Abstract
Objective
To assess whether abatacept as initial biological DMARD (bDMARD) in the treatment of RA, when compared with other bDMARDs, is associated with an increased risk of cancer overall and by specific cancer sites (breast, lung, lymphoma, melanoma and non-melanoma skin cancer).
Methods
We performed a population-based cohort study among patients newly treated with bDMARDs within the US-based Truven MarketScan population and Supplemental US Medicare from 2007 to 2014. Cox proportional hazards models were used to estimate hazard ratios and 95% CIs of any cancer (and specific cancers) associated with initiation of abatacept, compared with initiation of other bDMARDs, adjusted for age and deciles of the propensity score.
Results
The cohort included 4328 patients on abatacept and 59 860 on other bDMARDs, of whom 409 and 4197 were diagnosed with any cancer during follow-up (incidence rates 4.76 per 100 per year and 3.41 per 100 per year, respectively). Compared with other bDMARDs, the use of abatacept was associated with an increased incidence of cancer overall (hazard ratioadjusted 1.17; 95% CI 1.06, 1.30). Analyses by specific cancer sites showed a significantly increased incidence of non-melanoma skin cancer (hazard ratioadjusted 1.20; 95% CI 1.03, 1.39), but no significant difference for other specific cancer sites.
Conclusion
The use of abatacept as first bDMARD in the treatment of RA was associated with a slight increased risk of cancer overall and particularly non-melanoma skin cancer, compared with other bDMARDs. This potential signal needs to be replicated in other settings.
Keywords: cancer, non-melanoma skin cancer, breast cancer, abatacept, TNF inhibitors, biologic DMARDs, RA
Rheumatology key messages
Abatacept in RA was associated with a slight increased overall cancer risk relative to other biologic DMARDs.
The risk increase was for non-melanoma skin cancer but not breast, lung, lymphoma and melanoma.
These findings warrant replication particularly if prescribers are channelling higher risk patients to abatacept.
Introduction
Biologic DMARDs (bDMARDs) approved in the treatment of RA are known to induce partial immune incompetence and have been suspected to be associated with increased risks of cancers. However, the bulk of the evidence concerning anti-TNF-α bDMARDs to date remains reassuring. Abatacept, a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)-fusion protein that blocks T cell activation, has a distinct mechanism of action [1]. Therefore, abatacept could be associated with a different risk profile compared with other bDMARDs [2].
Among bDMARDs, most studies have assessed the risk of cancer associated with TNF-α inhibitors, suggesting that, compared with conventional synthetic DMARDs (csDMARDs), TNF-α inhibitors do not increase the risk of cancers in general, but may slightly increase the risk of skin cancer [melanoma or non-melanoma skin cancer (NMSC)] [3–6]. In contrast, there are very few data on the safety of abatacept, particularly in first-line utilization. Recently, a potential signal was reported for a higher risk of NMSC with abatacept compared with other bDMARDs [7–9]. However, one study showed evidence that physicians are prescribing abatacept to patients who are older, and have longer disease duration and more comorbid conditions [10]. This suggests that prescribers perceive a more favourable safety profile of abatacept in first line, thereby possibly channelling their use to populations at risk for cancers. Moreover, while the initiation of bDMARDs such as abatacept is usually combined with a csDMARD, primarily MTX, some patients with RA may receive bDMARDs as monotherapy, mainly because of MTX intolerance or noncompliance. While meta-analyses suggest that bDMARDs as monotherapy have generally similar efficacy as in combination with a csDMARD such as MTX, there are no data on the comparative safety of these two regimens [11].
We assessed whether abatacept, when used as the initial bDMARD for RA, is associated with an increased risk of overall cancer and of specific cancers, including breast, lung, lymphoma, melanoma and NMSC, compared with other bDMARDs. We also assessed whether the combination of bDMARDs and MTX is associated with an increased risk of cancer, compared with bDMARDs monotherapy.
Methods
Data source
We performed a population-based cohort study using the Truven Health MarketScan Commercial and Supplemental Medicare US databases [12]. The Truven MarketScan Commercial database is a US administrative claims database with patient information dating back to 2006 [12]. The database provides detailed information on >70 million privately insured patients <65 years of age, from >150 employers and 20 health plans. The MarketScan Medicare Supplemental database covers patients >65 years of age receiving Medicare coverage in the USA. This database records data on approximately 6 million patients, including demographics, drug information and enrolment information dating back to 2006. The study protocol was approved by the Research Ethics Board of the Jewish General Hospital, Montréal, Canada.
Study population
The base cohort consisted of all new users of a bDMARD, including abatacept, on or after 1 January 2007, through 31 December 2014. Thus, all patients with at least one prescription for a bDMARD aged 18 years or older on the date of the first bDMARD prescription (cohort entry date) were considered. New users were defined by no prior prescription of bDMARD during the 6 months before cohort entry. To be included in the cohort, patients were required to have been enroled in the database for at least 6 months before cohort entry. They were also required to have at least two inpatient or outpatient RA diagnostic codes (International Classification of Diseases, Ninth revision: 714.xx) at any time prior to and including the cohort entry date, or within 6 months after this date. This criterion for RA is based on MacLean’s positive predictive value of an administrative data-based algorithm for the identification of patients with RA [13]. We excluded patients with history of any cancer (including NMSC) in the 6 months before cohort entry, as well as patients with <6 months of follow-up after cohort entry. The latter was necessary for latency purposes as short duration exposures are unlikely to be associated with cancer. As the risk of cancer is also expected to be latent and continue beyond the period of exposure, follow-up started 6 months after cohort entry until a cancer diagnosis, end of enrolment in the database or end of data collection (31 December 2014), whichever occurred first, irrespective of whether there was a subsequent switch to another bDMARD.
Exposure definition
Exposure to abatacept and other bDMARDs (listed in supplementary Table S1, available at Rheumatology online) was defined from dispensed prescriptions. Treatments were identified using the National Drug Code for dispensed medications and procedure codes for injection or infusion. Patients were considered exposed to the first bDMARD prescribed at cohort entry until the end of follow-up, irrespective of whether there was a subsequent switch to another bDMARD (analogous to an intention-to-treat approach). For the primary objective, initiators of abatacept were compared with initiators of other bDMARDs. For the secondary objective, initiators of bDMARDs (including abatacept) concomitantly using MTX were compared with initiators of bDMARDs as monotherapy (including abatacept).
Outcomes
The primary outcome of interest was overall cancer. Outcome events of cancers were identified using International Classification of Diseases, Ninth revision diagnosis codes (supplementary Table S2, available at Rheumatology online). We also performed separate analyses for cancers of particular interest, namely breast cancer, lung cancer, lymphoma, melanoma and NMSC.
Statistical analysis
Descriptive statistics were used to compare baseline characteristics between patients initially prescribed abatacept vs other bDMARDs, as well as to compare bDMARDs with or without MTX. Overall rates of cancer were estimated using Poisson distribution by counting events and person-time of follow-up.
To control for potential confounding, we used multivariate logistic regression to estimate propensity scores of being exposed to abatacept vs other bDMARDs conditional on baseline covariates measured in the 6 months before cohort entry. These consisted of age, sex, year of cohort entry, comorbidities [hypertension, diabetes, hospitalized infections, asthma, chronic obstructive pulmonary disease, chronic kidney disease, leukopaenia, neutropaenia, peripheral arterial disease, hyperlipidaemia, cardiovascular disease, autoimmune diseases (excluding RA)] and medications [MTX, other csDMARDs (supplementary Table S1, available at Rheumatology online), parenteral antibiotics, oral and parenteral corticosteroids, and NSAIDs]. The propensity score distributions were then trimmed to include only patients with overlapping distributions.
For the primary objective, Cox proportional hazards regression models were used to estimate hazard ratios (HRs) with 95% CIs of cancer overall and specific cancers associated with initiation of abatacept, compared with initiation of other bDMARDs, adjusted for deciles of the propensity score and age. An intention-to-treat approach was used, based on the bDMARD prescription at cohort entry as the exposure definition. A latency period of 6 months was applied whereby any patient with an outcome event occurring during the first 6 months after the initiation of exposure was not included in the analysis. For the secondary objective, the same approach was used to compare all users of a bDMARD as add-on therapy to MTX with those similar patients using a bDMARD as monotherapy with respect to the incidence of all outcome events.
Sensitivity analyses
We conducted several sensitivity analyses to assess the robustness of our results. First, the baseline period of 6 months used to define new use and to measure the covariates was extended to 1 year. Second, the effect of the 6-month latency period was assessed by considering no latency, a 1-year latency and a 2-year latency period. Third, we used a definition requiring one inpatient cancer diagnostic code or two outpatient diagnostic codes to identify cancer, in which case, the date of the second diagnostic code was taken as the date of the event. Fourth, the comparator group was restricted to TNF-α inhibitors, namely infliximab, etanercept, adalimumab, certolizumab pegol and golimumab. Fifth, we excluded patients with a diagnosis of cancer at any time prior to cohort entry. Sixth, to estimate the potential effect of a switch from the first bDMARD to another bDMARD during follow-up, we repeated the primary analysis using a time-dependent exposure definition. Specifically, patients were considered exposed to the bDMARD prescribed at cohort entry (abatacept or other bDMARD) up until 6 months after the first switch (from abatacept to another bDMARD or from another bDMARD to abatacept), and were considered exposed to the latter until the end of follow-up (in a separate category). Seventh, we repeated the main analysis after matching each abatacept user to one bDMARD user on the propensity score. Finally, we performed stratified analyses by the source database, namely the commercial claims and Medicare Supplemental.
Statistical analyses were conducted using SAS, Version 9.2 (SAS institute, Inc., Cary, NC, USA) and R (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
Results
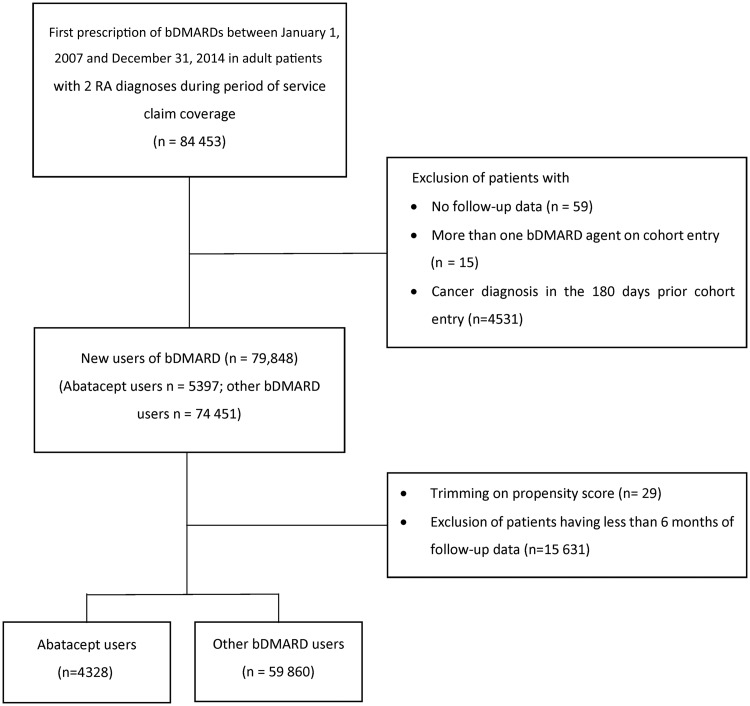
The base cohort included 84 453 new users of a bDMARD anytime between 2007 and 2014 (Fig. 1). After applying all inclusion and exclusion criteria, the study cohort included 64 188 patients (4328 on abatacept vs 59 860 on other bDMARDs). For the secondary objective, the study cohort included 2426 patients initiating bDMARD with MTX and 61 092 patients initiating bDMARD as monotherapy (supplementary Fig. S1, available at Rheumatology online).
Fig. 1.
Study flowchart for the primary objective
bDMARD: biologic DMARD.
Baseline characteristics differed between initiators of abatacept and initiators of other bDMARDs. Abatacept initiators were older, more frequently women and had more comorbidities (except for other autoimmune diseases) than the initiators of other bDMARDs (Table 1). However, fewer abatacept initiators were on MTX and NSAIDs at baseline. At cohort entry, other bDMARDs were mainly etanercept (40.8%), adalimumab (31.8%) and infliximab (13.9%). Initiators of bDMARD with MTX had fewer other autoimmune diseases than initiators of bDMARDs without MTX, but were more likely to have used MTX during the 180-day baseline period (supplementary Table S3, available at Rheumatology online). After cohort entry, 1046 patients (24%) switched from abatacept to another bDMARD, and 3730 patients (6%) switched from another bDMARD to abatacept.
Table 1.
Characteristics of patients initiating treatment with abatacept vs with other biologic DMARDs
| Abatacept | Other bDMARDs | |
|---|---|---|
| Number of patients | 4328 | 59 860 |
| Age at cohort entry, years, mean (s.d.) | 55.8 (12.9) | 52.2 (12.9) |
| 18–39 | 448 (10.4) | 9812 (16.4) |
| 40–49 | 787 (18.2) | 13 086 (21.9) |
| 50–59 | 1437 (33.2) | 20 159 (33.7) |
| 60–69 | 1035 (23.9) | 11 954 (20.0) |
| 70–79 | 483 (11.2) | 3679 (6.1) |
| 80+ | 138 (3.2) | 1170 (2.0) |
| Women, n (%) | 3613 (83.5) | 45 815 (76.5) |
| Cohort entry year, n (%) | ||
| 2007 | 598 (13.8) | 6942 (11.6) |
| 2008 | 518 (12.0) | 7959 (13.3) |
| 2009 | 550 (12.7) | 8338 (13.9) |
| 2010 | 484 (11.2) | 7664 (12.8) |
| 2011 | 563 (13.0) | 8565 (14.3) |
| 2012 | 709 (16.4) | 8178 (13.7) |
| 2013 | 588 (13.6) | 7684 (12.8) |
| 2014 | 318 (7.3) | 4530 (7.6) |
| Medications at cohort entry, n (%) | ||
| DMARDs | ||
| Abatacept | 4328 (100.0) | 0 (0.0) |
| Adalimumab | 0 (0.0) | 19 048 (31.8) |
| Anakinra | 0 (0.0) | 225 (0.4) |
| Certolizumab | 0 (0.0) | 1388 (2.3) |
| Etanercept | 0 (0.0) | 24 437 (40.8) |
| Golimumab | 0 (0.0) | 1571 (2.6) |
| Infliximab | 0 (0.0) | 8306 (13.9) |
| Rituximab | 0 (0.0) | 3646 (6.1) |
| Tocilizumab | 0 (0.0) | 740 (1.2) |
| Tofacitinib | 0 (0.0) | 499 (0.8) |
| MTX | 105 (2.4) | 2318 (3.9) |
| Other csDMARDs | 50 (1.2) | 930 (1.6) |
| Comorbidities at baseline, n (%) | ||
| Hypertension | 1405 (32.5) | 15 729 (26.3) |
| Type 2 diabetes | 587 (13.6) | 6581 (11.0) |
| Asthma | 279 (6.4) | 3186 (5.3) |
| Chronic obstructive pulmonary disease | 294 (6.8) | 3169 (5.3) |
| Chronic kidney disease | 97 (2.2) | 1024 (1.7) |
| Leukopaenia | 30 (0.7) | 299 (0.5) |
| Neutropaenia | 18 (0.4) | 195 (0.3) |
| Peripheral arterial disease | 54 (1.2) | 548 (0.9) |
| Hyperlipidaemia | 891 (20.6) | 11 524 (19.3) |
| Cardiovascular disease | 1076 (24.9) | 10 837 (18.1) |
| Autoimmune disease (excluding RA) | 771 (17.8) | 10 875 (18.2) |
| Hospitalized infection | 118 (2.7) | 985 (1.6) |
| Medications at baseline, n (%) | ||
| MTXa | 2116 (48.9) | 33 699 (56.3) |
| Other csDMARDsa | 1709 (39.5) | 20 652 (34.5) |
| Parenteral antibiotics | 208 (4.8) | 2423 (4.0) |
| Oral corticosteroids | 2506 (57.9) | 33 154 (55.4) |
| Other corticosteroids | 1167 (27.0) | 14 762 (24.7) |
| NSAIDs | 1767 (40.8) | 27 044 (45.2) |
| Cholesterol-lowering medication | 954 (22.0) | 11 747 (19.6) |
Not including prescription on cohort entry day. bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD.
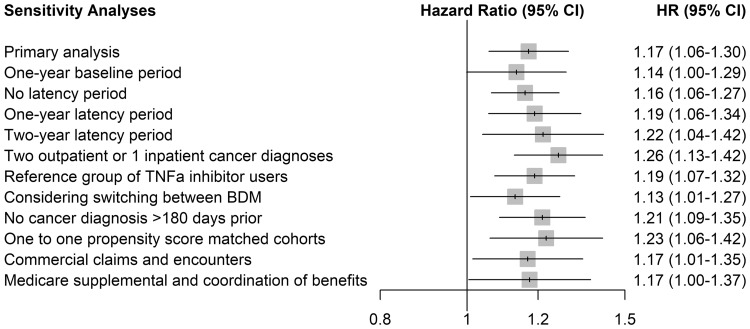
After allowing for the 6-month latency period, 409 patients were diagnosed with cancer among the 4328 patients on abatacept (incidence rate: 4.76 per 100 per year). In comparison, 4197 patients were diagnosed with cancer among the 59 860 on other bDMARDs (incidence rate: 3.41 per 100 per year). The adjusted HR (95% CI) of any cancer associated with abatacept use was 1.17 (1.06, 1.30) relative to other bDMARDs (Table 2). According to the Kaplan-Meier curve, there was no peak of increase early or late after initiation of abatacept (supplementary Fig. S2, available at Rheumatology online). The adjusted HR (95% CI) of any cancer, excluding NMSC, was 1.16 (1.01, 1.33). Analyses of specific cancer sites of interest showed a significant increased risk of NMSC (HR 1.20; 95% CI 1.03, 1.39). The HRs for the other specific cancer sites of interest generally showed a similar trend, although their CIs were wide and included unity. In particular, there was a trend towards an increased risk of breast cancer not reaching statistical significance (HR 1.25; 95% CI 0.94, 1.66). The results of the sensitivity analyses yielded findings consistent with those of the main analyses regarding the risk of cancer overall (Fig. 2). The stricter outcome definition (requiring two outpatient or one inpatient cancer diagnostic) generated HR that excluded the null (HR 1.26; 95% CI 1.13, 1.42). Compared with the use of TNF-α inhibitors only, use of abatacept was associated with a significant increase in the HR of cancer overall (HR 1.19; 95% CI 1.07, 1.32). Finally, when we considered switching between bDMARDs, the HR of any cancer remained significant (HR 1.13; 95% CI 1.01, 1.27).
Table 2.
Crude and adjusted HR of cancer overall and specific cancers associated with abatacept as the initial bDMARD compared with other bDMARDsa
| Initial treatment | Number of patients | Number of events | Person-years | Rate per 100 person-years | Crude HR | Adjusted HRb (95% CI) |
|---|---|---|---|---|---|---|
| Any cancer | ||||||
| Other bDMARD | 59 860 | 4197 | 123 254 | 3.41 | 1.00 | 1.00 (Reference) |
| Abatacept | 4328 | 409 | 8596 | 4.76 | 1.39 | 1.17 (1.06, 1.30) |
| Breast cancer | ||||||
| Other bDMARD | 45 815 | 491 | 94 967 | 0.52 | 1.00 | 1.00 (Reference) |
| Abatacept | 3613 | 53 | 7208 | 0.74 | 1.42 | 1.25 (0.94, 1.66) |
| Lung cancer | ||||||
| Other bDMARD | 59 860 | 216 | 123 254 | 0.18 | 1.00 | 1.00 (Reference) |
| Abatacept | 4328 | 21 | 8596 | 0.24 | 1.39 | 1.06 (0.67, 1.66) |
| Lymphoma | ||||||
| Other bDMARD | 59 860 | 234 | 123 254 | 0.19 | 1.00 | 1.00 (Reference) |
| Abatacept | 4328 | 21 | 8596 | 0.24 | 1.29 | 1.14 (0.73, 1.79) |
| Melanoma | ||||||
| Other bDMARD | 59 860 | 134 | 123 254 | 0.11 | 1.00 | 1.00 (Reference) |
| Abatacept | 4328 | 8 | 8596 | 0.09 | 0.86 | 0.77 (0.38, 1.59) |
| NMSC | ||||||
| Other bDMARD | 59 860 | 1798 | 123 254 | 1.46 | 1.00 | 1.00 (Reference) |
| Abatacept | 4328 | 182 | 8596 | 2.12 | 1.45 | 1.20 (1.03, 1.39) |
| Any cancer (excluding NMSC) | ||||||
| Other bDMARD | 59 860 | 2419 | 123 254 | 1.96 | 1.00 | 1.00 (Reference) |
| Abatacept | 4328 | 230 | 8596 | 2.68 | 1.36 | 1.16 (1.01, 1.33) |
Any patient with an outcome event occurring during the first 6 months after the initiation of exposure was not included in the analysis.
Adjusted for deciles of the propensity score and age. bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; NMSC: non-melanoma skin cancer; HR: hazard ratio.
Fig. 2.
Sensitivity analyses for the risk of cancer associated with abatacept as the initial bDMARD compared with other bDMARDs (primary objective)
bDMARD: biologic DMARD; HR: hazard ratio.
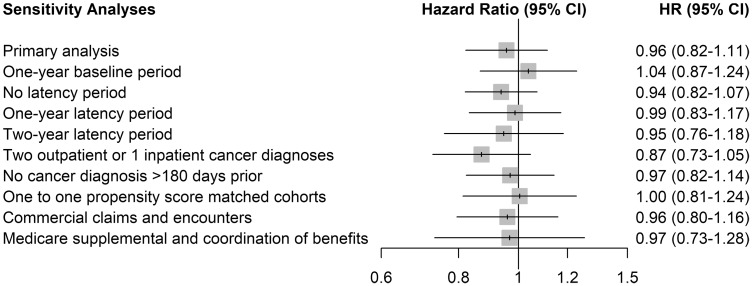
There were 176 patients with any cancer among the 2426 patients who initiated a bDMARD with MTX (incidence rate: 3.15 per 100 per year) and 4383 others among the 61 092 who initiated on bDMARD monotherapy (incidence rate: 3.49 per 100 per year). The adjusted HR of any cancer associated with the combination of bDMARD and MTX was 0.96 (95% CI 0.82, 1.11) relative to bDMARDs monotherapy (Table 3). The HRs for the specific cancer sites of interest showed a similar trend. The sensitivity analyses led to generally consistent results (Fig. 3).
Table 3.
Crude and adjusted HR of any cancer and specific cancers with the combination of bDMARD and MTX compared with bDMARD monotherapya
| Initial treatment | Number of patients | Number of events | Person-years | Rate per 100 person-years | Crude HR | Adjusted HRb (95% CI) |
|---|---|---|---|---|---|---|
| Any cancer | ||||||
| With MTX | 2426 | 176 | 5596 | 3.15 | 0.91 | 1.00 (Reference) |
| No MTX | 61 092 | 4383 | 125 441 | 3.49 | 1.00 | 0.96 (0.82, 1.11) |
| Breast cancer | ||||||
| No MTX | 46 989 | 517 | 97 201 | 0.53 | 1.00 | 1.00 (Reference) |
| With MTX | 1899 | 19 | 4298 | 0.44 | 0.84 | 0.87 (0.55, 1.37) |
| Lung cancer | ||||||
| No MTX | 61 092 | 226 | 125 441 | 0.18 | 1.00 | 1.00 (Reference) |
| With MTX | 2426 | 9 | 5596 | 0.16 | 0.89 | 0.96 (0.49, 1.87) |
| Lymphoma | ||||||
| No MTX | 61 092 | 245 | 125 441 | 0.20 | 1.00 | 1.00 (Reference) |
| With MTX | 2426 | 6 | 5596 | 0.11 | 0.55 | 0.59 (0.26, 1.33) |
| Melanoma | ||||||
| No MTX | 61 092 | 135 | 125 441 | 0.11 | 1.00 | 1.00 (Reference) |
| With MTX | 2426 | 6 | 5596 | 0.11 | 1.00 | 1.11 (0.48, 2.53) |
| NMSC | ||||||
| No MTX | 61 092 | 1894 | 125 441 | 1.51 | 1.00 | 1.00 (Reference) |
| With MTX | 2426 | 68 | 5596 | 1.22 | 0.81 | 0.85 (0.67, 1.09) |
| Any cancer (excluding NMSC) | ||||||
| No MTX | 61 092 | 2512 | 125 441 | 2.00 | 1.00 | 1.00 (Reference) |
| With MTX | 2426 | 108 | 5596 | 1.93 | 0.97 | 1.03 (0.85, 1.25) |
Any patient with an outcome event occurring during the first 6 months after the initiation of exposure was not included in the analysis. bAdjusted for deciles of the propensity score and age. bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; CI: confidence interval; NMSC: non-melanoma skin cancer; HR: hazard ratio.
Fig. 3.
Sensitivity analysis for the risk of cancer associated with the combination of bDMARD and MTX compared with bDMARD monotherapy (secondary objective)
bDMARD: biologic DMARD; CI: confidence interval; HR: hazard ratio.
Discussion
In this real-world cohort study of close to 64 000 patients with RA, patients initiating treatment with abatacept had a statistically significant 17% increased risk of overall cancer, compared with patients initiating treatment with other bDMARDs. There were no statistically significant differences for the specific cancers of interest (breast, lung, lymphoma and melanoma), with the exception of NMSC. Our findings for abatacept and cancer risk remained consistent in sensitivity analyses. The risks of cancer among the combination of a bDMARD and MTX were not elevated compared to those with bDMARD monotherapy. While these rates are suggestive of small increases in risk, they must be put into context with the limitations of real-world studies in order to understand their clinical significance.
To date, the three studies that evaluated the risk of cancer between bDMARDs when used as first-line treatment of RA did not find an increase in risk [9, 14, 15]. Cohort studies from Finland [14] and France [15] compared rituximab with TNF-α inhibitors, but were based on a small number of patients exposed to rituximab, 438 and 186 patients, respectively. The third study was a large prospective cohort study of the public health care system in Sweden that compared new users of non-TNF-α inhibitor bDMARDs (abatacept n = 2021, tocilizumab n = 1798 and rituximab n = 3586) and new users of TNF-α inhibitors (n = 15 129) [9]. In this study, abatacept was not associated with a significant increase in the risk of a first invasive solid or haematologic malignant neoplasm (excluding NMSC) compared with TNF-α inhibitors (HR 1.10, 95% CI 0.82, 1.48). However, the cohort of patients exposed to abatacept was smaller than ours; most patients starting treatment with abatacept had previously been treated with TNF inhibitors (81%). Our findings are consistent with those of this latter study for any malignancy excluding NMSC.
With respect to the risk of clinically relevant specific cancers, we found no association between abatacept use and the risk for breast cancer, lung cancer, lymphoma and melanoma. Since analyses for these specific cancer sites could be underpowered, further investigations are needed, especially for breast cancer where a numerical imbalance was found. A 20% increased risk of NMSC was observed, which is consistent with several previous observational studies. A first signal was found in a US registry for abatacept compared with csDMARDs for NMSC, but this signal was only based on two cases [8]. The Swedish study showed a higher risk of NMSC (HR 2.12; CI 95% 1.14, 3.95) [9] in abatacept users compared with TNF-α inhibitors [9]. Also, in a retrospective cohort study using Medicare data, patients treated with a combination of abatacept and MTX had a higher risk of NMSC recurrence compared with those receiving MTX monotherapy, although the increase was not statistically significant [7]. Finally, some cases of NMSC were also reported in clinical trials and in post-marketing studies [16–18].
CTLA-4 is an inhibitory molecule that plays a central role in down-regulating T cell activation. This provides the rationale for abatacept, a CTLA-4 analogue, in the treatment of autoimmune and inflammatory diseases. The role of CTLA-4 in cancer biology is more complex and includes weakened anti-tumour response and tumour progression [19], although there are conflicting data and the clinical significance remains uncertain [20]. Nevertheless, although we cannot rule out residual confounding from, for example, exposure to other immunosuppressant medications that could also contribute to cancer risk, the findings of this study are consistent with a possible role of CTLA-4 in allowing tumours to evade immune surveillance. While these findings require replication, it may be prudent to carefully monitor patients exposed to abatacept for NMSC, ensuring this is consistent with the package insert or prescribing recommendations.
Our study has several important strengths. First, our observational study is one of the largest studies to date on the risk of cancer in patients with RA initiating abatacept compared with patients initiating other bDMARDs. Second, we used relatively recent data to evaluate the risk of cancer in a population where abatacept is now frequently prescribed as first-line therapy. Third, this study involved a database that has been extensively used for pharmacoepidemiologic investigations, and previous studies indicate that these data are valid [21]. Fourth, we assessed the stability of our results in several sensitivity analyses, including various latency time-windows to explore the risk of cancer. Finally, with respect to exposure, we expect minimal misclassification bias since exposure was determined from dispensing records.
Despite the strengths of this study, our findings should be interpreted with consideration of its limitations. First, patients receiving abatacept anytime or as a first biologic still tend to be older and have more comorbid conditions. These data are limited in that clinical characteristics and severity of RA are not measurable. Second, although the MarketScan Database includes patients from the entire USA, it may not be entirely generalizable, since it contains patients more concentrated in the southern USA and a higher proportion of females. Moreover, patients with commercial insurance may not be representative of patients without insurance or those who receive government assistance (e.g. Medicaid) for medical care. Third, with respect to exposure, the 6-month baseline period may be too brief to confirm that the initiation of a bDMARD was truly based on the first ever bDMARD. However, the sensitivity analysis extending the baseline period to 1 year produced similar results. Fourth, this short baseline period may also be too brief to accurately identify whether all cancer cases were incident. In addition, older malignancies may not be recorded any longer and thus would not appear in the short baseline period. Fifth, with respect to the outcome measurement, identification of medical events is limited to data that are captured as part of the medical record or claims, and events were not linked to histopathologic findings. However, the sensitivity analysis defining a cancer by two separate diagnoses (or one inpatient cancer diagnostic) produced similar results. Sixth, while the cohort follow-up extended up to 8 years, the mean follow-up was only 2.1 years, which could underestimate the risk of adverse drug reactions such as cancers. Finally, despite the use of propensity score to balance the two comparison groups, these methods cannot eliminate residual confounding from unmeasured factors. Thus, there is the possibility that the results remain affected by unmeasured confounders, including smoking, sun exposure, obesity and alcohol consumption. However, we used several variables which could be considered as proxies, such as chronic obstructive pulmonary disease for smoking.
In our study conducted in a real-world US claims database, abatacept initiation in RA was associated with a slight increased risk of cancer overall relative to other bDMARDs. The increase was mainly for NMSC, with no significant increase for the other specific cancer sites. Add-on MTX on bDMARDs was not associated with a higher risk of cancer compared with a bDMARD monotherapy. Our results warrant replication in further large population-based studies. In the meantime, it may be prudent to carefully monitor patients exposed to abatacept for NMSC.
Funding: This research was funded in part by grants from the Canadian Institutes of Health Research (CIHR), Canadian Foundation for Innovation (CFI) and Bristol-Myers-Squibb. S.S. is the recipient of the James McGill Professorship award. F.M. received grants from La Fondation Pierre Deniker and Toulouse University Hospital (CHU Toulouse), France. L.A. is the recipient of a Chercheur-Boursier award from the Fonds de recherche du Québec – Santé and a William Dawson Scholar award. The sponsors were not involved in the conduct of the study. Bristol-Myers Squibb reviewed and approved the manuscript before its submission. The authors had ultimate control over the decision to publish and the final version of the manuscript submitted for publication. S.S. acts as guarantor of this manuscript.
Disclosure statement: S.S. has participated in advisory board meetings or as speaker or received research grants from AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol-Myers-Squibb and Novartis. All other authors declare no conflict of interest.
Supplementary Material
References
- 1. Korhonen R, Moilanen E.. Abatacept, a novel CD80/86-CD28 T cell co-stimulation modulator, in the treatment of rheumatoid arthritis. Basic Clin Pharmacol Toxicol 2009;104:276–84. [DOI] [PubMed] [Google Scholar]
- 2. Blair HA, Deeks ED.. Abatacept: a review in rheumatoid arthritis. Drugs 2017;77:1221–33. [DOI] [PubMed] [Google Scholar]
- 3. Askling J, Baecklund E, Granath F. et al. Anti-tumour necrosis factor therapy in rheumatoid arthritis and risk of malignant lymphomas: relative risks and time trends in the Swedish Biologics Register. Ann Rheum Dis 2009;68:648–53. [DOI] [PubMed] [Google Scholar]
- 4. Mercer LK, Green AC, Galloway JB. et al. The influence of anti-TNF therapy upon incidence of keratinocyte skin cancer in patients with rheumatoid arthritis: longitudinal results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2012;71:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raaschou P, Simard JF, Holmqvist M, Askling J.. Rheumatoid arthritis, anti-tumour necrosis factor therapy, and risk of malignant melanoma: nationwide population based prospective cohort study from Sweden. BMJ 2013;346:f1939. [DOI] [PubMed] [Google Scholar]
- 6. Saliba L, Moulis G, Abou Taam M. et al. Tumor necrosis factor inhibitors added to nonbiological immunosuppressants vs. nonbiological immunosuppressants alone: a different signal of cancer risk according to the condition. A disproportionality analysis in a nationwide pharmacovigilance database. Fundam Clin Pharmacol 2016;30:162–71. [DOI] [PubMed] [Google Scholar]
- 7. Scott FI, Mamtani R, Brensinger CM. et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol 2016;152:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon DH, Kremer JM, Fisher M. et al. Comparative cancer risk associated with methotrexate, other non-biologic and biologic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum 2014;43:489–97. [DOI] [PubMed] [Google Scholar]
- 9. Wadström H, Frisell T, Askling J.. Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical practice: a Nationwide Cohort Study from Sweden. JAMA Intern Med 2017;177:1605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim G, Barner JC, Rascati K, Richards K.. Factors associated with the initiation of biologic disease-modifying antirheumatic drugs in Texas Medicaid patients with rheumatoid arthritis. J Manag Care Spec Pharm 2015;21:401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buckley F, Finckh A, Huizinga TW, Dejonckheere F, Jansen JP.. Comparative efficacy of novel DMARDs as monotherapy and in combination with methotrexate in rheumatoid arthritis patients with inadequate response to conventional DMARDs: a network meta-analysis. J Manag Care Spec Pharm 2015;21:409–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MarketScan Databases. 2017. https://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases (22 November 2017, date last accessed).
- 13. MacLean C, Park G, Traina SB. et al. Positive predictive value (PPV) of an administrative data-based algorithm for the identification of patients with rheumatoid arthritis (RA). Arthritis Rheum 2001;44:106. [Google Scholar]
- 14. Aaltonen KJ, Joensuu JT, Virkki L. et al. Rates of serious infections and malignancies among patients with rheumatoid arthritis receiving either tumor necrosis factor inhibitor or rituximab therapy. J Rheumatol 2015;42:372–8. [DOI] [PubMed] [Google Scholar]
- 15. Slimani S, Lukas C, Combe B, Morel J.. Rituximab in rheumatoid arthritis and the risk of malignancies: report from a French cohort. Joint Bone Spine 2011;78:484–7. [DOI] [PubMed] [Google Scholar]
- 16.US FDA. Drug Approval Package—Orencia (Abatacept) Injectable (IV) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/125118_s0000_OrenciaTOC.cfm (2 october 2018, date last accessed).
- 17.Swiss Medic. Orencia (Abatacept)-Risque de carcinome épidermoide cutané, de papillome de la peau, de lymphome et de néoplasme malin du poumon 2017. https://www.swissmedic.ch/swissmedic/fr/home/medicaments-a-usage-humain/surveillance-du-marche/health-professional-communication–hpc-/dhpc-orencia-abatacept.html (2 October 2018, date last accessed).
- 18. Corcorran MA, Olson JM, Hecht C, Knezevich S, Vary JC.. Eruptive squamous cell carcinoma in a patient receiving abatacept for rheumatoid arthritis. J Am Acad Dermatol 2013;69:e178–9. [DOI] [PubMed] [Google Scholar]
- 19. Wang SD, Li HY, Li BH. et al. The role of CTLA-4 and PD-1 in anti-tumor immune response and their potential efficacy against osteosarcoma. Int Immunopharmacol 2016;38:81–9. [DOI] [PubMed] [Google Scholar]
- 20. Hu P, Liu Q, Deng G. et al. The prognostic value of cytotoxic T-lymphocyte antigen 4 in cancers: a systematic review and meta-analysis. Sci Rep 2017;7:42913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truven Health Analytics. Epidemiology MarketScan Research Databases 2017. http://truvenhealth.com/markets/life-sciences/expertise/market-knowledge/epidemiology (22 November 2017, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.