Abstract
Blunt thoracic aortic injuries are rare occurrences but carry an increased risk of mortality. Over the last two decades, however, major advances in diagnostic imaging, staging, and treatment have significantly improved outcomes. Modern imaging paved the way for a new staging system based on the anatomical layers of the aortic wall. This staging system, in turn, allowed for refinement of treatment, which now includes nonoperative management with anti-impulse therapy, endovascular intervention, and, if needed, open surgical repair. As is the case with any other rapidly evolving therapy, however, new challenges and controversies arise. The resolution of these challenges will rely on a broad, international, and multidisciplinary effort. (This is a review article based on the invited lecture of the 46th Annual Meeting of Japanese Society for Vascular Surgery.)
Keywords: aorta, trauma, surgery, endovascular, TEVAR
Introduction
Blunt thoracic aortic injury (BTAI) is a rare but lethal entity. Although the overall incidence is <1%, these injuries are the second leading cause of death in blunt trauma.1,2) Motor vehicle collisions are the most common (>70%) mechanism of injury, followed by motorcycle collisions, automobile–pedestrian collisions, and falls.3,4) Up to 80% of patients die before hospitalization, and those who survive often present with multiple associated injuries, including cardiac lesions, rib fractures, hemothoraces, and intra-abdominal injuries.5,6) Therefore, successful management of BTAI requires a circumspect approach, taking into consideration pathophysiology, diagnostic modalities, and contemporary therapeutic strategies. Given the recent shifts in therapeutic paradigms, an international, multidisciplinary effort is needed to establish evidence-based consensus guidelines.
Pathophysiology
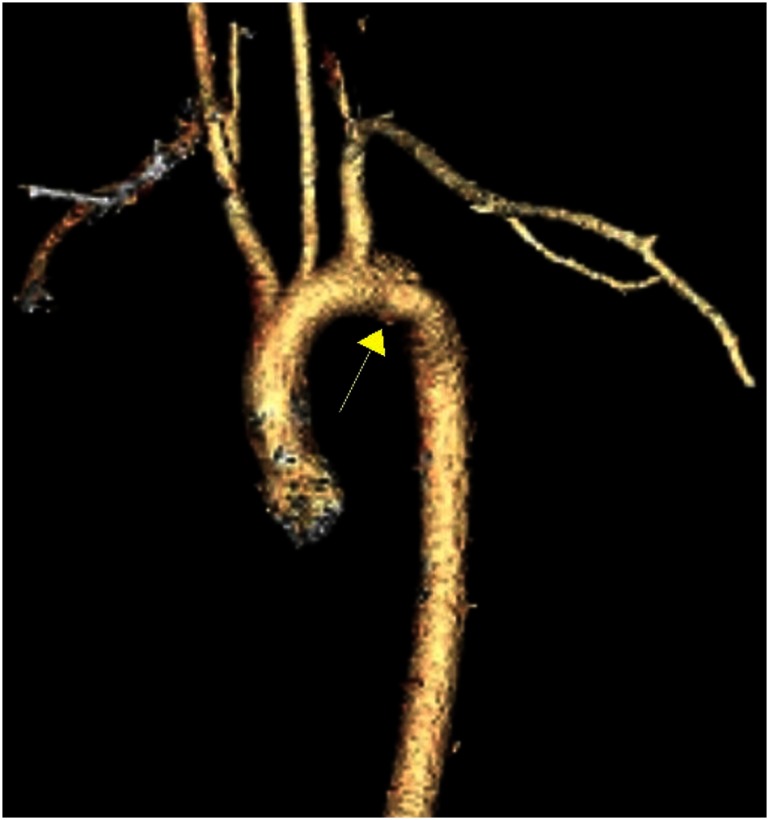
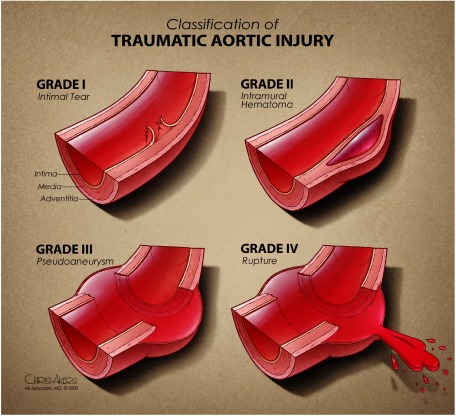
The most common mechanism of BTAI involves motor vehicle collisions (MVCs). Although varied hypotheses have been proposed, implicating shear, torsion, pinch, stretch, and hydrostatic forces, the reality likely involves a combination of these insults.7) Over 60% of blunt aortic injuries occur at the aortic isthmus, where the relatively fixed descending aorta meets the more mobile aortic arch (Fig. 1).5) Therefore, this junction bears considerable strain on sudden deceleration. Other segments of the aorta, however, may also be involved. These include ascending aorta (8–27%), aortic arch (8–18%), and distal descending aorta (11–21%), as well as abdominal aorta.5,6,8–11) Another important anatomical consideration is the multilayered aortic wall. BTAI represents a spectrum of lesions that is based on the anatomical layers involved: intimal tear (grade I), intramural hematoma (grade II), pseudoaneurysm (grade III), and rupture (grade IV) (Fig. 2).12) Understanding the pathophysiology facilitates diagnosis, staging, and treatment of aortic injuries.
Fig. 1 Pseudoaneurysm at the aortic isthmus.
Fig. 2 Classification of traumatic aortic injury. Reprinted from Reference 12 with permission from Azizzadeh A, et al.
Diagnosis
The diagnosis of BTAI starts with a thorough history and physical examination, and the initial evaluation conforms to Advanced Trauma Life Support guidelines. The history should focus on details of the mechanism and symptoms. The majority of injuries are related to MVCs; therefore, details regarding impact (e.g., head-on, side, or rear), seatbelt use, airbag deployment, ejection from the vehicle, steering wheel deformity, extent of vehicle damage, and extrication effort are important. If the mechanism is related to a fall, then the height of the fall is informative. Finally, eliciting information about possible associated injuries is critical. Patients may present in shock or with normal hemodynamics. Similarly, patients may report chest pain radiating to the back or remain asymptomatic. Important physical examination findings include distended neck veins, absent or muffled heart sounds, tracheal deviation, subcutaneous emphysema, chest wall instability or ecchymoses, abnormal breath sounds, and diminished peripheral pulses.
Imaging plays a central role in the diagnosis of BTAI. The initial imaging modality is a chest radiograph. Suggestive radiographic findings include a widened/abnormal mediastinum (may be seen in up to 93% of patients with traumatic aortic injuries), left pleural effusion, first and second rib fractures, tracheal deviation, a depressed left bronchus, an indistinct aortic knob, or apical capping.13,14) With reported sensitivities as low as 41%, however, a normal chest radiograph does not exclude BTAI.15) If there is clinical suspicion for BTAI, a computed tomographic angiogram (CTA) of the chest is necessary.
Although for nearly four decades aortography/angiography was considered the gold standard for diagnosis of blunt aortic injury, CTA is considered the diagnostic test of choice in the modern era.6) Reported sensitivities of CTA range from 95% to 100%, with negative predictive values ranging from 99% to 100%; however, specificities can be as low as 40%, with a positive predictive value of only 15%.16–18) Important false positive findings include an aortic spindle (fusiform dilation immediately distal to the isthmus), a ductus diverticulum, infundibula of the arch arteries, and an infundibulum of the right third intercostal artery (also known as the right intercostal–bronchial artery).19) Thus, CTA is a helpful tool for ruling out blunt aortic injuries but has some limitations. If CTA findings are equivocal, intravascular ultrasound (IVUS) can be a helpful adjunct.20) Finally, angiography is a potential diagnostic modality but, with the advent of CTA, has been relegated from a screening to a mainly therapeutic role. In addition to faster and more accurate diagnosis, advances in modern imaging also provided a more detailed analysis of aortic lesions and thus paved the way for improved staging and treatment.
Management
The management strategies for BTAI have undergone dramatic changes over the last two decades. These changes coincided with the development of a new BTAI staging system and the use of thoracic endovascular aortic repair (TEVAR) in trauma. Historically, the American Association for the Surgery of Trauma (AAST) classified thoracic vascular injuries based on the type of artery and the extent of arterial circumference involved.21) According to the AAST classification, all descending thoracic aortic lesions were classified as grade IV. This classification, however, failed to recognize the heterogeneity of injuries in the thoracic aorta. An improved grading system, which took into account the natural history of different types of lesions, was introduced in 2009.12) This grading system is based on anatomical layers of the aortic wall—intimal tear (grade I), intramural hematoma (grade II), pseudoaneurysm (grade III), rupture (grade IV)—and directly influences the management of blunt aortic injuries.
Medical Therapy
The treatment of BTAI starts with adequate blood pressure control. Depending on the grade of the injury, this intervention serves as either a definitive or a temporizing measure. On the basis of the Society for Vascular Surgery (SVS) clinical practice guidelines, expectant management with effective blood pressure control is sufficient for grade I lesions, as the majority of these lesions heal spontaneously.22) The primary goal of blood pressure control is to prevent progression of the lesion by reducing aortic wall stress. The risk of rupture has been shown to decrease from 12% to 1.5% with effective anti-impulse therapy.23) Although the optimal hemodynamic parameters are not well established, some studies suggest a goal systolic blood pressure of ≤100 mmHg, a mean arterial pressure of ≤80 mmHg, and a heart rate of ≤100 beats per minute.24) This is typically achieved with an intravenous beta-blocker (e.g., esmolol, labetalol) and can be supplemented with a vasodilator, if needed.
The goals of blood pressure therapy, however, need to be addressed within the context of associated injuries. In some instances, aggressive blood pressure control can be detrimental. Patients with concurrent traumatic brain or spinal cord injuries, for example, may require elevated blood pressures to maintain adequate tissue perfusion. This competing therapeutic goal may preclude nonoperative management of BTAI. Thus, treatment of aortic injuries in a polytrauma patient requires a comprehensive and multidisciplinary approach.
Medical management with anti-impulse therapy is the initial and, for some patients, definitive intervention. For grade II–IV lesions, however, the SVS clinical practice guidelines recommend urgent TEVAR.
Endovascular Intervention
TEVAR has emerged as the dominant therapy for BTAI. In the largest, multicenter BTAI analysis, 76.4% of patients were treated with TEVAR.25) Although the SVS clinical practice guidelines recommend urgent (<24 h) repair, some studies suggest that delayed therapy is well tolerated and may lead to improved outcomes. An analysis of patients undergoing early (<24 h) and delayed (>24 h) repair showed a significantly lower mortality rate in the delayed group compared to the early group (5.8% versus 16.5%).26) The delayed approach allows for the management of associated injuries and patient optimization before aortic intervention, which may account for improved outcomes. (Of note, grade IV lesions are not amenable to delayed therapy and require emergent intervention.)
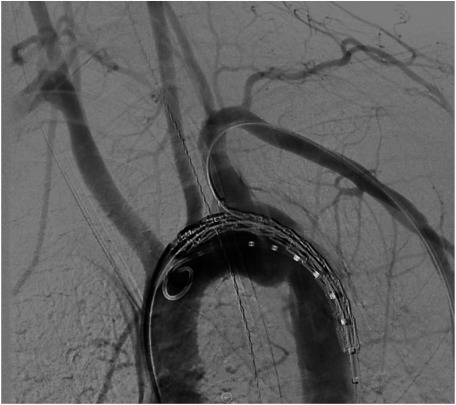
Once the timing of intervention is established, the patient is taken to the operating room for TEVAR. Typically, the procedure is performed in a hybrid operating room under general anesthesia. The abdomen and bilateral groins are prepped, and femoral access is gained via open or percutaneous techniques. Arch aortography is routinely performed (Fig. 3), along with evaluation of cerebrovascular anatomy, which is especially important if left subclavian artery coverage is anticipated. IVUS is performed selectively. The patient is anticoagulated using standard weight-based doses of heparin, but smaller doses are used in patients with severe injuries and contraindications. In most cases, a single 10 cm device provides adequate coverage, and care is taken to limit the graft size so as to minimize the risk of spinal ischemia. Up to 40% of patients may require left subclavian artery (LSA) coverage to obtain an adequate proximal landing zone. Intentional LSA coverage appears to be safe, however, without compromising functional outcomes.27) Finally, postdeployment balloon angioplasty is selectively performed to enhance proximal apposition or treat proximal type I endoleaks. At the conclusion of the procedure, heparin is reversed with protamine.
Fig. 3 Arch aortography, showing a grade III injury at the aortic isthmus.
Surgical Intervention
Endovascular intervention is not always feasible. A surgical repair is required if endovascular capabilities are unavailable or if a patient’s anatomy is unsuitable for TEVAR. Key technical considerations in an open repair include access to the thoracic cavity, vascular control, perfusion strategies, and spinal protection.
Access to the thoracic cavity is typically obtained through a left posterolateral thoracotomy in the fourth intercostal space, which provides optimal exposure around the aortic isthmus. Proximal vascular control is obtained by applying a clamp between the left common carotid and subclavian arteries; distal control is obtained by applying a clamp beyond the level of the lesion. Although a “clamp-and-sew” technique is an option, a perfusion strategy is generally used to minimize the risk of paraplegia. Distal aortic perfusion can be achieved using a left heart bypass, which provides pump inflow from the left atrium via the left inferior pulmonary vein and pump outflow via cannulation of the distal thoracic aorta. Alternatively, full cardiopulmonary bypass via femoral cannulation can be used.28) Although contemporary outcomes of surgical repair have improved, the overall and aortic-related mortalities remain relatively high (19.7% and 13.1%, respectively).25) Citing lower risks of death and spinal cord ischemia, the SVS clinical practice guidelines recommend TEVAR over open repair for all age groups with suitable anatomy.22)
Controversies in Management
The management of BTAI has evolved significantly over the last two decades, with major improvements in mortality and morbidity. As is the case with any other rapidly evolving therapy, however, new challenges and controversies arise. Recent studies have shown, for example, that grade II lesions can be managed nonoperatively, which conflicts with current SVS consensus guidelines.29) Other points of contention include long-term outcomes, device durability, natural history of disease, and optimal timing of intervention with consideration for associated injuries. In addition, alternative treatment algorithms have been proposed based on varying institutional experiences. Despite these lingering questions, however, the improvement in outcomes with recent advances is undeniable.
Outcomes
The largest multicenter analysis of BTAI was conducted by the Aortic Trauma Foundation (ATF) between 2008 and 2013.25) TEVAR was used in 76.4% of the 382 BTAI patients. The majority (50.3%) of injuries were grade III lesions, followed by grade I (24.6%), grade II (17.8%), and grade IV (7.3%) lesions. The overall in-hospital mortality was 18.8%, and the aortic-related mortality was 6.5%. On multivariate analysis, the use of TEVAR was the only protective variable against aortic-related mortality. Similar benefits were observed in the National Inpatient Sample. Ultee et al. analyzed 8,384 patients with traumatic thoracic aortic injuries between 2005 and 2011.30) The vast majority (60.2%) of patients were managed nonoperatively, whereas 29.7% and 10.1% underwent TEVAR and open repairs, respectively. The rates of TEVAR, however, increased dramatically from 6.5% of all interventions in 2005 to 86.5% of all interventions in 2011. Mortality in patients admitted with traumatic thoracic aortic injuries declined from 24.5% to 13.3% over the same study period. These studies demonstrate the increased prevalence of TEVAR as the primary intervention for BTAI and also highlight the continued improvement in outcomes.
Conclusion
BTAIs are rare occurrences but carry an increased risk of mortality. Over the last few decades, however, advances in imaging, development of a contemporary classification system, and the advent of TEVAR have all decreased the risk of mortality. Ongoing efforts to improve outcomes present new paradigms, questions, and challenges. These questions—the role for nonoperative management of higher grade lesions, long-term outcomes and device durability, natural history of disease, optimal timing of intervention with consideration for associated injuries, and alternate treatment algorithms—require a broad and multidisciplinary effort to resolve. To this end, ATF was established in 2014. With an international, multidisciplinary group of researchers and a nonindustry-driven prospective BTAI registry, ATF seeks to tackle these controversies and to ultimately improve outcomes in aortic trauma.
Disclosure Statement
The authors declare no competing interests.
Author Contributions
Data research: all authors
Discussion of the content: all authors
Editing: all authors
References
- 1).Arthurs ZM, Starnes BW, Sohn VY, et al. Functional and survival outcomes in traumatic blunt thoracic aortic injuries: an analysis of the National Trauma Databank. J Vasc Surg 2009; 49: 988-94. [DOI] [PubMed] [Google Scholar]
- 2).Smith RS, Chang FC. Traumatic rupture of the aorta: still a lethal injury. Am J Surg 1986; 152: 660-3. [DOI] [PubMed] [Google Scholar]
- 3).Fabian TC, Richardson JD, Croce MA, et al. Prospective study of blunt aortic injury: multicenter trial of the American Association for the Surgery of Trauma. J Trauma 1997; 42: 374-80; discussion, 380-3. [DOI] [PubMed] [Google Scholar]
- 4).Estrera AL, Miller CC 3rd, Guajardo-Salinas G, et al. Update on blunt thoracic aortic injury: fifteen-year single-institution experience. J Thorac Cardiovasc Surg 2013; 145 Suppl: S154-8. [DOI] [PubMed] [Google Scholar]
- 5).Teixeira PG, Inaba K, Barmparas G, et al. Blunt thoracic aortic injuries: an autopsy study. J Trauma 2011; 70: 197-202. [DOI] [PubMed] [Google Scholar]
- 6).Neschis DG, Scalea TM, Flinn WR, et al. Blunt aortic injury. N Engl J Med 2008; 359: 1708-16. [DOI] [PubMed] [Google Scholar]
- 7).Richens D, Field M, Neale M, et al. The mechanism of injury in blunt traumatic rupture of the aorta. Eur J Cardiothorac Surg 2002; 21: 288-93. [DOI] [PubMed] [Google Scholar]
- 8).Feczko JD, Lynch L, Pless JE, et al. An autopsy case review of 142 nonpenetrating (blunt) injuries of the aorta. J Trauma 1992; 33: 846-9. [DOI] [PubMed] [Google Scholar]
- 9).Parmley LF, Mattingly TW, Manion WC, et al. Nonpenetrating traumatic injury of the aorta. Circulation 1958; 17: 1086-101. [DOI] [PubMed] [Google Scholar]
- 10).Arajärvi E, Santavirta S, Tolonen J. Aortic ruptures in seat belt wearers. J Thorac Cardiovasc Surg 1989; 98: 355-61. [PubMed] [Google Scholar]
- 11).Burkhart HM, Gomez GA, Jacobson LE, et al. Fatal blunt aortic injuries: a review of 242 autopsy cases. J Trauma 2001; 50: 113-5. [DOI] [PubMed] [Google Scholar]
- 12).Azizzadeh A, Keyhani K, Miller CC 3rd, et al. Blunt traumatic aortic injury: initial experience with endovascular repair. J Vasc Surg 2009; 49: 1403-8. [DOI] [PubMed] [Google Scholar]
- 13).Woodring JH. The normal mediastinum in blunt traumatic rupture of the thoracic aorta and brachiocephalic arteries. J Emerg Med 1990; 8: 467-76. [DOI] [PubMed] [Google Scholar]
- 14).Mirvis SE, Bidwell JK, Buddemeyer EU, et al. Value of chest radiography in excluding traumatic aortic rupture. Radiology 1987; 163: 487-93. [DOI] [PubMed] [Google Scholar]
- 15).Gutierrez A, Inaba K, Siboni S, et al. The utility of chest X-ray as a screening tool for blunt thoracic aortic injury. Injury 2016; 47: 32-6. [DOI] [PubMed] [Google Scholar]
- 16).Gavant ML, Menke PG, Fabian T, et al. Blunt traumatic aortic rupture: detection with helical CT of the chest. Radiology 1995; 197: 125-33. [DOI] [PubMed] [Google Scholar]
- 17).Wicky S, Capasso P, Meuli R, et al. Spiral CT aortography: an efficient technique for the diagnosis of traumatic aortic injury. Eur Radiol 1998; 8: 828-33. [DOI] [PubMed] [Google Scholar]
- 18).Bruckner BA, DiBardino DJ, Cumbie TC, et al. Critical evaluation of chest computed tomography scans for blunt descending thoracic aortic injury. Ann Thorac Surg 2006; 81: 1339-46. [DOI] [PubMed] [Google Scholar]
- 19).Fisher RG, Sanchez-Torres M, Whigham CJ, et al. “Lumps” and “bumps” that mimic acute aortic and brachiocephalic vessel injury. Radiographics 1997; 17: 825-34. [DOI] [PubMed] [Google Scholar]
- 20).Azizzadeh A, Valdes J, Miller CC 3rd, et al. The utility of intravascular ultrasound compared to angiography in the diagnosis of blunt traumatic aortic injury. J Vasc Surg 2011; 53: 608-14. [DOI] [PubMed] [Google Scholar]
- 21).Moore EE, Malangoni MA, Cogbill TH, et al. Organ injury scaling. IV: thoracic vascular, lung, cardiac, and diaphragm. J Trauma 1994; 36: 299-300. [PubMed] [Google Scholar]
- 22).Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg 2011; 53: 187-92. [DOI] [PubMed] [Google Scholar]
- 23).Hemmila MR, Arbabi S, Rowe SA, et al. Delayed repair for blunt thoracic aortic injury: is it really equivalent to early repair? J Trauma 2004; 56: 13-23. [DOI] [PubMed] [Google Scholar]
- 24).Fabian TC, Davis KA, Gavant ML, et al. Prospective study of blunt aortic injury: helical CT is diagnostic and antihypertensive therapy reduces rupture. Ann Surg 1998; 227: 666-76; discussion, 676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).DuBose JJ, Leake SS, Brenner M, et al. Contemporary management and outcomes of blunt thoracic aortic injury: a multicenter retrospective study. J Trauma Acute Care Surg 2015; 78: 360-9. [DOI] [PubMed] [Google Scholar]
- 26).Demetriades D, Velmahos GC, Scalea TM, et al. Blunt traumatic thoracic aortic injuries: early or delayed repair—results of an American Association for the Surgery of Trauma prospective study. J Trauma 2009; 66: 967-73. [DOI] [PubMed] [Google Scholar]
- 27).McBride CL, Dubose JJ, Miller CC 3rd, et al. Intentional left subclavian artery coverage during thoracic endovascular aortic repair for traumatic aortic injury. J Vasc Surg 2015; 61: 73-9.e1. [DOI] [PubMed] [Google Scholar]
- 28).Cronenwett JL, Johnston KW. Rutherford's Vascular Surgery, 8th ed. Philadelphia, PA: Elsevier Saunders, 2014.
- 29).Sandhu HK, Leonard SD, Perlick A, et al. Determinants and outcomes of nonoperative management for blunt traumatic aortic injuries. J Vasc Surg 2018; 67: 389-98. [DOI] [PubMed] [Google Scholar]
- 30).Ultee KH, Soden PA, Chien V, et al. National trends in utilization and outcome of thoracic endovascular aortic repair for traumatic thoracic aortic injuries. J Vasc Surg 2016; 63: 1232-9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]