Abstract
Allometric scaling laws relate physiologic parameters to body weight. Genetically modified mice allow investigation of allometric scaling laws when fundamental cardiovascular components are altered. Elastin haploinsufficient (Eln+/−) mice have reduced elastin amounts, and fibulin-5 knockout (Fbln5−/−) mice have compromised elastic fiber integrity in the large arteries which may alter cardiovascular scaling laws. Previously published echocardiography data used to investigate aortic and left ventricular function in Eln+/− and Fbln5−/− mice throughout postnatal development and early adulthood were reanalyzed to determine cardiovascular scaling laws. Aortic diameter, heart weight, stroke volume, and cardiac output have scaling exponents within 1–32% of the predicted theoretical range, indicating that the scaling laws apply to maturing mice. For aortic diameter, Eln+/− and Eln+/+ mice have similar scaling exponents, but different scaling constants, suggesting a shift in starting diameter, but no changes in aortic growth with body weight. In contrast, the scaling exponent for aortic diameter in Fbln5−/− mice is lower than Fbln5+/+ mice, but the scaling constant is similar, suggesting that aortic growth with body weight is compromised in Fbln5−/− mice. For both Eln+/− and Fbln5−/− groups, the scaling constant for heart weight is increased compared to the respective control group, suggesting an increase in starting heart weight, but no change in the increase with body weight during maturation. The scaling exponents and constants for stroke volume and cardiac output are not significantly affected by reduced elastin amounts or compromised elastic fiber integrity in the large arteries, highlighting a robust cardiac adaptation despite arterial defects.
Keywords: elastin, fibulin-5, cardiovascular, allometric scaling, mice
Introduction
Allometric scaling laws describe the dependence of a biological variable on body mass. The scaling laws are generally of the form: , where Y is the biological variable of interest, Y0 is a scaling constant that depends on the organism, M is the body mass, and b is the scaling exponent. West et al. developed a quantitative model for allometric scaling in the cardiovascular system based on a space-filling fractal-like branching pattern, a size-invariant unit for the final branch in the circulatory system, and minimization of the energy required to distribute resources [1]. The predicted and previously observed exponents of the scaling laws for the aortic diameter, heart weight, stroke volume, and cardiac output are shown in Table 1. Numerous studies support the cardiovascular scaling laws and scaling exponent values for a variety of mammalian species [2–6].
Table 1.
Allometric scaling exponents for cardiovascular parameters
| Scaling exponent | ||||||
|---|---|---|---|---|---|---|
| Variable | Predicted | Previously observed | Eln+/+ | Eln+/− | Fbln5+/+ | Fbln5−/− |
| Aortic diameter | 0.375 | 0.360 [5] | 0.380–0.423 (N = 65) |
0.374–0.429 (N = 57) |
0.400–0.479
(N = 46) |
0.295–0.370a (N = 42) |
| Heart weight | 1.00 | 1.02 [2], 0.98 [6] | 0.936–1.08 (N = 61) |
0.860–1.02 (N = 57) |
1.04–1.28 (N = 39) |
0.924–1.10 (N = 43) |
| Stroke volume | 1.00 | 1.05 [4] | 0.875–1.09 (N = 51) |
0.858–1.08 (N = 47) |
1.01–1.26 (N = 37) |
0.924–1.10 (N = 37) |
| Cardiac output | 0.75 | 0.79 [2], 0.79 [4], 0.81 [6] | 0.734–0.943 (N = 50) |
0.770–1.04 (N = 47) |
0.858–1.12 (N = 37) |
0.835–1.10 (N = 37) |
Predicted exponents for allometric scaling laws of the form for cardiovascular variables are from West et al. [1]. References for previously observed values are provided individually. 95% CI intervals for the fitted scaling exponents from this study are presented. The number of mice used for each group and measurement are shown.
Indicates significant difference between Fbln5+/+ and Fbln5−/− scaling exponents for ASID.
Genetically modified mice allow investigation of the applicability of allometric scaling laws when fundamental cardiovascular components are altered. We previously showed that Murray's Law, which uses energy minimization principles to predict diameters of branching vessels [7], is maintained in small, surface arteries and veins of mice haploinsufficient for the elastin gene (Eln+/−) [8]. Elastin is a major extracellular matrix component of blood vessels that provides reversible elasticity during the cardiac cycle [9]. Elastin amounts decrease with vessel size [10], so small, surface arteries and veins may be affected differently by reductions in elastin expression compared to large elastic arteries, such as the aorta. The ascending aorta of Eln+/− mice is smaller than wild-type (WT) mice [11], prompting the hypothesis that cardiovascular scaling laws for the aorta may not be maintained in Eln+/− mice. Another genetically modified mouse model with normal elastin amounts, but compromised elastic fiber integrity due to the loss of fibulin-5 expression (Fbln5−/−), also has a smaller ascending aorta [12], suggesting that defects in the amount or integrity of elastic fibers may affect aortic scaling laws.
Genetic mutations in elastin and fibulin-5 are associated with supravalvular aortic stenosis [13] and cutis laxa [14], respectively, in humans. Humans and mice with reduced amounts of elastin or fibulin-5 have increased arterial stiffness, in addition to smaller arteries [11,15–19]. Increased arterial stiffness is linked to an increased risk of adverse cardiovascular events [20]. Arterial stiffening increases cardiac afterload and may compromise cardiac function. Hence, a lifetime of increased arterial stiffness due to a genetic mutation in elastin or fibulin-5 may affect scaling laws for cardiac parameters such as heart weight, stroke volume, and cardiac output. To investigate how reduced amount or integrity of elastic fibers affects allometric scaling laws in the cardiovascular system, previously published echocardiography data for Eln+/− [21] and Fbln5−/− [16] mice during postnatal development and early adulthood were reanalyzed as a function of body weight throughout maturation. Fitted scaling exponents and constants for cardiovascular parameters were compared to theoretical predictions and to the values obtained from the respective WT control mice.
Materials and Methods
Details of the mice and echocardiography protocols are provided in previous publications [16,21–23]. Briefly, male and female Eln+/−, Eln+/+, Fbln5−/−, and Fbln5+/+ mice at ages 7, 21, and 60 days were examined using a Vevo 770 High Resolution Imaging System (Visualsonics, Toronto, Canada) [16,21]. A 708 probe (max frequency = 82.5 MHz, axial resolution = 30 μm, lateral resolution = 70 μm) was used for 7-day-old mice and a 707B probe (max frequency = 45 MHz, axial resolution = 55 μm, lateral resolution = 115 μm) was used for 21- and 60-day-old mice. Mice were weighed, anesthetized with 1.5% isoflurane, and secured to an imaging platform. Chest hair was removed with a chemical depilatory cream. Body temperature was maintained with a heat lamp (7 days old) or with a feedback controlled heating pad (21 and 60 days old). Mice were examined using standard echocardiography protocols to determine aortic and left ventricular morphology and function from early postnatal development to young adulthood. For this study, previously published values [16,21] for the ascending aorta inner diameter at systole (ASID), heart weight (HW), stroke volume (SV), and cardiac output (CO) were plotted against body weight (BW) on a log-log scale to investigate allometric scaling relationships. Linear regression analyses were performed in graphpad prism 6 software to determine the slope and intercept of the fitted lines (equal to the scaling exponent and scaling constant in the allometric scaling law, respectively), the 95% confidence intervals (CIs), and significant differences between groups (P < 0.05). The total number of mice included in each group for each measurement is given in Table 1. As the measurements were either not significantly affected by sex or not affected by sex when scaled by body weight, males and females were combined as one group. Details of the number of males and females in each group can be found in the original publications [16,21].
Results and Discussion
Log-log graphs and linear fits for each cardiovascular parameter versus BW are shown in Fig. 1 for Eln+/− compared to Eln+/+ and Fbln5−/− compared to Fbln5+/+. The 95% CIs for the fitted scaling exponents are shown in Table 1. The 95% CIs in Table 1 encompass the predicted and previously observed values for the scaling exponents for 50% of the total measurement groups, and the mean scaling exponents in Fig. 1 are within 1–32% of the predicted values. The results indicate that allometric scaling laws that have been derived on theoretical principles [1] and experimentally determined for various mammalian species [2–5] are applicable to maturing mice. Differences between our experimental and predicted or previously observed scaling constants may be due to the smaller range of body weight for maturing mice compared to that for different species [24], variations in the scaling exponent for inter-species versus intra-species comparisons [25], differences in experimental conditions including the choice of anesthesia [2,4–6], and limitations in measurement resolution of the ultrasound probes.
Fig. 1.
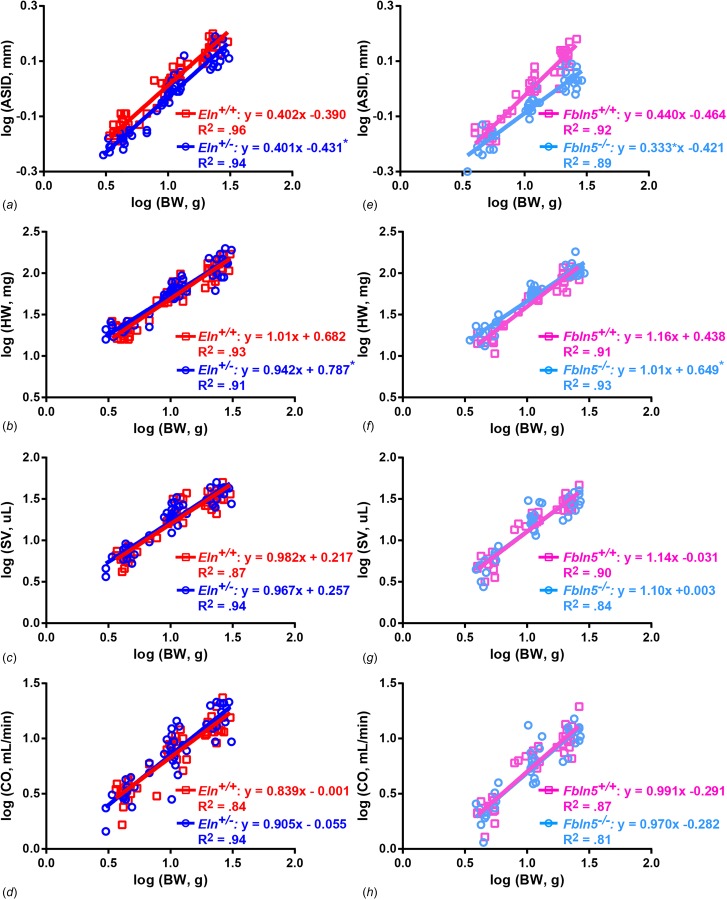
Experimental scaling laws for cardiovascular parameters in maturing mice. Log-log plots and linear regression analyses of cardiovascular parameters versus BW for Eln+/− compared to Eln+/+ (left column) and Fbln5−/− compared to Fbln5+/+ (right column) mice aged 7, 21, and 60 days. ASID (a, b), HW (c, d), SV (e, f), and CO (g, h) are shown. Best fit linear regression equation and R2 are given for each group. * = P < 0.05 for Eln+/− or Fbln5−/− compared to their respective WT control for slope or intercept. Original data are from Le et al. [16,21].
Reduced elastin amounts in Eln+/− mice and compromised elastic fiber integrity in Fbln5−/− mice affect the wall structure and mechanical behavior of the large arteries [11,12,15,17,26]. Our previous work showed that ASID in Eln+/− and Fbln5−/− mice is smaller than the respective WT controls throughout postnatal development and young adulthood [16,21]. Our current analyses demonstrate that the alterations in ASID occur through different mechanisms in the allometric scaling relationship for each genetic model. For Eln+/− mice, the ASID scaling exponent (slope) is similar to Eln+/+ mice, suggesting a similar change in aortic size with increasing body weight, but the scaling constant (y-intercept) is 10% smaller (P < 0.001), suggesting a different starting point due to reduced amounts of elastin available to build the aortic wall (Fig. 1(a)). For Fbln5−/− mice, the ASID scaling exponent is 25% smaller than Fbln5+/+ mice (P = 0.001), suggesting a different change in aortic size with increasing body weight, but the scaling constant is similar, suggesting a common starting point (Fig. 1(b)). It may be that the physiological consequences of compromised elastic fiber integrity do not become critical until later in maturation when the hemodynamic forces (including blood pressure and flow) reach a threshold value [16,27], necessitating alterations in aortic growth or remodeling. ASID is measured at systolic pressure, so lower systolic blood pressures may cause a smaller ASID. However, there are no differences between Fbln5−/− and Fbln5+/+ systolic blood pressure at each age [16] and there is a 6–10% increase in systolic blood pressure for Eln+/− mice compared to Eln+/+ at 21 and 60 days of age [15], indicating that the ASID differences are not due to reduced systolic blood pressure.
There are no significant differences in the scaling exponent for HW, but the scaling constant for Eln+/− compared to Eln+/+ mice is 15% larger (P = 0.04) (Fig. 1(c)) and for Fbln5−/− compared to Fbln5+/+ mice is 48% larger (P = 0.02) (Fig. 1(d)) compared to the respective WT controls indicating a shift in the organism specific starting point for HW, but no changes in the expected increase with body weight when the heart must pump blood out of a smaller, stiffer aorta. For the cardiac function parameters, SV and CO have similar scaling exponents and constants for Eln+/− and Fbln5−/− mice compared to the respective WT controls (Figs. 1(e)–1(h)), demonstrating that the left ventricle is able to adapt and maintain normal systolic function despite genetic alterations in elastin amount or integrity, a smaller aortic outlet, and increased aortic stiffness [11,15–17]. The cardiac results are consistent with our previous work that showed few significant differences in SV or CO, but a trend or significant increase in HW between groups at each age [16,21].
Aortic stiffness depends on the wall material properties, geometry (diameter and wall thickness), and physiologic loading (blood pressure). Although the ultrasound resolution is insufficient to measure physiologic wall thickness in mouse aorta (expected to be ∼35 μm [28]), we have measured the unloaded aortic wall thickness in previous work. There are no differences in unloaded wall thickness between Fbln5−/− and Fbln5+/+ aorta at each age [12]. There is a 10% decrease in unloaded wall thickness for Eln+/− aorta compared to Eln+/+ at 60 days of age only [15]. As discussed previously, systolic blood pressure is unaffected by the loss of fibulin-5 [16] and is increased by elastin haploinsufficiency at 21 and 60 days of age [15]. Coordinated changes in wall material properties, geometry, and loading conditions, as well as the definition of arterial stiffness being applied [29] will alter the associated stiffness comparison. For a variety of stiffness definitions, measured in vitro and in vivo, our group [11,12,15,16,21] and others [17,26] have shown a consistent increase in arterial stiffness due to the loss of fibulin-5 or haploinsufficiency of elastin. Despite the increase in arterial stiffness and resulting increased work load on the left ventricle, SV and CO are unchanged in Eln+/− and Fbln5−/− mice compared to the respective WT controls throughout maturation, demonstrating a robust cardiac adaptation.
Clinical cardiovascular measurements are often scaled by body size to aid in diagnostic and therapeutic decision making [30]. Mice are increasingly being used to investigate cardiovascular disease mechanisms, hence understanding how cardiovascular measurements in mice scale with body size is critical to interpreting observed differences (or the absence of differences) in cardiovascular function. Allometric scaling analyses can identify factors behind observed differences in cardiovascular measurements, such as altered starting point (scaling constant) versus altered growth or remodeling (scaling exponent) that may provide insight into disease mechanisms and suggest interventional strategies.
Conclusions
Allometric scaling laws for cardiovascular parameters can be determined theoretically [1] or experimentally and are consistent over a wide range of mammalian species [2–6]. Although the scaling laws are generally applied across species, they can also be applied to maturation within a species, such as from children to adults [24]. This study shows that scaling laws for aortic diameter, heart weight, stroke volume, and cardiac output in maturing mice are consistent with theoretical predictions and previously published values. The diameter scaling law for the ascending aorta is altered through reduction of the scaling constant in Eln+/− mice and of the scaling exponent in Fbln5−/− mice, indicating a smaller starting point for aortic size when less elastin is available and a reduced growth rate or remodeling of aortic size when elastic fiber integrity is compromised. The scaling constant for heart weight is increased in Eln+/− and Fbln5−/− mice, indicating a higher starting point for heart weight when elastin amount or integrity is compromised in the large arteries. Allometric scaling laws for stroke volume and cardiac output are not altered in Eln+/− and Fbln5−/− mice, demonstrating that cardiac function is maintained during maturation despite changes in aortic size and stiffness due to elastic fiber defects.
Funding Data
National Heart, Lung, and Blood Institute (HL-105314; Funder ID: 10.13039/100000050).
References
- [1]. West, G. B. , Brown, J. H. , and Enquist, B. J. , 1997, “ A General Model for the Origin of Allometric Scaling Laws in Biology,” Science, 276(5309), pp. 122–126. 10.1126/science.276.5309.122 [DOI] [PubMed] [Google Scholar]
- [2]. Lindstedt, S. L. , and Schaeffer, P. J. , 2002, “ Use of Allometry in Predicting Anatomical and Physiological Parameters of Mammals,” Lab. Animals., 36(1), pp. 1–19. 10.1258/0023677021911731 [DOI] [PubMed] [Google Scholar]
- [3]. Adolph, E. F. , 1949, “ Quantitative Relations in the Physiological Constitutions of Mammals,” Science, 109(2841), pp. 579–585. 10.1126/science.109.2841.579 [DOI] [PubMed] [Google Scholar]
- [4]. Holt, J. P. , Rhode, E. A. , and Kines, H. , 1968, “ Ventricular Volumes and Body Weight in Mammals,” Am. J. Physiol., 215(3), pp. 704–715. 10.1152/ajplegacy.1968.215.3.704 [DOI] [PubMed] [Google Scholar]
- [5]. Holt, J. P. , Rhode, E. A. , Holt, W. W. , and Kines, H. , 1981, “ Geometric Similarity of Aorta, Venae Cavae, and Certain of Their Branches in Mammals,” Am. J. Physiol., 241(1), pp. R100–R104. 10.1152/ajpregu.1981.241.1.R100 [DOI] [PubMed] [Google Scholar]
- [6]. Stahl, W. R. , 1967, “ Scaling of Respiratory Variables in Mammals,” J. Appl. Physiol., 22(3), pp. 453–460. 10.1152/jappl.1967.22.3.453 [DOI] [PubMed] [Google Scholar]
- [7]. Murray, C. D. , 1926, “ The Physiological Principle of Minimum Work—I: The Vascular System and the Cost of Blood Volume,” Proc. Natl. Acad. Sci. U. S. A., 12(3), pp. 207–214. 10.1073/pnas.12.3.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Sather, B. A. , Hageman, D. , and Wagenseil, J. E. , 2012, “ Murray's Law Holds True in Elastin Haploinsufficient (Eln+/-) and Wild-Type (WT) Mice,” ASME J. Biomech. Eng., 134(12), p. 124504. 10.1115/1.4023093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Wagenseil, J. E. , and Mecham, R. P. , 2009, “ Vascular Extracellular Matrix and Arterial Mechanics,” Physiol. Rev., 89(3), pp. 957–989. 10.1152/physrev.00041.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Dinardo, C. L. , Venturini, G. , Zhou, E. H. , Watanabe, I. S. , Campos, L. C. , Dariolli, R. , da Motta-Leal-Filho, J. M. , Carvalho, V. M. , Cardozo, K. H. , Krieger, J. E. , Alencar, A. M. , and Pereira, A. C. , 2014, “ Variation of Mechanical Properties and Quantitative Proteomics of VSMC Along the Arterial Tree,” Am. J. Physiol. Heart Circ. Physiol., 306(4), pp. H505–H516. 10.1152/ajpheart.00655.2013 [DOI] [PubMed] [Google Scholar]
- [11]. Wagenseil, J. E. , Nerurkar, N. L. , Knutsen, R. H. , Okamoto, R. J. , Li, D. Y. , and Mecham, R. P. , 2005, “ Effects of Elastin Haploinsufficiency on the Mechanical Behavior of Mouse Arteries,” Am. J. Physiol. Heart Circ. Physiol., 289(3), pp. H1209–H1217. 10.1152/ajpheart.00046.2005 [DOI] [PubMed] [Google Scholar]
- [12]. Le, V. P. , Cheng, J. K. , Kim, J. , Staiculescu, M. C. , Ficker, S. W. , Sheth, S. C. , Bhayani, S. A. , Mecham, R. P. , Yanagisawa, H. , and Wagenseil, J. E. , 2015, “ Mechanical Factors Direct Mouse Aortic Remodelling During Early Maturation,” J. R. Soc. Interface., 12(104), p. 20141350. 10.1098/rsif.2014.1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Li, D. Y. , Toland, A. E. , Boak, B. B. , Atkinson, D. L. , Ensing, G. J. , Morris, C. A. , and Keating, M. T. , 1997, “ Elastin Point Mutations Cause an Obstructive Vascular Disease, Supravalvular Aortic Stenosis,” Hum. Mol. Genet., 6(7), pp. 1021–1028. 10.1093/hmg/6.7.1021 [DOI] [PubMed] [Google Scholar]
- [14]. Loeys, B. , Van Maldergem, L. , Mortier, G. , Coucke, P. , Gerniers, S. , Naeyaert, J. M. , and De Paepe, A. , 2002, “ Homozygosity for a Missense Mutation in Fibulin-5 (FBLN5) Results in a Severe Form of Cutis Laxa,” Hum. Mol. Genet., 11(18), pp. 2113–2118. 10.1093/hmg/11.18.2113 [DOI] [PubMed] [Google Scholar]
- [15]. Le, V. P. , Knutsen, R. H. , Mecham, R. P. , and Wagenseil, J. E. , 2011, “ Decreased Aortic Diameter and Compliance Precedes Blood Pressure Increases in Postnatal Development of Elastin-Insufficient Mice,” Am. J. Physiol. Heart Circ. Physiol., 301(1), pp. H221–H229. 10.1152/ajpheart.00119.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Le, V. P. , Stoka, K. V. , Yanagisawa, H. , and Wagenseil, J. E. , 2014, “ Fibulin‐5 Null Mice With Decreased Arterial Compliance Maintain Normal Systolic Left Ventricular Function, But Not Diastolic Function During Maturation,” Physiol. Rep., 2(3), p. e00257. 10.1002/phy2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Wan, W. , Yanagisawa, H. , and Gleason, R. L., Jr. , 2010, “ Biomechanical and Microstructural Properties of Common Carotid Arteries From Fibulin-5 Null Mice,” Ann. Biomed. Eng., 38(12), pp. 3605–3617. 10.1007/s10439-010-0114-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Kozel, B. A. , Danback, J. R. , Waxler, J. L. , Knutsen, R. H. , de Las Fuentes, L. , Reusz, G. S. , Kis, E. , Bhatt, A. B. , and Pober, B. R. , 2014, “ Williams Syndrome Predisposes to Vascular Stiffness Modified by Antihypertensive Use and Copy Number Changes in NCF1,” Hypertension, 63(1), pp. 74–79. 10.1161/HYPERTENSIONAHA.113.02087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Papke, C. L. , and Yanagisawa, H. , 2014, “ Fibulin-4 and Fibulin-5 in Elastogenesis and Beyond: Insights From Mouse and Human Studies,” Matrix Biol., 37, pp. 142–149. 10.1016/j.matbio.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Mitchell, G. F. , Hwang, S. J. , Vasan, R. S. , Larson, M. G. , Pencina, M. J. , Hamburg, N. M. , Vita, J. A. , Levy, D. , and Benjamin, E. J. , 2010, “ Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study,” Circulation, 121(4), pp. 505–511. 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Le, V. P. , and Wagenseil, J. E. , 2012, “ Echocardiographic Characterization of Postnatal Development in Mice With Reduced Arterial Elasticity,” Cardiovasc. Eng. Technol., 3(4), pp. 424–438. 10.1007/s13239-012-0108-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Li, D. Y. , Faury, G. , Taylor, D. G. , Davis, E. C. , Boyle, W. A. , Mecham, R. P. , Stenzel, P. , Boak, B. , and Keating, M. T. , 1998, “ Novel Arterial Pathology in Mice and Humans Hemizygous for Elastin,” J. Clin. Invest., 102(10), pp. 1783–1787. 10.1172/JCI4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Yanagisawa, H. , Davis, E. C. , Starcher, B. C. , Ouchi, T. , Yanagisawa, M. , Richardson, J. A. , and Olson, E. N. , 2002, “ Fibulin-5 Is an Elastin-Binding Protein Essential for Elastic Fibre Development In Vivo,” Nature, 415(6868), pp. 168–171. 10.1038/415168a [DOI] [PubMed] [Google Scholar]
- [24]. Dawson, T. , 2014, “ Allometric Relations and Scaling Laws for the Cardiovascular System of Mammals,” System, 2(2), pp. 168–185. 10.3390/systems2020168 [DOI] [Google Scholar]
- [25]. Feldman, H. A. , and McMahon, T. A. , 1983, “ The 3/4 Mass Exponent for Energy Metabolism Is Not a Statistical Artifact,” Respir. Physiol., 52(2), pp. 149–163. 10.1016/0034-5687(83)90002-6 [DOI] [PubMed] [Google Scholar]
- [26]. Faury, G. , Pezet, M. , Knutsen, R. H. , Boyle, W. A. , Heximer, S. P. , McLean, S. E. , Minkes, R. K. , Blumer, K. J. , Kovacs, A. , Kelly, D. P. , Li, D. Y. , Starcher, B. , and Mecham, R. P. , 2003, “ Developmental Adaptation of the Mouse Cardiovascular System to Elastin Haploinsufficiency,” J. Clin. Invest., 112(9), pp. 1419–1428. 10.1172/JCI19028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Wagenseil, J. E. , Ciliberto, C. H. , Knutsen, R. H. , Levy, M. A. , Kovacs, A. , and Mecham, R. P. , 2010, “ The Importance of Elastin to Aortic Development in Mice,” Am. J. Physiol. Heart Circ. Physiol., 299(2), pp. H257–H264. 10.1152/ajpheart.00194.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Bellini, C. , Bersi, M. R. , Caulk, A. W. , Ferruzzi, J. , Milewicz, D. M. , Ramirez, F. , Rifkin, D. B. , Tellides, G. , Yanagisawa, H. , and Humphrey, J. D. , 2017, “ Comparison of 10 Murine Models Reveals a Distinct Biomechanical Phenotype in Thoracic Aortic Aneurysms,” J. R. Soc. Interface, 14(130), p. 20161036. 10.1098/rsif.2016.1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. O'Rourke, M. F. , Staessen, J. A. , Vlachopoulos, C. , Duprez, D. , and Plante, G. E. , 2002, “ Clinical Applications of Arterial Stiffness; Definitions and Reference Values,” Am. J. Hypertens., 15(5), pp. 426–444. 10.1016/S0895-7061(01)02319-6 [DOI] [PubMed] [Google Scholar]
- [30]. Dewey, F. E. , Rosenthal, D. , Murphy, D. J., Jr. , Froelicher, V. F. , and Ashley, E. A. , 2008, “ Does Size Matter? Clinical Applications of Scaling Cardiac Size and Function for Body Size,” Circulation, 117(17), pp. 2279–2287. 10.1161/CIRCULATIONAHA.107.736785 [DOI] [PubMed] [Google Scholar]


