Abstract
Expression levels and clinical significance of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients with diarrhea-type irritable bowel syndrome (D-IBS) were investigated. A retrospective analysis was performed on 46 tissue specimens (observation group) of the intestinal mucosa of patients with D-IBS, who were diagnosed in the Tongde Hospital of Zhejiang Province and received colonoscopy from March 2016 to December 2017, and 18 tissue specimens (control group) of the intestinal mucosa of healthy subjects who received physical examinations. The expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients in the observation and control group were detected by ELISA, and the relationship between 5-HT, 5-HT3R and the clinicopathological parameters of patients with D-IBS was analyzed. Pearson's correlation analysis was used to analyze the correlation of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients with D-IBS. The expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients in the observation group were significantly higher than those of the patients in the control group (344.86±67.52 ng/ml and 13.04±8.34 pg/ml) (P<0.001). There was a positive correlation between the expression level of 5-HT and the expression level of 5-HT3R in the intestinal mucosa tissue of patients with D-IBS (r=0.725, P<0.001). The expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients with D-IBS were both significantly higher than those of the healthy subjects. The expression levels of 5-HT and 5-HT3R in patients with D-IBS were correlated with age, sex and the history of gastrointestinal infection. 5-HT and 5-HT3R may be involved in the pathogenesis of D-IBS, and potentially used for clinical treatment.
Keywords: diarrhea-type irritable bowel syndrome, intestinal mucosa, 5-HT, 5-HT3R, clinical significance
Introduction
Irritable bowel syndrome (IBS) is a chronic intestinal disease. The main clinical signs are abdominal pain accompanied by the intermittent or persistent irregularity of defecation and the abnormal texture and shape of feces (1,2). Since this disease does not show obvious abnormalities of biochemical indicators, it is a functional disease (3). According to the statistics, the morbidity of IBS in the world is 5–20%, and the morbidity in Asia is 6.5–10.1% (4,5). The morbidity of IBS which conforms to Manning criteria in China is 22.8% and the morbidity is related to the stress of life and the pace of work (6). The patients with IBS are mainly young women (7). Although IBS does not threaten patients life, it still greatly affects their quality of life, its pathogenesis is inexplicable and patients cannot be treated in a specific manner (8).
At present, it is held by most medical researchers that the abnormality of gastrointestinal motility, the high sensitivity of viscus and the disorder of the brain-intestine axis function are involved in the pathogenesis of IBS. In addition, endocrine dysfunction, the stress response of mentality and immunoreaction are also considered to be involved in the pathogenesis of IBS (9,10).
5-HT, also known as serotonin, is an immunoregulatory factor and neurotransmitter. A great deal of 5-HT is intensively distributed in the gastrointestinal tract but only a small amount of 5-HT is distributed in the central nervous system (11). It has certain specificity and is of great significance in the physiological and pathological processes of the human body, such as the thermoregulation, the regulation of blood pressure, the production of algesia, as well as nausea and vomiting (12). 5-HT plays an important role in the brain-intestine axis which is between the intestinal tract and the central nervous system, and is also an important signal for maintaining intestinal balance; it regulates the intestinal motility, sensation and secretion of intestinal glands (13). The serotonin 3 receptor (5-HT3R) is an ion channel receptor found in the central and peripheral nervous systems (14). 5-HT3R is of great significance for the transmission of the information of digestive tract activity, regulation of intestinal peristalsis and secretion of intestinal glands (9). There are different clinical manifestations when 5-HT combines with different receptors. 5-HT mainly combines with 5-HT3R in patients with D-IBS, which leads to some intestinal dysfunctions, such as visceral hypersensitivity and abdominal discomfort (15). Most of 5-HT in the gastrointestinal tract is synthesized and stored by the chromaffin cells of the intestinal mucosa (16). When the intestinal tract is stimulated, it causes an increase of 5-HT in the chromaffin cells, and the combination of 5-HT and 5-HT3R which is in the nerve endings of exogenous primary afferent neurons, so that the visceral afferent nerve and the intestinal nervous system are in a hypersensitive state, which results in symptoms, such as diarrhea, abdominal pain and discomfort (17).
IBS includes four main types, which are diarrhea, constipation, mixed, and undefined type, with the diarrhea type being more common in clinical practice at present (18). This study aimed to investigate the expression levels and clinical significance of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients with diarrhea-type irritable bowel syndrome (D-IBS) and provide some references for the research and development of new drugs for the treatment of D-IBS.
Patients and methods
General data
The tissue specimens of the intestinal mucosa of patients with D-IBS, who were diagnosed in the Tongde Hospital of Zhejiang Province (Hangzhou, China) and received colonoscopy from March 2016 to December 2017, were retrospectively analyzed (observation group). The tissue specimens of the intestinal mucosa of healthy subjects who received physical examinations or volunteers were also retrospectively analyzed (control group). There were 46 patients in the observation group, including 19 males and 27 females, with an average age of 40.35±5.68 years. Eighteen patients in the control group included 5 males and 13 females, with an average age of 39.85±6.12 years. Inclusion criteria: all patients conformed with the Rome III diagnostic criteria of IBS (19); and the intestinal mucosa of all healthy subjects who received physical examinations, or volunteers, was normal. The intestinal cleanliness of all selected patients reached the standard, and patients received colonoscopy with complete clinical data. Exclusion criteria: patients with pregnancy, lactation, lesions of intestinal mucosa, diseases of digestive system, autoimmune diseases, diabetes, heart disease, mental illness and cancers. This study was approved by the Ethics Committee of the Tongde Hospital of Zhejiang Province. Patients who participated in this research had complete clinical data. Signed informed consents were obtained from the patients and/or guardians.
Detection methods
Intestinal mucosa tissue (1 g) of all subjects was removed. Cold phosphate-buffered saline (PBS) with a concentration of 0.02 mol/l and pH 7.0 was used to wash the tissue and wipe off the blood. Then 5 ml of pre-cooling PBS were added into the tissue, and the mixture was homogenized using a homogenizer (Omni International, Inc., Kennesaw, GA, USA). The homogenate was centrifuged for 5 min at 4,200 × g at 4°C and the supernatant was saved for use. The expression levels of 5-HT (Shanghai Huzheng Industrial Co., Ltd., Shanghai, China; item no. HZ-5-HT-Ge) and 5-HT3R (Shanghai Jianglai Biotechnology Co., Ltd., Shanghai, China; item no. 1532413532) in the intestinal mucosa tissue of the observation and control group were measured using enzyme-linked immunosorbent assay (ELISA). The reagent box and the sample to be tested were taken from the refrigerator 30 min earlier, so that the temperature was balanced with the room temperature. Wells were set up for the sample to be tested, standard and blank well, and all the experimental operations were strictly carried out in accordance with the instructions. Firstly, 100 µl of the sample diluent were added into the blank well. Then 100 µl of the sample diluent were respectively added into the standard well and the well of sample to be tested, and 100 µl of the test solution was added into each well after discarding the liquid and drying. The mixture was incubated for 1 h at 37°C, and the liquid was discarded and dried again. Then the mixture was rinsed 3 times using PBS. After drying, 100 µl of another test solution were added into the mixture and then the mixture was incubated for 1 h at 37°C again. The liquid was discarded and dried and then rinsed 3 times with PBS. A total of 90 µl of the substrate solution was added into the mixture, and color development was performed in the dark at 37°C. Finally, 50 µl of the stop solution were added to terminate the translation, and BioTek ELx800 automatic enzyme-labeled instrument (Beijing Taize Ruida Technology Co., Ltd., Beijing, China) was used to detect the OD value of each well at the wavelength of 450 nm and to calculate the concentration of 5-HT and 5-HT3R.
Statistical methods
The analysis was carried out using SPSS 20.0 statistical software (Shanghai Kabei Information Technology Co., Ltd., Shanghai, China). Enumeration data were expressed as n (%), and the Chi-square test was used for the comparison between groups. Mean ± SD was used to express the measurement data. The independent sample t-test was used for the comparison between two groups, while the paired t-test for the comparison within the group. Pearson's correlation analysis was applied to analyze the correlation of 5-HT and 5-HT3R in the D-IBS group; P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of general data
There was no significant difference in age, sex, body mass index (BMI), smoking, drinking, and place of residence between the observation and control group (P>0.05), which suggested that the groups were comparable. The difference in the history of gastrointestinal infection (history of acute gastrointestinal infection at least 1 day before the onset of D-IBS symptoms, cured with treatment for <5 days, with no recurrence) was statistically significant (P<0.05) (Table I).
Table I.
General data [mean ± SD, n (%)].
| Indicators | Observation group (n=46) | Control group (n=18) | t/χ2 | P-value |
|---|---|---|---|---|
| Age (years) | 40.35±5.68 | 39.85±6.12 | 0.310 | 0.758 |
| Sex | 1.010 | 0.396 | ||
| Male | 19 (41.30) | 5 (27.78) | ||
| Female | 27 (58.70) | 13 (72.22) | ||
| BMI (kg/m2) | 21.65±1.47 | 21.58±1.73 | 0.163 | 0.871 |
| Smoking | 2.046 | 0.246 | ||
| Yes | 19 (41.30) | 4 (22.22) | ||
| No | 27 (58.70) | 14 (77.78) | ||
| Drinking | 1.274 | 0.377 | ||
| Yes | 17 (36.96) | 4 (22.22) | ||
| No | 29 (63.04) | 14 (77.78) | ||
| Place of residence | 2.029 | 0.230 | ||
| Countryside | 12 (26.09) | 8 (44.44) | ||
| City | 34 (73.91) | 10 (55.56) | ||
| History of gastrointestinal infection | 9.847 | 0.003a | ||
| Yes | 40 (86.96) | 7 (38.89) | ||
| No | 6 (13.04) | 11 (61.11) |
P<0.05, compared within the group. BMI, body mass index.
Expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of the observation and control group
The expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients in the observation group (476.59±75.23 ng/ml and 28.69±15.62 pg/ml, respectively) were both significantly higher than those in the control group (344.86±67.52 ng/ml and 13.04±8.34 pg/ml, respectively), and the differences were statistically significant (P<0.001) (Fig. 1 and Table II).
Figure 1.
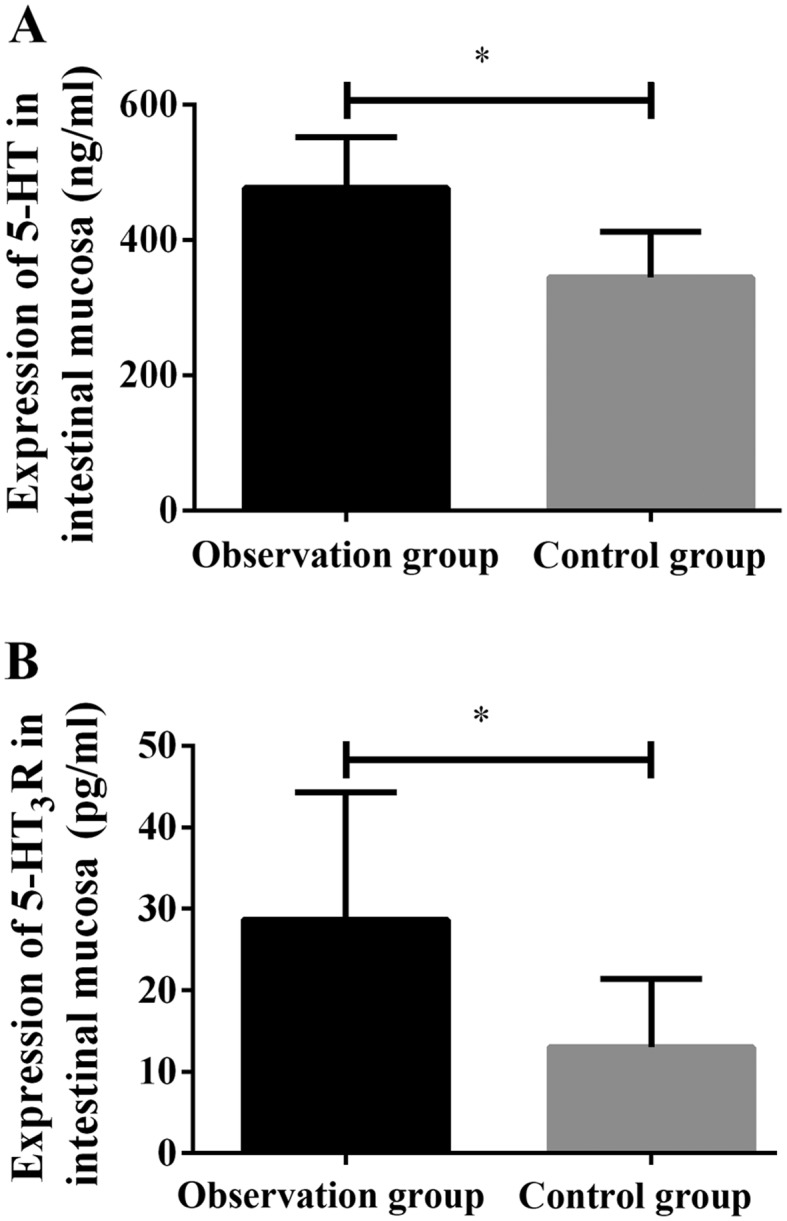
Expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of the observation and control group. (A) The results of ELISA showed that the expression level of 5-HT in the intestinal mucosa tissue of patients in the observation group was significantly higher than that in the control group, and the difference was statistically significant (*P<0.001). (B) The expression level of 5-HT3R in the intestinal mucosa tissue of patients in the observation group was significantly higher than that in the control group, and the difference was statistically significant (*P<0.001).
Table II.
Expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of the observation and control group (mean ± SD).
| Groups | n | 5-HT (ng/ml) | 5-HT3R (pg/ml) |
|---|---|---|---|
| Observation group | 46 | 476.59±75.23 | 28.69±15.62 |
| Control group | 18 | 344.86±67.52 | 13.04±8.34 |
| t | 6.473 | 4.019 | |
| P-value | <0.001 | <0.001 |
Correlation analysis of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients in the observation group
The expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients with D-IBS were positively correlated (r=0.725, P<0.001) (Fig. 2).
Figure 2.
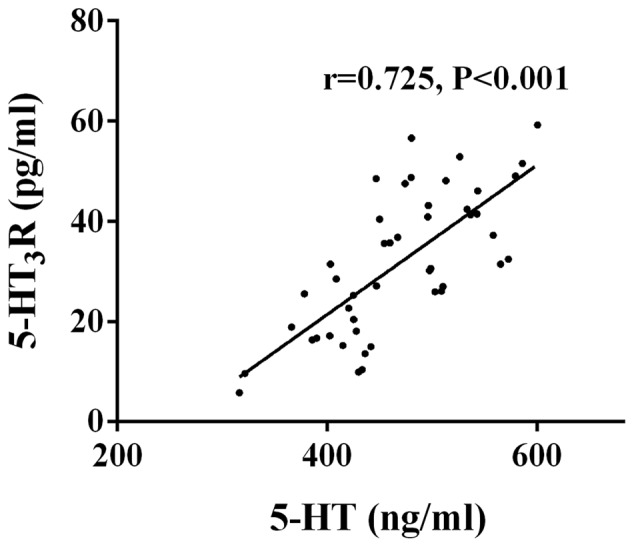
Correlation analysis of 5-HT and 5-HT3R in the intestinal mucosa tissue of the patients in the observation group. Pearson's correlation analysis showed that the expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients with D-IBS were positively correlated (r=0.725, P<0.001). D-IBS, diarrhea-type irritable bowel syndrome.
Relationship between 5-HT, 5-HT3R and clinicopathological parameters of D-IBS
The expression levels of 5-HT and 5-HT3R in patients with D-IBS were not significantly associated with the course of disease, smoking, drinking, and place of residence (P>0.05), but were associated with age, sex, and the history of gastrointestinal infection, and the differences were statistically significant (P<0.05). The expression levels of 5-HT and 5-HT3R in patients with D-IBS who were aged ≤40 years were significantly higher than those in patients who were >40 years of age, and the expression levels of 5-HT and 5-HT3R in female patients with D-IBS were significantly higher than those in male patients. The expression levels of 5-HT and 5-HT3R in patients with D-IBS who had history of gastrointestinal infection were significantly higher than those who had no history of gastrointestinal infection (Table III).
Table III.
Relationship between 5-HT, 5-HT3R and clinicopathological parameters of D-IBS (mean ± SD).
| Factors | n | 5-HT (ng/ml) | t | P-value | 5-HT3R (pg/ml) | t | P-value |
|---|---|---|---|---|---|---|---|
| Age (years) | 2.341 | 0.024a | 2.351 | 0.023a | |||
| >40 | 12 | 420.58±69.38 | 19.21±10.68 | ||||
| ≤40 | 34 | 478.59±75.23 | 30.69±15.62 | ||||
| Sex | 2.605 | 0.013a | 2.185 | 0.034a | |||
| Male | 19 | 418.26±67.31 | 19.06±13.47 | ||||
| Female | 27 | 473.69±73.56 | 29.66±17.85 | ||||
| Course of disease (years) | 1.225 | 0.227 | 0.964 | 0.341 | |||
| >2 | 28 | 475.09±73.85 | 29.52±13.67 | ||||
| ≤2 | 18 | 448.25±70.29 | 25.74±11.82 | ||||
| Smoking | 1.485 | 0.145 | 0.594 | 0.555 | |||
| Yes | 19 | 469.53±68.21 | 29.26±14.75 | ||||
| No | 27 | 440.67±62.53 | 26.52±15.83 | ||||
| Drinking | 1.300 | 0.201 | 0.730 | 0.470 | |||
| Yes | 17 | 473.29±72.55 | 30.15±15.23 | ||||
| No | 29 | 446.37±64.92 | 26.85±14.56 | ||||
| Place of residence | 1.795 | 0.080 | 0.902 | 0.372 | |||
| Countryside | 12 | 436.63±64.02 | 25.03±10.35 | ||||
| City | 34 | 480.34±75.14 | 29.53±16.08 | ||||
| History of gastrointestinal infection | 2.033 | 0.048a | 2.100 | 0.042a | |||
| Yes | 40 | 485.22±76.35 | 31.79±16.59 | ||||
| No | 6 | 418.25±65.88 | 17.01±11.28 |
P<0.05, compared within the group. D-IBS, diarrhea-type irritable bowel syndrome.
Discussion
The morbidity of irritable bowel syndrome (IBS), a common global disease, is correlated with the level of economic development, and is higher in cities with developed economy and a heavy pressure of work and life (20). The course of IBS is generally long and repetitive, and it requires patients to take a large number of oral medicines chronically, which increases patients economic and mental stress, and also greatly consumes the medical resources (21). According to Choung et al (22), D-IBS is affected by psychological and environmental factors, so clinical trials are more practical than animal experiments.
It is known that the complex 5-HT receptors are divided into 7 families, namely 5-HT1-7R, and 14 subtypes, including two major classes: ligand-gated ion channel receptors and G protein-coupled receptors (23). 5-HT binds to the 5-HT receptor to regulate complex functions, such as secretion, absorption, gastrointestinal motility and sensation, which lead to a series of typical symptoms of IBS, such as bowel movement abnormalities (24). According to a previous study (25), 5-HT in animal models and isolated tissues can promote the secretion of intestinal water and electrolytes, so it is considered that the symptoms of D-IBS patients, such as increased intestinal gland secretion, diarrhea and the improvement of digestive tract transport capacity may be related to the increase of intestinal motility and intestinal gland secretion by 5-HT. 5-HT3R is widely distributed in the digestive tract and is expressed in the submucosal plexus and intestinal myenteric plexus. The activation of 5-HT3R can lead to depolarization of the cell membrane, calcium influx, and thus excite the central and peripheral neurons, promoting the release of neurotransmitters, such as acetylcholine from parasympathetic nerve endings, which leads to the development of high sensitivity of the viscera and regulates contraction and relaxation of smooth muscle (26). Andresen and Hollerbach (27) found that 5-HT3 receptor antagonists can effectively treat D-IBS, but its clinical use is limited due to its adverse reactions.
The present study showed that the expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients in the observation group are significantly higher than those of patients in the control group, and the differences were found to be statistically significant (P<0.001). In accordance to these results, Sun et al (28), have revealed that the positive cells of 5-HT and the expression of 5-HT3R are both significantly higher than those in the normal control group. Another study has speculated that there is a direct relationship between the increase in colonic mucosal expression of 5-HT3R and the onset of D-IBS (29). Combined with the results of this study, 5-HT and 5-HT3R may be involved in the occurrence of D-IBS. After electroacupuncture and medicine treatment, the abnormal expression of 5-HT and 5-HT3R in D-IBS colonic mucosa has been shown to significantly decrease, and the levels of 5-HT and 5-HT3R in the electroacupuncture group are significantly lower than those in the medicine group, suggesting that the therapeutic effect is better than medicine (30). It is suggested that the expression changes of 5-HT and 5-HT3R may reflect the severity of D-IBS and the evaluation of curative effect. However, more research is needed to prove the specific conclusions. The interaction between 5-HT and 5-HT3R may regulate intestinal secretion, motor function, and pain level through the regulation information of central nervous system (31). It is speculated that 5-HT is associated with 5-HT3R. In the present study, after the correlation coefficient analysis, the results showed that the expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients with D-IBS are positively correlated (r=0.725, P<0.001). Spiller (32) has found that in the colonic mucosa of D-IBS patients, the synthesis and secretion of 5-HT increases, and the expression of 5-HT3 receptor is upregulated, suggesting that 5-HT and 5-HT3 receptors may be correlated. However, there are few related studies at present, and further research is needed to prove this.
The results of the present study also showed that the expression levels of 5-HT and 5-HT3R in the patients with D-IBS were not significantly correlated with the course of disease, smoking, drinking and place of residence (P>0.05), but were correlated with age, sex and the history of gastrointestinal infection, and the differences were statistically significant (P<0.05). Among the patients, the expression levels of 5-HT and 5-HT3R in patients with D-IBS who were aged ≤40 years were significantly higher than those in patients who were >40 years of age, and the expression levels of 5-HT and 5-HT3R in female patients with D-IBS were significantly higher than those in male patients. The expression levels of 5-HT and 5-HT3R in patients with D-IBS who had history of gastrointestinal infection were significantly higher than those of patients who did not have history of gastrointestinal infection. We speculate that the expression levels of 5-HT and 5-HT3R in D-IBS patients may be affected by hormone levels, resulting in a higher expression in females than males. The study of Thompson et al (33) has shown that the morbidity of female patients with IBS is higher than that of male patients, which may be related to the menstrual cycle and the hormone level changes of females, similar to the results of this study. Since both 5-HT and 5-HT3R exist in the central nervous system of the human body and the occurrence of IBS could also be affected by psychological factors (34,35), there are many reasons that may have caused the expression levels of 5-HT and 5-HT3R in the young subjects and patients with D-IBS who had history of gastrointestinal infection to be significantly higher than those in patients who were aged >40 years and did not have the history of gastrointestinal infection. These reasons include heavy pressure of work and life, psychological anxiety, the irregularity of diet, food hygiene, and the belated diagnosis caused by the personal negligent care (36,37).
In this study, the expression levels of 5-HT and 5-HT3R in D-IBS patients were studied in many aspects, which provided a reference for clinical research. However, there are limitations in this retrospective analysis, such as possible bias in design. The exact mechanism of 5-HT and 5-HT3R in the development of D-IBS remains to be further studied.
In summary, the expression levels of 5-HT and 5-HT3R in the intestinal mucosa tissue of patients with D-IBS are both significantly higher than those of healthy subjects, and the expression levels of 5-HT and 5-HT3R in patients with D-IBS are not significantly correlated with the course of disease, smoking, drinking, and place of residence, but are correlated with age, sex and the history of gastrointestinal infection. 5-HT and 5-HT3R may be involved in the pathogenesis of D-IBS, and potentially be used for clinical treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China Young Scientist Program (no. 81302957); the Zhejiang Provincial Natural Science Foundation of China (nos. LY17H270003 and LQ19H270051); the Zhejiang Province Institute of Science and Technology of Special Foundation (no. 2017F30044); the Zhejiang Province Key Research Project of Traditional Chinese Medicine (no. 2018ZZ005); the Zhejiang Medical and Health Science and Technology Plan Project (no. 2018KY326); and the Zhejiang Province Project of Traditional Chinese Medicine (no. 2018ZB030).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors contributions
RF and MC designed the study and drafted the manuscript. YC acquired the data. GM and SL analyzed the data and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Tongde Hospital of Zhejiang Province (Hangzhou, China). Patients who participated in this research had complete clinical data. Signed informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Harper L, Bold J. An exploration into the motivation for gluten avoidance in the absence of coeliac disease. Gastroenterol Hepatol Bed Bench. 2018;11:259–268. [PMC free article] [PubMed] [Google Scholar]
- 2.Fadgyas-Stanculete M, Buga AM, Popa-Wagner A, Dumitrascu DL. The relationship between irritable bowel syndrome and psychiatric disorders: From molecular changes to clinical manifestations. J Mol Psychiatry. 2014;2:4–11. doi: 10.1186/2049-9256-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao YF, Ma XQ, Wang R, Yan XY, Li ZS, Zou DW, He J. Epidemiology of functional constipation and comparison with constipation-predominant irritable bowel syndrome: The Systematic Investigation of Gastrointestinal Diseases in China (SILC) Aliment Pharmacol Ther. 2011;34:1020–1029. doi: 10.1111/j.1365-2036.2011.04809.x. [DOI] [PubMed] [Google Scholar]
- 4.Ma XP, Hong J, An CP, Zhang D, Huang Y, Wu HG, Zhang CH, Meeuwsen S. Acupuncture-moxibustion in treating irritable bowel syndrome: How does it work? World J Gastroenterol. 2014;20:6044–6054. doi: 10.3748/wjg.v20.i20.6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee IS, Preissl H, Enck P. How to perform and interpret functional magnetic resonance imaging studies in functional gastrointestinal disorders. J Neurogastroenterol Motil. 2017;23:197–207. doi: 10.5056/jnm16196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajendra S, Alahuddin S. Prevalence of irritable bowel syndrome in a multi-ethnic Asian population. Aliment Pharmacol Ther. 2004;19:704–706. doi: 10.1111/j.1365-2036.2004.01891.x. [DOI] [PubMed] [Google Scholar]
- 7.Harris LA, Umar SB, Baffy N, Heitkemper MM. Irritable bowel syndrome and female patients. Gastroenterol Clin North Am. 2016;45:179–204. doi: 10.1016/j.gtc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, Hong JY, Gupta A, Tillisch K, Ebrat B, et al. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155:137–149. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759–6773. doi: 10.3748/wjg.v20.i22.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundmann O, Yoon SL. Complementary and alternative medicines in irritable bowel syndrome: An integrative view. World J Gastroenterol. 2014;20:346–362. doi: 10.3748/wjg.v20.i2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman MM, Mahadeva S, Ghoshal UC. Epidemiological and clinical perspectives on irritable bowel syndrome in India, Bangladesh and Malaysia: A review. World J Gastroenterol. 2017;23:6788–6801. doi: 10.3748/wjg.v23.i37.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs. 2004;5:55–60. [PubMed] [Google Scholar]
- 13.Stasi C, Bellini M, Bassotti G, Blandizzi C, Milani S. Serotonin receptors and their role in the pathophysiology and therapy of irritable bowel syndrome. Tech Coloproctol. 2014;18:613–621. doi: 10.1007/s10151-013-1106-8. [DOI] [PubMed] [Google Scholar]
- 14.Glatzle J, Sternini C, Robin C, Zittel TT, Wong H, Reeve JR, Jr, Raybould HE. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- 15.Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285–1293. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–1101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mawe GM, Coates MD, Moses PL. Review article: Intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23:1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wu Z, Qiao H, Zhang Y. A genetic association study of single nucleotide polymorphisms in GNβ3 and COMT in elderly patients with irritable bowel syndrome. Med Sci Monit. 2014;20:1246–1254. doi: 10.12659/MSM.890315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endo Y, Shoji T, Fukudo S. Epidemiology of irritable bowel syndrome. Ann Gastroenterol. 2015;28:158–159. [PMC free article] [PubMed] [Google Scholar]
- 20.Luscombe FA. Health-related quality of life and associated psychosocial factors in irritable bowel syndrome: A review. Qual Life Res. 2000;9:161–176. doi: 10.1023/A:1008970312068. [DOI] [PubMed] [Google Scholar]
- 21.Salaga M, Binienda A, Tichkule RB, Thakur GA, Makriyannis A, Storr M, Fichna J. The novel peripherally active cannabinoid type 1 and serotonin type 3 receptor agonist AM9405 inhibits gastrointestinal motility and reduces abdominal pain in mouse models mimicking irritable bowel syndrome. Eur J Pharmacol. 2018;836:34–43. doi: 10.1016/j.ejphar.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Choung RS, Locke GR, III, Zinsmeister AR, Schleck CD, Talley NJ. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: A psychological component is the rule. Am J Gastroenterol. 2009;104:1772–1779. doi: 10.1038/ajg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiba T, Yamamoto K, Sato S, Suzuki K. Long-term efficacy and safety of ramosetron in the treatment of diarrhea-predominant irritable bowel syndrome. Clin Exp Gastroenterol. 2013;6:123–128. doi: 10.2147/CEG.S32721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Zhou H, Liu B. Current status of study on 5-HT in the pathogenesis of irritable bowel syndrome. Chin J Gastroenterol. 2011;16:445–448. (In Chinese) [Google Scholar]
- 25.Lee OY. Asian motility studies in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:120–130. doi: 10.5056/jnm.2010.16.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–2977. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 27.Andresen V, Hollerbach S. Reassessing the benefits and risks of alosetron: What is its place in the treatment of irritable bowel syndrome? Drug Saf. 2004;27:283–292. doi: 10.2165/00002018-200427050-00001. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Wu X, Meng Y, Cheng J, Ning H, Peng Y, Pei L, Zhang W. Electro-acupuncture decreases 5-HT, CGRP and increases NPY in the brain-gut axis in two rat models of Diarrhea-predominant irritable bowel syndrome (D-IBS) BMC Complement Altern Med. 2015;15:340. doi: 10.1186/s12906-015-0863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: Systematic review and meta-analysis. Am J Gastroenterol. 2009;104:1831–1843. doi: 10.1038/ajg.2009.223. [DOI] [PubMed] [Google Scholar]
- 30.Houghton LA, Foster JM, Whorwell PJ. Alosetron, a 5-HT3 receptor antagonist, delays colonic transit inpatients with irritable bowel syndrome and healthy volunteers. Aliment Pharmacol Ther. 2000;14:775–782. doi: 10.1046/j.1365-2036.2000.00762.x. [DOI] [PubMed] [Google Scholar]
- 31.Talley NJ. Review article: 5-hydroxytryptamine agonists and antagonists in the modulation of gastrointestinal motility and sensation: clinical implications. Aliment Pharmacol Ther. 1992;6:273–289. doi: 10.1111/j.1365-2036.1992.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 32.Spiller RC. Targeting the 5-HT(3) receptor in the treatment of irritable bowel syndrome. Curr Opin Pharmacol. 2011;11:68–74. doi: 10.1016/j.coph.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: First population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–235. doi: 10.1023/A:1013208713670. [DOI] [PubMed] [Google Scholar]
- 34.Qin HY, Cheng CW, Tang XD, Bian ZX. Impact of psychological stress on irritable bowel syndrome. World J Gastroenterol. 2014;20:14126–14131. doi: 10.3748/wjg.v20.i39.14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarcho JM, Chang L, Berman M, Suyenobu B, Naliboff BD, Lieberman MD, Ameen VZ, Mandelkern MA, Mayer EA. Neural and psychological predictors of treatment response in irritable bowel syndrome patients with a 5-HT3 receptor antagonist: A pilot study. Aliment Pharmacol Ther. 2008;28:344–352. doi: 10.1111/j.1365-2036.2008.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han SH, Lee OY, Bae SC, Lee SH, Chang YK, Yang SY, Yoon BC, Choi HS, Hahm JS, Lee MH, et al. Prevalence of irritable bowel syndrome in Korea: Population-based survey using the Rome II criteria. J Gastroenterol Hepatol. 2006;21:1687–1692. doi: 10.1111/j.1440-1746.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 37.Mulak A, Taché Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014;20:2433–2448. doi: 10.3748/wjg.v20.i10.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.


