Abstract
Inflammatory bowel disease (IBD) is a risk factor in colon cancer. Endoplasmic reticulum (ER) stress is associated with IBD and cancer. In the current study an azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced mouse colonic tumor model was established to analyze the expression of ER stress chaperone molecules. Female C57BL/6 mice were intraperitoneally injected with 12 mg/kg AOM. On the 7th day following AOM injection, mice were treated with 1% DSS supplemented to the drinking water for 7 days, then followed by 14 days of normal drinking water. The cycle of 7 days DSS plus 14 days normal water was repeated twice and colonic tumors were evaluated for their number and size. Mice in the control group were injected with saline and received normal drinking water for the course of the experiment. mRNA levels of cytokines, inositol-requiring enzyme (IRE)1α and 1β, their downstream targets X-box binding protein (XBP)1u, XBP1s and mucin (MUC) 2 and interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α were detected by reverse transcription-quantitative polymerase chain reaction. IRE1α, IRE1β and MUC2 protein expression was evaluated by immunohistochemistry, and IRE1α and IRE1β levels were further assessed by western blot analysis. It was observed that tumors developed in the distal colon of mice treated with AOM/DSS. IL-6, IL-8 and TNF-α mRNA levels were significantly increased in mice of the tumor group compared with mice of the control group. There were no significant differences in IRE1α mRNA and protein expression between the two groups and XBP1s mRNA levels were increased in the tumor compared with the control group. IRE1β and MUC2 mRNA levels were significantly decreased in the tumor compared with the control group (decreased by 42 and 30%, respectively). IRE1β and MUC2 proteins were predominately expressed in colonic epithelial cells and expression was decreased in the tumor compared with the control group. In conclusion, the downregulation of IRE1β and MUC2 may reduce the ability of colon tissues to resist inflammation, thus promoting the occurrence and development of colonic tumors.
Keywords: colitis, colorectal cancer, endoplasmic reticulum stress, inflammatory bowel disease, inositol-requiring enzyme 1, mucin
Introduction
Colorectal cancer (CRC) is the third most common type of cancer and the fourth-leading cause of cancer-associated mortality worldwide (1). Genetic and epigenetic genomic alterations, leading to gene amplification, the activation of oncogenes and the loss of tumor suppressor genes, are involved in the tumorigenesis of CRC (2). Risk factors associated with CRC include environmental and dietary mutagens, intestinal microorganisms and pathogens, and chronic intestinal inflammation (3). The connection between inflammation and tumorigenesis is well established. The processes of colitis-associated cancer (CAC) and non-inflammatory CRC feature common events, including the development of localized hyperplasia, polyps, adenoma and ultimately, carcinoma (4). CAC is the CRC subtype preceded by inflammatory bowel disease (IBD), Crohn's disease or ulcerative colitis (UC) (5). UC is associated with a cumulative risk of CAC of ≤20% within 30 years of disease onset (5,6). Azoxymethane (AOM) is a mutagenic agent that induces multiple colonic tumors following a single injection in chronic colitis mouse models within short periods (7). This mouse model of CAC is valuable for the understanding of the mechanisms of inflammation in tumorigenesis (8).
Endoplasmic reticulum (ER) stress is associated with both the pathogenesis of intestinal inflammation and tumorigenesis (9–11). The ER is crucial for the folding of secretory and membrane proteins, lipid biosynthesis, the regulation of intracellular Ca2+ and redox signaling. During ER stress, the disturbance of ER homeostasis due to oxidative stress, energy deprivation, altered metabolic status or inflammatory stimuli causes ER calcium depletion and the accumulation of unfolded and misfolded proteins in the ER lumen (12). In mammalian cells, there are three types of ER stress sensors: Protein-kinase-RNA-like-ER kinase, activating transcription factor 6 and inositol-requiring enzyme (IRE) 1. These transmembrane ER proteins regulate the unfolded protein response to address ER stress (13,14). IRE1 is the most evolutionally conserved among the three ER stress response sensors (15). There are two IRE1 paralogs, α and β, present in mammals (16). IRE1α is a ubiquitously expressed kinase and site-specific RNA endonuclease (17,18), that processes the mRNA encoding X-box binding protein (XBP) 1 to maintain ER homeostasis (19). IRE1α excises a 26-nucleotide-intron sequence from the unspliced XBP1u mRNA to produce spliced XBP-1s mRNA, resulting in the activation of XBP1 protein expression. The XBP1 protein is a transcription factor involved in tumor growth and survival (10,20). Although IRE1β is a paralog of IRE1α (21), its expression is restricted to the epithelium of the gastrointestinal and respiratory tracts (16,22), while IRE1α is expressed in a variety of tissues (23). IRE1α and IRE1β protect mice from dextran sulfate sodium (DSS)-induced colitis (16,24); however, these two IRE1 proteins have different functions in ER stress and varying substrate specificities (25). For example, IRE1β has a decreased XBP1u mRNA RNase activity compared with IRE1α (22) and IRE1β degrades 28S rRNA more efficiently than IRE1α (26). IRE1β overexpression in HeLa cells results in the cleavage of 28S rRNA, leading to cell apoptosis (27). IRE1β, but not IRE1α, regulates the secretory protein mucin (MUC) 2 in intestinal goblet cells (26,28). MUC2 is a putatively vital molecule in the innate immune defense of the colon; a lack of the MUC2 gene results in spontaneous colitis and colon cancer in mice (29–32).
The current study induced colonic tumor formation in mice using AOM and dextran sulfate sodium (DSS). It was observed that IRE1β and MUC2 expression was downregulated in the tumors. This indicated that the inhibition of the IRE1β-MUC2 signaling pathway may promote the occurrence and development of colonic tumors.
Materials and methods
Mice
Female C57BL/6 (B6) mice (Vital River Laboratory Animal Technology Co., Ltd., Beijing, China) were maintained in the animal experimental center of The First Affiliated Hospital of Henan University of Science and Technology. Mice were housed with a controlled temperature of 20–22°C, 50% humidity and a 12-h light/dark cycle; they were fed with rodent chow from Beijing HFK Bioscience Co., Ltd. (Beijing, China). The Animal Care and Use Committee of The First Affiliated Hospital of Henan University of Science and Technology (Luoyang, China) approved all the animal procedures in this study.
Colitis-mediated colon tumors were induced using a modified protocol previously described by Neufert et al (7). A total of 20 mice (age, 7–8 weeks; weight, 19–23 g) were randomly allocated into the control and tumor groups (n=10/group). Mice in the tumor group received an intraperitoneal injection of 1 mg/ml AOM (12 mg/kg; molecular weight (MW), 74.08 Da; cat. no. MFCD00126912; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and control mice received saline (12 ml/kg saline). In primary experiments, the high mortality of mice was associated with direct administration following intraperitoneal injection, so on the 7th day after intraperitoneal injection of AOM, mice in the tumor group received 1.0% DSS (MW 36–50 kDa; cat. no. 160110; MP Biomedicals, LLC, Santa Ana, CA, USA) for 7 days to induce colitis. The 1% DSS solution was prepared by dissolving fine-grain DSS powder (1 g) in 100 ml drinking water. DSS solution was freshly prepared prior to administration. Water bottles containing DSS were replaced at 5 days with fresh DSS solution for the remaining 3 days. On day 8, the DSS solution was replaced with normal drinking water for 14 days; mice in the control group did not receive DSS. The cycle of 7 days DSS/14 days normal drinking water was repeated three times. Control mice were provided with normal drinking water throughout the experiment. Mice in both groups had free access to food and water. The mice were monitored once every two days for total of 69 days to record body weight, stool consistency, rectal bleeding and ulceration.
Mice were euthanized at the end of the third cycle. The entire colon was excised and measured. Colons were cut open and laid flat, lumen-side up. The number and size of colonic tumors was assessed. Colon tissues and tumor tissues were frozen at −80°C for subsequent reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analyses. RNAlater Stabilization reagent (Qiagen GmbH, Hilden, Germany) was used to prevent mRNA degradation according to the manufacturers' instructions. Further samples from the colon and tumor tissue were fixed with 10% neutral-formalin for 24 h at room temperature and embedded in paraffin 62°C for 3 h. Sections were stained by hematoxylin and eosin (H&E) and immunohistochemistry (IHC) as described below. The severity of colitis was evaluated based on the disease activity index (DAI) using a previously described method (Table I) (33).
Table I.
Disease activity index score.
| Assigned score | |||||
|---|---|---|---|---|---|
| Characteristic | 0 | 1 | 2 | 3 | 4 |
| Body mass decrease (%) | 0 | 1–5 | 5–10 | 10–15 | >15 |
| Stool appearance | Normal | – | Loose | – | Diarrhea |
| Rectal bleeding | Normal | – | Hemoccult positive | – | Gross bleeding |
The disease activity index=(body mass decrease + stool character + rectal bleeding)/3.
Histopathology examination
Tissue slices (4-µm) were prepared from distal colon tissue samples and stained with H&E for histological examination. Staining was performed at room temperature and 1% Hematoxylin (stained for 1–10 min) and 0.5% Eosin (stained for 2–5 min) were used. Pathology and level of inflammation were scored in a blinded fashion using a previously described scoring system (34) to quantify: The extent of neutrophil and lymphocyte infiltration (0–3 points); Paneth and goblet cell degranulation (0–2 points); epithelium reactivity, including crypt distortion (0–3 points); and inflammatory foci (0–3 points).
IHC was conducted with sections mounted on poly-L-lysine-coated slides using a modified biotin-peroxidase complex method as previously described (35). Briefly, tissue sections were incubated overnight at 4°C with rabbit polyclonal antibodies against IRE1α (dilution, 1:150; cat. no. sc-20790; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), IRE1β (dilution, 1:100; cat. no. GTX87426; GeneTex, Inc., Irvine, CA, USA) and MUC2 (dilution, 1:100; cat. no. ab76774; Abcam, Cambridge, UK). Antigen-antibody complexes were detected with a biotinylated goat anti-rabbit antibody (dilution, 1:300; cat. no. SA1020; Boster Biological Technology Co., Ltd., Wuhan, China) conjugated with streptavidin-horseradish peroxidase (HRP) for 30 min at 37°C. At room temperature and visualized by reacting with the 3,3-diaminobenzidine reagent kit (Solarbio Science & Technology Co., Ltd., Beijing, China; cat. no. DA1010) at room temperature for 5 min. Sections were counterstained with 1% hematoxylin for 1–10 min at room temperature. Negative control sections were obtained by omitting the primary antibody or by using an unspecific antibody. Images at a magnification of ×400 were captured using a Nikon Ds-Fi2 500w light microscope, (Nikon Corporation, Tokyo, Japan). IHC staining was used to semi-quantitatively determine protein levels as previously described (35): A total of 10 fields were randomly selected on each slide and 100 cells per field were counted and scored for IRE1α, IRE1β and MUC2 staining. The semi-quantitative scores were obtained from the staining intensity (none, 0; weak, 1; moderate, 2; strong, 3) multiplied by the percentage of positively stained cells (≤5%, 0; 6–25%, 1; 26–50%, 2; 51–75%, 3; and >75%, 4).
RT-qPCR analysis
Total RNA from the colon tissue samples was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions. cDNA was synthesized using PrimeScript-RT Master mix (Takara Bio, Inc., Otsu, Japan) with the following temperature protocol: 37°C for 15 min, 85°C for 5 sec and 4°C for 10 min. qPCR was performed as described previously, using undiluted cDNA templates (35). Primer sequences (Table II) were designed with Primer 3.0 (36) and synthesized by Sangon Biotech Co., Ltd. (Zhengzhou, China). qPCR was performed using a CFX96 Real-Time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and SYBR green (Takara Bio, Inc.) was used to detect PCR products. The reaction followed an initial step at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, 58°C for 30 sec and 72°C for 30 sec. For annealing temperatures >60°C, a two-step method was used (37). Each sample was assayed in triplicate. Amplification efficiency was >97%. A melting curve analysis was performed for the PCR products of each target gene and β-actin to evaluate primer specificity. The relative abundance of target gene mRNA level was evaluated using 2−ΔΔCq method, normalized to β-actin (38). mRNA expression levels were presented as relative fold by comparing the quantity of mRNA between different groups. When Cq >35, the gene was considered to not be expressed.
Table II.
Reverse transcription-quantitative polymerase chain reaction primers.
| Primer (5′-3′) | ||
|---|---|---|
| Gene | Forward | Reverse |
| IL-6 | AACGATGATGCACTTGCAGA | TGGTACTCCAGAAGACCAGAGG |
| TNF-α | CCACCACGCTCTTCTGTCTACT | TGCTACGACGTGGGCTACA |
| IL-8 | CTAGGCATCTTCGTCCGTCC | TTGGGCCAACAGTAGCCTTC |
| XBP1u | GGTCTGCTGAGTCCGCAGCACTC | AGGCTTGGTGTATACATGG |
| XBP1s | GGTCTGCTGAGTCCGCAGCAGG | AGGCTTGGTGTATACATGG |
| IRE1α | GCATCACCAAGTGGAAGTATC | ACCATTGAGGGAGAGGCATAG |
| IRE1β | CACAACCTATCGCCGCTACT | CATCCTGGTGCCATGTGTAA |
| MUC2 | CTGACCAAGAGCGAACACAA | CATGACTGGAAGCAACTGGA |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
IL, interleukin; TNF, tumor necrosis factor; IRE, inositol-requiring enzyme; XBP, X-box binding protein; MUC, mucin.
Western blotting
Protein lysates were prepared from the extracted tissues in radioimmunoprecipitation assay lysis buffer (cat. no. R0020; Solarbio Science & Technology Co., Ltd.) on ice by homogenization with a grinder. The supernatant was obtained by centrifugation (15 min; 10,800 × g; 4°C. The bicinchoninic acid (cat. no. PC0020; Solarbio Science & Technology Co., Ltd.) method was used to determine protein concentration. Protein (30 µg) from each sample was denatured, resolved on 10% SDS-PAGE gels and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Rabbit polyclonal antibodies against IRE1α (dilution, 1:1,000; cat. no. 14C10; Cell Signaling Technology, Inc., Danvers, MA, USA) and IRE1β (dilution, 1:300; cat. no. ab135795; Abcam) and mouse monoclonal antibodies against GAPDH (dilution, 1:1,000; cat. no. CW0100A; CWBIO Technology Co. Ltd, Beijing, China) were incubated with the membranes at 4°C overnight, followed by HRP-conjugated anti-rabbit (dilution, 1:1,000; cat. no. BA1054) or anti-mouse IgG (dilution, 1:3,000; cat. no. BA1050; both Boster Biological Technology Co. Ltd.) for 1 h at room temperature, and enhanced chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc.) according to the manufacturers' protocols. GAPDH was used to normalize protein expression. A ChemiDoc XRS (Bio-Rad Laboratories, Inc.) was used to capture images, which were quantified using ImageJ software v1.48 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard deviation. Experiments were repeated at least three times. All statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Student's t-test or Mann-Whitney U test were used to determined significant differences between groups. Wilcoxon signed-rank test was used for non-parametric data. P<0.05 was considered to indicate a statistically significant difference.
Results
Treatment with AOM/DSS induces tumors in mouse colons
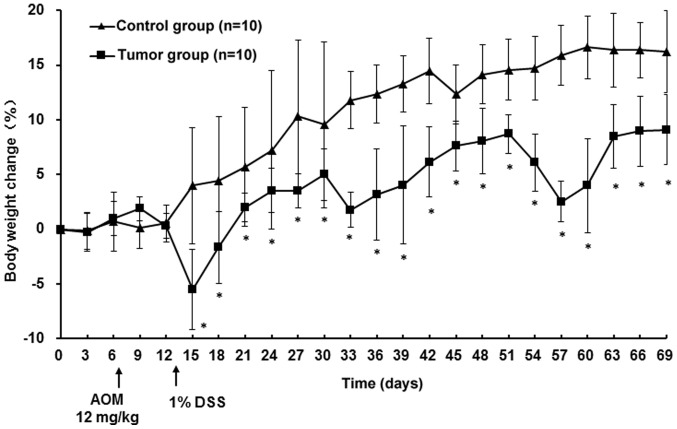
According to Thaker et al (39) and preliminary experiments, the most consistent results were observed using female mice; hence, only female mice were utilized in the current study. No signs of inflammation or colonic tumors were observed in the control group. Mice in the tumor group exhibited no sign of illness following AOM treatment and reduced appetite and drank less starting on day 6 of the 1% DSS treatment. By day 7, all mice of the tumor group presented with bloody stools and declining body weight; symptoms lasted until day 4 after 1% DSS treatment. The weight loss of the mice in the tumor group was the lowest with −5.5% during the first cycle. In the following two cycles, mice exhibited similar symptoms, but less severe. Body weights of mice in the tumor group increased, although slower compared with the control mice; particularly during DSS treatment. At the endpoint, there was a significant difference in body weight between the tumor and the control group (P<0.05; Fig. 1) and DAI scores in the tumor group were significantly higher compared with the control group (P<0.05; Table III). No mortality was observed in either group. The colon length in the tumor group was significantly decreased compared with the control group (P<0.05; Table III). A total of 36 (average number of tumors per mouse, 3.6; range, 2–5) colonic tumors were observed in mice of the tumor group. The mean tumor diameter was 3.1 mm (range, 1–4 mm). Histological examination revealed that the mucosa of the colons from the tumor group were disordered and the crypt structure was destroyed compared with the control group: Colonic epithelial cells were atypical, with decreased differentiation, increased cell size and large nuclei; the nucleus-to-cytoplasm ratio of the cells was increased, the number of goblet cells was decreased and the number of inflammatory cells was increased (Fig. 2A and B).
Figure 1.
Changes in body weight of the mice over the course of the experiment. At day 1, mice in the tumor group received an intraperitoneal injection of azoxymethane. On 7th day mice in the tumor group received drinking water containing 1% dextran sulfate sodium for 7 days, followed by normal drinking water for 14 days. This 7/14 day cycle was repeated three times. Mice in the control group were injected with saline and received normal drinking water for the duration of the experiment. *P<0.05 vs. control group.
Table III.
Disease activity index scores and colon length.
P<0.05 vs. control group. DAI, disease activity index.
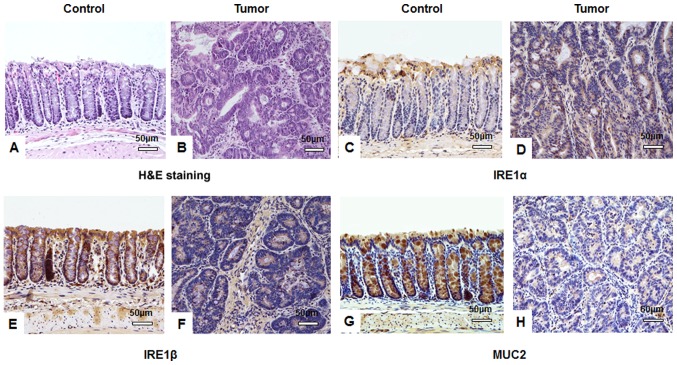
Figure 2.
IHC staining suggests that AOM/DSS treatment affects IRE1α, IRE1β and MUC2 expression in tissues. Following a one-off treatment with AOM and three cycles of DSS, tumor tissues from mice in the tumor group (n=10) and colon tissues from mice in the untreated control group (n=10) assessed by IHC. Hematoxylin and eosin staining of (A) control and (B) tumor tissues identifying typical tumor characteristics, including disordered mucosa, destroyed crypt structure, less differentiated cells, a larger cell and nuclei size, and an increased nucleus-to-cytoplasm ratio. IRE1α protein was stained using IHC in (C) control and (D) tumor tissues and expression in the cytoplasm of colon submucosa cells was observed. IHC visualized IRE1β protein in (E) control and (F) tumor tissues and identified cytoplasmic expression in colonic mucosa epithelial cells. MUC2 protein expression was visualized using IHC in goblet cells of (G) control and (H) tumor tissues. All images were obtained using a Nikon Ds-Fi2 500w [Ds-Fi2; light microscope (magnification, ×200)]; scale bar, 50 µm. IHC, immunohistochemistry; AOM, azoxymethane; DSS, dextran sulfate sodium; IRE, inositol-requiring enzyme; MUC, mucin.
mRNA expression differs between the control and tumor groups
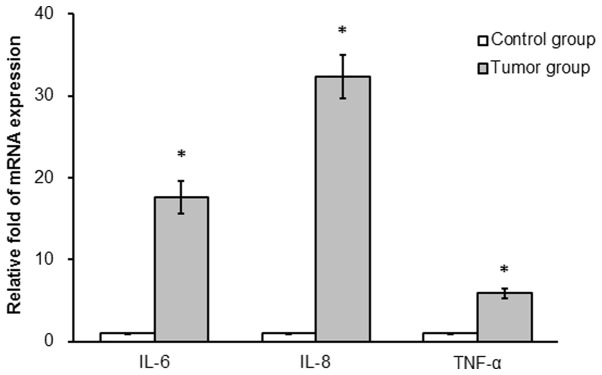
mRNA expression of IL-6, IL-8 and TNF-α was determined in colonic tissues from the two groups. IL-6, IL-8 and TNF-α mRNA expression was significantly increased in the tumor compared with the control group (17.6-, 32.3- and 5.9-fold, respectively; P<0.05; Fig. 3).
Figure 3.
AOM/DSS treatment increases mRNA levels of inflammatory cytokines in mice. Following a one-off treatment with AOM and three cycles of DSS, IL-6, IL-8 and TNF-α mRNA expression levels were determined in mice of the tumor and the untreated control group (n=10/group) using reverse transcription-quantitative polymerase chain reaction assays. *P<0.05 vs. control group. AOM, azoxymethane; DSS, dextran sulfate sodium; IL, interleukin; TNF, tumor necrosis factor.
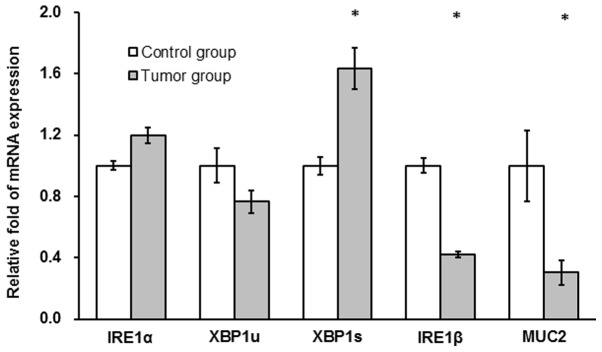
IRE1α and XBP1u mRNA expression levels were not significantly different between the tumor and the control group (P>0.05; Fig. 4). XBP1s mRNA levels were significantly increased in the tumor compared with the control group (~2-fold; P<0.05; Fig. 4) and IRE1β and MUC2 mRNA levels in the tumor group were significantly decreased compared with the control group (42 and 30%, respectively; P<0.05; Fig. 4).
Figure 4.
AOM/DSS treatment affects mRNA levels of XBP1s, IRE1β and MUC2 in colon tissues of mice. Following a one-off treatment with AOM and three cycles of DSS, mRNA expression of IRE1α, XBP1u, XBP1s, IRE1β and MUC2 were determined in tissues from mice in the untreated control and tumor groups (n=10/group) at the end point of the experiment using reverse transcription-quantitative polymerase chain reaction assays. *P<0.05 vs. control group. AOM, azoxymethane; DSS, dextran sulfate sodium; IRE, inositol-requiring enzyme; XBP, X-box binding protein; MUC, mucin.
IRE1β and MUC2 protein expression is downregulated in tumor tissues
As determined by IHC staining, there was no significant difference in IRE1α protein expression between the tumor and control group (P>0.05; Table IV). IRE1α was primarily expressed in the cytoplasm of colonic submucosa cells. A low level of IRE1α expression was observed in the cytoplasm of colonic epithelial cells, particularly in brush border cells (Fig. 2C and D). IRE1β protein expression in the tumor group was significantly decreased compared with the control group (P<0.05; Table IV). IRE1β protein was predominately expressed in the cytoplasm of colonic mucosa epithelial cells (Fig. 2E and F). Similar to IRE1β, the expression of MUC2 protein in the tumor group was significantly lower than in the control group (P<0.05; Table IV). MUC2 protein was primarily expressed in the cytoplasm of colonic mucosa epithelial cells, particularly in goblet cells in the normal mucosa (Fig. 2G and H).
Table IV.
IRE1α, IRE1β and MUC2 protein expression in colonic tissues determined by immunohistochemistry analysis.
| Group | N | IRE1α | IRE1β | MUC2 |
|---|---|---|---|---|
| Control | 10 | 1.50±0.23 | 5.26±0.38 | 4.77±0.47 |
| Tumor | 10 | 1.63±0.18 | 2.53±0.23a | 1.83±0.41a |
P<0.05 vs. control group. IRE, inositol-requiring enzyme; MUC, mucin.
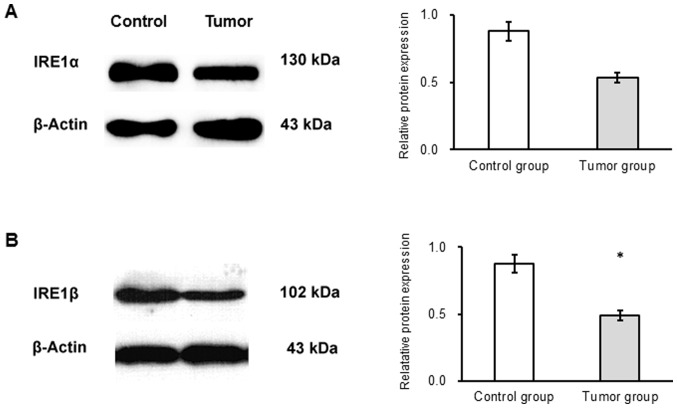
The results of western blot analysis revealed no significant difference in the expression of IRE1α protein between the groups (Fig. 5A). IRE1β protein expression was significantly decreased in the tumor compared with the control group (P<0.05; Fig. 5B).
Figure 5.
IRE1α and IRE1β protein expression is decreased in mice receiving AOM/DSS treatment. Following a one-off treatment with AOM and three cycles of DSS, tumor tissues from mice in the tumor group (n=10) and colon tissues from mice in the untreated control group (n=10) were collected. (A) IRE1α and (B) IRE1β protein expression was assessed by western blotting. The reduction of IRE1α expression was not significant, but the expression of IRE1β decreased significantly. *P<0.05 vs. control group. AOM, azoxymethane; DSS, dextran sulfate sodium; IRE, inositol-requiring enzyme.
Discussion
IRE1α serves a protective role against ER stress and colitis (24,40). The IRE1-XBP1 signaling pathway serves a pivotal role in cell survival under conditions of ER stress and is associated with tumorigenesis of various cancer types (41–44). In the current study, mRNA expression of IRE1α, XBP1u and XBP1s and IRE1α protein expression were compared in tumor and normal tissues. XBP1 mRNA, a signaling molecule in the IRE1α-XBP1 signaling pathway, is processed by IRE1α (19). XBP1s mRNA expression was significantly increased in tumor compared with control tissues. This suggested that the activity of the IRE1α-XBP1 pathway was increased, indicating that the ER stress response may be associated with tumorigenesis. The present study further observed that IRE1α mRNA levels were not increased in tumor tissues compared with the control, while XBP1s mRNA levels were. Under ER stress, IRE1α is activated through trans-autophosphorylation and dimerization, or oligomerization (45). It was speculated that activated IRE1α levels may be increased, while gene expression levels remained unchanged. Thus, the determination of phosphorylated-IRE1α levels requires future assessment.
IRE1α is a transmembrane RNAse that initiates the splicing of XBP1u to XBP1s. The latter encodes a transcription activator; its activity induces the expression of ER chaperones (46). IRE1α promotes cell survival-associated signal transduction and the transmission of apoptotic signals (47). It has been demonstrated that the IRE1-XBP1 signaling pathway is associated to the pathogenesis of IBD (48,49) and human cancer (50), leading to the consideration of the IRE1-XBP1 signaling pathway as a potential target for cancer therapy (51). A recent study suggested that IRE1α promotes cell survival by splicing XBP1 mRNA and promoting regulated IRE1-dependent decay (RIDD). During the RIDD process, IRE1 promotes the degradation of mRNAs that predominately encode ER-targeting proteins (52), which may be the mechanism for the involvement of the IRE1α-XBP1 signaling pathway in the pathogenesis of inflammation-mediated CRC. It was previously reported that IRE1α and XBP1 protein expression levels were unchanged in human CRC tissue compared with adjacent normal tissue (44). Therefore, the role of the IRE1-XBP1 signaling pathway in the pathogenesis of colonic tumors may be discrepant between humans and mice.
IRE1β, a homolog of IRE1α, protects from colitis and IRE1β−/− mice are more sensitive to experimentally induced colitis (16). In contrast to IRE1α, IRE1β affects different substrates, regulating the mRNA that encodes ER proteins to maintain ER homeostasis in highly differentiated secretory cells (28). Divergent effects of IRE1α and IRE1β on the cell fate may be implicated by their varying roles in tumorigenesis (25,46). Therefore, the current study analyzed IRE1β expression in tumor tissues. The results suggested that IRE1β mRNA and protein levels were decreased in the tumor compared with the control tissues. Although Tsuru et al (28) reported that IRE1β is specifically expressed in the ER of goblet cells, present IHC results revealed that IRE1β positive staining was observed in goblet and absorptive cells, indicating that IRE1β may affect multiple cells types. IRE1β acts to protect the intestine from inflammation (52). IRE1β expression in the epithelium of the gastrointestinal tract is consistent with promoting resistance to DSS-induced colitis at the epithelial cell level (16). When DSS is ingested, the gastrointestinal tract epithelial cells are the first to be exposed to the treatment (16). Toxicity likely has a primary role in the development of inflammation among colonic epithelia. DSS acts on the epithelial cell barrier, leading to reduced IRE1β expression in epithelial cells and weakening the protection against colonic inflammation (16). As IRE1β is a protective factor in colitis that can induce apoptosis (23) it is expected that a decrease in IRE1β expression is associated with tumorigenesis, as apoptosis is putatively associated with tumor occurrence (53). IRE1β has been reported to regulate the expression of microsomal triacylglycerol transfer protein, a regulator of lipid absorption in the colonic epithelium (54); however, it is unclear whether this function of IRE1β affects tumorigenesis. In an in vitro study, Dai et al (55) reported that IRE1β mRNA expression increases in undifferentiated and decreases in differentiated Caco-2 cells. IRE1β has been revealed to be involved in cell apoptosis (27). In a previous study, it was reported that IRE1β and MUC2 expression are downregulated in human colonic tissues (44). These results indicated that IRE1β serves an important role in the pathogenesis of colitis and tumors.
MUC2 is the major component of the intestinal mucus gel barrier, which serves an important role in the protection of intestinal function (31). Furthermore, MUC2 is putative to protecting the intestine against colitis and colorectal cancer (30). It has been demonstrated that MUC2-deficient mice spontaneously develop colon cancer (32). Consistent with this, the results of the current study revealed that MUC2 mRNA and protein levels were significantly decreased in tumor compared with normal tissue. It has been reported that IRE1β is involved in the ER homeostasis of colon goblet cells and the synthesis and secretion of MUC2 (28). IRE1β is believed to control the levels of translatable cytosolic MUC2 mRNA to maintain MUC production (28). An in vitro study has revealed that IRE1β regulates secretory proteins in cells via degrading mRNA in the ER (26). In addition, IRE1β is essential for airway epithelial MUC production (22). These data suggest that IRE1β may regulate MUC2 expression and the downregulation of IRE1β and MUC2 may reduce the protection of the colon to promote occurrence and development of tumors.
The current findings suggested that the ER stress-associated IRE1α-XBP1 signaling pathway was activated during the occurrence and development of CRC, and potentially contributed to these processes. The decreased expression of two protective molecules, IRE1β and MUC2, promoted the occurrence and development of inflammation and ultimately tumors in the colonic epithelium. The mechanism of these processes requires further analysis to identify potential therapeutic targets for the treatment or prevention of colitis-associated CRC.
Acknowledgements
The authors would like to thank Dr Lei Gao, Dr Tengfei Guo and Dr Dandan Feng from the Department of Gastroenterology and Hepatology, The First Affiliated Hospital, Henan University of Science and Technology (Luoyang, China) for providing reagents and materials, and for technical support.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81370487) and The Scientific Research Fund of Beijing Rehabilitation Hospital, Capital Medical University (grant no. 2017-004).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
FD performed the majority of experiments, analyzed the data and drafted the manuscript. SD, QX, PC and MC provided technical support, purchased reagents and analyzed the data. ZR and YF analyzed the data and provided their guidance for the study and manuscript writing. QG provided funding, designed the current study, analyzed the data and wrote the manuscript.
Ethics approval and consent to participate
The Animal Care and Use Committee of The First Affiliated Hospital of Henan University of Science and Technology approved all the animal procedures in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rabeneck L, Horton S, Zauber AG, Earle C. Colorectal cancer. JAMA J Am Med Assoc. 2015;270:2302. [Google Scholar]
- 2.Arvelo F, Sojo F, Cotte C. Biology of colorectal cancer. Ecancermedicalscience. 2015;9:520. doi: 10.3332/ecancer.2015.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raskov H, Pommergaard HC, Burcharth J, Rosenberg J. Colorectal carcinogenesis-update and perspectives. World J Gastroenterol. 2014;20:18151–18164. doi: 10.3748/wjg.v20.i48.18151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivennikov SI. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–244. doi: 10.1007/s00281-012-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114.e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Bye WA, Nguyen TM, Parker CE, Jairath V, East JE. Strategies for detecting colon cancer in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2017;9:CD000279. doi: 10.1002/14651858.CD000279.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 8.Kanneganti M, Mino-Kenudson M, Mizoguchi E. Animal models of colitis-associated carcinogenesis. J Biomed Biotechnol. 2011;2011:342637. doi: 10.1155/2011/342637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo K, Cao SS. Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease. Gastroenterol Res Pract. 2015;2015:328791. doi: 10.1155/2015/328791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dicks N, Gutierrez K, Michalak M, Bordignon V, Agellon LB. Endoplasmic reticulum stress, genome damage, and cancer. Front Oncol. 2015;5:11. doi: 10.3389/fonc.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao SS, Luo KL, Shi L. Endoplasmic reticulum stress interacts with inflammation in human diseases. J Cell Physiol. 2016;231:288–294. doi: 10.1002/jcp.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmar VM, Schröder M. Sensing endoplasmic reticulum stress. Adv Exp Med Biol. 2012;738:153–168. doi: 10.1007/978-1-4614-1680-7_10. [DOI] [PubMed] [Google Scholar]
- 13.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMillan DR, Gething MJ, Sambrook J. The cellular response to unfolded proteins: Intercompartmental signaling. Curr Opin Biotechnol. 1994;5:540–545. doi: 10.1016/0958-1669(94)90071-X. [DOI] [PubMed] [Google Scholar]
- 15.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-A. [DOI] [PubMed] [Google Scholar]
- 16.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manie SN, Lebeau J, Chevet E. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 3. Orchestrating the unfolded protein response in oncogenesis: An update. Am J Physiol Cell Physiol. 2014;307:C901–C907. doi: 10.1152/ajpcell.00292.2014. [DOI] [PubMed] [Google Scholar]
- 18.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, Koong AC. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 20.Koong AC, Chauhan V, Romero-Ramirez L. Targeting XBP-1 as a novel anti-cancer strategy. Cancer Biol Ther. 2006;5:756–759. doi: 10.4161/cbt.5.7.2973. [DOI] [PubMed] [Google Scholar]
- 21.Welihinda AA, Tirasophon W, Kaufman RJ. The cellular response to protein misfolding in the endoplasmic reticulum. Gene Expr. 1999;7:293–300. [PMC free article] [PubMed] [Google Scholar]
- 22.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, Ron D, O'Neal WK, Ribeiro CM. The ER stress transducer IRE1beta is required for airway epithelial mucin production. Mucosal Immunol. 2013;6:639–654. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Ya, Brandizzi F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013;23:547–555. doi: 10.1016/j.tcb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang HS, Chen Y, Fan L, Xi QL, Wu GH, Li XX, Yuan TL, He SQ, Yu Y, Shao ML, et al. The endoplasmic reticulum stress sensor IRE1α in intestinal epithelial cells is essential for protecting against colitis. J Biol Chem. 2015;290:15327–15336. doi: 10.1074/jbc.M114.633560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oikawa D, Kitamura A, Kinjo M, Iwawaki T. Direct association of unfolded proteins with mammalian ER stress sensor, IRE1β. PLoS One. 2012;7:e51290. doi: 10.1371/journal.pone.0051290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura D, Tsuru A, Ikegami K, Imagawa Y, Fujimoto N, Kohno K. Mammalian ER stress sensor IRE1β specifically down-regulates the synthesis of secretory pathway proteins. FEBS Lett. 2011;585:133–138. doi: 10.1016/j.febslet.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Iwawaki T, Hosoda A, Okuda T, Kamigori Y, Nomura-Furuwatari C, Kimata Y, Tsuru A, Kohno K. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol. 2001;3:158–164. doi: 10.1038/35055065. [DOI] [PubMed] [Google Scholar]
- 28.Tsuru A, Fujimoto N, Takahashi S, Saito M, Nakamura D, Iwano M, Iwawaki T, Kadokura H, Ron D, Kohno K. Negative feedback by IRE1β optimizes mucin production in goblet cells. Proc Natl Acad Sci USA. 2013;110:2864–2869. doi: 10.1073/pnas.1212484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawashima H. Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol Pharm Bull. 2012;35:1637–1641. doi: 10.1248/bpb.b12-00412. [DOI] [PubMed] [Google Scholar]
- 31.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 33.Jackson LN, Zhou Y, Qiu S, Wang Q, Evers BM. Alternative medicine products as a novel treatment strategy for inflammatory bowel disease. Am J Chin Med. 2008;36:953–965. doi: 10.1142/S0192415X08006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esworthy RS, Kim BW, Larson GP, Yip ML, Smith DD, Li M, Chu FF. Colitis locus on chromosome 2 impacting the severity of early-onset disease in mice deficient in GPX1 and GPX2. Inflamm Bowel Dis. 2011;17:1373–1386. doi: 10.1002/ibd.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Zhou Y, Zheng Y, Guo H, Gao L, Chen P, Feng D, Wu L, Yang M, Qi Y, et al. The gastric mucosa from patients infected with CagA+ or VacA+ Helicobacter pylori has a lower level of dual oxidase-2 expression than uninfected or infected with CagA-/VacA-H. pylori. Dig Dis Sci. 2016;61:2328–2337. doi: 10.1007/s10620-016-4144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Thaker AI, Shaker A, Rao MS, Ciorba MA. Modeling colitis-associated cancer with azoxymethane (AOM) and dextran sulfate sodium (DSS) J Vis Exp: 2012;(pii):4100. doi: 10.3791/4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C, Zhang X. IRE1alpha-XBP1 pathway promotes melanoma progression by regulating IL-6/STAT3 signaling. J Transl Med. 2017;15:42. doi: 10.1186/s12967-017-1147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujimoto T, Onda M, Nagai H, Nagahata T, Ogawa K, Emi M. Upregulation and overexpression of human X-box binding protein 1 (hXBP-1) gene in primary breast cancers. Breast Cancer. 2003;10:301–306. doi: 10.1007/BF02967649. [DOI] [PubMed] [Google Scholar]
- 43.Fujimoto T, Yoshimatsu K, Watanabe K, Yokomizo H, Otani T, Matsumoto A, Osawa G, Onda M, Ogawa K. Overexpression of human X-box binding protein 1 (XBP-1) in colorectal adenomas and adenocarcinomas. Anticancer Res. 2007;27:127–131. [PubMed] [Google Scholar]
- 44.Jiang Y, Zhou Y, Zheng Y, Guo H, Gao L, Chen P, Feng D, Qi R, Li X, Chang Y, et al. Expression of inositol-requiring enzyme 1β is downregulated in colorectal cancer. Oncol Lett. 2017;13:1109–1118. doi: 10.3892/ol.2017.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: Integrating stress signals through the stress sensor IRE1α. Physiol Rev. 2011;91:1219–1243. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 46.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lencer WI, DeLuca H, Grey MJ, Cho JA. Innate immunity at mucosal surfaces: The IRE1-RIDD-RIG-I pathway. Trends Immunol. 2015;36:401–409. doi: 10.1016/j.it.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaser A, Adolph TE, Blumberg RS. The unfolded protein response and gastrointestinal disease. Semin Immunopathol. 2013;35:307–319. doi: 10.1007/s00281-013-0379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero-Ramirez L, Cao H, Regalado MP, Kambham N, Siemann D, Kim JJ, Le QT, Koong AC. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Transl Oncol. 2009;2:31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadav RK, Chae SW, Kim HR, Chae HJ. Endoplasmic reticulum stress and cancer. J Cancer Prev. 2014;19:75–88. doi: 10.15430/JCP.2014.19.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coelho DS, Domingos PM. Physiological roles of regulated Ire1 dependent decay. Front Genet. 2014;5:76. doi: 10.3389/fgene.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moradi Marjaneh R, Hassanian SM, Ghobadi N, Ferns GA, Karimi A, Jazayeri MH, Nasiri M, Avan A, Khazaei M. Targeting the death receptor signaling pathway as a potential therapeutic target in the treatment of colorectal cancer. J Cell Physiol. 2018;233:6538–6549. doi: 10.1002/jcp.26640. [DOI] [PubMed] [Google Scholar]
- 54.Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, Ron D, Tabas I, Hussain MM. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008;7:445–455. doi: 10.1016/j.cmet.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dai K, Khatun I, Hussain MM. NR2F1 and IRE1beta suppress microsomal triglyceride transfer protein expression and lipoprotein assembly in undifferentiated intestinal epithelial cells. Arterioscler Thromb Vasc Biol. 2010;30:568–574. doi: 10.1161/ATVBAHA.109.198135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.