Abstract
A 69-year-old male patient was admitted to our hospital due to sudden right leg pain and paralysis when walking, which was suspected to be acute limb ischemia. Computed tomography and aortography revealed isolated abdominal aortic dissection with bilateral common iliac artery (CIA) stenosis. Endovascular therapy was performed. A Palmaz XL stent (20×40 mm) was deployed on the aortic dissection with intravascular ultrasound assistance. Subsequently, CIA lesions disappeared. Stent deployment at the aortic dissection primary tear resulted in occlusion of the false lumen of the distal dissection of the bilateral CIAs. Postoperatively, his symptoms disappeared, with no recurrence during 14 months of follow-up.
Keywords: isolated abdominal aortic dissection, acute limb ischemia, endovascular therapy
Introduction
Isolated abdominal aortic dissection (IAAD) is a rare aortic disease in which the dissection is limited to the abdominal aorta without concomitant thoracic dissection.1) IAAD accounts for 1.3% of all aortic dissections, with 87.5% of these being spontaneous dissections.2,3) Most patients present with abdominal pain as the initial symptom, whereas acute limb ischemia (ALI) is relatively rare. Therefore, it is sometimes difficult to diagnose this rare disease based only on limb symptoms like limb pain, and treatment strategies have not been established. However, recently, the effectiveness of endovascular therapy (EVT) has been reported.4,5)
We present a patient with limb ischemia caused by IAAD involving the bilateral iliac artery. We performed successful EVT for the lesions by deployment of a single stent at the abdominal initial flap.
Case Report
A 69-year-old male patient with a history of diabetes mellitus and dyslipidemia was admitted to our hospital with pain, weakness, hypoesthesia, and paralysis in his right leg when walking, but no symptoms were observed for the left leg. These symptoms disappeared after his arrival at our hospital.
There was no pallor or cyanosis on his foot. A score of 5 was obtained in the manual muscle test of both lower extremities. All femoral, popliteal, and dorsalis pedis arteries could be palpated. Blood examination showed elevated lactic acid (7.9 mmol/L) and D-dimer (2.4 µg/mL) levels. Creatine kinase (CK) level was not elevated (82 U/L) at the time of admission.
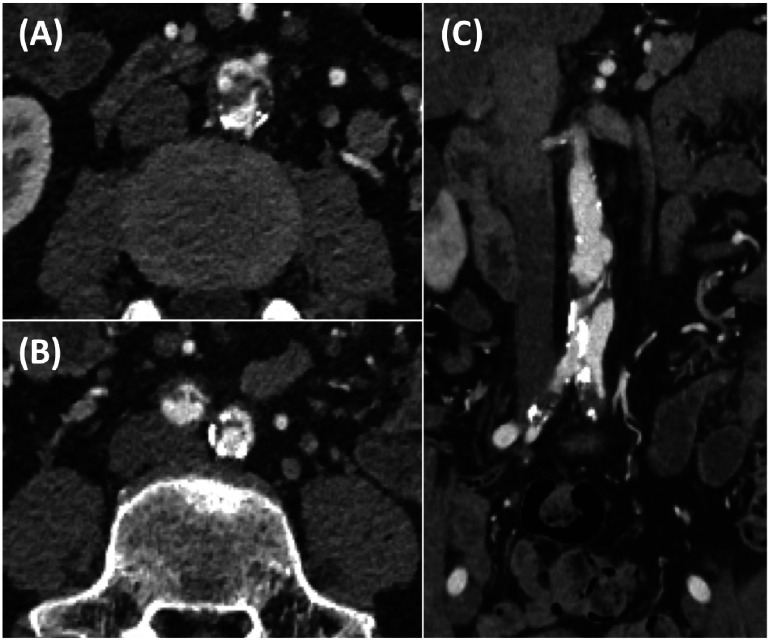
Lumbar magnetic resonance imaging showed no evidence of either spinal canal stenosis or spinal cord infarction. The ankle-brachial pressure index (ABPI) was significantly decreased on the right and left sides (0.50 and 0.59, respectively). Contrast computed tomography (CT) revealed IAAD and bilateral common iliac artery (CIA) stenosis (Fig. 1). The origin of the dissection was located on the infrarenal abdominal aorta without retrograde extension. There was no arterial stenosis below the external iliac artery.
Fig. 1 Preoperative contrast computed tomography of the abdominal aorta.
(A) Abdominal axial view revealed isolated dissection and presence of intimal flap. (B) Bilateral common iliac artery dissection and stenosis were noted in the axial view of the lower abdomen. (C) Abdominal coronal view showed isolated abdominal aortic dissection continuing to the common iliac arteries.
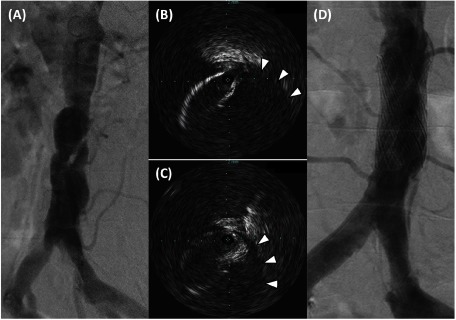
On day 2, blood test showed significantly elevated CK level (4,407 U/L) despite the absence of symptom recurrence and absence of change in physical findings. Because ALI was suspected, urgent catheterization was performed. Coronary angiography showed no significant stenosis in the coronary artery. Aortography revealed an intimal flap in the abdominal aorta and hazy stenotic lesion on the ostium of the right CIA (Fig. 2A). Although there was no obvious stenosis aortographically on the left CIA, a significant pressure gradient (≥15 mmHg) was detected during catheter pullback across the left CIA. We hypothesized that IAAD with bilateral CIA stenosis caused ALI and CK level elevation. After discussion with a cardiovascular surgeon, we concluded that EVT is preferable for this lesion as a revascularization because it is a minimally invasive and immediately proceedable treatment compared to surgery. In case of EVT failure, cardiovascular surgery standby was needed during the procedure. Thus, we performed EVT for those lesions immediately after the examination with on-site cardiovascular surgery.
Fig. 2 The catheterization and endovascular therapy.
(A) Aortography showed dissection flap and stenosis at the infrarenal abdominal aorta. Hazy stenosis was identified at the right common iliac artery. (B) Intravascular ultrasound of the abdominal aorta showed wire crossing through the true lumen, whereas the false lumen was on the opposite side (arrowheads). (C) Similar to the abdominal section, an intimal flap and false lumen (arrowheads) were observed in the intravascular ultrasound image of the right iliac artery. (D) After stent deployment, aortography revealed an enlarged true lumen in the abdominal aorta and disappearance of stenotic lesions in the common iliac artery.
First, we performed stent deployment on the abdominal aorta including the primary tear, and if necessary, additional stenting was planned for CIA lesions. Under local anesthesia, an introducer sheath of appropriate size for stenting (11 Fr) was placed at the right femoral artery. A 0.035-in hydrophilic wire (Radifocus Guidewire®, Terumo, Tokyo, Japan) was advanced through the sheath into the abdominal aorta, taking care to avoid entering the false lumen. Subsequently, intravascular ultrasound (IVUS) confirmed that the wire crossed the true lumen (Figs. 2B and 2C). The vessel diameter and lesion length were evaluated by IVUS for measurement of the appropriate stent size. A balloon-expandable bare metal stent (PALMAZ® XL stent 20×40 mm, Cordis, a Johnson & Johnson company, Miami Lakes, FL, USA) was deployed on the target lesion. After stent deployment, aortography revealed improvement in the aortic lesion and bilateral CIA lesions (Fig. 2D). Occlusion of the inferior mesenteric artery (IMA) was observed, although the patient showed no symptoms, including abdominal pain. IVUS showed absence of malapposition of the stent struts and absence of significant stenosis of the right CIA. Moreover, disappearance of the pressure gradient was confirmed by catheter pullback across the left CIA. The bilateral dorsalis pedis pulses were regular after the treatment.
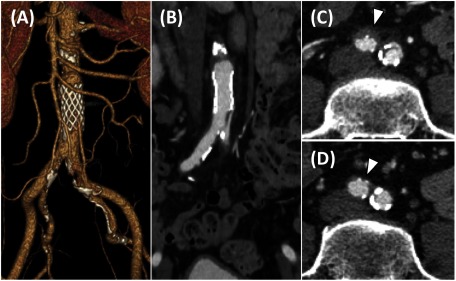
On day 3, contrast CT was performed (Figs. 3A, 3B and 3C). The false lumen of the abdominal aorta at the level of the stent was successfully occluded. Although the ostium of the IMA was obstructed, distal perfusion was preserved. The false lumen at the level of the ostium of the right CIA slightly persisted, whereas the true lumen significantly expanded on both sides of the CIA. The patient’s symptoms were completely resolved. The ABPI was significantly improved on the right (1.25) and left (1.19) sides.
Fig. 3 Postoperative contrast computed tomography scan of the abdominal aorta.
(A) Three-dimensional reconstruction of the abdominal aorta. (B) Abdominal axial view showed no significant stenosis from the abdominal aorta to the iliac artery. (C) False lumen was thrombosed but remained (arrowhead) in the right common iliac artery on the day after the procedure. (D) At the 11-month follow-up, the thrombosed false lumen in the right common iliac artery had disappeared (arrowhead).
On day 4, the patient was discharged. There was no recurrence of symptoms during the 14-month follow-up period. Contrast CT performed at 11 months after the procedure revealed no evidence of restenosis at the abdominal stent or bilateral CIA, whereas the false lumen of the right CIA disappeared (Fig. 3D).
Discussion
We describe a case of IAAD complicated with bilateral iliac artery stenosis. Urgent EVT, in which a single bare metal stent was deployed on the initial flap of the abdominal dissection, resulted in successful clinical outcomes.
Diagnosis for IAAD
IAAD is a relatively rare disease. A previous study reported that IAAD accounted for 1.3% of all aortic dissections, with a mean patient age of 67.7 years.3) Risk factors for IAAD include hypertension and preexisting atherosclerosis. Among the causes of IAAD, 87.5% were spontaneous, 6.25% were traumatic, and 6.25% were iatrogenic.2) As in our study, acute onset is noted in 72% of cases and that extending to CIA is observed in 16.4% of cases. Abdominal pain is the most common presentation (52.8%); however, 9.1% of patients with IAAD present with lower limb ischemia, such as in our study.
Regarding the patient’s symptom, some questions are still unanswered. The reason why ischemic symptoms developed only on his right limb, despite the bilateral ischemic state in our study, and how symptoms disappeared spontaneously upon arrival at our hospital are still unknown. We assume that the right iliac artery was once completely occluded by the dissection at the time of onset, and then reperfusion was achieved spontaneously upon arrival at our hospital, contributing to the improvement of the right limb symptoms.
Standard treatment for IAAD
Treatments for symptomatic IAAD include EVT, surgery, and conservative therapies.2) A review of the available literature revealed that 45%, 26%, and 29% of the patients with IAAD were treated with EVT, surgery, and conservative therapy, respectively.
Only 2% of the patients treated with EVT showed in-hospital mortality, and the causes of mortality were not associated with the procedures. EVT also showed a low complication rate during the follow-up, and the reintervention rate was 7%; most of these cases were successfully treated. These results revealed the superiority of EVT as a lower risk management strategy compared to surgery (5% in-hospital mortality and 6% perioperative complications) or conservative treatments (7% in-hospital mortality). Therefore, EVT may be developed as a standard treatment for IAAD.
Bare metal stent or covered stent
In this case, we selected a bare metal stent, whereas a covered stent was more commonly used for the treatment of IAAD in previous studies.2) However, this trend may be due to the use of covered stents for the treatment of other common aortic dissections.6) Compared to stenting on the aortic arch with curving morphology in which a balloon-expandable stent may be unsuitable for apposition, the abdominal aorta has a remarkably linear form that balloon-expandable bare metal stent deployment would be sufficient on the IAAD without aneurysmal change. In fact, there were some IAAD cases treated by bare metal stents in which no complication has been reported.4)
Regarding infrarenal aortic stenosis, known as an atherosclerotic disease, Monastiriotis et al. reported that 17 patients treated with bare metal stent showed 100% technical success rate and 100% primary assisted patency rate on a follow-up of 4.2 years.7) Similarly, Grimme et al. reported that patients with infrarenal aortic stenosis treated with covered stents showed a 100% technical success rate and 100% primary patency rate at the 2-year follow-up; however, in one case, stent graft infection developed, and surgery was required.8) Additionally, bare metal stent deployment seemed to show greater side branch patency compared to covered stents.9) Giribono et al. suggested that the use of bare metal stents should be considered as the first choice for IAAD if there is no aneurysm.4)
Treatment for symptomatic iliac artery stenosis
In cases of IAAD extending to the iliac artery, a bifurcated stent graft was preferred in previous reports.4) It was deployed for abdominal aortic aneurysms involving the iliac artery. Carroccio et al. reported that 3.7% of those graft limbs were occluded in a 4-year follow-up.10) The strongest risk factor of graft occlusion is smaller vessel diameter. In the case of aortic dissection, because the arterial diameter would be smaller compared to those of aortic aneurysms, the risk of graft occlusion could be higher. Wang et al. reported that among 25 patients with IAAD treated with a bifurcated stent graft, 2 patients (8%) showed stent graft occlusion at 6 and 11 months of follow-up,5) which is a relatively higher rate than the percentage of stent graft occlusion in patients with aneurysm as we mentioned above. Therefore, unnecessary bifurcated stent graft deployment should be avoided in cases of dissection.
Conclusion
We successfully performed stent deployment for focal abdominal aortic dissection and achieved complete resolution of stenosis, including that of bilateral CIA lesions. This was a difficult case with respect to treatment selection. Stent deployment at the site of a primary entry tear of the aortic dissection may be preferable in selected patients. Further investigation is required to establish a strategy for treatment of complex IAADs.
Disclosure Statement
All authors have no conflicts of interest to declare.
Author Contributions
Study conception: AM
Writing: MO
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Farber A, Wagner WH, Cossman DV, et al. Isolated dissection of the abdominal aorta: clinical presentation and therapeutic options. J Vasc Surg 2002; 36: 205-10. [DOI] [PubMed] [Google Scholar]
- 2).Dodos IA, Theodoridis P, Staramos D, et al. Endovascular repair of isolated abdominal aortic dissection. Literature review. Hellenic Journal of Surgery 2018; 90: 85-9. [Google Scholar]
- 3).Trimarchi S, Tsai T, Eagle KA, et al. Acute abdominal aortic dissection: insight from the International Registry of Acute Aortic Dissection (IRAD). J Vasc Surg 2007; 46: 913-9. [DOI] [PubMed] [Google Scholar]
- 4).Giribono AM, Ferrara D, Spalla F, et al. Endovascular treatment of spontaneous isolated abdominal aortic dissection. Acta Radiol Open 2016; 5: 2058460116681042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Wang D, Ma T, Guo D, et al. Endovascular treatment of acute and chronic isolated abdominal aortic dissection. Vascular 2018; 26: 418-24. [DOI] [PubMed] [Google Scholar]
- 6).Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med 1999; 340: 1546-52. [DOI] [PubMed] [Google Scholar]
- 7).Monastiriotis S, Loh S, Tassiopoulos A, et al. Clinical characteristics and outcome of isolated infrarenal aortic stenosis in young patients. J Vasc Surg 2018; 67: 1143-9. [DOI] [PubMed] [Google Scholar]
- 8).Grimme FA, Reijnen MM, Pfister K, et al. Polytetrafluoroethylene covered stent placement for focal occlusive disease of the infrarenal aorta. Eur J Vasc Endovasc Surg 2014; 48: 545-50. [DOI] [PubMed] [Google Scholar]
- 9).Sultan S, Hynes N. One-year results of the multilayer flow modulator stent in the management of thoracoabdominal aortic aneurysms and type B dissections. J Endovasc Ther 2013; 20: 366-77. [DOI] [PubMed] [Google Scholar]
- 10).Carroccio A, Faries PL, Morrissey NJ, et al. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg 2002; 36: 679-84. [PubMed] [Google Scholar]