Abstract
Correlation of miR-21 and B-type natriuretic peptide (BNP) with pregnancy-induced hypertension (PIH) complicated with heart failure and the diagnostic value was investigated. Sixty patients with PIH complicated with heart failure admitted to Affiliated Hospital of Chengde Medical University from July 2016 to July 2017 were enrolled as the experimental group, and 35 normal pregnant women as the control group. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) method was used to determine the expression level of plasma miR-21 expression level. An automatic biochemical analyzer was used to determine plasma BNP expression level. Spearmans correlation analysis was used for the correlation analysis of miR-21 and BNP. ROC curve was used for evaluating the diagnostic values of miR-21 and BNP for PIH complicated with heart failure. miR-21 and BNP expression levels were higher in patients with PIH complicated with heart failure than those in the normal individuals, and were increased in line with the heart failure grade (P<0.001). The plasma miR-21 expression was positively correlated with BNP in patients with PIH complicated with heart failure (r=0.685, P<0.001). Both miR-21 and BNP had higher diagnostic values for PIH complicated with heart failure, in the diagnosis, the best cut-off value [odds ratio (OR)] of miR-21 was 1.113, with an area under curve (AUC) of 0.889 and a 95% confidence interval (CI) of 82.05–95.76%; the OR of BNP was 123, with an AUC of 0.747 and a 95% CI of 64.95–84.38%. Blood pressure, six-minute walk test (6MWT), left ventricular ejection fraction (LVEF) and left ventricular end diastolic diameter (LVEDD) were independent risk factors for the occurrence of PIH complicated with heart failure (P<0.05). In conclusion, miR-21 and BNP, highly expressed in patients with PIH complicated with heart failure, are expected to become important biomarkers for diagnosing PIH complicated with heart failure and judging the degree of heart failure in the patients, and worthy of clinical popularization and application.
Keywords: pregnancy-induced hypertension, heart failure, miR-21, B-type natriuretic peptide
Introduction
As a common clinical complication during pregnancy that threatens maternal and fetal health, pregnancy-induced hypertension syndrome, referred to as pregnancy-induced hypertension (PIH), is one of high risk factors for death in pregnant women (1). The patients with PIH are mainly characterized by systemic small vasospasm that results in multiple organ damage throughout the body (2), and causes abnormal maternal uterine placenta function in pregnant women and thereby fetal hypoxia. As a result, the possibility of neonatal death is increased (3). PIH complicated with heart failure refers to the symptoms of myocardial damage in pregnant women with PIH. Heart failure, a severe complication of PIH and an important factor causing death in pregnant women, seriously threatens the safety of the pregnant woman and the fetus (4). At present, there are few biomarkers for the diagnosis and confirmation of PIH complicated with heart failure in clinical practice. This leads to the delayed and unclear diagnosis of the disease, delays the optimal treatment opportunity and endangers both the maternal and fetal life.
microRNA (miR) is a non-coding, single-stranded small RNA molecule, and has been considered to be involved in the occurrence of cardiovascular diseases (5). Currently, miR is widely used in the marker examination of malignant tumors (6), which is also manifested in blood according to a study as science and technology and scientific research levels continue to improve (7). miR-21, which plays an important role in the development of the cardiovascular system and the occurrence of diseases, is abnormally expressed in PIH complicated with heart failure, so it is expected to be a biomarker for the disease (8). A study has confirmed (9) that miR-21 is significantly differentially expressed in the serum of patients with heart failure and healthy individuals.
B-type natriuretic peptide (BNP) is a neurohormone secreted when the ventricular volume is enlarged and the pressure is overloaded (10). It is widely distributed in the tissues of heart, brain, spinal cord and lung, of which the content is highest in the heart. BNP has high sensitivity and specificity to the heart, and is not affected by subjective factors. In recent years, with continuous studies of its expression and role in heart diseases, BNP has been found to play an important role in evaluating heart failure (11). The study by McMurray et al (12) shows that BNP accurately reflects the degree of heart failure.
At present, there are few studies on the correlation of miR-21 and BNP with PIH complicated with heart failure and their diagnostic values. Therefore, in the present study, miR-21 and BNP expression levels in the plasma of patients were compared between the PIH group and the PIH complicated with the heart failure group, to evaluate the diagnostic values of miR-21 and BNP for PIH complicated with heart failure, in order to provide a basis for the early diagnosis and treatment of the disease.
Patients and methods
General information
Sixty patients with PIH complicated with heart failure admitted to Affiliated Hospital of Chengde Medical University (Chengde, China) from July 2016 to July 2017 were enrolled as the experimental group and divided into three grades based on the NYHA cardiac function classification (13). Cardiac function is generally divided into four grades, but heart failure into three grades by NYHA. Heart failure classification is slightly supplemented according to the NYHA classification. Fifty-two patients had a gestational week of ≤37 weeks and 8 patients had a gestational week of >37 weeks, with an average gestational week of 36.7±2.2 weeks. There were 18 patients in grade II, the daily physical activity of patients with heart disease is slightly restricted. Patients have no subjective symptoms when having a rest, but they have fatigue, palpitation and dyspnea or angina pectoris when carrying out normal activities, aged 23–35 years, with an average age of 27.15±2.2 years. There were 22 patients in grade III, the physical activity of patients with heart disease is obviously restricted, and patients have fatigue, palpitation, dyspnea or angina pectoris when carrying out slight activities, aged 23–36 years, with an average age of 28.7±3.5 years. There were 20 patients in grade IV, patients with heart disease have severe heart failure symptoms and are unable to engage in any physical activity. When having a rest, they have heart failure symptoms that are significantly aggravated after physical activities, aged 24–38 years, with an average age of 30.4±7.1 years. Thirty-five normal pregnant women were enrolled as the control group, aged 21–34 years, with an average age of 25.8±4.3 years. Patients with PIH complicated heart failure were in line with the diagnostic criteria for hypertensive heart failure during pregnancy (14).
Inclusion and exclusion criteria
The inclusion criteria were the following: i) Patients without complete left bundle branch block, acute myocardial infarction, pericardial effusion and hypertrophic cardiomyopathy were included; ii) patients without acute and chronic hepatitis, acute and chronic renal failure; iii) patients without pulmonary heart disease, pneumothorax and pulmonary infection; iv) patients who had not taken drugs affecting aldosterone metabolism in the body within 2 weeks; and v) patients without multiple organ failure. The exclusion criteria were the following: i) Patients who recently had acute myocardial infarction or recent acute myocarditis were excluded; ii) patients with acute and chronic infections; iii) patients with hyperthyroidism, sepsis, diabetes insipidus and tumors; iv) patients with primary aldosteronism; v) patients with bronchial asthma; and vi) patients with simple gestational hypertension or simple heart failure. The study was approved by the Ethics Committee of Affiliated Hospital of Chengde Medical University. Patients or their families signed a full informed consent form.
Equipment
Reagents and instruments
RNA extraction kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), spectrophotometer (Shanghai INESA Analytical Instrument Co., Ltd., Shanghai, China), RNA Reverse Transcription kit (Shanghai ShineGene Molecular Biotechnology Co., Ltd., Shanghai, China), Real-Time fluorescence quantification PCR kit (Aidlab Biotechnologies Co., Ltd., Beijing, China), U6 and miR-21 primer sequences (Sangon Biotech Co., Ltd., Shanghai, China), the automatic biochemical analyzer SQChemray240 of the laboratory medicine for the detection of BNP (Shanghai Huanxi Medical Devices Co., Ltd., Shanghai, China).
Methods
Total RNA extraction and cDNA synthesis
Total RNA was extracted in strict accordance with the instructions of the serum RNA extraction kit. A spectrophotometer was used to detect the optical density (OD), with the reference value of the qualified samples between 1.8 and 2.2. Reverse transcription was performed in accordance with the instructions of the Reverse Transcription kit. The RT reaction solution (20 µl of the reaction system) was prepared as follows: 2 µl of miScript Reverse Transcriptase Mix; 4 µl of 5X miScript HiSpec buffer; 5 µl of RNase-free water; 2 µl of 10X miScript Nucleics Mix; 7 µl of Template RNA (2 µg of RNA content per reaction system, the RNA required was calculated based on the RNA concentration extracted, and RNase-free water was used to make up to 7 µl). Reaction conditions were as follows: at 16°C for 30 min, at 42°C for 30 min and at 75°C for 15 min. After the reverse transcription, cDNA was placed in a refrigerator at −20°C for later use.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
With U6 as an internal reference gene, the reaction system was 20.00 µl: each 0.5 µl of positive and negative primers (Table I), 1.0 µl of cDNA product, 10 µl of 2X miR qPCR Mix, and DEPC were used to make up to 20 µl. Amplification conditions were: at 94°C for 3 min, at 94°C for 35 sec, at 60°C for 35 sec, and at 72°C for 60 sec, for a total of 40 cycles. The cycle number Cq value was obtained from the qPCR curve, and 2−ΔCq was used to calculate the miR-21 expression (15).
Table I.
Primer sequences.
| Genes | Upstream primers | Downstream primers |
|---|---|---|
| U6 internal reference | 5-ATTGGAACGATACAGAGAAGATT-3 | 5-GGAACGCTTCACGAATTTG-3 |
| miR-21 | 5-ACGTTGTGTAGCTTATCAGACTG-3 | 5-AATGGTTGTTCTCCACACTCTC-3 |
The automatic biochemical analyzer SQChemray240 of the laboratory medicine and supporting reagents were used to determine BNP with spectrophotometry in strict accordance with the instructions.
Statistical analysis
SPSS 17.0 (Keruanwang Technology Co., Ltd., Tianjin, China) statistical software was used for the statistical analysis of the research data. The t-test was used for the comparison between the two groups, and analysis of variance (ANOVA) and Least Significant Difference test was used for comparison among multiple groups. Measurement data were expressed as mean ± standard deviation (mean ± SD), and count data were expressed as rate (%), tested by χ2 test. Logistic univariate and multivariate regression analysis were used for disease risk factors, Spearmans correlation analysis for correlation and ROC curve for judging the predictive value and cut-point of indicators. P<0.05 indicates a statistically significant difference.
Results
General information
There were no significant differences in age, ethnicity, height, body weight, history of diabetes mellitus, smoking and drinking between the experimental group and the control group (P<0.05), but there were differences in hypoproteinemia, blood pressure, six-minute walk test (6MWT), left ventricular ejection fraction (LVEF) and left ventricular end diastolic diameter (LVEDD) (P>0.05) (Table II).
Table II.
Basic information of the experimental and control groups [n, (%)].
| Basic information | Experimental group (n=60) | Control group (n=35) | t/χ2 | P-value |
|---|---|---|---|---|
| Age (years) | 0.513 | 0.473 | ||
| <30 | 28 (46.67) | 19 (54.29) | ||
| ≥30 | 32 (53.33) | 16 (45.71) | ||
| Ethnicity | 0.118 | 0.732 | ||
| Han | 56 (93.33) | 32 (91.43) | ||
| Others | 4 (6.67) | 3 (8.57) | ||
| Height (cm) | 0.085 | 0.771 | ||
| <163 | 31 (51.67) | 17 (48.57) | ||
| ≥163 | 29 (48.33) | 18 (51.43) | ||
| Body weight (kg) | 1.688 | 0.194 | ||
| <50 | 34 (56.67) | 15 (42.86) | ||
| ≥50 | 26 (43.33) | 20 (57.14) | ||
| Hypoproteinemia | 4.231 | 0.040 | ||
| Yes | 13 (21.67) | 2 (5.71) | ||
| No | 47 (78.33) | 33 (94.29) | ||
| Anemia | 4.117 | 0.042 | ||
| Yes | 10 (16.67) | 1 (2.86) | ||
| No | 50 (83.33) | 34 (97.14) | ||
| History of diabetes mellitus | 0.073 | 0.788 | ||
| Yes | 8 (13.33) | 4 (11.43) | ||
| No | 52 (86.67) | 31 (88.57) | ||
| Smoking | 0.002 | 0.968 | ||
| Yes | 5 (8.33) | 3 (8.57) | ||
| No | 55 (91.67) | 32 (91.43) | ||
| Drinking | 0.137 | 0.711 | ||
| Yes | 7 (11.67) | 5 (14.29) | ||
| No | 53 (88.33) | 30 (85.71) | ||
| Blood pressure (mmHg) | ||||
| Systolic pressure | 159.78±18.32 | 123.71±11.34 | 10.520 | <0.001 |
| Diastolic pressure | 107.72±2.67 | 86.45±15.28 | 10.550 | <0.001 |
| 6MWT (m) | 298.56±70.93 | 526.79±102.61 | 12.79 | <0.001 |
| LVEF (%) | 40.26±17.54 | 59.67±12.43 | 5.735 | <0.001 |
| LVEDD (mm) | 62±6.39 | 53.01±1.21 | 8.220 | <0.001 |
6MWT, six-minute walk test; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter.
miR-21 expression level in the two groups of patients
The miR-21 expression level in the experimental group was 1.553±0.513 in grade II, 1.654±0.493 in grade III and 1.948±0.651 in grade IV, and that in the control group was 0.927±0.231. The miR-21 expression level in the control group was compared with that in grades II, III and IV in the experimental group, all P<0.05; that in grade II was compared with that in grade III, P>0.05; that in grade III was compared with that in grade IV, P<0.05; that in grade II was compared with that in grade IV, P<0.05 (Table III).
Table III.
miR-21 expression level in two groups of patients.
| Groups | n | miR-21 |
|---|---|---|
| Experimental group | ||
| Grade II | 18 | 1.553±0.513a |
| Grade III | 22 | 1.654±0.493a,c |
| Grade IV | 20 | 1.948±0.651a,b,d |
| Control group | 35 | 0.927±0.231 |
| F | – | 79.546 |
| P-value | – | <0.001 |
P<0.05 compared with the control group
P<0.05
P>0.05 compared with grade II in the experimental group
P<0.05 compared with grade III in the experimental group.
BNP expression level in two groups of patients
BNP expression level in the experimental group was 165.59±82.52 pg/ml in grade II, 786.75±121.99 pg/ml in grade III and 1018.48±109.54 pg/ml in grade IV, and that in the control group was 95.36±34.65 pg/ml. The BNP expression level in the control group was compared with that in grades II, III and IV in the experimental group, all P<0.05; that in grade II was compared with that in grade III, P<0.05; that in grade III was compared with that in grade IV, P<0.05; that in grade II was compared with that in grade IV, P<0.05 (Table IV).
Table IV.
BNP expression level in two groups of patients (pg/ml).
| Groups | n | BNP |
|---|---|---|
| Experimental group | ||
| Grade II | 18 | 165.59±82.52a |
| Grade III | 22 | 786.75±121.99a,b |
| Grade IV | 10 | 1018.48±109.54a–c |
| Control group | 35 | 95.36±34.65 |
| F | – | 46.670 |
| P-value | – | <0.001 |
P<0.05 compared with the control group
P<0.05 compared with grade II in the experimental group
P<0.05 compared with grade III in the experimental group. BNP, B-type natriuretic peptide.
ROC curve for evaluation of diagnostic value
By ROC curve fitting, in the diagnosis of PIH complicated with heart failure, the odds ratio (OR) of miR-21 was 1.113, the sensitivity was 88.53% and the specificity was 88.33%, with an area under curve (AUC) of 0.889 and a 95% CI of 82.05–95.76%; the OR of BNP was 123, the sensitivity was 88.57% and the specificity was 63.33%, with an AUC of 0.747 and a 95% CI of 64.95–84.38% (Fig. 1 and Table V).
Figure 1.
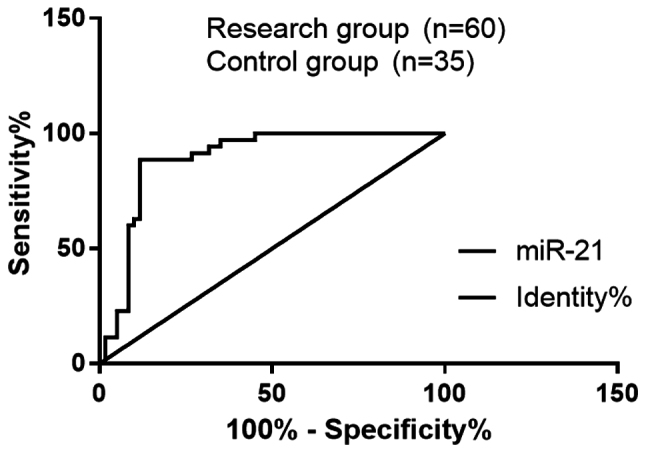
Diagnostic value of miR-21 and BNP for PIH complicated with heart failure. The analysis of ROC curve showed that in the diagnosis of PIH complicated with heart failure, the OR of miR-21 was 1.113, the sensitivity was 88.53% and the specificity was 88.33%, with an AUC of 0.889 and a 95% CI of 82.05–95.76%; the OR of BNP cut-off value was 123, the sensitivity was 88.57% and the specificity was 63.33%, with an AUC of 0.747 and a 95% CI of 64.95–84.38%. BNP, B-type natriuretic peptide; PIH, pregnancy-induced hypertension; ROC, receiver operating characteristic; AUC, area under curve; CI, confidence interval; OR, odds ratio.
Table V.
Diagnostic value of miR-21 and BNP for PIH complicated with heart failure.
| Items | AUC | OR | 95% CI (%) | P-value | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|---|---|
| miR-21 | 0.889 | 1.113 | 82.05–95.76 | <0.001 | 85.71 | 88.33 |
| BNP | 0.747 | 123 | 64.95–84.38 | <0.001 | 62.85 | 88.33 |
BNP, B-type natriuretic peptide; PIH, pregnancy-induced hypertension; AUC, area under curve; OR, odds ratio; CI, confidence interval.
Correlation analysis of miR-21 expression with BNP in PIH complicated with heart failure
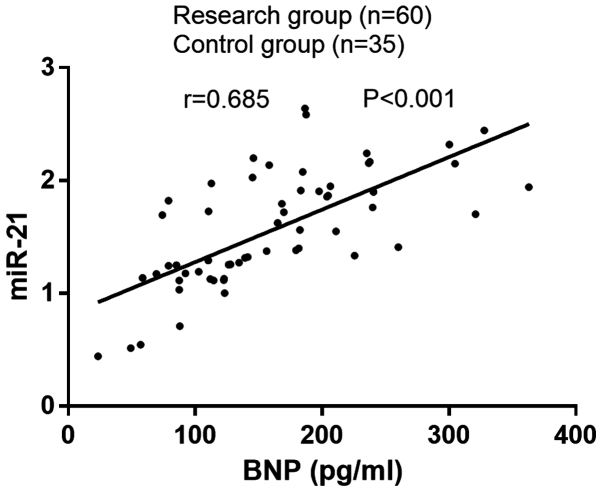
The plasma miR-21 expression was positively correlated with BNP in patients with PIH complicated with heart failure (r=0.685, P<0.001) (Fig. 2).
Figure 2.
Correlation analysis of miR-21 expression with BNP in PIH complicated with heart failure. The plasma miR-21 expression was positively correlated with BNP in patients with PIH complicated with heart failure (r=0.685, P<0.001). BNP, B-type natriuretic peptide; PIH, pregnancy-induced hypertension.
Analysis of risk factors for PIH complicated with heart failure
Logistic univariate analysis on risk factors for PIH complicated with heart failure showed that the blood pressure (P=0.018), 6MWT (P=0.006), LVEF (0.027) and LVEDD (0.036) of patients were correlated with PIH complicated with heart failure (Table VI). To control the influence of confounding factors on this outcome, logistic multivariate analysis was used on risk factors for PIH complicated with heart failure. The results showed that blood pressure (OR, 3.045; 95% CI, 0.935–11.442), 6MWT (OR, 9.547; 95% CI, 4.671–13.072), LVEF (OR, 8.665; 95% CI, 5.728–15.569) and LVEDD (OR, 8.665; 95% CI, 5.728–15.569) were independent risk factors for the disease (Table VII).
Table VI.
Logistic univariate analysis of risk factors for PIH complicated with heart failure.
| Clinical parameters | Coefficient | Standard error | Wald value | P-value | OR value | 95% CI |
|---|---|---|---|---|---|---|
| Blood pressure | 0.302 | 0.117 | 10.157 | 0.001 | 4.528 | 1.478–8.537 |
| 6MWT | 1.132 | 0.500 | 3.467 | 0.046 | 3.101 | 0.897–10.077 |
| Hypoproteinemia | 1.132 | 0.500 | 3.467 | 0.056 | 3.101 | 0.897–10.077 |
| LVEF | 1.431 | 0.495 | 6.491 | 0.043 | 4.485 | 1.471–11.436 |
| Anemia | 0.302 | 0.117 | 5.157 | 0.061 | 3.528 | 1.478–8.537 |
| LVEDD | 1.041 | 0.576 | 3.465 | 0.004 | 3.045 | 0.935–11.442 |
PIH, pregnancy-induced hypertension; OR, odds ratio; CI, confidence interval; 6MWT, six-minute walk test; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter.
Table VII.
Logistic multivariate analysis of risk factors for PIH complicated with heart failure.
| Clinical parameters | Coefficient | Standard error | Wald value | P-value | OR value | 95% CI |
|---|---|---|---|---|---|---|
| Blood pressure | 0.302 | 0.117 | 10.157 | 0.001 | 4.528 | 1.478–8.537 |
| 6MWT | 1.431 | 0.495 | 6.491 | 0.001 | 4.485 | 1.471–11.436 |
| LVEF | 1.041 | 0.576 | 3.465 | 0.001 | 3.045 | 0.935–11.442 |
| LVEDD | 1.954 | 0.392 | 20.329 | 0.001 | 9.547 | 4.671–13.072 |
PIH, pregnancy-induced hypertension; OR, odds ratio; CI, confidence interval; 6MWT, six-minute walk test; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter.
Discussion
Pregnancy-induced hypertension (PIH) complicated with heart failure is a common disease during pregnancy and characterized by no history of hypertension and heart-related diseases before pregnancy. Heart failure that is complicated with PIH, is mainly characterized by myocardial injury, which is a heart failure syndrome and the second cause of pregnant and maternal death (16). The main manifestation of such heart failure is arteriolar spasm, mainly systemic symptoms, resulting in the ischemia and hypoxia of the tissues and organs of the human body, and thereby myocardial ischemia and injury and an increase in cardiac load. Severe PIH easily cause this disease that leads to fetal distress, intrauterine growth retardation, fetal death and neonatal or maternal death (17).
At present, there is no precise factor or technical means for the early diagnosis of PIH complicated with heart failure. Kumarswamy et al (18) have pointed out that the miR-2 expression level in the plasma of patients with heart failure is significantly higher than that in the normal individuals. Berger et al (19) suggested that the BNP expression level in the plasma of patients with heart failure is significantly higher than that in the normal individuals, which is important for diagnosing and classifying heart failure. Du et al (20) also proposed that the BNP expression level in patients with PIH is significantly increased, with a diagnostic value. In the historical literature search, there are few studies on the correlation of miR-21 and BNP with PIH complicated with heart failure and their diagnostic values, which were detected and analyzed in the present study for clarification.
The present study found that the miR-21 expression level in patients with PIH complicated with heart failure was higher than that in the normal individuals, and increased in line with with the increase in the heart failure grade. When the human heart load is increased, the miR-21 expression level is also increased, which stimulates and activates the genetic program for cardiac fibrosis, and then fibrotic changes take place in myocardial cells that lose normal physiological functions (21). The study by Wu et al (22) also confirmed that miR-21 regulates and activates multiple factor signaling pathways, the expression level of which is significantly upregulated in cardiac fibroblasts of heart failure, and the signal transduction pathway of ERK-MAP kinase is thus enhanced, which causes the proliferation and fibrosis of cardiac fibroblasts. Adams et al (11) and Stokes et al (23) proposed in their studies that BNP expression in patients with heart failure is higher than that in normal individuals. This study showed that the BNP expression level in patients with PIH complicated with heart failure was significantly higher than that in normal individuals, which was significantly higher with the increase in heart failure grade. BNP is a peptide neurohormone mainly secreted by ventricular muscle cells. It is currently believed that the cardiac volume load is increased during heart failure, and acute decompensated high wall pressure in patients leads to a significant increase in the BNP expression level. This is consistent with the views of Kovács et al (24). The ROC curve was used for judging the predictive value and cut-point of indicators. The results showed that the OR of miR-21 was 1.113, the sensitivity was 88.53%, and the specificity was 88.33%, with an AUC of 0.889 and a 95% CI of 82.05–95.76%; the OR of BNP was 123, the sensitivity was 88.57%, and the specificity was 63.33%, with an AUC of 0.747 and a 95% CI of 64.95–84.38%. It is indicated that miR-21 and BNP have higher diagnostic values for PIH complicated with heart failure. Cavagna et al (25) and Cengiz et al (26), respectively pointed out that miR-21 and BNP have higher diagnostic values for PIH complicated with heart failure. This study also found that expression of miR-21 and BNP in patients with PIH complicated with heart failure was increased in line with the heart failure grade. The correlation analysis of miR-21 and BNP with PIH complicated with heart failure showed that miR-21 and BNP were positively correlated with PIH complicated with heart failure (r=0.685, P<0.001). In the present study, risk factors for the prognosis of PIH complicated with heart failure were also analyzed. The results showed that blood pressure, 6MWT, LVEF and LVEDD were independent risk factors for the disease, consistent with the views of Chambela et al (27).
Study subjects were screened in strict accordance with the inclusion and exclusion criteria in this investigation, and there were no differences between the experimental and control groups in terms of sex, age and lifestyle, which improve the authenticity and reliability of this study. In addition to detecting the miR-21 and BNP expression levels in patients with PIH complicated with heart failure, independent risk factors for this disease were also found. All-round observations and statistics, as well as the results were proven by the data, reflecting the rigor of this experiment. The experimental research is to extend the results obtained to a larger overall, so as to contribute to understanding the group and society. However, due to limited medical resources in Affiliated Hospital of Chengde Medical University, the number of enrolled patients is insufficient, so the results may lack extensive representation, and a future confirmation study is necessary.
In summary, miR-21 and BNP, highly expressed in patients with PIH complicated with heart failure, are expected to become important biomarkers for diagnosing PIH complicated with heart failure and judging the degree of heart failure in the patients, and worthy of clinical popularization and application.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding a uthor on reasonable request.
Authors contributions
CK, JC and JH were responsible for the total RNA extraction, cDNA synthesis and PCR. XJ, YZ and JZ collected and interpreted the patient general data. YG and XC reviewed the manuscript and contributed to performing the statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Affiliated Hospital of Chengde Medical University (Chengde, China). Patients who participated in this research, signed an informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2018;10:CD002252. doi: 10.1002/14651858.CD002252.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P, Magee LA, Audibert F, Bujold E, Côté A-M, Douglas MJ, et al. Canadian Hypertensive Disorders of Pregnancy Working Group Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J Obstet Gynaecol Can. 2014;36:416–441. doi: 10.1016/S1701-2163(15)30588-0. [DOI] [PubMed] [Google Scholar]
- 3.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gini R, Schuemie MJ, Mazzaglia G, Lapi F, Francesconi P, Pasqua A, Bianchini E, Montalbano C, Roberto G, Barletta V, et al. Automatic identification of type 2 diabetes, hypertension, ischaemic heart disease, heart failure and their levels of severity from Italian General Practitioners electronic medical records: A validation study. BMJ Open. 2016;6:e012413. doi: 10.1136/bmjopen-2016-012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Rana TM. Therapeutic targeting of microRNAs: Current status and future challenges. Nat Rev Drug Discov. 2014;13:622–638. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz-Culla M, Irizar H, Castillo-Triviño T, Sáenz-Cuesta M, Sepúlveda L, Lopetegi I, López de Munain A, Olascoaga J, Baranzini SE, Otaegui D. Blood miRNA expression pattern is a possible risk marker for natalizumab-associated progressive multifocal leukoencephalopathy in multiple sclerosis patients. Mult Scler. 2014;20:1851–1859. doi: 10.1177/1352458514534513. [DOI] [PubMed] [Google Scholar]
- 8.Kukreja RC, Yin C, Salloum FN. MicroRNAs: New players in cardiac injury and protection. Mol Pharmacol. 2011;80:558–564. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong X, Liu S, Zhang L, Yu S, Huo L, Qile M, Liu L, Yang B, Yu J. Downregulation of miR-21 is involved in direct actions of ursolic acid on the heart: Implications for cardiac fibrosis and hypertrophy. Cardiovasc Ther. 2015;33:161–167. doi: 10.1111/1755-5922.12125. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell A, Misialek JR, Folsom AR, Duprez D, Alonso A, Jerosch-Herold M, Sanchez OA, Watson KE, Sallam T, Konety SH. Usefulness of N-terminal pro-brain natriuretic peptide and myocardial perfusion in asymptomatic adults (from the multi-ethnic study of atherosclerosis) Am J Cardiol. 2015;115:1341–1345. doi: 10.1016/j.amjcard.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams KF, Jr, Giblin EM, Pearce N, Patterson JH. Integrating new pharmacologic agents into heart failure care: Role of heart failure practice guidelines in meeting this challenge. Pharmacotherapy. 2017;37:645–656. doi: 10.1002/phar.1934. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology; ESC Committee for Practice Guidelines ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 13.Bredy C, Ministeri M, Kempny A, Alonso-Gonzalez R, Swan L, Uebing A, Diller GP, Gatzoulis MA, Dimopoulos K. New York Heart Association (NYHA) classification in adults with congenital heart disease: Relation to objective measures of exercise and outcome. Eur Heart J Qual Care Clin Outcomes. 2018;4:51–58. doi: 10.1093/ehjqcco/qcx031. [DOI] [PubMed] [Google Scholar]
- 14.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Barasa A, Rosengren A, Sandstrom TZ, Ladfors L, Schaufelberger M. Heart failure in late pregnancy and postpartum: Incidence and long-term mortality in Sweden from 1997 to 2010. J Card Fail. 2017;23:370–378. doi: 10.1016/j.cardfail.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Theilen LH, Fraser A, Hollingshaus MS, Schliep KC, Varner MW, Smith KR, Esplin MS. All-cause and cause-specific mortality after hypertensive disease of pregnancy. Obstet Gynecol. 2016;128:238–244. doi: 10.1097/AOG.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8:706–713. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger R, Moertl D, Peter S, Ahmadi R, Huelsmann M, Yamuti S, Wagner B, Pacher R. N-terminal pro-B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol. 2010;55:645–653. doi: 10.1016/j.jacc.2009.08.078. [DOI] [PubMed] [Google Scholar]
- 20.Du Y, Fu J, Yao L, Qiao L, Liu N, Xing Y, Xue X. Altered expression of PPAR-γ and TRPC in neonatal rats with persistent pulmonary hypertension. Mol Med Rep. 2017;16:1117–1124. doi: 10.3892/mmr.2017.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120:3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Wang J, Ma H, Xiao Z, Dong X. MicroRNA-21 inhibits mitochondria-mediated apoptosis in keloid. Oncotarget. 2017;8:92914–92925. doi: 10.18632/oncotarget.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes MB, Bergin P, McGiffin D. Role of long-term mechanical circulatory support in patients with advanced heart failure. Intern Med J. 2016;46:530–540. doi: 10.1111/imj.12817. [DOI] [PubMed] [Google Scholar]
- 24.Kovács Á, Alogna A, Post H, Hamdani N. Is enhancing cGMP-PKG signalling a promising therapeutic target for heart failure with preserved ejection fraction? Neth Heart J. 2016;24:268–274. doi: 10.1007/s12471-016-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavagna L, Caporali R, Klersy C, Ghio S, Albertini R, Scelsi L, Moratti R, Bonino C, Montecucco C. Comparison of brain natriuretic peptide (BNP) and NT-proBNP in screening for pulmonary arterial hypertension in patients with systemic sclerosis. J Rheumatol. 2010;37:2064–2070. doi: 10.3899/jrheum.090997. [DOI] [PubMed] [Google Scholar]
- 26.Cengiz M, Yavuzer S, Kılıçkıran Avcı B, Yürüyen M, Yavuzer H, Dikici SA, Karataş OF, Özen M, Uzun H, Öngen Z. Circulating miR-21 and eNOS in subclinical atherosclerosis in patients with hypertension. Clin Exp Hypertens. 2015;37:643–649. doi: 10.3109/10641963.2015.1036064. [DOI] [PubMed] [Google Scholar]
- 27.Chambela MC, Mediano MFF, Ferreira RR, Japiassú AM, Waghabi MC, da Silva GMS, Saraiva RM. Correlation of 6-min walk test with left ventricular function and quality of life in heart failure due to Chagas disease. Trop Med Int Health. 2017;22:1314–1321. doi: 10.1111/tmi.12939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding a uthor on reasonable request.