Abstract
Hepatoid adenocarcinoma (HAC) is a rare and aggressive gastrointestinal tract cancer that is characterized by hepatic differentiation and production of alpha-fetoprotein (AFP). Cisplatin is mainly used to treat HAC, but the efficacy is poor. Recently, the histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), was approved as an anticancer agent. In this study, we investigated the anticancer effect of SAHA in combination with cisplatin in VAT-39 cells, a newly established HAC cell line. Cell viability and apoptosis were examined by MTT assay, flow cytometry and TUNEL assay. Expression of H3S10, cleaved caspase-3, Bax, and Bcl-2 were evaluated by immunohistochemistry and western blotting. AFP levels were examined in VAT-39 cells and culture medium. Combined treatment with cisplatin and SAHA efficiently inhibited cell proliferation and decreased cell viability. Apoptotic cells, but not necrotic cells, were significantly increased following the combined treatment, and an increase in the Bax/Bcl-2 ratio indicated that the combination of cisplatin and SAHA induced apoptosis through the mitochondrial pathway. VAT-39 cells treated with cisplatin and SAHA also partially lost their main characteristic of AFP production. We conclude that cisplatin and SAHA have a synergistic anticancer effect of inducing apoptosis, and that this combination treatment may be effective for HAC.
Keywords: HDAC inhibitor, SAHA, cisplatin, apoptosis, hepatoid adenocarcinoma
I. Introduction
Hepatoid adenocarcinoma (HAC) is a rare, aggressive cancer that is characterized by hepatic differentiation and alpha-fetoprotein (AFP) production [19]. Most cases occur in the stomach, but HAC is also found in the lung, pancreas, esophagus, ampulla of Vater, colon, urinary bladder, renal pelvis, ovary, and cervix [31, 34]. Clinically, HAC shows aggressive progression and a strong tendency to metastasize to lymph nodes and liver; and AFP production promotes neovascularization, high proliferative activity, and inhibition of apoptosis, which contribute to the poor prognosis [20, 33]. A recent meta-analysis found a 3-year survival rate of 7.36% and a median survival time of 10 months, indicating a poor prognosis [25]. Currently, treatment for HAC is surgical resection followed by adjuvant chemotherapy; however, the efficacy is poor [13, 29]. There are no standard treatment guidelines for HAC and development of a new treatment strategy is required.
Both genetic and epigenetic changes, including DNA methylation and histone modification, play essential roles in initiation and progression of HAC [7]. Post-translational modifications of histones regulate gene expression through methylation, acetylation, phosphorylation, and ubiquitination. Histone acetylation and deacetylation, which affect gene expression through changes in chromatin structure, are regulated by histone acetyltransferase and histone deacetylase (HDAC) enzymes [12]. Histone acetyltransferases promote transcriptional activity through addition of acetyl groups to lysine residues of histones [10], whereas HDAC removes acetyl groups from lysine, resulting in chromatin condensation and transcriptional repression [26]. Overexpression of HDACs is found in various cancers, and inhibition of HDAC activity is a promising strategy for cancer therapy [6, 38]. Thus, HDAC inhibitors are emerging as a new class of anticancer drugs [10]. These inhibitors increase acetylation of histones, leading to an open chromatin structure that allows access of DNA-targeted agents such as cisplatin, and also induce cell cycle arrest, apoptosis, and reactivation of epigenetically silenced tumor suppressor genes [11, 18, 28]. The HDAC inhibitor, suberoylanilide hydroxamic acid (SAHA), was recently used to treat patients with relapsed cutaneous T-cell lymphoma, and has synergistic effects when combined with chemotherapeutic drugs, such as cisplatin [1, 24]. Epigenetic changes such as altered histone acetylation occur in AFP-producing cancers, which suggests that HAC could be treated with SAHA [36]. However, the effects of SAHA in HAC are largely unknown.
In this study, we investigated the effects of a combination of cisplatin and SAHA in VAT-39 cells, a newly established HAC cell line [33]. Cell viability and apoptosis were examined by MTT assay, flow cytometry, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Expression of Bax, Bcl-2, cleaved caspase-3 and AFP were examined by immunohistochemistry and western blotting. The study revealed that cisplatin and SAHA have a synergistic anticancer effect involving induction of apoptosis and may be an effective treatment combination for HAC.
II. Materials and Methods
Cell culture
VAT-39 human HAC cells from the ampulla of Vater [33] were maintained in Dulbecco’s Modified Eagle’s Medium with L-glutamine and phenol red (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan). The medium was supplemented with 10% (v/v) heat-inactivated fetal bovine serum and penicillin-streptomycin-amphotericin B in a humidified atmosphere containing 5% CO2 at 37°C.
Cell viability assay
Cell viability was determined by MTT assay. VAT-39 cells were plated at a density of 3 × 103 cells/well in a 96-well plate for 48 hr at 37°C. The cells were then treated with cisplatin (2 or 5 μM) (Sigma-Aldrich, St. Louis, MO, USA) or SAHA (1 or 2 μM) (TCI, Tokyo, Japan) alone or with a combination of cisplatin and SAHA for 48 hr. MTT solution (5 mg/ml) (Nacalai Tesque Inc., Kyoto, Japan) was added to each well, and then the cells were incubated at 37°C for 2 hr. After removal of the MTT reagent, dimethyl sulfoxide was used to dissolve the formazan crystals. The resulting intracellular purple formazan was quantified with a spectrophotometer at an absorbance of 562 nm using an Immuno Mini NJ-2300 microplate reader (Nalge Nunc Int. Co. Ltd., Tokyo, Japan).
Immunohistochemistry
Immunohistochemistry was performed as reported previously [2, 5]. Cells were seeded on coverslips in 12-well plates at a density of 1 × 105 cells/well. After incubation with 5 μM cisplatin and 2 μM SAHA for 48 hr, the cells were washed with PBS, fixed with 4% paraformaldehyde in PBS for 15 min, and permeabilized with 0.2% Triton X-100 in PBS for 10 min at room temperature. Cellular endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol for 30 min, and nonspecific binding sites were blocked by incubation with 500 μg/ml normal goat IgG in 1% BSA/PBS for 60 min. Cells were incubated with primary antibody for 2 hr and washed in 0.075% Brij/PBS. For H3S10 (#9708, 1:100 diluted, Cell Signaling Technology, Danvers, MA, USA) staining, cell nuclei were counterstained with DAPI (Thermo Fisher Scientific, Waltham, MA, USA) [4]. For cleaved caspase-3 (#9664, 1:100 diluted, Cell Signaling Technology) and AFP (N1501, 1:20 diluted, Dako, Glostrup, Denmark), cells were incubated with HRP-conjugated goat anti-rabbit IgG (Dako) for 1 hr and washed with 0.075% Brij/PBS. After visualization with 3-amino-9-ethylcarbazole (Nichirei, Tokyo, Japan), cell nuclei were counterstained with hematoxylin [35]. As a negative control, normal rabbit IgG (Dako) was used at the same concentration instead of the primary antibodies in each experiment. Images were captured with an Olympus microscope (BX53, Tokyo, Japan).
Western blotting
Total cell lysates were prepared using hot sodium dodecyl sulfate (SDS) buffer containing 0.9% SDS, 15 mM EDTA, 8 mM unlabeled methionine, and a protease inhibitor cocktail. Lysates were boiled for 10 min, cooled, diluted in 0.3% SDS, adjusted to contain 33 mM Tris/acetate, pH 8.5, and 1.7% Triton X-100, and sonicated using a Bioruptor (Cosmo Bio Inc., Tokyo, Japan) [8]. Lysates were centrifuged at 15,000 rpm for 20 min at 4°C. The protein concentration was determined using a Pierce BCA protein assay kit (Thermo Fisher Scientific). Equal amounts of protein were mixed with loading buffer (0.2 M Tris-HCl, pH 8.0, 0.5 M sucrose, 5 mM EDTA, 0.01% bromophenol blue, 10% 2-mercaptoethanol, and 2.5% SDS), boiled for 5 min, separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% nonfat skim milk in Tris-buffered saline with 0.1% Tween 20 (TBST; 20 mM Tris buffer, pH 7.6, and 150 mM NaCl) for 1 hr at room temperature and then incubated overnight with primary antibody against Bcl-2 (N-19, 1:500), Bax (B-9, 1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), H3K9ac, H3K14ac, H3K18ac, H3K27ac (1:1,000), cleaved caspase-3 (1:500, Cell Signaling Technology), or β-actin (AC-15, 1:400,000 diluted, Sigma-Aldrich). The membranes were washed with TBST and incubated with HRP-conjugated goat anti-rabbit IgG (1:5,000) or HRP-conjugated goat anti-mouse IgG (1:5,000, Dako) at room temperature for 1 hr. Protein bands were detected using EZ west Lumi plus (Atto, Tokyo, Japan), and bands were measured using Image Quant TL software (GE Healthcare, Buckinghamshire, UK).
Flow cytometry
Cells were incubated with annexin V-FITC (Nacalai Tesque) and propidium iodide (PI) at room temperature for 15 min [22]. Apoptotic cells were analyzed by flow cytometry using a FACSCalibur (BD Biosciences, San Jose, CA, USA).
TUNEL assay
Apoptotic cells were detected with the a Mebstain apoptosis TUNEL kit direct (8445; Medical and Biological Laboratories, Nagoya, Japan). Briefly, cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 in PBS. The TUNEL reaction was performed for 1 hr at 37°C. Nuclei were counterstained with DAPI. Images were captured with a Zeiss LSM700 microscope (Zeiss, Jena, Germany).
Measurement of AFP concentration in culture medium
Culture medium was harvested at 48 hr, and the AFP concentration was measured with an Architect AFP assay using an Architecto i200SR (Abbott, Chiba, Japan) [33].
Quantitative analysis
For quantitative analysis of histone H3S10 and cleaved caspase-3, at least 2,000 cells were counted in random fields. Results are shown as the percentage of positive cells per total number of counted cells.
Statistical analysis
All analyses were performed with SPSS ver. 20 (SPSS Inc., Chicago, IL, USA). Data are shown as mean ± standard deviation (SD) from three independent experiments. Differences between groups were assessed by Student t-test. P < 0.05 was considered to be statistically significant.
III. Results
Cisplatin in combination with SAHA strongly inhibits cell proliferation in VAT-39 cells
Cell viability was examined by MTT assay to evaluate the antiproliferative effects of cisplatin and SAHA. Both drugs significantly decreased VAT-39 cell viability in a dose-dependent manner. Importantly, cisplatin in combination with SAHA decreased cell viability more efficiently than either treatment alone. Combinations of 2 μM cisplatin and 1 μM SAHA (Fig. 1A) and 5 μM cisplatin and 2 μM SAHA (Fig. 1B) decreased cell viability by 21.0 ± 6.5% and 43.9 ± 4.0%, respectively. Phosphorylated H3S10, a marker of cell mitosis, was also significantly decreased following combined treatment with cisplatin and SAHA compared to either treatment alone (Fig. 1C, D). These results indicate that cisplatin and SAHA have a synergistic effect in inhibiting proliferation of VAT-39 cells.
Fig. 1.
Effects of cisplatin and SAHA on VAT-39 cell proliferation. Cells were treated with (A) 2 μM cisplatin and 1 μM SAHA and (B) 5 μM cisplatin and 2 μM SAHA. After 48 h of treatment, cell viability was analyzed by MTT assay. (C) Immunohistochemical localization of H3S10 phosphorylation in cisplatin (5 μM) and SAHA (2 μM)-treated VAT-39 cells. Arrows indicate mitotic cells in the control group. (D) The number of H3S10-positive cells is shown in the bar graph. *P < 0.05, ***P < 0.001. Data are shown as the mean ± SD of three independent experiments. Bar = 50 μm.
SAHA increases histone H3 acetylation in VAT-39 cells
Transcriptional activation of genes is associated with acetylation of histone H3K9, H3K14, H3K18 and H3K27 [21, 39]. Therefore, the effects of cisplatin and SAHA on acetylation of histone H3 in VAT-39 cells were evaluated by western blotting. SAHA increased acetylation of H3K9, H3K14, H3K18, and H3K27 dose-dependently, but cisplatin had no such effects (Fig. 2A, B). These results show that a low concentration of SAHA (1–2 μM) was sufficient to induce histone H3 hyperacetylation. Based on these results, the combination dose of 5 μM cisplatin and 2 μM SAHA was used for further experiments.
Fig. 2.
Effects of cisplatin and SAHA on acetylation of histone H3 in VAT-39 cells. Western blot analysis of H3K9ac, H3K14ac, H3K18ac, and H3K27ac in VAT-39 cells treated with (A) 2 μM cisplatin and 1 μM SAHA and (B) 5 μM cisplatin and 2 μM SAHA. Isolated proteins (10 μg) were subjected to SDS-PAGE. Bands corresponding to H3K9ac (17 kDa), H3K14ac (17 kDa), H3K18ac (17 kDa), H3K27 (17 kDa), and β-actin (42 kDa) are shown. Data were obtained in three independent experiments.
Cisplatin and SAHA synergistically increase apoptotic cell death in VAT-39 cells
To analyze cell death, flow cytometry was performed to detect apoptotic and necrotic cells (Fig. 3A). Compared to control cells, the number of apoptotic cells was 2.2 times higher in cisplatin-treated cells, and 3.3 times higher in cells treated with cisplatin and SAHA in combination. There were no differences in the number of necrotic cells in all groups (Fig. 3B). Immunohistochemistry showed significantly increased cleaved caspase-3 expression in cisplatin and SAHA-treated cells (Fig. 4A), with a 12 times increase in cleaved caspase-3-positive cells compared to that in control cells (Fig. 4B). Western blotting confirmed these findings, including an increased cleaved caspase-3 level in cisplatin and SAHA-treated cells (Fig. 4C). Apoptosis was confirmed in a TUNEL assay (Fig. 4D). The number of TUNEL-positive cells was increased by cisplatin or SAHA alone compared to controls, but there was a marked increase in the number of TUNEL-positive cells in combination treatment with cisplatin and SAHA. These findings suggest that cisplatin and SAHA synergistically induce apoptosis in VAT-39 cells.
Fig. 3.
Effects of cisplatin and SAHA on cell death of VAT-39 cells. (A) Apoptosis and necrosis were analyzed by flow cytometry using annexin V-FITC and propidium iodide (PI). (B) Data were obtained from three independent experiments and results are shown in the bar graph. The annexin V-positive/PI-negative population are early apoptotic cells, and the annexin V-positive/PI-positive population are late apoptotic and/or necrotic cells. *P < 0.05. Data are shown as the mean ± SD.
Fig. 4.
Effect of cisplatin and SAHA on induction of apoptotic cell death in VAT-39 cells. (A) Immunohistochemical localization of cleaved caspase-3 in 5 μM cisplatin and 2 μM SAHA-treated VAT-39 cells. (B) Counts of cleaved caspase-3-positive cells are shown in the bar graph. (C) Western blot analysis detected double bands of cleaved caspase-3 (17 kDa and 19 kDa). Isolated proteins (20 μg) were subjected to SDS-PAGE. β-actin (42 kDa) was used as a loading control. (D) Cell death was examined by TUNEL assay using the Mebstain apoptosis TUNEL kit. Arrows indicate TUNEL-positive cells among cisplatin and SAHA-treated cells. ***P < 0.001. Data are shown as the mean ± SD of three independent experiments. Bar = 50 μm.
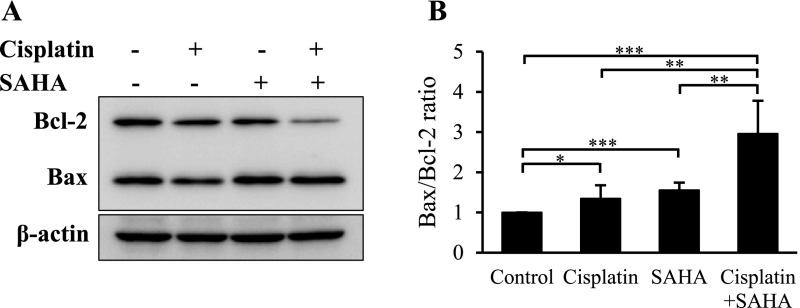
Cisplatin in combination with SAHA decreases Bcl-2 expression in VAT-39 cells
To investigate the mechanism of the induced apoptotic pathway, Bax and Bcl-2 expression was examined in cisplatin and SAHA-treated VAT-39 cells. A significant decrease in Bcl-2 expression was found in cells treated with the combination of cisplatin and SAHA (Fig. 5A), whereas Bax expression was unchanged in all groups. Densitometry showed that the Bax/Bcl-2 ratio was increased by 3 times in cells treated with cisplatin and SAHA in combination compared to control cells (Fig. 5B). These findings show that cisplatin and SAHA induce apoptosis through the mitochondrial pathway.
Fig. 5.
Bcl-2 and Bax expression in VAT-39 cells. (A) Western blot analysis of Bcl-2 and Bax in 5 μM cisplatin- and 2 μM SAHA-treated VAT-39 cells. Isolated proteins (10 μg) were subjected to SDS-PAGE. Rabbit anti-Bcl-2 and mouse anti-Bax antibodies were incubated with the same membrane. (B) Protein bands were analyzed with densitometry, and the result is shown as the Bax/Bcl-2 ratio. *P < 0.05, **P < 0.01, ***P < 0.001. Data are shown as the mean ± SD of three independent experiments.
Cisplatin in combination with SAHA inhibits AFP production in VAT-39 cells
AFP production is the main characteristic of HAC cells. Therefore, AFP expression was examined in VAT-39 cells after cisplatin and SAHA treatment. In control cells, strong expression of AFP was observed in the perinuclear area, which indicates AFP production (Fig. 6A). Decreased AFP expression was seen in cells treated with cisplatin alone or SAHA alone, and most VAT-39 cells lost AFP production with combined treatment with cisplatin and SAHA. The amount of secreted AFP was measured in supernatants of culture media (Fig. 6B). The AFP concentration decreased 3.3 times after combined treatment with cisplatin and SAHA. Culture medium without cells had an AFP level of 0 ng/ml (data not shown). Since the decrease in AFP production could be due to the decrease in cell number after cisplatin and SAHA treatment, western blotting analysis was performed to determine the relative amounts of AFP production in the groups (Fig. 6C). Densitometry revealed a significant decrease in AFP expression in cisplatin and SAHA-treated cells (Fig. 6D). Collectively, these results indicate that VAT-39 cells have partial loss of AFP production after combined treatment with cisplatin and SAHA.
Fig. 6.
Effects of cisplatin and SAHA on AFP expression in VAT-39 cells. (A) Immunohistochemical localization of AFP in 5 μM cisplatin and 2 μM SAHA-treated VAT-39 cells. (B) AFP concentration in culture medium. (C) Western blot analysis of AFP. (D) Densitometry analysis of AFP shown in a bar graph. Protein expression was normalized to β-actin (42 kDa). *P < 0.05, **P < 0.01, ***P < 0.001. Data are shown as the mean ± SD of four independent experiments. Bar = 20 μm.
IV. Discussion
In this study, we found a synergistic anticancer effect of apoptosis induction in combined treatment with cisplatin and SAHA in HAC cells. AFP production, a major characteristic of HAC, was markedly decreased after the combination treatment. These results indicate that cisplatin in combination with SAHA may be effective for treatment of HAC, an aggressive cancer with a poor prognosis. Cisplatin combined with SAHA decreased VAT-39 cell viability more strongly than each drug alone, and this synergistic antiproliferative effect occurred in a dose-dependent manner.
SAHA is a pan-HDAC inhibitor that may open the chromatin structure and increase accessibility of cisplatin to DNA in cancer cells [32]. Hyper- and hypoacetylation of histones are generally correlated with gene transcription that regulates many cellular processes. Acetylation at histone H3K9, H3K14, H3K18 and H3K27 is specifically associated with transcriptional activation of genes involved in apoptosis [23, 37]. Our western blotting results revealed that SAHA induces these hyperacetylation events, and this may support induction of apoptosis by cisplatin. The well-characterized molecular mechanism of cisplatin action involves crosslinking of purine bases on the DNA duplex, leading to DNA damage, disruption of DNA repair mechanisms, and subsequent induction of apoptosis of cancer cells [11]. Synergistic effects were observed at low doses of both cisplatin (5 μM) and SAHA (2 μM). Cisplatin is effective at a high dose, but patients experience side effects that include drug resistance, allergic reactions, and functional alteration of the gastrointestinal and urinary tracts [3]. Therefore, our findings may be helpful for development of efficient anticancer therapy with minimal side effects.
Apoptotic cells, but not necrotic cells, significantly increased after combined treatment with cisplatin and SAHA. Similarly, other studies of cisplatin in combination with SAHA showed increased apoptosis in larynx cancer cells and osteosarcoma cells [14, 17]. Disruption of the balance between anti-apoptotic Bcl-2 and pro-apoptotic Bax leads to apoptosis [30], and therefore, the Bax/Bcl-2 ratio is an important indicator of apoptosis [16, 27]. In our findings, this ratio increased 3 times in VAT-39 cells treated with cisplatin and SAHA compared to control cells, suggesting that this treatment affects the mitochondrial apoptosis pathway in VAT-39 cells.
Expression and production of AFP in VAT-39 cells were significantly decreased after combined treatment with cisplatin and SAHA. AFP is a well-known marker of cancer with hepatoid differentiation, neovascularization and high proliferative activity [9]. A high metastasis rate, aggressive phenotype, and poor prognosis are also related to AFP production in cancer cells [15]. A recent study of 22 patients with HAC found that only 3 had a good prognosis, 8 responded partially, and 11 did not respond to cisplatin-based chemotherapy [29]. Thus, addition of SAHA to this chemotherapy may be effective.
The current study has the limitation of use of a single HAC cell line, but promising anticancer effects were obtained by combined treatment with cisplatin and SAHA. These drugs act synergistically to induce apoptosis of HAC cells, and the characteristic AFP production of these cells was markedly decreased after the combination treatment.
V. Conflicts of Interest
The authors declare that there are no conflicts of interest.
VI. Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 16K08471 to Y. Hishikawa).
VII. References
- 1.Al-Yacoub N., Fecker L. F., Möbs M., Plötz M., Braun F. K., Sterry W. and Eberle J. (2012) Apoptosis induction by SAHA in cutaneous T-cell lymphoma cells is related to downregulation of c-FLIP and enhanced TRAIL signaling. J. Invest. Dermatol. 132; 2263–2274. [DOI] [PubMed] [Google Scholar]
- 2.Ali M. N., Choijookhuu N., Takagi H., Srisowanna N., Nguyen Nhat Huynh M., Yamaguchi Y., Synn Oo, P., Tin H. K. M., Sato K., Yamaguchi R. and Hishikawa Y. (2018) The HDAC inhibitor, SAHA, prevents colonic inflammation by suppressing pro-inflammatory cytokines and chemokines in DSS-induced colitis. Acta Histochem. Cytochem. 51; 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astolfi L., Ghiselli S., Guaran V., Chicca M., Simoni E., Olivetto E., Lelli G. and Martini A. (2013) Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: a retrospective evaluation. Oncol. Rep. 29; 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batchuluun K., Azuma M., Fujiwara K., Yashiro T. and Kikuchi M. (2017) Notch signaling and maintenance of sox2 expression in rat anterior pituitary cells. Acta Histochem. Cytochem. 50; 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batmunkh B., Choijookhuu N., Srisowanna N., Byambatsogt U., Synn O. P., Noor A. M., Yamaguchi Y. and Hishikawa Y. (2017) Estrogen accelerates cell proliferation through estrogen receptor α during rat liver regeneration after partial hepatectomy. Acta Histochem. Cytochem. 50; 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceccacci E. and Minucci S. (2016) Inhibition of histone deacetylases in cancer therapy: lessons from leukaemia. Br. J. Cancer 114; 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chervona Y. and Costa M. (2012) Histone modifications and cancer: biomarkers of prognosis? Am. J. Cancer Res. 2; 589–597. [PMC free article] [PubMed] [Google Scholar]
- 8.Choijookhuu N., Hino S., Oo P. S., Batmunkh B., Mohmand N. A., Kyaw M. T. and Hishikawa Y. (2015) Ontogenetic changes in the expression of estrogen receptor β in mouse duodenal epithelium. Clin. Res. Hepatol. Gastroenterol. 39; 499–507. [DOI] [PubMed] [Google Scholar]
- 9.Chun H. and Kwon S. J. (2011) Clinicopathological characteristics of alpha-fetoprotein-producing gastric cancer. J. Gastric Cancer 11; 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damaskos C., Garmpis N., Valsami S., Kontos M., Spartalis E., Kalampokas T., Kalampokas E., Athanasiou A., Moris D., Daskalopoulou A., Davakis S., Tsourouflis G., Kontzoglou K., Perrea D., Nikiteas N. and Dimitroulis D. (2017) Histone deacetylase inhibitors: an attractive therapeutic strategy against breast cancer. Anticancer Res. 37; 35–46. [DOI] [PubMed] [Google Scholar]
- 11.Dasari S. and Tchounwou P. B. (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 740; 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer N., Sechet E., Friedman R., Amiot A., Sobhani I., Nigro G., Sansonetti P. J. and Sperandio B. (2016) Histone deacetylase inhibition enhances antimicrobial peptide but not inflammatory cytokine expression upon bacterial challenge. Proc. Natl. Acad. Sci. U S A 113; E2993–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavrancic T. and Park Y. H. (2015) A novel approach using sorafenib in alpha fetoprotein-producing hepatoid adenocarcinoma of the lung. J. Natl. Compr. Canc. Netw. 13; 387–391. [DOI] [PubMed] [Google Scholar]
- 14.Grabarska A., Łuszczki J. J., Nowosadzka E., Gumbarewicz E., Jeleniewicz W., Dmoszyńska-Graniczka M., Kowalczuk K., Kupisz K., Polberg K. and Stepulak A. (2017) Histone deacetylase inhibitor SAHA as potential targeted therapy agent for larynx cancer cells. J. Cancer 8; 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman K., Beasley M. B. and Braman S. S. (2016) Hepatoid adenocarcinoma of the lung: Review of a rare form of lung cancer. Respir. Med. 119; 175–179. [DOI] [PubMed] [Google Scholar]
- 16.Hishikawa Y., Tamaru N., Ejima K., Hayashi T. and Koji T. (2004) Expression of keratinocyte growth factor and its receptor in human breast cancer: its inhibitory role in the induction of apoptosis possibly through the overexpression of Bcl-2. Arch. Histol. Cytol. 67; 455–464. [DOI] [PubMed] [Google Scholar]
- 17.Hou M., Huang Z., Chen S., Wang H., Feng T., Yan S., Su Y. and Zuo G. (2018) Synergistic antitumor effect of suberoylanilide hydroxamic acid and cisplatin in osteosarcoma cells. Oncol. Lett. 16; 4663–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insinga A., Monestiroli S., Ronzoni S., Gelmetti V., Marchesi F., Viale A., Altucci L., Nervi C., Minucci S. and Pelicci P. G. (2005) Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat. Med. 11; 71–76. [DOI] [PubMed] [Google Scholar]
- 19.Ishikura H., Kanda M., Ito M., Nosaka K. and Mizuno K. (1990) Hepatoid adenocarcinoma: a distinctive histological subtype of alpha-fetoprotein-producing lung carcinoma. Virchows Arch. A Pathol. Anat. Histopathol. 417; 73–80. [DOI] [PubMed] [Google Scholar]
- 20.Koide N., Nishio A., Igarashi J., Kajikawa S., Adachi W. and Amano J. (1999) Alpha-fetoprotein-producing gastric cancer: histochemical analysis of cell proliferation, apoptosis, and angiogenesis. Am. J. Gastroenterol. 94; 1658–1663. [DOI] [PubMed] [Google Scholar]
- 21.Kurdistani S. K., Tavazoie S. and Grunstein M. (2004) Mapping global histone acetylation patterns to gene expression. Cell 117; 721–733. [DOI] [PubMed] [Google Scholar]
- 22.Kwan Y. P., Saito T., Ibrahim D., Al-Hassan F. M., Ein Oon C., Chen Y., Jothy S. L., Kanwar J. R. and Sasidharan S. (2015) Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 54; 1223–1236. [DOI] [PubMed] [Google Scholar]
- 23.Minton K. (2016) Reading protein acetylation. Nat. Rev. Mol. Cell Biol. 17; 676–677. [DOI] [PubMed] [Google Scholar]
- 24.Ong P. S., Wang X. Q., Lin H. S., Chan S. Y. and Ho P. C. (2012) Synergistic effects of suberoylanilide hydroxamic acid combined with cisplatin causing cell cycle arrest independent apoptosis in platinum-resistant ovarian cancer cells. Int. J. Oncol. 40; 1705–1713. [DOI] [PubMed] [Google Scholar]
- 25.Qu B. G., Bi W. M., Qu B. T., Qu T., Han X. H., Wang H., Liu Y. X. and Jia Y. G. (2016) PRISMA-compliant article: clinical characteristics and factors influencing prognosis of patients with hepatoid adenocarcinoma of the stomach in China. Medicine (Baltimore) 95; e3399. doi: 10.1097/MD.0000000000003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richon V. M. (2006) Cancer biology: mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel histone deacetylase inhibitor. Br. J. Cancer 95; S2–S6. [Google Scholar]
- 27.Salakou S., Kardamakis D., Tsamandas A. C., Zolota V., Apostolakis E., Tzelepi V., Papathanasopoulos P., Bonikos D. S., Papapetropoulos T., Petsas T. and Dougenis D. (2007) Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo 21; 123–132. [PubMed] [Google Scholar]
- 28.Schrump D. S. (2009) Cytotoxicity mediated by histone deacetylase inhibitors in cancer cells: mechanisms and potential clinical implications. Clin. Cancer Res. 15; 3947–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simmet V., Noblecourt M., Lizée T., Morvant B., Girault S., Soulié P. and Capitain O. (2018) Chemotherapy of metastatic hepatoid adenocarcinoma: Literature review and two case reports with cisplatin etoposide. Oncol. Lett. 15; 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh L., Pushker N., Saini N., Sen S., Sharma A., Bakhshi S., Chawla B. and Kashyap S. (2014) Expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins in human retinoblastoma. Clin. Exp. Ophthalmol. 43; 259–267. [DOI] [PubMed] [Google Scholar]
- 31.Søreide J. A., Greve O. J., Gudlaugsson E. and Størset S. (2016) Hepatoid adenocarcinoma of the stomach—proper identification and treatment remain a challenge. Scand. J. Gastroenterol. 51; 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suraweera A., O’Byrne K. J. and Richard D. J. (2018) Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front. Oncol. 8; 92. doi: 10.3389/fonc.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi N., Aoyama F., Hiyoshi M., Kataoka H. and Sawaguchi A. (2014) Establishment and biological characterization of a novel cell line derived from hepatoid adenocarcinoma originated at the ampulla of Vater. Int. J. Oncol. 44; 1139–1145. [DOI] [PubMed] [Google Scholar]
- 34.Terracciano L. M., Glatz K., Mhawech P., Vasei M., Lehmann F. S., Vecchione R. and Tornillo L. (2003) Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am. J. Surg. Pathol. 27; 1302–1312. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchiya K., Ikeda T., Batmunkh B., Choijookhuu N., Ishizaki H., Hotokezaka M., Hishikawa Y. and Nanashima A. (2017) Frequency of CD4+CD161+ T cell and interleukin-10 expression in inflammatory bowel diseases. Acta Histochem. Cytochem. 50; 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamura N. and Kishimoto T. (2012) Epigenetic regulation of GATA4 expression by histone modification in AFP-producing gastric adenocarcinoma. Exp. Mol. Pathol. 93; 35–39. [DOI] [PubMed] [Google Scholar]
- 37.Yan X., Pan B., Lv T., Liu L., Zhu J., Shen W., Huang X. and Tian J. (2017) Inhibition of histone acetylation by curcumin reduces alcohol-induced fetal cardiac apoptosis. J. Biomed. Sci. 24; 1. doi: 10.1186/s12929-016-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H., Salz T., Zajac-Kaye M., Liao D., Huang S. and Qiu Y. (2014) Overexpression of histone deacetylases in cancer cells is controlled by interplay of transcription factors and epigenetic modulators. FASEB J. 28; 4265–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yerra V. G. and Advani A. (2018) Histones and heart failure in diabetes. Cell. Mol. Life Sci. doi: 10.1007/s00018-018-2857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]