnel plot of comparison: 1 RV1 versus placebo, outcome: 1.1 Rotavirus diarrhoea: severe (up to 1 year follow‐up).
1.1.2. All‐cause diarrhoea: severe
Severe all‐cause diarrhoea was reported as cases in six trials (RV1 Colgate 2016‐BGD; RV1 Li 2014‐CHN; RV1 Madhi 2010‐AF; RV1 Phua 2005‐SGP; RV1 Ruiz‐Palac 06‐LA/EU; RV1 Vesikari 2007a‐EU) and as episodes in two trials (RV1 Phua 2009‐AS; RV1 Ruiz‐Palac 06‐LA/EU). We have reported these data separately. Trials were performed in low‐mortality countries (RV1 Li 2014‐CHN; RV1 Phua 2005‐SGP; RV1 Phua 2009‐AS; RV1 Ruiz‐Palac 06‐LA/EU; RV1 Vesikari 2007a‐EU), and in high‐mortality countries (RV1 Colgate 2016‐BGD; RV1 Madhi 2010‐MWI; RV1 Madhi 2010‐ZAF).
Low‐mortality countries (WHO strata A and B)
RV1 reduced the number of severe cases of all‐cause diarrhoea by 41% at one year (RR 0.59, 95% CI 0.47 to 0.74; 28,051 participants, 3 trials; Analysis 1.3), and by 40% at two years (RR 0.60, 95% CI 0.36 to 1.02; 9417 participants, 3 trials; Analysis 1.4). Pooled results showed statistical heterogeneity at both one year (I2 statistic = 63%) and two years follow‐up (I2 statistic = 90%). RV1 reduced the rate of severe episodes of all‐cause diarrhoea by 40% at one year (rate ratio 0.60, 95% CI 0.50 to 0.72; 17,867 participants, 1 trial; Analysis 1.5), and by 37% at two years (rate ratio 0.63, 95% CI 0.56 to 0.71; 39,091 participants, 2 trials; Analysis 1.6). One trial reported on severe all‐cause diarrhoea after three years follow‐up (RV1 Phua 2009‐AS); RV1 reduced the number of severe cases by 27% (RR 0.73, 95% CI 0.61 to 0.88; 10,519 participants; data not shown).
Analysis 1.3.
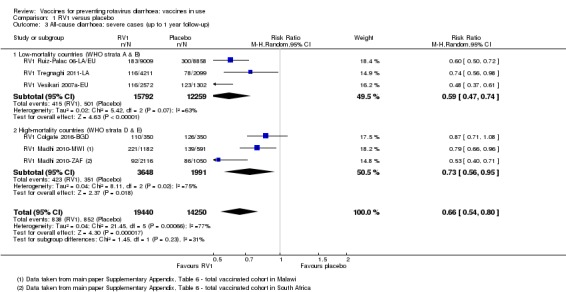
Comparison 1 RV1 versus placebo, Outcome 3 All‐cause diarrhoea: severe cases (up to 1 year follow‐up).
Analysis 1.4.
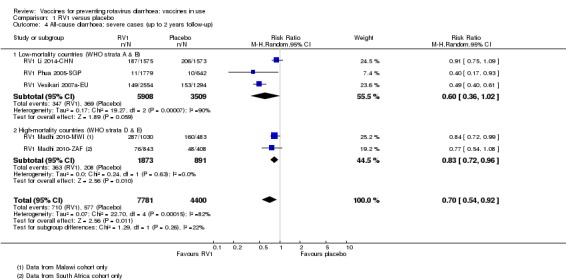
Comparison 1 RV1 versus placebo, Outcome 4 All‐cause diarrhoea: severe cases (up to 2 years follow‐up).
Analysis 1.5.
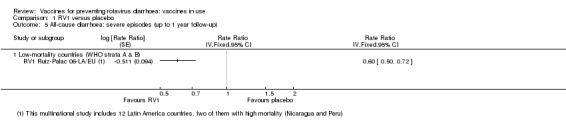
Comparison 1 RV1 versus placebo, Outcome 5 All‐cause diarrhoea: severe episodes (up to 1 year follow‐up).
Analysis 1.6.
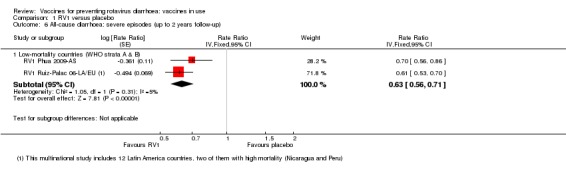
Comparison 1 RV1 versus placebo, Outcome 6 All‐cause diarrhoea: severe episodes (up to 2 years follow‐up).
High‐mortality countries (WHO strata D and E)
RV1 reduced the number of severe cases of all‐cause diarrhoea by 27% at one year follow‐up (RR 0.73, 95% CI 0.56 to 0.95; 5639 participants, 3 comparisons from 2 trials; Analysis 1.3), and by 17% at two years follow‐up (RR 0.83, 95% CI 0.72 to 0.96; 2764 participants, 2 comparisons from 1 trial; Analysis 1.4). Pooled results showed statistical heterogeneity at one‐year follow‐up (I2 statistic = 75%).
1.1.3. All‐cause death
Thirty trials reported on all‐cause death, either as the number of deaths (RV1 Bernstein 1999‐USA; RV1 Kim 2012‐KOR; RV1 Li 2013b‐CHN; RV1 Li 2014‐CHN; RV1 Madhi 2010‐AF; RV1 NCT00158756‐RUS; RV1 Phua 2005‐SGP; RV1 Phua 2009‐AS; RV1 Steele 2010a‐ZAF; RV1 Vesikari 2007a‐EU) or as the number of fatal serious adverse events (RV1 Anh 2011‐PHL; RV1 Anh 2011‐VNM; RV1 GSK[021] 2007‐PAN; RV1 GSK[033] 2007‐LA; RV1 GSK[041] 2007‐KOR; RV1 GSK[101555] 2008‐PHL; RV1 Kawamura 2011‐JPN; RV1 Kerdpanich 2010‐THA; RV1 Narang 2009‐IND; RV1 Omenaca 2012‐EU; RV1 Rivera 2011‐DOM; RV1 Ruiz‐Palac 06‐LA/EU; RV1 Salinas 2005‐LA; RV1 Steele 2008‐ZAF; RV1 Steele 2010b‐ZAF; RV1 Tregnaghi 2011‐LA; RV1 Vesikari 2004b‐FIN; RV1 Vesikari 2011‐FIN; RV1 Zaman 2009‐BGD). We pooled the number of deaths and fatal serious adverse events; see Analysis 1.7. We present details of causes of death for each trial in Appendix 9. Most trials were performed in low‐mortality countries, with eight trials in high‐mortality countries (RV1 Colgate 2016‐BGD; RV1 GSK[033] 2007‐LA; RV1 Madhi 2010‐AF; RV1 Narang 2009‐IND; RV1 Steele 2008‐ZAF; RV1 Steele 2010a‐ZAF; RV1 Steele 2010b‐ZAF; RV1 Zaman 2009‐BGD).
Analysis 1.7.
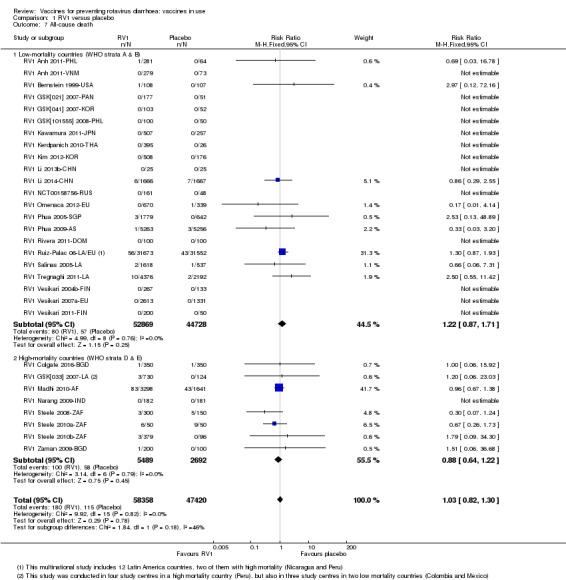
Comparison 1 RV1 versus placebo, Outcome 7 All‐cause death.
Low‐mortality countries (WHO strata A and B)
There was no statistically significant difference in all‐cause death between the two arms (RR 1.22, 95% CI 0.87 to 1.71; 97,597 participants, 22 trials).
High‐mortality countries (WHO strata D and E)
There was no statistically significant difference in all‐cause death between the two arms (RR 0.88, 95% CI 0.64 to 1.22; 8181 participants, 8 trials).
1.1.4. All serious adverse events
The total number of serious adverse events was reported in 31 trials, performed in low‐mortality countries (RV1 Anh 2011‐PHL; RV1 Anh 2011‐VNM; RV1 Bernstein 1998‐USA; RV1 Dennehy 2005‐NA; RV1 GSK[021] 2007‐PAN; RV1 GSK[041] 2007‐KOR; RV1 GSK[101555] 2008‐PHL; RV1 Kawamura 2011‐JPN; RV1 Kerdpanich 2010‐THA; RV1 Kim 2012‐KOR; RV1 Li 2013a‐CHN; RV1 Li 2014‐CHN; RV1 NCT00158756‐RUS; RV1 Omenaca 2012‐EU; RV1 Phua 2005‐SGP; RV1 Phua 2009‐AS; RV1 Rivera 2011‐DOM; RV1 Ruiz‐Palac 06‐LA/EU; RV1 Salinas 2005‐LA; RV1 Tregnaghi 2011‐LA; RV1 Vesikari 2004a‐FIN; RV1 Vesikari 2004b‐FIN; RV1 Vesikari 2007a‐EU; RV1 Vesikari 2011‐FIN), and in high‐mortality countries (RV1 GSK[033] 2007‐LA; RV1 Madhi 2010‐AF; RV1 Narang 2009‐IND; RV1 Steele 2008‐ZAF; RV1 Steele 2010a‐ZAF; RV1 Steele 2010b‐ZAF; RV1 Zaman 2009‐BGD); see Analysis 1.8.
Analysis 1.8.
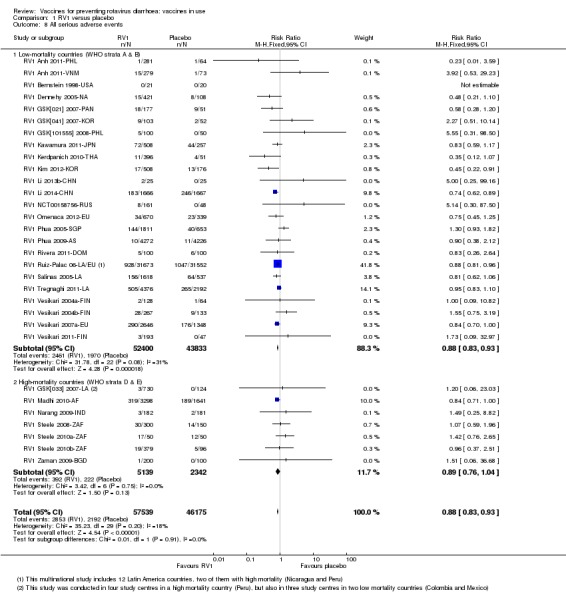
Comparison 1 RV1 versus placebo, Outcome 8 All serious adverse events.
Low‐mortality countries (WHO strata A and B)
Fewer children allocated to RV1 had serious adverse events compared with placebo (RR 0.88, 95% CI 0.83 to 0.93; 96,233 participants, 24 trials). In addition, in one trial (RV1 Li 2013a‐CHN) that vaccinated 25 older children (aged two to six years) with one‐dose RV1 there were no serious adverse events reported.
High‐mortality countries (WHO strata D and E)
There was no statistically significant difference in the number of serious adverse events between the two arms (RR 0.89, 95% CI 0.76 to 1.04; 7481 participants, 7 trials).
1.1.5. Serious adverse events: intussusception
Twenty‐one trials reported on intussusception, and 11 of these reported that no cases of intussuception had occurred. Trials were performed in low‐mortality countries (RV1 Dennehy 2005‐NA; RV1 GSK[041] 2007‐KOR; RV1 Kawamura 2011‐JPN; RV1 Kim 2012‐KOR; RV1 Phua 2005‐SGP; RV1 Phua 2009‐AS; RV1 Rivera 2011‐DOM; RV1 Ruiz‐Palac 06‐LA/EU; RV1 Salinas 2005‐LA; RV1 Tregnaghi 2011‐LA; RV1 Vesikari 2004b‐FIN; RV1 Vesikari 2007a‐EU; RV1 Vesikari 2011‐FIN), and in high‐mortality countries (RV1 Madhi 2010‐AF; RV1 Steele 2008‐ZAF; RV1 Steele 2010b‐ZAF; RV1 Zaman 2017‐BGD); see Analysis 1.9.
Analysis 1.9.
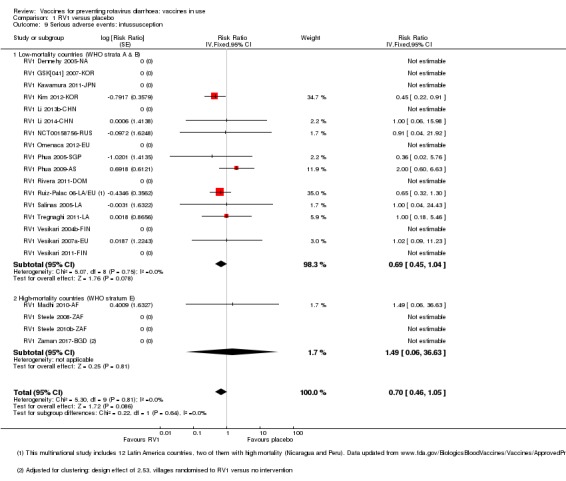
Comparison 1 RV1 versus placebo, Outcome 9 Serious adverse events: intussusception.
Low‐mortality countries (WHO strata A and B)
Twenty‐nine cases of intussusception were reported in a total of 49,355 children in the RV1 arm compared with 28 cases of intussusception in 42,477 children of the placebo arm. Pooled results showed no increased risk for intussusception in children receiving RV1 when compared to placebo (RR 0.69, 95% CI 0.45 to 1.04; 96,513 participants, 17 trials).
High‐mortality countries (WHO stratum E)
One case of intussusception was reported in a total of 3677 children in the RV1 arm compared with no cases of intussusception in 1737 children in the placebo or no‐intervention arm. Pooled results showed no increased risk for intussusception in children receiving RV1 when compared to placebo (RR 1.49, 95% CI 0.06 to 36.63; 10,460 participants, 4 trials).
1.2. Secondary outcomes
1.2.1 Serious adverse events: Kawasaki disease
Three trials reported four cases of Kawasaki disease among 7701 children allocated to RV1 compared to no cases in 5416 children allocated to placebo (RV1 Phua 2005‐SGP; RV1 Phua 2009‐AS; RV1 Salinas 2005‐LA). We did not observe a statistically significant difference between the intervention and placebo groups (RR 1.79, 95% CI 0.30 to 10.61; 13,117 participants, 3 trials; Analysis 1.10).
Analysis 1.10.
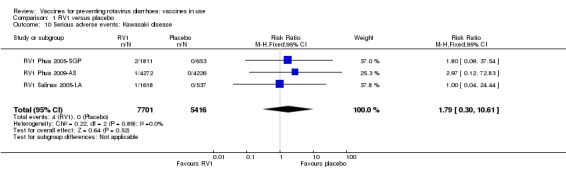
Comparison 1 RV1 versus placebo, Outcome 10 Serious adverse events: Kawasaki disease.
1.2.2. Serious adverse events requiring hospitalization
Two trials reported serious adverse events requiring hospitalization (RV1 Ruiz‐Palac 06‐LA/EU; RV1 Steele 2008‐ZAF) and found fewer events in the RV1 group than the placebo group (RR 0.88, 95% CI 0.81 to 0.96; 63,675 participants, 2 trials; Analysis 1.11).
Analysis 1.11.
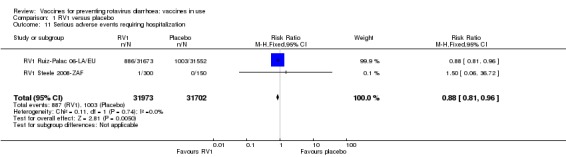
Comparison 1 RV1 versus placebo, Outcome 11 Serious adverse events requiring hospitalization.
1.2.3 Rotavirus diarrhoea of any severity
Eighteen trials provided data for the efficacy of RV1 to prevent rotavirus diarrhoea in children; see Analysis 1.12 for two‐months safety trial follow‐up, Analysis 1.13 for one‐year follow‐up and Analysis 1.14 for two‐year follow‐up. Trials were performed in low‐mortality countries (RV1 Anh 2011‐PHL; RV1 Anh 2011‐VNM; RV1 Bernstein 1999‐USA; RV1 GSK[041] 2007‐KOR; RV1 GSK[101555] 2008‐PHL; RV1 Kerdpanich 2010‐THA; RV1 Omenaca 2012‐EU; RV1 Phua 2005‐SGP; RV1 Rivera 2011‐DOM; RV1 Salinas 2005‐LA; RV1 Vesikari 2004b‐FIN; RV1 Vesikari 2007a‐EU; RV1 Vesikari 2011‐FIN), and in high‐mortality countries (RV1 Madhi 2010‐MWI; RV1 Madhi 2010‐ZAF; RV1 Narang 2009‐IND; RV1 Steele 2010a‐ZAF; RV1 Steele 2010b‐ZAF; RV1 Zaman 2009‐BGD). Data below are grouped accordingly.
Analysis 1.12.
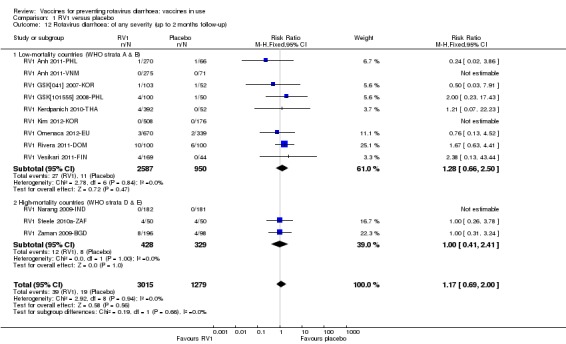
Comparison 1 RV1 versus placebo, Outcome 12 Rotavirus diarrhoea: of any severity (up to 2 months follow‐up).
Analysis 1.13.
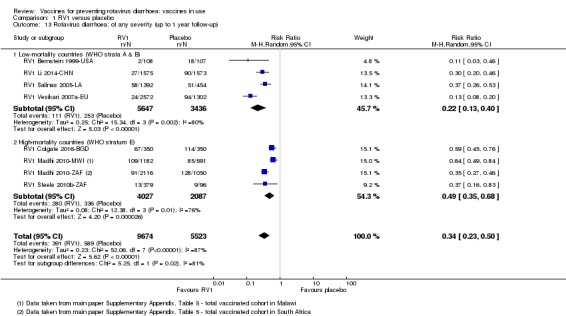
Comparison 1 RV1 versus placebo, Outcome 13 Rotavirus diarrhoea: of any severity (up to 1 year follow‐up).
Analysis 1.14.
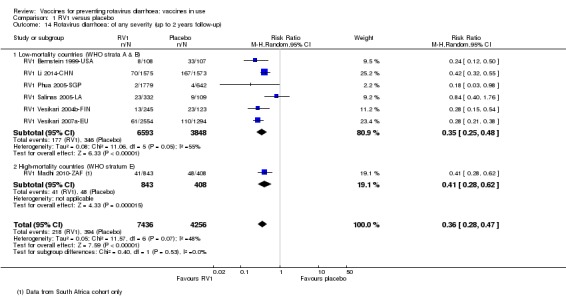
Comparison 1 RV1 versus placebo, Outcome 14 Rotavirus diarrhoea: of any severity (up to 2 years follow‐up).
Low‐mortality countries (WHO strata A and B)
Safety trials (up to two months follow‐up): RV1 was not superior to placebo in the prevention of rotavirus diarrhoea in the trials assessing outcomes up to two months after vaccination (RR 1.28, 95% CI 0.66 to 2.50; 3537 participants, 9 trials). These trials, although reporting cases of rotavirus diarrhoea, were not designed to measure efficacy.
Efficacy trials (one to three years follow‐up): RV1 reduced rotavirus diarrhoea by 78% at up to one year (RR 0.22, 95% CI 0.13 to 0.40; 9083 participants, 4 trials) and 65% at the second year of follow‐up (RR 0.35, 95% CI 0.25 to 0.48; 10,441 participants, 6 trials). Pooled results, however, showed statistical heterogeneity at one year (I2 statistic = 80%, Analysis 1.13) and two years (I2 statistic = 55%, Analysis 1.14) of follow‐up. At the third year of follow‐up, there were very few reported cases of rotavirus diarrhoea of any severity. Based on a single trial (RV1 Vesikari 2007a‐EU, 1590 participants), there was no difference between RV1 and placebo groups (data not shown).
High‐mortality countries (WHO strata D and E)
Safety trials (up to two months follow‐up): Three trials found no difference in the RV1 group compared to placebo when outcomes were assessed up to two months after vaccination (RR 1.00, 95% CI 0.41 to 2.41; 757 participants, 3 trials).
Efficacy trials (one to two years follow‐up): RV1 reduced rotavirus diarrhoea by 51% during the first year of follow‐up (RR 0.49, 95% CI 0.35 to 0.68; 6114 participants, 4 comparisons from 3 trials), and by 59% during the second year (RR 0.41, 95% CI 0.28 to 0.62; 1251 participants, 1 trial). Pooled results showed statistical heterogeneity at one‐year follow‐up (I2 statistic = 76%, Analysis 1.13).
1.2.4. All‐cause diarrhoea: of any severity
This outcome was reported as cases in 11 trials from low‐mortality countries (RV1 Anh 2011‐PHL; RV1 Anh 2011‐VNM; RV1 Kerdpanich 2010‐THA; RV1 Kim 2012‐KOR; RV1 Li 2014‐CHN; RV1 Omenaca 2012‐EU; RV1 Phua 2005‐SGP; RV1 Rivera 2011‐DOM; RV1 Salinas 2005‐LA; RV1 Vesikari 2004b‐FIN; RV1 Vesikari 2011‐FIN), in two trials from high‐mortality countries (RV1 Colgate 2016‐BGD; RV1 Steele 2010a‐ZAF), and as episodes in three trials from low‐mortality countries (RV1 Rivera 2011‐DOM; RV1 Salinas 2005‐LA; RV1 Vesikari 2004b‐FIN). We have reported these data separately.
Low‐mortality countries (WHO strata A and B)
Safety trials (up to two months follow‐up): RV1 was not better than placebo in reducing the number of cases of all‐cause diarrhoea at two months (RR 0.86, 95% CI 0.67 to 1.09; 3032 participants, 6 trials; Analysis 1.15).
Analysis 1.15.
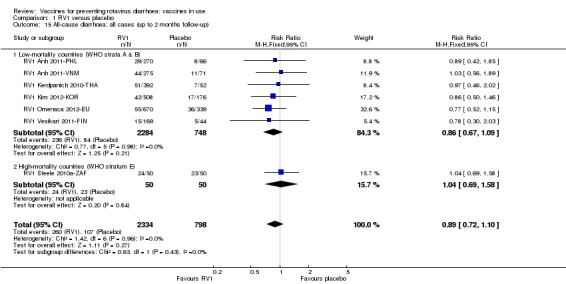
Comparison 1 RV1 versus placebo, Outcome 15 All‐cause diarrhoea: all cases (up to 2 months follow‐up).
Efficacy trials (one to two years follow‐up): RV1 was not better than placebo in reducing the number of cases of all‐cause diarrhoea at one year follow‐up (RR 0.92, 95% CI 0.82 to 1.03; 2204 participants, 2 trials, Analysis 1.16), or after two years (RR 0.93, 95% CI 0.87 to 1.00; 5937 participants, 3 trials; Analysis 1.17).Two trials reported the number of episodes, with no statistically significant benefit with RV1 when compared to placebo at one year (Rate Ratio 0.98, 95% CI 0.88 to 1.10; 2204 participants, 2 trials; Analysis 1.18) or at two years (Rate Ratio 1.02, 95% CI 0.78 to 1.33; 736 participants, 1 trial; Analysis 1.19).
Analysis 1.16.
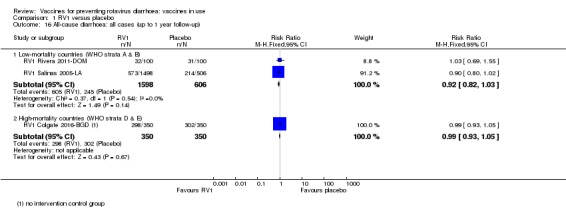
Comparison 1 RV1 versus placebo, Outcome 16 All‐cause diarrhoea: all cases (up to 1 year follow‐up).
Analysis 1.17.
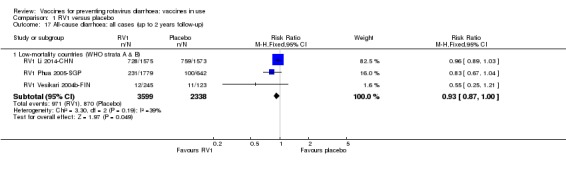
Comparison 1 RV1 versus placebo, Outcome 17 All‐cause diarrhoea: all cases (up to 2 years follow‐up).
Analysis 1.18.
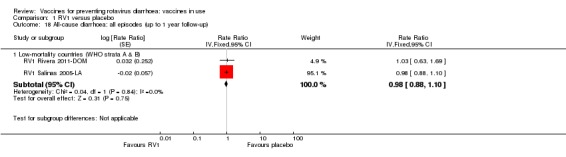
Comparison 1 RV1 versus placebo, Outcome 18 All‐cause diarrhoea: all episodes (up to 1 year follow‐up).
Analysis 1.19.
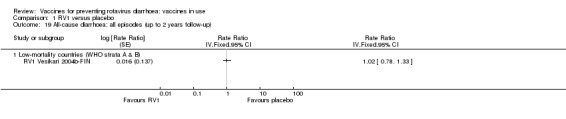
Comparison 1 RV1 versus placebo, Outcome 19 All‐cause diarrhoea: all episodes (up to 2 years follow‐up).
High‐mortality countries (WHO stratum E)
Safety trials (up to two months follow‐up): RV1 was not better than placebo in reducing the number of cases of all‐cause diarrhoea at two months (RR 1.04, 95% CI 0.69 to 1.58; 100 participants, 1 trial; Analysis 1.15).
Efficacy trials (one‐year follow‐up): RV1 was not better than no intervention in reducing the number of cases of all‐cause diarrhoea at one‐year follow‐up (RR 0.99, 95% CI 0.93 to 1.05; 700 participants, 1 trial; Analysis 1.16)
1.2.5. All‐cause hospitalizations
Two trials (RV1 Phua 2005‐SGP; RV1 Ruiz‐Palac 06‐LA/EU) provided data for the efficacy of RV1 to prevent all‐cause hospitalizations.
Low‐mortality countries (WHO stratum A)
RV1 was not better than placebo in reducing the number of hospitalizations at up to two years of follow‐up (RR 0.63, 95% CI 0.27 to 1.47; 65,646 participants, 2 trials; Analysis 1.20).
Analysis 1.20.
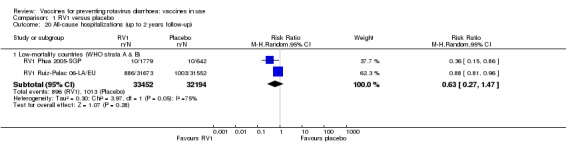
Comparison 1 RV1 versus placebo, Outcome 20 All‐cause hospitalizations (up to 2 years follow‐up).
1.2.6. Rotavirus diarrhoea: requiring hospitalization or medical attention
Rotavirus‐related hospitalizations were reduced by 82% after one year (RR 0.18, 95% CI 0.09 to 0.33; 48,718 participants, 8 trials), 85% at two years (RR 0.15, 95% CI 0.11 to 0.22; 35,331 participants, 7 trials), and 95% at three years (RR 0.05, 95% CI 0.02 to 0.16; 10,519 participants, 1 trial (RV1 Phua 2009‐AS, data not shown)); pooled results showed statistical heterogeneity at one year of follow‐up (I2 statistic = 55%); see Analysis 1.21.
Analysis 1.21.
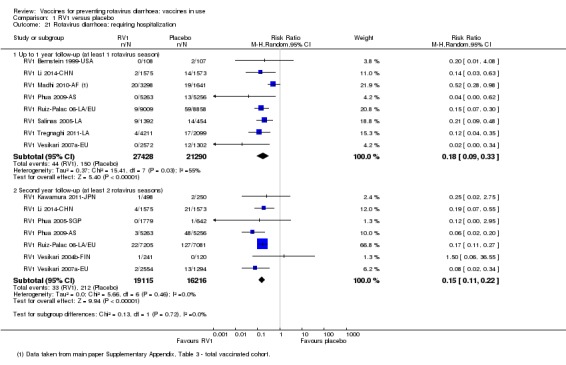
Comparison 1 RV1 versus placebo, Outcome 21 Rotavirus diarrhoea: requiring hospitalization.
RV1 reduced rotavirus‐related medical visits by 92% at one year (RR 0.08, 95% CI 0.04 to 0.16; 3874 participants, 1 trial) and 78% at two years (RR 0.22, 95% CI 0.16 to 0.31; 7017 participants, 3 trials); see Analysis 1.22.
Analysis 1.22.

Comparison 1 RV1 versus placebo, Outcome 22 Rotavirus diarrhoea: requiring medical attention.
1.2.7. All‐cause diarrhoea: requiring hospitalization
There was no significant difference between RV1 and placebo in cases of hospitalization for all‐cause diarrhoea at one‐year follow‐up (RR 0.43, 95% CI 0.17 to 1.11; 14,393 participants, 2 trials; Analysis 1.23). At two years follow‐up, RV1 reduced cases by 48% (RR 0.52, 95% CI 0.27 to 0.99; 14,367 participants, 2 trials; Analysis 1.23). RV1 Phua 2009‐AS reported that for hospitalizations due to all‐cause diarrhoea at three years of follow‐up, RV1 reduced hospitalizations by 28% (RR 0.72, 95% CI 0.59 to 0.86; 10,519 participants, data not shown). Pooled results showed statistical heterogeneity at one year (I2 statistic = 83%) and at two years follow‐up (I2 statistic = 77%).
Analysis 1.23.
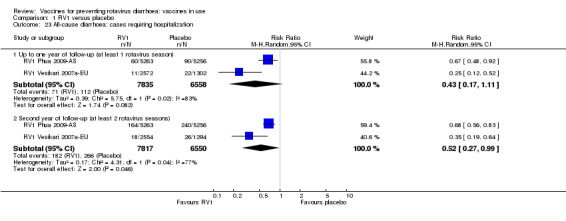
Comparison 1 RV1 versus placebo, Outcome 23 All‐cause diarrhoea: cases requiring hospitalization.
RV1 Ruiz‐Palac 06‐LA/EU presented data on the number of episodes (Analysis 1.24); RV1 reduced hospitalizations by 42% at one year (rate ratio 0.58, 95% CI 0.47 to 0.71; 17,867 participants, 1 trial) and 47% at two years (rate ratio 0.53, 95% CI 0.46 to 0.61; 14,286 participants, 1 trial).
Analysis 1.24.
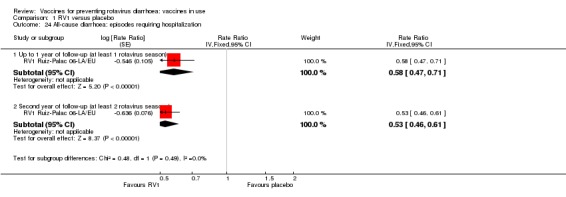
Comparison 1 RV1 versus placebo, Outcome 24 All‐cause diarrhoea: episodes requiring hospitalization.
1.2.8. Reactogenicity
The occurrence of fever (Analysis 1.25), diarrhoea (Analysis 1.26), and vomiting (Analysis 1.27) were evaluated at several time points: after the first dose, after the second dose, after the third dose, and at the end of the follow‐up period. Most trials contributed data to these outcomes. There were similar results for RV1 and placebo for each outcome and time point.
Analysis 1.25.
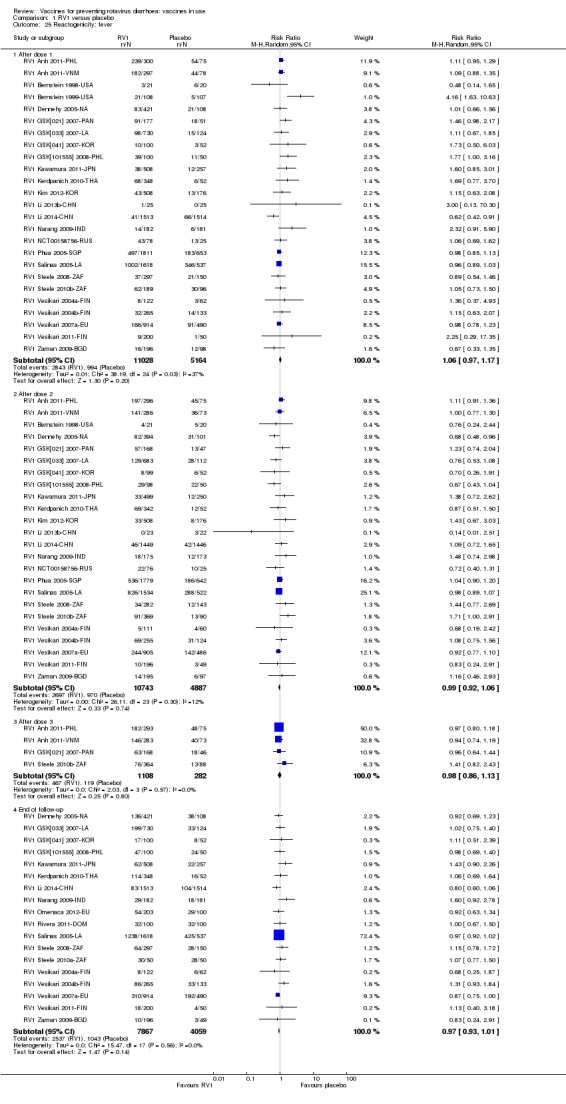
Comparison 1 RV1 versus placebo, Outcome 25 Reactogenicity: fever.
Analysis 1.26.
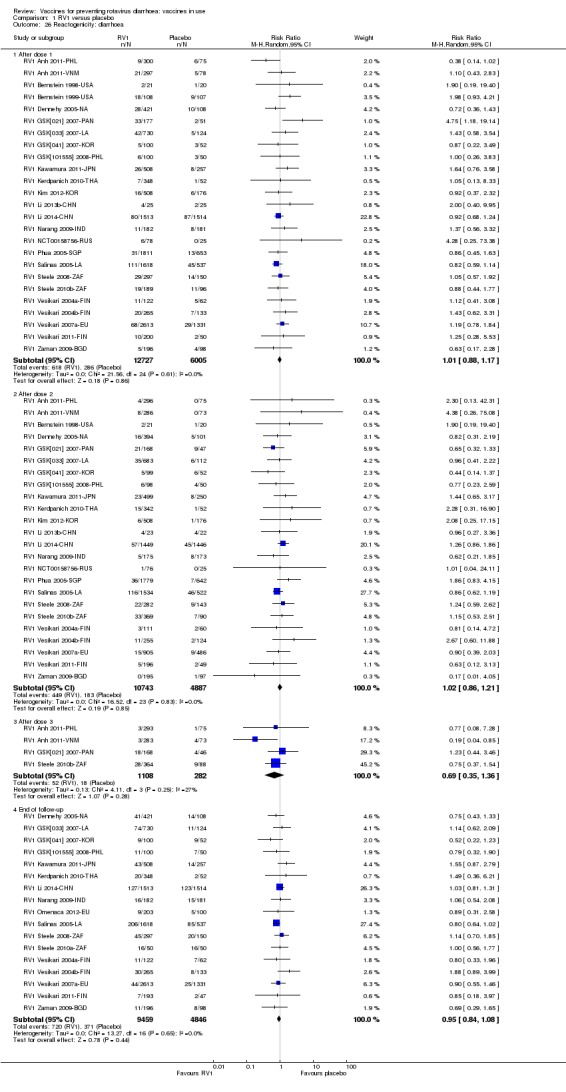
Comparison 1 RV1 versus placebo, Outcome 26 Reactogenicity: diarrhoea.
Analysis 1.27.
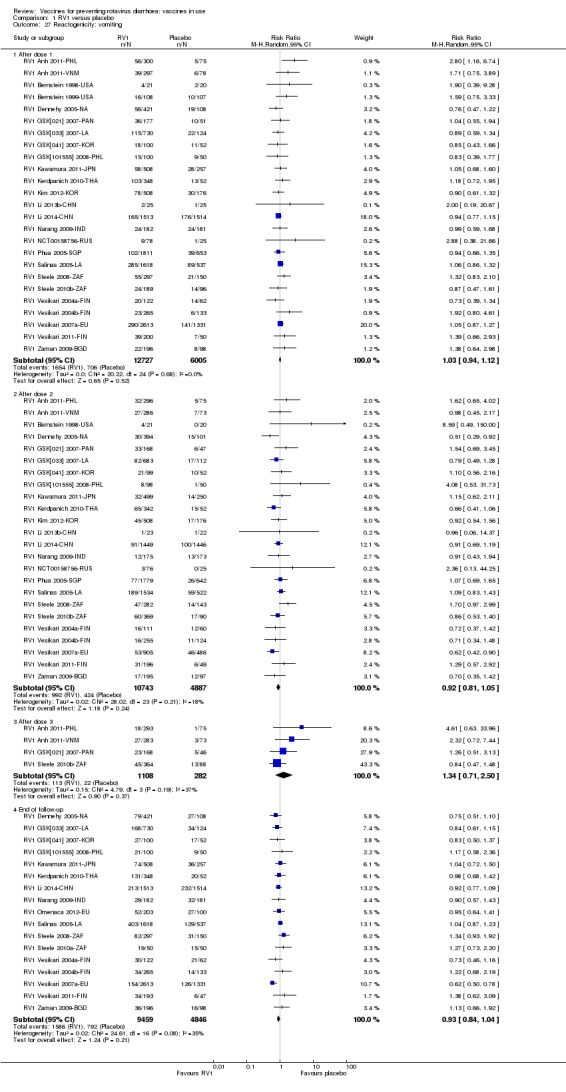
Comparison 1 RV1 versus placebo, Outcome 27 Reactogenicity: vomiting.
1.2.9. Adverse events that require discontinuation of vaccination schedule
There was no statistically significant difference between RV1 and placebo in the number of adverse events leading to discontinuation of the vaccination schedule (RR 1.03, 95% CI 0.83 to 1.26; 94,980 participants, 26 trials; Analysis 1.28).
Analysis 1.28.
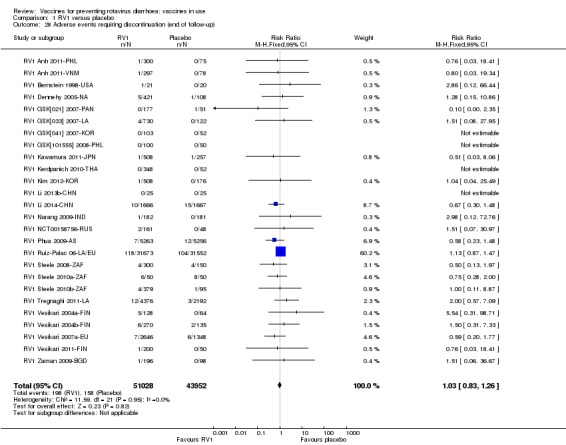
Comparison 1 RV1 versus placebo, Outcome 28 Adverse events requiring discontinuation (end of follow‐up).
1.3. Immunogenicity
Data on immunogenicity was not stratified by WHO strata. RV1 was more immunogenic than placebo when measured by vaccine virus shedding after the final vaccine dose (RR 10.94, 95% CI 4.90 to 24.43; 2638 participants, 16 trials), although the results showed statistical heterogeneity (I2 statistic = 76%, Analysis 1.29). RV1 was also more immunogenic when measured by seroconversion at all time points (Analysis 1.30); although the pooled data showed statistical heterogeneity after one dose (I2 statistic = 57%), after two doses (I2 statistic = 79%), and after three doses (I2 statistic = 51%).
Analysis 1.29.
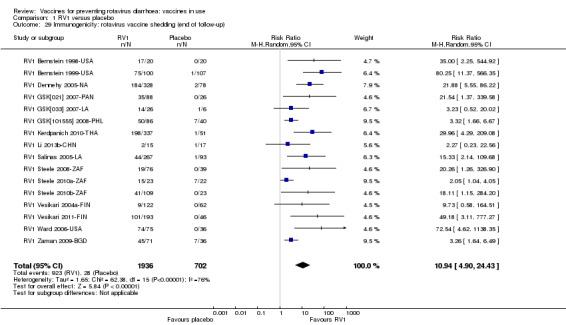
Comparison 1 RV1 versus placebo, Outcome 29 Immunogenicity: rotavirus vaccine shedding (end of follow‐up).
Analysis 1.30.
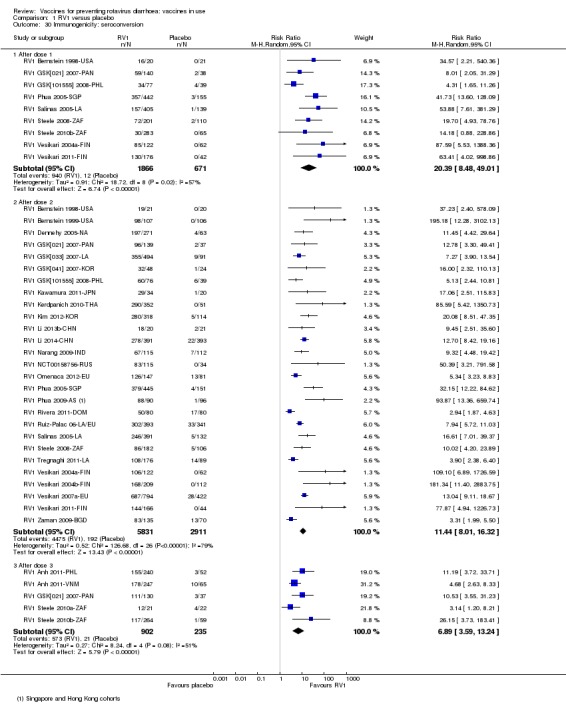
Comparison 1 RV1 versus placebo, Outcome 30 Immunogenicity: seroconversion.
1.4. Dropouts before the end of trial
Twenty‐eight trials reported on the number of participants who dropped out of the trial before it ended. Overall, there was no statistically significant difference between the RV1 and placebo or no‐intervention groups (RR 0.95, 95% CI 0.90 to 1.00; 93,106 participants, 28 trials; Analysis 1.31).
Analysis 1.31.
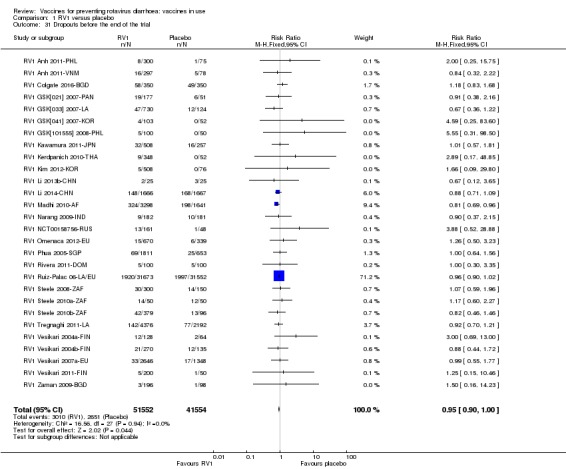
Comparison 1 RV1 versus placebo, Outcome 31 Dropouts before the end of the trial.
1.5. Subgroup analyses
1.5.1. G type
Rotavirus diarrhoea: of any severity
Six trials reported on rotavirus diarrhoea of any severity by different G types. There were significantly fewer episodes of rotavirus diarrhoea of any severity in the group receiving RV1 when compared to placebo, regardless of G type (G1, G2, G3, G4, or G9); however, the pooled data for G1 (I2 statistic = 81%) and G9 (I2 statistic = 63%) types showed statistical heterogeneity, see Analysis 1.32.
Analysis 1.32.
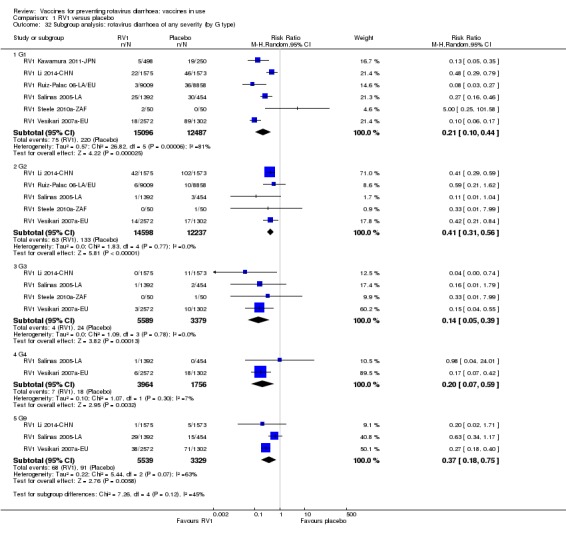
Comparison 1 RV1 versus placebo, Outcome 32 Subgroup analysis: rotavirus diarrhoea of any severity (by G type).
Rotavirus diarrhoea: severe
There were significantly fewer severe episodes of rotavirus diarrhoea in the RV1 groups compared with placebo in episodes attributed to the G1, G2, G3, G9, and G12 types; see Analysis 1.33. Results were not statistically significant for G4 and G8 types. The pooled data for G8 types showed statistical heterogeneity (I2 statistic = 63%).
Analysis 1.33.
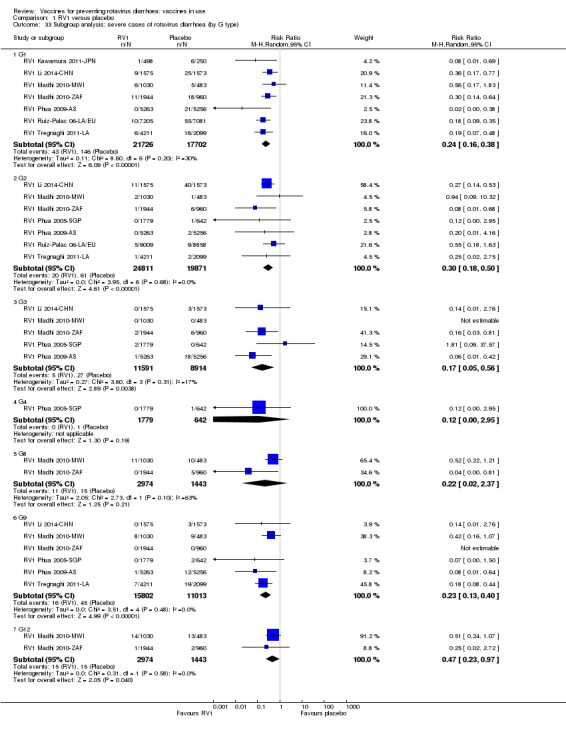
Comparison 1 RV1 versus placebo, Outcome 33 Subgroup analysis: severe cases of rotavirus diarrhoea (by G type).
1.5.2. Malnourished children
Rotavirus diarrhoea: of any severity
One trial provided data separately as the number of cases of rotavirus diarrhoea of any severity in a subgroup of malnourished children (RV1 Salinas 2005‐LA). RV1 was significantly better than placebo in preventing rotavirus diarrhoea for this subgroup at one year of follow‐up (RR 0.39, 95% CI 0.19 to 0.79; 287 participants, 1 trial, Analysis 1.34).
Analysis 1.34.
Comparison 1 RV1 versus placebo, Outcome 34 Subgroup analysis: rotavirus diarrhoea in malnourished children.
1.5.3. Children infected with HIV
Rotavirus diarrhoea: of any severity
One safety trial included only confirmed HIV‐positive, asymptomatic or mildly symptomatic children (RV1 Steele 2010a‐ZAF). At one‐month follow‐up, no statistically significant difference between the RV1 and placebo arms for rotavirus diarrhoea was reported (RR 1.00, 95% CI 0.26 to 3.78; 100 participants, 1 trial; Analysis 1.35).
Analysis 1.35.
Comparison 1 RV1 versus placebo, Outcome 35 Subgroup analysis: rotavirus diarrhoea in HIV‐infected children.
One efficacy trial included children who were infected with HIV or children that had been exposed to HIV, as long as they were not clinically immunosuppressed (e.g. AIDS) at the age of vaccination (six weeks) (RV1 Madhi 2010‐AF). HIV tests were performed on approximately 46% of children from Malawi and 23% of children from South Africa. We did not conduct a specific analysis for this population, but the authors stated that demographic characteristics and the proportion of children who were infected with HIV were similar across the study groups.
1.6 Sensitivity analysis
1.6.1 Primary outcomes with high heterogeneity according to allocation concealment
To investigate heterogeneity for primary outcomes with pooled results where I2 statistic > 75%, we planned to pool data only from studies with low risk of bias for allocation concealment in a sensitivity analysis. We rated all trials at low risk of bias for allocation concealment for the two outcomes where heterogeneity was high (I2 statistic > 75%); see Analysis 1.3 (I2 statistic = 75%) and Analysis 1.4 (I2 statistic = 90%).
1.6.2 Cluster‐randomised trials
Two outcomes (serious adverse events: intussusception, and rotavirus severe diarrhoea at two years) included one cluster‐randomised trial carried out in a high‐mortality country (RV1 Zaman 2017‐BGD). When we excluded data from this trial there was a small but non‐significant change to the effect estimate and 95% CI for Rotavirus diarrhoea: severe (up to 2 years follow‐up) (RR 0.58, 95% CI 0.42 to 0.79, 2764 participants, 1 trial; analysis not shown), and there were no changes to effect estimates or 95% CIs for serious adverse events: intussusception.
‘Summary of findings'
Summary of findings of primary outcomes according to country mortality rate (WHO strata A to E) are presented in Table 1 (RV1, low‐mortality countries), and in Table 2 (RV1, high‐mortality countries).
2. RV5
2.1. Primary outcomes
2.1.1. Rotavirus diarrhoea: severe
Seven trials provided data for the efficacy of RV5 to prevent severe rotavirus diarrhoea in children; see Analysis 2.1 for one‐year follow‐up and Analysis 2.2 for two years follow‐up. Trials were performed in low‐mortality countries (RV5 Clark 2004‐USA; RV5 Vesikari 2006a‐FIN; RV5 Vesikari 2006b‐INT; RV5 Block 2007‐EU/USA; RV5 Iwata 2013‐JPN; RV5 Mo 2017‐CHN), one trial was split between low‐mortality Vietnam in stratum B (RV5 Zaman 2010‐VNM) and high‐mortality Bangladesh in stratum D (RV5 Zaman 2010‐BGD), and another between high‐mortality Ghana and Mali in stratum D (RV5 Armah 2010‐GHA; RV5 Armah 2010‐MLI) and high‐mortality Kenya in stratum E (RV5 Armah 2010‐KEN). Data below are grouped accordingly.
Analysis 2.1.
Comparison 2 RV5 versus placebo, Outcome 1 Rotavirus diarrhoea: severe (up to 1 year follow‐up).
Analysis 2.2.
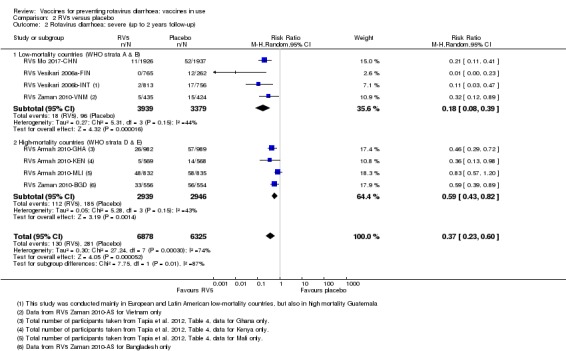
Comparison 2 RV5 versus placebo, Outcome 2 Rotavirus diarrhoea: severe (up to 2 years follow‐up).
Low‐mortality countries (WHO strata A and B)
RV5 reduced the number of severe rotavirus diarrhoea cases by 92% at one year (RR 0.08, 95% CI 0.03 to 0.22; 4132 participants, 5 trials) and 82% by two years (RR 0.18, 95% CI 0.08 to 0.39; 7318 participants, 4 trials). Pooled results showed statistical heterogeneity at two‐year follow‐up (I2 statistic = 44%); see Analysis 2.2.
High‐mortality countries (WHO strata D and E)
RV5 reduced the number of severe rotavirus diarrhoea cases by 57% at one year (RR 0.43, 95% CI 0.29 to 0.62; 5916 participants, 4 comparisons from 2 trials) and 41% at two years (RR 0.59, 95% CI 0.43 to 0.82; 5885 participants, 4 comparisons from 2 trials). Pooled results showed statistical heterogeneity at two‐year follow‐up (I2 statistic = 43%); see Analysis 2.2.
2.1.2. All‐cause diarrhoea: severe
Only two trials provided data for the efficacy of RV5 to prevent severe all‐cause diarrhoea in children; see Analysis 2.3 for one‐year follow‐up and Analysis 2.4 for two‐year follow‐up. Trials were performed in high‐mortality countries (RV5 Armah 2010‐GHA; RV5 Armah 2010‐KEN; RV5 Armah 2010‐MLI; RV5 Zaman 2010‐AS). We did not identify any trial that reported on this outcome that was performed in a low‐mortality country.
Analysis 2.3.
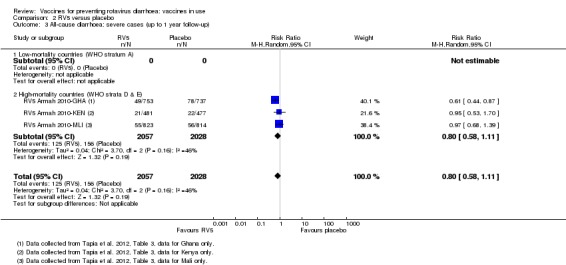
Comparison 2 RV5 versus placebo, Outcome 3 All‐cause diarrhoea: severe cases (up to 1 year follow‐up).
Analysis 2.4.
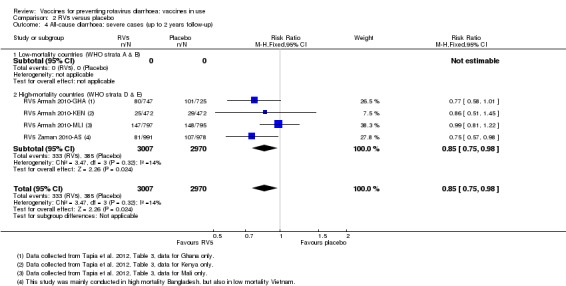
Comparison 2 RV5 versus placebo, Outcome 4 All‐cause diarrhoea: severe cases (up to 2 years follow‐up).
High‐mortality countries (WHO strata D and E)
There was no statistically significant difference between RV5 and placebo for all‐cause severe diarrhoea at one‐year follow‐up (RR 0.80, 95% CI 0.58 to 1.11; 4085 participants, 3 comparisons from 1 trial). At two‐year follow‐up, RV5 reduced severe cases by 15% (RR 0.85, 95% CI 0.75 to 0.98; 5977 participants, 4 comparisons from 2 trials). Pooled results showed statistical heterogeneity at one‐year follow‐up (I2 statistic = 46%); see Analysis 2.3.
2.1.3. All‐cause death
Eleven trials reported on all‐cause death, in most trials as the number of deaths (RV5 Armah 2010‐AF; RV5 Iwata 2013‐JPN; RV5 Lawrence 2012‐CHN; RV5 Levin 2017‐AF; RV5 Merck[009] 2005‐USA; RV5 Mo 2017‐CHN; RV5 Vesikari 2006a‐FIN; RV5 Vesikari 2006b‐INT; RV5 Zaman 2010‐AS), and in two trials as fatal serious adverse events (RV5 Block 2007‐EU/USA; RV5 Ciarlet 2009‐EU). We pooled the number of deaths and fatal serious adverse events; see Analysis 2.5. We present details of causes of death for each trial in Appendix 9. Most trials were performed in low‐mortality countries, with one trial split between low‐mortality Vietnam in stratum B (RV5 Zaman 2010‐VNM) and high‐mortality Bangladesh in stratum D (RV5 Zaman 2010‐BGD), and another between high‐mortality Ghana and Mali in stratum D (RV5 Armah 2010‐GHA; RV5 Armah 2010‐MLI) and high‐mortality Kenya in stratum E (RV5 Armah 2010‐KEN).
Analysis 2.5.
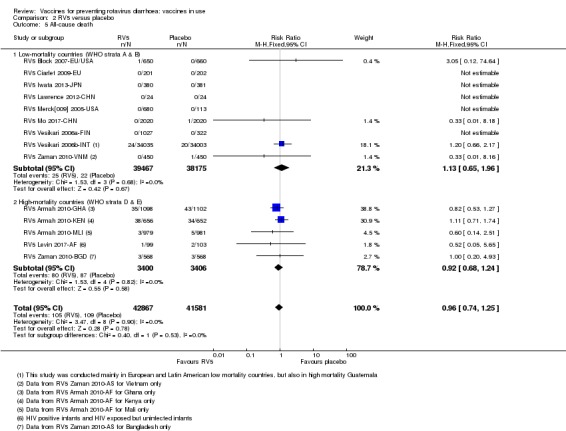
Comparison 2 RV5 versus placebo, Outcome 5 All‐cause death.
Low‐mortality countries (WHO strata A and B)
There was no statistically significant difference in all‐cause death between RV5 and placebo arm (RR 1.13, 95% CI 0.65 to 1.96; 77,642 participants, 9 trials; Analysis 2.5).
High‐mortality countries (WHO strata D and E)
There was no statistically significant difference in all‐cause death between the two arms (RR 0.92, 95% CI 0.68 to 1.24; 6806 participants, 5 comparisons from 3 trials; Analysis 2.5).
2.1.4. All serious adverse events
Serious adverse events were reported in 11 trials, in trials in low‐mortality countries (RV5 Block 2007‐EU/USA; RV5 Ciarlet 2009‐EU; RV5 Iwata 2013‐JPN; RV5 Kim 2008‐KOR; RV5 Lawrence 2012‐CHN; RV5 Mo 2017‐CHN; RV5 Vesikari 2006b‐INT; RV5 Zaman 2010‐VNM), and in high‐mortality countries (RV5 Armah 2010‐GHA; RV5 Armah 2010‐KEN; RV5 Armah 2010‐MLI; RV5 Dhingra 2014‐IND; RV5 Levin 2017‐AF; RV5 Zaman 2010‐BGD); see Analysis 2.6.
Analysis 2.6.
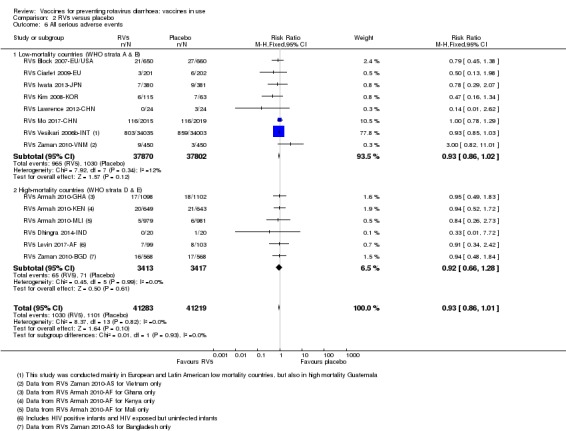
Comparison 2 RV5 versus placebo, Outcome 6 All serious adverse events.
Low‐mortality countries (WHO strata A and B)
Pooled results showed no statistically significant difference in the number of serious adverse events in the RV5 group compared with the placebo group (RR 0.93, 95% CI 0.86 to 1.02; 75,672 participants, 8 trials; Analysis 2.6). In addition, in a separate cohort of RV5 Lawrence 2012‐CHN that vaccinated 24 older children (aged two to six years) with one‐dose RV5 there were no serious adverse events reported.
High‐mortality countries (WHO strata D and E)
Pooled results showed no statistically significant difference in the number of serious adverse events in the RV5 group compared with the placebo group (RR 0.92, 95% CI 0.66 to 1.28; 6830 participants, 6 comparisons from 4 trials; Analysis 2.6).
2.1.5. Serious adverse events: intussusception
Thirteen trials reported cases of intussusception. Trials were performed in low‐mortality countries (RV5 Block 2007‐EU/USA; RV5 Ciarlet 2009‐EU; RV5 Clark 2003‐USA; RV5 Clark 2004‐USA; RV5 Iwata 2013‐JPN; RV5 Kim 2008‐KOR; RV5 Lawrence 2012‐CHN; RV5 Merck[009] 2005‐USA; RV5 Mo 2017‐CHN; RV5 Vesikari 2006a‐FIN; RV5 Vesikari 2006b‐INT; RV5 Zaman 2010‐VNM), and in high‐mortality countries (RV5 Armah 2010‐GHA; RV5 Armah 2010‐KEN; RV5 Armah 2010‐MLI; RV5 Zaman 2010‐BGD); see Analysis 2.7.
Analysis 2.7.
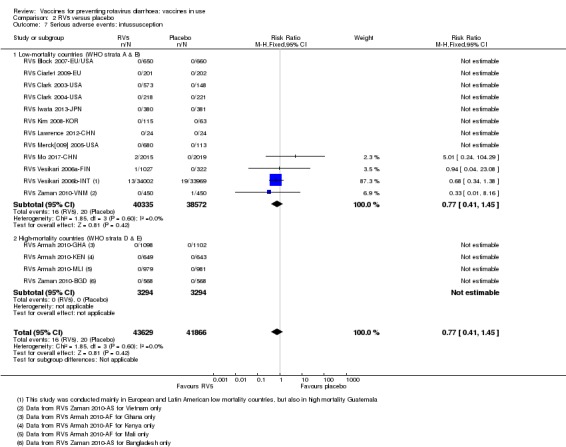
Comparison 2 RV5 versus placebo, Outcome 7 Serious adverse events: intussusception.
Low‐mortality countries (WHO strata A and B)
Fourteen cases of intussusception were reported in a total of 38,321 children in the RV5 arm compared with 20 cases of intussusception in 36,553 children in the placebo arm. Pooled results showed no increased risk of intussusception in children receiving RV5 when compared to placebo (RR 0.77, 95% CI 0.41 to 1.45; 78,907 participants, 12 trials; Analysis 2.7).
High‐mortality countries (WHO strata D and E)
There were no reported cases of intussusception in a total of 3294 children in the RV5 arm and 3294 children in the placebo arm (4 comparisons from 2 trials).
2.2. Secondary outcomes
2.2.1. Rotavirus diarrhoea: of any severity
Nine trials provided data for the efficacy of RV5 to prevent rotavirus diarrhoea of any severity in children; see Analysis 2.8 for one‐year follow‐up and Analysis 2.9 for two‐year follow‐up. Trials were performed in low‐mortality countries (RV5 Block 2007‐EU/USA; RV5 Clark 2003‐USA; RV5 Clark 2004‐USA; RV5 Iwata 2013‐JPN; RV5 Mo 2017‐CHN; RV5 Vesikari 2006a‐FIN; RV5 Vesikari 2006b‐INT), and in high‐mortality countries (RV5 Armah 2010‐GHA; RV5 Armah 2010‐KEN; RV5 Armah 2010‐MLI; RV5 Zaman 2010‐AS). Data below are grouped accordingly.
Analysis 2.8.
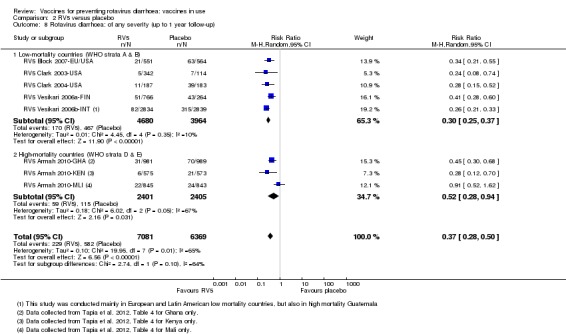
Comparison 2 RV5 versus placebo, Outcome 8 Rotavirus diarrhoea: of any severity (up to 1 year follow‐up).
Analysis 2.9.
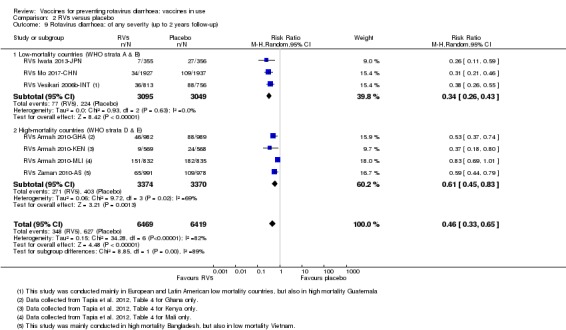
Comparison 2 RV5 versus placebo, Outcome 9 Rotavirus diarrhoea: of any severity (up to 2 years follow‐up).
Low‐mortality countries (WHO strata A and B)
RV5 reduced the number of cases of rotavirus diarrhoea by 70% at one year (RR 0.30, 95% CI 0.25 to 0.37; 8644 participants, 5 trials; Analysis 2.8) and by 66% during the second year (RR 0.34, 95% CI 0.26 to 0.43; 6144 participants, 3 trials; Analysis 2.9).
High‐mortality countries (WHO strata D and E)
RV5 reduced the number of cases of rotavirus diarrhoea by 48% at one year (RR 0.52, 95% CI 0.28 to 0.94; 4806 participants, 3 comparisons from 1 trial; Analysis 2.8) and by 39% during the second year (RR 0.61, 95% CI 0.45 to 0.83; 6744 participants, 4 comparisons from 2 trials; Analysis 2.9). Pooled results were significantly heterogenous at one‐year (I2 statistic = 67%; see Analysis 2.8) and at two‐year (I2 statistic = 69%; see Analysis 2.9) follow‐up.
2.2.2. All‐cause diarrhoea: of any severity
One trial performed in high‐mortality Kenya (RV5 Armah 2010‐KEN) provided data for the efficacy of RV5 to prevent all‐cause diarrhoea of any severity; see Analysis 2.10 for one‐year and Analysis 2.11 for two‐year follow‐up.
Analysis 2.10.
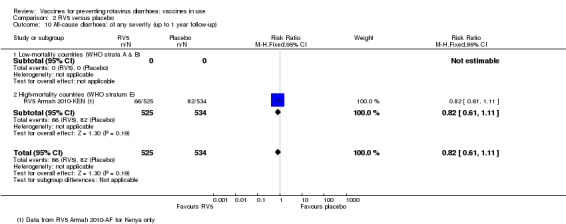
Comparison 2 RV5 versus placebo, Outcome 10 All‐cause diarrhoea: of any severity (up to 1 year follow‐up).
Analysis 2.11.
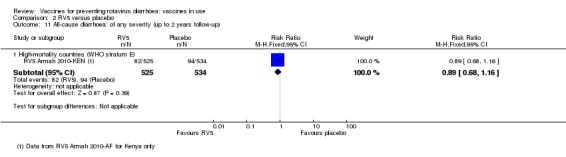
Comparison 2 RV5 versus placebo, Outcome 11 All‐cause diarrhoea: of any severity (up to 2 years follow‐up).
High‐mortality countries (WHO stratum E)
There was no statistically significant difference between RV5 and placebo for any severity all‐cause diarrhoea at one year (RR 0.82, 95% CI 0.61 to 1.11; 1059 participants, 1 trial; Analysis 2.10) or at two‐year follow‐up (RR 0.89, 95% CI 0.68 to 1.16; 1059 participants, 1 trial; Analysis 2.11).
All‐cause hospitalization
Data on all‐cause hospitalization were provided from one trial carried out in Botswana, Tanzania, Zambia, and Zimbabwe (RV5 Levin 2017‐AF).
There was no statistically significant difference between RV5 and placebo for all‐cause hospitalization at two‐year follow‐up (RR 1.21, 95% CI 0.42 to 3.49; 202 participants, 1 trial; Analysis 2.12).
Analysis 2.12.
Comparison 2 RV5 versus placebo, Outcome 12 All‐cause hospitalizations (up to 2 years follow‐up).
2.2.3. Rotavirus diarrhoea: requiring hospitalization or medical attention
RV5 reduced hospitalizations due to rotavirus diarrhoea episodes by 96% at one year of follow‐up (RR 0.04, 95% CI 0.02 to 0.10; 57,134 participants, 1 trial; Analysis 2.13).
Analysis 2.13.
Comparison 2 RV5 versus placebo, Outcome 13 Rotavirus diarrhoea: requiring hospitalization.
RV5 reduced the number of children requiring medical attention at one year of follow‐up by 93% compared to placebo (RR 0.07, 95% CI 0.04 to 0.12; 57,134 participants, 1 trial; Analysis 2.14).
Analysis 2.14.
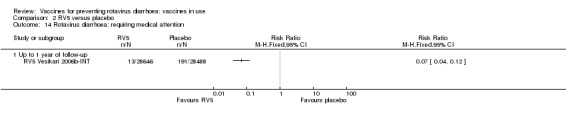
Comparison 2 RV5 versus placebo, Outcome 14 Rotavirus diarrhoea: requiring medical attention.
Data for medical attention and hospitalization rates due to all‐cause diarrhoea were not estimable.
2.2.4. Reactogenicity
The incidence of fever (Analysis 2.15), diarrhoea (Analysis 2.16), and vomiting (Analysis 2.17) were evaluated after the first dose, second dose, and third dose, and at the end of the follow‐up period. We found no statistically significant differences between the RV5 and placebo groups for any of the reactogenicity outcomes and time points. We noted significant heterogeneity for the pooled post‐first dose data on fever (I2 statistic = 61%).
Analysis 2.15.
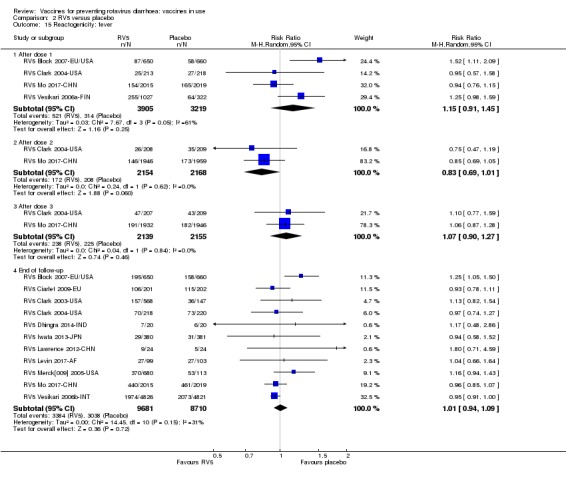
Comparison 2 RV5 versus placebo, Outcome 15 Reactogenicity: fever.
Analysis 2.16.
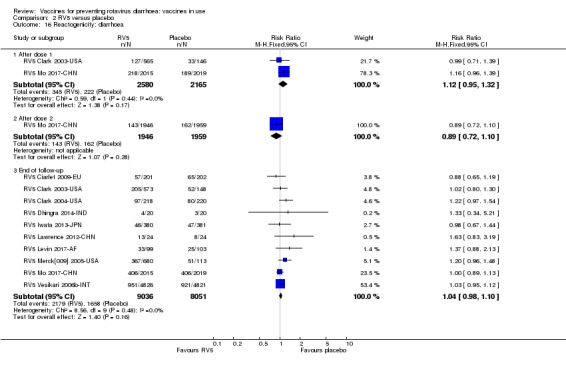
Comparison 2 RV5 versus placebo, Outcome 16 Reactogenicity: diarrhoea.
Analysis 2.17.
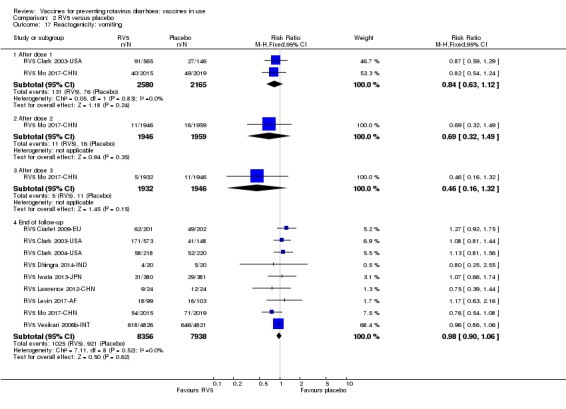
Comparison 2 RV5 versus placebo, Outcome 17 Reactogenicity: vomiting.
2.2.5. Adverse events that require discontinuation of vaccination schedule
Ten trials reported the number of adverse events leading to discontinuation of the vaccination schedule, with no statistically significant difference between RV5 and placebo (RR 0.89, 95% CI 0.57 to 1.39; 15,471 participants, 10 trials; Analysis 2.18).
Analysis 2.18.
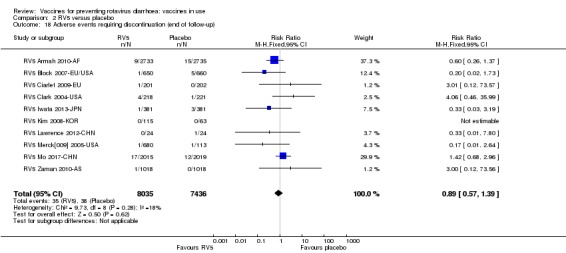
Comparison 2 RV5 versus placebo, Outcome 18 Adverse events requiring discontinuation (end of follow‐up).
2.3. Immunogenicity
RV5 immunogenicity was measured by rotavirus vaccine virus shedding (5 trials, Analysis 2.19) and seroconversion (10 trials, Analysis 2.20) after the third vaccine dose. We decided not to pool the data, however, because of significant heterogeneity (I2 statistic = 80% and 87%, respectively).
Analysis 2.19.
Comparison 2 RV5 versus placebo, Outcome 19 Immunogenicity: rotavirus vaccine shedding (after dose 3).
Analysis 2.20.
Comparison 2 RV5 versus placebo, Outcome 20 Immunogenicity: seroconversion.
2.4. Dropouts before the end of trial
Similar numbers of children taking RV5 and placebo dropped out from trials before they ended (RR 0.98, 95% CI 0.90 to 1.08; 85,855 participants, 13 trials; Analysis 2.21).
Analysis 2.21.
Comparison 2 RV5 versus placebo, Outcome 21 Dropouts before the end of the trial.
2.5. Subgroup analyses
2.5.1. G type
Rotavirus diarrhoea: of any severity
When the analyses were stratified by the G type (Analysis 2.22), there were fewer episodes of rotavirus diarrhoea in the RV5 group compared to the placebo group for the G1 type (RR 0.26, 95% CI 0.21 to 0.32; 11,022 participants, 4 trials), the G2 type (RR 0.35, 95% CI 0.16 to 0.78; 9907 participants, 3 trials), and the G9 type (RR 0.33, 95% CI 0.20 to 0.54; 9537 participants, 2 trials). The results were not statistically significant for G3 (RR 0.40, 95% CI 0.08 to 2.02; 11,022 participants, 4 trials) or for G4 (RR 0.41, 95% CI 0.13 to 1.33; 9907 participants, 3 trials).
Analysis 2.22.
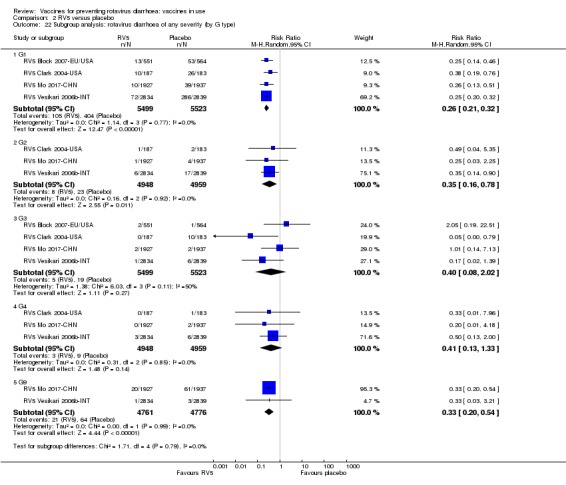
Comparison 2 RV5 versus placebo, Outcome 22 Subgroup analysis: rotavirus diarrhoea of any severity (by G type).
Rotavirus diarrhoea: severe
There were significantly fewer severe episodes of rotavirus diarrhoea in the RV5 groups for G4 (RR 0.12, 95% CI 0.03 to 0.46; 76,606 participants, 3 trials) and G9 (RR 0.13, 95% CI 0.05 to 0.34; 76,606 participants, 3 trials). Pooled results were not significant for G1 (RR 0.23, 95% CI 0.03 to 1.74; 76,606 participants, 3 trials), G2 (RR 0.41, 95% CI 0.13 to 1.37; 76,606 participants, 3 trials), and for G3 (RR 0.38, 95% CI 0.05 to 2.74; 76,606 participants, 3 trials). The pooled data for G1 (I2 statistic = 97%) and G3 (I2 statistic = 64%) types showed statistical heterogeneity.
2.5.2. HIV‐infected children
One trial (RV5 Armah 2010‐AF) performed HIV tests for 89% of participants and reported outcomes for HIV‐infected children (38/1158); another trial (RV5 Levin 2017‐AF) included and reported outcomes for HIV‐exposed but uninfected and HIV‐infected children. We included only HIV‐infected children from this study in this subgroup analysis (Analysis 2.24).
Analysis 2.24.
Comparison 2 RV5 versus placebo, Outcome 24 Subgroup analysis: HIV‐infected children.
Rotavirus diarrhoea: severe (up to two years of follow‐up)
1/21 children in the vaccine arm, and 0/17 children in the placebo arm had severe rotavirus diarrhoea at two‐year follow‐up; there was no statistically significant difference detected between the two treatment arms (1 trial).
All‐cause diarrhoea: severe (up to two years of follow‐up)
5/21 children in the vaccine arm, and 1/17 children in the placebo arm had severe all‐cause diarrhoea at two‐year follow‐up; there was no statistically significant difference detected between the two treatment arms (1 trial).
All‐cause death
9/58 children in the vaccine arm, and 6/56 children in the placebo arm died; there was no statistically significant difference between the two arms (2 trials).
Serious adverse events (1 ‐ 14 days after any dose)
10/58 children in the vaccine arm, and 6/55 children in the placebo arm had a serious adverse event; there was no statistically significant difference between the two arms (2 trials).
2.6 Sensitivity analysis
2.6.1 Primary outcomes with high heterogeneity according to allocation concealment
There were no primary outcomes with high heterogeneity (I2 statistic > 75%).
‘Summary of findings'
Summary of findings of primary outcomes according to country mortality rate (WHO strata A to E) are presented in Table 3 (RV5, low‐mortality countries), and in Table 4 (RV5, high‐mortality countries).
3. Rotavac
3.1. Primary outcomes
3.1.1. Rotavirus diarrhoea: severe
High‐mortality countries (WHO stratum D)
One trial conducted in India provided data for the efficacy of Rotavac to prevent severe rotavirus diarrhoea in children. Rotavac reduced severe rotavirus diarrhoea cases by 57% at one year (RR 0.43, 95% CI 0.30 to 0.60; 6799 participants, 1 trial; Analysis 3.1) and by 54% by two years (RR 0.46, 95% CI 0.35 to 0.60; 6541 participants, 1 trial; Analysis 3.2).
Analysis 3.1.
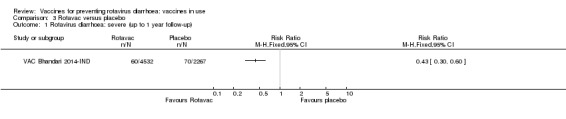
Comparison 3 Rotavac versus placebo, Outcome 1 Rotavirus diarrhoea: severe (up to 1 year follow‐up).
Analysis 3.2.
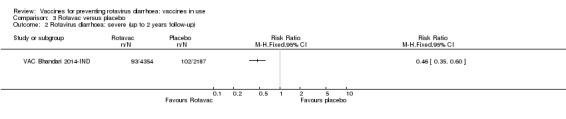
Comparison 3 Rotavac versus placebo, Outcome 2 Rotavirus diarrhoea: severe (up to 2 years follow‐up).
3.1.2. All‐cause diarrhoea: severe
High‐mortality countries (WHO stratum D)
One trial conducted in India provided data for the efficacy of Rotavac to prevent severe all‐cause diarrhoea in children. The trial showed a reduction in the number of severe cases of diarrhoea with Rotavac compared to placebo at one year by 16% (RR 0.84, 95% CI 0.71 to 0.98; 6799 participants, 1 trial; Analysis 3.3).
Analysis 3.3.
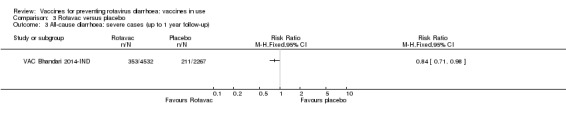
Comparison 3 Rotavac versus placebo, Outcome 3 All‐cause diarrhoea: severe cases (up to 1 year follow‐up).
3.1.3. All‐cause death
High‐mortality countries (WHO stratum D)
Two trials conducted in India reported on all‐cause death. There was no statistically significant difference in all‐cause death between Rotavac and placebo (RR 0.92, 95% CI 0.52 to 1.62; 8155 participants Analysis 3.4). We present details of causes of death for each trial in Appendix 9.
Analysis 3.4.
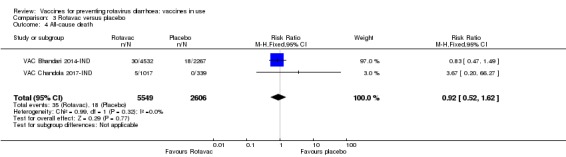
Comparison 3 Rotavac versus placebo, Outcome 4 All‐cause death.
3.1.4. All serious adverse events
High‐mortality countries (WHO stratum D)
Serious adverse events were reported in three trials conducted in India. Pooled results showed no statistically significant difference in the number of serious adverse events in the Rotavac group compared with the placebo group (RR 0.93, 95% CI 0.85 to 1.02; 8210 participants, 3 trials; Analysis 3.5).
Analysis 3.5.
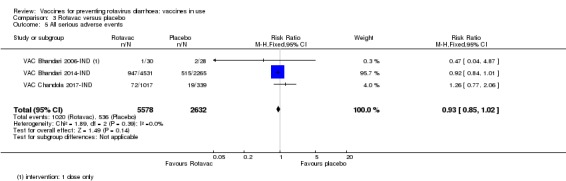
Comparison 3 Rotavac versus placebo, Outcome 5 All serious adverse events.
3.1.5. Serious adverse events: intussusception
High‐mortality countries (WHO stratum D)
Four trials conducted in India reported on cases of intussusception. Eight cases of intussusception were reported in a total of 5764 children in the Rotavac arm compared with three cases of intussusception in 2818 children in the placebo arm. Pooled results showed no increased risk of intussusception in children receiving Rotavac when compared to placebo (RR 1.33, 95% CI 0.35 to 5.02; 8582 participants, 4 trials; Analysis 3.6).
Analysis 3.6.
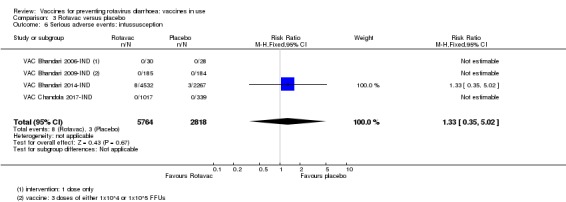
Comparison 3 Rotavac versus placebo, Outcome 6 Serious adverse events: intussusception.
3.2. Secondary outcomes
3.2.1. Rotavirus diarrhoea: of any severity
One trial provided data for the efficacy of Rotavac to prevent rotavirus diarrhoea of any severity in children. Rotavac reduced the number of cases of rotavirus diarrhoea of any severity by 34% at both one‐year (RR 0.66, 95% CI 0.56 to 0.78; 6799 participants, 1 trial; Analysis 3.7) and two‐year follow‐up (RR 0.66, 95% CI 0.57 to 0.76; 6541 participants, 1 trial; Analysis 3.8).
Analysis 3.7.
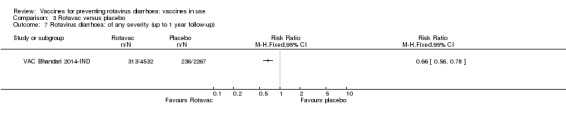
Comparison 3 Rotavac versus placebo, Outcome 7 Rotavirus diarrhoea: of any severity (up to 1 year follow‐up).
Analysis 3.8.
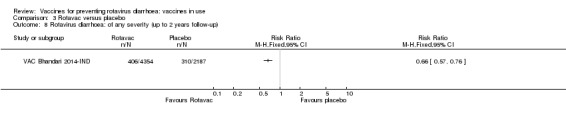
Comparison 3 Rotavac versus placebo, Outcome 8 Rotavirus diarrhoea: of any severity (up to 2 years follow‐up).
3.2.2. Rotavirus diarrhoea: requiring medical attention
Rotavac reduced the number of children requiring medical attention due to rotavirus diarrhoea at one year of follow‐up by 31% compared to placebo (RR 0.69, 95% CI 0.58 to 0.81; 6799 participants, 1 trial; Analysis 3.9).
Analysis 3.9.
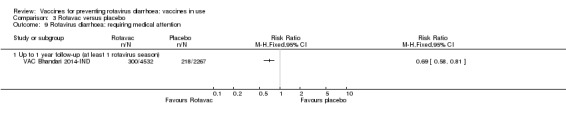
Comparison 3 Rotavac versus placebo, Outcome 9 Rotavirus diarrhoea: requiring medical attention.
3.2.3. Reactogenicity
The incidences of fever (Analysis 3.10), diarrhoea (Analysis 3.11), and vomiting (Analysis 3.12) were evaluated after the first dose in two trials, second dose in one trial, and third dose in one trial. We found no statistically significant differences between the Rotavac and placebo groups for most of the reactogenicity outcomes and time points, except for diarrhoea, which demonstrated an increase with Rotavac compared to placebo after the second dose (RR 1.55, 95% CI 1.00 to 2.41; 356 participants) and third dose (RR 4.09, 95% CI 2.11 to 7.92; 358 participants).
Analysis 3.10.
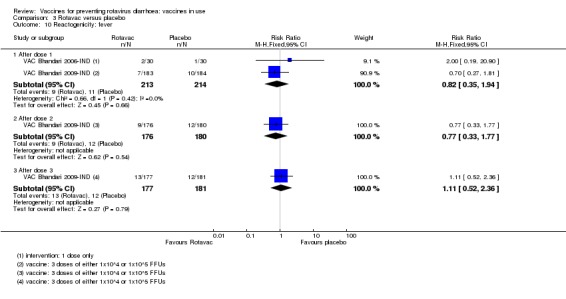
Comparison 3 Rotavac versus placebo, Outcome 10 Reactogenicity: fever.
Analysis 3.11.
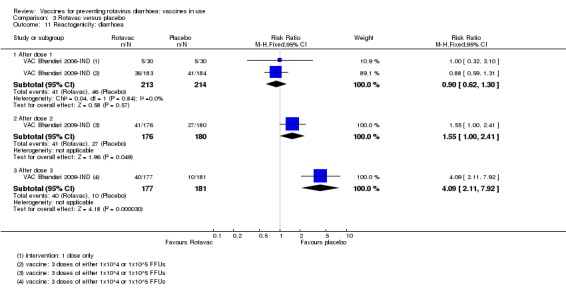
Comparison 3 Rotavac versus placebo, Outcome 11 Reactogenicity: diarrhoea.
Analysis 3.12.
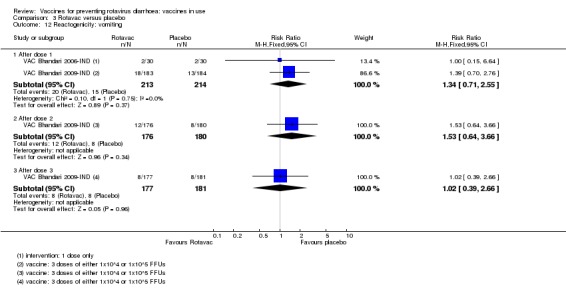
Comparison 3 Rotavac versus placebo, Outcome 12 Reactogenicity: vomiting.
3.2.4. Immunogenicity
Rotavac was more immunogenic than placebo when measured by vaccine virus shedding at the end of follow‐up (RR 9.86, 95% CI 2.58 to 37.63; 427 participants, 2 trials, Analysis 3.13). It was also more immunogenic when measured by seroconversion at all time points (Analysis 3.14): after the first dose (RR 3.58, 95% CI 2.03 to 6.29; 121 participants, 1 trial), after the second dose (RR 2.97, 95% CI 1.78 to 4.98; 117 participants, 1 trial), and after the third dose (RR 2.82, 95% CI 2.26 to 3.51; 1699 participants, 3 trials).
Analysis 3.13.
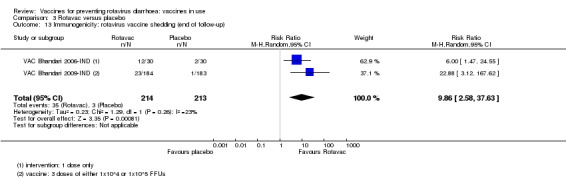
Comparison 3 Rotavac versus placebo, Outcome 13 Immunogenicity: rotavirus vaccine shedding (end of follow‐up).
Analysis 3.14.
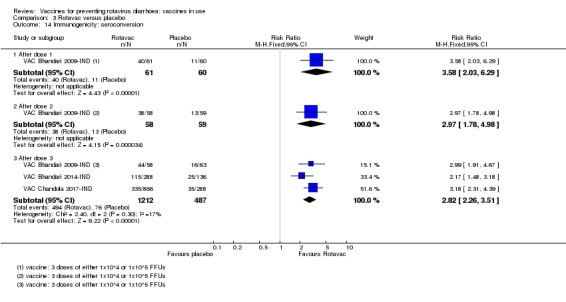
Comparison 3 Rotavac versus placebo, Outcome 14 Immunogenicity: seroconversion.
3.2.5. Dropouts before the end of trial
Similar numbers of children taking Rotavac or placebo dropped out from trials before they ended (RR 0.81, 95% CI 0.62 to 1.06; 8215 participants, 3 trials; Analysis 3.15).
Analysis 3.15.
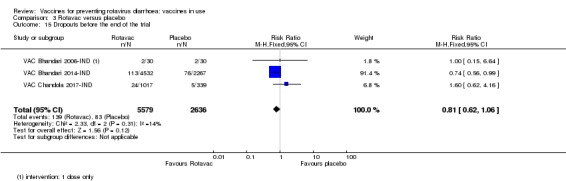
Comparison 3 Rotavac versus placebo, Outcome 15 Dropouts before the end of the trial.
3.3. Subgroup analyses
3.3.1. G type
Rotavirus diarrhoea: severe
One trial reported severe cases of rotavirus diarrhoea by G and P type (VAC Bhandari 2014‐IND).
At one‐year follow‐up (Analysis 3.16) there were significantly fewer severe episodes of rotavirus diarrhoea in the Rotavac groups for G2P[4] (RR 0.39, 95% CI 0.22 to 0.69; 6541 participants) and G12P[6] (RR 0.31, 95% CI 0.13 to 0.74; 6541 participants); results were not significantly different between Rotavac and placebo for G1P[8] (RR 0.66, 95% CI 0.36 to 1.20; 6541 participants) and G12P[8] (RR 0.30, 95% CI 0.07 to 1.26; 6541 participants).
Analysis 3.16.
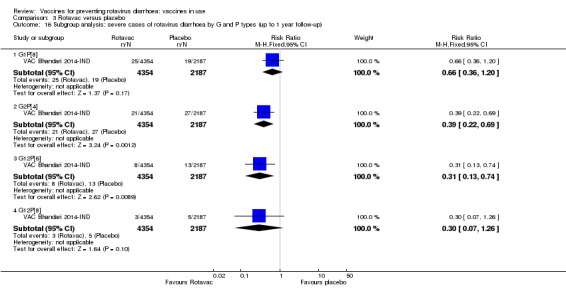
Comparison 3 Rotavac versus placebo, Outcome 16 Subgroup analysis: severe cases of rotavirus diarrhoea by G and P types (up to 1 year follow‐up).
At two‐year follow‐up (Analysis 3.17) there were significantly fewer severe episodes of rotavirus diarrhoea in the Rotavac groups for G1P[8] (RR 0.59, 95% CI 0.38 to 0.93; 6541 participants), G2P[4] (RR 0.37, 95% CI 0.23 to 0.62; 6541 participants), G12P[6] (RR 0.31, 95% CI 0.13 to 0.74; 6541 participants), and G12P[8] (RR 0.31, 95% CI 0.10 to 0.96; 6541 participants).
Analysis 3.17.
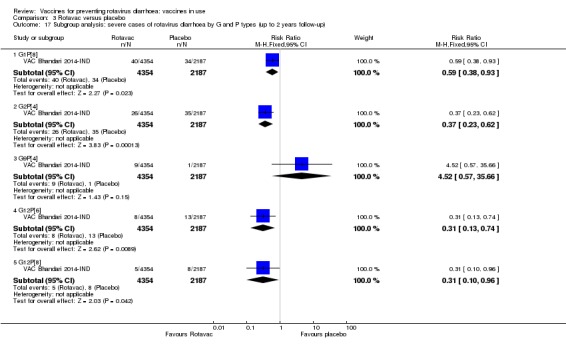
Comparison 3 Rotavac versus placebo, Outcome 17 Subgroup analysis: severe cases of rotavirus diarrhoea by G and P types (up to 2 years follow‐up).
The included Rotavac trials did not report separate data on immunocompromised or malnourished subgroups.
3.4 Sensitivity analyses
3.4.1 Primary outcomes with high heterogeneity according to allocation concealment
There were no primary outcomes with high heterogeneity (I2 statistic > 75%).
‘Summary of findings'
Summary of findings of primary outcomes are presented in Table 5 (Rotavac, high‐mortality countries),
Discussion
Rotavirus vaccines have been under development since the 1980s, and to date three have been prequalified by the WHO (RV1, RV5 and Rotavac). Three additional rotavirus vaccines are licensed for use in individual countries (LLR, Rotasiil, and Rotavin, see Appendix 10). RRV‐TV (RotaShield) has not been used since 1999. The three vaccines prequalified by the WHO (RV1, RV5, Rotavac), and currently in use, are the focus of this review.
Summary of main results
We included 55 trials with a total of 216,480 participants, that evaluated RV1 (36 trials), RV5 (15 trials), and Rotavac (4 trials). Our analysis stratified the primary outcomes by WHO mortality strata (high‐mortality countries, with high child mortality; and low‐mortality, with low or very low child mortality; WHO 1999).
The trials were not designed or powered to detect an effect on preventing death or on the occurrence of possible rare serious adverse events, such as intussusception.
1. RV1 in countries with low child mortality (WHO strata A and B)
Fourteen trials were conducted in Asia, six in Europe, four in Latin America, four in North America, and one in Europe and Latin America.
In infants under one year
RV1 prevents 84% of cases of severe rotavirus diarrhoea: RR 0.16, 95% CI 0.09 to 0.26; 43,779 participants, 7 trials; high‐certainty evidence.
RV1 prevents 41% of cases of severe all‐cause diarrhoea: RR 0.59, 95% CI 0.47 to 0.74; 28,053 participants, 3 trials; moderate‐certainty evidence.
In children up to two years
RV1 prevents 82% of cases of severe rotavirus diarrhoea: RR 0.18, 95% CI 0.14 to 0.23; 36,002 participants, 9 trials; high‐certainty evidence.
RV1 prevents 37% of severe all‐cause diarrhoea episodes: Rate ratio 0.63, 95% CI 0.56 to 0.71; 39,091 participants, 2 trials; moderate‐certainty evidence.
For all‐cause death, an effect of the vaccine has not been shown: RR 1.22, 95% CI 0.87 to 1.71; 97,597 participants, 22 trials; low‐certainty evidence.
For serious adverse events, children receiving RV1 had 12% fewer events than those receiving placebo: RR 0.88, 95% CI 0.83 to 0.93; 96,233 participants, 24 trials; high‐certainty evidence.
For intussusception, RV1 was not associated with a higher risk: RR 0.69, 95% CI 0.45 to 1.04; 96,513 participants, 17 trials; low‐certainty evidence.
See Table 1.
2. RV1 in countries with high child mortality (WHO strata D and E)
Two trials were conducted in Bangladesh, one in India, one in Peru, three in South Africa, and one in South Africa and Malawi.
In infants under one year
RV1 prevents 63% of cases of severe rotavirus diarrhoea: RR 0.37, 95% CI 0.23 to 0.60; 6114 participants, 3 trials; high‐certainty evidence.
RV1 prevents 27% of cases of severe all‐cause diarrhoea: RR 0.73, 95% CI 0.56 to 0.95; 5639 participants, 2 trials; high‐certainty evidence.
In children up to two years
RV1 prevents 35% of cases of severe rotavirus diarrhoea: RR 0.65, 95% CI 0.51 to 0.83; 13,768 participants, 2 trials; high‐certainty evidence.
RV1 prevents 17% of cases of severe all‐cause diarrhoea: RR 0.83, 95% CI 0.72 to 0.96; 2764 participants, 1 trial; moderate‐certainty evidence.
For all‐cause death, an effect of the vaccine has not been shown: RR 0.88, 95% CI 0.64 to 1.22; 8181 participants, 8 trials; low‐certainty evidence.
For serious adverse events, an effect of the vaccine has not been shown: RR 0.89, 95% CI 0.76 to 1.04; 7481 participants, 7 trials; high‐certainty evidence.
For intussusception, RV1 was not associated with a higher risk: RR 1.49, 95% CI 0.06 to 36.63; 17,492 participants, 4 trials; low‐certainty evidence.
See Table 2.
3. RV5 in countries with low child mortality (WHO strata A and B)
Three trials were conducted in Asia, two in Europe, three in North America, one in Europe and the USA, one in Europe and the Americas.
In infants under one year
RV5 prevents 92% of cases of severe rotavirus diarrhoea: RR 0.08, 95% CI 0.03 to 0.22; 4132 participants, 5 trials; moderate‐certainty evidence.
We found no RV5 trials that reported on severe all‐cause diarrhoea.
In children up to two years
RV5 prevents 82% of cases of severe rotavirus diarrhoea: RR 0.18, 95% CI 0.08 to 0.39; 7318 participants, 4 trials; moderate‐certainty evidence.
We found no RV5 trials that reported on severe all‐cause diarrhoea.
For all‐cause death, an effect of the vaccine has not been shown: RR 1.13, 95% CI 0.65 to 1.96; 77,642 participants, 9 trials; low‐certainty evidence.
For serious adverse events, an effect of the vaccine has not been shown: RR 0.93, 95% CI 0.86 to 1.02; 75,672 participants, 8 trials; high‐certainty evidence.
For intussusception, RV5 was not associated with a higher risk: RR 0.77, 95% CI 0.41 to 1.45; 78,907 participants, 12 trials; low‐certainty evidence.
See Table 3.
4. RV5 in countries with high child mortality (WHO strata D and E)
Two trials were conducted in Asia and two in Africa.
In infants under one year
RV5 prevents 57% of cases of severe rotavirus diarrhoea: RR 0.43, 95% CI 0.29 to 0.62; 5916 participants, 2 trials; high‐certainty evidence.
Data on severe all‐cause diarrhoea was reported in one trial. This suggested a protective effect, but the results were not statistically significant: RR 0.80, 95% CI 0.58 to 1.11; 4085 participants, 1 trial; moderate‐certainty evidence.
In children up to two years
RV5 prevents 41% of cases of severe rotavirus diarrhoea: RR 0.59, 95% CI 0.43 to 0.82; 5885 participants, 2 trials; high‐certainty evidence.
RV5 prevents 15% of cases of severe all‐cause diarrhoea: RR 0.85, 95% CI 0.75 to 0.98; 5977 participants, 2 trials; high‐certainty evidence.
For all‐cause death, an effect of the vaccine has not been shown: RR 0.92, 95% CI 0.68 to 1.24; 6806 participants, 3 trials; low‐certainty evidence.
For serious adverse events, an effect of the vaccine has not been shown: RR 0.92, 95% CI 0.66 to 1.28; 6830 participants, 4 trials; moderate‐certainty evidence.
For intussusception, RV5 was not associated with a higher risk: no cases were reported, 6588 participants, 2 trials; low‐certainty evidence.
See Table 4.
5. Rotavac in countries with high child mortality (WHO stratum D)
Four trials were conducted in India.
In infants under one year
Rotavac prevents 57% of cases of severe rotavirus diarrhoea: RR 0.43, 95% CI 0.30 to 0.60; 6799 participants, 1 trial; moderate‐certainty evidence.
In children up to two years
Rotavac prevents 54% of cases of severe rotavirus diarrhoea: RR 0.46, 95% CI 0.35 to 0.60; 6541 participants, 1 trial; moderate‐certainty evidence.
Rotavac prevents 16% of cases of severe all‐cause diarrhoea: RR 0.84, 95% CI 0.71 to 0.98; 6799 participants, one trial; moderate‐certainty evidence.
For all‐cause death, an effect of the vaccine has not been shown: RR 0.92, 95% CI 0.52 to 1.62; 8155 participants, 2 trials; very low‐certainty evidence.
For serious adverse events, an effect of the vaccine has not been shown: RR 0.93, 95% CI 0.85 to 1.02; 8210 participants, 3 trials; moderate‐certainty evidence.
For intussusception, Rotavac was not associated with a higher risk: RR 1.33, 95% CI 0.35 to 5.02; 8582 participants, 4 trials; very low‐certainty evidence.
See Table 5.
Overall completeness and applicability of evidence
We carried out this systematic review using RCTs. All the included trials were placebo‐controlled, except for two RV1 trials that compared vaccine to no intervention (RV1 Colgate 2016‐BGD; RV1 Zaman 2017‐BGD). We could not evaluate potential herd protection afforded by vaccination. The trials provided only limited data for special groups of children, such as malnourished or immunocompromised children.
Efficacy by setting
RV1 and RV5 were highly efficacious in reducing severe rotavirus diarrhoea episodes in low‐mortality countries; widespread roll‐out of rotavirus vaccines has led to major reductions in rotavirus hospitalizations in such settings (Hungerford 2015; Jonesteller 2017). In contrast, trials of RV1 and RV5 in high‐mortality countries in Africa and Asia demonstrated a relatively lower vaccine efficacy. However, because of the higher burden of rotavirus disease in such countries, the absolute number of events prevented by vaccination is greater than in low‐mortality countries (RV1 Madhi 2010‐AF).
Efficacy by age
Results from RV1 and RV5 found higher vaccine efficacy against severe rotavirus diarrhoea in the first year compared to the cumulative efficacy for the first and second years. The efficacy was lower but the differences between the first and second years were greater in high‐mortality (RV1: 63% up to one year versus 54% up to two years; RV5: 57% versus 41%) compared to low‐mortality countries (RV1: 84% up to one year versus 82% up to two years; RV5: 92% versus 82%). Trials with Rotavac were not carried out in any low‐mortality country.
Reduced vaccine efficacy in high‐mortality countries in trials reporting two years of follow‐up could be explained either by waning of vaccine‐induced immunity, or some protection in the placebo group resulting from more frequent exposure to natural rotavirus infection (RV1 Madhi 2010‐AF). Post‐introduction studies have shown reduced effectiveness in the second year of life in some, but not all, high‐burden settings (Bar‐Zeev 2015; Groome 2014). Additional vaccine doses have been explored to extend the duration of protection in high disease‐burden settings (Cunliffe 2016).
Efficacy by schedule
Children in trials performed in low‐mortality countries received the vaccines according to the country's immunization schedule. Trials performed in high‐mortality countries examined the efficacy of RV1 when administered at 10 to 14 weeks of age, a later age than is recommended in the Expanded Programme on Immunization (EPI) schedule. However, the 6‐ and 10‐week RV1 schedule used in EPI programmes has now been extensively evaluated following vaccine roll‐out in high‐mortality countries in Africa, with effectiveness comparable to efficacy trial estimates (Bar‐Zeev 2015).
All‐cause diarrhoea
The impact of rotavirus vaccination on severe all‐cause diarrhoea from a public health perspective is important, as laboratories in low‐income countries may not routinely test for rotavirus infection. The effect on all‐cause diarrhoea is a function of the contribution of rotavirus to all diarrhoea and the efficacy of the vaccine against rotavirus. Surprisingly, few trials reported vaccine efficacy against all‐cause diarrhoea. Vaccine efficacy against all‐cause diarrhoea of any severity was lower, meaning that vaccination may not have a noticeable impact on milder episodes of diarrhoea occurring in the community (Hungerford 2018).
Mortality data
The included trials were not individually powered to detect a mortality effect. This review did not detect a difference in the number of deaths for children receiving any of the vaccines or placebo. Two post‐vaccine implementation national surveillance studies from Mexico and Brazil reported that the introduction of RV1 into the national immunization programme was associated with a decline in the number of diarrhoea‐related deaths (Do Carmo 2011; Richardson 2010) in comparison with historical controls. A study from rural Malawi showed that diarrhoea deaths reduced by a third following RV1 introduction (Bar‐Zeev 2018).
Safety data
There was no detectable difference in the number of cases of intussusception for children receiving vaccine or placebo. While both RV1 and RV5 have been associated with a low risk of intussusception in post‐marketing studies in Europe, Americas and Australia, the benefits of vaccination are considered to outweigh the risk of vaccine‐associated intussusception (Yen 2016). However, the risk of intussusception after administration of RV1 was not higher than the background risk of intussusception in seven lower‐income sub‐Saharan African countries (Tate 2018).
Subgroup analyses
Rotavirus G‐types
All three rotavirus vaccines showed efficacy against most of the specific rotavirus G‐types that were assessed (G1, G2, G3, G4, G8, G9, and G12), although results were often inconsistent between different countries and imprecise due to few events.
Immunocompromised children
One RV1 trial and two RV5 trials reported on immunocompromised children, all exposed to or infected with HIV. We found no differences for efficacy or safety, but samples were not sufficiently powered. It is now strongly recommended that all HIV‐infected or HIV‐exposed infants be vaccinated with oral rotavirus vaccine, unless severely immunocompromised (Calles 2010). While we lack specific information on many immunodeficiencies, infants with known severe combined immunodeficiency should not receive live rotavirus vaccine (Pinto 2016; Vesikari 2015).
Children with malnutrition
One RV1 trial (RV1 Salinas 2005‐LA) found that RV1 was significantly better than placebo in preventing rotavirus diarrhoea in a subgroup of malnourished children.
Certainty of the evidence
The trials included in this updated review were placebo‐controlled (53 trials) or compared vaccine to no intervention (RV1 Colgate 2016‐BGD; RV1 Zaman 2017‐BGD), were conducted in Latin America, North America, Europe, Asia, and Africa, and the largest included over 60,000 children (RV1 Ruiz‐Palac 06‐LA/EU; RV5 Vesikari 2006b‐INT); we identified the need for such trials in the original version of the review (Soares‐Weiser 2004). However, most children were followed for safety outcomes only.
The certainty of the evidence for efficacy outcomes (rotavirus diarrhoea of any severity and severe, and all‐cause diarrhoea of any severity and severe) was either high or moderate. This was because most trials were assessed at low risk of bias, especially more recent trials, and pooled samples were usually large enough to generate more precise estimates. When we downgraded efficacy outcomes to moderate certainty, this was due to selective reporting bias (only half of the studies reporting on severe rotavirus diarrhoea reported on severe all‐cause diarrhoea), imprecision (low number of events), attrition bias (incomplete outcome data were not clearly reported), or indirectness (only one study carried out in one high‐mortality country or neighbouring high‐mortality countries makes it difficult to generalize to any high‐mortality country).
The certainty of the evidence for all‐cause mortality was low because the trials were not powered to detect an effect on mortality, and results were consequently imprecise with wide 95% CIs.
The certainty of the evidence for all serious adverse events was mostly high but downgraded to moderate for RV5 in high‐mortality countries due to imprecise results, and for Rotavac due to indirectness (all trials were carried out in India). For the rare serious adverse event intussusception, evidence was of low certainty for RV1 and RV5 due to imprecision because trials were not powered to detect an association between RV1 and intussusception. For Rotavac evidence on intussusception was of very low certainty, due to imprecision and indirectness as previously described.
Potential biases in the review process
We stratified all analyses by WHO mortality strata, which may not reflect the current situation in the member countries. The use of the strata may not be sensitive enough to show differences at the country level, and perhaps stratifying by prevalence/burden of rotavirus may be a better method to group the analyses. In addition, not all countries are represented by the studies performed, and some strata (e.g. C) are lacking sufficient data.
Agreements and disagreements with other studies or reviews
We identified three systematic reviews of RCTs evaluating RV1 or RV5 or both that have been conducted since the 2012 update of this Cochrane Review:
Lamberti 2016 included RCTs and observational studies and evaluated region‐specific effectiveness of RV1, RV5 and Rotavac. The systematic review found that rotavirus vaccination was both efficacious and effective in preventing rotavirus diarrhoea, severe rotavirus diarrhoea and rotavirus hospitalizations among children under five across all regions, with higher efficacy in more developed regions.
Velázquez 2017 included RCTs and post‐licensure observational studies from Latin America and the Caribbean, and found that RV1 reduced the risk of any‐severity rotavirus‐related gastroenteritis by 65% and of severe gastroenteritis by 82% versus placebo. Both RV1 and RV5 vaccines significantly reduced the risk of hospitalization and emergency visits by 85% for RV1 and by 90% for RV5. Vaccination with RV5 or RV1 did not increase the risk of death, intussusception, or other severe adverse events.
Buyse 2014 presented an integrated meta‐analysis of safety and reactogenicity data of 28 RV1 RCTs and found that RV1 has a reactogenicity and safety profile similar to placebo.
The findings of these systematic reviews agree with the findings of our review, although the scope of these reviews was narrower; they reviewed efficacy or safety only, or were limited to a specific geographical region, or reviewed only one of the vaccines. Consequently, we included more trials in our review. Finally, the major findings of this review update, including new evidence from 14 trials of RV1, RV5, and Rotavac, are not significantly different from the previous Soares‐Weiser 2012b review.
Relationship to current policies
The data in this review support the WHO's Strategic Advisory Group of Experts (SAGE) on Immunization’s recommendation for "the inclusion of rotavirus vaccination of infants into all national immunization programmes" with a stronger recommendation for countries where "diarrhoeal deaths account for ≥10% of mortality among children aged <5 years" (SAGE 2009).
Authors' conclusions
RV1, RV5 and Rotavac are efficacious vaccines in preventing rotavirus diarrhoea with comparable safety and efficacy profiles. The systematic review data support the global WHO rotavirus vaccine recommendation (SAGE 2009; SAGE 2012).
The data from the included RCTs exclude a risk of intussusception with RV1, RV5, and Rotavac of the magnitude observed with the first licensed vaccine (RRV‐TV, RotaShield). However, since the data cannot exclude a smaller risk of intussusception or other rare serious adverse events, routine vaccine introduction should be accompanied by safety surveillance (Buttery 2011; Patel 2011; Shui 2012; Weintraub 2014).
Placebo‐controlled efficacy trials of RV1 and RV5 have been undertaken in representative populations of low‐ and high‐mortality countries and do not require repetition; efficacy or effectiveness trials of Rotavac outside of India should be considered if Rotavac is introduced globally. Further research would be valuable in the following areas:
Continued post‐introduction studies to examine the impact and effectiveness of rotavirus vaccination, particularly in high‐mortality countries.
A greater understanding of the lower vaccine efficacy observed in high‐mortality countries compared to low‐mortality countries in Africa and Asia in the first and second years of life.
Studies to assess the potential benefit of alternative dosage schedules of rotavirus vaccine, especially in high‐mortality countries (e.g. neonatal dosing, additional dosing).
Continued post‐introduction studies in representative countries should examine vaccine safety with particular respect to intussusception and should analyze the risk/benefit of rotavirus vaccination (Patel 2011). Post‐introduction safety studies of Rotavac are currently lacking (Dutta 2017). Given the rarity of the event, data from different countries may need to be pooled (Escolano 2011; Escolano 2015), or self‐controlled case series analyses may need to be carried out (Carlin 2013; Stowe 2016; Tate 2018; Yih 2014).
Acknowledgements
The Academic Editor of this review update was Dr Hellen Gelband.
A number of people contributed to the preparation of the original review version and are acknowledged in the original review (Soares‐Weiser 2004). We thank Harriet MacLehose, Irit Ben‐Aharon, Sukrti Nagpal, and Elad Goldberg for their contributions as authors to previous updates of this review.
For the 2012 review update: we thank Dr Ana Maria Restrepo (Initiative for Vaccine Research, WHO) for support in acquiring information on trials methodology and Merck for providing the relevant information from their trials. We also thank Professor Paul Fine from the London School of Tropical Medicine for comments on the previous version of this review (2012, Issue 2). We thank Professor Paul Garner and Dr David Sinclair for their assistance and guidance in the preparation of the 2012 update.
For this review update: a part of this update is based on a systematic review commissioned by the Initiative for Vaccine Research, WHO that was carried out by Cochrane Response in 2017. We thank Artemisia Kakourou, Inga Mills, Rachel Wheeler, Nicola Maayan, Gemma Villanueva, Elise Cogo, and Katrin Probyn, all members of the Cochrane Response team, for help with screening and data extraction.
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | Embaseb | LILACSb | BIOSIS |
| 1 | rotavirus | rotavirus | rotavirus | rotavirus | rotavirus | rotavirus |
| 2 | diarrhoea | diarrhoea | ROTAVIRUS INFECTIONS | ROTAVIRUS | diarrhoea | diarrhoea |
| 3 | diarrhoea | diarrhoea | 1 or 2 | 1 or 2 | diarrhea | diarrhoea |
| 4 | gastroenteritis | gastroenteritis | diarrhoea | diarrhoea | gastroenteritis | gastroenteritis |
| 5 | 2 or 3 or 4 | 2 or 3 or 4 | gastroenteritis | gastroenteritis | 2 or 3 or 4 | 2 or 3 or 4 |
| 6 | 1 and 5 | 1 and 5 | 4 or 5 | 4 or 5 | 1 and 5 | 1 and 5 |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by Cochrane (Lefebvre 2011); upper case: MeSH or EMTREE heading; lower case: free‐text term.
Appendix 2. Trial type (efficacy or safety) and length of follow‐up
| Trial | Type: efficacy or safety | Follow‐up time |
| RV1 Anh 2011‐PHL | Safety | 1 month after last dose |
| RV1 Anh 2011‐VNM | Safety | 1 month after last dose |
| RV1 Bernstein 1998‐USA | Safety | 1 month |
| RV1 Bernstein 1999‐USA | Efficacy/Safety | 2 years |
| RV1 Colgate 2016‐BGD | Efficacy | 1 year |
| RV1 Dennehy 2005‐NA | Safety | 10 to 12 months |
| RV1 GSK[021] 2007‐PAN | Safety | 1 month after dose 3 |
| RV1 GSK[033] 2007‐LA | Safety | 1 month |
| RV1 GSK[041] 2007‐KOR | Safety | 2 months |
| RV1 GSK[101555] 2008‐PHL | Safety | 1 month |
| RV1 Kawamura 2011‐JPN | Efficacy/Safety | Up to the age of 2 years |
| RV1 Kerdpanich 2010‐THA | Safety | 2 months after last dose |
| RV1 Kim 2012‐KOR | Safety | 1 month after last dose |
| RV1 Li 2013a‐CHN | Safety | 1 month |
| RV1 Li 2013b‐CHN | Safety | 1 month |
| RV1 Li 2014‐CHN | Efficacy/Safety | 2 years |
| RV1 Madhi 2010‐AF | Efficacy/Safety | 2 years |
| RV1 Narang 2009‐IND | Safety | 1 month |
| RV1 NCT00158756‐RUS | Safety | 1 year |
| RV1 Omenaca 2012‐EU | Safety | At least 1 month after dose 2 |
| RV1 Phua 2005‐SGP | Efficacy/Safety | Until infant aged 18 months (ie 13 to 15 months) |
| RV1 Phua 2009‐AS | Efficacy/Safety | 3 years |
| RV1 Rivera 2011‐DOM | Safety | 17 weeks after each dose |
| RV1 Ruiz‐Palac 06‐LA/EU | Efficacy/Safety | 9 to 10 months |
| RV1 Salinas 2005‐LA | Efficacy/Safety | Up to 2 years |
| RV1 Steele 2008‐ZAF | Safety | Up to 6 months |
| RV1 Steele 2010a‐ZAF | Safety | 31 days after each dose, 42 days after the last dose |
| RV1 Steele 2010b‐ZAF | Safety | Up to 6 months |
| RV1 Tregnaghi 2011‐LA | Efficacy/Safety | Up to age 1 year |
| RV1 Vesikari 2004a‐FIN | Safety | 8 to 30 days after each dose |
| RV1 Vesikari 2004b‐FIN | Efficacy/Safety | 1 and 2 years (both reported) |
| RV1 Vesikari 2007a‐EU | Efficacy/Safety | 1 and 2 years (plus 3 years in Finland) |
| RV1 Vesikari 2011‐FIN | Safety | 2 months |
| RV1 Ward 2006‐USA | Safety | 7 days after each vaccination; 3 to 5 weeks after dose 2 |
| RV1 Zaman 2009‐BGD | Safety | 31 days |
| RV1 Zaman 2017‐BGD | Effectiveness | 2 years |
| RV5 Armah 2010‐AF | Efficacy/Safety | Up to 43 days for safety outcomes, up to 21 months for efficacy outcomes |
| RV5 Block 2007‐EU/USA | Efficacy/Safety | 42 days for safety/immunogenicity; 1 year for efficacy |
| RV5 Ciarlet 2009‐EU | Safety | 42 days |
| RV5 Clark 2003‐USA | Efficacy/Safety | 1 year |
| RV5 Clark 2004‐USA | Efficacy/Safety | 1 year |
| RV5 Dhingra 2014‐IND | Safety | 1 month |
| RV5 Iwata 2013‐JPN | Efficacy/Safety | 25 months |
| RV5 Kim 2008‐KOR | Safety | 42 days |
| RV5 Lawrence 2012‐CHN | Safety | 2 weeks after last dose |
| RV5 Levin 2017‐AF | Safety | 1 month |
| RV5 Merck[009] 2005‐USA | Safety | 42 days |
| RV5 Mo 2017‐CHN | Efficacy/Safety | 2 years |
| RV5 Vesikari 2006a‐FIN | Efficacy/Safety | 1 to 3 years |
| RV5 Vesikari 2006b‐INT | Efficacy/Safety | 43 days for safety; 2 years for efficacy |
| RV5 Zaman 2010‐AS | Efficacy/Safety | Up to 43 days for safety outcomes, up to 2 years for efficacy outcomes |
| VAC Bhandari 2006‐IND | Safety | 1 month |
| VAC Bhandari 2009‐IND | Safety | 12 weeks |
| VAC Bhandari 2014‐IND | Efficacy/Safety | up to 2 years of age |
| VAC Chandola 2017‐IND | Safety | 1 year |
Appendix 3. Efficacy outcome measures by trial
| Trial | Rotavirus diarrhoea (any severity) | All‐cause diarrhoea | ED visit | Hospitalization (all‐cause) | All‐cause death | Dropouts | |||
| All | Severe | Hospital | All | Severe | |||||
| RV1 Anh 2011‐PHL | X | ‐ | ‐ | X | ‐ | ‐ | ‐ | X | X |
| RV1 Anh 2011‐VNM | X | ‐ | ‐ | X | ‐ | ‐ | ‐ | X | X |
| RV1 Bernstein 1998‐USA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| RV1 Bernstein 1999‐USA | X | X | X | Xa | ‐ | Xa | ‐ | X | ‐ |
| RV1 Colgate 2016‐BGD | X | X | ‐ | X | X | ‐ | ‐ | X | X |
| RV1 Dennehy 2005‐NA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| RV1 GSK[021] 2007‐PAN | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 GSK[033] 2007‐LA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 GSK[041] 2007‐KOR | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 GSK[101555] 2008‐PHL | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 Kawamura 2011‐JPN | ‐ | X | X | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 Kerdpanich 2010‐THA | X | ‐ | ‐ | X | ‐ | ‐ | ‐ | X | X |
| RV1 Kim 2012‐KOR | X | ‐ | ‐ | X | ‐ | ‐ | ‐ | X | X |
| RV1 Li 2013a‐CHN | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 Li 2013b‐CHN | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| RV1 Li 2014‐CHN | X | X | X | X | X | ‐ | ‐ | X | X |
| RV1 Madhi 2010‐AF | X | X | X | ‐ | X | ‐ | ‐ | X | X |
| RV1 Narang 2009‐IND | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 NCT00158756‐RUS | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 Omenaca 2012‐EU | X | ‐ | ‐ | X | ‐ | ‐ | ‐ | ‐ | X |
| RV1 Phua 2009‐AS | Xa | X | X | Xa | X | Xa | X | ||
| RV1 Phua 2005‐SGP | X | X | X | X | X | X | X | X | X |
| RV1 Rivera 2011‐DOM | X | ‐ | ‐ | X | ‐ | ‐ | ‐ | ‐ | X |
| RV1 Ruiz‐Palac 06‐LA/EU | Xa | X | X | Xa | X | ‐ | Xa | X | Xa |
| RV1 Salinas 2005‐LA | X | X | X | X | Xa | ‐ | Xa | X | |
| RV1 Steele 2008‐ZAF | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 Steele 2010a‐ZAF | X | ‐ | ‐ | X | ‐ | ‐ | ‐ | X | X |
| RV1 Steele 2010b‐ZAF | X | X | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV1 Tregnaghi 2011‐LA | ‐ | X | ‐ | ‐ | Xa | ‐ | ‐ | X | X |
| RV1 Vesikari 2004a‐FIN | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | Xa | X |
| RV1 Vesikari 2004b‐FIN | X | X | X | X | ‐ | ‐ | ‐ | X | X |
| RV1 Vesikari 2007a‐EU | X | X | X | Xa | X | Xa | Xa | ‐ | ‐ |
| RV1 Vesikari 2011‐FIN | X | ‐ | ‐ | X | ‐ | ‐ | ‐ | X | X |
| RV1 Ward 2006‐USA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| RV1 Zaman 2009‐BGD | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | |
| RV1 Zaman 2017‐BGD | ‐ | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| RV5 Armah 2010‐AF | X | X | ‐ | X | X | ‐ | ‐ | X | X |
| RV5 Block 2007‐EU/USA | X | X | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV5 Ciarlet 2009‐EU | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | ‐ |
| RV5 Clark 2003‐USA | X | Xa | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X |
| RV5 Clark 2004‐USA | X | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X |
| RV5 Dhingra 2014‐IND | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X |
| RV5 Iwata 2013‐JPN | X | X | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV5 Kim 2008‐KOR | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| RV5 Lawrence 2012‐CHN | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV5 Levin 2017‐AF | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV5 Merck[009] 2005‐USA | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV5 Mo 2017‐CHN | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV5 Vesikari 2006a‐FIN | X | X | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
| RV5 Vesikari 2006b‐INT | X | X | X | ‐ | ‐ | Xa | Xa | X | X |
| RV5 Zaman 2010‐AS | X | X | ‐ | ‐ | X | ‐ | ‐ | X | X |
| VAC Bhandari 2006‐IND | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X |
| VAC Bhandari 2009‐IND | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| VAC Bhandari 2014‐IND | X | X | X | ‐ | X | ‐ | ‐ | X | X |
| VAC Chandola 2017‐IND | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X | X |
aReported as an outcome measure in trial, but no data available for analysis.
Appendix 4. Safety and immunogenicity outcomes measures by trial
AE: adverse events. aReported as an outcome measure in trial, but no data available for analysis.
Appendix 5. Trial location
| Trial | Year | Location | Sites | Country mortality rate | WHO mortality strata | Region |
| RV1 Anh 2011‐PHL | 2007 | Philippines | 1 | Low‐mortality | B | Asia |
| RV1 Anh 2011‐VNM | 2007 | Vietnam | 11 | Low‐mortality | B | Asia |
| RV1 Bernstein 1998‐USA | 1998 | USA | 1 | Low‐mortality | A | North America |
| RV1 Bernstein 1999‐USA | 1999 | USA | 2 | Low‐mortality | A | North America |
| RV1 Colgate 2016‐BGD | 2014 | Bangladesh | 1 | High‐mortality | D | Asia |
| RV1 Dennehy 2005‐NA | 2005 | USA and Canada | 41 | Low‐mortality | A | North America |
| RV1 GSK[021] 2007‐PAN | 2007 | Panama | 1 | Low‐mortality | B | Latin America |
| RV1 GSK[033] 2007‐LA | 2007 | Colombia, Mexico, and Peru | (2 in Colombia, 1 in Mexico, and 4 in Peru) | High‐mortalitya | B, D | Latin America |
| RV1 GSK[041] 2007‐KOR | 2007 | South Korea | 6 | Low‐mortality | B | Asia |
| RV1 GSK[101555] 2008‐PHL | 2008 | Philippines | 1 | Low‐mortality | B | Asia |
| RV1 Kawamura 2011‐JPN | 2009 | Japan | 18 | Low‐mortality | A | Asia |
| RV1 Kerdpanich 2010‐THA | 2005 | Thailand | 2 | Low‐mortality | B | Asia |
| RV1 Kim 2012‐KOR | 2010 | Republic of Korea | 19 | Low‐mortality | B | Asia |
| RV1 Li 2013a‐CHN | 2010 | China | 1 | Low‐mortality | B | Asia |
| RV1 Li 2013b‐CHN | 2010 | China | 1 | Low‐mortality | B | Asia |
| RV1 Li 2014‐CHN | 2012 | China | 4 | Low‐mortality | B | Asia |
| RV1 Madhi 2010‐AF | 2010 | South Africa and Malawi | 2 | High‐mortality | E | Africa |
| RV1 Narang 2009‐IND | 2009 | India | 4 | High‐mortality | D | Asia |
| RV1 NCT00158756‐RUS | 2006 | Russian Federation | 9 | Low‐mortality | C | Europe |
| RV1 Omenaca 2012‐EU | 2008 | France, Poland, Portugal, and Spain | Multiple sites in each country | Low‐mortality | A, B | Europe |
| RV1 Phua 2005‐SGP | 2005 | Singapore | 8 | Low‐mortality | A | Asia |
| RV1 Phua 2009‐AS | 2009 | Hong Kong, Singapore, and Taiwan | 3 | Low‐mortality | A | Asia |
| RV1 Rivera 2011‐DOM | 2008 | Dominican Republic | 1 | Low‐mortality | B | Latin America |
| RV1 Ruiz‐Palac 06‐LA/EU | 2006 | Argentina, Brazil, Chile, Colombia, Dominican Republic, Finland, Honduras, Mexico, Nicaragua, Panama, Peru, and Venezuela | Multiple | Low‐mortalityb | A, B, D | Latin America/Europe |
| RV1 Salinas 2005‐LA | 2005 | Brazil, Mexico, and Venezuela | 3 | Low‐mortality | B | Latin America |
| RV1 Steele 2008‐ZAF | 2007 | South Africa | 1 | High‐mortality | E | Africa |
| RV1 Steele 2010a‐ZAF | 2008 | South Africa | 5 | High‐mortality | E | Africa |
| RV1 Steele 2010b‐ZAF | 2007 | South Africa | 7 | High‐mortality | E | Africa |
| RV1 Tregnaghi 2011‐LA | 2008 | Argentina, Brazil, Colombia, Dominican Republic, Honduras, and Panama | Multiple sites in each country | Low‐mortality | B | Latin America |
| RV1 Vesikari 2004a‐FIN | 2004 | Finland | 2 | Low‐mortality | A | Europe |
| RV1 Vesikari 2004b‐FIN | 2004 | Finland | 6 | Low‐mortality | A | Europe |
| RV1 Vesikari 2007a‐EU | 2007 | Czech Republic, Finland, France, Germany, Italy, and Spain | 98 | Low‐mortality | A | Europe |
| RV1 Vesikari 2011‐FIN | 2005 | Finland | 5 | Low‐mortality | A | Europe |
| RV1 Ward 2006‐USA | 2006 | USA | 2 | Low mortality | A | North America |
| RV1 Zaman 2009‐BGD | 2005 | Bangladesh | 1 | High‐mortality | D | Asia |
| RV1 Zaman 2017‐BGD | 2011 | Bangladesh | 142 | High‐mortality | D | Asia |
| RV5 Armah 2010‐AF | 2009 | Ghana, Kenya, and Mali | 3 | High‐mortality | D, E | Africa |
| RV5 Block 2007‐EU/USA | 2007 | Finland and USA | 30 | Low‐mortality | A | Europe and North America |
| RV5 Ciarlet 2009‐EU | 2008 | Austria, Belgium, and Germany | 26 | Low‐mortality | A | Europe |
| RV5 Clark 2003‐USA | 2003 | USA | 19 | Low‐mortality | A | North America |
| RV5 Clark 2004‐USA | 2004 | USA | 10 | Low‐mortality | A | North America |
| RV5 Dhingra 2014‐IND | 2012 | India | 2 | High‐mortality | D | Asia |
| RV5 Iwata 2013‐JPN | 2009 | Japan | 32 | Low‐mortality | A | Asia |
| RV5 Kim 2008‐KOR | 2008 | South Korea | 8 | Low‐mortality | B | Asia |
| RV5 Lawrence 2012‐CHN | 2010 | China | Not reported | Low‐mortality | B | Asia |
| RV5 Merck[009] 2005‐USA | 2005 | USA | 10 | Low‐mortality | A | North America |
| RV5 Mo 2017‐CHN | 2015 | China | 5 | Low‐mortality | B | Asia |
| RV5 Vesikari 2006a‐FIN | 2006 | Finland | 4 | Low‐mortality | A | Europe |
| RV5 Vesikari 2006b‐INT | 2006 | Belgium, Costa Rica, Finland, Germany, Guatemala, Italy, Jamaica, Mexico, Puerto Rico, Sweden, Taiwan, and USA | 356 | Low‐mortalityb | A, B, D | Asia, Caribbean, Europe, Latin America, North America |
| RV5 Zaman 2010‐AS | 2009 | Bangladesh and Vietnam | Multiple | High‐mortalitya | B, D | Asia |
| VAC Bhandari 2006‐IND | 2005 | India | 1 | High‐mortality | D | Asia |
| VAC Bhandari 2009‐IND | 2006‐8 | India | 1 | High‐mortality | D | Asia |
| VAC Bhandari 2014‐IND | 2011‐13 | India | 3 | High‐mortality | D | Asia |
| VAC Chandola 2017‐IND | 2014‐15 | India | 1 | High‐mortality | D | Asia |
aThis study was conducted mainly in high‐mortality countries, but also in low‐mortality countries. bThis study was conducted mainly in low‐mortality countries, but also in high‐mortality countries.
Appendix 6. Vaccine schedules
| Trial | Number of doses | Time between doses (weeks) | Number of arms: vaccine/placebo | Infant vaccination status | Note |
| RV1 Anh 2011‐PHL | 2 | 4 or 8 | 2/1 | Commercially available diphtheria, tetanus, whole‐cell pertussis (DTPw), hepatitis B (HBV) and oral poliovirus (OPV) vaccines were administered concomitantly with the study vaccine/placebo as part of the routine Expanded Programme of Immunization (EPI) in the Philippines | Compares different schedules: (1) vaccine dose at month 1 and 2, and placebo at day 0; and (2) vaccine dose at day 0 and month 2, and placebo at month 1 |
| RV1 Anh 2011‐VNM | 2 | 4 or 8 | 2/1 | Commercially available diphtheria, tetanus, whole‐cell pertussis (DTPw), hepatitis B (HBV) and oral poliovirus (OPV) vaccines were administered concomitantly with the study vaccine/placebo as part of the routine Expanded Programme of Immunization (EPI) in Vietnam | Compares different schedules: (1) vaccine dose at day 0 and month 1, and placebo at month 2; and (2) vaccine dose at day 0 and month 2, and placebo at month 1 |
| RV1 Bernstein 1998‐USA | 2 | 6 to 10 | 1/1 | Rotavirus vaccine was separated from all other infant vaccines by at least 2 weeks | — |
| RV1 Bernstein 1999‐USA | 2 | 6 to 10 | 1/1 | Other vaccines separated from the trial vaccines by at least 2 weeks | — |
| RV1 Colgate 2016‐BGD | 2 | 7 | 1/1 (no RV1) | Alongside Rotarix at 10 and 17 weeks of age the polio vaccine intervention was the administration of an injected, inactivated polio vaccine (IPV) dose replacing the 4th dose of tOPV at 39 weeks of age. Study children also received all standard EPI vaccines (BCG at birth; pentavalent vaccine (DPT, HepB, Hib) at 6, 10, and 14 weeks; bivalent Measles‐Rubella at 40 weeks; and monovalent Measles at 65 weeks) | RV1 plus polio vacccine (IPV), observational control group only |
| RV1 Dennehy 2005‐NA | 2 | 7 | 2/1 | Vaccine or placebo given concomitantly with diphtheria‐tetanus‐acellular pertussis, inactivated poliovirus, H influenzae type b, and S pneumoniae conjugate vaccines for participants in USA or with a diphtheria‐tetanus‐acellular pertussis/inactivated poliovirus/H influenza type b combination vaccine for participants in Canada "Routine hepatitis B vaccinations were administered according to local practice." |
2 different PFUs compared |
| RV1 GSK[021] 2007‐PAN | 3 | 8 | 2/2 | Use of other vaccines not mentioned | Licensed formulation versus modified formulation |
| RV1 GSK[033] 2007‐LA | 2 | 8 | 3/1 | Use of other vaccines not mentioned | 3 ‘Lots’ of RV1 vaccine compared |
| RV1 GSK[041] 2007‐KOR | 2 | 8 | 1/1 | H influenzae type b vaccine administered concomitantly along with the 2 doses of vaccine/placebo and at 2 months after dose 2; other routine childhood vaccines were to be given at least 14 days before trial vaccine/placebo | — |
| RV1 GSK[101555] 2008‐PHL | 2 | 8 | 2/2 | No mention of whether infants received other vaccines | Data from the lyophilized formulation, which is not yet approved or marketed, are not reported |
| RV1 Kawamura 2011‐JPN | 2 | 4 | 1/1 | Combined diphtheria and tetanus toxoids and acellular pertussis (DTPa) and Hepatitis B (HBV) vaccines were allowed to be co‐administered along with RV1 vaccine/placebo | — |
| RV1 Kerdpanich 2010‐THA | 2 | 8 | 3/2 | Diphtheria toxoid, tetanus toxoid, acellular pertussis, inactivated polio and H influenzae type b combination vaccine (Infanrix™‐IPV/Hib) at 2 and 4 months of age and diphtheria toxoid, tetanus toxoid, acellular pertussis, hepatitis B, inactivated polio andH influenzae type b combination vaccine (Infanrix hexaTM) at 6 months of age | Compares: regular vaccine reconstituted in buffer; vaccine reconstituted in water; vaccine stored above recommended temperature; placebo reconstituted in water; placebo reconstituted in buffer |
| RV1 Kim 2012‐KOR | 2 | 4 | 1/1 | Routine childhood vaccines as recommended by the local vaccination schedule were allowed to be administered concomitantly with RIX4414/placebo. These vaccines included the combined diphtheria‐tetanus‐acellular pertussis vaccine, Hemophilus influenzae type b vaccine, inactivated poliovirus vaccine and pneumococcal vaccine. The infants had received the BCG vaccine and 2 doses of hepatitis B vaccine prior to study enrolment | — |
| RV1 Li 2013a‐CHN | 1 | ‐ | 1/1 | Children were allowed to receive routine childhood vaccinations according to local immunization practice during the study period, with a minimum interval of at least 7 days between the administration of routine vaccines and the study vaccine or placebo | Child arm (2 ‐ 6 years of age) of the same study as RV1 Li 2013b‐CHN |
| RV1 Li 2013b‐CHN | 1 | ‐ | 1/1 | Infants were allowed to receive routine childhood vaccinations according to local immunization practice during the study period, with a minimum interval of at least 7 days between the administration of routine vaccines and the study vaccine or placebo | Infant arm (6‐16 weeks of age) of the same study as RV1 Li 2013a‐CHN |
| RV1 Li 2014‐CHN | 2 | 4 | 2/2 | As part of the routine childhood vaccination according to the EPI recommendations in China, participants also received 3 doses of Infanrix™ vaccine and 3 doses of the oral poliovirus vaccine. The Infanrix™ and the OPV vaccines were administered independently of (Sub‐cohort 1) or concomitantly with (Sub‐cohort 2) the Rotarix™ vaccine. When administered concomitantly, participants received the 3 doses of Infanrix™ vaccine at months 1, 2 and 3, and the 3 doses of the OPV vaccine at Day 0, Month 1 and Month 2 | — |
| RV1 Madhi 2010‐AF | 2 or 3 | 5 to 10 | 2/1 | All participants received routine infant vaccinations according to EPI recommendations | — |
| RV1 Narang 2009‐IND | 2 | 8 | 1/1 | Routine vaccinations (diphtheria‐tetanus‐whole cell pertussis‐hepatitis b, H influenzae type b, and oral poliovirus vaccine) were administered at 6, 10, and 14 weeks of age (given with a 2‐week separation from the first and subsequent dose of the RV1 vaccine or placebo) | — |
| RV1 NCT00158756‐RUS | 3 | 6 | 5 | GlaxoSmithKline (GSK) Biologicals' Tritanrix™HepB and GSK Biologicals Kft's DTPwHBV vaccines as compared to concomitant administration of Commonwealth Serum Laboratory's (CSL's) DTPw (Triple Antigen™) and GSK Biologicals' HBV (Engerix™B), when coadministered With GSK Biologicals' Oral Live Attenuated Human Rotavirus (HRV) vaccine, to healthy infants at 3, 4½ and 6 months of age, after a birth dose of Hepatitis B vaccine | Hep B and DTPw‐HBV vaccines in combination with other vaccines/placebo were compared in the study arms |
| RV1 Omenaca 2012‐EU | 2 | 4 or 8 | 1/1 | All participants received routine infant vaccinations in accordance with the local National Plan of Immunization schedule in each of the respective participating countries | — |
| RV1 Phua 2005‐SGP | 2 | 4 | 3/1 | Hepatitis B vaccine, diphtheria‐tetanus‐acellular pertussis, poliovirus, and H influenzae type b co‐administered with interventions | 3 different PFUs compared |
| RV1 Phua 2009‐AS | 2 | 6 to 10 | 1/1 | Infants received other routine paediatric immunizations (combined diphtheria toxoid‐tetanus toxoid‐acellular pertussis (DTPa) – inactivated poliovirus [IPV] and H influenzae type B (Hib) vaccine and hepatitis B vaccine (HBV)) during the study period according to local schedules. Almost all infants received BCG dose at birth. If oral polio vaccine (OPV) was given as part of the routine schedule in the participating countries, a time interval of 2 weeks was observed between the OPV doses and RIX4414 vaccine/placebo doses | — |
| RV1 Rivera 2011‐DOM | 2 | 7 | 1/1 | All infants received 3 doses of combined diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus and H influenzae vaccine. | 1 complimentary dose of RV1 was administered to all infants enrolled in this study (both study groups) who were aged < 6 months at Visit 3 (Week 13) as a benefit to the placebo group for participation in the study |
| RV1 Ruiz‐Palac 06‐LA/EU | 2 | 4 or 8 | 1/1 | Routine immunizations according to local regulations; oral poliovirus vaccination at least 2 weeks before or after rotavirus vaccine | — |
| RV1 Salinas 2005‐LA | 2 | 8 | 3/1 | Oral polio vaccine given after 2 weeks, not together with RV1 | 3 different PFUs compared Main publication did not report that the trial included 2 subsets: 2 doses of human rotavirus or placebo subset: these participants received 2 oral doses of RV1 vaccine or placebo according to a 0, 2 months schedule, and routine vaccinations (DTPw‐ Hepatitis B vaccine (HBV) + Hib vaccine) at a 0, 2, and 4 months schedule 3 doses of RV1 or placebo subset: these participants received 3 oral doses of RV1 vaccine or placebo, and routine vaccinations (DTPw‐HBV + Hib vaccine) concomitantly with each dose of human rotavirus vaccine and placebo at a 0, 2, and 4 months schedule |
| RV1 Steele 2008‐ZAF | 2 | 4 | 3/1 | RV1 plus (1) oral polio vaccine (OPV) + diphtheria‐tetanus‐acellular pertussis/H influenzae type b (DTPA/HIB) vaccine; (2) OPV placebo + diphtheria‐tetanus‐acellular pertussis inactivated polio‐H influenzae type b (DTPA‐IPV/HIB) vaccine; or (3) OPV + DTPA/HIB vaccine | Compares different co‐administration combinations (see previous column) |
| RV1 Steele 2010a‐ZAF | 3 | 4 | 1/1 | RV1 vaccine was concomitantly administered with 3 doses of combined diphtheria, tetanus and whole‐cell pertussis, hepatitis B, and H influenzae type b vaccine (TritanrixHepBHib) and OPV (PolioSabin) | For infants who developed clinical symptoms of HIV (WHO stages III or IV disease) any time after enrolment, access to antiretroviral therapy (cotrimoxazole) according to the South African national guidelines was facilitated. Infants who needed treatment were referred to antiretroviral therapy centres by the investigators |
| RV1 Steele 2010b‐ZAF | 2 or 3 | 4 | 2/1 | Infants received routine vaccinations according to the local EPI schedule in South Africa. BCG and OPV vaccinations were given at birth; all other routine vaccinations (including diphtheria‐tetanus toxoids‐whole cell pertussis, hepatitis B, H influenzae type b, and OPV) were administered concomitantly with the study vaccine | Compares number of doses (2 or 3) |
| RV1 Tregnaghi 2011‐LA | 2 | 4 or 8 | 1/1 | All participants received routine infant vaccinations (Hepatitis B vaccine), diphtheria‐tetanus‐acellular pertussis, poliovirus, and H influenzae type b) according to EPI recommendations in each country. First 2 doses of routine EPI vaccinations were co‐administered with the RV1 vaccine or placebo doses; the 3ird routine EPI vaccination was administered 1 to 2 months later according to the national plan of immunization in each country |
— |
| RV1 Vesikari 2004a‐FIN | 2 | 8 | 3/1 | Infant routine vaccinations were separated from the study vaccines by 2 weeks | 3 different PFUs compared |
| RV1 Vesikari 2004b‐FIN | 2 | 8 | 1/1 | Infant routine vaccinations (diphtheria tetanus toxoids‐pertussis, H influenzae type b, and inactivated poliovirus vaccines) were separated from the study vaccines by at least 2 weeks | — |
| RV1 Vesikari 2007a‐EU | 2 | 4 or 8 | 1/1 | Concomitant vaccines included 7 valent pneumococcal polysaccharide conjugate vaccine (Prevenar) and meningococcal group c conjugate vaccine (Meningitec); Hepatitis B vaccine, diphtheria‐tetanus‐acellular pertussis, polio virus, and H influenzae type b vaccines were co‐administered | — |
| RV1 Vesikari 2011‐FIN | 2 | 4 | 2/2 | Routine childhood vaccinations were allowed according to local practice, but at least 14 days apart from each dose of study vaccine | Compares liquid and lyophilized vaccine formulations |
|
RV1 Ward 2006‐USA |
2 | 4 | 2/1 | Not specified | 2 different PFUs compared |
| RV1 Zaman 2009‐BGD | 2 | — | 2/2 | All children in the study received the standard EPI vaccines starting at 6 weeks of age. Oral polio vaccine (OPV) co‐administered in trial: either concomitantly with RV1 or 15 days before RV1. | Compared RV1 plus oral polio vaccine with RV1 alone |
| RV1 Zaman 2017‐BGD | 2 | 4 | 1/1 (no RV1 vaccine) | HRV was scheduled to be given along with other standard infant vaccines including OPV at the DTP1 and DTP2 immunization visits, recommended in Bangladesh to occur at 6 and 10 weeks of age | Cluster randomised trial |
| RV5 Armah 2010‐AF | 3 | 4 | 1/1 | All children in the study received the standard EPI vaccines (including oral poliovirus vaccine) starting at 6 weeks of age | — |
| RV5 Block 2007‐EU/USA | 3 | 4 to 10 | 1/1 | Use of oral poliovirus vaccine during the course of the study or within 42 days before first dose of vaccine/placebo was an exclusion criterion; administration of other vaccines permitted | — |
| RV5 Ciarlet 2009‐EU | 3 | 4 to 6 | 1/1 | Hepatitis B vaccine, diphtheria‐tetanus‐acellular pertussis, polio virus, and H influenzae type b co‐administered | — |
| RV5 Clark 2003‐USA | 3 | 6 to 8 | 1/1 | Children that had recently received oral polio vaccine were excluded from the study | Breastfed; infants in the vaccine control group (Group 1) received the reassortants as administered in previous studies within 30 mins of feeding Enfamil formula (30 ml) or Mylanta Double Strength (0.5 ml/kg). Infants in a corresponding placebo group (Group 2) were pre‐fed as in Group 1 |
| RV5 Clark 2004‐USA | 3 | 6 to 8 | 1/1 | Receipt of any other vaccines within 14 days was not allowed. | — |
| RV5 Dhingra 2014‐IND | 3 | 4 | 4/1 | Infants in Cohort 2 concomitantly received a combined DTPw‐HB‐Hib pentavalent vaccine and Trivalent Oral Polio Vaccine | BRV‐TV at 3 different concentrations, compared to RV5 or placebo |
| RV5 Iwata 2013‐JPN | 3 | 4 to 10 | 1/1 | No information about use of other vaccines | — |
| RV5 Kim 2008‐KOR | 3 | 4 to 10 | 1/1 | Infants excluded if they had or were to receive oral poliovirus vaccine at any time during the study or in the 42 days before the first dose; concomitant administration of other licensed vaccines and breastfeeding was not restricted | — |
| RV5 Lawrence 2012‐CHN | 3 | 4‐10 | 1/1 | Other live vaccines 14 days before or after study vaccine were not allowed | — |
| RV5 Levin 2017‐AF | 3 | 4‐10 | 1/1 | Enrolment was closed in participating countries when RV1 was added to national vaccine schedules. | — |
| RV5 Merck[009] 2005‐USA | 3 | 4 to 10 | 1/1 | Infants were excluded if they had or were to receive oral poliovirus vaccine at any time during the study or in the 42 days before the first dose; concomitant administration of other licensed vaccines and breastfeeding was not reported | — |
| RV5 Mo 2017‐CHN | 3 | 4 | 2/2 | The routine China EPI vaccines (oral poliovirus vaccine and diphtheria, tetanus, and acellular pertussis vaccine) either staggered or concomitantly with RV5 or placebo. | — |
| RV5 Vesikari 2006a‐FIN | 3 | 4 to 8 | 3/1 | Licensed vaccines could be administered throughout the study, but were not given on the same day as study vaccine; inactivated poliovirus vaccine was exclusively used in Finland at the time of the study | Compares different RV5 components: G1‐4, P1A; G1‐4; and P1A |
| RV5 Vesikari 2006b‐INT | 3 | 4 to 10 | 1/1 | Administration of other licensed childhood vaccines and breastfeeding were not restricted; for a subset of participants in the USA (U.A. concomitant use cohort), Merck also provided the licensed paediatric vaccines that were administered concomitantly (same day) with RV5 or placebo, which included Comvax, Infanrix, Ipol, and Prevnar | — |
| RV5 Zaman 2010‐AS | 3 | 4 | 1/1 | All children in the study received the standard EPI vaccines (including oral poliovirus vaccine) starting at 6 weeks of age | — |
| VAC Bhandari 2006‐IND | 1 | ‐ | 1/1 (/1) | Infants were vaccinated with DPT, Hep B and OPV separately from rotavirus vaccine | Included an additional vaccine arm for a rotavirus vaccine candidate (I321) that was not included for anaysis in this review |
| VAC Bhandari 2009‐IND | 3 | 4 | 2/2 | Infants received 3 doses of DTP; OPV; and Hep B at 6, 10, and 14 weeks of age; Rotavac was administered at 8, 12, and 16 weeks of age | Randomized participants to high‐ (1 x 105 ffu) and low‐dose (1 x 104 ffu) vaccine arms which were combined in this review |
| VAC Bhandari 2014‐IND | 3 | 4 | 1/1 | Other childhood vaccines (DTPw, Hib, Hep B, and OPV) given concurrently | — |
| VAC Chandola 2017‐IND | 3 | 4‐8 | 3/1 | Co‐administered with EPI vaccines: OPV and combined DPT, HepB and Hib | Randomized participants to 3 vaccine production lots as well as to placebo; we combined the different production lot arms in our analyses |
BCG: Bacille Calmette Guérin; EPI: Extended Programme of Immunization; FFU: focus‐forming unit;H influenzae: Haemophilus influenzae; PFU: plaque‐forming unit.
Appendix 7. Methods to collect adverse event data
Appendix 8. Ongoing studies: vaccine and location
| Trial | Rotavirus vaccine | Location | |
| Region | Country | ||
| OTHER ACTRN12610000525088 | RV3‐BB | Oceania | Australia |
| OTHER CTRI/2015/07/006034 | Rotasiil (Serum Institute of India Ltd.) | Asia | India |
| OTHER CTRI/2015/12/006428 | RV1; Rotavac (Bharat) | Asia | India |
| OTHER NCT01061658 | BRV‐TV | Asia | India |
| OTHER NCT02153866 | RV vaccine, type not reported | Asia | China |
| OTHER NCT02193061 | RV1; RV5 | America | Mexico |
| OTHER NCT02542462 | RV1; RV5 | America | USA |
| OTHER NCT02646891 | Trivalent P2VP8 | Africa | South Africa |
| OTHER NCT02847026 | RV1; RV5 | Asia | Bangladesh |
| OTHER NCT03462108 | Rotavirus vaccine (Bio Farma) | Asia | Indonesia |
| OTHER NCT03483116 | RV3‐BB | Africa | Malawi |
| RV1 ISRCTN86632774 | RV1 | Africa | South Africa |
| RV1 NCT02941107 | RV1 | Oceania | Australia |
| RV1 Tatochenko 2008 | RV1 | Not reported | Not reported |
| RV5 NCT02728869 | RV5 | Asia | Bangladesh |
Appendix 9. Deathsa: from published trials and from communication with trial authors
| Vaccine | Trial | No. of deaths | Cause of death | |||
| Vaccine | Placebo | Unclear | Total | |||
| RV1 | RV1 Anh 2011‐PHL | 1 | 0 | 0 | 1 | Salmonella gastroenteritis |
| RV1 Anh 2011‐VNM | 0 | 0 | 0 | 0 | — | |
| RV1 Bernstein 1998‐USA | 0 | 0 | 0 | 0 | — | |
| RV1 Bernstein 1999‐USA | 0 | 0 | 1 (1) | 1 | Pneumococcal sepsis | |
| RV1 Colgate 2016‐BGD | 1 | 1 | 0 | 2 | Reasons not reported | |
| RV1 GSK[021] 2007‐PAN | 0 | 0 | 0 | 0 | — | |
| RV1 Tregnaghi 2011‐LA | 10 | 2 | 0 | 12 | Meningitis bacterial (1 vaccine, 1 placebo), pneumonia (3 vaccine), aortic valve stenosis (1 vaccine), bronchiolitis (1 vaccine), dengue fever (1 vaccine), endocarditis bacterial (1 vaccine), intussusception (1 vaccine), multi‐organ failure (1 placebo), respiratory failure (1 vaccine), sepsis (2 vaccine) | |
| RV1 GSK[033] 2007‐LA | 3 | 0 | 0 | 3 | Gastroenteritis (1 vaccine), bronchopneumonia (1 vaccine), aspiration (1 vaccine) | |
| RV1 GSK[041] 2007‐KOR | 0 | 0 | 0 | 2 | Not reported | |
| RV1 GSK[101555] 2008‐PHL | 0 | 0 | 0 | 0 | — | |
| RV1 Kawamura 2011‐JPN | 0 | 0 | 0 | 0 | — | |
| RV1 Kerdpanich 2010‐THA | 0 | 0 | 0 | 0 | — | |
| RV1 Kim 2012‐KOR | 0 | 0 | 0 | 0 | — | |
| RV1 Li 2013a‐CHN | 0 | 0 | 0 | 0 | — | |
| RV1 Li 2013b‐CHN | 0 | 0 | 0 | 0 | — | |
| RV1 Li 2014‐CHN | 6 | 7 | 0 | 13 | Vaccine (6): Asphyxia, Drowning, Central nervous system infection, Bronchopneumonia, Cortical dysplasia, Intracranial Haemorrhage, Asphyxia, Meningitis, Multi‐organ failure, Hemotophagic histiocytosis, Acute lymphocytic leukemia, Multi‐organ failure Placebo (7): Diarrhea, Multi‐organ failure, Congenital heart disease, Respiratory failure, brain contusion, subarachnoid hemorrhage, skull fracture, cerebral hematoma, and brain herniation | |
| RV1 Madhi 2010‐AF | 83 | 43 | 0 | 126 | Reasons not stated | |
| RV1 Narang 2009‐IND | 0 | 0 | 0 | 0 | — | |
| RV1 NCT00158756‐RUS | 0 | 0 | 0 | 0 | — | |
| RV1 Phua 2005‐SGP | 3 | 0 | 0 | 3 | Leukaemia (1 vaccine); accident‐induced subarachnoid haemorrhage (1 vaccine); cardiorespiratory failure after acute viral pneumonitis (1 vaccine) | |
| RV1 Phua 2009‐AS | 1 | 3 | 0 | 4 | Aspiration and metabolic disorder, adenoviral pneumonia, interstitial pneumonia, and sudden infant death syndrome (not stated which group) | |
| RV1 Rivera 2011‐DOM | 0 | 0 | 0 | 0 | — | |
| RV1 Ruiz‐Palac 06‐LA/EU | 56 | 43 | 0 | 99 | Diarrhoea (4 vaccine, 2 placebo); pneumonia (16 vaccine, 6 placebo); other causes not mentioned | |
| RV1 Salinas 2005‐LA | 2 | 1 | 0 | 3 | Generalized visceral congestion (1 placebo); sepsis (1 vaccine); automobile accident (1 vaccine) | |
| RV1 Steele 2008‐ZAF | 3 | 5 | 0 | 8 | Bronchopneumonia (1 placebo), pneumonia (2 vaccine, 2 placebo), hepatic steatosis (1 placebo), brain oedema (1 vaccine, 1 placebo) | |
| RV1 Steele 2010a‐ZAF | 6 | 9 | 0 | 15 | Bronchopneumonia, sepsis, and gastroenteritis were the most common causes | |
| RV1 Steele 2010b‐ZAF | 3 | 0 | 0 | 3 | Bronchopneumonia and gastroenteritis (3 vaccines) | |
| RV1 Vesikari 2004b‐FIN | 0 | 0 | 0 | 0 | — | |
| RV1 Vesikari 2007a‐EU | 0 | 0 | 0 | 0 | — | |
| RV1 Vesikari 2011‐FIN | 0 | 0 | 0 | 0 | — | |
| RV1 Zaman 2009‐BGD | 1 | 0 | 0 | 1 | — | |
|
RV5 |
RV5 Armah 2010‐AF | 76 | 82 | 0 | 158 | Gastroenteritis (20 vaccine, 16 placebo); 11 deaths occurred in identified HIV‐infected participants in Kenya; sudden infant death syndrome (1 placebo); other causes not mentioned |
| RV5 Block 2007‐EU/USA | 1 | 0 | 0 | 1 | Sudden infant death syndrome (1 vaccine) | |
| RV5 Ciarlet 2009‐EU | 0 | 0 | 0 | 0 | — | |
| RV5 Iwata 2013‐JPN | 0 | 0 | 0 | 0 | — | |
| RV5 Lawrence 2012‐CHN | 0 | 0 | 0 | 0 | — | |
| RV5 Levin 2017‐AF | 1 | 2 | 0 | 3 | Pneumonia | |
| RV5 Merck[009] 2005‐USA | 0 | 0 | 0 | 0 | — | |
| RV5 Mo 2017‐CHN | 0 | 1 | 0 | 1 | Reasons not reported | |
| RV5 Vesikari 2006a‐FIN | 0 | 0 | 0 | 0 | — | |
| RV5 Vesikari 2006b‐INT | 24 | 20 | 0 | 44 | Sudden infant death syndrome (7 vaccine and 7 placebo), other causes not mentioned | |
| RV5 Zaman 2010‐AS | 3 | 4 | 0 | 7 | Not all causes reported, most common causes were drowning and sepsis | |
| Rotavac | VAC Bhandari 2014‐IND | 30 | 18 | 0 | 48 | The most common causes of death were infection and infestations followed by general disorders and administration site conditions. Days after vaccination not reported. None were considered to be vaccine‐related |
| VAC Chandola 2017‐IND | 5 | 0 | 0 | 5 | Cause of death: sepsis and aspiration (79 ‐ 141 days after Rotavac vaccination), unexplained sudden death (3 days after Rotavac vaccination). None were considered to be vaccine‐related | |
aNumbers in brackets are the number of deaths reported by the trial authors following personal communication with them, i.e. they are not in the published trial reports.
Appendix 10. Other licensed rotavirus vaccines in use
| Vaccine | Vaccination schedule | Vaccine antigens | Manufacturer | License information |
| Lanzhou lamb rotavirus (LLR) | 1 dose annually for children 2 months to 3 years and one booster dose at 3 to 5 years | Monovalent, live‐attenuated lamb G10 P[12] strain | Lanzhou Institute of Biological Products, China | 2000 (China), nationally licenced |
| Rotasiil, Bovine rotavirus‐pentavalent vaccine (BRV‐PV) | 3 doses at 6, 10 and 14 weeks | Pentavalent, bovine‐human reassortant vaccine containing serotypes G1, G2, G3, G4 and G9 | Serum Institute of India Ltd. | 2017 (India), nationally licenced |
| Rotavin‐M1 | 2 doses Minimum 6 weeks given at least 30 days apart |
Monovalent, live‐attenuated human G1 P[8] strain | Polyvac, Vietnam | 2007 (Vietnam), nationally licenced |
Data and analyses
Comparison 1.
RV1 versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rotavirus diarrhoea: severe (up to 1 year follow‐up) | 11 | 49893 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.14, 0.34] |
| 1.1 Low‐mortality countries (WHO strata A & B) | 7 | 43779 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.09, 0.26] |
| 1.2 High‐mortality countries (WHO strata D & E) | 4 | 6114 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.23, 0.60] |
| 2 Rotavirus diarrhoea: severe (up to 2 years follow‐up) | 12 | Risk Ratio (Fixed, 95% CI) | 0.34 [0.29, 0.41] | |
| 2.1 Low‐mortality countries (WHO strata A & B) | 9 | Risk Ratio (Fixed, 95% CI) | 0.18 [0.14, 0.23] | |
| 2.2 High‐mortality countries (WHO strata D & E) | 3 | Risk Ratio (Fixed, 95% CI) | 0.65 [0.51, 0.83] | |
| 3 All‐cause diarrhoea: severe cases (up to 1 year follow‐up) | 6 | 33690 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.54, 0.80] |
| 3.1 Low‐mortality countries (WHO strata A & B) | 3 | 28051 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.47, 0.74] |
| 3.2 High‐mortality countries (WHO strata D & E) | 3 | 5639 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.56, 0.95] |
| 4 All‐cause diarrhoea: severe cases (up to 2 years follow‐up) | 5 | 12181 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.54, 0.92] |
| 4.1 Low‐mortality countries (WHO strata A & B) | 3 | 9417 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.36, 1.02] |
| 4.2 High‐mortality countries (WHO strata D & E) | 2 | 2764 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.96] |
| 5 All‐cause diarrhoea: severe episodes (up to 1 year follow‐up) | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 5.1 Low‐mortality countries (WHO strata A & B) | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 All‐cause diarrhoea: severe episodes (up to 2 years follow‐up) | 2 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 6.1 Low‐mortality countries (WHO strata A & B) | 2 | Rate Ratio (Fixed, 95% CI) | 0.63 [0.56, 0.71] | |
| 7 All‐cause death | 30 | 105778 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.30] |
| 7.1 Low‐mortality countries (WHO strata A & B) | 22 | 97597 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.87, 1.71] |
| 7.2 High‐mortality countries (WHO strata D & E) | 8 | 8181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.22] |
| 8 All serious adverse events | 31 | 103714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.83, 0.93] |
| 8.1 Low‐mortality countries (WHO strata A & B) | 24 | 96233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.83, 0.93] |
| 8.2 High‐mortality countries (WHO strata D & E) | 7 | 7481 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 9 Serious adverse events: intussusception | 21 | Risk Ratio (Fixed, 95% CI) | 0.70 [0.46, 1.05] | |
| 9.1 Low‐mortality countries (WHO strata A & B) | 17 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.45, 1.04] | |
| 9.2 High‐mortality countries (WHO stratum E) | 4 | Risk Ratio (Fixed, 95% CI) | 1.49 [0.06, 36.63] | |
| 10 Serious adverse events: Kawasaki disease | 3 | 13117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.30, 10.61] |
| 11 Serious adverse events requiring hospitalization | 2 | 63675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.81, 0.96] |
| 12 Rotavirus diarrhoea: of any severity (up to 2 months follow‐up) | 12 | 4294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.69, 2.00] |
| 12.1 Low‐mortality countries (WHO strata A & B) | 9 | 3537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.66, 2.50] |
| 12.2 High‐mortality countries (WHO strata D & E) | 3 | 757 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.41, 2.41] |
| 13 Rotavirus diarrhoea: of any severity (up to 1 year follow‐up) | 8 | 15197 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.23, 0.50] |
| 13.1 Low‐mortality countries (WHO strata A & B) | 4 | 9083 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.13, 0.40] |
| 13.2 High‐mortality countries (WHO stratum E) | 4 | 6114 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.35, 0.68] |
| 14 Rotavirus diarrhoea: of any severity (up to 2 years follow‐up) | 7 | 11692 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.28, 0.47] |
| 14.1 Low‐mortality countries (WHO strata A & B) | 6 | 10441 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.25, 0.48] |
| 14.2 High‐mortality countries (WHO stratum E) | 1 | 1251 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.62] |
| 15 All‐cause diarrhoea: all cases (up to 2 months follow‐up) | 7 | 3132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.72, 1.10] |
| 15.1 Low‐mortality countries (WHO strata A & B) | 6 | 3032 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.09] |
| 15.2 High‐mortality countries (WHO stratum E) | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.69, 1.58] |
| 16 All‐cause diarrhoea: all cases (up to 1 year follow‐up) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Low‐mortality countries (WHO strata A & B) | 2 | 2204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.82, 1.03] |
| 16.2 High‐mortality countries (WHO strata D & E) | 1 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 17 All‐cause diarrhoea: all cases (up to 2 years follow‐up) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Low‐mortality countries (WHO strata A & B) | 3 | 5937 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 18 All‐cause diarrhoea: all episodes (up to 1 year follow‐up) | 2 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 18.1 Low‐mortality countries (WHO strata A & B) | 2 | Rate Ratio (Fixed, 95% CI) | 0.98 [0.88, 1.10] | |
| 19 All‐cause diarrhoea: all episodes (up to 2 years follow‐up) | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 19.1 Low‐mortality countries (WHO strata A & B) | 1 | Rate Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 All‐cause hospitalizations (up to 2 years follow‐up) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 Low‐mortality countries (WHO strata A & B) | 2 | 65646 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.47] |
| 21 Rotavirus diarrhoea: requiring hospitalization | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.1 Up to 1 year follow‐up (at least 1 rotavirus season) | 8 | 48718 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.09, 0.33] |
| 21.2 Second year follow‐up (at least 2 rotavirus seasons) | 7 | 35331 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.11, 0.22] |
| 22 Rotavirus diarrhoea: requiring medical attention | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 Up to 1 year follow‐up (at least 1 rotavirus season) | 1 | 3874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.04, 0.16] |
| 22.2 Second year follow‐up (at least 2 rotavirus seasons) | 3 | 7017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.16, 0.31] |
| 23 All‐cause diarrhoea: cases requiring hospitalization | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.1 Up to one year of follow‐up (at least 1 rotavirus season) | 2 | 14393 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.17, 1.11] |
| 23.2 Second year of follow‐up (at least 2 rotavirus seasons) | 2 | 14367 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 0.99] |
| 24 All‐cause diarrhoea: episodes requiring hospitalization | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 24.1 Up to 1 year of follow‐up (at least 1 rotavirus season) | 1 | Rate Ratio (Fixed, 95% CI) | 0.58 [0.47, 0.71] | |
| 24.2 Second year of follow‐up (at least 2 rotavirus seasons) | 1 | Rate Ratio (Fixed, 95% CI) | 0.53 [0.46, 0.61] | |
| 25 Reactogenicity: fever | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 25.1 After dose 1 | 25 | 16192 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.97, 1.17] |
| 25.2 After dose 2 | 24 | 15630 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.92, 1.06] |
| 25.3 After dose 3 | 4 | 1390 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.86, 1.13] |
| 25.4 End of follow‐up | 18 | 11926 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.93, 1.01] |
| 26 Reactogenicity: diarrhoea | 27 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 26.1 After dose 1 | 25 | 18732 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.88, 1.17] |
| 26.2 After dose 2 | 24 | 15630 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.86, 1.21] |
| 26.3 After dose 3 | 4 | 1390 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.35, 1.36] |
| 26.4 End of follow‐up | 17 | 14305 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 27 Reactogenicity: vomiting | 27 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 After dose 1 | 25 | 18732 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.94, 1.12] |
| 27.2 After dose 2 | 24 | 15630 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| 27.3 After dose 3 | 4 | 1390 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.71, 2.50] |
| 27.4 End of follow‐up | 17 | 14305 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.04] |
| 28 Adverse events requiring discontinuation (end of follow‐up) | 26 | 94980 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.83, 1.26] |
| 29 Immunogenicity: rotavirus vaccine shedding (end of follow‐up) | 16 | 2638 | Risk Ratio (M‐H, Random, 95% CI) | 10.94 [4.90, 24.43] |
| 30 Immunogenicity: seroconversion | 31 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 30.1 After dose 1 | 9 | 2537 | Risk Ratio (M‐H, Random, 95% CI) | 20.39 [8.48, 49.01] |
| 30.2 After dose 2 | 27 | 8742 | Risk Ratio (M‐H, Random, 95% CI) | 11.44 [8.01, 16.32] |
| 30.3 After dose 3 | 5 | 1137 | Risk Ratio (M‐H, Random, 95% CI) | 6.89 [3.59, 13.24] |
| 31 Dropouts before the end of the trial | 28 | 93106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.90, 1.00] |
| 32 Subgroup analysis: rotavirus diarrhoea of any severity (by G type) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 32.1 G1 | 6 | 27583 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.10, 0.44] |
| 32.2 G2 | 5 | 26835 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.31, 0.56] |
| 32.3 G3 | 4 | 8968 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.05, 0.39] |
| 32.4 G4 | 2 | 5720 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.07, 0.59] |
| 32.5 G9 | 3 | 8868 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.18, 0.75] |
| 33 Subgroup analysis: severe cases of rotavirus diarrhoea (by G type) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 33.1 G1 | 7 | 39428 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.16, 0.38] |
| 33.2 G2 | 7 | 44682 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.18, 0.50] |
| 33.3 G3 | 5 | 20505 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.56] |
| 33.4 G4 | 1 | 2421 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.00, 2.95] |
| 33.5 G8 | 2 | 4417 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.02, 2.37] |
| 33.6 G9 | 6 | 26815 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.13, 0.40] |
| 33.7 G12 | 2 | 4417 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.23, 0.97] |
| 34 Subgroup analysis: rotavirus diarrhoea in malnourished children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 34.1 Up to 1 year of follow‐up (at least 1 rotavirus season) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 35 Subgroup analysis: rotavirus diarrhoea in HIV‐infected children | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.78] |
Comparison 2.
RV5 versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rotavirus diarrhoea: severe (up to 1 year follow‐up) | 9 | 10048 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.22, 0.44] |
| 1.1 Low‐mortality countries (WHO strata A & B) | 5 | 4132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.22] |
| 1.2 High‐mortality countries (WHO strata D & E) | 4 | 5916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.29, 0.62] |
| 2 Rotavirus diarrhoea: severe (up to 2 years follow‐up) | 8 | 13203 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.23, 0.60] |
| 2.1 Low‐mortality countries (WHO strata A & B) | 4 | 7318 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.08, 0.39] |
| 2.2 High‐mortality countries (WHO strata D & E) | 4 | 5885 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.82] |
| 3 All‐cause diarrhoea: severe cases (up to 1 year follow‐up) | 3 | 4085 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.58, 1.11] |
| 3.1 Low‐mortality countries (WHO stratum A) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 High‐mortality countries (WHO strata D & E) | 3 | 4085 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.58, 1.11] |
| 4 All‐cause diarrhoea: severe cases (up to 2 years follow‐up) | 4 | 5977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.98] |
| 4.1 Low‐mortality countries (WHO strata A & B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 High‐mortality countries (WHO strata D & E) | 4 | 5977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.98] |
| 5 All‐cause death | 14 | 84448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.74, 1.25] |
| 5.1 Low‐mortality countries (WHO strata A & B) | 9 | 77642 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.65, 1.96] |
| 5.2 High‐mortality countries (WHO strata D & E) | 5 | 6806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.24] |
| 6 All serious adverse events | 14 | 82502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.01] |
| 6.1 Low‐mortality countries (WHO strata A & B) | 8 | 75672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.02] |
| 6.2 High‐mortality countries (WHO strata D & E) | 6 | 6830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.28] |
| 7 Serious adverse events: intussusception | 16 | 85495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.45] |
| 7.1 Low‐mortality countries (WHO strata A & B) | 12 | 78907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.45] |
| 7.2 High‐mortality countries (WHO strata D & E) | 4 | 6588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Rotavirus diarrhoea: of any severity (up to 1 year follow‐up) | 8 | 13450 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.28, 0.50] |
| 8.1 Low‐mortality countries (WHO strata A & B) | 5 | 8644 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.25, 0.37] |
| 8.2 High‐mortality countries (WHO strata D & E) | 3 | 4806 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.94] |
| 9 Rotavirus diarrhoea: of any severity (up to 2 years follow‐up) | 7 | 12888 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.33, 0.65] |
| 9.1 Low‐mortality countries (WHO strata A & B) | 3 | 6144 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.26, 0.43] |
| 9.2 High‐mortality countries (WHO strata D & E) | 4 | 6744 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.45, 0.83] |
| 10 All‐cause diarrhoea: of any severity (up to 1 year follow‐up) | 1 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.11] |
| 10.1 Low‐mortality countries (WHO strata A & B) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 High‐mortality countries (WHO stratum E) | 1 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.11] |
| 11 All‐cause diarrhoea: of any severity (up to 2 years follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 High‐mortality countries (WHO stratum E) | 1 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.68, 1.16] |
| 12 All‐cause hospitalizations (up to 2 years follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 High‐mortality countries (WHO strata D & E) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Rotavirus diarrhoea: requiring hospitalization | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 Up to 1 year of follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Rotavirus diarrhoea: requiring medical attention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.1 Up to 1 year of follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Reactogenicity: fever | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 After dose 1 | 4 | 7124 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.45] |
| 15.2 After dose 2 | 2 | 4322 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.69, 1.01] |
| 15.3 After dose 3 | 2 | 4294 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.90, 1.27] |
| 15.4 End of follow‐up | 11 | 18391 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.94, 1.09] |
| 16 Reactogenicity: diarrhoea | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 After dose 1 | 2 | 4745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.95, 1.32] |
| 16.2 After dose 2 | 1 | 3905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.72, 1.10] |
| 16.3 End of follow‐up | 10 | 17087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| 17 Reactogenicity: vomiting | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 After dose 1 | 2 | 4745 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.12] |
| 17.2 After dose 2 | 1 | 3905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.32, 1.49] |
| 17.3 After dose 3 | 1 | 3878 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.16, 1.32] |
| 17.4 End of follow‐up | 9 | 16294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.90, 1.06] |
| 18 Adverse events requiring discontinuation (end of follow‐up) | 10 | 15471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.57, 1.39] |
| 19 Immunogenicity: rotavirus vaccine shedding (after dose 3) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 20 Immunogenicity: seroconversion | 10 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 20.1 After dose 3 | 10 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Dropouts before the end of the trial | 13 | 85855 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.90, 1.08] |
| 22 Subgroup analysis: rotavirus diarrhoea of any severity (by G type) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 G1 | 4 | 11022 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.21, 0.32] |
| 22.2 G2 | 3 | 9907 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.16, 0.78] |
| 22.3 G3 | 4 | 11022 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 2.02] |
| 22.4 G4 | 3 | 9907 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.13, 1.33] |
| 22.5 G9 | 2 | 9537 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.20, 0.54] |
| 23 Subgroup analysis: severe cases of rotavirus diarrhoea (by G type) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.1 G1 | 3 | 76606 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 1.74] |
| 23.2 G2 | 3 | 76606 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.13, 1.37] |
| 23.3 G3 | 3 | 76606 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.05, 2.74] |
| 23.4 G4 | 3 | 76606 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.46] |
| 23.5 G9 | 3 | 76606 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.05, 0.34] |
| 24 Subgroup analysis: HIV‐infected children | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 Rotavirus diarrhoea: severe (up to two years follow‐up) | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.11, 56.68] |
| 24.2 All‐cause diarrhoea: severe (up to two years follow‐up) | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.05 [0.52, 31.43] |
| 24.3 All‐cause death | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.51, 3.21] |
| 24.4 Serious adverse events (up to 24 weeks) | 2 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.59, 3.97] |
Analysis 2.23.
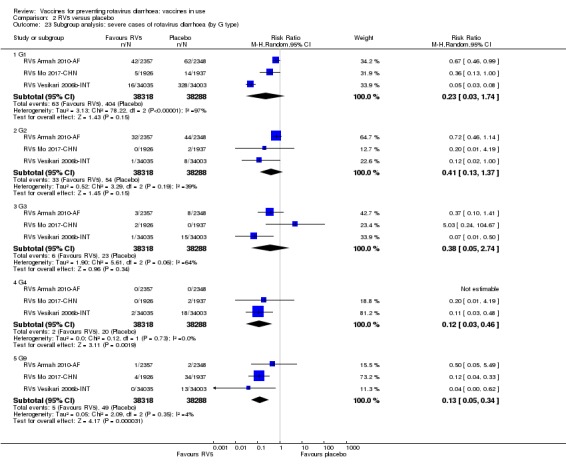
Comparison 2 RV5 versus placebo, Outcome 23 Subgroup analysis: severe cases of rotavirus diarrhoea (by G type).
Comparison 3.
Rotavac versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rotavirus diarrhoea: severe (up to 1 year follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Rotavirus diarrhoea: severe (up to 2 years follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 All‐cause diarrhoea: severe cases (up to 1 year follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 All‐cause death | 2 | 8155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.52, 1.62] |
| 5 All serious adverse events | 3 | 8210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.02] |
| 6 Serious adverse events: intussusception | 4 | 8582 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.35, 5.02] |
| 7 Rotavirus diarrhoea: of any severity (up to 1 year follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Rotavirus diarrhoea: of any severity (up to 2 years follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Rotavirus diarrhoea: requiring medical attention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Up to 1 year follow‐up (at least 1 rotavirus season) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Reactogenicity: fever | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 After dose 1 | 2 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.35, 1.94] |
| 10.2 After dose 2 | 1 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.33, 1.77] |
| 10.3 After dose 3 | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.52, 2.36] |
| 11 Reactogenicity: diarrhoea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 After dose 1 | 2 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.62, 1.30] |
| 11.2 After dose 2 | 1 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.00, 2.41] |
| 11.3 After dose 3 | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [2.11, 7.92] |
| 12 Reactogenicity: vomiting | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 After dose 1 | 2 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.71, 2.55] |
| 12.2 After dose 2 | 1 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.64, 3.66] |
| 12.3 After dose 3 | 1 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.39, 2.66] |
| 13 Immunogenicity: rotavirus vaccine shedding (end of follow‐up) | 2 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 9.86 [2.58, 37.63] |
| 14 Immunogenicity: seroconversion | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 After dose 1 | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [2.03, 6.29] |
| 14.2 After dose 2 | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [1.78, 4.98] |
| 14.3 After dose 3 | 3 | 1699 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [2.26, 3.51] |
| 15 Dropouts before the end of the trial | 3 | 8215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.62, 1.06] |
| 16 Subgroup analysis: severe cases of rotavirus diarrhoea by G and P types (up to 1 year follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 G1P[8] | 1 | 6541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.36, 1.20] |
| 16.2 G2P[4] | 1 | 6541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.22, 0.69] |
| 16.3 G12P[6] | 1 | 6541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.13, 0.74] |
| 16.4 G12P[8] | 1 | 6541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.07, 1.26] |
| 17 Subgroup analysis: severe cases of rotavirus diarrhoea by G and P types (up to 2 years follow‐up) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 G1P[8] | 1 | 6541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
| 17.2 G2P[4] | 1 | 6541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.23, 0.62] |
| 17.3 G9P[4] | 1 | 6541 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.52 [0.57, 35.66] |
| 17.4 G12P[6] | 1 | 6541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.13, 0.74] |
| 17.5 G12P[8] | 1 | 6541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 0.96] |
What's new
| Date | Event | Description |
|---|---|---|
| 19 March 2019 | New search has been performed | We amended the protocol to include only vaccines prequalified for use by the World Health Organization (WHO). We included 14 new studies from the April 2018 search, including four studies on a new vaccine (Rotavac). Nicholas Henschke joined the author team. |
| 19 March 2019 | New citation required but conclusions have not changed | This is the fourth update of the original rotavirus vaccines review (Soares‐Weiser 2004). This review concerns vaccines that have been prequalified for global use by the WHO (WHO 2018). In the previous versions of this review we included any rotavirus vaccine in use. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 5, 2010
| Date | Event | Description |
|---|---|---|
| 10 May 2012 | New search has been performed | No new trials were identified from the updated May 2012 search. |
| 10 May 2012 | New citation required but conclusions have not changed | Review updated to incorporate different country mortality strata and outcomes changed to reflect the different rotavirus vaccines’ efficacy and safety in countries with different mortality rates. |
| 8 January 2012 | New search has been performed | Review updated to include nine trials identified in a new literature search, which was conducted in October 2011 (MEDLINE via PubMed) and June 2011 (other databases). |
| 11 November 2011 | New citation required but conclusions have not changed | Hanna Bergman and Sukkrti Nagpal joined the author team. |
| 10 May 2010 | Amended | Minor typographical errors corrected. |
| 2 February 2010 | New citation required and conclusions have changed | A new search on 2 February 2010 identified 9 new potentially relevant studies. We independently assessed these studies and incorporated data from the eligible trials into the review. |
| 21 July 2009 | New search has been performed | The original rotavirus vaccines review (Soares‐Weiser 2004) was split into two reviews: rotavirus vaccines in use (this review); and other rotavirus vaccines, including those no longer in use or in development (Soares‐Weiser 2004). This involved a new search, revised inclusion criteria, updated review methods. All data from those trials also included in the original review were re‐extracted. New authors joined the review team for this review. |
Differences between protocol and review
This is the fourth update of the original rotavirus vaccines review (Soares‐Weiser 2004). This review concerns vaccines that have been prequalified for global use by the WHO (WHO 2018). In the previous versions of this review we included any rotavirus vaccine in use (Soares‐Weiser 2004; Soares‐Weiser 2010; Soares‐Weiser 2012a; Soares‐Weiser 2012b).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT Length of follow‐up: 1 month after last dose Adverse event data collection methods: not reported |
|
| Participants |
Number: 375 enrolled; ATP safety cohort: 345; ATP immunogenicity cohort: 292 Inclusion criteria: healthy infants aged 5 – 10 weeks at the time of the first study vaccination dose with a birth weight of > 2 kg Exclusion criteria: use of any investigational drug or vaccine other than the study vaccine or confirmed immunosuppression/immunodeficient conditions or allergy to RIX4414 vaccine/placebo components |
|
| Interventions | 1. 2 doses of RIX4414* plus 1 dose of placebo according to a PL‐V‐V schedule 2. 2 doses of RIX4414* plus 1 dose of placebo according to a V‐PL‐V schedule 3. 3 placebo doses * Human rotavirus (RV1) liquid vaccine, oral suspension (GSK Biologicals, Belgium), containing at least 106.0 median Cell Culture Infective Dose 50 percent (CCID50) of live attenuated RIX4414 human rotavirus strain (G1P[8]) Schedule: 3 doses according to a 0‐, 1‐, and 2‐month schedule |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity, including fever, diarrhoea and vomiting, 8 days after each dose (collected from GSK report) 2. Adverse events leading to discontinuation 3. Serious adverse events 4. Fatal serious adverse events 5. Dropouts 6. * Rotavirus diarrhoea, rotavirus antigen isolated from any of the stool samples collected from children with diarrhoea episodes, up to 1 month after last dose 7. * All‐cause diarrhoea, up to 1 month after last dose Outcomes to measure immunogenicity 8. Anti‐rotavirus IgA antibody seroconversion, ≥ 20 U/mL * Outcome reported as proportion (P) with 95% CI. Events (n) and totals (N) were estimated by using the values when 2 formulae for the standard error (SE) converged |
|
| Immunization status | Commercially‐available diphtheria, tetanus, whole‐cell pertussis (DTPw), hepatitis B (HBV) and oral poliovirus (OPV) vaccines were administered concomitantly with the study vaccine/placebo as part of the routine Expanded Programme of Immunization (EPI) in the Philippines | |
| Location | Philippines (single centre) WHO mortality stratum B |
|
| Notes | Study known as RIX GSK[063] 2008‐AS in previously published versions of this review Date: March to September 2007 Source of funding: GlaxoSmithKline Biologicals Study rationale: "This study will provide data on the immune response and safety of GSK Biologicals' HRV [human rotavirus] liquid vaccine when given along with the routine infant immunizations in Philippines." "The study also[...]explored the potential effect of scheduling of the HRV [human rotavirus] vaccine doses with respect to the existing routine vaccination schedules" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated Quote: "Block randomization scheme (2:2:1 ratio) with standard SAS program was used" |
| Allocation concealment (selection bias) | Low risk | Central allocation Quote: "Based on the block size, the vaccine doses were distributed to each of the study centers" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and key personnel were blinded Quote: "The study was double‐blind with respect to the RIX4414 oral suspension (liquid formulation), placebo and scheduling of doses. The parents/guardians of infants, investigators and study personnel were unaware of the study vaccine/ placebo administered" Quote: "The placebo was identical to the vaccine in composition" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced across groups with reasons for dropout/exclusion reported |
| Selective reporting (reporting bias) | Low risk | All prepublished outcomes included |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 1 month after last dose Adverse event data collection methods: not reported |
|
| Participants |
Number: 375 enrolled; ATP safety cohort: 352; ATP immunogenicity cohort: 330 Inclusion criteria: healthy infants aged 6 to 10 weeks at the time of the first study vaccination dose with a birth weight of > 2 kg Exclusion criteria: use of any investigational drug or vaccine other than the study vaccine or confirmed immunosuppression/immunodeficient conditions or allergy to RIX4414 vaccine/placebo components |
|
| Interventions | 1. 2 doses of RIX4414* plus 1 dose of placebo according to a V‐V‐PL schedule 2. 2 doses of RIX4414* plus 1 dose of placebo according to a V‐PL‐V schedule 3. 3 placebo doses * Human rotavirus [RV1] liquid vaccine, oral suspension (GSK Biologicals, Belgium), containing at least 106 median Cell Culture Infective Dose 50 percent (CCID50) of live attenuated RIX4414 human rotavirus strain (G1P[8]) Schedule: 3 doses according to a 0‐, 1‐, and 2‐month schedule |
|
| Outcomes |
Clinical outcome measures (Safety and Efficacy) 1. Reactogenicity, including fever, diarrhoea and vomiting, 8 days after each dose (collected from GSK report) 2. Adverse events leading to discontinuation 3. Serious adverse events 4. Fatal serious adverse events 5. Dropouts 6. * Rotavirus diarrhoea, rotavirus antigen isolated from any of the stool samples collected from children with diarrhoea episodes, up to 1 month after last dose (outcome not included in the prepublished protocol) 7. * All‐cause diarrhoea, up to 1 month after last dose (outcome not included in the prepublished protocol) Outcomes to measure immunogenicity 8. Anti‐rotavirus IgA antibody seroconversion, ≥ 20 U/ML * Outcome reported as proportion (P) with 95% CI. Events (n) and totals (N) were estimated by using the values when 2 formulae for the standard error (SE) converged |
|
| Immunization status | Commercially‐available diphtheria, tetanus, whole‐cell pertussis (DTPw), hepatitis B (HBV) and oral poliovirus (OPV) vaccines were administered concomitantly with the study vaccine/placebo as part of the routine Expanded Programme of Immunization (EPI) in Vietnam | |
| Location | Vietnam (11 satellite centres) WHO mortality stratum B |
|
| Notes | Study known as RIX GSK[051] 2008‐AS in previously published versions of this review Date: September 2006 to March 2007 Source of funding: GlaxoSmithKline Biologicals Study rationale: "To provide specific data on immunogenicity of GSK Biologicals' human rotavirus liquid vaccine, when co‐administered with the routine Expanded Program of Immunization (EPI) in Vietnam. The study will also assess reactogenicity and safety of the human rotavirus liquid vaccine relative to the placebo" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated Quote: "Block randomization scheme (2:2:1 ratio) with standard SAS program was used" |
| Allocation concealment (selection bias) | Low risk | Central allocation Quote: "Based on the block size, the vaccine doses were distributed to each of the study centers" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and key personnel were blinded Quote: "The study was double‐blind with respect to the RIX4414 oral suspension (liquid formulation), placebo and scheduling of doses. The parents/guardians of infants, investigators and study personnel were unaware of the study vaccine/ placebo administered" Quote: "The placebo was identical to the vaccine in composition" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced across groups with reasons for dropout/exclusion reported |
| Selective reporting (reporting bias) | Unclear risk | One outcome (rotavirus diarrhoea) not included in the prepublished protocol |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: outcomes measured up to 1 month after the second dose Adverse event data collection methods: participants or their parents filled out a diary card for 7 days after each dose (passive method) |
|
| Participants |
Number: 42 enrolled; 42 evaluable Inclusion criteria: all infants aged 6 to 26 weeks recruited from private practice offices in Cincinnati Exclusion criteria: not stated |
|
| Interventions | RV1 1. RIX4414 (RV1): 105 PFU; 21 participants 2. Placebo: 20 participants Schedule: 2 doses given 6 to 10 weeks apart |
|
| Outcomes |
Clinical outcome measures 1. Reactogenicity: diarrhoea defined as > 3 stools that were looser than normal in a 24‐hour period; fever defined as a temperature > 100.4 °F obtained rectally in infants 2. Serious adverse events 3. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 4. Vaccine virus shedding: rotavirus shedding after immunization; combined time points (review includes data from combined time points) 5. Seroconversion: ≥ 4‐fold rise in rotavirus IgA antibody (serum and stool) (review includes data from after dose 1 and dose 2) |
|
| Immunization status | Rotavirus vaccine was separated from all other infant vaccines by at least 2 weeks | |
| Location | Cincinnati, USA WHO mortality stratum A |
|
| Notes |
Date: August to November 1995 Source of funding: Virus Research Institute, Inc. (now Avant Immunotherapeutics Inc.) 1 participant in the placebo group did not complete the study because of persistent otitis media |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Trial report does not provide enough details |
| Methods | RCT Length of follow‐up: outcomes measured at 2 years Adverse event data collection methods: "diary card for 7 days after vaccine. All moderate to severe side effects were reported by the investigator to an independent study monitor on a continuous basis during the study" (passive method); "telephoned parents every 2 weeks after the first immunisation, and then weekly during the expected rotavirus season (Jan 1‐May 31) as a reminder and to collect data on any adverse events" (active method) |
|
| Participants |
Number: 215 randomized; 214 evaluable Age range: 3 to 6 months Inclusion criteria: healthy children aged 10 to 16 weeks at the time of the first dose Exclusion criteria: fever; premature labour; an immunosuppressed or pregnant individual in the same household; birth at < 36 weeks of gestation; participation in any other investigational clinical trial; or no telephone in the household |
|
| Interventions | 89‐12 (a precursor of RIX4414 (RV1) 1. 89‐12 (a precursor of RIX4414 (RV1)): 105 PFU; 2 doses given 6 to 10 weeks apart; 108 participants 2. Placebo: 105 PFU; 2 doses given 6 to 10 weeks apart; 107 participants "Infants received an oral dose of 1.0 mL vaccine (105 PFU) or placebo immediately after 2.0 mL of an antacid containing 160 mg aluminium hydroxide and 160 mg magnesium hydroxide to buffer stomach acid. The infant was not fed for 1 h before or after the immunisation" |
|
| Outcomes |
Clinical outcome measures 1. All‐cause diarrhoea: gastroenteritis defined as vomiting (> 1 hour after feeding), diarrhoea (≥ 3 looser than normal stools in a 24‐hour period), or both; measured up to 2 years 2. Severe rotavirus diarrhoea: severity assessed using a scoring system with a "20‐point scale identical to that used in previous rotavirus trials. In this system, points are assigned according to the duration and severity of diarrhoea and vomiting, the severity of fever, and the presence of dehydration or hospital admissions for each episode of gastroenteritis. A score greater than 8 was prospectively defined as severe, and a score more than 14 as very severe"; measured up to 2 years 3. Rotavirus diarrhoea: "An illness was classified as caused by rotavirus if a stool specimen collected no later than 7 days after resolution of symptoms contained rotavirus antigen. All episodes of rotavirus gastroenteritis occurring between the second vaccination and the end of the study were included"; measured up to 7 days 4. Reactogenicity: "Parents filled out a diary card for 7 days after each dose. Signs included were: daily (evening) rectal temperatures, diarrhoea, vomiting, and the number and consistency of all stools"; measured up to 7 days 5. All‐cause death; measured up to 2 years 6. Emergency department visit; measured up to 2 years 7. Rotavirus diarrhoea requiring hospitalization Outcomes to measure immunogenicity 8. Vaccine virus shedding (review includes after dose 2 data) 9. Immunogenicity (ELISA): "Serum samples were analysed for IgA and IgG antibody to rotavirus by an ELISA" and "neutralising antibody to the 89‐12 strains by an antigen reduction assay" (only rotavirus‐specific IgA results reported in this review from after dose 2 time point) |
|
| Immunization status | Other vaccines separated from the trial vaccines by at least 2 weeks | |
| Location | Cincinnati, Baltimore, and Sellersviller, USA WHO mortality stratum A |
|
| Notes |
Date: August 1997 to June 1998 Source of funding: Virus Research Institute, Inc. (now Avant Immunotherapeutics Inc.) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infants were assigned to receive either 89‐12 or placebo according to a computer‐generated randomization schedule (one/one) in blocks of ten provided by the sponsor. The intention‐to‐treat analysis included all participants who received at least one dose of study vaccine. Before the code was broken, all cases of rotavirus gastroenteritis and the severity of each episode were verified" |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind, no details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No impact on intervention effect estimate Quote: "Of the 215 children enrolled, 213 received both doses of vaccine or placebo, and 214 were followed up for gastrointestinal disease. One child in the vaccine group did not receive the vaccine because of persistent fever at the time of the scheduled revaccination, and one child in the placebo group was found to have a congenital tracheal malformation while in the trial and was not revaccinated" |
| Selective reporting (reporting bias) | Low risk | All expected outcomes included |
| Other bias | Unclear risk | Insufficient information |
| Methods | RCT, open‐label non‐placebo controlled trial Length of follow‐up: outcomes measured at 1 year Adverse event data collection methods: Passive: All adverse events following interventions were captured for 48 hours following each intervention and were scored for probable, possible, or unlikely relationship to each intervention. All missing protocol‐defined events were captured as protocol deviations and reported annually. Comprehensive safety reports were submitted semi‐annually to the study’s Independent Medical Monitor and to the Data and Safety Monitoring Board |
|
| Participants |
Number: 700 enrolled; 593 evaluable Age range: birth to age 7 days at enrolment, 10 ‐ 17 weeks at vaccine administration Inclusion criteria: Healthy infant aged 0 to 7 days, no obvious congenital abnormalities or birth defects, no abnormal (frequency and consistency) stools since birth, stable household with no plans to leave the area for the next one year Exclusion criteria: Parents are not willing to have child vaccinated at the field clinic or to have child's blood drawn, parents are planning to enrol child into another clinical study, mother not willing to have blood drawn and breast milk extracted, parents not willing to have field research assistant in home twice a week, history of seizures or other apparent neurologic disorders, infant received any vaccines before start of study, except Bacillus Calmette‐Guerin (BCG), infant has any sibling currently or previously enrolled in this study (including a twin) |
|
| Interventions | 1. RV1 dose 1 at 10 weeks, dose 2 at 17 weeks (350 enrolled participants) 2. No RV1 vaccine (350 enrolled participants) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Rotavirus diarrhoea (severe) 2. All‐cause diarrhoea (severe) 3. All‐cause deaths 4. Rotavirus diarrhoea (any severity) 5. All‐cause diarrhoea (any severity) 6. Dropouts from the trial |
|
| Immunization status | Along with Rotarix at 10 and 17 weeks of age, the polio vaccine intervention was the administration of an injected, inactivated polio vaccine (IPV) dose replacing the fourth dose of tOPV at 39 weeks of age. In addition to the vaccine interventions, study children received all standard EPI vaccines through the study clinic.The national Bangladesh Expanded Program on Immunizations (EPI) schedule includes BCG at birth; pentavalent vaccine (DPT, HepB, Hib) at 6, 10, and 14 weeks; bivalent Measles‐Rubella at 40 weeks; and monovalent Measles at 65 weeks | |
| Location | Single site, Bangladesh WHO mortality stratum D |
|
| Notes |
Date: May 2011 to November 2013 Source of funding: Bill and Melinda Gates Foundation Study rationale: The primary objective was to determine the efficacy of a 2‐dose Rotarix oral rotavirus vaccine (given at 10 and 17 weeks of age) to prevent rotavirus diarrhoea in the first year of life |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized using permuted blocks with random block size selection. |
| Allocation concealment (selection bias) | Low risk | All clinical investigators and laboratories were masked to vaccine arm, but medical officers were not. |
| Blinding (performance bias and detection bias) All outcomes | High risk | RV1 versus no intervention, unable to blind (no placebo) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary ITT analysis, moderate attrition. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes appear to be reported, protocol published |
| Other bias | Low risk | No other bias apparent |
| Methods | RCT Length of follow‐up: 10 to 12 months Adverse event data collection methods: "For the 15 days after each dose of vaccine, the parent or guardian maintained a daily record that included fever, irritability/fussiness, diarrhoea, vomiting, loss of appetite and cough/runny nose. In addition, the parent or guardian was asked to record any gastroenteritis episode occurring in the period from the first dose until 2 months after the second dose of vaccine." (passive method); "Subjects were also monitored for any serious adverse events occurring throughout participation in the study (10–12 months in total) and for unsolicited adverse events occurring within 43 days after each dose of vaccine or placebo" (active method) |
|
| Participants |
Number: 529 enrolled; 479 evaluable Age range: 1 to 3 months (beginning) Inclusion criteria: healthy infants aged 5 to 15 weeks at the time of the first dose. Vaccine administration delayed if acute illness present (fever > 38 °C/gastroenteritis/antibiotics within 7 days before scheduled vaccination) Exclusion criteria: premature labour (< 36 weeks); chronic condition; (chronic gastrointestinal disease, immunosuppressive diseases); household contact with immunosuppressed individuals/pregnant women |
|
| Interventions | RV1 1. RIX4414 (RV1) 1.1. 105.2; 212 participants 1.2. 106.4; 209 participants 2. Placebo: 108 participants Schedule: 2 doses given 7 weeks apart |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: fever, irritability/fussiness, diarrhoea, vomiting, loss of appetite and cough/runny nose; measured during 15 days post‐vaccination 2. Serious adverse events 3. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 4. Viral shedding: viral shedding in any stool specimen collected between first dose and 2 months after second vaccine dose (review includes after dose 2 data) 5. Seroconversion: anti‐rotavirus IgA ELISA ≥ 20 Units/mL in participants negative for rotavirus antibody before the first dose of vaccine (review includes data from 2 months after dose 2) |
|
| Immunization status | Vaccine or placebo given concomitantly with diphtheria‐tetanus‐acellular pertussis, inactivated poliovirus, H. influenzae type b, and Streptococcus pneumoniae conjugate vaccines for participants in USA or with a diphtheria‐tetanus‐acellular pertussis/inactivated poliovirus/H. influenza type b combination vaccine for participants in Canada "Routine hepatitis B vaccinations were administered according to local practice" |
|
| Location | 41 centres in USA and Canada WHO mortality stratum A |
|
| Notes |
Date: 13 December 2000 to 2 August 2002 Source of funding: GlaxoSmithKline Biologicals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Central allocation Quote: "double blind randomized unbalanced allocation scheme (2:2:1 ratio)" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and key personnel; Quote: "Study personnel and families were blinded to group assignment until study completion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups Quote: "Fifty‐nine subjects, who were proportionately distributed among vaccine groups, did not complete the entire 10‐ to 12‐month study" |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Other bias | Unclear risk | No details |
| Methods | RCT Length of follow‐up: 1 month after dose 3 Adverse event data collection methods: not reported |
|
| Participants |
Number: 228 enrolled; 203 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants, born after a normal gestation period of ≥ 36 weeks; 6 to 12 weeks of age at the time of the first dose of the study vaccination course; free of obvious health problems as established by medical history and clinical examination before entering into study Exclusion criteria: any clinically significant history of chronic gastrointestinal disease including any uncorrected congenital malformation of the gastrointestinal tract or other serious medical condition as determined by the investigator and previous confirmed occurrence of rotavirus gastroenteritis |
|
| Interventions | RV1 1. RIX4414 (RV1): 106.5 PFU*; 177 participants (randomized) 1.1 Received modified vaccine formulation 1.2 Received a licensed RV1 vaccine *Dose unclear; in the same study, some use 106.5 PFU and some 105 PFU 2. Placebo: 51 participants (randomized) 2.1 Received a placebo of the modified vaccine formulation 2.2 Received a placebo of the licensed RV1 vaccine Schedule: 3 doses at 2, 4, and 6 months of age |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: for each type of solicited symptom, occurrence of the symptom within the 8‐day (days 0 to 7) solicited follow‐up period after each dose; occurrence of unsolicited adverse events within 31 days (days 0 to 30) after each dose, according to MedDRA classification; measured up to 31 days after vaccine/placebo 2. Serious adverse events: occurrence throughout entire study period; measured up to 31 days after vaccine/placebo 3. Dropouts: measured up to 31 days after vaccine/placebo 4. All‐cause death 5. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 6. Viral shedding: number (%) of participants with rotavirus in at least 1 stool (review includes data from combined time points) 7. Seroconversion: appearance of anti‐rotavirus antibody concentration ≥ 20 U/mL in participants negative for rotavirus before vaccination (review includes data from 2 months after dose 1 and 2 months after dose 2, and 1 month after dose 3) |
|
| Immunization status | Use of other vaccines not mentioned | |
| Location | 1 centre in Panama WHO mortality stratum B |
|
| Notes |
Date: 23 August 2002 to 9 May 2003 Source of funding: GlaxoSmithKline Biologicals Study rationale: "to compare the immunogenicity and safety of a modified vaccine formulation to the licensed human rotavirus [Rotarix] vaccine" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parent/guardian and study personnel were not aware of the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 203/228 participants completed the study. Reasons for withdrawal were reported and balanced between groups. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Unclear risk | No details |
| Methods | RCT Length of follow‐up: 1 month after dose 2 Adverse event data collection methods: not reported |
|
| Participants |
Number: 854 enrolled; 795 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants, born after a normal gestation period of ≥ 36 weeks; 6 to 12 weeks of age at the time of the first dose of the study vaccination course, free of obvious health problems as established by medical history and clinical examination before entering into the study Exclusion criteria: any clinically significant history of chronic gastrointestinal disease including any uncorrected congenital malformation of the gastrointestinal tract or other serious medical condition as determined by the investigator and previous confirmed occurrence of rotavirus gastroenteritis |
|
| Interventions | RV1 1. RIX4414 (RV1): 106.5 PFU*; 730 participants (randomized) 1.1. Received RV1 vaccine Lot A 1.2. Received RV1 vaccine Lot B 1.3. Received RV1 vaccine Lot C *Dose unclear, some use 106.5 PFU and some 105 PFU 2. Placebo: 124 participants (randomized) Schedule: 2 oral doses given at 2 and 4 months; visits 1, 2, and 3 correspond to months 0, 2, and 4 in the schedule |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: for each type of solicited symptom, occurrence of the symptom within the 8‐day (days 0 to 7) solicited follow‐up period after each dose; occurrence of unsolicited adverse events within 31 days (days 0 to 30) after each dose, according to MedDRA classification; measured up to 31 days after vaccine/placebo 2. Serious adverse events: occurrence throughout entire study period; measured up to 31 days after vaccine/placebo 3. Dropouts: measured up to 31 days after vaccine/placebo 4. All‐cause death 5. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 6. Vaccine virus shedding: presence of rotavirus antigen in stool samples collected on day of vaccination and on planned days following each dose in a subset of participants [review includes data from combined time points] 7. Seroconversion: appearance of serum anti‐rotavirus IgA antibody concentrations ≥ 20 U/mL [review includes data from 2 months after dose 2] |
|
| Immunization status | Use of other vaccines not mentioned | |
| Location | 7 study centres (2 in Colombia, 1 in Mexico, and 4 in Peru) WHO mortality strata B, D |
|
| Notes |
Date: 8 August 2003 to 29 January 2004 Source of funding: GlaxoSmithKline Biologicals Study rationale: "to assess the clinical consistency of 3 production lots of human rotavirus vaccine in terms of immunogenicity and safety when given to healthy infants at 2 and 4 months of age" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parent/guardian and study personnel were not aware of the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 795/854 completed the study. Reasons for dropping out were reported and were balanced between study groups |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Unclear risk | No details |
| Methods | RCT Length of follow‐up: 2 months after dose 2 Adverse event data collection methods: not reported |
|
| Participants |
Number: 155 enrolled; 151 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: full‐term infants; healthy infants aged between 6 and 12 weeks (42 to 90 days) at the time of the first vaccination for whom the vaccination history was available Exclusion criteria: previous confirmed occurrence of rotavirus gastroenteritis |
|
| Interventions | RV1 1. RIX4414 (RV1): 106.5 PFU; 103 participants (randomized) 2. Placebo: 52 participants (randomized) Schedule: 2 oral doses starting at about 2 months of age; second dose at 4 months of age |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: for each type of solicited symptom, occurrence of the symptom within the 15‐day (days 0 to 14) solicited follow‐up period after each dose; occurrence of unsolicited adverse events within 43 days (days 0 to 42) after each dose, according to MedDRA classification; up to 43 days after vaccine/placebo 2. Serious adverse events: no definition; occurrence throughout the entire study period (up to 2 months after dose 2) 3. Dropouts: measured up to 2 months after dose 2 4. Rotavirus diarrhoea: presence of rotavirus in gastroenteritis episode stools collected from dose 1 of vaccine/placebo up to 2 months after dose 2 5. All‐cause death 6. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 7. Seroconversion: appearance of anti‐rotavirus immunoglobulin A antibody concentration 20 U/mL in participants who were seronegative before vaccination (review includes data from 2 months after dose 2) |
|
| Immunization status | H. influenzae type b vaccine administered concomitantly along with the 2 doses of vaccine/placebo and at 2 months after dose 2; other routine childhood vaccines were to be given at least 14 days before trial vaccine/placebo | |
| Location | 6 centres in Korea WHO mortality stratum B |
|
| Notes |
Date: 15 July 2005 to 11 May 2006 Registration number: NCT00134732 Source of funding: GlaxoSmithKline Biologicals Study rationale: "to assess immunogenicity and safety of 2 doses of the HRV [human rotavirus] vaccine in Korean infants aged approximately 2 months at the time of the first dose" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parent/guardian and study personnel were not aware of the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/103 participants in the vaccine arm did not complete the study |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Unclear risk | No details |
| Methods | RCT Length of follow‐up: outcomes measured 1 month after last dose of vaccine/placebo Adverse event data collection methods: not reported |
|
| Participants |
Number: 150 enrolled; 145 evaluable Age range: 6 to 12 weeks Inclusion criteria: healthy, full‐term infants aged 6 to 12 weeks; male or female infants between, and including, 6 and 12 weeks of age at the time of the first vaccination, free of obvious health problems, born after a normal gestation period (between 36 and 42 weeks) or with a birth weight > 2000 g Exclusion criteria: infants with previous confirmed occurrence of rotavirus gastroenteritis |
|
| Interventions | RV1 1. RIX4414 (RV1): 106.5; 100 participants* 1.1 Licensed formulation 1.2 Lyophilized formulation 2. Placebo: 50 participants* 2.1 Normal placebo 2.2 Lyophilized formulation Schedule: 2 doses starting at 6–12 weeks of age according to a 0, 2 month schedule *Data from the lyophilized formulation, which is not yet approved or marketed, are not reported in review |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: for each type of solicited symptom, occurrence of the symptom within the 15‐day (day 0 to 14) solicited follow‐up period after each dose; occurrence of unsolicited adverse events within 31 (day 0 to 30) days after any doses of RV1 vaccine or placebo, according to MedDRA classification 2. Serious adverse events: occurrence throughout entire study period (up to 31 days after final dose of vaccine/placebo) 3. Dropouts: measured up to 31 days after final dose of vaccine/placebo 4. Rotavirus diarrhoea: presence of rotavirus in gastroenteritis stools collected until 1 month after dose 2 5. All‐cause death 6. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 7. Vaccine viral shedding in stool (review includes data from combined time points) 8. Seroconversion: appearance of anti‐rotavirus IgA antibody concentration ≥ 20 U/mL in participants initially (i.e. before first dose of vaccine/placebo) negative for rotavirus (review includes data from 2 months after dose 1, 1 month after dose 2, and combined dose 1 and 2 at 1 month after dose 2) |
|
| Immunization status | Use of other vaccines not mentioned | |
| Location | 1 study centre in the Philippines WHO mortality stratum B |
|
| Notes |
Date: 11 May 2004 to 13 September 2004 Source of funding: GlaxoSmithKline Biologicals Trial objective: "To assess the immunogenicity and safety of 2 different formulations of live attenuated HRV [human rotavirus] vaccine given as a two‐dose primary vaccination in healthy infants previously uninfected with HRV" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The ATP cohort for immunogenicity included all vaccinated subjects: – who had received at least one dose of study vaccine/control according to their random assignment, – for whom the randomization code had not been broken" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details; Quote: "Double‐blind with respect to each HRV [RV1] vaccine formulation and its respective placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/100 participants withdrawn from the vaccine group |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Unclear risk | No details |
| Methods | RCT Length of follow‐up: up to the age of 2 years Adverse event data collection methods: not reported |
|
| Participants |
Number: 765 Age range: 6 to 14 weeks Inclusion criteria: full‐term healthy infants aged 6 to 14 weeks at the time of the first dose Exclusion criteria: use of any other investigational or non‐registered product (drug or vaccine) within 30 days preceding the first dose of human rotavirus vaccine; history of use of experimental rotavirus vaccine; chronic administration of immunosuppressants or other immune‐modifying drugs since birth; concurrently participating in another clinical study; any clinically significant history of a serious medical condition; previous confirmed occurrence of rotavirus gastroenteritis |
|
| Interventions | 1. RV1, 508 participants 2. Placebo, 257 participants Schedule: 2 doses according to a 0‐, 1‐month schedule |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Any rotavirus gastroenteritis leading to medical intervention and caused by the circulating wild‐type rotavirus strains, from 2 weeks after dose 2 up to 2 years of age, stool sample collected as soon as possible but preferably not later than 7 days after the start of the episode 2. Severe rotavirus gastroenteritis (≥ 11 on the Vesikari scale) leading to a medical intervention and caused by the circulating wild‐type rotavirus strains (a) of G1 type, (b) of non‐G1 types, from 2 weeks after dose 2 up to 2 years of age 3. Each type of solicited symptom (including: cough, diarrhoea, fever, irritability, loss of appetite and vomiting) during the 8‐day follow‐up period after each dose 4. Adverse events leading to discontinuation of the trial 5. Serious adverse events, including intussusception, up to 2 years of age 6. Fatal serious adverse events 7. Dropouts before the end of the trial Outcomes to measure immunogenicity 8. Seroconversion in terms of anti‐rotavirus IgA antibody, from 2 months after dose 2. Seroconversion was defined as the appearance of anti‐rotavirus immunoglobulin A antibody concentration over 20 units (U)/millilitre (mL) in infants initially (i.e. prior to the first dose of RV1) seronegative |
|
| Immunization status | Combined diphtheria and tetanus toxoids and acellular pertussis (DTPa) and Hepatitis B (HBV) vaccines were allowed to be co‐administered along with RV1 vaccine/placebo | |
| Location | Japan WHO mortality stratum A |
|
| Notes |
Date: June 2007 to November 2009 Source of funding: GlaxoSmithKline Registration number: NCT00480324 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parent/guardian and study personnel were not aware of the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition/exclusions balanced between groups |
| Selective reporting (reporting bias) | Low risk | Protocol published a priori, all prepublished outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 2 months post‐dose 2 Adverse event data collection methods: passive; "Diary cards were provided to the parents/guardians of infants to record the solicited general symptoms occurring during the 15 day follow up period after each vaccine dose. The solicited general symptoms were loss of appetite, fussiness/irritability, fever, diarrhoea, vomiting and cough/runny nose. The intensity of each of these symptoms was graded on a 3‐point scale where “0” indicates normal and “3” indicates severe" |
|
| Participants |
Number: 450 enrolled; ATP safety cohort: 447; ATP immunogenicity cohort: 339 Inclusion criteria: healthy infants aged 6 to 12 weeks at the time of the first vaccination Exclusion criteria: any other investigational drug or vaccine; a history of gastrointestinal disease or rotavirus gastroenteritis; allergy to any of the vaccine components; a history of immunosuppressive or immunodeficient condition |
|
| Interventions | 1. RIX4414* vaccine reconstituted in buffer stored at 2 °C – 8 °C, n = 174 2. RIX4414* vaccine reconstituted in water stored at 2° C – 8 °C, n = 174 3. RIX4414* vaccine reconstituted in buffer stored at 37 °C for 7 days, n = 50 4. Placebo reconstituted in buffer, n = 26 5. Placebo reconstituted in water, n = 26 * Lyophilized formulation containing at least 106.0 CCID50 of the RIX4414 strain Schedule: 2 doses at month 0 and 2 |
|
| Outcomes |
Clinical outcome measures 1. * Rotavirus diarrhoea, stool sample collected during diarrhoea episode, up to 2 months post‐dose 2 2. * All‐cause diarrhoea, up to 2 months post‐dose 2 3. Reactogenicity, including fever, vomiting and diarrhoea, 15‐day follow‐up period after each dose (collected from GSK report) 4. Serious adverse events, up to 2 months post‐dose 2 5. Fatal serious adverse events 6. Adverse events resulting in discontinuation (collected from GSK report) 7. Dropouts: measured up to 2 months after dose 2 (collected from GSK report) Outcomes to measure immunogenicity 8. Seroconversion, anti‐rotavirus IgA antibody levels (cut off: ≥ 20 U/mL by ELISA ), 2 months post‐dose 2 9. Rotavirus antigen shedding in stool (review includes data from combined time points) (collected from GSK report) * Outcome reported as proportion (P) with 95% CI. Events (n) and totals (N) were estimated by using the values when 2 formulae for the standard error (SE) converged |
|
| Immunization status | "During the study period, participating infants were offered commercially available GSK Biologicals’ diphtheria toxoid, tetanus toxoid, acellular pertussis, inactivated polio and H. influenzae type b combination vaccine (InfanrixTM‐IPV/Hib) at two and four months of age and diphtheria toxoid, tetanus toxoid, acellular pertussis, hepatitis B, inactivated polio and H. influenzae type b combination vaccine (Infanrix hexaTM) at six months of age" | |
| Location | 2 centres in Thailand WHO mortality stratum B |
|
| Notes | Study known as RIX GSK[039] 2007‐AS, in previously published versions of this review Date: March to December 2005 Source of funding: GSK Biologicals Study rationale: This study evaluated the stability of lyophilized RIX4414 vaccine in terms of immunogenicity when reconstituted in water instead of regular buffer, and when stored at tropical room temperature (37 °C) for 7 days before reconstitution |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Partially blind study. Quote: “Single blind”, not reported whether personnel or participants were blinded Quote: “The placebo was identical in appearance and composition to the active vaccine” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced across groups with reasons for withdrawal reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 1 month post‐dose 2 Adverse event data collection methods: Passive: Adverse events were recorded during the 8‐day and 31‐day follow‐up period after each dose of RIX4414/placebo, respectively. SAEs were recorded during the entire study period |
|
| Participants |
Number: 684 enrolled; 642 evaluable Age range: 6 to 12 weeks Inclusion criteria: Infants who the investigator believes that their parents/guardians can and will comply with the requirements of the protocol should be enrolled in the study: male or female between, and including, 6 to 12 weeks of age at the time of the first dose of the vaccination, healthy infants as established by medical history and clinical examination, born after a normal gestation period of between 37 and 41 weeks + 6 days inclusive, available vaccination history from vaccination diary cards or medical charts Exclusion criteria: Use of any investigational or non‐registered product (drug or vaccine) other than the study vaccine(s) within 30 days preceding the dose of study vaccine, or planned use during the study period, chronic administration (defined as more than 14 days) of immunosuppressants or other immune‐modifying drugs since birth, planned administration/ administration of a vaccine not foreseen by the study protocol within 30 days of the first dose of vaccine, with the exception of the routine infant vaccines, concurrently participating in another clinical study, confirmed or suspected immunosuppressive or immunodeficient condition, clinically significant history of chronic gastrointestinal disease including any uncorrected congenital malformation of the gastrointestinal tract or other serious medical condition as determined by the investigator, history of allergic disease or reactions likely to be exacerbated by any component of the vaccine, acute disease at the time of enrolment, administration of immunoglobulins or any blood products, or both, since birth or planned administration during the study period, gastroenteritis (GE) within 7 days preceding the study vaccine administration, previous confirmed occurrence of RV GE, previous vaccination with rotavirus vaccine or planned use during the study period |
|
| Interventions | 1. RV1 2. Placebo Schedule: 2 oral doses according to a 0‐, 1‐, or 2‐month schedule |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause deaths 2. All serious adverse events 3. Serious adverse events: intussusception 4. Rotavirus diarrhoea: of any severity (up to 2 months follow‐up) 5. All‐cause diarrhoea: of any severity (up to 2 months follow‐up) 6. Reactogenicity: vomiting, diarrhoea, fever 7. Adverse events requiring discontinuation 8. Dropouts from the trial Outcomes to measure immunogenicity 9. Seroconversion |
|
| Immunization status | Routine childhood vaccines as recommended by the local vaccination schedule were allowed to be administered concomitantly with RIX4414/placebo. These vaccines included the combined diphtheria‐tetanus‐acellular pertussis vaccine, Haemophilus influenzae type b vaccine, inactivated poliovirus vaccine and pneumococcal vaccine. The infants had received the BCG vaccine and 2 doses of hepatitis B vaccine prior to study enrolment | |
| Location | 19 sites, Republic of Korea WHO mortality stratum B |
|
| Notes |
Date: August 2009 to July 2010 Source of funding: GlaxoSmithKline Study rationale: To evaluate Immunogenicity, Reactogenicity and Safety of Rotarix™ Vaccine in Korean Infants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All infants receiving RIX4414 or placebo were allocated into their respective groups using an internet based randomization tool SBIR (Internet based randomization system) according to 3:1 ratio" Quote: "A standard SAS® program generated a randomization list used to number the vaccines. A randomized (3:1) blocking scheme maintained the balance between the two treatments where a unique treatment number identified the study vaccine to be administered to the infants." |
| Allocation concealment (selection bias) | Low risk | The person in charge of the vaccination accessed the randomization system on Internet. Upon providing a participant number and the age (6 ‐ 12 weeks) for the infant, the randomization system used the minimization algorithm to determine the treatment number to be used for the participant. The actual treatment number used for first vaccination of the participant was recorded by the investigator in the eCRF (Randomisation/Treatment Allocation Section) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Each dose of RIX4414 or placebo was administered in a blinded manner where the parents/guardians and the physicians were unaware of the vaccine administered" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 462/684 completed the study, reasons for attrition provided |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting bias |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 1 month Adverse event data collection methods: Passive: diary cards were provided to participants or their parents/guardians to record solicited adverse events for 8 days after each vaccination (day 0 – 7). Serious adverse events were recorded for the duration of the study |
|
| Participants |
Number: 50 enrolled; 50 evaluable Age range: 2 to 6 years old Inclusion criteria: participants were required to be of Chinese origin, in good health and free of obvious health problems |
|
| Interventions | 1. single dose of GlaxoSmithKline (GSK) Biologicals’ human rotavirus (HRV) vaccine (444563). Each 1.5 ml dose of the liquid human RV vaccine contained at least (CCID50) of the live attenuated RIX4414 human RV strain 2. single dose placebo |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events |
|
| Immunization status | Children were allowed to receive routine childhood vaccinations according to local immunization practice during the study period, with a minimum interval of at least 7 days between the administration of routine vaccines and the study vaccine or placebo | |
| Location | Single site, China WHO mortality stratum B |
|
| Notes |
Date: March 2010 to April 2010 Source of funding: GlaxoSmithKline Study rationale: To assess the safety of a single oral dose of HRV vaccine when compared to placebo group, in terms of solicited adverse events (AEs) in healthy children aged 2 to 6 years. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation at the investigator site was performed using an internet‐based randomization system (SBIR) |
| Allocation concealment (selection bias) | Low risk | Treatment allocation at the investigator site was performed using an internet‐based randomization system (SBIR) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was conducted in a double‐blind manner with respect to HRV vaccine and placebo. The parents/LARs of the infants, the study personnel and the investigator were unaware of the study vaccine administered (liquid HRV vaccine or placebo). The laboratory in charge of the laboratory testing was blinded to the treatment, and codes were used to link the participant and study (without any link to the treatment attributed to the participant) to each sample |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | Planned outcomes fully reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 1 month after second dose Adverse event data collection methods: Passive: diary cards were provided to participants or their parents/guardians to record solicited adverse events for 8 days after each vaccination (day 0 – 7). Serious adverse events were recorded for the duration of the study |
|
| Participants |
Number: 50 enrolled; 50 evaluable Age range: 6 to 16 weeks Inclusion criteria: Infants were required to be aged 6 – 16 weeks at the time of first vaccination. Participants were required to be of Chinese origin, in good health and free of obvious health problems |
|
| Interventions | 1. RV1, each 1.5 ml dose of the liquid HRV vaccine contained at least 106.0 median cell culture infective dose (CCID50) of the live attenuated RIX4414 human RV strain 2. Placebo Schedule: 2 oral doses according to a 0‐, 1‐month schedule |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause deaths 2. Serious adverse events 3. Intussusception 4. Reactognicity: fever, diarrhoea, vomiting 5. Dropouts before the end of the trial 6. Adverse event requiring discontinuation Outcomes to measure immunogenicity 7. Vaccine shedding 8. Seroconversion |
|
| Immunization status | Infants were allowed to receive routine childhood vaccinations according to local immunization practice during the study period, with a minimum interval of at least 7 days between the administration of routine vaccines and the study vaccine or placebo | |
| Location | Single site, China WHO mortality stratum B |
|
| Notes |
Date: April to June 2010 Source of funding: GlaxoSmithKline Study rationale: To assess the safety of a single oral dose of HRV vaccine when compared to placebo group, in terms of solicited adverse events (AEs) in healthy infants aged 6‐16 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation at the investigator site was performed using an internet‐based randomization system (SBIR) |
| Allocation concealment (selection bias) | Low risk | Treatment allocation at the investigator site was performed using an internet‐based randomization system (SBIR) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was conducted in a double‐blind manner with respect to HRV vaccine and placebo. The parents/LARs of the infants, the study personnel and the investigator were unaware of the study vaccine administered (liquid HRV vaccine or placebo). The laboratory in charge of the laboratory testing was blinded to the treatment, and codes were used to link the participant and study (without any link to the treatment attributed to the participant) to each sample |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | Planned outcomes fully reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 2 years Adverse event data collection methods: (not reported if active or passive) serious adverse events were recorded throughout the study period |
|
| Participants |
Number: 3333 enrolled; 3148 evaluable Age range: 6 to 16 weeks Inclusion criteria: participants who the investigator believes that their parents/LARs can and will comply with the requirements of the protocol, male or female infant of Chinese origin between, and including, 6 and 16 weeks of age at the time of the first vaccination, healthy infants as established by medical history and clinical examination before entering into the study, born after a gestation period of 36 to 42 weeks inclusive Exclusion criteria: child in care; use of any investigational or non‐registered product other than the study vaccine within 30 days preceding the first dose of study vaccine, or planned use during the study period; any clinically significant history of gastrointestinal disease; any confirmed or suspected immunosuppressive or immunodeficient condition; history of confirmed rotavirus gastroenteritis; acute disease and/or fever at the time of enrolment; gastroenteritis within 7 days preceding the study vaccine or placebo administration |
|
| Interventions | 2 cohorts 1. 1st RV season RIX4414 (1575 participants) or placebo (1573 participants) 2. 2nd RV season RIX4414 (1500 participants) or placebo (1479 participants) Schedule: 2 doses of Rotarix™ vaccine, liquid formulation, at day 0 and at month 1 |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause diarrhoea, severe and any severity 2. Rotavirus diarrhoea, severe and any severity 3. Rotavirus diarrhoea requiring hospitalization 4. All‐cause mortality 5. Serious adverse events 6. Intussusception 7. Reactogenicity: fever, diarrhoea, vomiting 8. Adverse events requiring discontinuation 9. Dropouts before end of the trial Outcomes to measure immunogenicity 10. Seroconversion |
|
| Immunization status | As part of the routine childhood vaccination according to the Expanded Program of Immunization (EPI) recommendations in China, participants also received 3 doses of Infanrix™ vaccine and 3 doses of the oral poliovirus vaccine manufactured by the Institute of Medical Biology of the Chinese Academy of Medical Sciences (OPV). The Infanrix™ and the OPV vaccines were administered independently of (sub‐cohort 1) or concomitantly with (sub‐cohort 2) the Rotarix™ vaccine. When administered concomitantly, participants received the 3 doses of Infanrix™ vaccine at months 1, 2, and 3, and the 3 doses of the OPV vaccine at day 0, month 1 and month 2. The Rotarix™ and OPV vaccines were administered orally; the Infanrix™ vaccine was administered intramuscularly in the left anterolateral thigh | |
| Location | 4 sites, China WHO mortality stratum B |
|
| Notes |
Date: August 2010 to May 2012 Source of funding: GlaxoSmithKline Study rationale: The aim of this study was to assess the efficacy, immunogenicity and safety of two doses of GSK Biologicals’ HRV vaccine in healthy Chinese infants aged between 6 and 16 weeks at the time of the first dose of vaccination. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence generated using software (MATEX developed for SAS) |
| Allocation concealment (selection bias) | Low risk | Treatment allocation at the investigator site was performed using SBIR (internet randomization tool) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Concealed from parents/guardians, study personnel, and investigators, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for attrition provided |
| Selective reporting (reporting bias) | Low risk | Planned outcomes fully reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: outcomes measured 2 weeks after last dose to 1 year of age, and at 2 years Adverse event data collection methods: active surveillance for all gastroenteritis episodes was conducted by members of the study staff through weekly visits to parents or guardians to collect diary cards and through the collection of data from health clinics that served the study populations |
|
| Participants |
Number: 4939 enrolled; 4417 evaluable Age range: 1 to 6 months Inclusion criteria: healthy infants aged 6 to 10 weeks for the group receiving 3 doses and 10 to 14 weeks for the group receiving 2 doses of RV1 Exclusion criteria: children HIV‐positive that were immunosuppressed at < 6 weeks before vaccination |
|
| Interventions | RV1 1. RIX4414 (RV1): dose same as commercial; 3298 participants 1.1 2 doses 1.2 3 doses 2. Placebo: 1641 participants 2.1 Normal placebo Schedule: 2 to 3 doses given 1 month apart |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause diarrhoea 2. Rotavirus diarrhoea: stool samples were tested for rotavirus with the use of an enzyme‐linked immunosorbent assay (ELISA) (Rotaclone, Meridian Bioscience) 3. Severe rotavirus diarrhoea: the severity of each episode of gastroenteritis was evaluated with the use of the Vesikari scale 13 (on which scores range from 1 to 20, with higher scores indicating greater severity) and was categorized as severe if the score was 11 or more * 4. Severe all‐cause diarrhoea: the severity of each episode of gastroenteritis was evaluated with the use of the Vesikari scale 13 (on which scores range from 1 to 20, with higher scores indicating greater severity) and was categorized as severe if the score was 11 or more 5. All‐cause mortality: all serious adverse events including deaths were recorded for the period between the date the first dose of vaccine or placebo was administered and the date the child reached 1 year of age 6. Serious adverse events: all serious adverse events including deaths were recorded for the period between the date the first dose of vaccine or placebo was administered and the date the child reached 1 year of age Outcomes to measure immunogenicity 7. Immunogenicity: ELISA ‐ 1 month after the last dose to determine the serum concentrations of antirotavirus IgA antibody |
|
| Immunization status | Vaccines that are administered routinely according to the guidelines of the Expanded Programme on Immunization (EPI) were concomitantly administered with the vaccine or placebo, including oral polio vaccine | |
| Location | South Africa and Malawi WHO mortality stratum E |
|
| Notes | This trial was conducted in Malawi and South Africa, with data reported separately by country available under RV1 Madhi 2010‐MWI and RV1 Madhi 2010‐ZAF Date: October 2005 to February 2007 (South Africa); October 2006 to July 2007 (Malawi) Source of funding: PATH Rotavirus Vaccine Programme and GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization list was generated at GSK Biologicals, Rixensart, using a standard SAS® (Statistical Analysis System) programme and this was used to number the vaccines |
| Allocation concealment (selection bias) | Low risk | The vaccine doses were distributed to each study centre while respecting the randomizations block size |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The site investigator was unaware of the group assignments of the children |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: outcomes measured 2 weeks after last dose to 1 year of age, and at 2 years Adverse event data collection methods: active surveillance for all gastroenteritis episodes was conducted by members of the study staff through weekly visits to parents or guardians to collect diary cards and through the collection of data from health clinics that served the study populations |
|
| Participants |
Number: 1773 enrolled Age range: 1 to 6 months Inclusion criteria: healthy infants aged 6 to 10 weeks for the group receiving 3 doses and 10 to 14 weeks for the group receiving 2 doses of RV1 Exclusion criteria: children HIV‐positive that were immunosuppressed at < 6 weeks before vaccination |
|
| Interventions | RV1 1. RIX4414 (RV1): dose same as commercial; 1182 participants 1.1 2 doses 1.2 3 doses 2. Placebo: 591 participants 2.1 Normal placebo Schedule: 2 to 3 doses given 1 month apart |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause diarrhoea 2. Rotavirus diarrhoea: stool samples were tested for rotavirus with the use of an ELISA (Rotaclone, Meridian Bioscience) 3. Severe rotavirus diarrhoea: the severity of each episode of gastroenteritis was evaluated with the use of the Vesikari scale 13 (on which scores range from 1 to 20, with higher scores indicating greater severity) and was categorized as severe if the score was 11 or more* 4. Severe all‐cause diarrhoea: the severity of each episode of gastroenteritis was evaluated with the use of the Vesikari scale 13 (on which scores range from 1 to 20, with higher scores indicating greater severity) and was categorized as severe if the score was 11 or more 5. All‐cause mortality: all serious adverse events including deaths were recorded for the period between the date the first dose of vaccine or placebo was administered and the date the child reached 1 year of age 6. Serious adverse events: all serious adverse events including deaths were recorded for the period between the date the first dose of vaccine or placebo was administered and the date the child reached 1 year of age Outcomes to measure immunogenicity 7. Immunogenicity: ELISA ‐ 1 month after the last dose to determine the serum concentrations of antirotavirus IgA antibody |
|
| Immunization status | Vaccines that are administered routinely according to the guidelines of the Expanded Programme on Immunization (EPI) were concomitantly administered with the vaccine or placebo, including oral polio vaccine | |
| Location | Malawi WHO mortality stratum E |
|
| Notes | This trial was conducted in Malawi and South Africa. This part presents data reported for the Malawi cohort, while data reported for South Africa can be found under RV1 Madhi 2010‐ZAF, data reported for both countries under RV1 Madhi 2010‐AF Date: October 2006 to July 2007 Source of funding: PATH Rotavirus Vaccine Programme and GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization list was generated at GSK Biologicals, Rixensart, using a standard SAS® (Statistical Analysis System) program and this was used to number the vaccines |
| Allocation concealment (selection bias) | Low risk | The vaccine doses were distributed to each study centre while respecting the randomizations block size |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The site investigator was unaware of the group assignments of the children |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: outcomes measured 2 weeks after last dose to 1 year of age, and at 2 years (only Cohort 2) Adverse event data collection methods: active surveillance for all gastroenteritis episodes was conducted by members of the study staff through weekly visits to parents or guardians to collect diary cards and through the collection of data from health clinics that served the study populations |
|
| Participants |
Number: 3166 enrolled Age range: 1 to 6 months Inclusion criteria: healthy infants aged 6 to 10 weeks for the group receiving 3 doses and 10 to 14 weeks for the group receiving 2 doses of RV1 Exclusion criteria: children HIV‐positive that were immunosuppressed at < 6 weeks before vaccination |
|
| Interventions | RV1 1. RIX4414 (RV1): dose same as commercial; 2116 participants 1.1 2 doses 1.2 3 doses 2. Placebo: 1050 participants 2.1 Normal placebo Schedule: 2 to 3 doses given 1 month apart |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause diarrhoea 2. Rotavirus diarrhoea: stool samples were tested for rotavirus with the use of an ELISA (Rotaclone, Meridian Bioscience) 3. Severe rotavirus diarrhoea: the severity of each episode of gastroenteritis was evaluated with the use of the Vesikari scale 13 (on which scores range from 1 to 20, with higher scores indicating greater severity) and was categorized as severe if the score was 11 or more* 4. Severe all‐cause diarrhoea: the severity of each episode of gastroenteritis was evaluated with the use of the Vesikari scale 13 (on which scores range from 1 to 20, with higher scores indicating greater severity) and was categorized as severe if the score was 11 or more 5. All‐cause mortality: all serious adverse events including deaths were recorded for the period between the date the first dose of vaccine or placebo was administered and the date the child reached 1 year of age 6. Serious adverse events: all serious adverse events including deaths were recorded for the period between the date the first dose of vaccine or placebo was administered and the date the child reached 1 year of age Outcomes to measure immunogenicity 7. Immunogenicity: ELISA ‐ 1 month after the last dose to determine the serum concentrations of antirotavirus IgA antibody *G types for severe rotavirus diarrhoea for the first year follow‐up were reported and added to the analyses, G types for any rotavirus diarrhoea were reported for the second year only, and were not added to the analysis |
|
| Immunization status | Vaccines that are administered routinely according to the guidelines of the Expanded Programme on Immunization (EPI) were concomitantly administered with the vaccine or placebo, including oral polio vaccine | |
| Location | South Africa WHO mortality stratum E |
|
| Notes | This trial was conducted in Malawi and South Africa. This part presents data reported for the South Africa cohorts, data reported for Malawi can be found under RV1 Madhi 2010‐MWI, and data reported for both countries under RV1 Madhi 2010‐AF Date: October 2005 to February 2007 Source of funding: PATH Rotavirus Vaccine Programme and GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization list was generated at GSK Biologicals, Rixensart, using a standard SAS® (Statistical Analysis System) program and this was used to number the vaccines |
| Allocation concealment (selection bias) | Low risk | The vaccine doses were distributed to each study centre while respecting the randomizations block size |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The site investigator was unaware of the group assignments of the children |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 1 month after dose 2 Adverse event data collection methods: passive, parents/guardians filled in diary cards of any symptoms |
|
| Participants |
Number: 363 enrolled; 344 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy male or female infants between and including 8 to 10 weeks of age at the time of first vaccination; free of obvious health problems as established by medical history and clinical examination before entering into the study; Exclusion criteria: history of confirmed rotavirus gastroenteritis or with prior administration of experimental rotavirus vaccine |
|
| Interventions | RV1 1. RIX4414 (RV1): 106.5 PFU; 182 participants (randomized) 2. Placebo: 181 participants (randomized) Schedule: 2 oral doses given at age 2 and 4 months |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: for each type of solicited symptom, occurrence of the symptom within the 8‐day (days 0 to 7) solicited follow‐up period after each dose; occurrence of unsolicited adverse events within 31 days (days 0 to 30) after each dose, according to MedDRA classification; measured up to 31 days after vaccine/placebo 2. Serious adverse events: no definition; occurrence throughout entire study period (up to 31 days after vaccine/placebo) 3. Dropouts: no definition; measured up to 31 days after vaccine/placebo 4. Rotavirus diarrhoea: presence of rotavirus in gastroenteritis episode stools collected from dose 1 of RV1 vaccine/placebo up to 2 months after dose 2; measured up to 31 days after vaccine/placebo 5. All‐cause death 6. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 7. Seroconversion: appearance of anti‐rotavirus immunoglobulin A (IgA) antibody concentration ≥ 20 U/mL in participants who were seronegative before vaccination (review includes data from 1 month after dose 2) |
|
| Immunization status | Routine vaccinations (diphtheria‐tetanus‐whole cell pertussis‐hepatitis b, H. influenzae type b, and oral poliovirus vaccine) were administered at 6, 10, and 14 weeks of age (given with a 2‐week separation from the first and subsequent dose of the RV1 vaccine or placebo) | |
| Location | 4 centres in India WHO mortality stratum D |
|
| Notes |
Date: 10 February 2006 to 8 September 2006 Source of funding: GlaxoSmithKline Biologicals Study rationale: "to assess the immunogenicity and safety of 2 doses of oral live attenuated human rotavirus vaccine in healthy infants in India" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Likely to be adequate: treatment masked to investigators Quote: "a treatment number identified uniquely the vaccine doses to be administered to the same subject" and "subjects were administered the vaccine dose with the lowest treatment number available at the study centre" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parent/guardian and study personnel were not aware of the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition/exclusions balanced between groups |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 1 year Adverse event data collection methods: Not reported |
|
| Participants |
Number: 308 enrolled; 209 evaluated (1 study arm was not included in analyses of this review) Age range: 11 to 17 weeks of age at the time of the first vaccination Inclusion criteria: infants who the investigator believes that their parent/guardian can and will comply with the requirements of the protocol, administration of 1 dose of hepatitis B vaccine at birth, male or female between and including 11 and 17 weeks of age at the time of the first DTPw vaccination, free of obvious health problems as established by medical history and clinical examination before entering into the study Exclusion criteria: use of any investigational or non‐registered product (drug or vaccine) other than the study vaccine(s) within 30 days preceding the first dose of study vaccine, or planned use during the study period, chronic administration of immunosuppressants or other immune‐modifying drugs since birth, any confirmed or suspected immunosuppressive or immunodeficient condition based on medical history and physical examination (no laboratory testing is required), administration of immunoglobulins or any blood products, or both, since birth or planned administration during the study period |
|
| Interventions | 1. RV1 at 3 and 4½ months + DTPw‐HBV at 3, 4½ and 6 months (80 participants) 2. Placebo at 3 and 4½ months + DTPw‐HBV at 3, 4½ and 6 months (25 participants) 3. RV1 at 3 and 4½ months + DTPw‐HBV Kft. at 3, 4½ and 6 months (81 participants) 4. Placebo at 3 and 4½ months + DTPw‐HBV Kft. at 3, 4½ and 6 months (23 participants) 5. DTPwcsl + HBV at 3, 4½ and 6 months (99 participants), this group was not included in analyses of this review |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity 2. Serious adverse events 3. All‐cause death 4. Intussusception 5. Dropouts Outcomes to measure immunogenicity 6. Seroconversion |
|
| Immunization status | GlaxoSmithKline (GSK) Biologicals' Tritanrix™HepB and GSK Biologicals Kft's DTPwHBV Vaccines as compared to concomitant administration of Commonwealth Serum Laboratory's (CSL's) DTPw (Triple Antigen™) and GSK Biologicals' HBV (Engerix™B), when co‐administered with GSK Biologicals' oral live attenuated Human Rotavirus (HRV) vaccine, to healthy infants at 3, 4½ and 6 months of age, after a birth dose of Hepatitis B vaccine | |
| Location | 9 sites, Russian Federation WHO mortality strata: C |
|
| Notes |
Date: September 2005 to November 2006 Source of funding: GlaxoSmithKline Study rationale: To compare the 2 formulations of GSK Biologicals' DTPw‐HBV vaccine to concomitant administration of CSL's DTPw vaccine and GSK Biologicals' HBV with respect to the antibody response to the diphtheria antigen after a 3‐dose primary vaccination course. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized (4:1:4:1:5) using GSK Biologicals central randomization system (SBIR) |
| Allocation concealment (selection bias) | Low risk | Cental allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was conducted in a double‐blind manner with respect to the Rotarix and placebo groups and in single‐blinded manner with respect to the Tritanrix‐HepB and Zilbrix groups. The study was open with respect to the Triple Antigen + Engerix‐B group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 30 to 83 days after dose 2 Adverse events data collection methods: active surveillance: at each study visit parents were asked about AEs; passive surveillance: throughout the trial, parents were asked to immediately report AEs to the investigator |
|
| Participants |
Number: 1009 Age range: 6 to 12 weeks of age at the time of the first study vaccination Inclusion criteria: medically stable pre‐term infants, born within a gestational period of 27 ‐ 36 weeks, planned to be discharged from hospital's neonatal stay on or before the day of the first human rotavirus vaccine/placebo administration Exclusion criteria: use of any investigational or non‐registered product (drug or vaccine) other than the human rotavirus vaccine within 30 days preceding the first dose of human rotavirus vaccine; any clinically significant history of chronic gastrointestinal disease; any confirmed or suspected immunosuppressive or immunodeficient condition; history of allergic disease; major congenital defects or serious chronic illness Each study group is further stratified into 2 subgroups depending on the gestational age at birth of the participant: Stratum I: very pre‐term infants, born after a gestational period of 27 to 30 weeks (189 to 216 days) (20% of enrolment); Stratum II: mild pre‐term infants born after a gestational period of 31 to 36 weeks (217 to 258 days) (80% of enrolment) |
|
| Interventions | 1. RV1, 670 participants 2. Placebo, 339 participants Schedule: 2 oral doses of vaccine or placebo, 1 dose at day 0 and 1 dose at months 1 or 2, depending on the country |
|
| Outcomes |
Clinical outcome measures 1. Serious adverse events, including fatal events and intussusception, from day 0 up to 83 days after dose 2 of RV1 vaccine/placebo 2. Solicited symptoms, within 15 days after each RV1 vaccine/placebo dose. Solicited symptoms included diarrhoea (3 or more looser than normal stools/day), fever (axillary temperature over 37.5 °C), irritability, loss of appetite, and vomiting 3. All‐cause gastroenteritis and rotavirus gastroenteritis, from dose 1 up to 83 days after dose 2 of RV1 vaccine/placebo. Gastroenteritis: diarrhoea with or without vomiting. Rotavirus gastroenteritis: a gastroenteritis episode was a rotavirus gastroenteritis episode if a stool sample taken during or not later than 7 days after the episode was rotavirus positive by ELISA 4. Dropouts before the end of the trial Outcomes to measure immunogenicity 5. Seroconversion to anti‐rotavirus IgA antibody, at Visit 3, 1 month after Dose 2 of RV1 vaccine/placebo. Number of participants with anti‐rotavirus IgA antibody concentration over 20 units/mL |
|
| Immunization status | In accordance with the local National Plan of Immunisation schedule in each of the respective participating countries, GSK Biologicals' Infanrix Hexa® (DTPa‐HBV‐IPV/Hib), Infanrix Quinta® (DTPa‐IPV‐Hib), Infanrix®+IPV+Hib (DTPa+IPV+Hib) and/or Engerix‐B® (HBV) will be co‐administered (at a maximum interval of 2 days from each other) with each human rotavirus vaccine or placebo dose Hepatitis B and BCG vaccines at birth are allowed if included in the local National Plan of Immunisation schedule in participating countries At the discretion of the investigator the following vaccines may be administered during each infant's study participation:
|
|
| Location | France, Poland, Portugal, Spain WHO mortality strata A, B |
|
| Notes | Study known as RV1 NCT00420745 2009‐EU in previously published versions of this review Date: January 2007 to March 2008 Source of funding: GlaxoSmithKline Registration number: NCT00420745 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomizations |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parent/guardian and study personnel were not aware of the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced between groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes included |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: until infants aged 18 months (i.e. about 13 to 15 months of follow‐up) Adverse events data collection methods: "diary cards during a 15‐day follow‐up period after each vaccine dose was administered, and the symptoms were graded according to severity. AEs occurring up to 42 days after administration of each study vaccine was recorded" (passive method) |
|
| Participants |
Number: 2464 enrolled; 2365 evaluable Age range: 3 to 6 months Inclusion criteria: male or female infants, born after a normal gestation period of 36 to 42 weeks; aged 11 to 17 weeks at time of first dose of study vaccine; free of obvious health problems as established by medical history and clinical examination before entering into the study Exclusion criteria: "Subjects with previous confirmed occurrence of rotavirus gastroenteritis, previous vaccination against or history of diphtheria, tetanus, pertussis, polio and/or Hib, had a history of allergic reaction to any vaccine component, were immunocompromised or had contact with immunosuppressed individual or pregnant women in their household, had any clinically significant history of chronic gastrointestinal (GI) disease including any uncorrected congenital malformation of GI tract or subjects with use of antibiotics within 7 days preceding Dose 1" |
|
| Interventions | RV1 1. RIX4414 (RV1) 1.1. 104.7 FFU; 510 participants 1.2. 105.2 FFU; 648 participants 1.3. 106.1 FFU; 653 participants 2. Placebo; 653 participants All vaccines given in 2 doses with a 1‐month interval Outcomes measured at ˜15 months (efficacy data from 2 weeks after second dose to 18 months of age) |
|
| Outcomes |
Clinical outcome measures 1. All‐cause diarrhoea: episodes of acute gastroenteritis; parents instructed to record (diary cards) body temperature, the number of episodes of vomiting, the number of looser‐than‐normal stools, and whether they sought medical intervention or medication, and were asked to obtain at least 2 stool samples on 2 different days within 7 days of the onset of symptoms; measured at 2 weeks to 18 months 2. Rotavirus diarrhoea: see all‐cause diarrhoea; "Rotavirus gastroenteritis was confirmed if at least 1 of the 2 stool specimens was found to be positive for rotavirus by ELISA. Rotavirus isolates were G‐typed by use of reverse‐transcriptase polymerase chain reaction (RT‐PCR)"; measured at 2 weeks to 18 months 3. Severe all‐cause diarrhoea: severity of each episode of gastroenteritis graded using a 20‐point scoring system described by Ruuska 1990 4. Severe rotavirus diarrhoea: see severe all‐cause diarrhoea 5. All‐cause death 6. All‐cause hospital admission 7. Emergency department visit 8. Serious adverse events 9. Reactogenicity: fever if rectal temperature > 38 ºC 10. Adverse events requiring discontinuation 11. Rotavirus diarrhoea requiring hospitalization 12. Dropouts Outcomes to measure immunogenicity 11. Shedding of vaccine virus: in stool samples on day of each vaccination and on days 7 and 15 after each vaccination (from 50 participants/group, the "stool sample subset") (review includes data from 1 month after dose 1 and 1 month after dose 2) 12. Seroconversion: serum anti‐rotavirus IgA antibody seroconversion rate; "seroconversion" "defined by an anti‐rotavirus IgA antibody concentration of ≥ 20 U/mL, for infants who were initially (i.e. before administration of the first vaccine dose) seronegative for anti‐rotavirus IgA antibodies (i.e. a concentration of <20 U/mL) and/or who had a stool sample that was negative for rotavirus antigen. Any detection of RIX4414 antigen in stool samples was taken as evidence of a vaccine response" |
|
| Immunization status | Hepatitis B vaccine, diphtheria‐tetanus‐acellular pertussis, poliovirus, and H. influenzae type b co‐administered with interventions | |
| Location | 8 centres in Singapore WHO mortality stratum A |
|
| Notes |
Date: 4 January 2001 to 15 April 2003 Funding: GlaxoSmithKline Biologicals Other: 93% of population were Asian |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Parent/guardian and study personnel were not aware of the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data imputed appropriately |
| Selective reporting (reporting bias) | Unclear risk | Reasons for low number of rotavirus gastroenteritis; "A smaller number of rotavirus‐related gastroenteritis cases than expected were documented during the study. For 41% (160/387) of the reported gastroenteritis episodes, stool samples were not available for determination of the etiology of the gastroenteritis. No results were available for 6% (24/387) of the gastroenteritis episodes because of an insufficient quantity of stool samples collected or because of invalid results" |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 2 weeks post‐dose 2 to 3 years Adverse events data collection methods: passive method, using diary cards |
|
| Participants |
Number: 10,708 enrolled; 10,519 evaluable Age range: 3 to 6 months Inclusion criteria: healthy infants 6 to 12 weeks of age in Hong Kong and Taiwan, or 11 to 17 weeks of age in Singapore at the time of the first dose Exclusion criteria: "they did not have a history of chronic administration of immunosuppressants since birth, any confirmed or suspected immunosuppressive or immunodeficient condition, history of allergic disease or reaction likely to be exacerbated by any vaccine component, had not received any investigational drugs/vaccines from 30 days before Dose 1 or planned use during the study, had not received immunoglobulins and/or blood products since birth or planned administration during the study period, did not have any clinically significant history of chronic gastrointestinal disease including any uncorrected congenital malformation of the gastrointestinal tract or other serious medical condition as determined by the investigator, and did not have first or second degree of consanguinity of parents" |
|
| Interventions | RV1 1. RIX4414 (RV1) 106 FFU; 5359 participants 2. Placebo; 5349 participants All vaccines given in 2 doses with a 1 to 2 month interval |
|
| Outcomes |
Clinical outcome measures 1. All‐cause diarrhoea: a gastroenteritis episode was defined as occurrence of diarrhoea with or without vomiting (diarrhoea was defined as the passage of 3 or more looser‐than‐normal stool within a 24‐hour period) 2. Severe all‐cause diarrhoea: severe gastroenteritis was defined as an episode of diarrhoea with or without vomiting that required overnight hospitalization or rehydration therapy, or both (equivalent to WHO plan B or C) in a medical facility and with a score of 11 points on the 20‐point Vesikari scale 3. Rotavirus diarrhoea: stool samples collected during gastroenteritis episodes were tested for the presence of rotavirus using ELISA method (RotacloneTM, Meridian Bioscience) at GlaxoSmithKline Biologicals’ laboratories in Rixensart, Belgium. All rotavirus‐positive stool samples were tested by reverse transcriptase polymerase chain reaction (RT‐PCR) followed by reverse hybridization assay, and optional sequencing, at Delft Diagnostic Laboratory, The Netherlands, to determine G and P types, and differentiation of G1P[8] vaccine type 4. Severe rotavirus diarrhoea*: see above 5. Emergency department visit: active surveillance was conducted at hospitals and medical facilities in the study area to capture gastroenteritis episodes requiring hospitalization and/or re‐hydration therapy (equivalent to WHO plan B or C) in a medical facility from day of the first vaccine or placebo dose until the follow‐up visit at 24 months of age 6. Serious adverse events: intussusception and SAEs were followed during the study duration. A case of definite intussusception required confirmation at surgery or autopsy or by using imaging techniques such as gas or liquid contrast enema or abdominal ultrasound. Abstractable data for all serious adverse events and Kawasaki disease were only provided for the third year of follow‐up. Intussusception data for the third year follow‐up was not included in the analysis as the follow‐up population was smaller (RV1: 2/4272; placebo: 1/4226) 7. All‐cause deaths Outcomes to measure immunogenicity None *G types for severe rotavirus diarrhoea up to two years follow‐up was reported and added to the analyses, data for the third year was reported but not included in the analysis as the follow‐up population was smaller" |
|
| Immunization status | Infants received other routine paediatric immunizations (combined diphtheria toxoid‐tetanus toxoid‐acellular pertussis (DTPa) inactivated poliovirus (IPV) and H. influenzae type b (HiB) vaccine and hepatitis B vaccine (HBV)) during the study period according to local schedules. Almost all infants received BCG dose at birth. If oral polio vaccine (OPV) was given as part of the routine schedule in the participating countries, a time interval of 2 weeks was observed between the OPV doses and RIX4414 vaccine/placebo doses. One dose of oral polio vaccine (OPV) was given at birth in Hong Kong (99.8% participants) and Taiwan (0.7% participants). However, during the study period, > 95% of infants in the 3 countries received DTPa‐IPV‐HiB concomitantly with both doses of RIX4414 vaccine/placebo as per local schedules. 50.9% of participants were male and the study population was predominantly Chinese (76.3%). | |
| Location | Hong Kong, Singapore, Taiwan WHO mortality stratum A |
|
| Notes |
Date: 8 December 2003 to 31 August 2005 Funding: GlaxoSmithKline Other: all enrolled infants received the first dose of RIX4414 vaccine or placebo, and 10,551 (98.5%) received both doses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization list was generated at GSK Biologicals, Rixensart, using a standard SAS® programme and was used to number the vaccines |
| Allocation concealment (selection bias) | Low risk | A randomization blocking scheme was used to ensure that the balance between treatments was maintained. Treatment allocation at the investigator sites was performed using a central randomization system on the Internet |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Data analysis was performed at GSK Biologicals. The treatment code remains masked, except for statisticians and the database administrator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analysis of efficacy was performed from 2 weeks post‐dose 2 until 2 years of age on the ATP cohort that included participants who completed the full 2‐dose vaccination course and complied with the protocol. The total vaccinated cohort was used to calculate vaccine efficacy starting from the first dose onwards |
| Selective reporting (reporting bias) | Low risk | All expected outcomes included |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 17 weeks Adverse events data collection methods: not reported |
|
| Participants |
Number: 200 Age range: 6 to 14 weeks of age at the time of the first study vaccination Inclusion criteria: healthy infants with a live twin living in the same household who is also enrolled in this study, born after a gestation period of over 32 weeks Exclusion criteria: use of any investigational or non‐registered product other than the study vaccine(s); any confirmed or suspected immunosuppressive or immunodeficient condition; any clinically significant history of chronic gastrointestinal disease; history of allergic disease; acute disease at time of enrolment; gastroenteritis within 7 days preceding the first study vaccine administration; documented HIV‐positive infant |
|
| Interventions | 1. RV1 (RIX 4414) Vaccine, 100 participants 2. Placebo, 100 participants Schedule: both vaccine and placebo 2 doses at Day 0 (Visit 1) and Week 7 (Visit 2) Notes: 1 complimentary dose of RV1 was administered to all infants enrolled in this study (both study groups) who are aged less than 6 months at Visit 3 (Week 13) as a benefit to the placebo group for participation in the study |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Gastroenteritis, up to week 17 2. Rotavirus gastroenteritis, up to week 13. Rotavirus gastroenteritis episodes were defined as gastroenteritis episodes for which the stool sample temporally closest to the onset day of the gastroenteritis episode was positive for rotavirus by ELISA 3. Serious adverse events, including fatal serious adverse events and intussusception, up to week 17 4. Dropouts from the study Outcomes to measure immunogenicity 5. Anti‐rotavirus IgA antibody seroconversion and concentration in each group, at visit 3 |
|
| Immunization status | All infants received 3 doses of combined diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus and H. influenzae vaccine | |
| Location | Dominican Republic WHO mortality stratum B |
|
| Notes | Study known as RV1 NCT00396630 2009‐LA in previously published versions of this review. Date: January 2007 to February 2008 Source of funding: GlaxoSmithKline Registration number: NCT00396630 Aim: "to explore horizontal transmission of the HRV [human rotavirus] vaccine strain within a family from the twin vaccinated with Rotarix to the twin receiving placebo" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomization list was generated at GlaxoSmithKline (GSK) Biologicals, Rixensart, using a standard SAS® program. A randomization blocking scheme (1:1 ratio, block size = 2) was used to ensure balance between the treatment arms; a treatment number uniquely identified the vaccine doses to be administered to the same infant" |
| Allocation concealment (selection bias) | Low risk | Quote: "No investigator or any person involved in the clinical trial (including laboratory personnel, statisticians and data management) was aware of the treatment groups during the course of the study" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The study was double‐blinded and the parents/guardians of infants, investigator and the study personnel were unaware of the study vaccine administered" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition/exclusions balanced between groups |
| Selective reporting (reporting bias) | Low risk | Trial report does not provide enough details |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 9 to 10 months Adverse events data collection methods: active surveillance system established at hospital and medical facilities in study areas to capture intussusceptions and severe gastroenteritis episodes (active method) |
|
| Participants |
Number: 63,225 enrolled for safety and 20,169 enrolled for efficacy; 59,308 evaluable for safety, and 17,882 evaluable for first‐year efficacy and 14,615 for second‐year efficacy Age range: 1 to 3 months (start) and 3 to 6 months (end) Inclusion criteria: healthy infants aged 6 to 12 weeks (in all countries except Chile) or 6 to 13 weeks (in Chile) at time of first dose of RV1 or placebo; "healthy infants 6‐13 weeks of age at the time of the first study vaccination whose parent/guardian sign a written informed consent and whose parents/guardians can and will comply with the requirements of the protocol (e.g., completion of the diary cards, return for follow‐up visits)" Exclusion criteria (from NCT00140673): use of any investigational or non‐registered product (drug or vaccine) other than the study vaccine(s) within 30 days preceding the first dose of study vaccine or placebo, or planned use during the study period; chronic administration (defined as > 14 days) of immunosuppressants or other immune‐modifying drugs since birth (topical steroids allowed); child unlikely to remain in the study area for the duration of the study; any confirmed or suspected immunosuppressive or immunodeficient condition, including HIV infection; history of allergic disease or reaction likely to be exacerbated by any component of the vaccine; administration of immunoglobulins or blood products or both since birth or planned administration during the study period; any clinically significant history of chronic gastrointestinal disease including any uncorrected congenital malformation of the gastrointestinal tract or other serious medical condition as determined by the investigator |
|
| Interventions | RV1 1. RIX4414 (RV1): 106.5 PFU; 31,673 participants (safety), 10,159 participants (efficacy) 2. Placebo; 31,552 participants (safety), 10,010 participants (efficacy) Both vaccine and placebo given in 2 doses with 4 to 8 weeks interval Both vaccine and placebo reconstituted in 1.3 mL of liquid calcium carbonate buffer |
|
| Outcomes |
Clinical outcome measures 1. Serious adverse events: "defined as any new health‐related problems that resulted in death, were life‐threatening, necessitated hospitalization or prolongation of existing hospitalization, or resulted in disability or incapacity"; "case of definite intussusception required confirmation at surgery or autopsy or with the use of imaging techniques, such as imaging with gas‐ or liquid‐contrast enema or abdominal ultrasonography"; measured up to 30 days after vaccination and during the first year follow‐up for efficacy; intussusception measured up to 100 days after dose 1. Final intussusception results taken from CDC report (CDC 2010) 2. Severe all‐cause diarrhoea: severe gastroenteritis measured as an "episode of diarrhoea with or without vomiting that required hospitalization and/or re‐hydration therapy (equivalent to WHealth O plan B or C) in a medical facility"; measured from 2 weeks after second dose up to 2 years follow‐up 3. All‐cause diarrhoea; measured from 2 weeks after second dose up to 2 years follow‐up 4. Rotavirus diarrhoea; measured from 2 weeks after second dose up to 2 years follow‐up 5. Severe rotavirus diarrhoea: severe rotavirus gastroenteritis defined as an "an episode of severe gastroenteritis occurring at least 2 weeks after the full vaccination course in which rotavirus other than vaccine strain was identified in a stool sample collected during the episode of severe gastroenteritis"; measured from 2 weeks after second dose up to 2 years follow‐up 6. All‐cause death; measured up to 30 days after vaccination 7. All‐cause hospital admission; from 2 weeks after second dose up to 2 years follow‐up 8. Reactogenicity; up to 30 days after vaccination 9. Dropouts; measured up to 2 years follow‐up 11. Rotavirus diarrhoea requiring hospitalizations 12. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 13. Seroconversion: serum rotavirus IgA antibody concentrations in a subset of 100 participants per country (except in Finland) at Visits 1 and 3 (data not included in review because it was not a random sample) Outcomes measured up to 30 days after second dose of vaccine (safety outcomes) and up to 2 years (efficacy outcomes) |
|
| Immunization status | Routine immunizations according to local regulations; oral poliovirus vaccination at least 2 weeks before or after rotavirus vaccine | |
| Location | Latin America and Europe (Argentina, Brazil, Chile, Colombia, Dominican Republic, Finland, Honduras, Mexico, Nicaragua, Panama, Peru, and Venezuela); second year follow‐up in all locations except Finland and Peru WHO mortality strata A, B, D |
|
| Notes |
Date: 5 August 2003 to 20 October 2005 Source of funding: GlaxoSmithKline Biologicals Data extracted from appendix accompanying main report and GlaxoSmithKline companion reports |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "GlaxoSmithKline Biologicals provided vaccine supplies that were numbered with a computer‐generated randomization list. We used a blocking scheme randomization. GSK did the masking and concealment" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was done by a central Internet randomization system" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Treatment allocation remained concealed from investigators and parents of participating infants throughout the study. GSK did the masking and concealment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "full GSK report account for all withdrawals regardless of reason" |
| Selective reporting (reporting bias) | High risk | The trial reported only on severe episodes of rotavirus diarrhoea and all‐cause diarrhoea, and not on diarrhoea of any severity, which is unusual in these trials |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 2 years (stated in GlaxoSmithKline report) Adverse event data collection methods: diary cards were supplied to the parents to record occurrence of specific solicited symptoms for 15 days after each vaccination (passive method); any other unsolicited symptoms were recorded during 43 days after each vaccination (passive method); serious adverse events were recorded throughout the study |
|
| Participants |
Number: 2155 enrolled; 2004 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants, born after a normal gestation period of 36 to 42 weeks or with a birth weight > 2000 g; aged 6 to 12 weeks at the time of the first vaccination; free of obvious health problems as established by medical history and clinical examination before entering into the study Exclusion criteria: previous confirmed occurrence of rotavirus gastroenteritis; previous vaccination against or history of diphtheria, tetanus, pertussis, polio and/or H. influenzae type b vaccine (HiB); any clinically significant history of chronic gastrointestinal disease including any uncorrected congenital malformation of gastrointestinal tract; use of antibiotics within 7 days preceding dose 1; immunocompromised or were in household contact with an immunosuppressed individual or pregnant woman |
|
| Interventions | RV1 1. RIX4414 (RV1) 1.1. 104.7 PFU; 538 participants (randomized) 1.2. 105.2 PFU; 540 participants (randomized) 1.3. 105.8 PFU; 540 participants (randomized) 2. Placebo: 537 participants (randomized) Schedule: 2 doses given every 2 months An additional 200 participants were randomized to RV1 x placebo to receive 3 doses. This is not mentioned in the main publication, only in the GlaxoSmithKline report (no data available) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events: no definition; measured during follow‐up (2 years) 2. Reactogenicity: no definition; measured up to 43 days after vaccination 3. All‐cause diarrhoea: gastroenteritis defined as diarrhoea characterized by ≥ 3 looser than normal stools within a day; minimum of 5 days required between episodes for them to be considered as separate events; measured during follow‐up (2 years) 4. Severe all‐cause diarrhoea: information on diary cards was used to assess the severity of each gastroenteritis episode according to a 20‐point scoring system; measured during follow‐up (2 years) 5. Rotavirus diarrhoea: all rotavirus‐positive specimens were tested by RT‐PCR at GlaxoSmithKline to determine the G type; any G1 rotavirus detected until 2 months after the second dose were analyzed to differentiate between vaccine strain and wild G1 strains; only gastroenteritis episodes in which wild rotavirus other than the vaccine strain was identified in a stool specimen were included in the efficacy analysis; measured during follow‐up (2 years) 6. Severe rotavirus diarrhoea: see above; measured during follow‐up (2 years) 7. All‐cause hospital admission: no definition; measured during follow‐up (2 years) 8. All‐cause mortality: no definition; measured during follow‐up (2 years) 9. Rotavirus diarrhoea resulting in hospitalization Outcomes to measure immunogenicity 10. Vaccine take: rotavirus shedding in stool specimens (review includes data from day 7 after dose 2) 11. Seroconversion: "percentages of infants with post‐antirotavirus IgA antibody concentration 20 units/mL in infants who were negative for rotavirus before the first dose of RIX4414 or placebo" (review includes data from 2 months after dose 1 and 2 months after dose 2) |
|
| Immunization status | Oral polio vaccine given after 2 weeks, not together with RV1 | |
| Location | Belem (Brazil), Mexico City (Mexico), Valencia (Venezuela) WHO mortality stratum B |
|
| Notes |
Date: 25 May 2001 to 8 November 2003 Source of funding: GlaxoSmithKline Biologicals Malnutrition: reported in Journal of Infectious Disease, 2007, 196(4): 537‐40 Other: main publication did not report that the trial included 2 subsets:
Immunogenicity sampling: "A subset of infants (N 800) provided blood samples 2 months after the first dose (serology for antirotavirus IgA antibodies) and 2 months after the second dose (serology for antirotavirus IgA antibodies and antibodies against antigens of routine infant vaccines). The first 200 enrolled infants in each participating country constituted this subset, and the remaining 200 infants were included according to the order of enrolment irrespective of country". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated Quote:"The participating infants were randomly assigned to one of the 4 study groups (3 vaccine groups and a placebo group) following a 1:1:1:1 allocation ratio according to a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double blinding was maintained during the entire study period" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | High risk | Not all prespecified outcomes reported |
| Other bias | Unclear risk | GlaxoSmithKline final report stated that part of the population received 3 doses of rotavirus vaccine. This was not mentioned on the original published report |
| Methods | RCT Length of follow‐up: up to 6 months after last vaccine given Adverse event data collection methods: "The infants were monitored for at least 30 min after each vaccination. Parents received a diary card to record information daily about solicited general symptoms (fever, fussiness/irritability, diarrhoea, vomiting, loss of appetite or cough/runny nose) for 15 days after each dose of RIX4414 or placebo, and any other adverse events occurring until the next study visit. Weekly supervision was done by Health Care Workers from Madibeng District Health Centre. The study physician or his staff questioned the parents on their child’s health and verified the completed diary card at each visit" |
|
| Participants |
Number: 450 enrolled; 406 evaluable 2 cohorts were vaccinated: 1st cohort before the rotavirus season (271 participants); 2nd cohort after the rotavirus season (179) participants Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants, born after a normal gestation period of ≥ 36 weeks; 5 to 10 weeks of age at the time of the first study visit; free of obvious health problems as established by medical history and clinical examination before entering into the study. There were no restrictions on feeding the infants before or after vaccination Exclusion criteria: infants were excluded if they had a clinically significant history of gastrointestinal disease or malformation, had received vaccines or treatment prohibited by the protocol, were immuno‐compromised or were in household contact with an immunosuppressed individual or pregnant woman. BCG and OPV vaccinations at birth were allowed according to the local EPI schedule. Vaccination was postponed if the infant had fever (≥ 37.5 ºC axillary or ≥ 38 ºC rectal) or gastroenteritis within the previous 7 days |
|
| Interventions | RV1 1. RIX4414 (RV1): 105 FFU; 2 doses given 1 month apart; 300 participants (randomized) 1.1. RV1 vaccine + oral polio vaccine + diphtheria‐tetanus‐acellular pertussis/H. influenzae type b vaccine 1.2. RV1 vaccine + oral polio vaccine placebo + diphtheria‐tetanus‐acellular pertussis inactivated polio‐H. influenzae type b vaccine 1.3. RV1 placebo + diphtheria‐tetanus‐acellular pertussis inactivated polio‐H. influenzae type b vaccine 2. Placebo: 2 doses given 1 month apart; 150 participants (randomized) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity (see Adverse event data collection methods above) 2. Serious adverse events: Infants who experienced a serious adverse event and required hospitalization were admitted at the local district hospital in the study sites or at Ga‐Rankuwa Hospital, the referral hospital for the study site and surrounding areas. Parents were informed on the symptoms of intussusception and were instructed to contact the study physician or clinic if any signs of intussusception became apparent. Any suspected cases were immediately referred to Ga‐Rankuwa Hospital. All serious adverse events were reported to the sponsor and the Ethics committees and followed up until resolved. Parents were contacted 6 months after the second dose of RIX4414 or placebo to obtain information on any serious adverse events since the final study visit. All serious adverse events were reviewed periodically by an independent safety monitoring committee 3. All‐cause death 4. Dropouts 5. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 6. Vaccine virus shedding: vaccine virus in stool sample (review includes data from combined time points) 7. Seroconversion: appearance of anti‐rotavirus IgA antibody (concentration ≥ 20 U/mL) in participants negative for rotavirus before vaccination (review includes data from 289 participants) |
|
| Immunization status | Diphtheria‐tetanus‐acellular pertussis, polio virus, and H. influenzae type b co‐administered in trial | |
| Location | Madibeng District, North West Province, South Africa WHO mortality stratum E |
|
| Notes |
Date: 1st cohort started from 22 November 2001; 2nd cohort from 23 October 2002 to 15 October 2003 Source of funding: The study (e‐Track 444563‐014/NCT00346892) was sponsored by a public‐private partnership RAPID and GSK Biologicals. The RAPID partnership consists of public sector partners (including the WHO, US Agency for International Development, National Institutes of Health, Children’s Vaccine Programme and the Centers for Disease Control), academic institutions (International Centre for Diarrhoeal Disease Research, Bangladesh and Medical University of Southern Africa) and GlaxoSmithKline Biologicals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Very likely Quote: "This study was conducted under the WHO RAPID (Rotavirus Action Partnership for Immunization and Development) programme that facilitates conduct of rotavirus vaccine trials in developing countries, specifically in Africa and Asia, to address specific developing country needs. The RAPID partnership consists of public sector partners (including the WHO, US Agency for International Development, National Institutes of Health, Children’s Vaccine Programme and the Centers for Disease Control), academic institutions (International Centre for Diarrhoeal Disease Research, Bangladesh and Medical University of Southern Africa) and GlaxoSmithKline Biologicals" |
| Allocation concealment (selection bias) | Low risk | Likely to be adequate: treatment masked to investigators Quote: "a unique randomization number identified the vials to be administered to the same subject" and "subjects were administered the vaccine dose with the lowest treatment number available at the study centre" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of oral polio vaccine co‐administration not completely blinded Quote: "OPV and its placebo used in the first cohort were identical in appearance allowing for double blinding while this was not possible in the second cohort due to differences in appearance of OPV and its placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All infants who had received at least one dose of RIX4414 or placebo (total vaccinated cohort) were included in the primary analysis of reactogenicity" |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 31 days after each vaccine dose and 42 days after the last vaccine dose Adverse event data collection methods: all solicited general symptoms (fever, fussiness /irritability, diarrhoea, vomiting, loss of appetite, cough/runny nose) and unsolicited symptoms were recorded during the 15‐day and 31‐day postvaccination follow‐up period after each RIX4414/placebo dose, respectively. The intensity of adverse events was assessed on a 4‐point scale, where '0' indicated no symptoms; '1' mild; '2' moderate; and '3' severe symptoms. Symptoms of Grade 3 intensity were defined as follows: rectal temperature ≥ 39.5 °C (fever), ≥6 looser‐than‐normal stools a day (diarrhoea), ≥ 3 episodes of vomiting a day (vomiting), refusing food intake (loss of appetite), and preventing normal activity (cough/runny nose, fussiness/irritability). Grade 2 symptoms were defined as rectal temperature of 38.5 °C to 39.5 °C (fever), 4 to 5 looser‐than‐normal stools a day (diarrhoea), 2 episodes of vomiting a day (vomiting), eating lesser than usual, which interfered with normal activity (loss of appetite), and interfering with normal activity (cough/runny nose, fussiness /irritability). Occurrence of SAEs was recorded throughout the study period |
|
| Participants |
Number: 100 enrolled; 100 evaluable for safety, 50 for immunogenicity Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: only HIV‐positive infants (confirmed at screening) who were clinically asymptomatic or mildly symptomatic (clinical stages I and II according to WHO classification) and aged 6 to 10 weeks at the time of Dose 1 of RIX4414/placebo were enrolled. There were no restrictions on feeding the infants before or after vaccination Exclusion criteria: infants were not included in the study if they were confirmed HIV‐negative, had received any other investigational drug or vaccine 30 days before receiving the first dose of study vaccine, or had a history of chronic gastroenteritis or previous documented rotavirus gastroenteritis |
|
| Interventions | 1. RV1: 3 doses at least 106.0 CCID50 viral concentration 2. Placebo |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity (see Adverse event data collection methods above) 2. All‐cause diarrhoea; A gastroentiritis episode was defined as diarrhoea (3 or more, looser‐than‐normal stools a day) with or without vomiting. Stool samples were collected on days 0, 7, 15, and 22 of Doses 1 and 2 and on days 0, 7, 15, 30, 45, and 60 of Dose 3 3. Rotavirus diarrhoea; measured from 1 week after second dose up to 2 months' follow‐up 4. Serious adverse events: infants who experienced a serious adverse event and required hospitalization were admitted at the local district hospital in the study sites or at Ga‐Rankuwa Hospital, the referral hospital for the study site and surrounding areas. Parents were informed on the symptoms of intussusception and were instructed to contact the study physician or clinic if any signs of intussusception became apparent. Any suspected cases were immediately referred to Ga‐Rankuwa Hospital. All serious adverse events were reported to the sponsor and the Ethics committees and followed up until resolved. Parents were contacted 6 months after the second dose of RIX4414 or placebo to obtain information on any serious adverse events since the final study visit. All serious adverse events were reviewed periodically by an independent safety monitoring committee 5. All‐cause death 6. Dropouts Outcomes to measure immunogenicity 7. Vaccine take: defined as serum antirotavirus IgA concentration 20 U/mL in post‐vaccination sera or rotavirus vaccine shedding in any stool sample collected from dose 1 to 2 months post‐dose 3 for infants initially negative for rotavirus 8. Seroconversion: appearance of anti‐rotavirus IgA antibody (concentration ≥ 20 U/mL) in participants negative for rotavirus before vaccination (review includes data from 289 participants) |
|
| Immunization status | RV1 vaccine was concomitantly administered with 3 doses of combined diphtheria, tetanus and whole‐cell pertussis, hepatitis B, and H. influenzae type b vaccine (TritanrixHepBHib) and OPV (PolioSabin) | |
| Location | Pretoria, South Africa WHO mortality stratum E |
|
| Notes |
Registration number: ISRCTN11877362/NCT00263666 Source of funding: RAPID trials (USA); WHO (Switzerland) and GlaxoSmithKline Biologicals For infants who developed clinical symptoms of HIV (WHO stages III or IV disease) anytime after enrolment, access to antiretroviral therapy (cotrimoxazole) according to the South African national guidelines was facilitated. Infants who needed treatment were referred to antiretroviral therapy centres by the investigators. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Very likely Quote: "This study was conducted under the WHO RAPID (Rotavirus Action Partnership for Immunization and Development) programme that facilitates conduct of rotavirus vaccine trials in developing countries, specifically in Africa and Asia, to address specific developing country needs. The RAPID partnership consists of public sector partners (including the WHO, US Agency for International Development, National Institutes of Health, Children’s Vaccine Programme and the Centers for Disease Control), academic institutions (International Centre for Diarrhoeal Disease Research, Bangladesh and Medical University of Southern Africa) and GlaxoSmithKline Biologicals". |
| Allocation concealment (selection bias) | Unclear risk | 1:1 randomization, no further details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The placebo was similar to RIX4414 in appearance and contained the same constituents as the active vaccine except that it did not contain the vaccine virus" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All infants who had received at least one dose of RIX4414 or placebo (total vaccinated cohort) were included in the primary analysis of reactogenicity" |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 6 months after last dose of vaccine or placebo Adverse event data collection methods: "The infants were monitored for at least 30 min after each vaccination. Parents received a diary card to record information daily about solicited general symptoms (fever, fussiness/irritability, diarrhoea, vomiting, loss of appetite or cough/runny nose) for 15 days after each dose of RIX4414 or placebo, and any other adverse events occurring until the next study visit. Weekly supervision was done by Health Care Workers from Madibeng District Health Centre. The study physician or his staff questioned the parents on their child’s health and verified the completed diary card at each visit" |
|
| Participants |
Number: 475 participants enrolled; 420 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants, born after a normal gestation period of ≥ 36 weeks; 6 to 10 weeks of age at the time of the first study visit; free of obvious health problems as established by medical history and clinical examination before entering into the study, and mothers had confirmed negative HIV status Exclusion criteria: infants were excluded if they had a clinically significant history of gastrointestinal disease or malformation, had received vaccines or treatment prohibited by the protocol, were immuno‐compromised or were in household contact with an immuno‐suppressed individual or pregnant woman. BCG and OPV vaccinations at birth were allowed according to the local EPI schedule. Infants with acute disease at the time of enrolment or gastroenteritis (diarrhoea) within 7 days before administration of the study vaccine were also excluded. In addition, vaccination was postponed if the infant had fever (≥ 37.5 °C axillary or ≥ 38 °C rectal) or gastroenteritis within the previous 7 days |
|
| Interventions | RV1 1. RIX4414 (RV1): at least 106.0 PFU CCID50 1.1. 2 doses, 1 month apart (at 10 and 14 weeks) plus 1 dose of placebo (at 6 weeks); 190 participants (randomized) 1.2. 3 doses, 1 month apart (at 6, 10, and 14 weeks of age); 189 participants (randomized) 2. Placebo: 3 doses, 1 month apart (at 6, 10, and 14 weeks of age); 96 participants (randomized) Schedule: Visits 1 (Dose 1), 2 (Dose 2), 3 (Dose 3), 4 and 5 correspond to months 0, 1, 2, 4, and 8 to 11 in the schedule |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: for each type of solicited symptom, occurrence of the symptom within the 15‐day (days 0 to 14) solicited follow‐up period after each dose; occurrence of unsolicited adverse events within 43 days (days 0 to 42) after each dose, according to MedDRA classification; measured up to 43 days after vaccine/placebo 2. Serious adverse events: occurrence throughout entire study period; measured up to 6 months 5. All‐cause death: fatal adverse events measured up to 6 months 6. Dropouts: measured up to 6 months 7. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 8. Viral shedding: presence of rotavirus in any stool sample (review includes data from combined time points (these combined data for 2 and 3 doses)) 9. Seroconversion: appearance of anti‐rotavirus IgA antibody concentration ≥ 20 U/mL in participants negative for rotavirus before first dose (review includes data from 1 month after dose 1 and 2 months after dose 3) |
|
| Immunization status | Infants received routine vaccinations according to the local EPI schedule in South Africa. BCG and OPV vaccinations were given at birth; all other routine vaccinations (including diphtheria‐tetanus toxoids‐whole cell pertussis, hepatitis B, H. influenzae type b, and OPV) were administered concomitantly with the study vaccine. All of the infants received a dose of OPV concomitantly with each dose of study vaccine or placebo at all administration times | |
| Location | 7 centres in South Africa WHO mortality stratum E |
|
| Notes | Study known as RIX GSK[013] 2007‐AF in previously published versions of this review Date: 5 September 2003 to 25 October 2004 Source of funding: GlaxoSmithKline Biologicals Study rationale: "The aim of this study was to determine if there was a difference in immune response between the two different schedules that were tested" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Very likely. This study was conducted under the auspices of WHO (eTrack 444563/013/NCT00383903) |
| Allocation concealment (selection bias) | Low risk | Likely to be adequate: treatment masked to investigators Quote: "a randomization number uniquely identified the three vials to be administered to the same subject" and "subjects were administered the vaccine dose with the lowest number available at the study centre" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The placebo was similar to RIX4414 in appearance and contained the same constituents as the active vaccine except that it did not contain the vaccine virus" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All infants who had received at least one dose of RIX4414 or placebo (total vaccinated cohort) were included in the primary analysis of reactogenicity" |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 1 year of age Adverse event data collection methods: not reported |
|
| Participants |
Number: 6568 enrolled; 6349 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: boys or girls between and including 6 and 12 weeks (42 to 90 days) of age at the time of the first vaccination according to the country recommendations for the routine vaccination schedules; free of obvious health problems as established by medical history and clinical examination before entering into the study Exclusion criteria: history of chronic gastrointestinal disease including any uncorrected congenital malformation of the gastrointestinal tract or other serious medical condition as determined by the investigator |
|
| Interventions | RV1 1. RIX4414 (RV1): 106.5 PFU; 2 doses at 1 or 2 months; 4376 participants (randomized) 2. Placebo: 2 doses at 1 or 2 months; 2192 participants (randomized) Schedule: both groups received RV1 vaccine or placebo vaccine orally; first dose at month 0 then second dose at month 1 or month 2 2 cohorts: there were two periods of enrolment, each with its own visit schedule:
|
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Rotavirus diarrhoea: occurrence of severe rotavirus gastroenteritis (requiring hospitalizations or rehydration therapy or both in a medical facility) caused by the wild rotavirus strains during the period starting from 2 weeks after dose 2 until 1 year of age; measured up to 1 year after vaccine/placebo 2. Serious adverse events: occurrence throughout the entire study period; measured up to 1 year after vaccine/placebo 3. Dropouts: measured up to 1 year after vaccine/placebo 4. All‐cause death: fatal serious adverse events; measured up to 1 year after vaccine/placebo 5. Adverse events resulting in discontinuation 6. All‐cause diarrhoea – severe Outcomes to measure immunogenicity 7. Seroconversion: serum rotavirus immunoglobulin A (IgA) antibody concentrations 1 to 2 months after second study vaccine dose (at visit 3) in a subset of 300 participants enrolled in year 2003 ‐ 2004 (review includes data from 1 to 2 months after dose 2) |
|
| Immunization status | All participants received routine infant vaccinations (Hepatitis B vaccine), diphtheria‐tetanus‐acellular pertussis, poliovirus, and H. influenzae type b) according to Expanded Programme of Immunization (EPI) recommendations in each country First 2 doses of routine EPI vaccinations were co‐administered with the RV1 vaccine or placebo doses; the third routine EPI vaccination was administered 1 to 2 months later according to the national plan of immunization in each country |
|
| Location | Multiple sites in 6 countries in Latin America (Argentina, Brazil, Colombia, Dominican Republic, Honduras, and Panama) WHO mortality stratum B |
|
| Notes |
Date: 3 December 2003 to 20 March 2007 Source of funding: GlaxoSmithKline Biologicals Study rationale: "to evaluate the efficacy, immunogenicity and safety of 2 doses of oral live attenuated human rotavirus [RV1] vaccine given concomitantly with routine EPI vaccinations (including DTPw [licensed combined diphtheria and tetanus toxoids and whole‐cell pertussis vaccine], HBV [licensed hepatitis type B vaccine], Hib [licensed H. influenzae type b vaccine] and OPV [oral polio vaccine]) in healthy infants" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parent/guardian and study personnel were not aware of the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96.7% completed the study |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Unclear risk | No details |
| Methods | RCT Length of follow‐up: 8 to 30 days after each dose Adverse event data collection methods: diary cards provided to participants or participants' parents/guardians to record solicited general symptoms on the day of each vaccination and for 7 subsequent days (passive method) |
|
| Participants |
Number: 192 enrolled; 178 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants, born after a normal gestation period of 36 to 42 weeks; 6 to 12 weeks of age at the time of the first dose of the study vaccination course; free of obvious health problems as established by medical history and clinical examination before entering into the study Exclusion criteria: participating in any other clinical trial; acute disease; history of allergic reaction to any vaccine component; history of chronic gastrointestinal disease or other serious medical condition; undergone immunosuppressive therapy; received antibiotics within 14 days preceding the study vaccine administration and during the first 7 days after vaccine administration; any confirmed or suspected immunosuppressive or immunodeficient condition, had received any immunoglobulin therapy or blood products before start or during the trial; abnormal stool pattern or household contact with an immunosuppressed individual or pregnant woman; for the infants, previous confirmed occurrence of rotavirus gastroenteritis |
|
| Interventions | RV1 1. RIX4414 (RV1) 1.1. 104.1 PFU; 32 participants (randomized) 1.2. 104.7 PFU; 64 participants (randomized) * 1.3. 105.8 PFU; 32 participants (randomized) 2. Placebo: 64 participants (randomized) Schedule: 2 doses given 2 months apart *Half of infants receiving 104.7 PFU of RV1 were tested with prior administration of Mylanta as buffer; in the other half vaccine was diluted in a buffer containing calcium carbonate Feeding was not allowed for an hour before and after study vaccine administration |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Adverse events requiring discontinuation: no definition; measured at 31‐day follow‐up after each dose 2. Serious adverse events: no definition; measured at 31‐day follow‐up after each dose 3. Reactogenicity: no definition; measured at 31‐day follow‐up after each dose 4. Dropouts: no definition; measured at 31‐day follow‐up after each dose 5. All‐cause mortality: no definition; measured at 31‐day follow‐up after each dose Outcomes to measure immunogenicity 6. Rotavirus shedding in stool (review includes data from day 7 to 9 after dose 2) 7. Seroconversion: appearance of serum anti‐rotavirus IgA antibody to rotavirus in post‐vaccination sera at a titre of ≥ 20 U/mL in previously uninfected infants; measured in infants only (review includes data from 2 months after dose 1 and 1 month after dose 2) |
|
| Immunization status | Infant routine vaccinations were separated from the study vaccines by 2 weeks | |
| Location | 2 centres in Finland WHO mortality stratum A |
|
| Notes |
Date: 29 May to 18 December 2000 Source of funding: GlaxoSmithKline Biologicals Trial report also includes results for a study in adults and in previously rotavirus‐infected children; neither included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Likely to be adequate: treatment masked to investigators Quote: "A randomisation or subject number identified uniquely the vaccine dose to be administered to the subject", and "subjects were administered the vaccine dose with the lowest number available at the study site" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The study was performed under double‐blind with respect to the groups within each study part" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14/192 participants dropped out of the study, balanced between groups with reasons provided. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Unclear risk | No information |
| Methods | RCT Unbalanced randomization (2:1) Length of follow‐up: 1 and 2 years of follow‐up are reported Adverse event data collection methods: to assess reactogenicity, parents recorded daily on diary cards rectal temperature, any diarrhoea, vomiting, irritability, and loss of appetite for 15 days after each vaccination. Any other symptoms or signs occurring during a 43‐day follow‐up period after each vaccination were recorded as unsolicited symptoms (or signs) (passive method) |
|
| Participants |
Number: 405 enrolled; 372 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants, born after a normal gestation period of 36 to 42 weeks; 6 to 12 weeks of age at the time of the first dose of the study vaccination course; free of obvious health problems as established by medical history and clinical examination before entering into the study Exclusion criteria: premature labour; vaccination was delayed if infant had fever (rectal temperature > 38 °C) or had gastroenteritis within the previous 7 days |
|
| Interventions | RV1 1. RIX4414 (RV1): 104.7 PFU; 2 doses given 2 months apart; 270 participants (randomized) 2. Placebo: 2 doses given 2 months apart; 135 participants (randomized) Feeding was not allowed for 1 hour before administration of the study vaccine |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Rotavirus diarrhoea: occurrence of rotavirus gastroenteritis during the period starting from 2 weeks after dose 2 until the end of the first rotavirus season following vaccination as detected by RT‐PCR in stool samples; occurrence of asymptomatic rotavirus infections during the period starting from 1 month after dose 2 until the end of each rotavirus season following vaccination; G type of the wild rotavirus strain by RT‐PCR; measured at 1 year (first report) and 2 years (second report) 2. Reactogenicity: for each type of solicited symptom, occurrence of the symptom within the 15‐day solicited follow‐up period after each dose; measured at 15 days after each dose 3. Adverse events requiring discontinuation: occurrence of unsolicited symptoms within 42 days after each dose, according to WHO's classification; measured 42 days after each dose 4. Serious adverse events: no definition; measured at all follow‐ups 5. All‐cause diarrhoea: gastroenteritis was defined as diarrhoea (≥ 3 looser‐than‐normal stools within any day) and/or vomiting (≥ 1 episodes of forceful emptying of partially digested stomach contents > 1 hour after feeding within any day); 2 occurrences of gastroenteritis were classified as separate episodes if there were ≥ 5 symptom‐free days between them 6. Severe rotavirus diarrhoea: score of < 7 prospectively defined as mild; score of 7 to 10 as moderate; and a score > 11 as severe 7. Rotavirus diarrhoea resulting in hospitalization 8. All‐cause death 9. Dropouts Outcomes to measure immunogenicity 10. Seroconversion: anti‐rotavirus antibody IgA concentration of ≥ 20 units/mL in infants negative for this before the first dose (review includes data from 1 month after dose 2) |
|
| Immunization status | Infant routine vaccinations (diphtheria tetanus toxoids‐pertussis, H. influenzae type b, and inactivated poliovirus vaccines) were separated from the study vaccines by at least 2 weeks | |
| Location | 6 centres in Finland WHO mortality stratum A |
|
| Notes |
Date: 21 August 2000 to 11 July 2002 Source of funding: GlaxoSmithKline Biologicals Other: GSK 444663/004 (rota‐004annex) reports a second year extension of the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible infants were randomly assigned (2:1 ratio) to 2 study groups according to a computer‐generated randomization list to receive the vaccine or placebo by mouth" |
| Allocation concealment (selection bias) | Low risk | Likely to be adequate: treatment masked to investigators Quote: "A randomisation or subject number identified uniquely the vaccine dose to be administered to each subject", and "subjects were administered the vaccine dose with the lowest number available at the study site" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The placebo had the same constituents and identical appearance as the active vaccine, but did not contain the vaccine virus" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33/405 participants dropped out of the study, balanced between groups with reasons provided |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Unclear risk | No information |
| Methods | RCT Length of follow‐up: 1 and 2 years of follow‐up in all countries, and a third year follow‐up in Finland (GSK109810) Adverse event data collection methods: "active surveillance for gastroenteritis episodes and serious adverse events from the day of the first vaccine or placebo dose (8 September 2004) until the follow‐up visit at the end of the second rotavirus epidemic season (10 August 2006) ... Study staff contacted parents every week" (active method); "During every episode, we asked parents to record in a daily diary card the number of looser than normal stools, axillary or rectal temperature, number of vomiting episodes, any rehydration or other medication administered, and any medical attention (defined as medical personnel contact, advice, or visit; emergency room contact or visit; or admission)" (passive method) |
|
| Participants |
Number: 3994 enrolled; 3848 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants aged 6 to 14 weeks who weighed > 2000 g at birth Exclusion criteria: acute disease at the time of enrolment; history of chronic administration of immunosuppressants since birth; received any vaccines or treatments prohibited by the protocol; or had any disorders or illnesses excluded by the protocol |
|
| Interventions | RV1 1. RIX4414 (RV1): 106.5 PFU; 2 doses given 1 or 2 months apart; 2646 participants (randomized) 2. Placebo: 2 doses given 1 or 2 months apart; 1348 participants (randomized) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause diarrhoea: gastroenteritis defined as diarrhoea characterized by at least 3 looser‐than‐normal stools within a day, with or without vomiting; measured 2 weeks after dose 2 until end of 2 years follow‐up 2. Rotavirus diarrhoea: trialists deemed a gastroenteritis episode to be caused by rotavirus if a rotavirus strain was identified in a stool sample collected during the episode or within 7 days after resolution of symptoms, or before the next episode if fewer than 7 days had fallen between the end of 1 episode and the start of the next, in cases of multiple episodes; measured 2 weeks after dose 2 until end of 2 years follow‐up 3. Severe rotavirus diarrhoea: score < 7 was defined prospectively as mild, score of 7 to 10 as moderate, and a score of ≥ 11 as severe 4. Severe all‐cause diarrhoea: as for severe rotavirus diarrhoea 5. Emergency department visit: no definition 6. All‐cause hospitalization admission: no definition 7. Serious adverse events: no definition 8. Rotavirus diarrhoea resulting in hospitalization 9. Rotavirus diarrhoea requiring medical attention (defined as "medical personnel contact, advice, or visit; emergency room contact or visit; or admission") 10. Reactogenicity Outcomes to measure immunogenicity 11. Seroconversion: appearance of anti‐rotavirus IgA antibody concentration ≥ 20 U/mL in participants seronegative for rotavirus before vaccination (review includes data from 1 to 2 months after dose 2) |
|
| Immunization status | Concomitant vaccines included 7 valent pneumococcal polysaccharide conjugate vaccine (Prevenar) and meningococcal group c conjugate vaccine (Meningitec); Hepatitis B vaccine, diphtheria‐tetanus‐acellular pertussis, polio virus, and H. influenzae type b vaccines were co‐administered | |
| Location | 98 centres in 6 European countries (Czech Republic, Finland, France, Germany, Italy, and Spain) WHO mortality stratum A |
|
| Notes |
Date: 12 February 2007 to 08 August 2007 Source of funding: funded by GlaxoSmithKline Biologicals Other: vaccination postponed if baby either had a temperature of ≥ 37.5 °C (axillary) or of 38.0 °C (rectal) or had gastroenteritis within 7 days before planned vaccination Study aim: "to assess the efficacy and safety of HRV [RV1] vaccine during the 3rd year of age in subjects primed with a 2‐dose schedule in study 102247, with the first dose administered at the age of 6 to 14 weeks" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "GSK Biologicals provided vaccine supplies that were numbered with a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was done by a central Internet randomization system. Infants were randomly allocated in a 2/1 ratio two doses of either RIX4414 or placebo" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Treatment allocation remained concealed from investigators and the parents of participating infants throughout the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data imputed appropriately |
| Selective reporting (reporting bias) | Unclear risk | Data are provided only for rotavirus gastroenteritis and for severe gastroenteritis, not for all gastroenteritis episodes |
| Other bias | Unclear risk | No information |
| Methods | RCT Length of follow‐up: 2 months Adverse event data collection methods: passive. “Parents/guardians of infants were provided diary cards to record solicited general symptoms (loss of appetite, fussiness/irritability, fever, diarrhoea, vomiting, and cough/runny nose) during a 15‐day post‐vaccination follow‐up period. The intensity of each adverse event was assessed using a 4‐point scale where "0" refers to ‘absent’ and "3" refers to ‘severe’" |
|
| Participants |
Number: 250 enrolled and randomized; ATP safety cohort: 240; ATP immunogenicity cohort: 237 Inclusion criteria: healthy infants aged 6 to 10 weeks with a birth weight > 2 kg. Exclusion criteria: any other investigational drug or vaccine 30 days prior to the administration of the first dose of the study vaccine; a history of allergy; rotavirus gastroenteritis; infants with acute illness at the time of enrolment could not receive the vaccine until the condition was resolved |
|
| Interventions | 1. Liquid formulation of RIX4414*/(RV1), 1.5 mL (n=100) 2. Placebo corresponding to liquid vaccine formulation (n=25) 3. Lyophilized formulation RIX4414*/(RV1), 1 mL (n=100) 4. Placebo corresponding to lyophilized vaccine formulation (n=25) * vaccine containing at least 106 median CCID50 of live attenuated RIX4414 human rotavirus strain Schedule: 2 oral doses at month 0 and 1 (minimum time interval between doses: 14 days) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity, occurrence of the symptom within the 15‐day solicited follow‐up period after each dose (collected from GSK report) 2. Serious adverse events, occurrence throughout study period 3. * Rotavirus diarrhoea, stool samples collected during diarrhoea episodes tested for rotavirus strains 4. * All‐cause diarrhoea, up to 1 month post‐dose 2 5. Dropouts: up to 2 months after dose 2 (collected from GSK report) 6. All‐cause death (collected from GSK report) 7. Adverse events resulting in discontinuation (collected from GSK report) Outcomes to measure immunogenicity 8. Seroconversion, antirotavirus IgA antibody concentration > 20 U/mL, 1 month after each dose (collected from GSK report) 9. Rotavirus vaccine virus shedding in stools, reported at peak (day 7 post‐dose 1) * Outcome reported as proportion (P) with 95% CI. Events (n) and totals (N) were estimated by using the value when 2 formulae for the standard error (SE) converged |
|
| Immunization status | Routine childhood vaccinations were allowed according to local practice, but at least 14 days apart from each dose of study vaccine | |
| Location | 5 centres in Finland WHO mortality stratum A |
|
| Notes | Study known as RIX GSK[048] 2007‐EU in previously published versions of this review Date: August to November 2005 Source of funding: GlaxoSmithKline Biologicals Study rationale: the immunogenicity, reactogenicity and safety of the RV1 liquid formulation were compared with lyophilized formulation and placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated Quote: "A standard SAS® program was used for generating the randomization list and a block randomization was used in order to ensure that the balance between the treatment arms were maintained" |
| Allocation concealment (selection bias) | Low risk | Likely to be adequate: treatment masked to investigators Quote: "a unique randomization number identified the vials to be administered to the same subject" and "subjects were administered the vaccine dose with the lowest treatment number available at the study centre" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and key personnel were blinded as far as technically possible Quote: "The study was double blind with respect to each of the vaccine formulation and their respective placebo; however, blinding between the two vaccine formulations was not technically possible because of the difference in appearance of the vaccines". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced across study groups with reasons for dropout/exclusion reported |
| Selective reporting (reporting bias) | Low risk | All pre‐published outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 7 days following each vaccination; 3 to 5 weeks after second vaccination Adverse event data collection methods: unclear |
|
| Participants |
Number: 117 enrolled; 111 evaluable Age range: 3 to 6 months (beginning); 3 to 6 months (end) Inclusion criteria: not specified Exclusion criteria: not specified |
|
| Interventions | RV1 1. RIX4414 (RV1) 1.1. 1 x 105 dose; 41 participants (randomized) 1.2. 1 x 106 dose; 39 participants (randomized) 2. Placebo: 37 participants Schedule: 2 doses given at a 6‐ to 10‐week interval |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity*: symptoms of rotavirus illness, including fever, diarrhoea, and vomiting; measured for 7 days after each dose *Although mentioned in the methods, no results are presented Outcomes to measure immunogenicity 2. Vaccine take: faecal shedding of rotavirus antigen (review includes data from after either dose 1 or 2) 3. Seroconversion: serum rotavirus IgA responses (increases in level of serum rotavirus IgA ≥ 4 fold) (review includes data from after either dose 1 or 2) |
|
| Immunization status | Not specified | |
| Location | Cincinnati and Baltimore, USA WHO mortality stratum A |
|
| Notes |
Date: July to December 1996 Source of funding: "Avant Immunotherapeutics, to which the 89‐12 vaccine candidate was licensed and which sublicensed its product to GlaxoSmithKline (which developed Rotarix from 89‐12)." 89‐12 was the precursor to RV1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "double‐blinded, placebo‐controlled study designed" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐blinded, placebo‐controlled study designed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No impact on intervention effect estimate Quote: "Of the 80 vaccine recipients in this trial, 2 had evidence of natural rotavirus infection before administration of the first dose, determined on the basis of rotavirus IgA in their serum. These, along with the 3 who received only 1 dose of vaccine, were eliminated from further analyses". |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Methods | RCT Length of follow‐up: 31 days after each vaccination (total of 14 weeks) Adverse event data collection methods: "active surveillance for reactogenicity and safety was conducted via daily home visits by study personnel for 8 days after each dose of vaccine or placebo dose and bi‐weekly home visits thereafter until one month after last dose" (active method); "During every episode, parents were asked to record in a daily diary card the number of looser than normal stools, axillary or rectal temperature, number of vomiting episodes, any rehydration or other medication administered, and any medical attention (defined as medical personnel contact, advice, or visit; emergency room contact or visit; or admission)" (passive method); serious adverse events were reviewed periodically by an independent committee |
|
| Participants |
Number: 300 enrolled; 290 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants aged 6 to 7 weeks Exclusion criteria: acute disease at the time of enrolment; malnourished children; history of chronic administration of immunosuppressants since birth; received any vaccines or treatments prohibited by the protocol; or had any disorders or illnesses excluded by the protocol |
|
| Interventions | RV1 1. RIX4414 (RV1) 1.1. 1 x 106.5 dose + OPV; 100 participants (randomized) 1.2. 1 x 106.5 dose; 100 participants (randomized) 2. Placebo: 2.1. Placebo + OPV; 50 participants (randomized) 2.2. Placebo; 50 participants (randomized) Schedule: 2 doses given at a 6‐ to 12‐week interval |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: for each type of solicited symptom, occurrence of the symptom within the 8‐day (Day 0 to 7) solicited follow‐up period after each dose; occurrence of unsolicited adverse events within 31 days (Day 0 to 30) after each dose, according to MedDRA classification; measured up to 31 days after vaccine/placebo 2. Serious adverse events: occurrence throughout entire study period (up to 105 days after vaccine/placebo) 3. Dropouts: measured up to 105 days after vaccine/placebo 4. Rotavirus diarrhoea: presence of rotavirus in gastroenteritis episode stools collected from dose 1 of vaccine/placebo up to 2 months after dose 2; measured up to 105 days after vaccine/placebo 5. All‐cause death 6. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 7. Viral shedding: % participants with rotavirus antigen in stool samples collected at predetermined time points (ATP cohort for immunogenicity, stool analysis subset) (review includes data from combined time points) 8. Seroconversion: appearance of anti‐rotavirus immunoglobulin A antibody concentration ≥ 20 U/mL in participants who were negative for rotavirus before vaccination (review includes data from 1 month after dose 2) |
|
| Immunization status | All children in the study received the standard EPI vaccines starting at 6 weeks of age, including oral polio vaccine for 1 RV1 vaccine arm and 1 placebo arm | |
| Location | Single site in urban Dhaka at Mirpur, Bangladesh WHO mortality stratum D |
|
| Notes |
Date: June 2005 to January 2006 Source of funding: funded by GlaxoSmithKline Biologicals and the Rotavirus Vaccine Program (RVP) at the Program for Appropriate Technology in Health (PATH) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, using a SAS programme |
| Allocation concealment (selection bias) | Low risk | Likely to be adequate: treatment masked to investigators Quote: "A treatment number identified uniquely the vaccine doses to be administered to the same subject", and "subjects were administered the study vaccine dose (HRV vaccine or placebo) with the lowest number available at the study site" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Parent/guardian and study personnel were not aware of the treatment administered |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data imputed appropriately |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Other bias | Unclear risk | No information |
| Methods | Cluster‐RCT, open‐label, cluster‐randomized (by village), parallel‐group field trial with an observed‐only control group Length of follow‐up: 2 years Adverse event data collection methods: (not reported if active of passive)"Serious adverse events among infants vaccinated with HRV were assessed by the principal investigator or trained study physicians and followed to resolution" |
|
| Participants |
Number: 12,318 enrolled; 11,004 evaluable Age range: 6 to 20 weeks Inclusion criteria: 6 to 20 weeks of age, having primary residence at the time of DTP1 receipt in a village selected for introduction of HRV, and having a parent or guardian provide written informed consent Exclusion criteria: history of intussusception, hypersensitivity to the active substance or any component in the vaccine, uncorrected congenital malformation of the gastrointestinal tract, or known or suspected immunodeficiency. Infants with an acute febrile illness were temporarily excluded from HRV vaccination only if that illness was severe enough to warrant postponement of other EPI vaccinations. Infants with current diarrhoea or vomiting or both were not excluded unless the illness met the aforementioned temporary exclusion criterion |
|
| Interventions | 1. RV1; 1‐ml dose of HRV (Rotarix; GSK Biologicals, Rixensart, Belgium) (n=71 villages with 6527 age‐eligible infants) 2. Non‐placebo controlled (observed only controls) (n=71 villages with 5791 age‐eligible infants) Schedule: at 6 and 10 weeks of age |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Severe rotavirus diarrhoea 2. Serious adverse events |
|
| Immunization status | HRV was scheduled to be given along with other standard infant vaccines including OPV at the DTP1 and DTP2 immunization visits, recommended in Bangladesh to occur at 6 and 10 weeks of age. | |
| Location | 142 study sites (cluster‐randomized villages), Bangladesh WHO mortality stratum D |
|
| Notes |
Date: September 2008 to March 2011 Source of funding: GAVI and PATH Study rationale: The primary objective of the trial was to estimate the overall effectiveness of an HRV vaccination programme in reducing the risk of presenting with acute rotavirus diarrhoea to a treatment facility among all children who had been age‐eligible for vaccination with HRV during the vaccination programme |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Villages were randomized in a 1:1 ratio for introduction of HRV or not. Prior to study initiation, PATH computer‐generated the allocation sequences using block randomization with block sizes of 12 |
| Allocation concealment (selection bias) | Unclear risk | The generated allocation sequences were securely transferred to the principal investigator, who distributed the sequences to the field supervisors who oversaw HRV vaccinations |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was conducted open‐label without masking, and field staff conducting the vaccinations were unblinded. Medical staff collecting clinical data on diarrhoeal presentations and laboratory personnel conducting assays on stools were not informed of previous HRV receipt of participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcome data available for 11,004/12,318 enrolled participants |
| Selective reporting (reporting bias) | High risk | Online registration of trial ( NCT00737503) indicates all‐cause diarrhoea as an outcome but results were not reported for this outcome in the study report |
| Other bias | Unclear risk | Cluster‐randomized trial. |
| Methods | RCT Length of follow‐up: up to 43 days for safety outcomes, and up to 21 months for efficacy outcomes Adverse event data collection methods: "Study physicians reported and documented all serious adverse events occurring within 14 days of any dose and deaths or vaccine‐related serious adverse events occurring at any time during the study" A subset had active surveillance: "A subset of 300 participants enrolled in Kenya was followed up for 42 days for all adverse events, including vomiting, diarrhoea, and high temperature. Home visits were attempted on days 3, 5, 7, 14, 21, and 42 after all vaccinations". |
|
| Participants |
Number: 5560 enrolled; 5468 randomized, 5225 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants aged 4 to 12 weeks; "no symptoms of active gastrointestinal disease and could be adequately followed up for safety by home visit or telephone contact (1 week and 2 weeks after any dose of vaccine or placebo)"; breast‐feeding was not restricted; no enrolment restrictions based on HIV status ‐ infants in Kenya were offered routine HIV testing, and a subset were followed up for safety All children exposed to or infected with HIV were referred for appropriate HIV care and treatment; voluntary counselling and testing were also offered to mothers of infants exposed to HIV Exclusion criteria: see above Special group: HIV‐infected participants |
|
| Interventions | RV5 1. WC3 (RV5): 2 mL (every dose had an estimated potency of 107 infectious units per reassortant rotavirus); 3 doses given 4 weeks apart; 2733 participants (randomized) 2. Placebo: 2 mL; 3 doses given 4 weeks apart; 2735 participants (randomized) Schedule: 3 doses given at a 4‐week interval |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events (including intussusception) 2. Death due to serious adverse events 3. Rotavirus diarrhoea: case definition for rotavirus gastroenteritis required participants to meet both of the following criteria: (1) ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, and (2) rotavirus detected by enzyme immunoassay (EIA) in a stool specimen taken within 14 days after the onset of symptoms 4. Severe rotavirus diarrhoea: an established clinical scoring system based on the intensity and duration of fever, vomiting, diarrhoea, and changes in behaviour used to categorize episodes of rotavirus gastroenteritis on a 20‐point severity scale; scores > 11 were considered to indicate severe disease; measured up to 2 years follow‐up 5. All‐cause diarrhoea 6. All‐cause diarrhoea – severe 7. Reactogenicity*: symptoms of rotavirus illness, including fever, diarrhoea, and vomiting; measured for 7 days after each dose (review includes data from for the end of follow‐up) *Data on fever and vomiting are provided only on figure 2 and data could not be extracted reliably Outcomes to measure immunogenicity 8. Seroconversion: serum rotavirus IgA responses (increases in level of serum rotavirus IgA ≥ 4‐fold) (review includes data from after dose 2) |
|
| Immunization status | All children in the study received the standard EPI vaccines (including oral poliovirus vaccine) starting at 6 weeks of age | |
| Location | Sites in rural Kassena‐Nankana district (Ghana), rural Karemo division, Siaya district (Kenya), and urban area of Bamako (Mali) WHO mortality strata D, E |
|
| Notes | This trial was conducted in Ghana, Kenya and Mali; data reported separately by country can be found under RV5 Armah 2010‐GHA; RV5 Armah 2010‐KEN and RV5 Armah 2010‐MLI. Date: 28 April 2007 to 31 March 2009 Source of funding: funded by PATH (GAVI Alliance grant) and Merck Registration number: NCT00362648 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Unique allocation numbers were designated at Merck as pentavalent rotavirus vaccine or placebo with computer generated block randomization, with block sizes of six" |
| Allocation concealment (selection bias) | Low risk | Quote: "Vaccine and placebo packages were then labelled with allocation numbers and provided to sites in identical presentations. Sites were instructed to assign allocation numbers to participants in sequential order as they were enrolled" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff Quote: "Participants were enrolled by study staff, who remained masked to treatment assignment throughout the trial" Researchers Quote: "The statistician from Merck who analysed the data and the Merck and PATH protocol teams were masked to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 43 days for safety outcomes, and up to 21 months for efficacy outcomes Adverse event data collection methods: "Study physicians reported and documented all serious adverse events occurring within 14 days of any dose and deaths or vaccine‐related serious adverse events occurring at any time during the study". |
|
| Participants |
Number: 2200 randomized Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants aged 4 to 12 weeks; "no symptoms of active gastrointestinal disease and could be adequately followed up for safety by home visit or telephone contact (1 week and 2 weeks after any dose of vaccine or placebo)"; breast‐feeding was not restricted; no enrolment restrictions based on HIV status All children exposed to or infected with HIV were referred for appropriate HIV care and treatment; voluntary counselling and testing were also offered to mothers of infants exposed to HIV Exclusion criteria: see above |
|
| Interventions | RV5 1. WC3 (RV5): 2 mL (every dose had an estimated potency of 107 infectious units per reassortant rotavirus); 3 doses given 4 weeks apart; 1098 participants (randomized) 2. Placebo: 2 mL; 3 doses given 4 weeks apart; 1102 participants (randomized) Schedule: 3 doses given at a 4‐week interval |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events (including intussusception) 2. Death due to serious adverse events 3. Rotavirus diarrhoea: case definition for rotavirus gastroenteritis required participants to meet both of the following criteria: (1) ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, and (2) rotavirus detected by EIA in a stool specimen taken within 14 days after the onset of symptoms 4. Severe rotavirus diarrhoea: an established clinical scoring system based on the intensity and duration of fever, vomiting, diarrhoea, and changes in behaviour used to categorize episodes of rotavirus gastroenteritis on a 20‐point severity scale; scores > 11 were considered to indicate severe disease; measured up to 2 years follow‐up 5. All‐cause diarrhoea 6. All‐cause diarrhoea – severe 7. Reactogenicity*: symptoms of rotavirus illness, including fever, diarrhoea, and vomiting; measured for 7 days after each dose (review includes data from for the end of follow‐up) *Data on fever and vomiting are provided only on figure 2 and data could not be extracted reliably Outcomes to measure immunogenicity 8. Seroconversion: serum rotavirus IgA responses (increases in level of serum rotavirus IgA ≥ 4‐fold) (review includes data from after dose 2) |
|
| Immunization status | All children in the study received the standard EPI vaccines (including oral poliovirus vaccine) starting at 6 weeks of age | |
| Location | Sites in rural Kassena‐Nankana district, Ghana WHO mortality stratum D |
|
| Notes | This trial was conducted in Ghana, Kenya and Mali; this part presents data for the Ghana cohort. Data reported separately for the other countries can be found under RV5 Armah 2010‐KEN and RV5 Armah 2010‐MLI data reported for all countries under RV5 Armah 2010‐AF Date: 28 April 2007 to 31 March 2009 Source of funding: funded by PATH (GAVI Alliance grant) and Merck Registration number: NCT00362648 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Unique allocation numbers were designated at Merck as pentavalent rotavirus vaccine or placebo with computer generated block randomization, with block sizes of six" |
| Allocation concealment (selection bias) | Low risk | Quote: "Vaccine and placebo packages were then labelled with allocation numbers and provided to sites in identical presentations. Sites were instructed to assign allocation numbers to participants in sequential order as they were enrolled" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff Quote: "Participants were enrolled by study staff, who remained masked to treatment assignment throughout the trial" Researchers Quote: "The statistician from Merck who analysed the data and the Merck and PATH protocol teams were masked to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 43 days for safety outcomes, and up to 21 months for efficacy outcomes Adverse event data collection methods: "Study physicians reported and documented all serious adverse events occurring within 14 days of any dose and deaths or vaccine‐related serious adverse events occurring at any time during the study" A subset had active surveillance: "A subset of 300 participants enrolled in Kenya was followed up for 42 days for all adverse events, including vomiting, diarrhoea, and high temperature. Home visits were attempted on days 3, 5, 7, 14, 21, and 42 after all vaccinations". |
|
| Participants |
Number: 1322 enrolled; 1308 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants aged 4 to 12 weeks; "no symptoms of active gastrointestinal disease and could be adequately followed up for safety by home visit or telephone contact (1 week and 2 weeks after any dose of vaccine or placebo)"; breast‐feeding was not restricted; no enrolment restrictions based on HIV status ‐ infants in Kenya were offered routine HIV testing, and a subset were followed up for safety All children exposed to or infected with HIV were referred for appropriate HIV care and treatment; voluntary counselling and testing were also offered to mothers of infants exposed to HIV Exclusion criteria: see above Special group: HIV‐infected participants |
|
| Interventions | RV5 1. WC3 (RV5): 2 mL (every dose had an estimated potency of 107 infectious units per reassortant rotavirus); 3 doses given 4 weeks apart; 656 participants (received at least one dose) 2. Placebo: 2 mL; 3 doses given 4 weeks apart; 652 participants (received at least one dose) Schedule: 3 doses given at a 4 week interval |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events (including intussusception) 2. Death due to serious adverse events 3. Rotavirus diarrhoea: case definition for rotavirus gastroenteritis required participants to meet both of the following criteria: (1) ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, and (2) rotavirus detected by EIA in a stool specimen taken within 14 days after the onset of symptoms 4. Severe rotavirus diarrhoea: an established clinical scoring system based on the intensity and duration of fever, vomiting, diarrhoea, and changes in behaviour used to categorize episodes of rotavirus gastroenteritis on a 20‐point severity scale; scores > 11 were considered to indicate severe disease; measured up to 2 years follow‐up 5. All‐cause diarrhoea 6. All‐cause diarrhoea – severe 7. Reactogenicity*: symptoms of rotavirus illness, including fever, diarrhoea, and vomiting; measured for 7 days after each dose (review includes data from for the end of follow‐up) *Data on fever and vomiting are provided only on figure 2 and data could not be extracted reliably Outcomes to measure immunogenicity 8. Seroconversion: serum rotavirus IgA responses (increases in level of serum rotavirus IgA ≥ 4‐fold) (review includes data from after dose 2) |
|
| Immunization status | All children in the study received the standard EPI vaccines (including oral poliovirus vaccine) starting at 6 weeks of age | |
| Location | Sites in rural Karemo division, Siaya district, Kenya WHO mortality stratum E |
|
| Notes | This trial was conducted in Ghana, Kenya and Mali; this part presents data for the Kenya cohort. Data reported separately for the other countries can be found under RV5 Armah 2010‐GHA and RV5 Armah 2010‐MLI, and for all countries under RV5 Armah 2010‐AF Date: 28 April 2007 to 31 March 2009 Source of funding: funded by PATH (GAVI Alliance grant) and Merck Registration number: NCT00362648 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Unique allocation numbers were designated at Merck as pentavalent rotavirus vaccine or placebo with computer generated block randomization, with block sizes of six" |
| Allocation concealment (selection bias) | Low risk | Quote: "Vaccine and placebo packages were then labelled with allocation numbers and provided to sites in identical presentations. Sites were instructed to assign allocation numbers to participants in sequential order as they were enrolled" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff Quote: "Participants were enrolled by study staff, who remained masked to treatment assignment throughout the trial" Researchers Quote: "The statistician from Merck who analysed the data and the Merck and PATH protocol teams were masked to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 43 days for safety outcomes, and up to 21 months for efficacy outcomes Adverse event data collection methods: "Study physicians reported and documented all serious adverse events occurring within 14 days of any dose and deaths or vaccine‐related serious adverse events occurring at any time during the study". |
|
| Participants |
Number: 2011 enrolled; 1960 randomized and evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants aged 4 to 12 weeks; "no symptoms of active gastrointestinal disease and could be adequately followed up for safety by home visit or telephone contact (1 week and 2 weeks after any dose of vaccine or placebo)"; breast‐feeding was not restricted; no enrolment restrictions based on HIV status. All children exposed to or infected with HIV were referred for appropriate HIV care and treatment; voluntary counselling and testing were also offered to mothers of infants exposed to HIV Exclusion criteria: see above |
|
| Interventions | RV5 1. WC3 (RV5): 2 mL (every dose had an estimated potency of 107 infectious units per reassortant rotavirus); 3 doses given 4 weeks apart; 979 participants (randomized) 2. Placebo: 2 mL; 3 doses given 4 weeks apart; 981 participants (randomized) Schedule: 3 doses given at a 4 week interval |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events (including intussusception) 2. Death due to serious adverse events 3. Rotavirus diarrhoea: case definition for rotavirus gastroenteritis required participants to meet both of the following criteria: (1) ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, and (2) rotavirus detected by EIA in a stool specimen taken within 14 days after the onset of symptoms 4. Severe rotavirus diarrhoea: an established clinical scoring system based on the intensity and duration of fever, vomiting, diarrhoea, and changes in behaviour used to categorize episodes of rotavirus gastroenteritis on a 20‐point severity scale; scores > 11 were considered to indicate severe disease; measured up to 2 years follow‐up 5. All‐cause diarrhoea 6. All‐cause diarrhoea – severe 7. Reactogenicity *: symptoms of rotavirus illness, including fever, diarrhoea, and vomiting; measured for 7 days after each dose (review includes data from for the end of follow‐up) * Data on fever and vomiting are provided only on figure 2 and data could not be extracted reliably Outcomes to measure immunogenicity 8. Seroconversion: serum rotavirus IgA responses (increases in level of serum rotavirus IgA ≥ 4‐fold) (review includes data from after dose 2) |
|
| Immunization status | All children in the study received the standard EPI vaccines (including oral poliovirus vaccine) starting at 6 weeks of age | |
| Location | Sites in urban area of Bamako, Mali WHO mortality stratum D |
|
| Notes | This trial was conducted in Ghana, Kenya and Mali; this part presents data for the Mali cohort. Date: 28 April 2007 to 31 March 2009 Source of funding: funded by PATH (GAVI Alliance grant) and Merck Registration number: NCT00362648 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Unique allocation numbers were designated at Merck as pentavalent rotavirus vaccine or placebo with computer generated block randomization, with block sizes of six" |
| Allocation concealment (selection bias) | Low risk | Quote: "Vaccine and placebo packages were then labelled with allocation numbers and provided to sites in identical presentations. Sites were instructed to assign allocation numbers to participants in sequential order as they were enrolled" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff Quote: "Participants were enrolled by study staff, who remained masked to treatment assignment throughout the trial" Researchers Quote: "The statistician from Merck who analysed the data and the Merck and PATH protocol teams were masked to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 42 days for safety/immunogenicity; up to 1 year for efficacy Adverse event data collection methods: parents or guardians contacted by the study site on day 7, day 14, and day 42 after each vaccination and asked about serious adverse events (active method); parents or guardians were provided diary cards and were instructed to record daily temperatures for the infant for 7 days after each vaccination (passive method) |
|
| Participants |
Number: 1312 enrolled; 1200 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants, 6 through 12 weeks of age, who had no known history of congenital abdominal disorders, intussusception, or abdominal surgery; no known or suspected impairment of immunological function; no known hypersensitivity to any component of the rotavirus vaccine; no prior receipt of any rotavirus vaccine; no fever, with a rectal temperature ≥ 38.1 °C (≥ 100.5 °F) at the time of immunization; no history of known prior rotavirus disease, chronic diarrhoea, or failure to thrive; no clinical evidence of active gastrointestinal illness; no receipt of intramuscular, oral, or intravenous corticosteroid treatment within the 2 weeks before vaccination; did not reside in a household with an immunocompromised person; no prior receipt of a blood transfusion or blood products, including immunoglobulins; no receipt of oral poliovirus vaccine during the course of the study or within 42 days before first dose of vaccine/placebo; any infant who could not be adequately followed for safety by telephone or home visit; and no condition, which, in the opinion of the investigator, may have interfered with the evaluation of the study objectives Exclusion criteria: see above |
|
| Interventions | RV5 1. WC3 (RV5): 1.1 x 107 PFU; 651 participants (randomized) 2. Placebo: 661 participants (randomized) Schedule: 3 doses given 4 to 10 weeks apart |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events: potential cases of intussusception were adjudicated by an independent blinded committee; all study personnel remained blinded to the treatment arm and adjudication results of the potential intussusception cases; data on cases of intussusception, deaths, or other serious adverse events determined to be vaccine‐related by the investigator were collected throughout the trial; measured up to 42 days, and up to 1 year (for vaccine‐related serious adverse events) 2. Reactogenicity: no definition; measured up to 42 days 3. Dropouts: no definition: measured up to 1 year 4. Rotavirus diarrhoea: case of rotavirus gastroenteritis defined as meeting both of the following criteria: (a) > 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both; and (b) rotavirus antigen detection by EIA in the stool sample. Primary analysis of efficacy included only cases caused by naturally‐occurring rotavirus of serotypes G1, G2, G3, or G4 as confirmed by RT‐PCR occurring at least 14 days after the third dose 5. Severe rotavirus diarrhoea: each episode graded on a 24‐point scale, where a score < 8 designated as mild, > 8 as moderate‐and‐severe, and > 16 as a severe disease 6. All‐cause death 7. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 8. Seroconversion: pre‐vaccination and post‐vaccination sera analyzed for serotype‐specific rotavirus neutralizing antibody and for serum anti‐rotavirus immunoglobulin A (IgA) (review includes data from after dose 3) |
|
| Immunization status | Use of oral poliovirus vaccine during the course of the study or within 42 days before first dose of vaccine/placebo was an exclusion criterion; administration of other vaccines permitted | |
| Location | 30 sites; 27 in USA, and 3 in Finland WHO mortality stratum A |
|
| Notes |
Date: 24 September 2002 (first participant in) to 11 February 2004 Source of funding: Merck & Co., Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled infants were randomly assigned 1:1 by using computer‐generated allocation schedules to receive either vaccine or visibly indistinguishable placebo in a sucrose citrate buffer administered orally as three 2‐mL doses 4 to 10 weeks apart" |
| Allocation concealment (selection bias) | Low risk | Sequential identical containers (see quote above) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"This randomized, clinical trial blinded to investigator, parent or guardian, and sponsor" "The placebo was identical to the vaccine except that it did not contain the rotavirus reassortants or trace trypsin" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | High risk | Key expected outcome (episodes of gastroenteritis) not included |
| Other bias | Unclear risk | Relevant information needed for assessment not provided |
| Methods | RCT Length of follow‐up: up to 42 days after last dose Adverse event data collection methods: see outcome measures; passive method used for reactogenicity, and active method used for serious adverse events |
|
| Participants |
Number: 403 enrolled; 403 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants, aged 6 to 12 weeks; mothers negative for hepatitis B surface antigen; no known history of congenital abdominal disorders; intussusception, or abdominal surgery; no known or suspected impairment of immunological function; no history of seizure with or without fever; no known hypersensitivity to any component of rotavirus vaccine or INFANRIX hexa; no prior receipt of any rotavirus, DTaP, DTP, H. influenzae type b, Hepatitis B, injectable poliovirus vaccine, or oral polio vaccine during the course of the study, within 42 days before first dose of RV5 or before final blood draw (42 days after dose 3); no fever, with a rectal temperature < 38.1 °C (< 100.5 °F) at the time of immunization; no history of known rotavirus disease, chronic diarrhoea, or failure to thrive; no clinical evidence of active gastrointestinal illness; no prior receipt of intramuscular, oral, or intravenous corticosteroids treatment within 2 weeks before vaccination; did not reside in a household with an immunocompromised person; no receipt of a blood transfusion or blood products, including immunoglobulin; did not participate in another clinical study within 42 days before or during current study; could be adequately followed for safety Exclusion criteria: as above |
|
| Interventions | RV5 1. WC3 (RV5) plus Infanrix hexa: RV5 (2 mL; 3 doses given 4 to 6 weeks apart); 201 participants (randomized) 2. Placebo plus Infanrix hexa: placebo (2 mL; 3 doses given 4 to 6 weeks apart); 202 participants (randomized) Infanrix hexa: comes in 2 parts; first part is a white, milky liquid (0.5 mL) in a pre‐filled syringe that consists of the combined diphtheria, tetanus, pertussis, hepatitis b, and inactivated poliovirus vaccine; second part is the H. influenzae type b vaccine and is a white pellet in a separate glass vial; both parts mixed together before being injected intramuscularly |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: in both groups, at each study visit, parents/legal guardians received Vaccination Report Cards (VRCs) which they completed for 7 days with information on fever, diarrhoea, and vomiting starting from the day of office visit and returned completed VRCs to the study site at the next visit 2. Serious adverse events: parents/legal guardians of all participants were contacted by telephone or home visit on approximately day 14 after each office visit in either group for safety follow‐up and asked about all serious adverse experiences; measured up to 42 days 3. All‐cause death 4. Adverse events resulting in discontinuation Outcomes to measure immunogenicity None specific to review |
|
| Immunization status | Hepatitis B vaccine, diphtheria‐tetanus‐acellular pertussis, polio virus, and H. influenzae type b co‐administered | |
| Location | 26 study sites in Austria, Belgium, and Germany WHO mortality stratum A |
|
| Notes |
Date: 22 February 2006 to 13 November 2006 Source of funding: Merck & Co., Inc. Other: only data about serious adverse events and adverse events leading to discontinuation are provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomized 1:1 to receive hexavalent vaccine concomitantly with either RV5 (RotaTeq) or placebo (Merck 2012) |
| Allocation concealment (selection bias) | Low risk | Allocation numbers were generated for participants, investigators, adults, and parents/guardians of children were blinded throughout trial (Merck 2012) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | RV5 was visibly indistinguishable from placebo, investigators, parents/guardians and study personnel (internal and external) were blinded throughout trial (Merck 2012) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In both treatment groups (RV5+Hexavalent and Placebo+Hexavalent), ˜84% of the infants reported 1 or more adverse events within 14 days after vaccination. One subject discontinued in the concomitant‐use group because of abdominal pain (considered non‐serious)" (Merck 2012) |
| Selective reporting (reporting bias) | High risk | Not all prespecified outcomes reported |
| Other bias | Unclear risk | No details |
| Methods | RCT Length of follow‐up: up to 1 year Adverse event data collection methods: parents/guardians recorded temperatures 4 to 6 hours after each dose and then daily thereafter for 7 days and the number of episodes of vomiting and diarrhoea daily for 7 days (passive method); also recorded any behavioural or systemic adverse experience on a VRC and was asked to report any serious adverse experience immediately to the study site; telephone call made to each parent/guardian 14 days after each dose to verify that no serious adverse experiences had occurred (active) |
|
| Participants |
Number: 731 enrolled; 681 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Special groups: breast‐fed; infants in the vaccine control group (Group 1) received the reassortants as administered in previous studies within 30 minutes of feeding Enfamil formula (30 ml) or Mylanta Double Strength (0.5 ml/kg). Infants in a corresponding placebo group (Group 2) were pre‐fed as in Group 1 Inclusion criteria: healthy infants 2 to 4 months of age Exclusion criteria: known hypersensitivity to any component of the rotavirus vaccine; known or suspected immunologic impairment; prior administration of any rotavirus vaccine; fever at the time of vaccination; history of chronic diarrhoea; failure to thrive or gastrointestinal illness; recent receipt of oral polio vaccine or blood products; residence in the household with an immunocompromised person; and failure to fast for 1 hour before vaccination |
|
| Interventions | RV5 1. WC3 (RV5): 107 PFU; 581 participants (randomized) 2. Placebo: 150 participants (randomized) Schedule: 3 doses given 42 to 56 days apart |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: parents/guardians recorded temperatures 4 to 6 hours after each dose and then daily thereafter for 7 days and the number of episodes of vomiting and diarrhoea daily for 7 days; fever defined as 38.1 °C (rectal) or 37.5 °C (oral, otic, or axillary); measured up to 42 days after vaccine/placebo 2. Rotavirus diarrhoea: case of rotavirus gastroenteritis defined as ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, occurring at least 14 days after the third dose of vaccine/placebo and detection by ELISA of wild‐type G1 or G2 rotavirus or both in a stool specimen collected within 14 days of symptom onset; measured up to 1 year 3. Severe rotavirus diarrhoea: clinical scoring system used to assess severity of illness for each episode of rotavirus acute gastroenteritis; measured up to 1 year 4. Serious adverse events: defined as: death; life‐threatening events; experiences that resulted in hospitalization, persistent disability, or that prolonged a hospitalization; and other important medical events. Data on deaths or any serious adverse experiences judged to be vaccine‐related were collected for the duration of the study; measured up to 1 year 5. Intussusception, data from correspondence with Merck (Merck 2012) 6. Dropouts Outcomes to measure immunogenicity 7. Viral shedding: at least a 3‐fold rise in serum‐neutralizing antibody to total stool IgA (review includes data from after dose 3) 8. Seroconversion: at least a 3‐fold rise in serum‐neutralizing antibody to serum IgA (review includes data from after dose 3) |
|
| Immunization status | Children that had recently received oral polio vaccine were excluded from the study | |
| Location | 19 centres in the USA WHO mortality stratum A |
|
| Notes |
Date: September 1997 through September 1998 Source of funding: Merck & Co., Inc. Other: active surveillance for cases of rotavirus gastroenteritis at each study site began when the local laboratory confirmed at least 3 cases of rotavirus gastroenteritis or on 31 January 1998, whichever came first |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details Quote: "Children who met all eligibility criteria were randomized to one of eight treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and key personnel Quote: "Parents of participating infants and study personnel were blinded to receipt of vaccine/placebo but not to the volume administered or to the prefeeding requirement" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions |
| Selective reporting (reporting bias) | High risk | Not all prespecified outcomes reported Quote: "Because there were relatively few confirmed cases of RV [rotavirus] caused by serotypes G1 and G2, the evidence is insufficient to declare that the efficacy of any buffered formulation is > 0.0%" |
| Other bias | High risk | Poor reporting of efficacy data |
| Methods | RCT Length of follow‐up: up to 1 year (season) Adverse event data collection methods: episodes of fever (subjective assessment of fever), vomiting, diarrhoea, behavioural changes, and any other adverse experiences during the 14 days after each dose were also reported on the diary card (passive method); parents were asked to report any serious adverse experience immediately to the study site (passive method); telephone call made to each participant 14 days after each vaccination to ask about serious adverse experiences (active method) |
|
| Participants |
Number: 439 enrolled; 416 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants approximately 2 to 6 months of age were enrolled and followed for episodes of acute gastroenteritis Exclusion criteria: known hypersensitivity to any component of the rotavirus vaccine; known or suspected immunologic impairment; prior administration of any rotavirus vaccine; fever at time of vaccination (> 38.1 °C rectal); history of chronic diarrhoea or failure to thrive; clinical evidence of gastrointestinal illness; receipt of any other vaccines within 14 days; immunocompromised resident in the home; or any condition, which, in the opinion of the investigator, might interfere with the evaluation of the study objectives |
|
| Interventions | RV5 1. WC3 (RV5): 107 PFU; 3 doses at 6 to 8 week intervals; 218 participants (randomized) 2. Placebo: 3 doses at 6 to 8 week intervals; 221 participants (randomized) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Rotavirus diarrhoea: case of rotavirus disease in a study participant defined as ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, occurring at least 14 days after the third dose of vaccine/placebo and identification of rotavirus in a stool specimen obtained within 14 days of symptom onset; measured up to 1 year 2. Severe rotavirus diarrhoea: based on a clinical scoring system for evaluating the severity of an episode of infant acute gastroenteritis (0 to 24 points) they consider severe above 16 points; measured up to 1 year 3. Dropouts: measured up to 1 year 4. Serious adverse events: serious adverse experiences included death, life‐threatening events, and experiences that resulted in hospitalization, persistent disability, or that prolonged a hospitalization; deaths or any serious adverse experiences judged to be vaccine‐related were recorded for the duration of the study; measured up to 1 year, including intussusception (data from correspondence with Merck, Merck 2012). 5. Reactogenicity: all participants were followed for clinical adverse experiences for 14 days after each vaccination 6. Adverse events requiring discontinuation; measured up to 1 year Outcomes to measure immunogenicity 7. Viral shedding: stools were collected to evaluate vaccine strain shedding among subsets of infants at different time periods after each dose (review includes data from after dose 3) 8. Seroconversion: pre‐vaccination and post‐vaccination sera assayed for anti‐rotavirus immunoglobulin A (IgA) and anti‐rotavirus IgG (units/mL, based on pooled human serum standards); ≥ 3‐fold rise in titre from baseline to after dose 3 (review includes data from after dose 3) |
|
| Immunization status | Receipt of any other vaccines within 14 days was not allowed. | |
| Location | 10 study sites in the USA WHO mortality stratum A |
|
| Notes |
Date: August 1993 to June 1994 Source of funding: Merck & Co., Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Infants who met all eligibility criteria were randomly assigned in a 1:1 ratio". No further details. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The vials of vaccine and placebo were visibly indistinguishable" Quote: "The placebo was identical to the vaccine except that it did not contain the rotavirus reassortants". Investigators, study personnel (internal and external), and parents/guardians were blinded throughout trial. (Merck 2012) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions |
| Selective reporting (reporting bias) | High risk | ≥ 1 outcome of interest reported incompletely Quote: "Only wild‐type (ie, non‐vaccine related) rotavirus cases were considered for the primary case definition" |
| Other bias | Unclear risk | Not enough detail to make a judgement |
| Methods | RCT Length of follow‐up: 28 days after 3rd dose Adverse event data collection methods: Active and passive: "participants were observed for 30 min post vaccination for immediate adverse events at the study site. Subsequently, the subject’s parents/guardians were given a thermometer, a Symptom Diary (SD) covering Days 0–6 and a second SD covering Days 7–27 for safety follow up following each of the three doses. They were instructed to observe and record their child’s axillary temperature twice daily as well as any AEs up to 7 days after each dose in the first SD, and from day 7 to day 27 in the second SD. Parents/guardians were instructed to bring the study infants to the study clinic on Day 7 and Day 28 after each administration of the BRV‐TV vaccine/RotaTeq/Placebo as an outpatient and whenever any symptoms developed.The diary card contained list of solicited events and blank spaces to capture any unsolicited events" |
|
| Participants |
Number: 100 enrolled; 100 evaluated Age range: 6 ‐ 8 weeks of age at time of enrolment Inclusion criteria: Healthy infants, of either sex, 6 ‐ 8 weeks of age at time of enrolment; born after a gestational period of 36 ‐ 42 weeks with birth weight > 2 kg Exclusion criteria: History of congenital abdominal disorders, intussusception, or abdominal surgery; infants exhibiting signs of severe malnutrition; known or suspected impairment of immunological function in participant or immediate family; developmental delay or neurological disorder; known hypersensitivity to any component of the rotavirus vaccine; fever; history of known rotavirus disease, chronic diarrhoea, or failure to thrive; any conditions which, in the opinion of the investigator, might interfere with the evaluation of the study objectives |
|
| Interventions | 1. RV5 (2.0 mL) 2. BRV‐TV (2.0 mL), antigen concentration (105.0 FFU per serotype per dose) 3. BRV‐TV (2.0 mL), antigen concentration (105.8 FFU per serotype per dose) 4. BRV‐TV (2.0 mL), antigen concentration (106.4 FFU per serotype per dose) 5. Placebo (2.0 mL) Schedule: 3 doses of vaccines/comparator/placebo were administered at 6 – 8, 10 – 12 and 14 – 16 weeks of age |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All serious adverse events 2. Reactogenicity: fever, diarrhoea, vomiting 3. Dropouts before the end of the trial Outcomes to measure immunogenicity 4. Rotavirus vaccine shedding |
|
| Immunization status | Infants concomitantly received a combined Diphtheria, Tetanus, Whole‐cell pertussis, Hepatitis B and Haemophilus influenzae type b (DTPwHB‐Hib) pentavalent vaccine and Trivalent Oral Polio Vaccine | |
| Location | 2 sites, India WHO mortality stratum D |
|
| Notes | Alongside the infant cohort, the study also included an additional cohort of healthy adult volunteers Date: July 2012 ‐ not reported Source of funding: Shantha Biotechnics Limited Study rationale: study was carried out with the long‐term aim to produce a locally licensed vaccine which is equally safe and immunogenic as compared to available licensed vaccines |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization. |
| Allocation concealment (selection bias) | Low risk | Likely to be adequate Quote: "Pre‐numbered or coded identical containers" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blind, participant and outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data presented for all 100 participants |
| Selective reporting (reporting bias) | Low risk | No indication of selective outcome reporting |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 25 months Adverse event data collection methods: any death, vaccine‐related serious adverse events and intussusception were collected during the study period; parents/guardians asked to record adverse events on a standardized VRC during 14 days after each vaccination |
|
| Participants |
Number: 762 Age range: 6 to 12 weeks Inclusion criteria: healthy Japanese Infants Exclusion criteria: history of known prior rotavirus gastroenteritis; infants who are concurrently participating in or are anticipated to participate in other studies of investigational products at any time during the study period |
|
| Interventions | 1. Rotavirus vaccine, live, oral, pentavalent [RV5], 381 participants 2. Placebo (unspecified), 381 participants Schedule: 3 doses, 28 to 70 days apart, with 14 days of safety follow‐up after each vaccination, and follow‐up for acute gastroenteritis episodes until the end of the study |
|
| Outcomes | 1. Efficacy against rotavirus gastroenteritis of any severity, at least 14 days following the 3rd vaccination 2. Efficacy against moderate to severe and severe rotavirus gastroenteritis, at least 14 days following the 3rd vaccination 3. Serious adverse events, including intussusception (data from correspondence with Merck; Merck 2012). 4. Reactogenicity (fever, vomiting, diarrhoea) 5. Dropouts before the end of the trial 6. Adverse events leading to discontinuation of the trial 7. Number of deaths (data from correspondence with Merck; Merck 2012) |
|
| Immunization status | No information about other vaccines given | |
| Location | 32 sites in Japan WHO mortality stratum A |
|
| Notes |
Date: August 2008 to September 2009 Registration number: NCT00718237 Source of funding: Merck Sharp & Dohme Corp Rationale: "to evaluate whether V260 is effective and well tolerated in Japanese healthy infants" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation number was assigned and the subject was randomized to the group receiving RV5 or the group receiving placebo in a 1:1 ratio according to the randomization code prepared by a computer at the US Merck Headquarters Office" (Merck 2012) |
| Allocation concealment (selection bias) | Low risk | Allocation numbers were generated and allocated centrally for participants (Merck 2012) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | RV5 was visibly indistinguishable from placebo, investigators, study personnel (internal and external) and parents/guardians were blinded throughout trial (Merck 2012) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition/exclusions balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 42 days after last dose Adverse event data collection methods: diary cards (passive method) |
|
| Participants |
Number: 178 enrolled; 171 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants; 6 to 12 weeks of age Exclusion criteria: history of congenital abdominal disorders, intussusception, or abdominal surgery; known or suspected impairment of immunological function; known hypersensitivity to any component of the rotavirus vaccine; prior receipt of any rotavirus vaccine; fever, with a rectal temperature ≥ 38.1 °C (≥ 100.5 °F) at the time of immunization; history of known prior rotavirus disease, chronic diarrhoea, or failure to thrive; clinical evidence of active gastrointestinal illness (infants with gastro‐oesophageal reflux disease were permitted to participate in the study as long as the gastro‐oesophageal reflux disease was well controlled with or without medication); receipt of intramuscular, oral, or intravenous corticosteroid treatment between the 2 weeks before first vaccination and 2 weeks after last vaccination; reside in a household with an immunocompromised person; prior receipt of a blood transfusion or blood products, including immunoglobulins; receipt of OPV during the course of the study or within 42 days before first dose of vaccine/placebo; and condition, which, in the opinion of the investigator, may have interfered with the evaluation of the study objectives |
|
| Interventions | RV5 1. WC3 (RV5): 6.9 to 8.6 x 107 PFU; 3 doses given 4 to 10 weeks apart; 115 participants (randomized) 2. Placebo: 3 doses given 4 to 10 weeks apart; 63 participants (randomized) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events: no definition; measured up to 42 days 2. Reactogenicity: no definition; measured up to 14 days 3. Adverse events resulting in discontinuation Outcomes to measure immunogenicity 4. Seroconversion: sero‐response serum anti‐rotavirus immunoglobulin A (IgA) defined as an increase in antibody titre by a factor of ≥ 3 from baseline (data could not be extracted for review) |
|
| Immunization status | Infants excluded if they had or were to receive oral poliovirus vaccine at any time during the study or in the 42 days before the first dose; concomitant administration of other licensed vaccines and breast‐feeding was not restricted | |
| Location | 8 study centres in South Korea WHO mortality stratum B |
|
| Notes |
Date: 2 August 2005 (first participant in) to 25 May 2006 (last dose given); last participant completed follow‐up on 5 July 2006 Source of funding: Merck & Co., Inc. Other: most of the outcome data are not provided in the reports |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomized 2:1 to receive hexavalent vaccine concomitantly with either RV5 (RotaTeq) or placebo (Merck 2012) |
| Allocation concealment (selection bias) | Low risk | Allocation numbers were generated for participants, investigators, adults, and parents/guardians of children were blinded throughout trial (Merck 2012) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | RV5 was visibly indistinguishable from placebo, investigators, study personnel (internal and external), and parents/guardians were blinded throughout trial (Merck 2012) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reason related to outcome |
| Selective reporting (reporting bias) | High risk | Key expected outcome not included |
| Other bias | Unclear risk | Information not provided |
| Methods | RCT Length of follow‐up: 2 weeks after last dose Adverse event data collection methods: not reported |
|
| Participants |
Number: Infant cohort: 48 enrolled and randomized, child cohort: 48 enrolled and randomized Inclusion criteria: healthy infants aged 6 to 12 weeks, and healthy children aged 2 to 6 years, there was also a cohort of adults (not reported in this review) Exclusion criteria: receiving other live vaccines 14 days before or after study vaccine; prior administration of any rotavirus vaccine; elevated temperature, with axillary temperature ≥ 37.1 °C 24 hours before study vaccine; prior or active gastrointestinal illnesses; immunodeficiency |
|
| Interventions | 1. 2.0 mL RV5 (V260) administered orally. The vaccine consists of an oral solution of 5 live human‐bovine reassortant rotaviruses (24 infants, 24 children) 2. 2.0 mL matching placebo to RV5 administered orally (24 infants, 24 children) Schedule: infant cohort: 3 doses of RV5/placebo at 3 separate visits scheduled 28 to 70 days apart. The third dose was administered by 32 weeks of age; child cohort: one dose |
|
| Outcomes |
Clinical outcome measures 1. Serious adverse events, up to 14 days post‐vaccination, including intussusception (data from correspondence with Merck; Merck 2012). 2. Adverse events requiring discontinuation 3. Dropouts from the trial 4. Number of deaths (data from correspondence with Merck; Merck 2012). 5. Reactogenicity Outcomes to measure immunogenicity 6. Vaccine virus shedding in stools, day 3 to day 7 following each of the 3 doses of RV5/placebo |
|
| Immunization status | Other live vaccines 14 days before or after study vaccine were not allowed | |
| Location | China WHO mortality stratum B |
|
| Notes |
Date: September 2009 to March 2010 Source of funding: Merck Sharp & Dohme Corp Study rationale: "This study will assess the safety and tolerability of RV5 (V260) in the healthy Chinese populations. Approximately 144 participants will be enrolled and equally stratified into three age cohorts, Cohort I ages 19‐47 years, Cohort II ages 2‐6 years, and Cohort III ages 6‐12 weeks" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants were randomized according to a computer‐generated allocation schedule (Merck 2012) |
| Allocation concealment (selection bias) | Low risk | Allocation numbers were generated for participants; investigators, adults, and parents/guardians of children were blinded throughout trial (Merck 2012) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | RV5 was visibly indistinguishable from placebo; investigators, study personnel (internal and external) and parents/guardians were blinded throughout trial (Merck 2012) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced across groups with reasons reported for withdrawal |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 6 weeks after last dose Adverse event data collection methods: Active: At each visit, data were recorded on adverse events observed by the caretaker and investigator, including signs/symptoms ≥ grade 1 and new clinically significant diagnoses |
|
| Participants |
Number: 202 enrolled; 202 evaluable Age range: infants 2 to < 15 weeks Inclusion criteria: Participant was born to an HIV‐infected mother; presence or absence of HIV RNA or DNA in the blood of the infant; CD4% documented at screening Exclusion criteria: concurrent participation in any study of an investigational drug or vaccine, except for studies for prevention of perinatal HIV transmission; gastrointestinal illness or fever; any condition, which would, in the opinion of the site investigator, place the participant at an unacceptable risk of injury or render the participant unable to meet the requirements of the protocol |
|
| Interventions | 1. RV5, 2 mL solution of live reassortant rotaviruses, containing G1, G2, G3, G4 and P1A which contains a minimum of 2.0 2.8 x 106 infectious units (IU) per individual reassortant dose, depending on the serotype, and not greater than 116 x 106 IUs per aggregate dose in 62 HIV‐uninfected but exposed and 37 HIV‐infected participants 2. Placebo in 64 HIV‐uninfected but exposed and 39 HIV‐infected participants Schedule: 3 doses of RV5 or placebo at intervals of 4 ‐ 10 weeks with the third dose administered by 32 weeks of age |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause deaths 2. All‐cause serious adverse events 3. Hospitalization 4. Reactiogenicity: fever, diarrhoea, vomiting Outcomes to measure immunogenicity 4. Rotavirus vaccine shedding (after 3rd dose) 5. Seroconversion |
|
| Immunization status | Enrolment was closed in participating countries when RV1 was added to national vaccine schedules | |
| Location | Botswana (2 sites), United Republic of Tanzania (1 site) , Zambia (1 site) and Zimbabwe (2 sites) WHO mortality stratum E |
|
| Notes |
Date: December 2009 ‐ January 2014 Source of funding: Merck & Co., Inc. and the International Maternal, Pediatric, and Adolescent AIDS Clinical Trial Network (IMPAACT) through the National Institute of Health Study rationale: evaluate the safety and immunogenicity of the Rotavirus vaccine RotaTeq, in HIV infected and uninfected children born to HIV infected mothers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study reported to be randomized, but no details provided on the randomization process |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo‐controlled but no details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition, reasons provided |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Unclear risk | Nine infants were unblinded after their first or second dose when rotavirus vaccine became available at their site. The 4 infants found to be on RV5 continued to receive their remaining study doses. Of the 5 infants on placebo, 2 were given the 2 recommended doses of Rotarix, but 3 were too old to receive Rotarix |
| Methods | RCT Length of follow‐up: up to 42 days after vaccination Adverse event data collection methods: not reported |
|
| Participants |
Number: 793 enrolled; 706 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants; 6 to 12 weeks of age Exclusion criteria: history of congenital abdominal disorders, intussusception, or abdominal surgery; known or suspected impairment of immunological function; known hypersensitivity to any component of the rotavirus vaccine; prior receipt of any rotavirus vaccine; fever, with a rectal temperature ≥ 38.1 °C (≥ 100.5 °F) at the time of immunization; history of known prior rotavirus disease, chronic diarrhoea, or failure to thrive; clinical evidence of active gastrointestinal illness (infants with gastro‐oesophageal reflux disease were permitted to participate in the study as long as the gastro‐oesophageal reflux disease was well controlled with or without medication); receipt of intramuscular, oral, or intravenous corticosteroid treatment between the 2 weeks before first vaccination and 2 weeks after last vaccination; reside in a household with an immunocompromised person; prior receipt of a blood transfusion or blood products, including immunoglobulins; receipt of oral polio vaccine during the course of the study or within 42 days before first dose of vaccine/placebo; and condition, which, in the opinion of the investigator, may have interfered with the evaluation of the study objectives |
|
| Interventions | RV5 1. WC3 (RV5): 2 mL (10.7 PFU); 3 doses given at 4 to 10 week intervals; 680 participants (randomized) 2. Placebo: 3 doses given at 28 to 70 day intervals; 113 participants (randomized) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Reactogenicity: no definition; measured 7 days after vaccination 2. Dropouts: measured up to 42 days 3. Adverse events requiring discontinuations: measured up to 42 days, (data from correspondence with Merck; Merck 2012) 4. Serious adverse events: not defined; measured up to 42 days, including intussusception (data from correspondence with Merck; Merck 2012) 5. Number of deaths (data from correspondence with Merck; Merck 2012) Outcomes to measure immunogenicity None |
|
| Immunization status | Infants were excluded if they had or were to receive oral poliovirus vaccine at any time during the study or in the 42 days before the first dose; concomitant administration of other licensed vaccines and breast‐feeding was not reported | |
| Location | 10 centres in USA WHO mortality stratum A |
|
| Notes |
Date: 9 May 2003 to 13 August 2004 Source of funding: Merck & Co., Inc. Study objective: "Comparison of the Immunogenicity and Safety of Three Consistency Lots of RotaTeq in Healthy Infants" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization to 1 of 4 treatment groups. A randomization scheme of 2:2:2:1, with a blocking factor of 14 was used, and participants received either 1 of 3 lots of RV5 or placebo (Merck 2012) |
| Allocation concealment (selection bias) | Low risk | Allocation numbers were generated for participants; investigators, adults, and parents/guardians of children were blinded throughout trial (Merck 2012) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | RV5 was visibly indistinguishable from placebo; investigators, study personnel (internal and external) and parents/guardians were blinded throughout trial (Merck 2012) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | RCT Length of follow‐up: 2 years Adverse event data collection methods: Passive: All adverse events were collected for 30 days following each dose. |
|
| Participants |
Number: 4040 enrolled; 4040 evaluable Age range: 6 – 12 weeks (at start of study) Inclusion criteria: Healthy infants at least 6 weeks and up to 12 weeks of age at the time of the first study vaccination Exclusion criteria: History of congenital abdominal disorders, prior rotavirus gastroenteritis, chronic diarrhoea, failure to thrive, or abdominal surgery; history of intussusception; impairment of immunological function; acute disease, severe chronic disease, or chronic disease during the acute period; participation in another interventional study; any condition which, in the opinion of the investigator, may interfere with the evaluation of the study objectives |
|
| Interventions | 1. RV5, 2 mL (n=2020 randomized) 1.1 RV5 alongside staggered EPI (OPV administered as a 1 g oral solution at age ˜2½, 3½, and 4½ months, and DTaP administered as a 0.5 mL intramuscular injection at age ˜3½, 4½, and 5½ months) 1.2.RV5 with concomitant EPI (OPV administered as a 1 g oral solution at age ˜2, 3, and 4 months, and DTaP administered as a 0.5 mL intramuscular injection at age ˜3, 4, and 5 months) 2. Placebo (n=2020 randomized) 2.1 placebo alongside staggered EPI (OPV administered as a 1 g oral solution at age ˜2½, 3½, and 4½ months, and DTaP administered as a 0.5 mL intramuscular injection at age ˜3½, 4½, and 5½ months) 2.2 placebo with concomitant EPI (OPV administered as a 1 g oral solution at age ˜2, 3, and 4 months, and DTaP administered as a 0.5 mL intramuscular injection at age ˜3, 4, and 5 months) Schedule: RV5 or placebo at age 2, 3, and 4 months |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Severe Rotavirus diarrhoea 2. All‐cause deaths 3. Serious adverse events 4. Intussusception 5. Rotavirus diarrhoea (any severity) 6. Reactogenicity: fever, diarrhoea, vomiting 7. Adverse events due to discontinuation 8. Dropouts from the trial |
|
| Immunization status | Routine EPI vaccines (OPV, DTaP) either staggered or concomitantly with RV5 or placebo | |
| Location | 5 sites, China WHO mortality stratum B |
|
| Notes |
Date: May 2014 ‐ June 2015 Source of funding: Merck Sharp & Dohme Corp. Study rationale: assess the efficacy, safety, and immunogenicity of a 3 dose regimen of RotaTeq™ (V260) in healthy Chinese infants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study reported to be randomized, but no details provided on the randomization process |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded for vaccine versus placebo, not for staggered versus concomitant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition and reasons provided |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 1 to 3 rotavirus seasons (1 to 3 years) Adverse event data collection methods: diary cards (passive method); telephone calls to parents/legal guardians to ask about serious adverse events (active method) Note: the per‐protocol population used for the primary efficacy analysis included 1496 participants after exclusion of 450 participants (23.1%). The modified intention‐to‐treat population used in a secondary efficacy analysis consisted of the 1647 participants, including protocol violators, who had any valid post‐dose 3 efficacy data |
|
| Participants |
Number: 1946 enrolled; 1496 evaluable (after 2 years) Age range: 3 to 6 months (beginning); > 6 months (end) Inclusion criteria: healthy infants between 2 and 8 months of age Exclusion criteria: not described |
|
| Interventions | RV5 1. WC3 (RV5) 1.1. G1‐4, P1A (2.69 x 107, 7.92 x 106, 2.41 x 106); 3 doses given 4 to 8 weeks apart; 1027 participants (randomized) 1.2. G1‐4 (2.9 x 107); 3 doses given 4 to 8 weeks apart; 270 participants (randomized) 1.3. P1A (9.24 x 107); 3 doses given 4 to 8 weeks apart; 327 participants (randomized) 2. Placebo: 3 doses given 4 to 8 weeks apart; 322 participants (randomized) We excluded the 2 arms dealing with different G or P serotypes and compared a single arm to placebo |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Rotavirus diarrhoea: case definition for rotavirus gastroenteritis required: (1) ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both; and (2) rotavirus antigen detection by EIA. The primary analysis of efficacy considered episodes as positive only when caused by wild‐type rotavirus with a vaccine G serotype (G1, G2, G3, or G4) confirmed by PCR occurring at least 14 days after the third dose of vaccine; measured 1 to 3 years 2. Severe rotavirus diarrhoea: clinical scoring system based on the intensity and duration of symptoms of fever, vomiting, diarrhoea, and behavioural changes was used to rate the severity of gastroenteritis, using a 24‐point severity scale where a score of 1 to 8 was designated as mild, > 8 was designated as moderate‐and‐severe, and > 16 was designated as severe; measured 1 to 3 years 3. Reactogenicity: not defined other than all participants were followed for clinical adverse events for 42 days after each dose of vaccine or placebo; parents/guardians were provided with diary cards to record adverse events 4. Serious adverse events: not defined; noted that they were to be reported immediately. Parents/legal guardians were contacted by phone approximately 14 days after each dose and asked about serious adverse events. Data on deaths and serious adverse events judged by the investigator to be vaccine‐related were collected for the duration of the study (up to 42 days) 5. All‐cause death Outcomes to measure immunogenicity 6. Seroconversion: prevaccination and post‐vaccination sera assayed for rotavirus‐specific IgA by ELISA with seroconversion defined as ≥ 3‐fold rise in antibody titre from baseline to 2 weeks after dose 3 (review includes data from 14 days after dose 3) |
|
| Immunization status | Licensed vaccines could be administered throughout the study, but were not given on the same day as study vaccine; inactivated poliovirus vaccine was exclusively used in Finland at the time of the study | |
| Location | 4 sites (Tampere, Espoo, Lahti, Pori) in Finland WHO mortality stratum A |
|
| Notes |
Date: June 1998 and June 2001 Source of funding: Merck & Co., Inc. Other: in total, 1946 infants (1300 in the first year and 646 in the second year of the study) were enrolled in the study and received at least the first dose of 1 of the 5 active vaccines or placebo. Overall, 1813 (93.2%) participants received 3 doses and were followed for ≥ 42 days after the final dose. 1800 participants (92.5%) were followed through the first rotavirus season after vaccination; 1740 participants (89.4%) were followed through a second rotavirus season. Of the 1300 participants enrolled in the first year, 880 (67.7%) were followed through a third rotavirus season |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (Merck 2012) |
| Allocation concealment (selection bias) | Low risk | Allocation numbers were generated for participants; investigators and parents/guardians were blinded throughout trial (Merck 2012) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sequential identical containers Quote: "The vials containing either vaccine or placebo were visibly indistinguishable." Participants and key personnel Quote: "This randomized clinical trial blinded to subject, investigator, parent/legal guardian, and sponsor. The placebo was identical to the vaccine except that it did not contain rotavirus reassortants or trace trypsin" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions |
| Selective reporting (reporting bias) | High risk | ≥1 outcome of interest reported incompletely |
| Other bias | Unclear risk | Insufficient information to assess |
| Methods | RCT Length of follow‐up: up to 43 days for safety outcomes, and up to 2 years for efficacy outcomes Adverse event data collection methods: active surveillance was used to obtain safety data; parents or legal guardians were contacted on days 7, 14, and 42 after each dose and every 6 weeks thereafter for 1 year after the first dose with respect to intussusception and serious adverse events (active method) |
|
| Participants |
Number: 70,301 enrolled and 69,274 randomized (efficacy study subpopulation of 5673); 57,134 evaluable for safety outcomes; for efficacy outcomes, 4512 evaluable in year 1 and 1569 evaluable in year 2 Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants between 6 and 12 weeks of chronological age were eligible regardless of gestational age; no known history of congenital abdominal disorders, intussusception, or abdominal surgery; no known or suspected impairment of immunological function; no known hypersensitivity to any component of the rotavirus vaccine; no prior receipt of any rotavirus vaccine; no fever, with a rectal temperature ≥ 38.1 °C (≥ 100.5 °F) at the time of immunization; no history of known prior rotavirus disease, chronic diarrhoea, or failure to thrive; no clinical evidence of active gastrointestinal illness; no receipt of intramuscular, oral, or intravenous corticosteroid treatment within the 2 weeks before vaccination; did not reside in a household with an immunocompromised person; no prior receipt of a blood transfusion or blood products, including immunoglobulins; no receipt of oral poliovirus vaccine during the course of the study or within 42 days prior to the first dose of vaccine/placebo Exclusion criteria: see above for details Special group: infants born at < 36 weeks of gestational age were considered premature and infants born at < 32 weeks of gestational age were considered extremely premature; no formal safety or efficacy hypotheses were prespecified for premature infants |
|
| Interventions | RV5 1. WC3 (RV5): 2 mL (6.7 to 12.4 x 107 PFU); 3 doses given 4 to 10 weeks apart; 34,644 participants (randomized) 2. Placebo: 2 mL; 3 doses given 4 to 10 weeks apart; 34,630 participants (randomized) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Rotavirus diarrhoea: case definition for rotavirus gastroenteritis required participants to meet both of the following criteria: (1) ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, and (2) rotavirus detected by EIA in a stool specimen taken within 14 days after the onset of symptoms. Only naturally‐occurring "rotavirus AGEs" caused by the composite of the human rotavirus G‐serotypes in the vaccine (G1, G2, G3, and G4) occurring through the first rotavirus season that began at least 14 days following the third vaccination were included in the primary analysis; measured up to 2 years follow‐up 2. Severe rotavirus diarrhoea: an established clinical scoring system based on the intensity and duration of fever, vomiting, diarrhoea, and changes in behaviour used to categorize episodes of rotavirus gastroenteritis on a 24‐point severity scale; scores > 16 were considered to indicate severe disease; measured up to 2 years follow‐up 3. Emergency department visit: hospitalizations and emergency department visits for acute gastroenteritis; measured up to 1 year of follow‐up 4. All‐cause hospital admission: see above; measured up to 1 year of follow‐up 5. All‐cause mortality: measured up to 1 year of follow‐up 6. Dropouts: no definition; measured up to 2 years follow‐up 7. Serious adverse events: monitored for at least 42 days after each dose for serious adverse events, including intussusception. All suspected cases of intussusception were reported to an independent, blinded adjudication committee, which included a paediatric surgeon, a paediatric radiologist, and a paediatrician with extensive experience in emergency medicine. The committee adjudicated potential cases of intussusception according to a prespecified case definition that required confirmation of the diagnosis by radiography or at surgery or autopsy; measured up to 1 year of follow‐up. Final intussusception results taken from CDC report (CDC 2010) 8. Reactogenicity: not defined; measured up to 43 days after vaccine 9. Adverse events requiring discontinuation: not defined; measured up to 1 year of follow‐up 10. Rotavirus diarrhoea resulting in hospitalization Outcomes to measure immunogenicity 11. Seroconversion: defined as an increase in the antibody titre by a factor of ≥ 3 from baseline (review includes data from 14 days after dose 3) |
|
| Immunization status | Administration of other licensed childhood vaccines and breast‐feeding were not restricted; for a subset of participants in the USA (U.A. concomitant use cohort), Merck also provided the licensed paediatric vaccines that were administered concomitantly (same day) with RV5 or placebo, which included Comvax, Infanrix, Ipol, and Prevnar | |
| Location | 356 primary study sites in Belgium, Costa Rica, Finland, Germany, Guatemala, Italy, Jamaica, Mexico, Puerto Rico, Sweden, Taiwan, and the USA WHO mortality strata A, B, D |
|
| Notes |
Date: 12 January 2001 to 6 October 2004 Source of funding: Merck & Co., Inc. Other: there is a full report on premature babies that will be data‐extracted separately |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomized 1:1 to receive either RV5 (RotaTeq) or placebo (Merck 2012) |
| Allocation concealment (selection bias) | Low risk | Allocation numbers were generated for participants; investigators and parents/guardians were blinded throughout trial (Merck 2012) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and key personnel Quote: "Randomized, multicenter, double blinded (operated under in‐house blinding procedures), placebo controlled, safety and efficacy trial. The placebo was an exact match minus the virus" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Unclear risk | Difficult to judge, as some important information about randomization/allocation concealment are not provided |
| Methods | RCT Length of follow‐up: up to 43 days for safety outcomes, and up to 2 years for efficacy outcomes Adverse event data collection methods: active surveillance was used to obtain safety data; parents or legal guardians were contacted on the first 14 days after each dose and every month thereafter for 1 year after the first dose with respect to intussusception and serious adverse events (active method). "Serious adverse events were classified with the US regulatory definition, in line with ICH guidance, and identified by monthly query and parental reporting at any time or identification by study staff in hospitals or clinics. Intussusception at any time was assessed with an additional detailed protocol. All these events were monitored by an independent, unmasked, data and safety monitoring board that met about twice a year during the course of the investigation. The board also provided guidance about enrolment and severity scoring" |
|
| Participants |
Number: 2119 enrolled; 2036 randomized, 2016 evaluable Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants aged 4 to 12 weeks. Breast‐feeding was not restricted and there was no enrolment restrictions based on HIV status, although HIV testing was not done Exclusion criteria: see above |
|
| Interventions | RV5 1. WC3 (RV5): 2 mL (6.7 to 12.4 x 107 PFU); 3 doses given 4 weeks apart; 1018 participants (randomized) 2. Placebo: 2 mL; 3 doses given 4 weeks apart; 1018 participants (randomized) Schedule: 3 doses given at 4‐week intervals |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events 2. Death due to serious adverse events 3. Rotavirus diarrhoea: case definition for rotavirus gastroenteritis required participants to meet both of the following criteria: (1) ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, and (2) rotavirus detected by EIA in a stool specimen taken within 14 days after the onset of symptoms 4. Severe rotavirus diarrhoea: an established clinical scoring system based on the intensity and duration of fever, vomiting, diarrhoea, and changes in behaviour used to categorize episodes of rotavirus gastroenteritis on a 20‐point severity scale; scores > 11 were considered to indicate severe disease; measured up to 2 years follow‐up 5. All‐cause diarrhoea 6. All‐cause diarrhoea – severe 7. Reactogenicity *: symptoms of rotavirus illness, including fever, diarrhoea, and vomiting; measured for 7 days after each dose (review includes data from for the end of follow‐up) Data on fever and vomiting are provided only on figure 2 and data could not be extracted reliably Outcomes to measure immunogenicity 8. Seroconversion: serum rotavirus IgA responses (increases in level of serum rotavirus IgA ≥ 4‐fold) (review includes data from after dose 2) |
|
| Immunization status | All children in the study received the standard EPI vaccines (including oral poliovirus vaccine) starting at 6 weeks of age | |
| Location | Sites in rural Matlab (Bangladesh) and urban and peri‐urban Nha Trang (Vietnam) WHO mortality strata B, D |
|
| Notes | This trial was conducted in Bangladesh and Vietnam; data reported separately by country can be found under RV5 Zaman 2010‐BGD and RV5 Zaman 2010‐VNM. Date: March 29, 2007 to March 31, 2009 Source of funding: funded by PATH (GAVI Alliance grant) and Merck |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Unique allocation numbers were designated at Merck as pentavalent rotavirus vaccine or placebo with computer generated block randomization, with block sizes of six" |
| Allocation concealment (selection bias) | Low risk | Quote: "Vaccine and placebo packages were then labelled with allocation numbers and provided to sites in identical presentations. Sites were instructed to assign allocation numbers to participants in sequential order as they were enrolled" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff Quote: "Participants were enrolled by study staff, who remained masked to treatment assignment throughout the trial" Researchers Quote: "The statistician from Merck who analysed the data and the Merck and PATH protocol teams were masked to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 43 days for safety outcomes, and up to 2 years for efficacy outcomes Adverse event data collection methods: active surveillance was used to obtain safety data; parents or legal guardians were contacted on the first 14 days after each dose and every month thereafter for 1 year after the first dose with respect to intussusception and serious adverse events (active method). "Serious adverse events were classified with the US regulatory definition, in line with ICH guidance, and identified by monthly query and parental reporting at any time or identification by study staff in hospitals or clinics. Intussusception at any time was assessed with an additional detailed protocol. All these events were monitored by an independent, unmasked, data and safety monitoring board that met about twice a year during the course of the investigation. The board also provided guidance about enrolment and severity scoring". |
|
| Participants |
Number: 1136 randomized Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants aged 4 to 12 weeks. Breast‐feeding was not restricted and there were no enrolment restrictions based on HIV status, although HIV testing was not done Exclusion criteria: see above |
|
| Interventions | RV5 1. WC3 (RV5): 2 mL (6.7 to 12.4 x 107 PFU); 3 doses given 4 weeks apart; 568 participants (randomized) 2. Placebo: 2 mL; 3 doses given 4 weeks apart; 568 participants (randomized) Schedule: 3 doses given at a 4‐week interval |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events 2. Death due to serious adverse events 3. Rotavirus diarrhoea: case definition for rotavirus gastroenteritis required participants to meet both of the following criteria: (1) ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, and (2) rotavirus detected by EIA in a stool specimen taken within 14 days after the onset of symptoms 4. Severe rotavirus diarrhoea: an established clinical scoring system based on the intensity and duration of fever, vomiting, diarrhoea, and changes in behaviour used to categorize episodes of rotavirus gastroenteritis on a 20‐point severity scale; scores > 11 were considered to indicate severe disease; measured up to 2 years follow‐up 5. All‐cause diarrhoea 6. All‐cause diarrhoea – severe 7. Reactogenicity *: symptoms of rotavirus illness, including fever, diarrhoea, and vomiting; measured for 7 days after each dose (review includes data from for the end of follow‐up) Data on fever and vomiting are provided only on figure 2 and data could not be extracted reliably Outcomes to measure immunogenicity 8. Seroconversion: serum rotavirus IgA responses (increases in level of serum rotavirus IgA ≥ 4 fold) (review includes data from after dose 2) |
|
| Immunization status | All children in the study received the standard EPI vaccines (including oral poliovirus vaccine) starting at 6 weeks of age | |
| Location | Sites in rural Matlab, Bangladesh WHO mortality stratum D |
|
| Notes | This trial was conducted in Bangladesh and Vietnam; this part presents data for the Bangladesh cohort, data reported separately for Vietnam can be found under RV5 Zaman 2010‐VNM and data for both countries under RV5 Zaman 2010‐AS Date: March 29, 2007 to March 31, 2009 Source of funding: funded by PATH (GAVI Alliance grant) and Merck |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Unique allocation numbers were designated at Merck as pentavalent rotavirus vaccine or placebo with computer generated block randomization, with block sizes of six" |
| Allocation concealment (selection bias) | Low risk | Quote: "Vaccine and placebo packages were then labelled with allocation numbers and provided to sites in identical presentations. Sites were instructed to assign allocation numbers to participants in sequential order as they were enrolled" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff Quote: "Participants were enrolled by study staff, who remained masked to treatment assignment throughout the trial" Researchers Quote: "The statistician from Merck who analysed the data and the Merck and PATH protocol teams were masked to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 43 days for safety outcomes, and up to 2 years for efficacy outcomes Adverse event data collection methods: active surveillance was used to obtain safety data; parents or legal guardians were contacted on the first 14 days after each dose and every month thereafter for 1 year after the first dose with respect to intussusception and serious adverse events (active method). "Serious adverse events were classified with the US regulatory definition, in line with ICH guidance, and identified by monthly query and parental reporting at any time or identification by study staff in hospitals or clinics. Intussusception at any time was assessed with an additional detailed protocol. All these events were monitored by an independent, unmasked, data and safety monitoring board that met about twice a year during the course of the investigation. The board also provided guidance about enrolment and severity scoring". |
|
| Participants |
Number: 900 randomized Age range: 1 to 3 months (beginning); 3 to 6 months (end) Inclusion criteria: healthy infants aged 4 to 12 weeks. Breast‐feeding was not restricted and there were no enrolment restrictions based on HIV status, although HIV testing was not done Exclusion criteria: see above |
|
| Interventions | RV5 1. WC3 (RV5): 2 mL (6.7 to 12.4 x 107 PFU); 3 doses given 4 weeks apart; 450 participants (randomized) 2. Placebo: 2 mL; 3 doses given 4 weeks apart; 450 participants (randomized) Schedule: 3 doses given at 4‐week intervals |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Serious adverse events 2. Death due to serious adverse events 3. Rotavirus diarrhoea: case definition for rotavirus gastroenteritis required participants to meet both of the following criteria: (1) ≥ 3 watery or looser‐than‐normal stools within a 24‐hour period or forceful vomiting, or both, and (2) rotavirus detected by EIA in a stool specimen taken within 14 days after the onset of symptoms 4. Severe rotavirus diarrhoea: an established clinical scoring system based on the intensity and duration of fever, vomiting, diarrhoea, and changes in behaviour used to categorize episodes of rotavirus gastroenteritis on a 20‐point severity scale; scores > 11 were considered to indicate severe disease; measured up to 2 years follow‐up 5. All‐cause diarrhoea 6. All‐cause diarrhoea – severe 7. Reactogenicity*: symptoms of rotavirus illness, including fever, diarrhoea, and vomiting; measured for 7 days after each dose (review includes data from for the end of follow‐up) Data on fever and vomiting are provided only on figure 2 and data could not be extracted reliably Outcomes to measure immunogenicity 8. Seroconversion: serum rotavirus IgA responses (increases in level of serum rotavirus IgA ≥ 4‐fold) (review includes data from after dose 2) |
|
| Immunization status | All children in the study received the standard EPI vaccines (including oral poliovirus vaccine) starting at 6 weeks of age | |
| Location | Sites in urban and peri‐urban Nha Trang, Vietnam WHO mortality stratum B |
|
| Notes | This trial was conducted in Bangladesh and Vietnam; this part presents data for the Vietnam cohort. Data reported separately for Bangladesh can be found under RV5 Zaman 2010‐BGD and data for both countries under RV5 Zaman 2010‐AS Date: March 29, 2007 to March 31, 2009 Source of funding: funded by PATH (GAVI Alliance grant) and Merck |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Unique allocation numbers were designated at Merck as pentavalent rotavirus vaccine or placebo with computer generated block randomization, with block sizes of six" |
| Allocation concealment (selection bias) | Low risk | Quote: "Vaccine and placebo packages were then labelled with allocation numbers and provided to sites in identical presentations. Sites were instructed to assign allocation numbers to participants in sequential order as they were enrolled" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff Quote: "Participants were enrolled by study staff, who remained masked to treatment assignment throughout the trial" Researchers Quote: "The statistician from Merck who analysed the data and the Merck and PATH protocol teams were masked to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data balanced across groups |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | No apparent other bias |
| Methods | Phase I RCT Length of follow‐up: 28 days Adverse event data collection methods: Caregivers reported any symptoms or illnesses on diary cards or to physician on‐call 24 hours; physicians and field investigators visited participants twice daily the first 14 days |
|
| Participants |
Number: 90 enrolled, 90 randomized, 83 evaluable Age range: 8 weeks at enrollment and first dose Inclusion criteria: healthy, non‐malnourished infants Exclusion criteria: Evidence of renal, cardiovascular, liver or other reticuloendothelial, neurological, gastrointestinal, haematologic, rheumatologic or immunologic disease |
|
| Interventions | Rotavac 1. Rotavac vaccine (116E) (105 FFU), n = 30 2. Rotavirus vaccine candidate I321, n = 30 3. Placebo, n = 30 Schedule: 1 dose given at 8 weeks of age |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause death 2. Intussusception 3. Serious adverse events 4. Reactogenicity (up to 14 days) Outcomes to measure immunogenicity 5. Immunogrnicity: seroconversion (4‐fold rise in titre of IgA) 6. Immunogenicity: shedding |
|
| Immunization status | Infants were vaccinated with DPT, Hep B and OPV separately from rotavirus vaccine | |
| Location | 1 site (Delhi) in India WHO mortality stratum D |
|
| Notes |
Date: January to May 2005 Registration number: NCT00280111; ISRCTN57452882 Source of funding: Bharat Biotech International Ltd. Notes: study arm administered vaccine candidate I321 was excluded from data analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For randomisation, a sequence of codes was generated using Stata, version 8 (Statacorp, College Station, TX, USA) by a statistician not otherwise involved with the trial." |
| Allocation concealment (selection bias) | Low risk | Quote: "Two copies of the randomisation code were prepared; one was sent to the Division of Microbiology and Infectious Diseases (DMID) at the NIH under sealed cover, and the second was given to a physician, not otherwise involved in the study, for reconstituting the vaccine/placebo at the time of enrolment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double‐blind" Quote: "The placebo was constituted by adding a crystal of potassium permanganate to sodium bicarbonate buffer and appeared identical to the vaccines but did not contain the virus." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition, reasons for loss to follow‐up were reported and evenly spread across groups |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, all outcomes in the trial register reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 12 weeks Adverse event data collection methods: Caregivers reported any symptoms or illnesses to physician on‐call 24 hours; infants were visited at home daily the first 14 days after each administration |
|
| Participants |
Number: 369 enrolled and randomized, 367 received at least one dose Age range: 8 to 9 weeks Inclusion criteria: healthy infants Exclusion criteria: family without access to a telephone, unavailable for follow‐up, weight‐for‐height z score of < 3 standard deviations, resided with an immunocompromised individual, born at a gestational age of < 37 weeks, major congenital abnormality, history of hospitalization for sepsis, pneumonia, or meningitis, diarrhoea in the previous 7 days, blood in stools any time after birth, need for daily medication, cardiovascular or neurological disease |
|
| Interventions | Rotavac 1. Rotavac vaccine (116E) (1 x 104 (low dose) or 1 x 105 FFU (high dose)), n = 185 2. Placebo, n = 184 Schedule: 3 doses given at 4‐week intervals at 8, 12, and 16 weeks of age |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause death 2. Intussusception (level 1 Brighton definition) 3. Serious adverse events 4. Reactogenicity (up to 14 days) Outcomes to measure immunogenicity 5. Immunogenicity: shedding 6. Immunogenicity: seroconversion (4‐fold increase in IgA antibody titer to rotavirus) |
|
| Immunization status | Infants received 3 doses of DTP; OPV; and Hep B at 6, 10, and 14 weeks of age | |
| Location | 1 site (New Delhi) in India WHO mortality stratum D |
|
| Notes |
Date: November 2006 to February 2008 Registration number: NCT00439660; ISRCTN57452882 Source of funding: Department of Biotechnology, Government of India and PATH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were assigned to either the vaccine or placebo groups in a 1:1 ratio with use of a randomization sequence generated by a statistician not otherwise involved with the study (Stata software, version 8.0) with a fixed block length of 4 |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by using serially‐numbered sealed opaque envelopes. One set of envelopes was available with the independent vaccine‐dispensing team and another with the study data safety monitoring board |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study reported to be double‐blind but no further details were reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intussusception data reported for all enrolled participants, immunogenicity and reactogenicity were not reported for all participants and the reason was not clear |
| Selective reporting (reporting bias) | Low risk | No indication of selective outcome reporting |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: up to 2 years of age Adverse event data collection methods: All participants were contacted weekly at home by trained field workers to identify gastroenteritis, signs and symptoms of suspected intussusception, hospitalizations, and other illnesses. In addition, families reported any adverse events |
|
| Participants |
Number: 6799 enrolled, randomized and received at least one dose Age range: 6 to 7 weeks at recruitment Inclusion criteria: parents consented to participation and had no plans to move out of the study area during the next 24 months Exclusion criteria: infants were excluded if they had received a rotavirus vaccine, had documented immunodeficiency or chronic gastroenteritis or any other condition judged by the investigator as an exclusion criterion. Presence of any illness requiring hospital referral and diarrhoea on the day of enrolment was a temporary exclusion |
|
| Interventions | Rotavac 1. Rotavac (ORV 116E) vaccine (1 x 105 FFU), n = 4532 2. Placebo, n = 2267 Schedule: 3 doses given at 4‐week intervals (6 to 7 weeks, ≥ 10 weeks, and ≥ 14 weeks of age) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. Severe rotavirus gastroenteritis (≥ 11 on the 20‐point Vesikari scoring scale) 2. All‐cause death 3. Intussusception (Brighton criteria level 1) 4. Serious adverse events 5. Severe all‐cause diarrhoea 6. Rotavirus diarrhoea: any severity Outcomes to measure immunogenicity 7. Seroconversion (4‐fold rise in titre from paired serum samples) |
|
| Immunization status | Other childhood vaccines (DTPw, Hib, Hep B, and OPV) given concurrently | |
| Location | 3 sites: Delhi, Pune, and Vellore in India WHO mortality stratum D |
|
| Notes |
Date: March 2011 to November 2012 Registration number: NCT01305109; CTRI/2010/091/000102 Source of funding: The Department of Biotechnology, and Biotechnology Industry Research Assistance Council, Government of India; the Bill & Melinda Gates Foundation to PATH; Research Council of Norway; Department for International Development, UK; National Institutes of Health, USA; Bharat Biotech International Ltd. Moved from ongoing Other NCT01305109 and Other CTRI‐091‐000102. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by Cenduit, LLC, Germany, with stratification by site, and a block size of 12 |
| Allocation concealment (selection bias) | Low risk | The letter code on the vaccine/placebo vial was masked with the participant identification number before sending the vial to the clinical co‐ordinator administering the test article to the enrolled infant |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The placebo was identical in content, packaging, and appearance to the vaccine but did not contain the virus |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 1% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, all outcomes in the trial register reported |
| Other bias | Low risk | No apparent other bias |
| Methods | RCT Length of follow‐up: 1 year Adverse event data collection methods: Daily contacts through telephone calls or home visit for 14 days after each dose. Thereafter, weekly contacts were made until infants were 1 year of age |
|
| Participants |
Number: 1356 enrolled and randomized, 1327 completed 1 year follow‐up Age range: 6 to 8 weeks Inclusion criteria: healthy infants whose parents were willing to participate and had no plans for moving away were eligible for enrolment Exclusion criteria: had already received the first dose of the childhood vaccines or any other rotavirus vaccine, had immunodeficiency disease or chronic gastroenteritis disease, and/or any condition warranting exclusion by the investigator |
|
| Interventions | Rotavac 1. Rotavac vaccine, 1 x 104 FFU, in 3 production lots, n = 1017 2. Placebo, n= 339 Schedule: 3 doses given at a 4‐ to 8‐week intervals (6 – 7 weeks, 10 ‐ < 14, and 14 ‐ < 18 weeks of age) |
|
| Outcomes |
Clinical outcome measures (safety and efficacy) 1. All‐cause death 2. Serious adverse events 3. Intussusception (level 1 Brighton criteria) 4. Reactogenicity Outcomes to measure immunogenicity 5. Immunogenicity: seroconversion (≥4 fold rise in IgA antibody titer to rotavirus) |
|
| Immunization status | Co‐administered with EPI vaccines: OPV and combined DPT, HepB and Hib | |
| Location | 1 site in Delhi, India WHO mortality stratum D |
|
| Notes |
Date: May 2014 to August 2015 Registration number: CTRI/2014/05/004592 Source of funding: PATH, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by Diagnosearch Life Sciences Pvt. Ltd. and the randomization list was available with an independent biostatistician" |
| Allocation concealment (selection bias) | Low risk | Central allocation Quote: "Randomization was done by Diagnosearch Life Sciences Pvt. Ltd. and the randomization list was available with an independent biostatistician" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The placebo was identical in content, packaging, and appearance to the vaccine. The study team received ROTAVAC® or placebo vials labeled with the subject Identification (ID) number to maintain blinding. The study team, vaccine administrators and laboratory personnel were not aware of the treatment status." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat population was analyzed for safety outcomes. Less than 5% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting, all outcomes in the trial register reported |
| Other bias | Low risk | No apparent other bias |
ATP: according to protocol; BCG: bacillus Calmette‐Guerin; eCRF: electronic case report form; ELISA: Enzyme Linked Immunosorbent Assay; FF: focus‐forming unit; ITT: intention‐to‐treat; LAR: legally acceptable representative; MedDRA: Medical Dictionary for Regulatory Activities; OPV: oral poliovirus; PFU: plaque‐forming unit; RCT: randomized controlled trial; RT‐PCR: reverse transcriptase‐polymerase chain reaction; (S)AE: (serious) adverse event; VRC: vaccine report card
Immunogenicity: only data for review‐relevant outcomes listed in these tables.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| OTHER Armah 2013 | RCT of withdrawn RV vaccine RRV‐TV |
| OTHER Bines 2015 | Neonatal RV vaccine RV3‐BB in development |
| OTHER Bines 2018 | RCT of unlicensed neonatal RV3‐BB rotavirus vaccine (ACTRN12612001282875) |
| OTHER Bucardo 2018 | Prospective cohort study |
| OTHER Bucher 2012 | Diagnostic test accuracy study |
| OTHER Chatterjee 2012 | RCT, not rotavirus vaccine |
| OTHER Cowley 2017 | RCT of unlicensed neonatal RV3‐BB rotavirus vaccine |
| OTHER CTRI/2009/091/000821 | RCT of Rotasiil versus placebo |
| OTHER Dang 2012 | RCT evaluating safety and immunogenicity of vaccine licensed in Vietnam (NCT01377571); vaccine not prequalified by the WHO |
| OTHER de Palma 2010 | Case‐control study |
| OTHER Dickson 2017 | Brief narrative report |
| OTHER Diness 2010 | Study of vitamin A supplementation with Bacille Calmette‐Guerin vaccine for rotavirus diarrhoea outcomes |
| OTHER Dutta 2011 | RCT, not rotavirus vaccine |
| OTHER Ella 2018 | All infants received rotavirus vaccine, and were randomized to Rotavac (116E) with or without buffering agent. (CTRI/2014/04/004548) |
| OTHER Friedrich 2017 | Editorial on Rotasiil rotavirus vaccine |
| OTHER Gagneur 2011 | Observational study (IVANHOE) |
| OTHER Groome 2017 | RCT in infants of RV vaccine in development: parenteral P2‐VP8‐P[8] subunit RV vaccine (NCT02109484) |
| OTHER Hiramatsu 2018 | Prospective cohort study |
| OTHER Isanaka 2017‐NER | Reporting on an RCT (NCT02145000) that evaluates safety and efficacy in a vaccine licensed in India but not prequalified by the WHO |
| OTHER Kempe 2007 | Survey of paediatricians about rotavirus disease and rotavirus vaccines |
| OTHER Kulkarni 2017 | Reporting on an RCT (NCT02133690) that evaluates safety and efficacy in a vaccine licensed in India but not prequalified by the WHO |
| OTHER Muhsen 2010 | Case‐control study |
| OTHER NCT00981669 | RCT included adults aged 18 ‐ 40 years |
| OTHER NCT01195844 | Observational study, prematurely terminated for poor recruitment |
| OTHER NCT01236066 | Ongoing observational study |
| OTHER NCT01375907 | Ongoing study with adult participants |
| OTHER NCT01571505 | RCT in infants comparing RV vaccine administered with IPV or OPV |
| OTHER Rivera 2011 | RCT, no placebo comparison |
| OTHER Thyagarajan 2011 | Procedural codes for rotavirus vaccination in the USA |
| OTHER Yin 2017 | Oral RV vaccine (not specified, could be both RV1 and RV5) was administered before versus after other injected vaccines to compare injection site pain of the other vaccines |
| OTHER Zade 2014a‐IND | Reporting on an RCT that evaluates safety in a vaccine licensed in India but not prequalified by the WHO |
| OTHER Zade 2014b‐IND | Reporting on an RCT (CTRI/2010/091/003064) that evaluates safety in a vaccine licensed in India but not prequalified by the WHO |
| RV1 / RV5 Libster | RCT of RV1 and RV5 combined in different sequences |
| RV1 Ali 2014 | Comparing different age schedules of RV1 |
| RV1 Armah 2016 | Comparing alternative dosing schedules |
| RV1 Buyse 2014 | Integrated analysis |
| RV1 Correia 2010 | Case‐control study |
| RV1 CTRI/2012/02/002454 | Ongoing RCT with no placebo group |
| RV1 Dennehy 2008 | RCT of RV1 vaccine, but no placebo group reported |
| RV1 Emperador 2016 | No placebo group: RV1 on a staggered versus concomitant schedule with other vaccines |
| RV1 GSK[107077‐057] 2008 | RCT of RV1 vaccine, but no placebo group reported |
| RV1 GSK[107876‐061] 2008 | RCT of RV1 vaccine, but no placebo group reported |
| RV1 GSK[444563‐020] 2007 | RCT, but excluded because report mentioned that "4 groups received an investigational vaccination regimen", but no details are provided about this vaccine (may be related to GlaxoSmithKline's RV1 vaccine) |
| RV1 Herrera 2013 | Not an RCT |
| RV1 Kazi 2017 | 1 arm of an RCT (RV1 Ali 2014) was included in this sub‐study analysing histo‐blood group antigens |
| RV1 Kompithra 2014 | No placebo group: immunogenicity for 3 versus 5 doses RV1 |
| RV1 Lazarus 2017 | All received RV vaccine with or without zinc and/or probiotic supplements |
| RV1 Lu 2013 | Not an RCT |
| RV1 NCT00353366 | Ongoing non‐randomized study |
| RV1 NCT00382772 2008 | RCT comparing RV1 liquid formulation to lyophilized formulation, no placebo |
| RV1 NCT00653198 | Ongoing case‐control study |
| RV1 NCT00655187 | Ongoing case‐control study |
| RV1 NCT01162590 | Ongoing study with adult participants |
| RV1 NCT01177826 | Ongoing observational study |
| RV1 NCT01273077 | Ongoing observational study |
| RV1 NCT01339221 | Ongoing observational study |
| RV1 Plosker 2011 | Economic analysis |
| RV1 Ramani 2016 | No placebo group: RV1 co‐administered with IPV or with OPV was compared. |
| RV1 Rojas 2007 | Viral conversion on the same population of RV1 Ruiz‐Palac 06‐LA/EU (included trial) |
| RV1 Rongsen‐Chandola 2014 | Infants were breastfed versus not breastfed 30 mins prior and post RV1 administration. No placebo group. |
| RV1 Suryakiran 2011 | Not RCT, integrated safety summary |
| RV1 Taddio 2015 | To assess pain at injection site of other vaccines, participants were randomised to 1. oral RV1 then other injected vaccines then oral sucrose, or to 2. oral sucrose then other injected vaccines then oral RV1 |
| RV1 Zaman 2016 | Study investigated co‐administration of Measles‐rubella vaccines with RV vaccine |
| RV5 / BRV‐TV Saluja 2017 | RCT of BRV‐TV versus RV5 |
| RV5 ACTRN12611000559910 | Ongoing observational study |
| RV5 Ciarlet 2008 | RCT of RV5 vaccine, but no placebo group reported |
| RV5 El Khoury 2011 | Mathematical model in Brazil |
| RV5 El Khoury 2011a | Mathematical model in six Asian countries |
| RV5 Martinon‐Torres 2017 | RCT comparing standard versus alternative formulation of RV5 |
| RV5 McGrath 2014 | Not an RCT |
| RV5 NCT00130832 2010 | Not RCT; open‐label study investigating different schedules of rotavirus and polio vaccine combinations without placebo |
| RV5 NCT00496054 | Ongoing non‐randomized study |
| RV5 NCT01926015 | Staggered versus concomitant administration of DTP‐IPV with RV5 |
| RV5 Saleh 2018 | Standard versus alternative schedule RV5 (NCT01960725) |
| RV5 Tugcu 2009 | RCT of RV5 vaccine, no placebo group reported |
| RV5 Uprety 2017 | Sub‐study of RV5 Levin 2017‐AF, this sub‐study only included participants in the vaccine arm and comparied HIV‐positive to HIV‐exposed but uninfected infants. |
| RV5 Vesikari 2011 | RCT of RV5 and MenCC vaccines ‐ concomitant or sequential administration, no placebo group reported |
| RV5 Weinberg 2017 | Sub‐study of selected participants from RV5 Levin 2017‐AF, reporting only irrelevant outcomes for this review. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | "A Phase 1 double‐blind, randomized study to compare the safety, tolerability and immunogenicity of oral RV3‐BB rotavirus vaccine and placebo in infants, children and male adults" |
| Methods | "Randomized controlled trial, parallel assignment" |
| Participants |
Number: 60 (target) Description: cohort 3: infants (male and female) aged 6 to 8 weeks inclusive, in good health |
| Interventions | 1 mL oral dose administered once 1. live attenuated human rotavirus vaccine RV3‐BB 2. Placebo |
| Outcomes | 1. Adverse events 2. Serologic markers of rotavirus immunity (immunoglobulin G (IgG) and immunoglobulin A (IgA), neutralizing antibodies (NAs)) 3. Presence of RV3‐BB rotavirus vaccine in faecal extracts |
| Starting date | 27 January 2010 Completion: not stated |
| Contact information | Dr Carl Kirkwood, Murdoch Childrens Research Institute 4th Floor, Front Entry Building Royal Children’s Hospital Flemington Road Parkville, Victoria 3052, Australia carl.kirkwood@mcri.edu.au |
| Notes |
Location: Australia Registration number: ACTRN12610000525088 (Australian New Zealand Clinical Trials Registry) Source of funding: Murdoch Childrens Research Institute |
| Trial name or title | "Clinical trial on Rotavirus vaccine to check consistency of different lots of vaccines manufactured and to check vaccine interference with other childhood vaccines given under universal immunization program in India" |
| Methods | Randomized, parallel‐group, multiple arm trial |
| Participants |
Number: 1500 Description: Healthy infants, age 6‐8 weeks |
| Interventions | 1.3 doses Rotasiil/BRV‐PV 2. 3 doses RV1 2 mL orally with routine vaccinations at 6, 4 and 10 weeks of age |
| Outcomes | 1. Rotavirus Immunogenicity 2. Immunogenicity of other vaccines 3. Immediate adverse events |
| Starting date | November 2015 Completion: not stated |
| Contact information | Dr Prasad Kulkarni; drpsk@seruminstitute.com |
| Notes |
Location: India Registration number: CTRI/2015/07/006034 Source of funding: Serum Institute of India Pvt Ltd. |
| Trial name or title | "Randomized open label study to compare immunogenicity and safety of ROTAVAC® and ROTARIX® rotavirus vaccine" |
| Methods | Randomized, parallel‐group, active controlled trial |
| Participants |
Number: 464 Description: Healthy infants, age 6 ‐ 8 weeks |
| Interventions | 1. 3 doses ROTAVAC®: 0.5 mL single dose containing NLT 105.0 FFU of live rotavirus116E 2. 2 doses RV1: Each 1‐mL dose contains a suspension of at least 106.0 median Cell Culture Infective Dose (CCID50) Schedule: 4‐week interval between doses |
| Outcomes | 1. Immunogenicity (GMTs) 2. Safety solicited for 7 days 3. SAEs throughout the study period |
| Starting date | December 2015 Completion: not stated |
| Contact information | Dr Binod Sah, binod3161@bharatbiotech.com |
| Notes |
Location: India Registration number: CTRI/2015/12/006428 Source of funding: Bharat Biotech |
| Trial name or title | "Phase I/II, Randomized, Double‐blind, Placebo‐controlled, Dosage Selection (10e5.5 or 10e6.25 FFU of Each Constituent Serotype Per 0.5 mL) Study to Evaluate the Safety, Tolerability, and Immunogenicity of a 3‐dose Series of Live Attenuated Tetravalent (G1‐G4) Bovine‐Human Reassortant Rotavirus Vaccine [BRV‐TV] Administered to Healthy Indian Infants" |
| Methods | "Randomized, Placebo Control, Safety Study, Parallel Assignment, Double Blind (Subject, Caregiver, Investigator)" |
| Participants |
Number: 90 (target) Description: healthy infants of either sex, 6 to 8 weeks of age at time of enrolment |
| Interventions | 1. Live attenuated tetravalent (G1 ‐ G4) bovine‐human reassortant rotavirus vaccine 2. Placebo |
| Outcomes | 1. Reactogenicity 2. Adverse events 3. Shedding of vaccine rotavirus in stool samples 4. Seroconversion rate 5. Sero‐response rate 6. GMT of serum IgA antibody against rotavirus |
| Starting date | 1 July 2010 Completion: not stated |
| Contact information | Gagandeep Kang, MD PhD, gkang@cmcvellore.ac.in |
| Notes |
Location: India Registration number: NCT01061658 Source of funding: Shantha Biotechnics Limited |
| Trial name or title | "The Safety and Immunogenicity Study of Rotavirus Vaccine Simultaneously Vaccinated With MR or MMR Vaccine" |
| Methods | Randomized, open label |
| Participants |
Number: 2800 (target) Description: 8 ˜ 9 months healthy child |
| Interventions | 1. RV vaccine 2. measles‐rubella vaccine 3. measles‐mumps‐rubella vaccine 4. RV + measles‐rubella vaccine 5. RV + measles‐mumps‐rubella vaccine |
| Outcomes | 1. General reactions 2. Severe adverse events 3. Antibody geometric mean titres |
| Starting date | December 2013 Completion: August 2014 |
| Contact information | Rui Ao, Sichuan Center for Disease Control and Prevention |
| Notes |
Location: China Registration number: NCT02153866 Source of funding: Sichuan Center for Disease Control and Prevention |
| Trial name or title | "Randomized, Controlled Single‐blind Clinical Study to Assess Vaccine Interchangeability Between RV5 and RV1 Using Seven Combined Anti‐rotavirus Prevention Programs" |
| Methods | Randomized, controlled, single‐blind |
| Participants |
Number: 1498 (target) Description: healthy infants 6 ‐ 10 weeks old |
| Interventions | 1. 1 dose RV1 2. 1 dose RV5 3. 1 dose RV1 + 2 doses RV5 4. 1 dose RV5 + 2 doses RV1 5. 2 doses RV5 + 1 dose RV1 6. 1 dose RV5 + 1 dose RV1 + 1 dose RV5 7. 1 dose RV1 + 1 dose RV5 + 1 dose RV1 |
| Outcomes | 1. Temperature 2. Evacuations |
| Starting date | November 2013 Completion: November 2017 |
| Contact information | Mercedes Macias Parra, MSc, National Institute of Pediatrics, Mexico |
| Notes |
Location: Mexico Registration number: NCT02193061 Source of funding: National Institute of Pediatrics, Mexico; Centro Nacional para la Salud de la Infancia y la Adolescencia; Merck Sharp & Dohme Corp. |
| Trial name or title | "Potential Mechanisms for Intussusception After Rotavirus Vaccine‐Pilot Study" |
| Methods | Prospective randomized clinical trial , phase 4 |
| Participants |
Number: 101 Description: Healthy infants aged 6 ‐ 13 weeks |
| Interventions | 1. RV1, single oral dose of licensed rotavirus vaccine, given alone 2. RV1, with other routine vaccines 3. RV5, single oral dose of licensed rotavirus vaccine given alone 4. RV5, with other routine vaccines |
| Outcomes | 1. The effects of RV1 and RV5 with or without other routine immunizations on gastrointestinal anatomy 2. The feasibility of conducting a larger‐scale study as determined by study recruitment rates and percentage of completed study visits |
| Starting date | November 2015 Completion: May 2017 (actual primary completion date), May 2018 (estimated study completion date) |
| Contact information | Mary A. Staat, MD, MPH Children's Hospital Medical Center, Cincinnati Ohio, United States, 45219 |
| Notes |
Location: USA Registration number: NCT02542462 Source of funding: Children's Hospital Medical Center, Cincinnati, USA |
| Trial name or title | "Safety and Immunogenicity Study of Trivalent P2‐VP8 Subunit Rotavirus Vaccine in Adults, Toddlers and Infants" |
| Methods | Phase I/II double‐blind, randomized, placebo‐controlled trial |
| Participants |
Number: 609 Description: Healthy adults (≥ 18 and ≤ 45 years), toddlers (≥ 2 and ≤ 3 years), and infants (≥ 6 and ≤ 8 weeks) |
| Interventions | 1. Trivalent P2VP8 (15 mcg) 2. Trivalent P2VP8 (30 mcg) 3. Trivalent P2VP8 (90 mcg) 4. Placebo |
| Outcomes | 1. Serious adverse events 2. Adverse events 3. Participants with vaccine‐related reactogenicity events 4. Proportion of infants with anti‐P2VP8 IgG sero‐responses 5. Proportion of infants with anti‐P2VP8 IgA sero‐responses 6. Proportion of infants with neutralizing antibody responses |
| Starting date | February 2016 Completion: January 2018 |
| Contact information | Michelle Groom, MBBCh Chris Hani Baragwanath Hospital |
| Notes |
Location: South Africa Registration number: NCT02646891 Source of funding: PATH |
| Trial name or title | "Fractional Inactivated Poliovirus Vaccine Booster and Rotavirus Study (fIPV)" |
| Methods | Open‐label phase IV, randomized controlled trial |
| Participants |
Number: 1144 Description: Infants 6 weeks of age (range: 42 ‐ 48 days) |
| Interventions | 1. RV1 at 6 and 10 weeks of age 1.1 RV1 + full dose of IPV at 14 and 22 weeks of age 1.2 RV1 + full dose of IPV at 14 weeks of age and a fractional dose IPV at 22 weeks of age 1.3 RV1 + full dose of IPV at 6 weeks of age and a fractional dose IPV at 22 weeks of age 1.4 RV1 + fractional doses of IPV at 6, 14, and 22 weeks of age 2. RV5 at 6, 10, and 14 weeks of age 2.1 RV5 + full dose of IPV at 14 and 22 weeks of age 2.2 RV5 + full dose of IPV at 14 weeks of age and a fractional dose IPV at 22 weeks of age 2.3 RV5 + full dose of IPV at 6 weeks of age and a fractional dose IPV at 22 weeks of age 2.4 RV5 + fractional doses of IPV at 6, 14, and 22 weeks of age |
| Outcomes | 1. Seroconversion 4. Rotavirus IgA geometric mean titres 5. Rotavirus IgA seroconversion and geometric mean titres by secretor status, Lewis and salivary ABO blood group phenotype |
| Starting date | September 2016 Completion: December 2017 |
| Contact information | Centers for Disease Control and Prevention |
| Notes |
Location: Bangladesh Registration number: NCT02847026 Source of funding: Centers for Disease Control and Prevention |
| Trial name or title | "Safety and Immunogenicity of Rotavirus (Bio Farma) Vaccine in Adults, Children & Neonates" |
| Methods | Phase 1, mixed methods study; double‐blind, randomized study (neonates); open‐label study (adults and children) |
| Participants |
Number: 100 Description: Adults, children and neonates |
| Interventions | 1. Rotavirus (Bio Farma) Vaccine 2. Placebo |
| Outcomes | 1. Solicited symptoms 2. Adverse events 3. Serious adverse events 4. Number of infants who have abnormality value of routine haematology and biochemical evaluation that probably related to the vaccination 5. Excretion of rotavirus in stools in neonates group 6. Number of infants with ≥ 3 times increasing antibody from baseline to post‐investigational product dosing 7. Serum anti‐rotavirus immunoglobulin (Ig)A 8. Serum neutralizing antibody 9. Geometric mean titre |
| Starting date | April 2018 Completion: December 2018 (estimated) |
| Contact information | Novilia Sjafri Bachtiar; novilia@biofarma.co.id |
| Notes |
Location: Indonesia Registration number: NCT03462108 Source of funding: PT Bio Farma |
| Trial name or title | "A Phase II Randomized, Double Blind, Parallel Group Dose‐ranging Study of Oral RV3‐BB Rotavirus Vaccine" |
| Methods | Phase II randomized, controlled trial. Double‐blind |
| Participants |
Number: 688 Description: up to 18 weeks (Child) |
| Interventions | 1. RV3‐BB 2. Placebo |
| Outcomes | 1.Cumulative anti‐rotavirus serum IgA response 2. Cumulative vaccine take and components of vaccine take (serum anti rotavirus IgA response or shedding of RV3‐BB) 3. Adverse events 4. Serious adverse events 5. Diarrhoea |
| Starting date | April 2018 Completion: May 2019 (primary completion date estimated), August 2019 (Estimated study completion date) |
| Contact information | Julie Bines, MD, +61393454107, julie.bines@mcri.edu.au |
| Notes |
Location: Malawi Registration number: NCT03483116 Source of funding: Murdoch Childrens Research Institute |
| Trial name or title | "A phase II, double blind randomized, placebo controlled study to assess the safety reactogenicity and immunogenicity of three doses of GSK Biologicals (South Africa)" |
| Methods | "randomized, controlled study with three parallel groups with balanced allocation (1:1:1)" |
| Participants | Target number: 271 Description: participants' parents/guardians who could comply with the protocol requirements (e.g. completion of diary cards, return for follow‐up visits); male or female aged 6 to 10 weeks of age at the time of first vaccination; written informed consent from parents/guardians; born after a gestation period of 36 to 42 weeks |
| Interventions | 1. RIX4414 (RV1): 2 doses vaccine at 106.5 CCID50 viral concentration plus 1 dose of placebo 2. Placebo: 3 doses |
| Outcomes | 1. Seroprotection for each polio serotype (primary) 2. Vaccine take 3. Viral shedding 4. Presence of rotavirus in diarrhoeal stools 5. Anti‐poliovirus antibody titres 6. Serum anti‐rotavirus immunoglobulin A (IgA) antibody titres 7. Solicited symptoms 8. Unsolicited adverse events 9. Serious adverse events |
| Starting date | 1 January 2001 Anticipated end date: 1 January 2003, completed |
| Contact information | Dr Duncan Steele (steeled@who.int), WHO |
| Notes |
Location: South Africa Registration number: ISRCTN86632774 Source of funding: RAPID trials (USA); WHO (Switzerland) |
| Trial name or title | "Optimising Rotavirus Vaccine in Aboriginal Children" |
| Methods | Phase 4, double‐blind, randomized controlled trial |
| Participants |
Number: 1000 Description: infants aged ≥ 6 months and < 12 months |
| Interventions | 1. RV1 2. Placebo |
| Outcomes | 1.Time to medical attendance (hospitalization, emergency department or medical clinic presentation) for which primary reason for presentation is presumed or confirmed acute gastroenteritis or acute diarrhoea illness before age 36 months 2. Anti‐rotavirus IgA seroconversion 3.Time to hospitalization for which the primary coded reason for admission is presumed or confirmed acute gastroenteritis or acute diarrhoea illness before age 36 months 4. Time to hospitalization for which rotavirus confirmed diarrhoea illness occurs before age 36 months 5. Rotavirus infection meeting the jurisdictional case definition 6. Change in anti‐rotavirus IgA log titre between administration of intervention (RV1/placebo) and 28 to 55 days post‐dose 7. The occurrence of intussusception fulfilling Brighton criteria 8. Serious adverse events |
| Starting date | March 2018 Completion: December 2020 (estimated) |
| Contact information | Tom Snelling, tom.snelling@telethonkids.org.au Carly McCallum, carly.foulis@telethonkids.org.au |
| Notes |
Location: Australia Registration number: NCT02941107 Source of funding: Telethon Kids Institute |
| Trial name or title | Co‐administration of a human rotavirus vaccine Rix4414 with DTPw‐HBv vaccines: immunogenicity and reactogenicity in healthy infants |
| Methods | Randomized controlled trial |
| Participants |
Number: 308 Description: healthy infants 11 to 17 weeks of age |
| Interventions | 1. RIX4414 vaccine 2. Placebo |
| Outcomes | 1. Immunogenicity 2. Safety |
| Starting date | Not reported |
| Contact information | GlaxoSmithKline |
| Notes |
Location: not reported Registration number: not reported Source of funding: GlaxoSmithKline |
| Trial name or title | "Safety, Reactogenicity and Immunogenicity of Heat‐stable Rotavirus Vaccine (HSRV) in Adults and Infants" |
| Methods | Phase I/II, randomized, single‐blind trial |
| Participants |
Number: 100 Description: Healthy infants of either sex, 6 ‐ 8 weeks of age; healthy adults |
| Interventions | 1. Hilleman Labs heat stable pentavalent vaccine 2. RV5 Schedule: 3 doses at 4‐week intervals |
| Outcomes | 3. Any adverse event 4. Serious adverse events 5. Anti‐Rotavirus IgA sero‐response rate 7. Viral shedding |
| Starting date | June 2016 Completion: April 2017 |
| Contact information | K Zaman, MBBS, PhD; International Center for Diarrheal Disease Research, Bangladesh |
| Notes |
Location: Bangladesh Registration number: NCT02728869 Source of funding: MSD Wellcome Trust Hilleman Laboratories Pvt. Ltd. |
BRV: bovine‐human reassortant vaccine; GMT: geometric mean titre; SAE: serious adverse event
Contributions of authors
Hanna Bergman: created ‘Summary of findings' tables, screened references, extracted, input and analyzed data, including ‘Risk of bias' assessments, and updated the review text for the 2012 update and this review update.
Nigel Cunliffe: provided guidance on inclusion criteria, review structure and content; and commented on ‘Summary of findings' and review drafts. He updated the Background and Discussion sections, and commented on ‘Summary of findings' and review drafts for this review update.
Femi Pitan: piloted data extraction form, provided guidance on inclusion criteria, and helped write the Background. He commented on review drafts for this review update.
Nicholas Henschke: screened abstracts and full texts, extracted and analyzed data, assessed risk of bias, and reviewed ‘Summary of findings' tables and the manuscript for this review update.
Karla Soares‐Weiser: updated review methods, designed data forms, took the lead in extracting and analyzing data, including ‘Risk of bias' assessments; and wrote the review. She commented on review drafts for this review update.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Project number 300342‐104
-
Initiative for Vaccine Research (IVR), World Health Organization (WHO), Switzerland.
A large part of this review update is based on a systematic review of RCTs and observational studies that was funded by the IVR department, WHO
Declarations of interest
Hanna Bergman: received payment for work on this review from Cochrane Response, an evidence services unit operated by the Cochrane Collaboration. Cochrane Response was contracted by the WHO to produce a systematic review upon which a part of this review update is based (see ‘Sources of support').
Nigel Cunliffe: received research grant support and honoraria for participation in Data Safety Monitoring Boards from GlaxoSmithKline Biologicals.
Femi Pitan: none known.
Nicholas Henschke: received payment for work on this review from Cochrane Response, an evidence services unit operated by the Cochrane Collaboration. Cochrane Response was contracted by the WHO to produce a systematic review upon which a part of this review update is based (see ‘Sources of support').
Karla Soares‐Weiser: has received payment in the past (not for the current update) to conduct this review from the DFID UK via the Effective Health Care Research Programme Consortium (see ‘Sources of support').
Unchanged
References
References to studies included in this review
- Anh DD, Carlos CC, Thiem DV, Hutagalung Y, Gatchalian S, Bock HL, et al. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 (Rotarix) oral suspension (liquid formulation) when co‐administered with expanded program on immunization (EPI) vaccines in Vietnam and the Philippines in 2006‐2007. Vaccine 2011;29(11):2029‐36. [DOI] [PubMed] [Google Scholar]; GlaxoSmithKline[109216‐063]. A phase II, randomized, double‐blind, placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Philippines. www.gsk‐studyregister.com/study/3204 (accessed 12 December 2018). ; NCT00432380. Immunogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' oral live attenuated human rotavirus (HRV) liquid vaccine (GSK 357941A) in healthy infants. clinicaltrials.gov/show/NCT00432380 (first received 7 February 2007).
- Anh DD, Carlos CC, Thiem DV, Hutagalung Y, Gatchalian S, Bock HL, et al. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 (Rotarix) oral suspension (liquid formulation) when co‐administered with expanded program on immunization (EPI) vaccines in Vietnam and the Philippines in 2006‐2007. Vaccine 2011;29(11):2029‐36. [DOI] [PubMed] [Google Scholar]; Anh DD, Thiem VD, Hutagalung Y, Bock HL, Suryakiran P, Delem A, et al. Immunogenicity, reactogenicity and safety of the oral live attenuated human rotavirus vaccine RIX4414 (Rotarix™) oral suspension (liquid formulation) in Vietnamese Infants. 13th International Congress on Infectious Diseases Abstracts, Poster Presentations. Kuala Lumpur, Malaysia; June 19–22, 2008. [Google Scholar]; GlaxoSmithKline[105722‐051]. A phase II, randomized, double‐blind, placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Vietnam. www.gsk‐studyregister.com/study/3012 (accessed 12 December 2018). ; NCT00345956. A placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GSK Bio oral live attenuated human rotavirus (HRV) liquid vaccine, when given to healthy infants, in Vietnam. clinicaltrials.gov/show/NCT00345956 (first received 29 June 2006).
- Bernstein DI, Smith VE, Sherwood JR, Schiff GM, Sander DS, DeFeudis D, et al. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89‐12. Vaccine 1998;16(4):381‐7. [DOI] [PubMed] [Google Scholar]
- Bernstein DI, Sack DA, Reisinger K, Rothstein E, Ward RL. Second‐year follow‐up evaluation of live, attenuated human rotavirus vaccine 89‐12 in healthy infants. Journal of Infectious Diseases 2002;186(10):1487‐9. [DOI] [PubMed] [Google Scholar]; Bernstein DI, Sack DA, Rothstein E, Reisinger K, Smith VE, O'Sullivan D, et al. Efficacy of live, attenuated, human rotavirus vaccine 89‐12 in infants: a randomised placebo‐controlled trial. Lancet 1999;354(9175):287‐90. [DOI] [PubMed] [Google Scholar]
- Colgate ER, Haque R, Dickson DM, Carmolli MP, Mychaleckyj JC, Nayak U, et al. Delayed dosing of oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Clinical Infectious Diseases 2016;63(5):634‐41. [DOI] [PubMed] [Google Scholar]; Kirkpatrick BD, Colgate ER, Mychaleckyj JC, Haque R, Dickson DM, Carmolli MP, et al. The "Performance of Rotavirus and Oral Polio Vaccines in Developing Countries" (PROVIDE) study: description of methods of an interventional study designed to explore complex biologic problems. American Journal of Tropical Medicine and Hygiene 2015;92(4):744‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee B, Dickson DM, DeCamp AC, Colgate ER, Diehl SA, Uddin MI, et al. Histo‐blood group antigen phenotype determines susceptibility to genotype‐specific rotavirus infections and impacts measures of rotavirus vaccine efficacy. Journal of Infectious Diseases 2018;217(9):1399‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee B, Diehl SA, Colgate ER, Dickson DM, Uddin MI, Sharmin S, et al. Lewis antigen and secretor status mediate susceptibility to p‐genotype specific rotavirus infections but do not affect rotavirus vaccine performance among infants in Bangladesh. American Journal of Tropical Medicine and Hygiene 2017;97:226. [Google Scholar]; NCT01375647. Exploration of the biologic basis for underperformance of oral polio and rotavirus vaccines in Bangladesh PROVIDE. clinicaltrials.gov/show/NCT01375647 (first received 17 June 2011). ; Rogawski ET, Platts‐Mills JA, Colgate ER, Haque R, Zaman K, Petri WA, et al. Quantifying the impact of natural immunity on rotavirus vaccine efficacy estimates: a clinical trial in Dhaka, Bangladesh (PROVIDE) and a simulation study. Journal of Infectious Diseases 2018;217(6):861‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy PH. A short report on the highlights of world‐wide development of RIX4414: a North American experience comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine (RIX4414) in infants in the United States and Canada. Vaccine 2006;24(18):3780‐1. [DOI] [PubMed] [Google Scholar]; Dennehy PH, Brady RC, Halperin SA, Ward RL, Alvey JC, Fischer FH Jr, et al. Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatric Infectious Disease Journal 2005;24(6):481‐8. [DOI] [PubMed] [Google Scholar]; GlaxoSmithKline[444563‐005]. A phase II, double‐blind, randomized, placebo‐controlled study of two doses of GlaxoSmithKline Biologicals’ live attenuated human rotavirus (HRV) vaccine at different virus concentrations (10 5.2 and 10 6.4 ffu) in healthy infants (approximately 2 months of age at first dose) following a 0, 2 month schedule and previously uninfected with human rotavirus. www.gsk‐studyregister.com/study/6783 (accessed 12 December 2018). ; NCT00729001. Study of two doses of GSK Biologicals' live attenuated HRV vaccine (two different formulations) in healthy infants. clinicaltrials.gov/ct2/show/NCT00729001 (accessed 6 August 2008).
- GlaxoSmithKline[444563‐021]. A phase II, double‐blind, randomized, placebo‐controlled clinical study to assess the immunogenicity and reactogenicity of three doses of a modified vaccine formulation versus GlaxoSmithKline (GSK) Biologicals’ live attenuated human rotavirus (HRV) vaccine when orally administered to healthy infants at 2, 4 and 6 months of age. www.gsk‐studyregister.com/study/6789 (accessed 12 December 2018).
- GlaxoSmithKline[444563‐033]. A phase III, randomized, double‐blind and placebo‐controlled study to assess the clinical consistency of three production lots of GSK Biologicals’ HRV vaccine in terms of immunogenicity and safety when given to healthy infants at 2 and 4 months of age. www.gsk‐studyregister.com/study/6794 (accessed 12 December 2018). ; López P, Galan Herrera JF, Cervantes Y, Costa Clemens SA, Aguirre F, Yarzabal JP. Three consecutive production lots of the human monovalent RIX4414 G1P(8) rotavirus vaccine, Rotarix™ induce a consistent immune response in Latin American infants. [Poster]. 4th World Congress of The World Society for Pediatric Infectious Diseases; 2005 September 01‐04; Warsaw, Poland. 2005. [Not available for review] [Google Scholar]; NCT00757770. Assessment of Clinical Consistency of Three Production Lots of GSK Biologicals' HRV Vaccine. clinicaltrials.gov/ct2/show/NCT00757770 (first received 23 September 2008).
- GlaxoSmithKline[103478‐041]. A phase IIIb, double‐blind, randomized, placebo‐controlled, multicentre study to assess the immunogenicity, safety and reactogenicity of 2 doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants (6‐12 weeks of age at first dose) previously uninfected with human rotavirus. www.gsk‐studyregister.com/study/2714 (accessed 12 December 2018). ; NCT00134732. Assess the immunogenicity, safety & reactogenicity of 2 doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants (6‐12 weeks of age at first dose) previously uninfected with human rotavirus. clinicaltrials.gov/ct2/show/record/NCT00134732 (first receiveded 25 August 2005).
- GlaxoSmithKline[101555]. A phase II, double‐blind, randomized, placebo‐controlled study to compare the immunogenicity, reactogenicity and safety of 2 different formulations of GlaxoSmithKline (GSK) Biologicals’ live attenuated human rotavirus (HRV) vaccine given as a two‐dose primary vaccination in healthy infants previously uninfected with HRV. www.gsk‐studyregister.com/study/2632 (accessed 12 December 2018).
- GlaxoSmithKline [107625 (Rota‐056)]. Efficacy, safety, reactogenicity and immunogenicity study of the lyophilised formulation of Rotarix vaccine in healthy Japanese infants. www.gsk‐studyregister.com/study/3131 (accessed 12 December 2018). ; Kawamura N, Tokoeda Y, Mori S, Ohshima M, Okahata H, Ueda D, et al. Efficacy of human rotavirus vaccine RIX4414 in Japanese infants from 2 weeks post dose 2 up to data lock point. 28th Meeting of European Society for Paediatric Infectious Diseases (ESPID); 2010 May 04‐08; Nice, France. 2010. [Google Scholar]; Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Doorn LJ, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011;29(37):6335‐41. [DOI] [PubMed] [Google Scholar]; NCT00480324. Efficacy, safety, reactogenicity and immunogenicity study of the lyophilised formulation of Rotarix vaccine in healthy Japanese infants. clinicaltrials.gov/show/NCT00480324 (first received 17 November 2010).
- GlaxoSmithKline[103477‐039]. A phase IIIb, partially blind, randomized, placebo‐controlled study to asses the effect on immunogenicity of administration of vaccine without buffering agent and to assess heat stability in terms of immunogenicity, reactogenicity and safety of GlaxoSmithKline Biologicals’ oral live attenuated human rotavirus (HRV) vaccine following a 0, 2 month schedule, in healthy infants previously uninfected with human rotavirus. www.gsk‐studyregister.com/study/2713 (accessed 12 December 2018). ; Kerdpanich A, Chokephaibulkit K, Watanaveeradej V, Vanprapar N, Hutagalung Y, Han HH, et al. Exposure to elevated temperature of 37°C for 7 days does not affect immunogenicity and reactogenicity of RIX4414. [Poster]. dsRNA Virus Meeting; 2006 October 21‐26; Cape Town, South Africa. 2006. [Not available for review] [Google Scholar]; Kerdpanich A, Chokephaibulkit K, Watanaveeradej V, Vanprapar N, Simasathien S, Phavichitr N, et al. Immunogenicity of a human rotavirus vaccine (RIX4414) after storage at 37 degrees C for seven days. Human Vaccine 2011;7(1):74‐80. [DOI] [PubMed] [Google Scholar]; Kerdpanich A, Chokephaibulkit K, Watanaveeradej V, Vanprapar N, Simasathien S, Phavichitr N, et al. Immunogenicity of a live‐attenuated human rotavirus RIX4414 vaccine with or without buffering agent. Human Vaccines 2010;6(3):254‐62. [DOI] [PubMed] [Google Scholar]; NCT00169455. Assess the effect on immunogenicity of administration of vaccine without buffering agent & assess heat stability in terms of immunogenicity, reactogenicity & safety of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine following a 0,2 m schedule, in healthy infants previously uninfected with human rotavirus. clinicaltrials.gov/show/NCT00169455 (first received 15 September 2005).
- GlaxoSmithKline[112269‐068]. Immunogenicity, reactogenicity and safety study to evaluate two doses of the lyophilised formulation of the human rotavirus (HRV) vaccine when administered to healthy Korean infants previously uninfected with HRV. www.gsk‐studyregister.com/study/3589 (accessed 12 December 2018). ; Kim JS, Bae CW, Lee KY, Park MS, Choi YY, Kim KN, et al. Immunogenicity, reactogenicity and safety of a human rotavirus vaccine (RIX4414) in Korean infants: a randomized, double‐blind, placebo‐controlled, phase IV study. Human Vaccines and Immunotherapeutics 2012;8(6):806‐12. [DOI] [PubMed] [Google Scholar]; NCT00969228. Study to Evaluate Immunogenicity, Reactogenicity and Safety of Rotarix™ Vaccine in Korean Infants. clinicaltrials.gov/ct2/show/NCT00969228 (first received 1 September 2009).
- GlaxoSmithKline[113552‐073]. Reactogenicity and safety of a single dose of GlaxoSmithKline (GSK) Biologicals' human rotavirus (HRV) vaccine (444563) in healthy children. www.gsk‐studyregister.com/study/3957 (accessed 12 December 2018). ; Li RC, Li YP, Mo ZJ, Luo D, Huang T, Kong JL, et al. Reactogenicity and safety of a liquid human rotavirus vaccine (RIX4414) in healthy adults, children and infants in China: randomized, double‐blind, placebo‐controlled Phase I studies. Human Vaccines and Immunotherapeutics 2013;9(8):1638‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01086436. Study to evaluate the safety of Rotarix in Chinese children. clinicaltrials.gov/ct2/show/NCT01086436 (first received on 15 March 2010).
- GlaxoSmithKline[113518‐074]. Reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated liquid human rotavirus (HRV) vaccine 444563, in healthy infants. www.gsk‐studyregister.com/study/3947 (accessed 12 December 2018). ; Li RC, Li YP, Mo ZJ, Luo D, Huang T, Kong JL, et al. Reactogenicity and safety of a liquid human rotavirus vaccine (RIX4414) in healthy adults, children and infants in China: randomized, double‐blind, placebo‐controlled Phase I studies. Human Vaccines and Immunotherapeutics 2013;9(8):1638‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01107587. Study to assess the safety of GSK Biologicals' liquid human rotavirus vaccine in healthy infants. clinicaltrials.gov/ct2/show/NCT01107587 (accessed 21 April 2010).
- GlaxoSmithKline[113808‐075]. Efficacy, immunogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' Oral Live Attenuated Liquid Human Rotavirus (HRV) Vaccine (444563), in healthy infants. www.gsk‐studyregister.com/study/4036 (accessed 12 December 2018). ; Li RC, Huang T, Li Y, Luo D, Tao J, Fu B, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo‐controlled trial in China. Human Vaccines and Immunotherapeutics 2014;10(1):11‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li RC, Huang T, Li Y, Wang LH, Tao J, Fu B, et al. Immunogenicity and reactogenicity of the human rotavirus vaccine, RIX4414 oral suspension, when co‐administered with routine childhood vaccines in Chinese infants. Human Vaccines and Immunotherapeutics 2016;12(3):785‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01171963. Efficacy, immunogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' oral live attenuated liquid human rotavirus (HRV) vaccine (444563), in healthy infants. clinicaltrials.gov/show/NCT01171963 (first received 29 July 2010).
- Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, Turner AM, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double‐blind, placebo controlled trial. Vaccine 2012;30(Suppl 1):A36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]; Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New England Journal of Medicine 2010;362(4):289‐98. [DOI] [PubMed] [Google Scholar]; Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, et al. Efficacy and immunogenicity of two or three dose rotavirus‐vaccine regimen in South African children over two consecutive rotavirus seasons: a randomized, double‐blind, placebo‐controlled trial. Vaccine 2012;30(Suppl 1):A44‐51. [DOI] [PubMed] [Google Scholar]; NCT00241644. Multi‐center study to assess the efficacy, safety and immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00241644 (first received 19 October 2005). ; NCT00598468. Multi‐center study to assess efficacy, safety & immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00598468 (first received 19 October 2005). ; Steele AD, Neuzil KM, Cunliffe NA, Madhi SA, Bos P, Ngwira B, et al. Human rotavirus vaccine Rotarix provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infectious Diseases 2012;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, Turner AM, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double‐blind, placebo controlled trial. Vaccine 2012;30(Suppl 1):A36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]; Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New England Journal of Medicine 2010;362(4):289‐98. [DOI] [PubMed] [Google Scholar]; NCT00241644. Multi‐center study to assess the efficacy, safety and immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00241644 (first received 19 October 2005). ; NCT00598468. Multi‐center study to assess efficacy, safety & immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00598468 (first received 19 October 2005). ; Steele AD, Neuzil KM, Cunliffe NA, Madhi SA, Bos P, Ngwira B, et al. Human rotavirus vaccine Rotarix provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infectious Diseases 2012;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New England Journal of Medicine 2010;362(4):289‐98. [DOI] [PubMed] [Google Scholar]; Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, et al. Efficacy and immunogenicity of two or three dose rotavirus‐vaccine regimen in South African children over two consecutive rotavirus seasons: a randomized, double‐blind, placebo‐controlled trial. Vaccine 2012;30(Suppl 1):A44‐51. [DOI] [PubMed] [Google Scholar]; NCT00241644. Multi‐center study to assess the efficacy, safety and immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00241644 (first received 19 October 2005). ; NCT00598468. Multi‐center study to assess efficacy, safety & immunogenicity of 2 or 3 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations in healthy infants. clinicaltrials.gov/show/NCT00598468 (first received 19 October 2005). ; Steele AD, Neuzil KM, Cunliffe NA, Madhi SA, Bos P, Ngwira B, et al. Human rotavirus vaccine Rotarix provides protection against diverse circulating rotavirus strains in African infants: a randomized controlled trial. BMC Infectious Diseases 2012;12:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GlaxoSmithKline[103792‐044]. A phase IIIb, randomised, multicentre double‐blind, placebo‐controlled study of the immunogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414) as primary dosing in healthy infants in India of approximately 8 weeks of age at the first dose. www.gsk‐studyregister.com/study/2723 (accessed 12 December 2018). ; NCT00289172. A multicenter study of the immunogenicity & safety of 2 doses of GSK Biologicals’ oral live attenuated human rotavirus vaccine (RIX4414) as primary dosing of healthy infants in India aged approximately 8 wks at the time of the first dose. clinicaltrials.gov/ct2/show/record/NCT00289172 (first received 9 February 2006). ; Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Human Vaccines 2009;5(6):414‐9. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline[104021‐DTPw CSL‐HBVGOD‐005]. A phase III, partially blind, randomized study to evaluate the immunogenicity, safety and reactogenicity of GlaxoSmithKline (GSK) Biologicals’ Tritanrix™‐HepB and GSK Biologicals Kft’s DTPw‐HBV vaccines as compared to concomitant administration of Commonwealth Serum Laboratory’s (CSL’s) DTPw (Triple Antigen™) and GSK Biologicals’ HBV (Engerix™‐B), when co‐administered with GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine, to healthy infants at 3, 4½ and 6 months of age, after a birth dose of hepatitis B vaccine. www.gsk‐studyregister.com/study/2753 (accessed 12 December 2018). ; NCT00158756. Assess immunogenicity & reactogenicity of 2 formulations of GSK's DTPw‐HBV vaccines vs concomitant admn of CSL's DTPw & GSK's HBV vaccine, co‐admnd with GSK's rotavirus vaccine, to infants at 3, 4½ & 6 mths, after birth dose of HBV vaccine. clinicaltrials.gov/show/NCT00158756 (first received 12 September 2005).
- GlaxoSmithKline[106481‐054]. Phase IIIb, Double Blind, Randomised, Placebo‐Controlled, Multi‐Country/Centre, Study to Assess Safety, Reactogenicity & Immunogenicity of 2 Doses of GSK Biologicals' Oral Live Attenuated Human Rotavirus (HRV) Vaccine in Pre‐Term Infants. www.gsk‐studyregister.com/study/3064 (accessed 12 December 2018). ; NCT00420745. Phase IIIb, double blind, randomised, placebo‐controlled, multi‐country/centre, study to assess safety, reactogenicity & immunogenicity of 2 doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine in pre‐term infants. clinicaltrials.gov/show/NCT00420745 (first received 11 January 2007). ; Omenaca F, Sarlangue J, Szenborn L, Nogueira M, Suryakiran PV, Smolenov IV, et al. Safety, reactogenicity and immunogenicity of the human rotavirus vaccine in preterm European infants: a randomized phase IIIb study. Pediatric Infectious Disease Journal 2012;31(5):487‐93. [DOI] [PubMed] [Google Scholar]
- Vos B, Gillard P, Cheuvart B. RIX4414 vaccine efficacy against rotavirus gastroenteritis due to G2P[4] strain. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2006 September 27‐30; San Francisco, USA. 2006. [Not available for review] [Google Scholar]; Vos B, Vesikari T, Linhares AC, Salinas B, Perez‐Schael I, Ruiz‐Palacios GM, et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatric Infectious Disease Journal 2004;23(10 Suppl):179‐82. [No data for review] [DOI] [PubMed] [Google Scholar]; Emmanuel B, et al. Immunogenicity of an acellular pertussis combination vaccine co‐administered with a novel rotavirus vaccine in Singaporean infants. Asian Congress on Pediatric Infectious Diseases (ACPID); 2‐4 September 2004; Kota Kinabula, Malaysia2004. [Not available for review]; Emmanuel S, Phua KB, Goh P, Quak SH, Datta SK, Han HH, et al. Immunogenicity of an acellular pertussis combination vaccine co‐administered with a novel rotavirus vaccine in Singaporean infants. 23rd Annual Meeting of European Society for Paediatric Infectious Diseases (ESPID); 2005 May 18‐20; Valencia, Spain. 2005. [Not available for review] [Google Scholar]; GlaxoSmithKline[444563‐007]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine at different viral concentrations (104.7, 105.2 and 106.1 foci forming units (ffu)) in healthy infants previously uninfected with human rotavirus and approximately three months of age, when administered concurrently with DTPa‐IPV/Hib and HBV vaccines. www.gsk‐studyregister.com/study/6785 (accessed 12 December 2018). ; NCT00429481. A study to assess the efficacy, immunogenicity and safety of 2 doses of oral live attenuated human rotavirus vaccine (Rotarix) at different viral concentrations in healthy infants. clinicaltrials.gov/ct2/show/record/NCT00429481 (first received 31 January 2007). ; Phua KB, Emmanuel SC, Goh P, Quak SH, Lee BW, Han HH, et al. A rotavirus vaccine for prophylaxis of infant rotavirus gastroenteritis: The Asian experience. Annals of the Academy of Medicine, Singapore 2006;35(1):38‐44. [PubMed] [Google Scholar]; Phua KB, Quak SH, Emmanuel S, Goh PS, Han HH, Hardt K, et al. A short report on highlights of worldwide development of RIX4414: a Singaporean experience. Vaccine 2006;24(18):3782‐3. [DOI] [PubMed] [Google Scholar]; Phua KB, Quak SH, Emmanuel S, Goh PSC, Han HH, Hardt K, et al. Highlights of world‐wide development: A Singapore experience. 3rd International Conference on Vaccines for Enteric Diseases (VED) 28‐30 April 2004; Montego Bay, Jamaica. 2004. [Not available for review] [Google Scholar]; Phua KB, Quak SH, Lee BW, Emmanuel SC, Goh P, Han HH, et al. Evaluation of RIX4414, a live, attenuated rotavirus vaccine, in a randomized, double‐blind, placebo‐controlled phase 2 trial involving 2464 Singaporean infants. Journal of Infectious Diseases 2005;192(Suppl 1):6‐16. [DOI] [PubMed] [Google Scholar]; Phua KB, Quak SH, Lim FS, Goh P, Teoh YL, Datta SK, et al. Immunogenicity, reactogenicity and safety of a diphtheria‐tetanus‐acellular pertussis‐inactivated polio and Haemophilus influenzae type b combination vaccine in a placebo‐controlled rotavirus vaccine study. Annals of the Academy of Medicine, Singapore 2008;37(7):546‐53. [PubMed] [Google Scholar]; Phua KB, et al. Immunogenicity and reactogenicity of two doses of an oral human rotavirus (HRV) vaccine at different concentrations in healthy infants from Singapore. 11th Asia Pacific Congress of Pediatrics and the 3rd Asia Pacific Congress of Pediatric Nursing; 2003; Bangkok, Thailand. 2003. [Not available for review] [Google Scholar]; Phua KB, et al. Intussusception in children: a seven‐year experience in Singapore. 24th International Congress of Pediatrics (ICP); 2004 August 15‐20; Cancun, Mexico. 2004. [Not available for review] [Google Scholar]; Vesikari T. RIX4414: A new attenuated human rotavirus vaccine. 23rd Annual Meeting of European Society for Paediatric Infectious Diseases (ESPID); 2005 May 18‐20; Valencia, Spain2005. [Not available for review]; Vesikari T, et al. High efficacy of two doses of Rotarix™ (RIX4414) against rotavirus disease in Europe, Latin‐America and Asia. Asian Congress on Pediatric Infectious Diseases (ACPID); 2006 March 07‐10; Cebu, Philippines. 2006. [Not available for review] [Google Scholar]
- Lau YL, Nelson EA, Poon KH, Chan PK, Chiu S, Sung R, et al. Efficacy, safety and immunogenicity of a human rotavirus vaccine (RIX4414) in Hong Kong children up to three years of age: a randomized, controlled trial. Vaccine 2013;31(18):2253‐9. [DOI] [PubMed] [Google Scholar]; NCT00197210. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy and safety of two doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine in healthy infants. clinicaltrials.gov/show/NCT00197210 (first received 20 September 2005). ; NCT00329745. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy and safety of two doses of GSK Biologicals' oral live attenuated human rotavirus (HRV) vaccine in healthy infants. clinicaltrials.gov/show/NCT00329745 (first received 25 May 2006). ; Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: A randomized clinical trial in an Asian population. Vaccine 2012;30(30):4552‐7. [DOI] [PubMed] [Google Scholar]; Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double‐blind, controlled study. Vaccine 2009;27(43):5936‐41. [DOI] [PubMed] [Google Scholar]; Phua KB, Lim FS, Quak SH, Lee BW, Teoh YL, Suryakiran PV, et al. Efficacy, immunogenicity and safety of a human rotavirus vaccine RIX4414 in Singaporean infants. Annals of the Academy of Medicine, Singapore 2016;45(2):44‐50. [PubMed] [Google Scholar]
- EUCTR2015‐001542‐29. Immunization of infants 6‐14 weeks of age, with GlaxoSmithKline Biologicals Rotavirus vaccine to explore the existence of transmission of rotavirus vaccine strain between twins in a family. clinicaltrialsregister.eu/ctr‐search/trial/2015‐001542‐29/results (accessed 31 August 2017). ; NCT00396630. A Phase IIIb, randomized, double‐blind, placebo‐controlled study to explore the existence of horizontal transmission of the RIX4414 vaccine strain between twins within a family. clinicaltrials.gov/show/NCT00396630 (first received 7 November 2006). ; Rivera L, Peña LM, Stainier I, Gillard P, Cheuvart B, Smolenov I, et al. Horizontal transmission of a human rotavirus vaccine strain‐‐a randomized, placebo‐controlled study in twins. Vaccine 2011;29(51):9508‐13. [DOI] [PubMed] [Google Scholar]
- Baay M, Bollaerts K, Struchiner C, Verstraeten T. Background rates of disease in Latin American children from a rotavirus vaccine study. Human Vaccines and Immunotherapeutics 2017;13(8):1916‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]; Costa Clemens SA, et al. Operational organization of a large scale phase III clinical trial of rotavirus vaccine in multiple sites and countries in Latin America. International Congress of Pediatrics (ICP); 2004 August 15‐20; Cancun, Mexico. 2004. [Not available for review] [Google Scholar]; Vos B, et al. Rotarix™: an effective way to prevent rotavirus diarrhoea and vomiting. 9th Congress of the Asian Pan Pacific Society of Paediatric Gastroenterology, Hepatology and Nutrition & 27th Annual Congress of the Malaysian Paediatric Association; 2005 June 16‐19; Kuala Lumpur, Malaysia. 2005. [Not available for review] [Google Scholar]; GlaxoSmithKline[444563‐023‐pt1]. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants. (Efficacy data from Visit 1 to Visit 4). www.gsk‐studyregister.com/study/6791 (accessed 12 December 2018). ; GlaxoSmithKline[444563‐023‐pt2]. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants. (2nd year efficacy from Visit 4 to Visit 6). www.gsk‐studyregister.com/study/6791 (accessed 12 December 2018). ; GlaxoSmithKline[444563‐023‐pt3]. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants. (Safety for the period between Visit 1 and Visit 3). www.gsk‐studyregister.com/study/6791 (accessed 12 December 2018). ; Justino MC, Araújo EC, Doorn LJ, Oliveira CS, Gabbay YB, Mascarenhas JD, et al. Oral live attenuated human rotavirus vaccine (Rotarix) offers sustained high protection against severe G9P[8] rotavirus gastroenteritis during the first two years of life in Brazilian children. Memorias do Instituto Oswaldo Cruz 2012;107(7):846‐53. [DOI] [PubMed] [Google Scholar]; Linhares AC, Velazquez FR, Perez‐Schael I, Saez‐Llorens X, Abate H, Espinoza F, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double‐blind, placebo‐controlled phase III study. Lancet 2008;371(9619):1181‐9. [DOI] [PubMed] [Google Scholar]; Lopez P, Linhares A, Pérez‐Schael I, Ruiz‐Palacios G, Costa Clemens S, Sanchez N. Early protection against severe rotavirus gastroenteritis ‐ RIX4414 experience in Latin America. 24th Annual Meeting of European Society for Paediatric Infectious Diseases (ESPID); 2006 May 03‐05; Basel, Switzerland. 2006. [Not available for review] [Google Scholar]; Macias M, Lopez P, Velazquez FR, Vergara RF, Salmeron J, Tavares JL. The rotavirus vaccine RIX4414 (Rotarix) is not associated with intussusception in one year old infants. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2005 December 16‐19; Washington DC, USA. 2005. [Not available for review] [Google Scholar]; NCT00139347. A multi‐country & multi‐center study to assess the efficacy, immunogenicity & safety of two doses of GSK Biologicals' oral live attenuated HRV vaccine given concomitantly with routine EPI vaccinations including OPV in healthy infants. clinicaltrials.gov/ct2/show/NCT00139347 (first received 31 August 2005). [Trial registration document (no data for review)]; NCT00140673. Placebo‐controlled, multi‐country & multi‐center study to assess the efficacy, safety & immunogenicity of 2 doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants. clinicaltrials.gov/ct2/show/NCT00140673 (first received 1 September 2005). [Trial registration document (no data for review)]; Perez‐Schael I, Linhares AC, Vesikari T. Two doses of the human attenuated rotavirus vaccine RIX4414 (Rotarix) show heterotypic protection in Latin America and Europe. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2005 December 16‐19; Washington DC, USA. 2005. [Not available for review] [Google Scholar]; Pike M. New rotavirus vaccine is effective and appears to have no increased risk of intussusception. Clinical Research Abstracts For Pediatricians 2006;149(1):143. [DOI] [PubMed] [Google Scholar]; Ruiz‐Palacios G, Aranza C, Velazquez FR, Richardson V, Nandi E, Macias M. Two doses of the human‐attenuated monovalent G1P[8] rotavirus vaccine, Rotarix show high efficacy in Mexican children against severe rotavirus gastroenteritis and severe overall gastroenteritis. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2005 December 16‐19; Washington DC, USA. 2005. [Not available for review] [Google Scholar]; Ruiz‐Palacios GM, Perez‐Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. New England Journal of Medicine 2006;354(1):11‐22. [DOI] [PubMed] [Google Scholar]; Velázquez FR, Abat H, Clemens S‐A Costa, Espinoza F, Gillard P, Linhares AC, et al. The human monovalent G1P[8] rotavirus vaccine, Rotarix is highly efficacious and provides crossprotection against G1 and non‐G1 serotypes. 23rd Annual meeting of European Society for Paediatric Infectious Diseases (ESPID); 2005 May 18‐20; Valencia, Spain. May 2005. [Google Scholar]; Velázquez RF, Abate H, Bouckenooghe A. RIX4414, the human G1P[8] rotavirus vaccine is highly efficacious during the second year of life. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2006 September 27‐30; San Francisco, USA. 2006. [Not available for review] [Google Scholar]; Vesikari T, O’ryan M, Abate H, Costa Clemens SA, Espinoza F, Gillard P. Overcoming the safety hurdle: the rotavirus vaccine RIX4414 is not associated with intussusception. 23rd Annual Meeting of European Society for Paediatric Infectious Diseases (ESPID); 2005 May 18‐20; Valencia, Spain. 2005. [Not available for review] [Google Scholar]
- Araujo EC, Clemens SA, Oliveira CS, Justino MC, Rubio P, Gabbay YB, et al. Safety, immunogenicity, and protective efficacy of two doses of RIX4414 live attenuated human rotavirus vaccine in healthy infants. Jornal de Pediatria 2007;83(3):217‐24. [DOI] [PubMed] [Google Scholar]; Vos B, Hardt K, Linhares AC, Ruiz‐Palacios G, Guerrero L, Salinas B. Efficacy of two doses of a human monovalent rotavirus vaccine, Rotarix™ in preventing gastro‐enteritis due to G1 and Non‐G1 rotavirus in Brazil, Mexico and Venezuela [Presentation]. 8th International Symposium on Double‐Stranded RNA Viruses; 2003 September 13‐18; Lucca, Italy. 2003. [Not available for review] [Google Scholar]; Vos B, Vesikari T, Linhares AC, Salinas B, Perez‐Schael I, Ruiz‐Palacios GM, et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatric Infectious Disease Journal 2004;23(10 Suppl):179‐82. [DOI] [PubMed] [Google Scholar]; GlaxoSmithKline[444563‐006‐Annex]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' live attenuated human rotavirus (HRV) vaccine at different virus concentrations (104.7, 105.2 and 105.8 foci forming units [ffu]) in healthy infants (approximately 2 months of age at first dose) following a 0, 2 month schedule and previously uninfected with HRV, when administered concurrently with DTPw‐HBV and Hib vaccines. [This summary presents results for the second and combined efficacy periods and results from the 3‐Dose subset. Results from the first efficacy period are presented in 444563/006 (Rota‐006) summary.]. www.gsk‐studyregister.com/study/6784 (accessed 12 December 2018). ; GlaxoSmithKline[444563‐006]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GlaxoSmithKline (GSK) Biologicals' live attenuated human rotavirus (HRV) vaccine at different virus concentrations (104.7, 105.2 and 105.8 foci forming units [ffu]) in healthy infants (approximately 2 months of age at first dose) following a 0, 2 month schedule and previously uninfected with HRV, when administered concurrently with DTPw‐HBV and Hib vaccines. www.gsk‐studyregister.com/study/6784 (accessed 12 December 2018). ; Linhares A, Perez‐Schael I, Ruiz‐Palacios M, Pernambuco E, Jacquet J, Vos B. Immunogenicity and reactogenicity of an oral human rotavirus (HRV) vaccine in Latin American infants [Poster]. 3rd World Congress of Pediatric Infectious Diseases; 2002 November 19‐23; Santiago, Chile. 2002. [Not available for review] [Google Scholar]; Linhares AC, Ruiz‐Palacios GM, Guerrero ML, Salinas B, Perez‐Schael I, Clemens SA, et al. A short report on highlights of world‐wide development of RIX4414: a Latin American experience. Vaccine 2006;24(18):3784‐5. [DOI] [PubMed] [Google Scholar]; Linhares AC, Verstraeten T, Wolleswinkel‐van den Bosch J, Clemens R, Breuer T. Rotavirus serotype G9 is associated with more‐severe disease in Latin America. Clinical Infectious Diseases 2006;43(3):312‐4. [Not available for review] [DOI] [PubMed] [Google Scholar]; NCT00385320. To assess the efficacy, immuno & safety of 2 doses of GSK HRV vaccine at different virus concentrations in healthy infants aged 2 months & previously uninfected with HRV, concurrently given with DTPw‐HBV, Hib. clinicaltrials.gov/show/NCT00385320 (first received 9 October 2006). ; Perez‐Schael I, Salinas B, Linhares A, Guerrero M, Ruiz‐Palacios G, Clemens S, et al. Protective efficacy of an oral human rotavirus (HRV) vaccine in Latin American infants [Poster]. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy ( ICAAC); San Diego, 27‐30 September 2002. 2002:18, LB‐23. [Not available for review] [Google Scholar]; Perez‐Schael I, Salinas B, Tomat M, Linhares AC, Guerrero ML, Ruiz‐Palacios GM, et al. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. Journal of Infectious Diseases 2007;196(4):537‐40. [DOI] [PubMed] [Google Scholar]; Ruiz‐Palacios G, Guerrero L, Linhares AC, et al. Efficacy of an oral human rotavirus (HRV) vaccine in preventing diarrhoea due to G1 and non‐G1 rotavirus [Presentation]. 3rd World Congress of Pediatric Infectious Diseases; 2002 November 19‐23; Santiago, Chile. 2002. [Not available for review] [Google Scholar]; Ruiz‐Palacios G, Guerrero ML, Linhares AC, et al. Two‐year efficacy of GlaxoSmithKline Biologicals' live attenuated rotavirus vaccine in Latin American children [Poster]. 24th International Congress of Pediatrics; 2004 August 15‐20; Cancun, Mexico. 2004. [Not available for review] [Google Scholar]; Ruiz‐Palacios GM. Impact of maternal antibodies on the immune response to an oral human rotavirus (HRV) vaccine in Mexican infants [Presentation]. 3rd World Congress of Pediatric Infectious Diseases; 2002 November 19‐23; Santiago, Chile. 2002. [Not available for review] [Google Scholar]; Ruiz‐Palacios GM, Guerrero ML, Bautista‐Marquez A, Ortega‐Gallegos H, Tuz‐Dzib F, Reyes‐Gonzalez L, et al. Dose response and efficacy of a live, attenuated human rotavirus vaccine in Mexican infants. Pediatrics 2007;120(2):e253‐61. [Not available for review] [DOI] [PubMed] [Google Scholar]; Salinas B, Perez Schael I, Linhares AC, Ruiz Palacios GM, Guerrero ML, Yarzabal JP, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: A randomized, placebo‐controlled trial in Latin American infants. Pediatric Infectious Disease Journal 2005;24(9):807‐16. [DOI] [PubMed] [Google Scholar]; Salinas B, Perez‐Schael I, Ruiz‐Palacios G, et al. Early protection by GlaxoSmithKline Biologicals' live attenuated rotavirus vaccine in Latin American children. [Poster]. 24th International Congress of Pediatrics; 2004 August 15‐20; Cancun, Mexico. 2004. [Not available for review] [Google Scholar]; Salinas B, Tomat MA, Yarzabal JP, et al. Efficacy of the rotavirus vaccine (RIX4414) among infants in Carabobo State, Venezuela. [Poster]. Asocación Venezolana para el Avance de la Cienca (AsoVAC); 2004; Venezuela. 2004. [Not available for review] [Google Scholar]
- GlaxoSmithKline[444563‐014]. A phase II, double‐blind before the 2002 rotavirus season and single blind with respect to OPV after, randomised, placebo‐controlled study of the safety, reactogenicity and immunogenicity of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414 at 105 ffu) co‐administered with either oral polio vaccine (OPV) or inactivated polio vaccine (IPV) in healthy infants (approximately 5‐10 weeks old) in South Africa. www.gsk‐studyregister.com/study/6787 (accessed 12 December 2018). ; ISRCTN37373664. A double blind, randomised placebo controlled study of the safety, reactogenicity and immunogenicity of two doses of orally administered human rotavirus vaccine (RIX4414) in healthy infants in South Africa. www.controlled‐trials.com/ISRCTN37373664 (first received 25 November 2005). ; NCT00346892. Reactogenicity & immunogenicity study of two doses of GSK Biologicals’ oral live attenuated HRV vaccine co‐administered with either OPV or IPV in healthy infants (approximately 5‐10 weeks old) in South Africa. clinicaltrials.gov/show/NCT00346892 (first received 30 June 2006). ; Steele AD, Tumbo J, Armah G, Reynders J, Scholtz F, Bos P, et al. Immunogenicity and reactogenicity of a new live attenuated oral rotavirus vaccine (RIX4414) when administered concurrently with poliovirus vaccines in African infants. 24th International Congress of Pediatrics; 2004 August 15‐20; Cancun, Mexico. 2004. [Not available for review] [Google Scholar]; Steele AD, Tumbo JM, Armah GE, Reynders J, Scholtz F, Bos P, et al. Concomitant administration of a live‐attenuated oral rotavirus vaccine (RIX4414) with poliovirus vaccines in African infants. 23rd Annual Meeting of the European Society for Pediatric Infectious Diseases‐ESPID; 2005 May 18‐20; Valencia, Spain2005. [Not available for review]; Steele AD, De Vos B, Tumbo J, Reynders J, Scholtz F, Bos P, et al. Co‐administration study in South African infants of a live‐attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine 2010 ;28(39):6542‐8. [DOI] [PubMed] [Google Scholar]
- EUCTR2015‐001484‐39. A phase II study to assess the safety and immunogenicity of GlaxoSmithKline Biologicals rotavirus vaccine, RIX4414 when administered to HIV infected infants in South Africa. www.clinicaltrialsregister.eu/ctr‐search/trial/2015‐001484‐39/results (first received 11 June 2015). ; GlaxoSmithKline[444563‐022]. A phase II, double‐blind, randomized, placebo‐controlled study to assess the safety, reactogenicity and immunogenicity of three doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine. www.gsk‐studyregister.com/study/6790 (accessed 12 December 2018). ; ISRCTN11877362. A phase II, double‐blind, randomised, placebo‐controlled study to assess the safety, reactogenicity and immunogenicity of three doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414 at 106.5 CCID50) administered to human immunodeficiency virus (HIV) infected infants at 6, 10 and 14 weeks of age in South Africa. www.controlled‐trials.com/ISRCTN11877362 (first received 25 November 2005). ; NCT00263666. A study of safety, reactogenicity and immunogenicity of HRV vaccine in HIV infected infants in South Africa. clinicaltrials.gov/ct2/show/results/NCT00263666 (first received on 9 December 2005). ; Steele AD, Madhi SA, Louw CE, Bos P, Tumbo JM, Werner CM, et al. Safety, reactogenicity, and immunogenicity of human rotavirus vaccine RIX4414 in human immunodeficiency virus‐positive infants in South Africa. Pediatric Infectious Diseases Journal 2011;30(2):125‐30. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline[444563‐013]. A phase II, double‐blind before the 2002 rotavirus season and single blind with respect to OPV after, randomised, placebo‐controlled study of the safety, reactogenicity and immunogenicity of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414 at 105 ffu) co‐administered with either oral polio vaccine (OPV) or inactivated polio vaccine (IPV) in healthy infants (approximately 5‐10 weeks old) in South Africa. www.gsk‐studyregister.com/study/6786 (accessed 12 December 2018). ; ISRCTN37373664. A double blind, randomised placebo controlled study of the safety, reactogenicity and immunogenicity of two doses of orally administered human rotavirus vaccine (RIX4414) in healthy infants in South Africa. controlled‐trials.com/ISRCTN37373664 (first received 25 November 2005). ; NCT00383903. A study of the safety, reactogenicity and immunogenicity of 2 or 3 doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants (approximately 5‐10 weeks old) in South Africa. clinicaltrials.gov/show/NCT00383903 (accessed 4 October 2006). ; Steele AD, Reynders J, Scholtz F, Bos P, De Beer MC, Tumbo J, et al. Comparison of 2 different regimens for reactogenicity, safety, and immunogenicity of the live attenuated oral rotavirus vaccine RIX4414 coadministered with oral polio vaccine in South African infants. Journal of Infectious Diseases 2010;202(Suppl):S93‐100. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline[444563‐024]. A phase III, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, immunogenicity and safety of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine given concomitantly with routine EPI vaccinations including OPV in healthy infants. www.gsk‐studyregister.com/study/6792 (accessed 12 December 2018). ; Gonzalez Ayala S, Rivera L, Rivera‐Medina DM, Lopez P, Valencia A, León T, et al. Co‐administration with rotavirus vaccine rix4414 (Rotarix™) does not interfere with the immunogenicity of oral polio vaccine (OPV). [Poster]. World Society for Pediatric Infectious Diseases (WSPID); 2007 November 15‐18; Bangkok, Thailand. 2007. [Not available for review] [Google Scholar]; Tregnaghi MW, Abate HJ, Valencia A, Lopez P, Silveira TR, Rivera L, et al. Human rotavirus vaccine is highly efficacious when coadministered with routine expandedprogram of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatric Infectious Disease Journal 2011;30(6):e103‐e8. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline[444563‐003]. A phase II, double‐blind, randomized, placebo‐controlled, dose‐escalating, stepwise study to assess safety, reactogenicity and immunogenicity of GlaxoSmithKline Biologicals’ live attenuated human rotavirus (HRV) vaccine in healthy infants previously uninfected with human rotavirus. www.gsk‐studyregister.com/study/6781 (accessed 12 December 2018). ; Vesikari T, Karvonen A, Korhonen T, Espo M, Lebacq E, Forster J, et al. Safety and immunogenicity of RIX4414 live attenuated human rotavirus vaccine in adults, toddlers and previously uninfected infants. Vaccine 2004;22(21‐2):2836‐42. [DOI] [PubMed] [Google Scholar]
- Vos B, Vesikari T, Linhares AC, Salinas B, Perez‐Schael I, Ruiz‐Palacios GM, et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatric Infectious Disease Journal 2004;23(10 Suppl):179‐82. [DOI] [PubMed] [Google Scholar]; GlaxoSmithKline[444563‐004‐Annex]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants approximately 2 months of age and previously uninfected with HRV. [This summary presents results for the second efficacy period (from the end of the first rotavirus season post‐vaccination until the end of the second rotavirus season) and for the two consecutive rotavirus seasons.]. www.gsk‐studyregister.com/study/6782 (accessed 12 December 2018). ; GlaxoSmithKline[444563‐004]. A phase IIb, double‐blind, randomized, placebo‐controlled study to assess the efficacy, immunogenicity, reactogenicity and safety of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants approximately 2 months of age and previously uninfected with HRV. [Results for the first efficacy period (starting from 2 weeks after Dose 2 until the end of the first RV season following vaccination)]. www.gsk‐studyregister.com/study/6782 (accessed 12 December 2018). ; NCT00425737. A study to assess the efficacy, immunogenicity and safety of two doses of oral live attenuated human rotavirus (HRV) vaccine (Rotarix) in healthy infants. clinicaltrials.gov/show/NCT00425737 (first receiveded 23 January 2007). ; Vesikari T, Karvonen A, Espo M, Korhonen T, Delem A, Vos B. Efficacy evaluation of an oral human rotavirus (HRV) vaccine in previously uninfected Finnish infants. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2002 September 27‐30; San Diego, California. 2002. [Not available for review] [Google Scholar]; Vesikari T, Karvonen A, Puustinen L, Zeng SQ, Szakal ED, Delem A, et al. Efficacy of RIX4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatric Infectious Disease Journal 2004;23(10):937‐43. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline[102247‐036‐Annex]. A phase IIIb, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants in co‐administration with specific childhood vaccinations. [This summary presents results for the second and combined efficacy periods]. www.gsk‐studyregister.com/study/2669 (accessed 12 December 2018). ; GlaxoSmithKline[102247‐036‐Yr3]. A phase IIIb, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants in co‐administration with specific childhood vaccinations. [A phase IIIb open study to assess the long‐term efficacy and safety of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine approximately three years after vaccination in healthy infants aged 6‐12 weeks at the time of first vaccination in the Rota‐036 study (eTrack No.102247) in Finland.]. www.gsk‐studyregister.com/study/2669 (accessed 12 December 2018). ; GlaxoSmithKline[102247‐036]. A phase IIIb, double‐blind, randomized, placebo‐controlled, multi‐country and multi‐center study to assess the efficacy, safety and immunogenicity of two doses of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants in co‐administration with specific childhood vaccinations. [This summary presents results from the first efficacy period.] [102247‐036]. www.gsk‐studyregister.com/study/2669 (accessed 12 December 2018). ; NCT00140686. A multi‐country & multi‐center study to assess the efficacy, safety & immunogenicity of 2 doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine in healthy infants in co‐administration with specific childhood vaccines. clinicaltrials.gov/ct2/show/record/NCT00140686 (first received 1 September 2005). [Trial registration document]; NCT00420316. To assess long‐term efficacy & safety of subjects approximately 3 years after priming with 2 doses of GlaxoSmithKline (GSK) Biologicals' oral live attenuated human rotavirus (HRV) vaccine (Rotarix) in the primary vaccination study (102247). clinicaltrials.gov/show/NCT00420316 (first received 11 January 2007). ; Schuster V, Otto W, Cohen R. RotarixTM An oral human Rotavirus vaccine, is highly immunogenic when co‐administered with a Streptococcus pneumoniae conjugate vaccine (PrevenarTM) in healthy infants from France and Germany. 24th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); 2006 June 15‐18; Basel, Swizterland. 2006. [Not available for review] [Google Scholar]; Tejedor JC, Diez‐Delegado J, Aristegui J. Rotarix (RIX4414), an oral Human Rotavirus vaccine, is highly immunogenic when co‐administered with a Neisseria meningitides Serogroup C vaccine (MeningitecTM) in healthy infants from Spain. 24th Annual Meeting Meeting of the European Society for Paediatric Infectious Diseases (ESPID); 2006 June 15‐18; Basel, Switzerland. 2006. [Not available for review] [Google Scholar]; Vesikari T, Karvonen A, Ferrante SA, Kuter BJ, Ciarlet M. Sustained efficacy of the pentavalent rotavirus vaccine, RV5, up to 3.1 years following the last dose of vaccine. Pediatric Infectious Disease Journal 2010;29(10):957‐63. [DOI] [PubMed] [Google Scholar]; Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double‐blind controlled study. Lancet 2007;370(9601):1757‐63. [DOI] [PubMed] [Google Scholar]; Vesikari T, Prymula R, Schuster V. Early protection against Rotavirus, Rotarix, experience in an European setting. 12th International Congress for Infectious Diseases; 2006 June 15‐18; Lisbon, Portugal. 2006. [Not available for review] [Google Scholar]; Vesikari T, Prymula R, Schuster V. Rotarix (RIX4414), an oral human rotavirus vaccine, is highly immunogenic when co‐administered with diphteria‐tetanus‐pertussis (DTPA)‐combined vaccines in healthy infants from Europe. 24th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); 2006 June 15‐18; Basel, Switzerland. 2006. [Not available for review] [Google Scholar]; Vesikari T, Prymula R, Schuster V, Tejedor JC, Cohen R, Bouckenooghe A, et al. Efficacy and immunogenicity of live‐attenuated human rotavirus vaccine in breast‐fed and formula‐fed European infants. Pediatric Infectious Disease Journal 2012;31(5):509‐13. [DOI] [PubMed] [Google Scholar]; Vesikari T, et al. Human rotavirus vaccine Rotarix is highly efficacious in Europe. 24th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); 2006 June 15‐18; Basel, Switzerland. 2006. [Not available for review] [Google Scholar]; Vesikari T, et al. Rotarix, an oral human Rotavirus vaccine, is highly immunogenic in healthy infants from 6 European countries. 24th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); 2006 June 15‐18; Basel, Switzerland. 2006. [Not available for review] [Google Scholar]; Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Thollot F, et al. Immunogenicity and safety of the human rotavirus vaccine Rotarix co‐administered with routine infant vaccines following the vaccination schedules in Europe. Vaccine 2010;28(32):5272‐9. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline[104480‐048]. A phase II, double‐blind, randomized, placebo controlled study to compare the immunogenicity, reactogenicity and safety of two different formulations of GlaxoSmithKline (GSK) Biologicals’ live attenuated human rotavirus (HRV) vaccine given as a two‐dose primary vaccination in healthy infants previously uninfected with HRV. www.gsk‐studyregister.com/study/2783 (accessed 12 December 2018). ; NCT00137930. Compare the immunogenicity, reactogenicity & safety of 2 different formulations of GSK Biologicals’ live attenuated human rotavirus (HRV) vaccine given as a two‐dose primary vaccination in healthy infants previously uninfected with HRV. clinicaltrials.gov/ct2/show/record/NCT00137930 (first received 30 August 2005). ; Vesikari T, Karvonen A, Bouckenooghe A, Suryakiran PV, Smolenov I, Han HH. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 oral suspension (liquid formulation) in Finnish infants. Vaccine 2011;29(11):2079‐84. [DOI] [PubMed] [Google Scholar]; Vesikari T, et al. Immunogenicity of liquid formulation of the oral live attenuated human rotavirus vaccine (Rotarix™) [Poster]. Sociedad Latinoamericana de Infectología Pediátrica (SLIPE). San Jose, Costa Rica 8‐11 May 2007. [Not available for review]
- Ward RL, Kirkwood CD, Sander DS, Smith VE, Shao M, Bean JA, et al. Reductions in cross‐neutralizing antibody responses in infants after attenuation of the human rotavirus vaccine candidate 89‐12. Journal of Infectious Diseases 2006;194(12):1729‐36. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline[103992‐045]. A phase II, randomised, double‐blind, placebo‐controlled study to evaluate the immunogenicity, reactogenicity and safety of two doses of GSK Biologicals’ oral live attenuated human rotavirus (HRV) vaccine (RIX4414 at 106.5 CCID50) when given concomitantly with OPV versus when given alone (HRV vaccine dose given 15 days after the OPV dose) in healthy infants in Bangladesh. www.gsk‐studyregister.com/study/2748 (accessed 12 December 2018). ; NCT00139334. Evaluate immunogenicity, reactogenicity & safety of 2 doses of GSK Biologicals’ oral live attenuated HRV vaccine (RIX4414 at 106.5 CCID50) when given concomitantly with OPV versus given alone (HRV vaccine dose given 15 days after the OPV dose) in healthy infants in Bangladesh. clinicaltrials.gov/show/NCT00139334 (first received 31 August 2005). ; Zaman K, Sack DA, Yunus M, Arifeen SE, Podder G, Azim T, et al. Bangladesh Rotavirus Vaccine study group. Successful co‐administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2‐dose schedule at 12 and 16 weeks of age. Vaccine 2009;27(9):1333‐9. [DOI] [PubMed] [Google Scholar]
- Zaman K, Sack DA, Neuzil KM, Yunus M, Moulton LH, Sugimoto JD, et al. Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster‐randomized trial. PLoS Medicine 2017;14(4):e1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armah GE, Breiman RF, Tapia MD, Dallas MJ, Neuzil KM, Binka FN, et al. Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine 2012;30(Suppl 1):A86‐93. [DOI] [PubMed] [Google Scholar]; Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub‐Saharan Africa: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):606‐14. [DOI] [PubMed] [Google Scholar]; Breiman RF, Zaman K, Armah G, Sow SO, Anh DD, Victor JC, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine 2012;30(Suppl 1):A24‐9. [DOI] [PubMed] [Google Scholar]; Gruber JF, Hille DA, Liu GF, Kaplan SS, Nelson M, Goveia MG, et al. Heterogeneity of rotavirus vaccine efficacy among infants in developing countries. Pediatric Infectious Disease Journal 2017;36(1):72‐8. [DOI] [PubMed] [Google Scholar]; Heylen E, Zeller M, Ciarlet M, Lawrence J, Steele D, Ranst M, et al. Comparative analysis of pentavalent rotavirus vaccine strains and G8 rotaviruses identified during vaccine trial in Africa. Science Reports 2015;5:14658. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00362648. Efficacy, safety, and immunogenicity of RotaTeqTM among infants in Asia and Africa. clinicaltrials.gov/ct2/show/record/NCT00362648 (first received 10 August 2006). ; Tapia MD, Armah G, Breiman RF, Dallas MJ, Lewis KD, Sow SO, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub‐Saharan Africa. Vaccine 2012;30(Suppl 1):A79‐85. [DOI] [PubMed] [Google Scholar]
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub‐Saharan Africa: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):606‐14. [DOI] [PubMed] [Google Scholar]; Tapia MD, Armah G, Breiman RF, Dallas MJ, Lewis KD, Sow SO, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub‐Saharan Africa. Vaccine 2012;30(Suppl 1):A79‐85. [DOI] [PubMed] [Google Scholar]
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub‐Saharan Africa: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):606‐14. [DOI] [PubMed] [Google Scholar]; Feikin DR, Laserson KF, Ojwando J, Nyambane G, Ssempijja V, Audi A, et al. Efficacy of pentavalent rotavirus vaccine in a high HIV prevalence population in Kenya. Vaccine 2012;30(Suppl 1):A52‐60. [DOI] [PubMed] [Google Scholar]; Laserson KF, Nyakundi D, Feikin DR, Nyambane G, Cook E, Oyieko J, et al. Safety of the pentavalent rotavirus vaccine (PRV), RotaTeq((R)), in Kenya, including among HIV‐infected and HIV‐exposed infants. Vaccine 2012;30(Suppl 1):A61‐70. [DOI] [PubMed] [Google Scholar]; Tapia MD, Armah G, Breiman RF, Dallas MJ, Lewis KD, Sow SO, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub‐Saharan Africa. Vaccine 2012;30(Suppl 1):A79‐85. [DOI] [PubMed] [Google Scholar]
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub‐Saharan Africa: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):606‐14. [DOI] [PubMed] [Google Scholar]; Sow SO, Tapia M, Haidara FC, Ciarlet M, Diallo F, Kodio M, et al. Efficacy of the oral pentavalent rotavirus vaccine in Mali. Vaccine 2012;30(Suppl 1):A71‐8. [DOI] [PubMed] [Google Scholar]; Tapia MD, Armah G, Breiman RF, Dallas MJ, Lewis KD, Sow SO, et al. Secondary efficacy endpoints of the pentavalent rotavirus vaccine against gastroenteritis in sub‐Saharan Africa. Vaccine 2012;30(Suppl 1):A79‐85. [DOI] [PubMed] [Google Scholar]
- Block SL, Vesikari T, Goveia MG, Rivers SB, Adeyi BA, Dallas MJ, et al. Efficacy, immunogenicity, and safety of a pentavalent human‐bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics 2007;119(1):11‐8. [DOI] [PubMed] [Google Scholar]; Merck[PN‐007]. Study of the efficacy, safety, and immunogenicity of RotaTeq™ at expiry potency NCT00092443 (PN 007). www.clinicalstudyresults.org/drugdetails/?indication_id=523&sort=c.company_name&page=1&drug_id=9002008. ; NCT00092443. Study of the efficacy, safety, and immunogenicity of V260 at expiry. clinicaltrials.gov/ct2/show/NCT00092443 (first received 27 September 2004). [Trial registration document]
- Ciarlet M, He S, Lai S, Petrecz M, Yuan G, Liu GF, et al. Concomitant use of the 3‐dose oral pentavalent rotavirus vaccine with a 3‐dose primary vaccination course of a diphtheria‐tetanus‐acellular pertussis‐hepatitis B‐inactivated polio‐Haemophilus influenzae type b vaccine: immunogenicity and reactogenicity. Pediatric Infectious Disease Journal 2009;28(3):177‐81. [DOI] [PubMed] [Google Scholar]; Merck[PN‐010]. Safety and immunogenicity of concomitant use of RotaTeq™ and INFANRIX™ hexa in healthy infants NCT00258154 (PN 010) [Merck & Co., Inc. Study Synopsis]. www.clinicalstudyresults.org/drugdetails/?indication_id=1101&sort=c.company_name&page=1&drug_id=28232008. ; NCT00258154. Safety and immunogenicity of concomitant use of V260 and INFANRIX(Tm) hexa in healthy infants. clinicaltrials.gov/show/NCT00258154 (first received 24 November 2005). [Trial registration document (no data for review)]
- Clark HF, Burke CJ, Volkin DB, Offit P, Ward RL, Bresee JS, et al. Safety, immunogenicity and efficacy in healthy infants of G1 and G2 human reassortant rotavirus vaccine in a new stabilizer/buffer liquid formulation. Pediatric Infectious Disease Journal 2003;22(10):914‐20. [DOI] [PubMed] [Google Scholar]
- Clark H, White C, Offit P, Stinson D, Eiden J, Weaver S, et al. Preliminary evaluation of safety and efficacy of quadrivalent human‐bovine reassortant rotavirus vaccine [abstract]. Pediatric Research 1995;37:172A. [No data for review] [Google Scholar]; Clark HF, Bernstein DI, Dennehy PH, Offit P, Pichichero M, Treanor J, et al. Safety, efficacy, and immunogenicity of a live, quadrivalent human‐bovine reassortant rotavirus vaccine in healthy infants. Journal of Pediatrics 2004;144(2):184‐90. [DOI] [PubMed] [Google Scholar]; Clark HF, Offit PA, Ellis RW, Eiden JJ, Krah D, Shaw AR, et al. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. Journal of Infectious Diseases 1996;174(Suppl 1):73‐80. [No data for review] [DOI] [PubMed] [Google Scholar]; Ward RL, Bernstein DI, Smith VE, Sander DS, Shaw A, Eiden JJ, et al. Rotavirus immunoglobulin a responses stimulated by each of 3 doses of a quadrivalent human/bovine reassortant rotavirus vaccine. Journal of Infectious Diseases 2004;189(12):2290‐3. [DOI] [PubMed] [Google Scholar]
- CTRI/2012/07/002820. A study to evaluate safety of Rotavirus vaccine in healthy adult volunteers followed by safety, tolerability and immunogenicity evaluation in healthy infants. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4929&EncHid=&userName=CTRI/2012/07/002820 2012 (accessed 11 June 2017). ; Dhingra MS, Kundu R, Gupta M, Kanungo S, Ganguly N, Singh MP, et al. Evaluation of safety and immunogenicity of a live attenuated tetravalent (G1‐G4) Bovine‐Human Reassortant Rotavirus vaccine (BRV‐TV) in healthy Indian adults and infants. Vaccine 2014;32 Suppl 1:A117‐23. [DOI] [PubMed] [Google Scholar]
- Iwata S, Nakata S, Ukae S, Koizumi Y, Morita Y, Kuroki H, et al. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double‐blind, placebo‐controlled, multicenter trial. Human Vaccines and Immunotherapeutics 2013;9(8):1626‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00718237. A Phase III randomized, placebo‐controlled clinical trial to study the efficacy and safety of V260 in healthy infants in Japan. clinicaltrials.gov/ct2/show/record/NCT00718237 (accessed 18 July 2008).
- Kim DS, Lee TJ, Kang JH, Kim JH, Lee JH, Ma SH, et al. Immunogenicity and safety of a pentavalent human‐bovine (WC3) reassortant rotavirus vaccine in healthy infants in Korea. Pediatric Infectious Disease Journal 2008;27(2):177‐8. [DOI] [PubMed] [Google Scholar]; Merck[PN‐013]. Immunogenicity and safety of RotaTeq® in healthy infants in Korea ‐ NCT00166517 (PN 013). www.clinicalstudyresults.org/drugdetails/?indication_id=523&sort=c.company_name&page=1&drug_id=21482008. ; NCT00166517. Immunogenicity and safety of V260 in healthy infants in Korea. clinicaltrials.gov/show/NCT00166517 (first received 14 September 2005). [Trial registration document (no data for review)]
- Lawrence J, He S, Hille D, Shen C, Kuter B, Schodel F, et al. A study of RotaTeqTM (pentavalent rotavirus vaccine) in Chinese healthy adults, children and infants. International Journal of Infectious Diseases 2012;16(1):e307. [Google Scholar]; NCT00953056. A Study of V260 in Healthy Chinese Adults, Children and Infants. clinicaltrials.gov/ct2/show/study/NCT00953056 (accessed 6 August 2009).
- Levin MJ, Lindsey JC, Kaplan SS, Schimana W, Lawrence J, McNeal MM, et al. Safety and immunogenicity of a live attenuated pentavalent rotavirus vaccine in HIV‐exposed infants with or without HIV infection in Africa. AIDS 2017;31(1):49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00880698. Safety and immunogenicity of a live, attenuated rotavirus (RotaTeqTM) in HIV‐1 infected and uninfected children born to HIV‐1‐infected mothers. clinicaltrials.gov/ct2/show/study/NCT00880698 (first received 14 April 2009). ; Uprety P, Lindsey J, Levin M, Rainwater‐Lovett K, Ziemniak C, Kaplan S, et al. Enhanced inflammation and rotavirus vaccine responses in perinatal HIV‐1 infection. Topics in Antiviral Medicine; 23rd Conference on Retroviruses and Opportunistic Infections, CROI; 2016. USA. 2016; Vol. 24(E‐1):347. [Google Scholar]
- Merck[PN‐009]. Protocol 009 ‐ Comparison of the immunogenicity and safety of three consistency lots of RotaTeq™ in healthy infants (NCT00092456). www.clinicalstudyresults.org/drugdetails/?indication_id=523&sort=c.company_name&page=1&drug_id=13572005. ; NCT00092456. Comparison of the immunogenicity and safety of three consistency lots of V260 in healthy infants. clinicaltrials.gov/ct2/show/record/NCT00092456 (first received 27 September 2004). [Trial registration document (no data for review)]
- Mo Z, Mo Y, Li M, Tao J, Yang X, Kong J, et al. Efficacy and safety of a pentavalent live human‐bovine reassortant rotavirus vaccine (RV5) in healthy Chinese infants: A randomized, double‐blind, placebo‐controlled trial. Vaccine 2017;35(43):5897‐904. [DOI] [PubMed] [Google Scholar]; NCT02062385. Efficacy, Safety, and Immunogenicity of V260 in Healthy Chinese Infants (V260‐024). clinicaltrials.gov/show/NCT02062385 (first received 13 February 2014).
- Vesikari T, Clark HF, Offit P, Schodel F, Dallas M, Heaton P, et al. The effect of dose and composition of a pentavalent rotavirus reassortant vaccine [RotaTeq (R)] in safety, efficacy, and immunogenicity in healthy infants. Pediatric Research 2003;53(4 Suppl):307A. [Google Scholar]; Vesikari T, Clark HF, Offit PA, Dallas MJ, DiStefano DJ, Goveia MG, et al. Effects of the potency and composition of the multivalent human‐bovine (WC3) reassortant rotavirus vaccine on efficacy, safety and immunogenicity in healthy infants. Vaccine 2006;24(22):4821‐9. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chang MH, Lin TY, Lee HC, Hsieh WS, Lee PI. Experience of pentavalent human‐bovine reassortant rotavirus vaccine among healthy infants in Taiwan. Journal of the Formosan Medical Association 2009;108(4):280‐5. [DOI] [PubMed] [Google Scholar]; Christie CD, Duncan ND, Thame KA, Onorato MT, Smith HD, Malcolm LG, et al. Pentavalent rotavirus vaccine in developing countries: safety and health care resource utilization. Pediatrics 2010;126(6):e1499‐506. [DOI] [PubMed] [Google Scholar]; Dennehy PH, Goveia MG, Dallas MJ, Heaton PM. The integrated phase III safety profile of the pentavalent human‐bovine (WC3) reassortant rotavirus vaccine. International Journal of Infectious Diseases 2007;11(Suppl 2):36‐42. [No data available for review] [DOI] [PubMed] [Google Scholar]; Goveia MG, DiNubile MJ, Dallas MJ, Heaton PM, Kuter BJ, REST Study Team. Efficacy of pentavalent human‐bovine (WC3) reassortant rotavirus vaccine based on breastfeeding frequency. Pediatric Infectious Disease Journal 2008;27(7):656‐8. [DOI] [PubMed] [Google Scholar]; Goveia MG, Rodriguez ZM, Dallas MJ, Itzler RF, Boslego JW, Heaton PM, et al. Safety and efficacy of the pentavalent human‐bovine (WC3) reassortant rotavirus vaccine in healthy premature infants. Pediatric Infectious Disease Journal 2007;26(12):1099‐104. [No data available for review] [DOI] [PubMed] [Google Scholar]; Goveia MG, Suprun L, Itzler RF, McFetridge R, Dallas MJ, Kuter BJ. Efficacy and safety of pentavalent human‐bovine reassortant rotavirus vaccine when administered with greater than 10 weeks between doses. Pediatric Infectious Disease Journal 2010;29(3):263‐5. [DOI] [PubMed] [Google Scholar]; Grant L, Watt J, Moulton L, Weatherholtz R, Reid R, Santosham M, et al. Lack of nonspecific protection against all‐cause nonrotavirus gastroenteritis by vaccination with orally administered rotavirus vaccine. Journal of Pediatric Gastroenterology and Nutrition 2013;56(6):635‐40. [DOI] [PubMed] [Google Scholar]; Grant LR, Watt JP, Weatherholtz RC, Moulton LH, Reid R, Santosham M, et al. Efficacy of a pentavalent human‐bovine reassortant rotavirus vaccine against rotavirus gastroenteritis among American Indian children. Pediatric Infectious Disease Journal 2012;31(2):184‐8. [DOI] [PubMed] [Google Scholar]; Itzler R, Koch G, Matson DO, Gothefors L, Damme P, Dinubile MJ, et al. Robustness of the healthcare utilization results from the Rotavirus Efficacy and Safety Trial (REST) evaluating the human‐bovine (WC3) reassortant pentavalent rotavirus vaccine (RV5). BMC Pediatrics 2010;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]; Merck[PN‐006]. Safety and efficacy of pentavalent (G1, G2, G3, G4, and P1) human‐bovine reassortant rotavirus vaccine in healthy infants NCT00090233 (PN 006). www.clinicalstudyresults.org/drugdetails/?indication_id=523&sort=c.company_name&page=1&drug_id=11442008. [No data available for review]; NCT00090233. Safety and efficacy of pentavalent (G1, G2, G3, G4, and P1) human‐bovine reassortant rotavirus vaccine in healthy infants. clinicaltrials.gov/ct2/show/record/NCT00090233 (first received 27 August 2004). [Trial registration document]; Rodriguez ZM, Goveia MG, Stek JE, Dallas MJ, Boslego JW, DiNubile MJ, et al. Concomitant use of an oral live pentavalent human‐bovine reassortant rotavirus vaccine with licensed parenteral pediatric vaccines in the United States. Pediatric Infectious Disease Journal 2007;26(3):221‐7. [DOI] [PubMed] [Google Scholar]; Vesikari T, Itzler R, Karvonen A, Korhonen T, Damme P, Behre U, et al. RotaTeq, a pentavalent rotavirus vaccine: efficacy and safety among infants in Europe. Vaccine 2009;28(2):345‐51. [DOI] [PubMed] [Google Scholar]; Vesikari T, Itzler R, Matson DO, Santosham M, Christie CD, Coia M, et al. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus‐associated health care utilization across three regions (11 countries). International Journal of Infectious Diseases 2007;11(Suppl 2):29‐35. [DOI] [PubMed] [Google Scholar]; Vesikari T, Karvonen A, Ferrante SA, Ciarlet M. Efficacy of the pentavalent rotavirus vaccine, RotaTeq(R), in Finnish infants up to 3 years of age: the Finnish Extension Study. European Journal of Pediatrics 2010;169(11):1379‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vesikari T, Matson DO, Dennehy P, Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human‐bovine (WC3) reassortant rotavirus vaccine. New England Journal of Medicine 2006;354(1):23‐33. [DOI] [PubMed] [Google Scholar]; Vesikari T, Karvonen A, Ferrante SA, Kuter BJ, Ciarlet M. Sustained efficacy of the pentavalent rotavirus vaccine, RV5, up to 3.1 years following the last dose of vaccine. Pediatric Infectious Disease Journal 2010;29(10):957‐63. [DOI] [PubMed] [Google Scholar]
- Breiman RF, Zaman K, Armah G, Sow SO, Anh DD, Victor JC, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine 2012;30(Suppl 1):A24‐9. [DOI] [PubMed] [Google Scholar]; Gruber JF, Hille DA, Liu GF, Kaplan SS, Nelson M, Goveia MG, et al. Heterogeneity of rotavirus vaccine efficacy among infants in developing countries. Pediatric Infectious Disease Journal 2017;36(1):72‐8. [DOI] [PubMed] [Google Scholar]; NCT00362648. Efficacy, safety, and immunogenicity of RotaTeqTM among infants in Asia and Africa. clinicaltrials.gov/ct2/show/record/NCT00362648 (accessed 10 August 2006). ; Shin S, Anh DD, Zaman K, Yunus M, Mai le TP, Thiem VD, et al. Immunogenicity of the pentavalent rotavirus vaccine among infants in two developing countries in Asia, Bangladesh and Vietnam. Vaccine 2012;30(Suppl 1):A106‐13. [DOI] [PubMed] [Google Scholar]; Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):615‐23. [DOI] [PubMed] [Google Scholar]
- Feller AJ, Zaman K, Lewis KD, Hossain I, Yunus M, Sack DA. Malnutrition levels among vaccinated and unvaccinated children between 2 and 3 years of age following enrollment in a randomized clinical trial with the pentavalent rotavirus vaccine (PRV) in Bangladesh. Vaccine 2012;30(Suppl 1):A101‐5. [DOI] [PubMed] [Google Scholar]; Zaman K, Yunus M, Arifeen SE, Azim T, Faruque AS, Huq E, et al. Methodology and lessons‐learned from the efficacy clinical trial of the pentavalent rotavirus vaccine in Bangladesh. Vaccine 2012;30(Suppl 1):A94‐100. [DOI] [PubMed] [Google Scholar]; Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):615‐23. [DOI] [PubMed] [Google Scholar]
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;376(9741):615‐23. [DOI] [PubMed] [Google Scholar]
- Bhandari N, Sharma P, Glass RI, Ray P, Greenberg H, Taneja S, et al. Safety and immunogenicity of two live attenuated human rotavirus vaccine candidates, 116E and I321, in infants: results of a randomised controlled trial. Vaccine 2006;24(31):5817‐23. [DOI] [PubMed] [Google Scholar]; ISRCTN57452882. A double‐blind randomised placebo controlled dose escalating phase Ib/IIa study to evaluate the safety and immunogenicity of live attenuated rotavirus vaccine 116E in healthy non‐malnourished infants eight to 20 weeks of age. isrctn.com/ISRCTN57452882 (first received 26 July 2006). ; NCT00280111. Safety and immunogenicity study of live attenuated Indian rotavirus vaccine candidate strains 116E and I321 in infants. clinicaltrials.gov/ct2/show/NCT00280111 (first received 20 January 2006).
- Bhandari N, Sharma P, Taneja S, Kumar T, Rongsen‐Chandola T, Appaiahgari MB, et al. A dose‐escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double‐blind, placebo‐controlled trial. Journal of Infectious Diseases 2009;200(3):421‐9. [DOI] [PubMed] [Google Scholar]; ISRCTN57452882. A double‐blind randomised placebo controlled dose escalating phase Ib/IIa study to evaluate the safety and immunogenicity of live attenuated rotavirus vaccine 116E in healthy non‐malnourished infants eight to 20 weeks of age. isrctn.com/ISRCTN57452882 (first received 26 July 2006). ; NCT00439660. Dose escalation study to evaluate oral rotavirus vaccine 116E live attenuated in healthy infants 8 to 20 weeks old. clinicaltrials.gov/ct2/show/NCT00439660 (first received 26 February 2007).
- Bhandari N, Rongsen‐Chandola T, Bavdekar A, John J, Antony K, Taneja S, et al. Efficacy of a monovalent human‐bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine 2014;32 Suppl 1:A110‐6. [DOI] [PubMed] [Google Scholar]; Bhandari N, Rongsen‐Chandola T, Bavdekar A, John J, Antony K, Taneja S, et al. Efficacy of a monovalent human‐bovine (116E) rotavirus vaccine in Indian infants: a randomised, double‐blind, placebo‐controlled trial. Lancet 2014;383(9935):2136‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]; CTRI/2010/091/000102. A phase III clinical trial to evaluate the protective efficacy of three doses of oral rotavirus vaccine (ORV) 116E. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1317 (first received 22 November 2010). ; John J, Kawade A, Rongsen‐Chandola T, Bavdekar A, Bhandari N, Taneja S, et al. Active surveillance for intussusception in a phase III efficacy trial of an oral monovalent rotavirus vaccine in India. Vaccine 2014;32 Suppl 1:A104‐9. [DOI] [PubMed] [Google Scholar]; John TJ. Why was there no vaccine‐associated intussusception in Indian rotavirus vaccine trial?. Indian Pediatrics 2015;52(10):906. [PubMed] [Google Scholar]; NCT01305109. A Phase III, randomized, double blind, placebo controlled trial to evaluate the protective efficacy of three doses of oral rotavirus vaccine (ORV) 116E, against severe rotavirus gastroenteritis in infants. clinicaltrials.gov/show/NCT01305109 (first received 28 February 2011).
- CTRI/2014/05/004592. A phase III study to evaluate the non‐interference in the immune response of rotavac to childhood vaccines and to assess the clinical lot consistency. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=5870&EncHid=&modid=&compid=%27,%275870det%27 (first received 13 May 2014). ; Chandola TR, Taneja S, Goyal N, Antony K, Bhatia K, More D, et al. ROTAVAC does not interfere with the immune response to childhood vaccines in Indian infants: a randomized placebo controlled trial. Heliyon 2017;3(5):e00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Armah GE, Kapikian AZ, Vesikari T, Cunliffe N, Jacobson RM, Burlington DB, et al. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV‐TV in Ghana with the first dose administered during the neonatal period. Journal of Infectious Diseases 2013;208(3):423‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACTRN12611001212943. RV3‐BB rotavirus vaccine phase IIa clinical trial of immunogenicity and safety. anzctr.org.au/ACTRN12611001212943.aspx (first received 24 November 2011). ; Bines JE, Danchin M, Jackson P, Handley A, Watts E, Lee KJ, et al. RV3 Rotavirus Vaccine Program. Safety and immunogenicity of RV3‐BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double‐blind, placebo‐controlled trial. Lancet Infectious Diseases 2015;15(12):1389‐97. [DOI] [PubMed] [Google Scholar]
- Bines JE, At Thobari J, Satria CD, Handley A, Watts E, Cowley D, et al. Human neonatal rotavirus vaccine (RV3‐BB) to target rotavirus from birth. New England Journal of Medicine 2018;378(8):719‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucardo F, Nordgren J, Reyes Y, Gonzalez F, Sharma S, Svensson L. The Lewis A phenotype is a restriction factor for Rotateq and Rotarix vaccine‐take in Nicaraguan children. Scientific Reports 2018;8(1):1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher A, Rivara G, Briceno D, Huicho L. [Use of a rapid rotavirus test in prescription of antibiotics in acute diarrhea in pediatrics: an observational, randomized, controlled study]. Revista de Gastroenterologia del Peru 2012;32(1):11‐5. [PubMed] [Google Scholar]
- Chatterjee A, O'Keefe C, Varman M, Klein NP, Luber S, Tomovici A, et al. Comparative immunogenicity and safety of different multivalent component pertussis vaccine formulations and a 5‐component acellular pertussis vaccine in infants and toddlers: a randomized, controlled, open‐label, multicenter study. Vaccine2012; Vol. 30, issue 23:3360‐8. [DOI] [PubMed]
- Cowley D, Boniface K, Bogdanovic‐Sakran N, Kirkwood CD, Bines JE. Rotavirus shedding following administration of RV3‐BB human neonatal rotavirus vaccine. Human Vaccines and Immunotherapeutics 2017;13(8):1908‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CTRI‐2009‐091‐000821. A randomized, double‐blind, placebo controlled study to assess the safety and tolerability of RotaVac vaccine (live attenuated bovine‐human (UK) reassortant pentavalent rotavirus vaccine). ctri.in/Clinicaltrials/ViewTrial.jsp?trialno=1302 (first received 15 October 2009).
- Dang DA, Nguyen VT, Vu DT, Nguyen TH, Nguyen DM, Yuhuan W. A dose‐escalation safety and immunogenicity study of a new live attenuated human rotavirus vaccine (Rotavin‐M1) in Vietnamese children. Vaccine 2012;30(Suppl 1):A114‐21. [DOI] [PubMed] [Google Scholar]; NCT01377571. A dose‐escalating study to evaluate the immunogenicity and safety of rotavin‐M1 vaccine in healthy infants. clinicaltrials.gov/ct2/show/NCT01377571 (first received 21 June 2011).
- Palma O, Cruz L, Ramos H, de Baires A, Villatoro N, Pastor D, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case‐control study. BMJ 2010;340:c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson I. Diarrhoea: low‐cost rotavirus vaccine shows efficacy in Niger. Nature Reviews. Gastroenterology & Hepatology 2017;14(5):260. [DOI] [PubMed] [Google Scholar]
- Diness BR, Christoffersen D, Pedersen UB, Rodrigues A, Fischer TK, Andersen A, et al. The effect of high‐dose vitamin A supplementation given with bacille Calmette‐Guerin vaccine at birth on infant rotavirus infection and diarrhea: a randomized prospective study from Guinea‐Bissau. Journal of Infectious Disease 2010;202(Suppl):S243‐51. [DOI] [PubMed] [Google Scholar]
- Dutta P, Mitra U, Dutta S, Rajendran K, Saha TK, Chatterjee MK. Randomised controlled clinical trial of Lactobacillus sporogenes (Bacillus coagulans), used as probiotic in clinical practice, on acute watery diarrhoea in children. Tropical Medicine and International Health 2011;16(5):555‐61. [DOI] [PubMed] [Google Scholar]
- CTRI/2014/04/004548. A phase IV immunogenicity and safety study of BBILs oral rotavirus vaccine 116E (ROTAVAC). ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=7937&EncHid=&modid=&compid=%27,%277937det%27 (first receiveded 17 April 2014).
- Friedrich MJ. Freeze‐dried rotavirus vaccine shows promise. JAMA 2017;317(19):1941. [DOI] [PubMed] [Google Scholar]
- Gagneur A, Nowak E, Lemaitre T, Segura JF, Delaperriere N, Abalea L, et al. IVANHOE investigators. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: The IVANHOE study. Vaccine 2011;29(21):3753‐9. [DOI] [PubMed] [Google Scholar]
- Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, et al. Safety and immunogenicity of a parenteral P2‐VP8‐P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double‐blind, placebo‐controlled trial. Lancet Infectious Diseases 2017;17(8):843‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu H, Suzuki R, Nagatani A, Boda H, Miyata M, Hattori F, et al. Rotavirus vaccination can be performed without viral dissemination in the neonatal intensive care unit. Journal of Infectious Diseases 2018;217(4):589‐96. [DOI] [PubMed] [Google Scholar]
- Isanaka S, Djibo A, Grais RF. Heat‐stable oral rotavirus vaccine. New England Journal of Medicine 2017;377(3):302. [DOI] [PubMed] [Google Scholar]; Isanaka S, Guindo O, Langendorf C, Grais R, ROSE Trial Study Team. Efficacy and safety of a low‐cost, heat‐stable oral roatvirus vaccine agains severe rotavirus gastroenteritis in Niger. American Journal of Tropical Medicine and Hygiene 2017;95:242. [Google Scholar]; Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, Sayinzoga‐Makombe N, et al. Efficacy of a low‐cost, heat‐stable oral rotavirus vaccine in Niger. New England Journal of Medicine 2017;376(12):1121‐30. [DOI] [PubMed] [Google Scholar]; NCT02145000. Efficacy and safety of a pentavalent rotavirus vaccine (BRV‐PV) against severe rotavirus gastroenteritis in Niger (ROSE). clinicaltrials.gov/ct2/show/NCT02145000 (accessed 22 May 2014).
- Kempe A, Daley MF, Parashar UD, Crane LA, Beaty BL, Stokley S, et al. Will pediatricians adopt the new rotavirus vaccine?. Pediatrics 2007;119(1):1‐10. [DOI] [PubMed] [Google Scholar]
- CTRI/2013/05/003667. A clinical trial to study the effect and safety of Rotavirus Vaccine against Severe Rotavirus Gastroenteritis in healthy Indian Infants. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=5518&EncHid=&modid=&compid=%27,%275518det%27 (first received 23 May 2013). ; Kulkarni PS, Desai S, Tewari T, Kawade A, Goyal N, Garg BS, et al. A randomized Phase III clinical trial to assess the efficacy of a bovine‐human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine 2017;35(45):6228‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT02133690. A clinical trial to study the effect and safety of rotavirus vaccine against severe rotavirus gastroenteritis in healthy Indian infants. clinicaltrials.gov/ct2/show/NCT02133690 (first received 8 May 2014). ; Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine rotavirus pentavalent vaccine development in India. Vaccine 2014;32(Suppl 1):A124‐8. [DOI] [PubMed] [Google Scholar]
- Muhsen K, Shulman L, Kasem E, Rubinstein U, Shachter J, Kremer A, et al. Effectiveness of rotavirus vaccines for prevention of rotavirus gastroenteritis‐associated hospitalizations in Israel: a case‐control study. Human Vaccines 2010;6(6):450‐4. [DOI] [PubMed] [Google Scholar]
- NCT00981669. Evaluation of rotavirus vaccine produced by Butantan Institute. Phase I ‐ safety, tolerability and immunogenicity evaluation. clinicaltrials.gov/show/NCT00981669 (first received 22 September 2009).
- NCT01195844. Rotavirus gastroenteritis in children less than 5 years‐old. Surveillance performed in hospitals from four Brazilian regions. clinicaltrials.gov/show/NCT01195844 (first received 6 September 2010).
- NCT01236066. A study on the impact of rotavirus vaccination on hospitalisations for rotavirus gastroenteritis in children aged <5 years in Australia. clinicaltrials.gov/show/NCT01236066 (first received 8 November 2010).
- NCT01375907. A Phase 1 study to evaluate safety and reactogenicity of a Vietnamese rotavirus vaccine (Rotavin‐M1 at 10e6.3FFU/Dose) among healthy adults in Vietnam. clinicaltrials.gov/show/NCT01375907 (first received 17 June 2011).
- NCT01571505. Exploration of the biologic basis for underperformance of oral polio and rotavirus vaccines in India (PROVIDE). clinicaltrials.gov/show/NCT01571505 (first received 5 April 2012).
- Rivera L, Peña LM, Stainier I, Gillard P, Cheuvart B, Smolenov I, et al. Horizontal transmission of a human rotavirus vaccine strain‐‐a randomized, placebo‐controlled study in twins. Vaccine 2011;29(51):9508‐13. [DOI] [PubMed] [Google Scholar]
- Thyagarajan V, Glass R, Rodgers K, Quinlan S, Holick CN, Rosillon D, et al. Validity of current procedural terminology codes for rotavirus vaccination in two commercially‐insured US populations. Pharmacoepidemiology and Drug Safety 2011;20:S242. [DOI] [PubMed] [Google Scholar]
- Yin H, Shih W, Lee H, Yang H, Chen Y, Cheng S, et al. Comparison of iatrogenic pain between rotavirus vaccination before and after vaccine injection in 2‐month‐old infants. Human Vaccines and Immunotherapeutics 2017;13(5):1136‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine rotavirus pentavalent vaccine development in India. Vaccine 2014 Aug 11;32(Suppl 1):A124‐8. [DOI] [PubMed] [Google Scholar]
- CTRI/2010/091/003064. A randomized, double‐blind, placebo controlled study to assess safety and tolerability of RotaVac vaccine (Live Attenuated Bovine‐Human (UK) Reassortant Pentavalent Rotavirus Vaccine). ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=2458&EncHid=&modid=&compid=%27,%272458det%27 (first received 04 January 2011). ; Zade JK, Kulkarni PS, Desai SA, Sabale RN, Naik SP, Dhere RM. Bovine rotavirus pentavalent vaccine development in India. Vaccine 2014;32(Suppl 1):A124‐8. [DOI] [PubMed] [Google Scholar]
- Libster R, McNeal M, Walter EB, Shane AL, Winokur P, Cress G, et al. Safety and immunogenicity of sequential rotavirus vaccine schedules. Pediatrics 2016;137(2):e20152603. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01266850. Safety and Immunogenicity of Sequential Rotavirus Vaccine Schedule. clinicaltrials.gov/ct2/show/NCT01266850 (first received 24 December 2010).
- Ali A, Kazi AM, Cortese MM, Fleming JA, Moon S, Parashar UD, et al. Correction: Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine ‐ a randomized trial. PLoS One 2015;10(12):e0145568. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ali A, Kazi AM, Cortese MM, Fleming JA, Moon S, Parashar UD, et al. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine‐‐a randomized trial. PLoS One 2015;10(6):e0127622. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ali SA, Kazi AM, Cortese MM, Fleming JA, Parashar UD, Jiang B, et al. Impact of different dosing schedules on the immunogenicity of the human rotavirus vaccine in infants in Pakistan: a randomized trial. Journal of Infectious Diseases 2014;210(11):1772‐9. [DOI] [PubMed] [Google Scholar]; NCT01199874. The immunogenicity of rotavirus vaccine under different age schedules and the impact of withholding breast feeding around the time of vaccination on the immunogenicity of Rotarix vaccine. clinicaltrials.gov/show/NCT01199874 (first received 13 September 2010).
- Armah G, Lewis KD, Cortese MM, Parashar UD, Ansah A, Gazley L, et al. A randomized, controlled trial of the impact of alternative dosing schedules on the immune response to human rotavirus vaccine in rural Ghanaian infants. Journal of Infectious Diseases 2016;213(11):1678‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01575197. Evaluation of the human rotavirus vaccine when given at varying schedules in rural Ghana. clinicaltrials.gov/show/NCT01575197 (first received 11 April 2012).
- Buyse H, Vinals C, Karkada N, Han HH. The human rotavirus vaccine Rotarix in infants: an integrated analysis of safety and reactogenicity. Human Vaccines and Immunotherapeutics 2014;10(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia JB, Patel MM, Nakagomi O, Montenegro FM, Germano EM, Correia NB, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. Journal of Infectious Diseases 2010;201(3):363‐9. [DOI] [PubMed] [Google Scholar]
- CTRI/2012/02/002454. Comparison of immunogenicity of a 3 dose and a 5 dose schedule of oral rotavirus vaccine in south Indian infants. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4262 (first received 28 May 2012).
- Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu‐Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics 2008;122(5):e1062‐6. [DOI] [PubMed] [Google Scholar]; NCT00334607. Assess the immunogenicity of 3 doses of Pediarix®, Prevnar® & ActHIB® given to healthy infants when administered with GSK Biologicals’ 2 dose oral live attenuated human rotavirus vaccine given during the same vaccination visit or separately. clinicaltrials.gov/show/NCT00334607 (first received 8 June 2006).
- Emperador DM, Velasquez DE, Estivariz CF, Lopman B, Jiang B, Parashar U, et al. Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent rotavirus vaccine immunogenicity in rural Bangladesh. Clinical Infectious Diseases 2016;62(2):150‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GlaxoSmithKline[107077‐057]. A phase III, open, randomized study to assess the immunogenicity, reactogenicity and safety of two different formulations of GlaxoSmithKline (GSK) Biologicals’ live attenuated human rotavirus (HRV) vaccine, given as a two‐dose primary vaccination, in healthy infants previously uninfected with HRV. ctr.gsk.co.uk/Summary/Vaccine_Rotavirus/III_107077.pdf (accessed before 09 October 2018).
- GlaxoSmithKline[107876‐061]. A phase III, randomised study to evaluate the clinical consistency in terms of immunogenicity and reactogenicity of three production lots of the liquid formulation of GlaxoSmithKline (GSK) Biologicals’ oral live attenuated human rotavirus (HRV) vaccine and to evaluate the liquid formulation as compared to the lyophilised formulation of the HRV vaccine in terms of immunogenicity, reactogenicity and safety when administered as a two‐dose primary vaccination in healthy infants previously uninfected with human rotavirus. ctr.gsk.co.uk/Summary/Vaccine_Rotavirus/III_107876.pdf (accessed before 09 October 2018).
- GlaxoSmithKline[444563‐020]. A phase II, double‐blind randomised, placebo controlled clinical dose‐range study to assess the immunogenicity and reactogenicity of an investigational vaccination regimen, and to assess the immunogenicity of OPV orally co‐administered to healthy infants at 2, 4 and 6 months of age. ctr.gsk.co.uk/Summary/Vaccine_Rotavirus/II_444563_020.pdf (accessed before 09 October 2018).
- Herrera D, Vásquez C, Corthésy B, Franco MA, Angel J. Rotavirus specific plasma secretory immunoglobulin in children with acute gastroenteritis and children vaccinated with an attenuated human rotavirus vaccine. Human Vaccines and Immunotherapeutics 2013;9(11):2409‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi AM, Cortese MM, Yu Y, Lopman B, Morrow AL, Fleming JA, et al. Secretor and salivary ABO blood group antigen status predict rotavirus vaccine take in infants. Journal of Infectious Diseases 2017;215(5):786‐9. [DOI] [PubMed] [Google Scholar]
- Kompithra RZ, Paul A, Manoharan D, Babji S, Sarkar R, Mathew LG, et al. Immunogenicity of a three dose and five dose oral human rotavirus vaccine (RIX4414) schedule in south Indian infants. Vaccine 2014;32 Suppl 1:A129‐33. [DOI] [PubMed] [Google Scholar]
- Lazarus RP, John J, Shanmugasundaram E, Rajan AK, Thiagarajan S, Giri S, et al. The effect of probiotics and zinc supplementation on the immune response to oral rotavirus vaccine: a randomized, factorial design, placebo‐controlled study among Indian infants. Vaccine 2018;36(2):273‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01616693. Zinc and/or probiotic supplementation of rotavirus and oral polio virus vaccines. clinicaltrials.gov/ct2/show/NCT01616693 (first received 12 June 2012). ; Parker EP, Praharaj I, Zekavati A, Lazarus RP, Giri S, Operario DJ, et al. Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India. Vaccine 2018;36(2):264‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CY, Chang LY, Shao PL, Suryakiran PV, Han HH, Huang LM. Immunogenicity, reactogenicity, and safety of a human rotavirus vaccine, Rotarix, in Taiwanese infants who received a dose of hepatitis B immunoglobulin after birth. Journal of the Formosan Medical Association 2013;112(9):574‐7. [DOI] [PubMed] [Google Scholar]
- NCT00353366. A study to evaluate the safety & reactogenicity of GSK Bio's live attenuated oral HRV vaccine, Rotarix when administered according to prescribing information, in Filipino subjects aged between 6 weeks & 14 weeks at first vaccination. clinicaltrials.gov/show/NCT00353366 (first received 18 July 2006).
- NCT00382772. Study to evaluate clinical consistency of the liquid formulation of GSK Biologicals' HRV vaccine and to evaluate liquid formulation compared to lyophilised formulation of the HRV vaccine administered as a two‐dose primary vaccination. clinicaltrials.gov/ct2/show/record/NCT00382772 (first received 02 October 2006).
- NCT00653198. Hospital‐based, case‐control study to assess the vaccine effectiveness of Rotarix™ against rotavirus severe gastroenteritis (RV SGE) among hospitalised children born after 1 March 2006 and at least 12 weeks of age, in Panama. clinicaltrials.gov/show/NCT00653198 (first received 4 April 2008).
- NCT00655187. Hospital‐based, case‐control study to assess the vaccine effectiveness of Rotarix™ against rotavirus severe gastroenteritis (RV SGE) among hospitalised children < 5 years of age in KK Hospital, Singapore. clinicaltrials.gov/show/NCT00655187 (first received 9 April 2008).
- NCT01162590. Reactogenicity and safety of a single dose of GlaxoSmithKline (GSK) Biologicals' human rotavirus (HRV) vaccine (444563) in healthy adults. clinicaltrials.gov/show/NCT01162590 (first received 14 July 2010).
- NCT01177826. Case‐control study to evaluate the vaccine effectiveness of GlaxoSmithKline (GSK) Biologicals' Live Attenuated Human Rotavirus (HRV) Vaccine (Rotarix™) against community‐acquired rotavirus severe gastroenteritis (RV SGE) among hospitalised children born after 1 October 2006, in Belgium. clinicaltrials.gov/show/NCT01177826 (first received 9 August 2010).
- NCT01273077. Evaluation of universal rotavirus vaccination program. clinicaltrials.gov/show/NCT01273077 (first received 10 January 2011).
- NCT01339221. Epidemiological, observational, post marketing study of the genetic stability of GSK Biologicals' Rotavirus Vaccine (Rotarix™) in children <5 years of age diagnosed with severe gastroenteritis, in Belgium. clinicaltrials.gov/show/NCT01339221 (first received 20 April 2011).
- Plosker GL. Rotavirus vaccine RIX4414 (Rotarix): A pharmacoeconomic review of its use in the prevention of rotavirus gastroenteritis in developed countries. Pharmacoeconomics 2011;29(5):439‐54. [DOI] [PubMed] [Google Scholar]
- Ramani S, Mamani N, Villena R, Bandyopadhyay AS, Gast C, Sato A, et al. Rotavirus serum IgA immune response in children receiving rotarix coadministered with bOPV or IPV. Pediatric Infectious Disease Journal 2016;35(10):1137‐9. [DOI] [PubMed] [Google Scholar]
- Rojas OL, Caicedo L, Guzman C, Rodriguez LS, Castaneda J, Uribe L, et al. Evaluation of circulating intestinally committed memory B cells in children vaccinated with attenuated human rotavirus vaccine. Viral Immunology 2007;20(2):300‐11. [DOI] [PubMed] [Google Scholar]
- Rongsen‐Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine 2014 Aug 11;32(Suppl 1):A134‐9. [DOI] [PubMed] [Google Scholar]; Rongsen‐Chandola T, Winje BA, Goyal N, Rathore SS, Mahesh M, Ranjan R, et al. Compliance of mothers following recommendations to breastfeed or withhold breast milk during rotavirus vaccination in North India: a randomized clinical trial. Trials 2014;15:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryakiran PV, Vinals C, Vanfraechem K, Han HH, Guerra Y, Buyse H. The human rotavirus vaccine rix4414 in infants: an integrated safety summary (ISS). Acta Paediatrica, International Journal of Paediatrics 2011;100:56‐7. [Google Scholar]
- Taddio A, Flanders D, Weinberg E, Lamba S, Vyas C, Ilersich AF, et al. A randomized trial of rotavirus vaccine versus sucrose solution for vaccine injection pain. Vaccine 2015;33(25):2939‐43. [DOI] [PubMed] [Google Scholar]
- NCT01700621. Coadministration of measles‐rubella and rotavirus vaccines. clinicaltrials.gov/ct2/show/NCT01700621 (first received on 4 October 2012). ; Zaman K, Fleming JA, Victor JC, Yunus M, Bari TI, Azim T, et al. Noninterference of rotavirus vaccine with measles‐rubella vaccine at 9 months of age and improvements in antirotavirus immunity: a randomized trial. Journal of Infectious Diseases 2016;213(11):1686‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CTRI/2014/08/004893. A study to evaluate immune non‐inferiority and safety of tetravalent Rotavirus Vaccine (BRV‐TV) in comparison to licensed vaccine (RotaTeq) in healthy infants. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=9950&EncHid=&userName=CTRI/2014/08/004893 (first received 20 August 2014). ; Saluja T, Palkar S, Misra P, Gupta M, Venugopal P, Sood AK, et al. Live attenuated tetravalent (G1‐G4) bovine‐human reassortant rotavirus vaccine (BRV‐TV): randomized, controlled phase III study in Indian infants. Vaccine 2017;35(28):3575‐81. [DOI] [PubMed] [Google Scholar]
- ACTRN 12611000559910. An observational, cross sectional, cohort study to assess the impact of rotavirus vaccine introduction on severe gastroenteritis in South Australian children. anzctr.org.au/ACTRN12611000559910.aspx (first received 31 May 2011).
- Ciarlet M, Sani‐Grosso R, Yuan G, Liu GF, Heaton PM, Gottesdiener KM, et al. Concomitant use of the oral pentavalent human‐bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatric Infectious Disease Journal 2008;27(10):874‐80. [DOI] [PubMed] [Google Scholar]
- Khoury AC, Mast TC, Ciarlet M, Markson L, Goveia MG, Munford V, et al. Projecting the effectiveness of RotaTeq against rotavirus‐related hospitalisations in Brazil. Memorias do Instituto Oswaldo Cruz 2011;106(5):541‐5. [DOI] [PubMed] [Google Scholar]
- Khoury AC, Mast TC, Ciarlet M, Markson LE, Goveia MG. Projecting the effectiveness of RotaTeq against rotavirus‐related hospitalizations and deaths in six Asian countries. Human Vaccines 2011;7(5):506‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon‐Torres F, Greenberg D, Varman M, Killar JA, Hille D, Strable EL, et al. Safety, tolerability and immunogenicity of pentavalent rotavirus vaccine manufactured by a modified process. Pediatric Infectious Disease Journal 2017;36(4):417‐22. [DOI] [PubMed] [Google Scholar]
- McGrath EJ, Thomas R, Duggan C, Asmar BI. Pentavalent rotavirus vaccine in infants with surgical gastrointestinal disease. Journal of Pediatric Gastroenterology and Nutrition 2014;59(1):44‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00130832. Concomitant use and staggered use of vaccine and oral poliovirus (OPV) in healthy infants. clinicaltrials.gov/ct2/show/results/NCT00130832 (first received 16 August 2005).
- NCT00496054. Evaluation of safety, tolerability and immunogenicity of vaccination with Rotateq (V260) in healthy infants in India. clinicaltrials.gov/show/NCT00496054 (first received 4 July 2007).
- NCT01926015. Immunogenicity and safety of concomitant administration of RotaTeq™ (V260) and the diphtheria, tetanus, pertussis and inactivated poliovirus vaccine (DTP‐IPV) in healthy Japanese infants (V260‐060). clinicaltrials.gov/ct2/show/NCT01926015 (first received on 20 August 2013).
- Saleh E, Eichner B, Clark DW, Gagliano ME, Troutman JM, Harrington L, et al. Open‐label pilot study to compare the safety and immunogenicity of pentavalent rotavirus vaccine (RV5) administered on an early alternative dosing schedule with those of RV5 administered on the recommended standard schedule. Journal of the Pediatric Infectious Diseases Society 2018;7(1):82‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugcu U, Sahin F, Bozdayi G, Aksakal FN, Alp G, Rota S, et al. Clinical efficacy of rotavirus vaccine in Turkish infants. Journal of Clinical Virology2009; Vol. 46, issue Suppl:S15–S61.
- Uprety P, Lindsey JC, Levin MJ, Rainwater‐Lovett K, Ziemniak C, Bwakura‐Dangarembizix M, et al. Inflammation and immune activation in antiretroviral‐treated human immunodeficiency virus type 1‐infected African infants and rotavirus vaccine responses. Journal of Infectious Diseases 2017;215(6):928‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Vesikari T, Karvonen A, Borrow R, Kitchin N, Baudin M, Thomas S, et al. Results from a randomized clinical trial of coadministration of RotaTeq, a pentavalent rotavirus vaccine, and NeisVac‐C, a meningococcal serogroup C conjugate vaccine. Clinical Vaccine Immunology 2011 May;18(5):878‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00443846. An open‐label, randomised, comparative, multi‐centre study of the immunogenicity and safety of the concomitant use of a live pentavalent rotavirus vaccine (RotaTeq®) and a meningococcal Group C conjugate (MCC) vaccine in healthy infants. clinicaltrials.gov/ct2/show/record/NCT00443846 (first received 6 March 2007).
- Weinberg A, Lindsey J, Bosch R, Persaud D, Sato P, Ogwu A, et al. B and T cell phenotypic profiles of African HIV‐infected and HIV‐exposed uninfected infants: associations with antibody responses to the pentavalent rotavirus vaccine. Frontiers in Immunology 2017;8:2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- ACTRN12610000525088. A Phase 1 double‐blind, randomized study to compare the safety, tolerability and immunogenicity of oral RV3‐BB rotavirus vaccine and placebo in infants, children and male adults. anzctr.org.au/ACTRN12610000525088.aspx (first received 22 June 2010).
- CTRI/2015/07/006034. Clinical trial on rotavirus vaccine to check consistency of different lots of vaccines manufactured and to check vaccine interference with other childhood vaccines given under universal immunization program in India. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=10177&EncHid=&modid=&compid=%27,%2710177det%27 (first received 20 July 2015).
- CTRI/2015/12/006428. Randomized open label study to compare immunogenicity and safety of ROTAVAC® and ROTARIX® rotavirus vaccine. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=13227&EncHid=&modid=&compid=%27,%2713227det%27 (first received 8 December 2015).
- NCT01061658. Phase I/II, randomized, double‐blind, placebo‐controlled, dosage selection (10e5.5 or 10e6.25 FFU of each constituent serotype per 0.5 mL) study to evaluate the safety, tolerability, and immunogenicity of a 3‐dose Series of live attenuated tetravalent (G1‐G4) bovine‐human reassortant rotavirus vaccine [BRV‐TV] administered to healthy Indian infants. clinicaltrials.gov/show/NCT01061658 (accessed 3 February 2010).
- NCT02153866. The safety and immunogenicity study of rotavirus vaccine simultaneously vaccinated with MR or MMR vaccine. clinicaltrials.gov/ct2/show/NCT02153866 (first received 3 June 2014).
- NCT02193061. Randomized, controlled single‐blind clinical study to assess vaccine interchangeability between RV5 and RV1 using seven combined anti‐rotavirus prevention programs. clinicaltrials.gov/ct2/show/NCT02193061 (first received 17 July 2014).
- NCT02542462. Potential mechanisms for intussusception after rotavirus vaccine‐pilot study. clinicaltrials.gov/ct2/show/NCT02542462 (first received 7 September 2015).
- NCT02646891. Safety and immunogenicity study of trivalent P2‐VP8 subunit rotavirus vaccine in adults, toddlers and infants. clinicaltrials.gov/ct2/show/NCT02646891 (first received 6 January 2016).
- NCT02847026. Fractional inactivated poliovirus vaccine booster and rotavirus study (fIPV). clinicaltrials.gov/ct2/show/NCT02847026 (accessed 24 April 2018) (first received 2 July 2016).
- NCT03462108. Safety and immunogenicity of Rotavirus (Bio Farma) vaccine in adults, children & neonates. clinicaltrials.gov/ct2/show/NCT03462108 (first received 12 March 2018).
- NCT03483116. A Phase II randomized, double blind, parallel group dose‐ranging study of oral RV3‐BB rotavirus vaccine. clinicaltrials.gov/ct2/show/NCT03483116 (first received 30 March 2018).
- ISRCTN86632774. A phase II, double blind randomised, placebo controlled study to assess the safety reactogenicity and immunogenicity of three doses of GSK Biologicals (South Africa). controlled‐trials.com/ISRCTN86632774 (first received 25 November 2005).
- NCT02941107. Optimising rotavirus vaccine in Aboriginal children. clinicaltrials.gov/ct2/show/NCT02941107 (first received 21 October 2016).
- Tatochenko VK, Romanenko VV, Kharit SM, Smolenov I, LeFevre I, Clercq N, et al. Co‐administration of a human rotavirus vaccine Rix4414 with DTPw‐HBv vaccines: immunogenicity and reactogenicity in healthy infants. 13th International Congress on Infectious Diseases, Kuala Lumpur, Malaysia; June 19–22. 2008. [Google Scholar]
- NCT02728869. Safety, reactogenicity and immunogenicity of heat‐stable rotavirus vaccine (HSRV) in adults and infants. clinicaltrials.gov/ct2/show/NCT02728869 (first received 5 April 2016).
Additional references
- Bar‐Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza‐Gomara M, Nakagomi O, et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll‐out: an observational and case‐control study. Lancet Infectious Diseases 2015;15(4):422‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐Zeev N, King C, Phiri T, Beard J, Mvula H, Crampin AC, et al. Impact of monovalent rotavirus vaccine on diarrhoea‐associated post‐neonatal infant mortality in rural communities in Malawi: a population‐based birth cohort study. Lancet Global Health 2018;6(9):e1036‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bines JE. Rotavirus vaccines and intussusception risk. Current Opinion in Gastroenterology 2005;21(1):20‐5. [PubMed] [Google Scholar]
- Buttery JP, Danchin MH, Lee KJ, Carlin JB, McIntyre PB, Elliott EJ, et al. Intussusception following rotavirus vaccine administration: Post‐marketing surveillance in the National Immunization Program in Australia. Vaccine 2011;29(16):3061‐66. [DOI] [PubMed] [Google Scholar]
- Buyse H, Vinals C, Karkada N, Han HH. The human rotavirus vaccine Rotarix™ in infants: an integrated analysis of safety and reactogenicity. Human Vaccines and Immunotherapeutics 2014;10(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bányai K, László B, Duque J, Steele AD, Nelson EA, Gentsch JR, et al. Systematic review of regional and temporal trends in global rotavirus strain diversity in the prerotavirus vaccine era: insights for understanding the impact of rotavirus vaccination programs. Vaccine 2012;30(Suppl 1):A122‐30. [DOI] [PubMed] [Google Scholar]
- Calles NR, Schutze GE. Immunizations for children with HIV. Module for HIV Curriculum available from: bipai.org/sites/bipai/files/20‐Immunizations.pdf (accessed 25 Oct 2018).
- Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia's National Immunization Program. Clinical Infectious Diseases 2013;57:1427‐34. [DOI] [PubMed] [Google Scholar]
- Advisory Committee on Immunization Practices, Department of Health and Human Services, Center for Disease Control and Prevention. Summary Report, October 27‐28, 2010. cdc.gov/vaccines/recs/acip/downloads/min‐archive/min‐oct10.pdf (URL no longer available) (accessed 02 March 2012).
- Advisory Committee on Immunization Practices. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and Reports 1999;48(RR‐2):1‐20. [PubMed] [Google Scholar]
- Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M. Rotavirus infection. Nature Reviews Disease Primers 2017;3:17083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe NA, Kang G. Can changes to scheduling enhance the performance of rotavirus vaccines in low‐income countries?. Journal of Infectious Diseases 2016;213(11):1673‐5. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C, Holmgren J. Vaccines against enteric infections for the developing world. Philosophical Transactions of the Royal Society of London 2015;370(1671):pii: 20150142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellepiane N, Wood D. Twenty‐five years of the WHO vaccines prequalification programme (1987‐2012): lessons learned and future perspectives. Vaccine 2015;33(1):52‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennehy PH, Brady RC, Halperin SA, Ward RL, Alvey JC, Fischer FH Jr, et al. North American Human Rotavirus Vaccine Study Group. Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatric Infectious Disease Journal 2005;24(6):481‐8. [DOI] [PubMed] [Google Scholar]
- Do Carmo GM, Yen C, Cortes J, Siqueira AA, De Oliveira WK, Cortez‐Escalante JJ, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time‐series analysis. PLoS Medicine 2011;8(4):e1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta AK. Rotavirus vaccination in India ‐ need for surveillance of intussusception. Indian Journal of Pediatrics 2017;84(2):95‐6. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Summary of Product Characteristics: Rotarix. ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000639/WC500054789.pdf(accessed November 2011).
- Escolano S, Farrington CP, Hill C, Tubert‐Bitter P. Intussusception after rotavirus vaccination‐‐spontaneous reports. New England Journal of Medicine 2011;365(22):2139. [DOI] [PubMed] [Google Scholar]
- Escolano S, Hill C, Tubert‐Bitter P. Intussusception risk after RotaTeq vaccination: evaluation from worldwide spontaneous reporting data using a self‐controlled case series approach. Vaccine 2015;33(8):1017‐20. [DOI] [PubMed] [Google Scholar]
- Fischer Walker C, Black R. Rotavirus vaccine and diarrhea mortality: quantifying regional variation in effect size. BMC Public Health 2011;11(Suppl 3):S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, Rehman AM, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. New England Journal of Medicine 2011;365(4):337‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed before 09 October 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
- Groome MJ, Page N, Cortese MM, Moyes J, Zar HJ, Kapongo CN, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case‐control study. Lancet Infectious Diseases 2014;14(11):1096‐1104. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency is preferable to testing for heterogeneity in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017) Cochrane, 2017. Available from www.training.cochrane.org/handbook.
- Hungerford D, Smith K, Tucker A, Iturriza‐Gómara M, Vivancos R, McLeonard C, et al. Population effectiveness of the pentavalent and monovalent rotavirus vaccines: a systematic review and meta‐analysis of observational studies. BMC Infectious Diseases 2017;17(1):569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerford D, Vivancos R, Read JM, Iturriza‐Gόmara M, French N, Cunliffe NA. Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time‐series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK. BMC Medicine 2018;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006‐2016. Clinical Infectious Diseases 2017;65(5):840‐50. [DOI] [PubMed] [Google Scholar]
- Lamberti LM, Ashraf S, Walker CL, Black RE. A systematic review of the effect of rotavirus vaccination on diarrhea outcomes among children younger than 5 years. Pediatric Infectious Disease Journal 2016;35(9):992‐8. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biology 2010;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhares AC, Velázquez FR, Pérez‐Schael I, Sáez‐Llorens X, Abate H, Espinoza F, et al. Human Rotavirus Vaccine Study Group. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double‐blind, placebo‐controlled phase III study. Lancet 2008;371(9619):1181‐9. [DOI] [PubMed] [Google Scholar]
- Merck. WHO list of vaccines for purchase by UN agencies as of November 2008. www.who.int/immunization_standards/vaccine_quality/pq_suppliers/en/index.html. Press release (accessed 21 November 2008).
- Merck, Co. Inc. Quality details for 9 RV5 included trials. Correspondence, 12 March 2012.
- Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. New England Journal of Medicine 2001;344(8):564‐72. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Glass RI. Public health. Progress toward rotavirus vaccines. Science 2006;312(5775):851‐2. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Alexander JP, Glass RI, Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and Reports 2006;55(RR‐12):1‐13. [PubMed] [Google Scholar]
- Patel MM, Lopez‐Collada VR, Bulhoes MM, Oliveira LH, Marquez AB, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. New England Journal of Medicine 2011;364(24):2283‐92. [DOI] [PubMed] [Google Scholar]
- Patel MM, Clark AD, Sanderson CF, Tate J, Parashar UD. Removing the age restrictions for rotavirus vaccination: a benefit‐risk modeling analysis. PLoS Medicine 2012;9(10):e1001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATH. Country National Immunization Program (NIP) Introductions of Rotavirus Vaccine. www.vaccineresources.org/files/PATH‐Country‐Introduction‐Table‐EN‐2016.05.01.pdf (accessed 27 July 2018).
- Pinto MV, Bihari S, Snape MD. Immunisation of the immunocompromised child. Journal of Infection 2016;72 Suppl:S13‐22. [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Richardson V, Hernandez‐Pichardo J, Quintanar‐Solares M, Esparza‐Aguilar M, Johnson B, Gomez‐Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. New England Journal of Medicine 2010;362(4):299‐305. [DOI] [PubMed] [Google Scholar]
- ROTA council. Global Introduction Status. rotacouncil.org/vaccine‐introduction/global‐introduction‐status/ (accessed 27 July 2018).
- Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for severity of diarrheal episodes. Scandinavian Journal of Infectious Diseases 1990;22(3):259‐67. [DOI] [PubMed] [Google Scholar]
- SAGE. Meeting of the immunization Strategic Advisory Group of Experts, April 2009‐‐conclusions and recommendations. Weekly Epidemiological Record 2009;84(23):220‐36. [PubMed] [Google Scholar]
- SAGE. Meeting of the immunization Strategic Advisory Group of Experts, April 2012 ‐ conclusions and recommendations. Weekly Epidemiological Record 2012;21(87):201‐16. [PubMed] [Google Scholar]
- Sanderson C, Clark A, Taylor D, Bolanos B. Global review of rotavirus morbidity and mortality data by age and region. www.who.int/immunization/sage/meetings/2012/april/Sanderson_et_al_SAGE_April_rotavirus.pdf 2011 (accessed 25 October 2018).
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.cochrane.training.org/handbook.
- Shui IM, Baggs J, Patel M, Parashar UD, Rett M, Belongia EA, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in US infants. JAMA 2012;307(6):598‐604. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on RotaShield and intussusception: the role of age at the time of vaccination. Journal of Infectious Diseases 2005;192(Suppl 1):36‐43. [DOI] [PubMed] [Google Scholar]
- Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England: A self‐controlled case‐series evaluation. Vaccine 2016;34(32):3684‐9. [DOI] [PubMed] [Google Scholar]
- Tate JE, Burton AH, Boschi‐Pinto C, Steele AD, Duque J, Parashar UD, WHO‐coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus‐associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta‐analysis. Lancet Infectious Diseases 2012;12(2):136‐41. [DOI] [PubMed] [Google Scholar]
- Tate JE, Mwenda JM, Armah G, Jani B, Omore R, Ademe A, et al. Evaluation of intussusception after monovalent rotavirus vaccination in Africa. New England Journal of Medicine 2018;378(16):1521‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter‐Campbell S, et al. Rotavirus infection in infants as protection against subsequent infections. New England Journal of Medicine 1996;335(14):1022‐8. [DOI] [PubMed] [Google Scholar]
- Velázquez RF, Linhares AC, Muñoz S, Seron P, Lorca P3, DeAntonio R, et al. Efficacy, safety and effectiveness of licensed rotavirus vaccines: a systematic review and meta‐analysis for Latin America and the Caribbean. BMC Pediatrics 2017;17(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T. Rotavirus vaccines against diarrhoeal disease. Lancet 1997;350(9090):1538‐41. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Damme P, Giaquinto C, Dagan R, Guarino A, Szajewska H, et al. European Society for Paediatric Infectious Diseases consensus recommendations for rotavirus vaccination in Europe. Pediatric Infectious Disease Journal 2015;34(6):635‐43. [DOI] [PubMed] [Google Scholar]
- Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination. New England Journal of Medicine 2014;370(6):513‐9. [DOI] [PubMed] [Google Scholar]
- World Health Organization. List of Member States by WHO region and mortality stratum. www.who.int/whr/2003/en/member_states_182‐184_en.pdf (accessed on 04 April 2012).
- World Health Organization. Rotavirus vaccines WHO position paper – January 2013. Weekly Epidemiological Record 2013;88(5):49‐64. 23424730 [Google Scholar]
- World Health Organization. WHO Prequalified Vaccines. extranet.who.int/gavi/PQ_Web/ (accessed 2 July 2018).
- Yen C, Tate JE, Hyde TB, Cortese MM, Lopman BA, Jiang B, et al. Rotavirus vaccines: current status and future considerations. Human Vaccines and Immunotherapeutics 2014;10(6):1436‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C, Healy K, Tate JE, Parashar UD, Bines J, Neuzil K, et al. Rotavirus vaccination and intussusception – Science, surveillance, and safety: a review of evidence and recommendations for future research priorities in low and middle income countries. Human Vaccines and Immunotherapeutics 2016;12(10):2580‐2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill‐Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. New England Journal of Medicine 2014;370(6):503‐12. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Soares‐Weiser K, Goldberg E, Tamimi G, Pitan OC, Leibovici L. Rotavirus vaccine for preventing diarrhoea. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002848.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares‐Weiser K, MacLehose H, Ben‐Aharon I, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD008521] [DOI] [PubMed] [Google Scholar]
- Soares‐Weiser K, MacLehose H, Bergman H, Ben‐Aharon I, Nagpal S, Goldberg E, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008521.pub2] [DOI] [PubMed] [Google Scholar]
- Soares‐Weiser K, MacLehose H, Bergman H, Ben‐Aharon I, Nagpal S, Goldberg E, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD008521.pub3] [DOI] [PubMed] [Google Scholar]


