Abstract
Objective: To investigate whether a finger-mounted tissue oximeter is useful in evaluating limb blood flow in patients with peripheral arterial disease (PAD).
Materials and Methods: Seventy-two patients with PAD were included, and the ankle-brachial index (ABI), transcutaneous oxygen pressure (TcPO2), and skin perfusion pressure (SPP) were measured. The regional tissue oxygenation saturation (rSO2) was measured using a finger-mounted tissue oximeter at the ankle, dorsal foot, and each dorsal and plantar toe. Correlations between rSO2 and ABI and between TcPO2 and SPP were analyzed. The patients were divided into three groups: Fontaine IIa (F-IIa), IIb (F-IIb), and III and IV (F-III/IV) groups. The difference in rSO2 between each group was analyzed.
Results: Significant correlations were observed between rSO2 and TcPO2 and between rSO2 and SPP. TcPO2 and SPP in the F-III/IV group were significantly lower than those in the F-IIa group. rSO2 in the F-IIb and F-III/IV groups was significantly lower than that in the F-IIa group.
Conclusion: The measurement of rSO2 using finger-mounted tissue oximetry is quick, simple, and painless. It can be used on any skin area and is useful to evaluate limb circulation in patients with PAD.
Keywords: limb blood flow, near-infrared spectroscopy, peripheral arterial disease, regional hemoglobin oxygen saturation
Introduction
The prevalence of peripheral arterial disease (PAD) has been reported to be in the range of 3–10%, increasing to 15–20% in persons over 70 years, and 0.1% of the population develops new cases of critical limb ischemia (CLI) every year in Europe and North America.1–3) In Japan, the prevalence of PAD has been reported to range from 1–3%,4–6) and the incidence is increasing. Clinical symptoms of PAD progress from intermittent claudication, rest pain, and skin ulcers to gangrene of the limbs. CLI, which is defined by the presence of rest pain, skin ulcers, or gangrene, is the most severe stage of PAD and is associated with high rates of mortality and amputation.1) Evaluating limb blood flow in the management of patients with PAD is essential. Several traditional diagnostic modalities, such as the measurement of the ankle-brachial index (ABI),1) skin perfusion pressure (SPP),7) and transcutaneous oxygen pressure (TcPO2),8,9) have been developed to evaluate limb blood flow and are widely accepted.2,3,8) However, these methods have some limitations.8) ABI measurement is painful and is limited to the area above the ankle; it is also unreliable for incompressible calcified vessels.8) SPP measurement, which is performed with a laser Doppler and pressure cuff, has been developed to evaluate more areas in the peripheral limbs.7) However, patients often experience intolerable pain caused by the cuff pressure. TcPO2 measurement requires 15 min to warm the sensor’s electrode, a flat skin surface for the placement of sensors, and a steady, stable position.9) Therefore, using traditional diagnostic modalities in patients with CLI who have rest pain or skin ulcers is difficult. Hence, the development of a new method, which can be used on any skin area and is quick, simple, precise, and painless, is essential.
Near-infrared spectroscopy (NIRS) is a simple, painless, and complication-free technique to evaluate tissue oxygenation.10,11) However, NIRS is not reliable in evaluating limb blood flow and is not recommended for the management of PAD, as per the present guidelines.2,3,8) Improvement of the NIRS device is required to evaluate limb blood flow. Recently, a finger-mounted tissue oximeter using the NIRS technique (Toccare: Astem Co., Ltd., Kawasaki, Japan) was developed. This device measures the regional tissue oxygenation saturation (rSO2) using a spatially resolved NIRS technique, and animal studies using mice have shown a correlation between the rSO2 in the craniofacial site and the blood pH or inspired oxygen fraction.11) Moreover, this device was reported to be useful to evaluate the condition of a fetus transvaginally and the condition of infants by measuring rSO2 at the scalp.12,13) This study aimed to investigate, for the first time, whether a finger-mounted tissue oximeter is useful in evaluating limb blood flow in patients with PAD.
Materials and Methods
Study approval
Informed consent was obtained from all participants. This study was approved by the Ethical Committee of Hamamatsu University School of Medicine (approval number: 16-057). The study protocol was registered at the UMIN Clinical Trials Registry (UMIN-CTR; ID: UMIN000025021).
Participants
This was a single-center observational study. Between July 2016 and July 2018, a total of 72 consecutive patients with PAD (mean age 73.8±8.9 years; men=53, women=19) who were treated at the outpatient vascular surgery clinic at the University Hospital of Hamamatsu University School of Medicine were included in this study. Table 1 shows the patients’ characteristics. The diagnosis of PAD was defined as an ABI <0.9. Data on each patient’s demographics, clinical symptoms, comorbid conditions, and vascular risk factors (Fontaine classification,14) age, sex, body mass index, smoking history, hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, cerebrovascular disease, and hemodialysis) were recorded. The definition of each condition was as follows: smoking history (either current or in the past) was present; hypertension was defined as the use of medications for hypertension or a systolic blood pressure >140 mmHg and/or a diastolic blood pressure >90 mmHg; hyperlipidemia was defined as the use of medications for hyperlipidemia or a total serum cholesterol concentration>220 mg/dL; diabetes mellitus was defined as the present or past use of medication for diabetes; coronary artery disease was defined as the present use of medication, past percutaneous coronary intervention, or coronary artery bypass grafting history; and cerebrovascular disease was defined as a history of stroke, transient ischemic attacks, or hemodialysis treatment.
Table 1 Baseline characteristics of patients with peripheral arterial disease.
| Fontaine classification | |||||
|---|---|---|---|---|---|
| IIa (n=19) | IIb (n=12) | III (n=10) | IV (n=31) | P value | |
| Age (mean±SD; years) | 74.1±6.4 | 73.9±6.2 | 77.3±12.4 | 73.8±8.9 | N.S |
| Male sex | 16 (84) | 7 (58) | 7 (70) | 23 (74) | N.S |
| Body mass index (mean±SD) | 22.9±2.5 | 20.5±3.0 | 21.1±4.0 | 21.1±3.5 | N.S |
| Smoking (%) | 14 (74) | 8 (67) | 6 (60) | 20 (65) | N.S |
| Hypertension (%) | 18 (95) | 8 (67) | 8 (80) | 25 (81) | N.S |
| Hyperlipidemia (%) | 8 (42) | 5 (42) | 6 (60) | 15 (48) | N.S |
| Diabetes mellitus (%) | 14 (74) | 6 (50) | 5 (50) | 21 (68) | N.S |
| Coronary artery disease (%) | 6 (32) | 6 (50) | 3 (30) | 16 (52) | N.S |
| Cerebrovascular disease (%) | 3 (16) | 2 (17) | 4 (40) | 4 (13) | N.S |
| Hemodialysis (%) | 2 (11) | 2 (17) | 4 (40) | 21 (68) | < 0.001 |
SD: standard deviation; N.S: not significant
Evaluation of limb blood flow using traditional diagnostic modalities
ABI, TcPO2, SPP, and rSO2 values were measured in both limbs of participants at their first admission. All procedures and measurements were performed in the supine position. The temperature of the laboratory was maintained at 25°C. As in the procedure reported previously, systolic blood pressures in the brachial and posterior tibial arteries were measured using an 8-MHz Doppler probe (BP-203, Omron, Kyoto, Japan), and the ABI was calculated. The TcPO2 at the ankle and dorsal foot was measured using a transcutaneous monitor (TCM 4 series, Radiometer, Copenhagen, Denmark), according to the procedure reported previously.15) The SPP at the ankle and dorsal foot was measured using a SPP system (SensiLase PAD 3000, Vasamed Eden Prairie, MN, USA).7)
Near-infrared spectroscopy technique
A finger-mounted tissue oximeter (Toccare)11–13) (Fig. 1A) was used to measure the rSO2 at the ankle and dorsal foot in both limbs of patients with PAD. This tissue oximeter probe consists of near-infrared light-emitting diodes (LEDs) and detector photodiodes (Fig. 1A). The detector photodiodes are located 6 and 8 mm away from the LEDs. This device was set to measure the rSO2, up to a depth of 0–5 mm from the oximeter probe.13) The rSO2 was calculated as the oxyhemoglobin level divided by a combination of oxyhemoglobin and deoxyhemoglobin levels.12) The obtained rSO2 values were saved automatically to a micro SD card. The advantages of this device include the light weight of the module (0.1 kg), its mobility, and the short sampling time (0.5 s). At this time, the cost of this device is approximately JPY 400,000.
Fig. 1 Measurement of the regional hemoglobin oxygen saturation (rSO2) using a finger-mounted tissue oximeter.
(A) A finger-mounted tissue oximeter using the near-infrared spectroscopy technique (white arrow: optical source; arrow head: optical detector). (B) rSO2 measurement at the dorsal side of the fourth toe near the ulcer (white arrow: rSO2 value).
Comparison between the groups of patients with PAD
Patients were divided into four groups according to the Fontaine classification: Fontaine IIa group (F-IIa), Fontaine IIb group (F-IIb), Fontaine III group (F-III), and Fontaine IV group (F-IV). F-IIa and F-IIb were defined as those with claudication symptoms after >200 m and <200 m of walking, respectively. Patients classified as F-IIa or F-IIb were assigned to the claudication group, and patients classified as Fontaine III or IV were assigned to the CLI group. In the affected limbs of patients with PAD, the rSO2 was also measured at each dorsal and plantar toe (Fig. 1B), which was difficult to measure when using traditional diagnostic modalities. ABI, TcPO2, SPP, and rSO2 values at each area were measured in the affected limbs and expressed as medians and interquartile ranges. These values were compared between the claudication group (i.e., F-IIa/IIb) and the CLI group (i.e., F-III/IV), and among the three groups (F-IIa, IIb, and III/IV).
Statistical analysis
Scatter plots comparing the rSO2 values and those of ABI, TcPO2, or SPP in both limbs were generated. The correlations between these values were analyzed using the nonparametric Spearman rank correlation test, and positive correlations were classified as poor, r=0–0.2; weak, r=0.2–0.4; moderate, r=0.4–0.7; or strong, r=0.7–1.0. A significant correlation was defined as P<0.05.
The characteristics of each group of patients with PAD were analyzed using the χ2 test. ABI, TcPO2, SPP, and rSO2 values in patients with PAD were expressed as medians. The differences in these values between the claudication group (i.e., F-IIa/IIb) and the CLI group (i.e., F-III/IV) were assessed with Mann–Whitney U tests, and the differences in these values among the three groups (F-IIa, IIb, and III/IV) were compared using the Kruskal–Wallis test with the Bonferroni–Dunn post hoc analysis because the assumption of homogeneity of variance was violated. A P value <0.05 was considered statistically significant. All analyses were performed using IBM SPSS version 25.0 software (IBM Corp., Armonk, NY, USA).
Results
Examination of limb blood flow using each evaluation modality
Six limbs of patients with PAD had already been amputated at the first admission. Therefore, the ABI, TcPO2, SPP, and rSO2 were measured in 138 limbs. However, measuring the ABI in 11 limbs (nine PAD limbs, two non-PAD limbs) was impossible because of intolerable pain with cuff pressure during the measurement. Moreover, at the ankle, measuring TcPO2 in four limbs (all limbs with PAD) and SPP in 13 limbs (all limbs with PAD) was impossible owing to involuntary movements, pain, difficulty in maintaining a stable position, and intolerable pain with cuff pressure. At the dorsal foot, TcPO2 could be measured in all limbs; however, it was impossible to measure SPP in 13 limbs (all limbs with PAD) owing to involuntary movements and intolerable pain with cuff pressure. In contrast, the rSO2 could be measured in all limbs at both the ankle and dorsal foot. Therefore, the examination success rate was 92.0% (127 limbs) for the ABI, 97.1% (134 limbs) for TcPO2 at the ankle, 100% (138 limbs) for TcPO2 at the dorsal foot, 90.6% (125 limbs) for SPP at the ankle, 90.6% (125 limbs) for SPP at the dorsal foot, and 100% (138 limbs) for rSO2 at both the ankle and dorsal foot.
Relationships between rSO2 and traditional diagnostic modalities
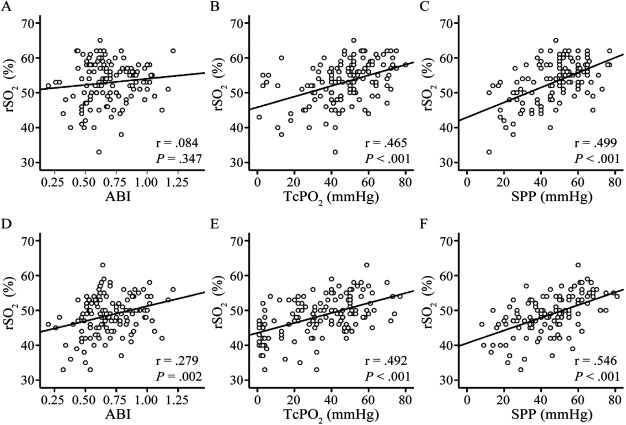
Figure 2 shows the relationships between the rSO2 values and the traditional diagnostic modalities (measurement of the ABI, TcPO2, and SPP): rSO2 at the ankle and ABI (n=127, P=0.347, r=0.084, Fig. 2A), rSO2 and TcPO2 at the ankle (n=134, P<0.001, r=0.465, Fig. 2B), rSO2 and SPP at the ankle (n=125, P<0.001, r=0.499, Fig. 2C), rSO2 at the dorsal foot and ABI (n=127, P=0.002, r=0.279, Fig. 2D), rSO2 and TcPO2 at the dorsal foot (n=138, P<0.001, r=0.492, Fig. 2E), and rSO2 and SPP at the dorsal foot (n=125, P<0.001, r=0.546, Fig. 2F). With regard to the relationship between rSO2 and ABI, neither ankle nor dorsal rSO2 correlated significantly with the ABI. Significant positive correlations were found between rSO2 and TcPO2 and between rSO2 and SPP at the ankle and dorsal foot.
Fig. 2 Relationship between the regional hemoglobin oxygen saturation (rSO2) and the value of traditional diagnostic modalities at the ankle and dorsal foot.
Scatter plots of the values of (A) the ankle-brachial index (ABI) and rSO2 at the ankle (n=127) (P=0.347, r=0.084), (B) transcutaneous oxygen pressure (TcPO2) and rSO2 at the ankle (n=134) (P<0.001, r=0.465), (C) skin perfusion pressure (SPP) and rSO2 at the ankle (n=125) (P<0.001, r=0.499), (D) ABI and rSO2 at the dorsal foot (n=127) (P=0.002, r=0.279), (E) TcPO2 and rSO2 at the dorsal foot (n=138) (P<0.001, r=0.492), and (F) SPP and rSO2 at the dorsal foot (n=125) (P<0.001, r=0.546) (Spearman’s test).
Comparison between the groups of patients with PAD
The 72 patients were divided into four groups: 19 in the F-IIa group, 12 in the F-IIb group, 10 in the F-III group, and 31 in the F-IV group (Table 1). Therefore, 31 patients were categorized into the claudication group (i.e., F-IIa/IIb) and 41 patients into the CLI group (i.e., F-III/IV). The ABI and TcPO2 at the ankle and dorsal foot, SPP at the ankle and dorsal foot, and rSO2 at the ankle, dorsal foot, and each dorsal and plantar toe were measured in 72 limbs with PAD. However, the ABI, TcPO2, and SPP were impossible to measure in all patients because of intolerable pain, difficulty to maintain a stable position, and involuntary movements. Therefore, the ABI was measured in 66 limbs with PAD, TcPO2 at the ankle in 70 limbs, TcPO2 at the dorsal foot in 72 limbs, SPP at the ankle in 66 limbs, and SPP at the dorsal foot in 65 limbs. rSO2 at the ankle and dorsal foot were measured in all patients with PAD. There was no significant difference in the ABI value between the claudication and CLI groups or among the groups of patients with PAD (Fig. 3A). However, significant differences were observed between the TcPO2, SPP, and rSO2 values at the ankle and dorsal foot between the claudication and CLI groups. Moreover, significant differences were also observed between the F-IIa and F-III/IV groups in the TcPO2 values at the ankle and dorsal foot (ankle, P=0.005; dorsal foot, P=0.013) (Figs. 3B and 3E). Significant differences were also observed between the F-IIa and F-III/IV groups in the SPP values at the ankle and dorsal foot (ankle, P=0.013; dorsal foot, P<0.001) (Figs. 3C and 3F). However, neither TcPO2 nor SPP showed significant differences between the F-IIa and F-IIb groups at either the ankle or the dorsal foot. In contrast, the rSO2 showed significant differences at the ankle and the dorsal foot between the F-IIa and F-IIb groups (ankle, P=0.038; dorsal foot, P=0.001). The rSO2 also showed significant differences between the F-IIa and F-III/IV groups (ankle, P<0.001; dorsal foot, P<0.001) (Figs. 3D and 3G).
Fig. 3 Comparison of traditional diagnostic values and the regional hemoglobin oxygen saturation (rSO2) among each group of patients with peripheral arterial disease.
The median and interquartile range (IQR) of the diagnostic values of the ankle-brachial index (ABI) (A) (P=0.209), transcutaneous oxygen pressure (TcPO2) at the ankle (B) (P=0.003), skin perfusion pressure (SPP) at the ankle (C) (P=0.016), rSO2 at the ankle (D) (P<0.001), TcPO2 at the dorsal foot (E) (P=0.011), SPP at the dorsal foot (P=0.001) (F), and rSO2 at the dorsal foot (G) (P<0.001) in each group (*P<0.05, ** P<0.01).
The rSO2 at each dorsal and plantar toe was measured easily in all participants in less than one second. rSO2 measurements in the first toes of three patients, the second toes of one patient, the third toes of two patients, the fourth toes of four patients, and the fifth toes of two patients were impossible because of necrosis. The rSO2 was measured in all toes, even those with ulcers, but not in those with necrosis. Significant differences between the claudication and CLI groups were observed in the rSO2 values at all dorsal and plantar toes. Moreover, significant differences were observed between the F-IIa and F-IIb groups in the rSO2 values at the dorsal and plantar toes (dorsal first toe, P=0.025; dorsal second toe, P=0.044; dorsal fourth toe, P=0.035; dorsal fifth toe, P=0.041; plantar first toe, P=0.026; plantar second toe, P=0.042; plantar third toe, P=0.037, plantar fourth toe, P=0.024) and the F-IIa and F-III/IV groups (all skin areas, P<0.001) (Fig. 4).
Fig. 4 Comparison of the regional hemoglobin oxygen saturation (rSO2) among each group of patients with peripheral arterial disease.
Left figures represent the measurement skin area (A, 1d–5d: 1st–5th dorsal toe) (B, 1p–5p: 1st–5th plantar toe). The median and interquartile range of the rSO2 in each measurement skin area are shown (*P<0.05, ** P<0.01).
Discussion
We showed the usefulness of a new finger-mounted tissue oximeter using the NIRS technique to evaluate limb ischemia. The use of traditional diagnostic modalities, such as the measurement of the ABI, SPP, and TcPO2, was limited because of the long measurement time, pain during measurement, skin lesions, or maintaining a posture during the measurement. In our study, measuring the ABI or SPP was impossible in about 10% of the cases because of intolerable cuff pressure pain during measurement, and measuring the TcPO2 at the ankle was impossible in about 3% of the cases because patients with PAD have difficulty maintaining a stable position. On the contrary, measuring the rSO2 using the finger-mounted tissue oximeter was successful in all ischemic limbs. Even in patients with rest pain or skin ulcers, the rSO2 could be measured quickly, simply, painlessly, and repeatedly at the bedside and on any skin area.
NIRS was developed in the 1980s and was used mostly to assess cerebral circulation.16) Cheatle et al. first reported decreased oxygen consumption in patients with PAD using the NIRS technique,17) and the usefulness of NIRS devices for the evaluation of patients with PAD was reported.10,16,18,19) Previous NIRS devices, which were developed to assess adult cerebral circulation, were set to measure the rSO2 1–3 cm deep from the oximeter probe and to evaluate mainly the skeletal muscle oxygenation in the lower limbs.19–21) Evaluating skin oxygenation at the toe is important for the management of patients with CLI who tend to develop toe ulcers. However, previous NIRS devices were not appropriate for the evaluation of toe skin oxygenation because the NIRS measurement depth was too great for the toe.
The rSO2 measured by previous NIRS devices was not recommended for the management of PAD based on the guidelines,2,3,7,8) because the correlation between rSO2 values using previous NIRS devices and the ABI was reported to be poor22) and was not even compared with TcPO2 or SPP, which represents skin perfusion. Because of the measurement depth of the previous NIRS devices, the rate of oxygen resaturation or recovery time after exercise was considered to be a proper parameter for NIRS devices to evaluate claudicants,10,16) and these parameters correlated mostly with the ABI.16,18,20,21) These reports suggested that previous NIRS devices were useful for the evaluation of muscle oxygenation after exercise in patients with PAD and claudication,23) but not for that of skin oxygenation. Thus, they have not been clinically applied to patients with CLI to evaluate skin perfusion. In contrast, the finger-mounted tissue oximeter used in this study, which was originally manufactured to measure the rSO2 through the fetal scalp, is set to measure the rSO2 5 mm deep from the probe.11–13) Therefore, this NIRS device can evaluate skin and subcutaneous tissue oxygenation at rest. As a result, the rSO2 measured with the device correlated significantly with data obtained with other conventional devices, such as TcPO2 and SPP. These results suggest that the rSO2 measured with the device is reliable to assess skin perfusion.
Measuring skin perfusion at each dorsal or plantar toe using conventional diagnostic modalities, such as measuring TcPO2, SPP, or previous NIRS devices, has not been applied routinely for two reasons. First, conventional device probes were not manufactured to be attached to these skin areas, but to be placed on the thigh, calf, or dorsal foot; second, the depth of the NIRS in previous devices was 1–3 cm deep. Thus, the skin/subcutaneous tissue of the toe might be too thin to measure because bones or tendons are present at that depth. In this study, the finger-mounted tissue oximeter enabled the measurement of the rSO2 at any skin area including ulcer regions without necrosis. Significant differences in rSO2 values between the groups of patients with PAD were observed at any skin area in our study. The rSO2 value was measured precisely at the toe where skin perfusion was difficult to measure using previous modalities.
Evaluating limb blood flow is essential to determine indications for revascularization,1) which is highly recommended for patients with CLI who have rest pain, ulcers, and/or necrosis (F-III/IV).24) In this study, we divided patients with claudication into the two subgroups of F-IIa and F-IIb. According to the literature, 80% of patients with claudication are stable for five years after the start of symptoms; however, the remaining 20% of patients advance to CLI, in which the F-IIb patients are more likely to develop CLI than F-IIa.1) Other literature also recommended revascularization of patients with PAD and severe claudication.24–26) Therefore, it is meaningful to differentiate between F-IIb and F-IIa patients not only from patient interviews about walking distances but also from objective values of the limb perfusion status. Unfortunately, previous measures, such as TcPO2 and SPP as well as previous NIRS devices, failed to show the differences between the two groups. Furthermore, for patients with diabetes with/without dialysis, assessing the perfusion status at the more peripheral regions, such as the toes, is rather important. The NIRS device in this study enabled this assessment to be performed with ease for the first time and succeeded in differentiating between the two groups.
The measurement of TcPO2 or SPP is useful for the management of PAD. Normal TcPO2 levels are approximately 60 mmHg, while levels of 20 mmHg or less strongly suggest that revascularization will be required to achieve healing. Normal SPP levels of 50–70 mmHg are decreased to 10–20 mmHg in limbs with severe ischemia.8,15) In this study, rSO2 levels of 45% at the ankle or dorsal foot were almost equivalent to the above-mentioned critical levels of TcPO2 or SPP according to the scatter plots and linear formula. However, further studies are needed to determine the cut-off values of rSO2 for wound healing.
This study had some limitations. First, the sample size was small, especially patients with mild PAD without CLI (F-IIa and F-IIb groups). Second, the relationship between wound healing and the rSO2 value was not clarified in this study, and the cut-off value for wound healing could not be decided. Third, although rSO2 values are considered to be influenced by skin pigmentation, we performed NIRS oximetry only in Japanese subjects; consequently, the data are representative of only Japanese patients.27)
This device was originally developed to evaluate fetal brain blood flow; therefore, it is set to measure the rSO2 5 mm deep from the probe.13) It is unclear whether this depth is also optimal for the evaluation of lower limb blood flow of patients with PAD. Further studies are needed to determine the cut-off rSO2 values for wound healing in patients with CLI using this device.
Conclusion
The use of a finger-mounted tissue oximeter enables the measurement of rSO2 values in any skin area quickly, simply, precisely, and painlessly at the bedside. The rSO2 value is correlated with the value of traditional diagnostic modalities and is significantly decreased in limbs with severe PAD. We showed the usefulness and reliability of the finger-mounted tissue oximeter to evaluate limb ischemia. This new device may be useful in determining therapeutic strategies for patients with PAD and may become one of the most useful devices to evaluate tissue oxygenation in patients with PAD.
Acknowledgments
This work was supported by the project “Development of Medical Devices and Systems for Advanced Medical Services” from the Division of Medical Device Research in the Japan Agency for Medical Research and Development, AMED (awarded to Naoki Unno).
Disclosure Statement
All authors declare that no competing interests exist.
Author Contributions
Study conception: TY, MS, KI, NU
Data collection: TY, TK, EN, KK, YY, NU
Analysis: TY, MS, NU
Investigation: TY, MS, NY, KI, TS
Writing: TY, MS, NU
Funding acquisition: NU
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45 Suppl S: S5-67. [DOI] [PubMed] [Google Scholar]
- 2).Conte MS, Pomposelli FB, Clair DG, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg 2015; 61 Suppl: 2S-41S. [DOI] [PubMed] [Google Scholar]
- 3).Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017; 69: 1465-508. [DOI] [PubMed] [Google Scholar]
- 4).Fujiwara T, Saitoh S, Takagi S, et al. Prevalence of asymptomatic arteriosclerosis obliterans and its relationship with risk factors in inhabitants of rural communities in Japan: Tanno-Sobetsu study. Atherosclerosis 2004; 177: 83-8. [DOI] [PubMed] [Google Scholar]
- 5).Ohnishi H, Sawayama Y, Furusyo N, et al. Risk factors for and the prevalence of peripheral arterial disease and its relationship to carotid atherosclerosis: the Kyushu and Okinawa Population Study (KOPS). J Atheroscler Thromb 2010; 17: 751-8. [DOI] [PubMed] [Google Scholar]
- 6).Usui T, Ninomiya T, Nagata M, et al. Albuminuria as a risk factor for peripheral arterial disease in a general population: the Hisayama study. J Atheroscler Thromb 2011; 18: 705-12. [DOI] [PubMed] [Google Scholar]
- 7).Castronuovo JJ Jr, Adera HM, Smiell JM, et al. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg 1997; 26: 629-37. [DOI] [PubMed] [Google Scholar]
- 8).Cao P, Eckstein HH, De Rango P, et al. Chapter II: Diagnostic methods. Eur J Vasc Endovasc Surg 2011; 42 Suppl 2: S13-32. [DOI] [PubMed] [Google Scholar]
- 9).Kalani M, Brismar K, Fagrell B, et al. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care 1999; 22: 147-51. [DOI] [PubMed] [Google Scholar]
- 10).Boezeman RPE, Moll FL, Ünlü Ç, et al. Systematic review of clinical applications of monitoring muscle tissue oxygenation with near-infrared spectroscopy in vascular disease. Microvasc Res 2016; 104: 11-22. [DOI] [PubMed] [Google Scholar]
- 11).Uchida T, Kanayama N, Kawai K, et al. Craniofacial tissue oxygen saturation is associated with blood pH using an examiner’s finger-mounted tissue oximetry in mice. J Biomed Opt 2016; 21: 40502. [DOI] [PubMed] [Google Scholar]
- 12).Kanayama N, Niwayama M. Examiner’s finger-mounted fetal tissue oximetry. J Biomed Opt 2014; 19: 067008. [DOI] [PubMed] [Google Scholar]
- 13).Mukai M, Uchida T, Itoh H, et al. Tissue oxygen saturation levels from fetus to neonate. J Obstet Gynaecol Res 2017; 43: 855-9. [DOI] [PubMed] [Google Scholar]
- 14).Fontaine R, Kim M, Kieny R. Die chirurgische Behandlung der peripheren Durchblutungsstörungen. Helv Chir Acta 1954; 21: 499-533. (in German) [PubMed] [Google Scholar]
- 15).Faglia E, Clerici G, Caminiti M, et al. Predictive values of transcutaneous oxygen tension for above-the-ankle amputation in diabetic patients with critical limb ischemia. Eur J Vasc Endovasc Surg 2007; 33: 731-6. [DOI] [PubMed] [Google Scholar]
- 16).Vardi M, Nini A. Near-infrared spectroscopy for evaluation of peripheral vascular disease. A systematic review of literature. Eur J Vasc Endovasc Surg 2008; 35: 68-74. [DOI] [PubMed] [Google Scholar]
- 17).Cheatle TR, Potter LA, Cope M, et al. Near-infrared spectroscopy in peripheral vascular disease. Br J Surg 1991; 78: 405-8. [DOI] [PubMed] [Google Scholar]
- 18).Wolf U, Wolf M, Choi JH, et al. Localized irregularities in hemoglobin flow and oxygenation in calf muscle in patients with peripheral vascular disease detected with near-infrared spectrophotometry. J Vasc Surg 2003; 37: 1017-26. [DOI] [PubMed] [Google Scholar]
- 19).Boezeman RPE, Becx BP, van den Heuvel DAF, et al. Monitoring of foot oxygenation with near-infrared spectroscopy in patients with critical limb ischemia undergoing percutaneous transluminal angioplasty: a pilot study. Eur J Vasc Endovasc Surg 2016; 52: 650-6. [DOI] [PubMed] [Google Scholar]
- 20).Komiyama T, Shigematsu H, Yasuhara H, et al. Near-infrared spectroscopy grades the severity of intermittent claudication in diabetics more accurately than ankle pressure measurement. Br J Surg 2000; 87: 459-66. [DOI] [PubMed] [Google Scholar]
- 21).Mesquita RC, Putt M, Chandra M, et al. Diffuse optical characterization of an exercising patient group with peripheral artery disease. J Biomed Opt 2013; 18: 57007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Manfredini F, Lamberti N, Rossi T, et al. A toe flexion NIRS assisted test for rapid assessment of foot perfusion in peripheral arterial disease: feasibility, validity, and diagnostic accuracy. Eur J Vasc Endovasc Surg 2017; 54: 187-94. [DOI] [PubMed] [Google Scholar]
- 23).Manfredini F, Malagoni AM, Mandini S, et al. Near-infrared spectroscopy assessment following exercise training in patients with intermittent claudication and in untrained healthy participants. Vasc Endovascular Surg 2012; 46: 315-24. [DOI] [PubMed] [Google Scholar]
- 24).Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med 2001; 344: 1608-21. [DOI] [PubMed] [Google Scholar]
- 25).Stecko W, Rogala W, Feldo M, et al. Results of endovascular treatment of iliac and femoral symptomatic lesions. Identification of re-intervention risk factors. Acta Angiologica 2017; 23: 115-23. [Google Scholar]
- 26).van Haelst STW, Haitjema S, Derksen W, et al. Atherosclerotic plaque characteristics are not associated with future cardiovascular events in patients undergoing iliofemoral endarterectomy. J Vasc Surg 2018; 67: 809-16.e1. [DOI] [PubMed] [Google Scholar]
- 27).Wassenaar EB, Van den Brand JGH. Reliability of near-infrared spectroscopy in people with dark skin pigmentation. J Clin Monit Comput 2005; 19: 195-9. [DOI] [PubMed] [Google Scholar]