Abstract
Neuregulin-1 (NRG-1) is considered to be a potential therapeutic agent for cardiovascular diseases due to its diverse protective effects. The aim of the present study was to investigate the effect of NRG-1 on cardiac electrophysiology in rats with myocardial infarction (MI). The rats were randomly divided into three groups: The sham operation group (SO; n=8); MI group (n=8); and the MI with recombinant human NRG (rhNRG)-1 administration group (NRG-1 group; 10 µg/kg; n=8). A rat MI model was established via ligation of the left anterior descending coronary artery. The rats in the NRG-1 group received a 10 µg/kg rhNRG-1 injection through the tail vein 30 min prior to ligation. Following 24 h of intervention, the field potential (FP) parameters, including the interspike interval (ISI), field potential duration (FPD), FPrise, FPmin, FPmax and conduction velocity (CV), were measured using microelectrode array technology. Subsequently, burst pacing was performed to assess ventricular arrhythmia (VA) susceptibility in the left ventricle. FP parameters in the MI group were significantly different when compared with those observed in the SO group. ISI, FPD, FPrise and FPmax in the infarct, peri-infarct and normal zones, as well as the CV of the infarct and peri-infarct zones, were all significantly decreased, and FPmin in the normal zone was increased (P<0.05). However, when compared with the MI group, NRG-1 prolonged the ISI and FPD in the 3 zones, and increased FPrise in the infarct zone, FPmax in the normal zone and CV in the peri-infarct zone; it also decreased FPmin in the normal zone (P<0.05). Furthermore, the incidence of VA was significantly reduced in the NRG-1 group when compared with the MI group (P<0.05). In conclusion, NRG-1 improved cardiac electrophysiological properties and reduced VA susceptibility in acute MI.
Keywords: neuregulin-1, myocardial infarction, cardiac electrophysiology, microelectrode array technology, ventricular arrhythmias
Introduction
Neuregulin-1 (NRG-1), which belongs to the epidermal growth factor family, is critical for cardiac development and functional maintenance of the mature myocardium (1). It was first detected in neural tissue, where it was reported to promote Schwann cell proliferation (2). Subsequent studies have revealed that NRG-1, through a group of associated protein-tyrosine kinase receptors known as ErbB (ErbB1-ErbB4) receptors (via the NRG-ErbB signaling axis) (3–6), serves vital roles in the cardiovascular system: It promotes cardiac performance, improves cardiac function, attenuates disease markers and prolongs survival. The cardioprotective effect of NRG-1 was first reported to prevent anthracycline-induced myofilament injury (7,8). Subsequently, animal experiments and preclinical trials have demonstrated that it serves important roles in maintaining cardiomyocyte structure and cardiac pumping functionality (9–12). It has also been identified to promote the proliferation and differentiation of cardiomyocytes in the developing or injured heart, thereby protecting against remodeling, fibrosis, apoptosis, myofibrillar disarray and scar formation (13–16). Therefore, it may represent a promising therapeutic strategy for heart failure, ischemia, dilation and hypertrophic cardiomyopathy (17,18). Although NRG-1 has been confirmed to have multi-target advantages in the cardiovascular system, there are limitations in our understanding of the effect of NRG-1 on cardiac electrophysiology. In a genetic predictors study, namely the Oregon Sudden Unexpected Death Study, a missense variant in NRG-1 was identified to be associated with ventricular fibrillation-induced sudden cardiac death (SCD) (19,20). Following a review of the current literature, it was noted that NRG-1 affected cellular electrophysiology in the central and peripheral nervous system. Therefore, the effects of NRG-1 on cardiac electrophysiology should not be overlooked (21). Previous studies have also demonstrated that NRG-1 protects the heart from acute ischemic injury (22–24). In the present study, the aim was to investigate the effects of NRG-1 pretreatment on cardiac electrophysiology in rats with acute myocardial infarction (MI).
Materials and methods
Experimental animals and grouping
A total of 24 Sprague- Dawley rats (13 males and 11 females; age, 8–10 weeks; weight, 250–300 g) were used for the experiments. All rats were housed at 20–25°C with 60% humidity under 12-h light/dark cycles with free access to food and water throughout the experiment. They were randomly assigned into one of the following groups: Sham operation (SO); MI; and MI with recombinant human NRG (rhNRG)-1 administration groups (n=8/group). Animals in the SO or MI groups received either sham surgery or ligature of the left anterior descending (LAD) coronary artery, respectively. Rats in the NRG-1 group received a 10 µg/kg rhNRG-1 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) injection through the tail vein 30 min prior to the LAD coronary artery ligature. The MI group was injected with saline following the LAD operation.
Animal handling was performed in accordance with the Wuhan Directive for Animal Research and the Guide for the Care and Use of Laboratory Animals. All procedures conducted in the present study were approved by the Ethics Committee of Wuhan University (Hubei, China).
Establishment of the rat MI model
Rats were anesthetized with an intraperitoneal injection of 1.5% pentobarbital sodium (Sigma-Aldrich; Merck KGaA) with a dose of 30 mg/kg body weight. Left intercostal thoracotomy was performed to expose the heart and the LAD coronary artery was ligated using a polypropylene suture under controlled ventilation (13). Successful induction of MI was observed by the operators when the anterior and apical left ventricular myocardium gradually turned a dull, pale color, accompanied by a weakened pulse. In addition, the electrocardiogram exhibited a gradual ST segment elevation in the I, II and avL leads. Rats in the SO group underwent thoracotomy and pericardiotomy, but without LAD ligation. Following this, the chest was closed and the animals were allowed to recover in a warm and clean cage.
Evaluation of infarct size
All rats were euthanized by cervical dislocation after all cardiac electrophysiology examinations. The heart was quickly excised, frozen at −20°C and sliced into four 2.0-mm-thick sections perpendicular to the long axis of the heart from apex to base. The heart sections were incubated with 2% triphenyltetrazolium chloride (TTC) at 37°C for 20 min in the dark and then fixed with 4% paraformaldehyde for 30 min at room temperature. Red parts in the heart stained by TTC indicated viable myocardium, whereas negatively stained areas represented infarcted myocardium and were pale in appearance. The infarct area was measured using ImageJ software 1.40 (National Institutes of Health, Bethesda, MD, USA) and the size of infarction area was expressed as a percentage of the whole left ventricle area.
Examination of cardiac electrophysiology in vivo
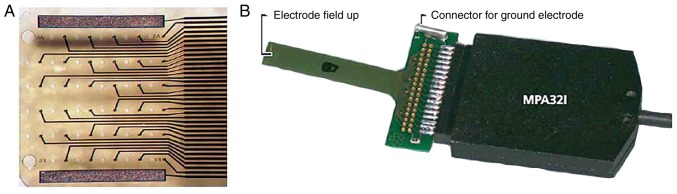
Microelectrode array technology (MEA; Multi Channel Systems GmbH, Kusterdingen, Germany) was employed prior to and 24 h after the sham or ligation procedures, recording the extracellular field potential (FP) at a multiplicity of defined points in the ventricle. The flexible microelectrode array, FlexMEA36 electrode (Multi Channel Systems GmbH), was controlled using a micromanipulator to tightly attach on to the free wall of the left ventricle at baseline to record the amplitude, the duration and the conduction velocity (CV) of FP. A total of 24 h post-surgery, following the secondary thoracotomy, the FlexMEA36 electrode was placed on the infarct, peri-infarct and normal zones of the left ventricle in the MI and NRG-1 groups, and the corresponding zone in the SO group. The infarct zone was visually identified by its pale color. The peri-infarct zone was defined as the zone within 3 mm of the marginal zone between the infarct and non-infarct zones (25). The grid of the FlexMEA36 electrode was a 6×6 array, including 32 recording electrodes, 2 reference electrodes and 2 ground electrodes, with a 30-µm electrode diameter and a 300-µm electrode spacing (Fig. 1).
Figure 1.
MEA technology adopted in the present study. (A) Magnified image of the electrode arrays of FlexMEA36. (B) Image of the flexible FlexMEA36 and the connector. MEA, microelectrode array.
Selection of FP parameters
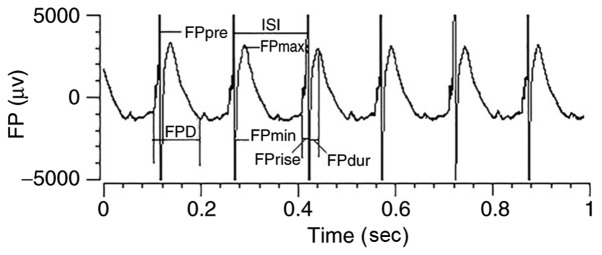
According to previous analyses and interpretations of the FP made by Banach and other researchers (26–28), the present study selected the following FP parameters to characterize the ventricular epicardial electrophysiology and to observe the impact of changing propagation directions among single electrodes of the MEA. The depicted parameters analyzed were: The interspike interval (ISI); FPmin, defined as the size of the largest negative peak in a cycle; FPmax, defined as the size of the largest positive peak in a cycle; FPD, defined as the duration of the FP, in turn describing the interval between FPmin and FPmax; FPrise, defined as the time from the onset of the FP baseline to FPmin; FPpre, defined as the first positive peak in a cycle; and CV, defined as the first active spot to the last active spot in a contact region cycle. All the recorded signals were magnified using the amplifier and saved to a computer using a data acquisition system. The data were analyzed offline with cardio2D+ software (Fig. 2).
Figure 2.
FP parameters. Schematic illustration of FP parameters analyzed in the present study to characterize the ventricular epicardial electrophysiology. FP, field potential; FPD, field potential duration; ISI, interspike interval.
Determination of ventricular arrhythmia (VA) susceptibility
Following the completion of FP detection, burst pacing was conducted to determine the VA susceptibility at the left ventricular anterior free wall and apex of each heart, which consisted of 2 msec pulses at 50 Hz and the duration was 2 sec. This protocol was repeated three times following a 3 min rest period. VAs were defined as a series of consecutive 2 sec or more premature ventricular contractions. VA susceptibility was evaluated based on the incidence of VAs.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (IBM, Corp, Armonk, NY, USA). All data are presented as the mean ± standard error. The differences of infarct sizes between MI and NRG-1 groups were performed using the unpaired Student's t-test. Comparisons of FP parameters between the three groups were conducted using one-way analysis of variance. Post hoc testing was performed for inter-group comparisons using the Student-Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
Animal model establishment and infarct size
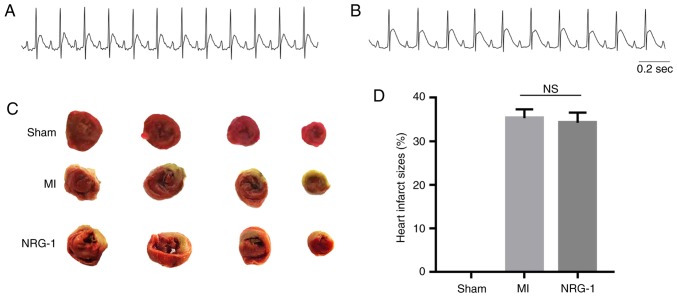
A successful MI model was confirmed by ST segment elevation in leads I, II and avL (Fig. 3A and B). Once MEA and burst pacing in the cardiac electrophysiology tests were performed, the infarct sizes in the MI and NRG-1 groups were measured by TTC. (Fig. 3C and D). The results revealed that there was no statistical significance between these two groups. These infarct sizes were 35.30±2.05 and 34.25±2.34%, respectively.
Figure 3.
Electrocardiogram of the II lead and measurement of infarct size. (A) Electrocardiogram prior to MI. (B) Electrocardiogram of MI. (C) Triphenyltetrazolium chloride staining of heart sections demonstration the pale infarct area of myocardial infarct. (D) Graph of heart infarct sizes. There was no statistical significance between MI and NRG-1 groups. MI, myocardial infarction; ns, not significant; NRG-1, neuregulin-1.
FP parameters at baseline
Prior to the sham or ligature surgeries, the FPs in the free wall of the left ventricle were recorded at the baseline. FPs that were demonstrated simultaneously from the 32 recording microelectrode spots were analyzed after a 15-min equilibrium in the signal stability. The mean values of ISI, FPD, FPrise, FPmax, FPmin, and CV are presented in Table I. There were no statistically significant differences among the SO, MI and NRG-1 groups (Table I).
Table I.
FP parameters at baseline.
| Groups | ISI (msec) | FPD (msec) | FPrise (msec) | FPmax (mv) | FPmin (mv) | CV (cm/sec) |
|---|---|---|---|---|---|---|
| SO | 174.0±5.76 | 103.5±4.07 | 20.5±2.39 | 7.5±0.51 | 7.9±0.83 | 80.54±6.37 |
| MI | 172.5±8.14 | 102.5±8.65 | 20.3±1.49 | 7.6±0.61 | 7.5±0.71 | 79.79±4.81 |
| NRG-1 | 170.8±5.39 | 102.5±12.40 | 20.4±2.77 | 7.8±0.66 | 7.4±0.74 | 80.91±4.91 |
Data represent mean ± standard error of the mean of various FP parameters in SO, MI and NRG-1 groups, respectively. SO, shame operation; MI, myocardium infarction; NRG-1, neuregulin-1; FP, field potential; ISI, interspike interval; CV, conduction velocity; FPD, field potential duration.
Effects of NRG-1 on FP parameters in MI
Synchronous, multifocal FP recordings were performed on the infarct, peri-infarct and normal zones of the left ventricle in the MI and NRG-1 groups, and the corresponding zone in the SO group. The ISI, measured as the time between the neighboring individual FPpre, reflected the heart rate. Compared with the SO group, the ISI in the three observation zones was shortened significantly in the MI group (P<0.05). However, in the NRG-1 group, NRG-1 pretreatment in rats with MI resulted in a reduced heart rate, measured as an increase in the ISI, when compared with the MI group (P<0.05). NRG-1 administration produced no statistically significant differences in the ISI when compared with the SO group (Table II).
Table II.
ISI of the infarct, peri-infarct and normal zone.
| Group | Infarct zone (ISI, msec) | Peri-infarct zone (ISI, msec) | Normal zone (ISI, msec) |
|---|---|---|---|
| SO | 169.20±11.87 | 171.92±8.32 | 174.16±12.45 |
| MI | 142.11±5.09a | 149.98±8.35a | 151.15±7.74a |
| NRG-1 | 158.34±14.26b | 160.01±14.35b | 165.03±17.50b |
Data represent mean ± standard error of the mean of ISI of the infarct, peri-infarct and normal zone in SO, MI and NRG-1 groups, respectively.
P<0.05 vs. SO group
P<0.05 vs. MI group. SO, shame operation; MI, myocardium infarction; NRG-1, neuregulin-1; ISI, interspike interval.
FPD refers to the duration of FP that corresponds to the action potential (AP) duration. It is measured as the time between the FPmin and FPmax, and thus between the depolarization peak (at FPmin) to the repolarization peak (at FPmax), with an indexed AP duration at 90% of repolarization (28). Compared with the SO group, the FPD of the infarct, peri-infarct and normal zones was shortened significantly in the MI group (P<0.05). Furthermore, in the NRG-1 group, the FPD was significantly longer compared with that observed in the MI group (P<0.05), and its mean value was similar to that of the SO group (Table III).
Table III.
FPD of the infarct, peri-infarct and normal zone.
| Group | Infarct zone (FPD, msec) | Peri-infarct zone (FPD, msec) | Normal zone (FPD, msec) |
|---|---|---|---|
| SO | 103.17±11.84 | 101.24±14.51 | 98.41±13.78 |
| MI | 91.01±7.21a | 89.35±7.45a | 88.85±14.87a |
| NRG-1 | 102.48±12.93b | 99.87±13.78b | 98.09±24.07b |
Data represent mean ± standard error of the mean of FPD of the infarct, peri-infarct and normal zone in SO, MI and NRG-1 group, respectively.
P<0.05 vs. SO group
P<0.05 vs. MI group. SO, shame operation; MI, myocardium infarction; NRG-1, Neuregulin-1; FPD, field potential duration.
FPrise was measured as the time span between the decline of the voltage from the baseline to the FPmin, which is correlated with the AP upstroke time (27). Compared with the SO group, the FPrise of the infarct, peri-infarct and normal zones significantly decreased in the MI group (P<0.05). The mean FPrise was significantly prolonged in the infarct zone of the NRG-1 group (P<0.05), with an increased tendency for prolongation in the peri-infarct and normal zones when compared with the MI group. There was no statistically significant difference in the three observation zones between the SO and NRG-1 groups (Table IV).
Table IV.
FPrise of the infarct, peri-infarct and normal zone.
| Group | Infarct zone (FPrise, msec) | Peri-infarct zone (FPrise, msec) | Normal zone (FPrise, msec) |
|---|---|---|---|
| SO | 19.11±5.24 | 17.67±3.97 | 18.96±6.65 |
| MI | 12.10±1.98a | 13.77±1.43a | 14.62±2.56a |
| NRG-1 | 18.66±24.55b | 14.58±4.89 | 16.87±2.45 |
Data represent mean ± standard error of the mean of FPrise of the infarct, peri-infarct and normal zone in SO, MI and NRG-1 group, respectively.
P<0.05 vs. SO group
P<0.05 vs. MI group. SO, shame operation; MI, myocardium infarction; NRG-1, neuregulin-1; FP, field potential.
FPmax was measured as the amplitude of the largest positive peak in a cycle, which was produced by tissue repolarization where the electrode was attached (28). Compared with the SO group, the amplitude of the FPmax of the infarct, peri-infarct and normal zones in the MI group, and that of the infarct and peri-infarct zones in the NRG-1 group, decreased significantly (P<0.05). NRG-1 administration only significantly raised FPmax in the normal zone of MI rats (P<0.05). In the infarct and peri-infarct zones, the amplitude of FPmax exhibited no statistically significant greater tendency in the NRG-1 group when compared with that in the MI group (Table V).
Table V.
FPmax of the infarct, peri-infarct and normal zone.
| Group | Infarct zone (FPmax, mv) | Peri-infarct zone (FPmax, mv) | Normal zone (FPmax, mv) |
|---|---|---|---|
| SO | 8.40±2.08 | 6.78±1.245 | 7.10±3.02 |
| MI | 3.36±2.03a | 2.44±1.41a | 3.68±3.11a |
| NRG-1 | 3.90±2.482a | 3.33±2.08a | 6.78±2.08b |
Data represent mean ± standard error of the mean of FPrise of the infarct, peri-infarct and normal zone in SO, MI and NRG-1 group, respectively.
P<0.05 FPmax vs. SO group
P<0.05 vs. MI group. SO, shame operation; MI, myocardium infarction; NRG-1, neuregulin-1; FP, field potential.
FPmin was measured as the amplitude of the largest negative peak in a cycle, which was produced by determining the depolarization of tissues attached to the electrode, as well as the association with the sodium ion current (28). Compared with the SO group, the amplitude of FPmin in the normal zone of the MI group significantly increased (P<0.05), and in the NRG-1 group it was smaller than the FPmin of the MI group in the normal zone (P<0.05). There was no significant difference in FPmin in the infarct and peri-infarct zones among the three groups (Table VI).
Table VI.
FPmin of the infarct, peri-infarct and normal zone.
| Group | Infarct zone (FPmin, mv) | Peri-infarct zone (FPmin, mv) | Normal zone (FPmin, mv) |
|---|---|---|---|
| SO | −8.43±3.30 | −7.19±4.03 | −7.86±3.40 |
| MI | −9.15±4.65 | −7.65±3.91 | −11.27±4.92a |
| NRG-1 | −7.25±5.51 | −7.06±4.88 | −9.05±3.62b |
Data represent mean ± standard error of the mean of FPmin of the infarct, peri-infarct and normal zone in SO, MI and NRG-1 group, respectively.
P<0.05 vs. SO group
P<0.05 vs. MI group. SO, shame operation; MI, myocardium infarction; NRG-1, neuregulin-1; FP, field potential.
The multiplicity grid of the microelectrode points of FlexMEA36 facilitates the simultaneous location of FPs to investigate alterations in local activation and excitation spread (29). The CV from the three areas during the sinus rhythm was analyzed using cardio2D+ software to calculate the origin and spread of excitation in a cycle from the first active spot to the last active spot of the contact region. Compared with the SO group, the CV of the infarct and peri-infarct zones in the MI and NRG-1 groups decreased significantly (P<0.05). Pretreatment with NRG-1 improved the excitation spread and increased the CV in the peri-infarct zone to a greater extent when compared with the MI group (P<0.05); however, no significant differences were observed in the infarct and normal zones between the two groups (Table VII).
Table VII.
CV of the infarct, peri-infarct and normal zone.
| Group | Infarct zone (CV, cm/sec) | Peri-infarct zone (CV, cm/sec) | Normal zone (CV, cm/sec) |
|---|---|---|---|
| SO | 106.35±9.70 | 105.92±9.93 | 105.05±5.03 |
| MI | 30.02±7.70a | 84.77±39.36a | 103.31±33.06 |
| NRG-1 | 31.95±10.78a | 95.96±20.73a,b | 104.55±24.85 |
Data represent mean ± standard error of the mean of CV of the infarct, peri-infarct and normal zone in SO, MI and NRG-1 group, respectively.
P<0.05 vs. SO group
P<0.05 vs. MI group. SO, shame operation; MI, myocardium infarction; NRG-1, neuregulin-1; CV, conduction velocity.
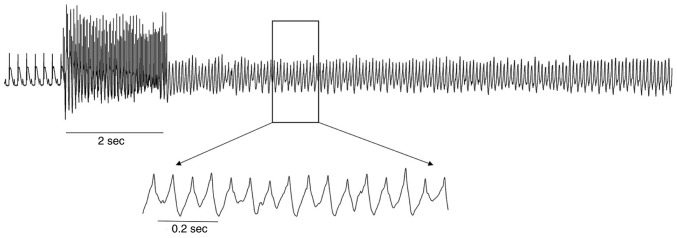
In addition, NRG-1 reduced VA susceptibility. During the burst pacing protocol, no VAs were elicited in the 8 rats of the SO group; however, they were significantly induced in 7 of the MI group rats (0.0 vs. 87.5%, respectively; P<0.05). Compared with the MI group, the incidence of VAs was lower in the NRG-1 group, as VAs were only recorded in 4 rats (87.5 vs. 50.0%, respectively; P<0.05; Fig. 4).
Figure 4.
Electrocardiogram of ventricular arrhythmias.
Discussion
NRG-1 is derived from the endocardial and microvasculature endothelium, and binds to the ErbB family of receptors in the heart, which activate a number of cellular processes in cardiac development and cardiac functional maintenance (8). Several studies on exogenous NRG-1 pretreatment have reported that NRG-1/ErbB signaling protects cardiomyocytes from apoptosis, promotes progenitor cell recruitment and increases the proliferation and regeneration of cardiomyocytes to repair cardiac injury (30,31). In several animal studies, experimental therapy with NRG-1 resulted in the inhibition of cardiac remodeling and the induction of tissue revascularization (10,32). Two completed clinical trials revealed that rhNRG-1 produced favorable acute hemodynamic effects at 2 h post-rhNRG-1 infusion and preserved remodeling reversal effects to restore cardiac function (12,17). NRG-1 can therefore be considered as a therapeutic alternative for heart failure. However, the previously performed studies rarely investigated its effects on cardiac electrophysiology. Several studies reported that NRG-1 increased pacemaker current and the calcium transient gradient, without affecting L-type calcium current in cardiomyocytes; it also inhibited the acetylcholine (Ach)-activated potassium current in embryonic chick atrial myocytes (33–35). Thus, to effectively evaluate exogenous NRG-1 for clinical use, its effects on cardiac electrophysiology require investigation.
The present study investigated the effects of NRG-1 pretreatment on cardiac electrophysiology in a rat MI model. Multielectrode recording was performed with MEA, which is a novel, sensitive and efficient technology that allows for simultaneous analysis of spatial electrophysiological differences (28). The MEA technology allows for broad applications of different excitable multicellular preparations, including cultured cardiomyocytes, heart sections and the myocardium in vivo (26,36–38). The present study used MEA to analyze different FP parameters in order to characterize the electrophysiological properties that were affected by NRG-1 in the infarct, peri-infarct and normal zones in the MI model.
ISI is inversely correlated with heart rate; in the present study, administration of NRG-1 increased ISI 24 h post-ligation. Notably, increased heart rate at rest is considered to be an independent risk factor for cardiovascular morbidity and mortality in several heart diseases (39). The effect of NRG-1 on ISI, as reflected by the heart rate in the MI model, demonstrated that it has beneficial effects on cardiac function. The direct impact of NRG-1 on the mature sinus node remains unknown; however, several previous studies have suggested that NRG-1 may serve potential roles (8,40,41). NRG-1/ErbB signaling was associated with both sympathetic and parasympathetic nerve activation (5). Furthermore, NRG-1 has previously exhibited sympathoinhibitory effects in the brain cardiovascular control center and was also involved in Ach-mediated synaptic transmission in the autonomic ganglia (5,42). One reported result of NRG-1 microinjection into the rostral ventrolateral medulla was a decrease in heart rate (6). Another study revealed that NRG-1 promoted cardiomyocyte differentiation into conduction system cells, as reflected by the increase in pacemaker current density during prenatal development (33).
The other FP parameters observed in the present study reflect the electrical activity, the spread of excitation and the electrophysiological alterations of myocardial cells. The relevant intrinsic characteristics of the FP parameters have been indicated to exhibit a linear association with APs by a number of previous reports (28). According to previous studies, the parameters FPrise and FPD were correlated with the AP upstroke time and AP duration (APD), FPmin and FPmax corresponded individually to the depolarization and repolarization of electrode attached tissues, and FPmin was critically dependent on the Na+ current (27). In the MI group, the shortened FPD and decreased FPrise and FPmax were consistent with the values noted in previous investigations in ischemic AP in other animal models, which were considered to correspond to the generation of arrhythmias in MI (43,44).
Following pretreatment with NRG-1 in the MI rats, the results of the present study demonstrated that NRG-1 prolonged FPD in the infarct, peri-infarct and normal zones. Reversal of the shortened FPD by NRG-1 suggested that NRG-1 could prolong APD in the acute ischemic myocardium. However, to the best of our knowledge, no previous studies have reported on the impact of NRG-1 on cardiac myocyte APs. As it is accepted that shortened APD in acute ventricular ischemia increases the susceptibility to arrhythmia, pretreatment with NRG-1 appeared to have a stabilizing effect on the cardiac electrophysiology in acute MI models.
FPrise associated with AP upstroke was significantly increased in the infarct zone following NRG-1 administration in the present study. The voltage-gated Na+ channel is responsible for the AP upstroke in cardiomyocytes and drives a critical determinant of electrical propagation; it may also serve a crucial role in arrhythmogenesis (45). NaV1.5, the membrane protein primarily present in cardiac myocytes, is a voltage-gated Na+ channel subunit, which mediates the fast influx of Na+ ions across the membrane (46). Although there are no studies on the effect of NRG-1 on the cardiac Na+ current, two previous studies have demonstrated that myoblast treatment with NRG-1 further increased NaV1.5 in skeletal myocytes (47,48).
FPmax and FPmin were produced by the repolarization or depolarization of the recording tissues, respectively, which were generated by the capacitance of the membrane attaching the microelectrode. Pretreatment with NRG-1 significantly reversed the amplitude changes in the FPmax and FPmin of the normal zone following LAD ligation. To the best of our knowledge, no previous studies have investigated the effects of NRG-1 on repolarization and the associated ion current in cardiomyocytes. In the central and peripheral nervous systems, NRG-1 has been reported to regulate the potassium and calcium current in neurons (49,50), indicating that NRG-1 may modulate the ion current in cardiomyocytes.
CV is an important parameter for excitation propagation. Using MEA technology for recording the present study results provided a satisfactory strategy for measuring the local myocardial CV, particularly for differentiating the varied areas during ischemia. It was observed that CV decreased during ischemia as the ischemic tissue became unexcitable, which is associated with spatial non-uniform propagation and re-entrant circuit in acutely ischemic myocardium, leading to arrhythmia (51,52). In the present study, CV significantly decreased in the infarct and peri-infarct zones of the MI and NRG-1 groups when compared with the SO group. However, the NRG-1 group exhibited a significant improvement in CV in the peri-infarct zone when compared with the MI group. Short-term NRG-1 treatment in an experimental model of volume overload-induced heart failure revealed that NRG-1 enabled the recovery of gap junction structure and improved connexin 43 expression (53), giving rise to the hypothesis that pretreatment with NRG-1 may improve electrical conduction.
Except for the abovementioned electrophysiology data, the reduction of VA incidence in the NRG-1 group further confirmed that NRG-1 was advantageous for improving cardiac electrophysiology in MI. A prospective study of out-of-hospital SCD demonstrated a strong association with a common missense variant in NRG-1 and identified ventricular fibrillation in SCD (20), which suggested that NRG-1 may exert an inhibitory effect on the occurrence of VA. As several results in phase II clinical trials have reported the advantages of NRG-1 administration in chronic heart failure, evaluating the influence of NRG-1 on cardiac electrophysiology may provide comprehensive understanding to enable the use of NRG-1 as a therapeutic agent in cardiovascular disease.
In conclusion, NRG-1 is an endogenous multifunctional modulator in the cardiovascular system. The results of the present study demonstrated that NRG-1 pre-treatment improved cardiac electrophysiology in an MI rat model. However, the underlying mechanisms of NRG-1, including its impact on APs and ion channels in cardiac myocytes, require further investigation.
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Key Basic Research Development Program, the ‘973’ Program (grant no. 2012CB518604), National Natural Science Foundation of China (grant no. 81772044, no. 81500255), Program for Innovative Research Team in Hainan Province (grant no. 2016CXTD012), Science Foundation of Wuhan Health and Family Planning Commission (grant no. WX17B09), Natural Science Foundation of Hubei Province (grant no. 2017CFC824) and Wuhan University Young Teachers Project (grant no. 2042017kf0138).
Availability of data and materials
The materials included in the manuscript, including all relevant raw data, may be made freely available to any researchers who wish to use them for non-commercial purposes. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
PR, ZL, HD, SD and HL designed the study and performed the experiments. PR, ZL, LZ and XW analyzed the data. PR, ZL, LW and XW prepared the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Wuhan University (Hubei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Falls DL. Neuregulins: Functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/S0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 2.Marchionni MA, Goodearl AD, Chen MS, Bermingham- McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 3.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Müller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsukawa R, Hirooka Y, Ito K, Honda N, Sunagawa K. Central neuregulin-1/ErbB signaling modulates cardiac function via sympathetic activity in pressure overload-induced heart failure. J Hypertens. 2014;32:817–825. doi: 10.1097/HJH.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 6.Matsukawa R, Hirooka Y, Nishihara M, Ito K, Sunagawa K. Neuregulin-1/ErbB signaling in rostral ventrolateral medulla is involved in blood pressure regulation as an antihypertensive system. J Hypertens. 2011;29:1735–1742. doi: 10.1097/HJH.0b013e32834937d6. [DOI] [PubMed] [Google Scholar]
- 7.Montaigne D, Hurt C, Neviere R. Mitochondria death/survival signaling pathways in cardiotoxicity induced by anthracyclines and anticancer-targeted therapies. Biochem Res Int. 2012;2012:951539. doi: 10.1155/2012/951539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rupert CE, Coulombe KL. The roles of neuregulin-1 in cardiac development, homeostasis, and disease. Biomark Insights. 2015;10(Suppl 1):S1–S9. doi: 10.4137/BMI.S20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill MF, Patel AV, Murphy A, Smith HM, Galindo GL, Pentassuglia L, Peng X, Lenneman CG, Odiete O, Friedman DB, et al. Intravenous glial growth factor 2 (GGF2) isoform of neuregulin-1β improves left ventricular function, gene and protein expression in rats after myocardial infarction. PLoS One. 2013;8:e55741. doi: 10.1371/journal.pone.0055741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y, Li X, Zhou M. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55:1907–1914. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 12.Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, Amor R, Liu X, Li XY, Zhou MD, et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13:83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- 13.Xiao J, Li B, Zheng Z, Wang M, Peng J, Li Y, Li Z. Therapeutic effects of neuregulin-1 gene transduction in rats with myocardial infarction. Coron Artery Dis. 2012;23:460–468. doi: 10.1097/MCA.0b013e32835877da. [DOI] [PubMed] [Google Scholar]
- 14.Formiga FR, Pelacho B, Garbayo E, Imbuluzqueta I, Díaz-Herráez P, Abizanda G, Gavira JJ, Simón-Yarza T, Albiasu E, Tamayo E, et al. Controlled delivery of fibroblast growth factor-1 and neuregulin-1 from biodegradable microparticles promotes cardiac repair in a rat myocardial infarction model through activation of endogenous regeneration. J Control Release. 2014;173:132–139. doi: 10.1016/j.jconrel.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Gu X, Liu X, Xu D, Li X, Yan M, Qi Y, Yan W, Wang W, Pan J, Xu Y, et al. Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res. 2010;88:334–343. doi: 10.1093/cvr/cvq223. [DOI] [PubMed] [Google Scholar]
- 16.Galindo CL, Kasasbeh E, Murphy A, Ryzhov S, Lenihan S, Ahmad FA, Williams P, Nunnally A, Adcock J, Song Y, et al. Anti-remodeling and anti-fibrotic effects of the neuregulin-1β glial growth factor 2 in a large animal model of heart failure. J Am Heart Assoc. 2014;3:e000773. doi: 10.1161/JAHA.113.000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendes-Ferreira P, De Keulenaer GW, Leite-Moreira AF, Brás-Silva C. Therapeutic potential of neuregulin-1 in cardiovascular disease. Drug Discov Today. 2013;18:836–842. doi: 10.1016/j.drudis.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Parodi EM, Kuhn B. Signalling between microvascular endothelium and cardiomyocytes through neuregulin. Cardiovasc Res. 2014;102:194–204. doi: 10.1093/cvr/cvu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huertas-Vazquez A, Teodorescu C, Reinier K, Uy-Evanado A, Chugh H, Jerger K, Ayala J, Gunson K, Jui J, Newton-Cheh C, et al. A common missense variant in the neuregulin 1 gene is associated with both schizophrenia and sudden cardiac death. Heart Rhythm. 2013;10:994–998. doi: 10.1016/j.hrthm.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napolitano C. Heart, brain, and the risk of sudden death. Heart Rhythm. 2013;10:999–1000. doi: 10.1016/j.hrthm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Liu Z, Duan HN, Wang L. Therapeutic potential of neuregulin in cardiovascular system: Can we ignore the effects of neuregulin on electrophysiology? Mini Rev Med Chem. 2016;16:867–871. doi: 10.2174/1389557516666160223122039. [DOI] [PubMed] [Google Scholar]
- 22.Hedhli N, Huang Q, Kalinowski A, Palmeri M, Hu X, Russell RR, Russell KS. Endothelial-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123:2254–2262. doi: 10.1161/CIRCULATIONAHA.110.991125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang X, Ding Y, Zhang Y, Chai YH, He J, Chiu SM, Gao F, Tse HF, Lian Q. Activation of NRG1-ERBB4 signaling potentiates mesenchymal stem cell-mediated myocardial repairs following myocardial infarction. Cell Death Dis. 2015;6:e1765. doi: 10.1038/cddis.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morano M, Angotti C, Tullio F, Gambarotta G, Penna C, Pagliaro P, Geuna S. Myocardial ischemia/reperfusion upregulates the transcription of the Neuregulin1 receptor ErbB3, but only postconditioning preserves protein translation: Role in oxidative stress. Int J Cardiol. 2017;233:73–79. doi: 10.1016/j.ijcard.2017.01.122. [DOI] [PubMed] [Google Scholar]
- 25.Wen HZ, Xie P, Zhang F, Ma Y, Li YL, Xu SK. Neuropilin 1 ameliorates electrical remodeling at infarct border zones in rats after myocardial infarction. Auton Neurosci. 2018;214:19–23. doi: 10.1016/j.autneu.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Banach K, Halbach MD, Hu P, Hescheler J, Egert U. Development of electrical activity in cardiac myocyte aggregates derived from mouse embryonic stem cells. Am J Physiol Heart Circ Physiol. 2003;284:H2114–H2123. doi: 10.1152/ajpheart.01106.2001. [DOI] [PubMed] [Google Scholar]
- 27.Kienast R, Stöger M, Handler M, Hanser F, Baumgartner C. Alterations of field potentials in isotropic cardiomyocyte cell layers induced by multiple endogenous pacemakers under normal and hypothermal conditions. Am J Physiol Heart Circ Physiol. 2014;307:H1013–H1023. doi: 10.1152/ajpheart.00097.2014. [DOI] [PubMed] [Google Scholar]
- 28.Halbach M, Egert U, Hescheler J, Banach K. Estimation of action potential changes from field potential recordings in multicellular mouse cardiac myocyte cultures. Cell Physiol Biochem. 2003;13:271–284. doi: 10.1159/000074542. [DOI] [PubMed] [Google Scholar]
- 29.Hescheler J, Halbach M, Egert U, Lu ZJ, Bohlen H, Fleischmann BK, Reppel M. Determination of electrical properties of ES cell-derived cardiomyocytes using MEAs. J Electrocardiol. 2004;37(Suppl):110–116. doi: 10.1016/j.jelectrocard.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 30.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Fang SJ, Wu XS, Han ZH, Zhang XX, Wang CM, Li XY, Lu LQ, Zhang JL. Neuregulin-1 preconditioning protects the heart against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Chin Med J (Engl) 2010;123:3597–3604. [PubMed] [Google Scholar]
- 32.Cohen JE, Purcell BP, MacArthur JW, Jr, Mu A, Shudo Y, Patel JB, Brusalis CM, Trubelja A, Fairman AS, Edwards BB, et al. A bioengineered hydrogel system enables targeted and sustained intramyocardial delivery of neuregulin, activating the cardiomyocyte cell cycle and enhancing ventricular function in a murine model of ischemic cardiomyopathy. Circ Heart Fail. 2014;7:619–626. doi: 10.1161/CIRCHEARTFAILURE.113.001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruhparwar A, Er F, Martin U, Radke K, Gruh I, Niehaus M, Karck M, Haverich A, Hoppe UC. Enrichment of cardiac pacemaker-like cells: Neuregulin-1 and cyclic AMP increase I(f)-current density and connexin 40 mRNA levels in fetal cardiomyocytes. Med Biol Eng Comput. 2007;45:221–227. doi: 10.1007/s11517-007-0164-3. [DOI] [PubMed] [Google Scholar]
- 34.Brero A, Ramella R, Fitou A, Dati C, Alloatti G, Gallo MP, Levi R. Neuregulin-1beta1 rapidly modulates nitric oxide synthesis and calcium handling in rat cardiomyocytes. Cardiovasc Res. 2010;88:443–452. doi: 10.1093/cvr/cvq238. [DOI] [PubMed] [Google Scholar]
- 35.Ford BD, Liu Y, Mann MA, Krauss R, Phillips K, Gan L, Fischbach GD. Neuregulin-1 suppresses muscarinic receptor expression and acetylcholine-activated muscarinic K+ channels in cardiac myocytes. Biochem Biophys Res Commun. 2003;308:23–28. doi: 10.1016/S0006-291X(03)01319-6. [DOI] [PubMed] [Google Scholar]
- 36.Bussek A, Wettwer E, Christ T, Lohmann H, Camelliti P, Ravens U. Tissue slices from adult mammalian hearts as a model for pharmacological drug testing. Cell Physiol Biochem. 2009;24:527–536. doi: 10.1159/000257528. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Yan H, Wugeti N, Guo Y, Zhang L, Ma M, Guo X, Jiao C, Xu W, Li T. Microelectrode array measurement of potassium ion channel remodeling on the field action potential duration in rapid atrial pacing rabbits model. Int J Clin Exp Med. 2015;8:249–256. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Guzadhur L, Jeevaratnam K, Salvage SC, Matthews GD, Lammers WJ, Lei M, Huang CL, Fraser JA. Arrhythmic substrate, slowed propagation and increased dispersion in conduction direction in the right ventricular outflow tract of murine Scn5a+/− hearts. Acta Physiol (Oxf) 2014;211:559–573. doi: 10.1111/apha.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brito Díaz B, Alemán Sánchez JJ, Cabrera de León A. Resting heart rate and cardiovascular disease. Med Clin (Barc) 2014;143:34–38. doi: 10.1016/j.medcli.2013.05.034. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Li B, Zhang C, Zhang J, Zeng M, Zheng Z. Effect of NRG-1/ErbB signaling intervention on the differentiation of bone marrow stromal cells into sinus node-like cells. J Cardiovasc Pharmacol. 2014;63:434–440. doi: 10.1097/FJC.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 41.Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci USA. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HG, Cho SM, Lee CK, Jeong SW. Neuregulin 1 as an endogenous regulator of nicotinic acetylcholine receptors in adult major pelvic ganglion neurons. Biochem Biophys Res Commun. 2015;463:632–637. doi: 10.1016/j.bbrc.2015.05.113. [DOI] [PubMed] [Google Scholar]
- 43.Di Diego JM, Antzelevitch C. Acute myocardial ischemia: Cellular mechanisms underlying ST segment elevation. J Electrocardiol. 2014;47:486–490. doi: 10.1016/j.jelectrocard.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazzara R, Scherlag BJ. Electrophysiologic basis for arrhythmias in ischemic heart disease. Am J Cardiol. 1984;53:1B–7B. doi: 10.1016/0002-9149(84)90493-4. [DOI] [PubMed] [Google Scholar]
- 45.Jeevaratnam K, Guzadhur L, Goh YM, Grace AA, Huang CL. Sodium channel haploinsufficiency and structural change in ventricular arrhythmogenesis. Acta Physiol (Oxf) 2016;216:186–202. doi: 10.1111/apha.12577. [DOI] [PubMed] [Google Scholar]
- 46.Veerman CC, Wilde AA, Lodder EM. The cardiac sodium channel gene SCN5A and its gene product NaV1.5: Role in physiology and pathophysiology. Gene. 2015;573:177–187. doi: 10.1016/j.gene.2015.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martínez-Mármol R, David M, Sanches R, Roura-Ferrer M, Villalonga N, Sorianello E, Webb SM, Zorzano A, Gumà A, Valenzuela C, Felipe A. Voltage-dependent Na+ channel phenotype changes in myoblasts. Consequences for cardiac repair. Cardiovasc Res. 2007;76:430–441. doi: 10.1016/j.cardiores.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Corfas G, Fischbach GD. The number of Na+ channels in cultured chick muscle is increased by ARIA, an acetylcholine receptor-inducing activity. J Neurosci. 1993;13:2118–2125. doi: 10.1523/JNEUROSCI.13-05-02118.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chae KS, Martin-Caraballo M, Anderson M, Dryer SE. Akt activation is necessary for growth factor-induced trafficking of functional K (Ca) channels in developing parasympathetic neurons. J Neurophysiol. 2005;93:1174–1182. doi: 10.1152/jn.00796.2004. [DOI] [PubMed] [Google Scholar]
- 50.Castillo C, Malavé C, Martínez JC, Núñez J, Hernández D, Pasquali F, Villegas GM, Villegas R. Neuregulin-1 isoform induces mitogenesis, KCa and Ca2+ currents in PC12 cells. A comparison with sciatic nerve conditioned medium. Brain Res. 2006;1110:64–75. doi: 10.1016/j.brainres.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 51.Janse MJ, Kleber AG, Capucci A, Coronel R, Wilms-Schopman F. Electrophysiological basis for arrhythmias caused by acute ischemia. Role of the subendocardium. J Mol Cell Cardiol. 1986;18:339–355. doi: 10.1016/S0022-2828(86)80898-7. [DOI] [PubMed] [Google Scholar]
- 52.Kléber AG, Janse MJ, Wilms-Schopmann FJ, Wilde AA, Coronel R. Changes in conduction velocity during acute ischemia in ventricular myocardium of the isolated porcine heart. Circulation. 1986;73:189–198. doi: 10.1161/01.CIR.73.1.189. [DOI] [PubMed] [Google Scholar]
- 53.Wang XH, Zhuo XZ, Ni YJ, Gong M, Wang TZ, Lu Q, Ma AQ. Improvement of cardiac function and reversal of gap junction remodeling by Neuregulin-1β in volume-overloaded rats with heart failure. J Geriatr Cardiol. 2012;9:172–179. doi: 10.3724/SP.J.1263.2012.03271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The materials included in the manuscript, including all relevant raw data, may be made freely available to any researchers who wish to use them for non-commercial purposes. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.