Abstract
Objectives
To examine the incidence, predictors and outcomes associated with severe gastrointestinal (GI) disease in a large inception SSc cohort.
Methods
SSc subjects with <2 years of disease duration were identified from two multicentre cohorts. Severe GI disease was defined as: malabsorption, hyperalimentation, pseudo-obstruction and/or ⩾10% weight loss in association with the use of antibiotics for bacterial overgrowth or oesophageal stricture. Kaplan–Meier, multivariate logistic regression and Cox proportional hazard analyses were performed to determine the cumulative incidence rate, independent clinical correlates and mortality rate associated with severe GI disease. A longitudinal mixed model was used to assess the impact of severe GI disease on the Short Form Health Survey.
Results
In this inception SSc cohort, the probability of developing severe GI disease was estimated at 9.1% at 2 years and 16.0% at 4 years. In multivariate analysis, severe GI disease was associated with inflammatory myositis (odds ratio 4.68, 95% CI 1.65, 13.24), telangiectasias (odds ratio 2.45, 95% CI 1.19, 5.04) and modified Rodnan skin score (odds ratio 1.03, 95% CI 1.01, 1.07). Severe GI disease was associated with a >2-fold increase in the risk of death (hazard ratio 2.27, 95% CI 1.27, 4.09) and worse health-related quality of life [Short Form Health Survey physical (β = −2.37, P = 0.02) and mental (β = −2.86, P = 0.01) component summary scores].
Conclusion
Severe GI disease is common in early SSc and is associated with significant morbidity and increased mortality. More research is needed to understand, prevent and mitigate severe GI disease in SSc.
Keywords: scleroderma, systemic sclerosis, gastrointestinal manifestations, mortality, health-related quality of life
Rheumatology key messages
Severe gastrointestinal involvement was common in this large inception cohort of scleroderma subjects.
Development of severe gastrointestinal disease was associated with muscle inflammation, skin fibrosis and vasculopathy.
Severe gastrointestinal involvement was associated with increased mortality and impairment in health-related quality of life.
Introduction
SSc is an autoimmune disorder characterized by vasculopathy, immunologic dysregulation, excessive collagen deposition and fibrosis affecting both skin and visceral organs [1]. SSc-related mortality remains very high in the current era [2–4]. The gastrointestinal (GI) tract is the most commonly involved internal organ, affecting up to 75–90% of patients [5–7]. In the largest study to date, severe GI disease was reported to affect 8% of SSc subjects and was associated with significant mortality, with only 15% of patients alive after 9 years of disease [8]. However, that study had some limitations: it was a single-centre study from a referral centre, and it included only subjects with diffuse cutaneous disease and subjects with prevalent disease (30% had >2 years of disease duration). Thus, the results were possibly affected by selection and survival biases.
The objective of this study was to investigate the incidence, predictors and outcomes of severe GI disease in a large, multicentre cohort of subjects with early SSc. We hypothesized that onset of severe GI involvement in early SSc was associated with increased morbidity and mortality.
Methods
Patient source
Subjects were SSc patients enrolled in either the Canadian Scleroderma Research Group (CSRG) registry or the Australian Scleroderma Cohort Study (ASCS). Briefly, subjects in the CSRG are recruited from 14 sites across Canada and must have a diagnosis of SSc verified by an experienced rheumatologist, be ⩾18 years of age and be fluent in either English or French. Over 98% of the subjects in the CSRG meet the 2013 ACR/EULAR classification criteria for SSc [9]. Patients in the ASCS are recruited by Australian Scleroderma Interest Group (ASIG) investigators from 12 Australian centres specializing in the care of patients with SSc, according to similar inclusion criteria. All patients included in this study fulfilled either the 1980 ACR [10] or Leroy and Medsger criteria for SSc [11]. Seven subjects from the ASCS database did not meet the ACR/EULAR classification criteria [12].
The inception cohort was defined as a subset of subjects with disease duration <2 years since the onset of the first non-RP disease symptom attributable to SSc at the time of their baseline study visit. We included all adult (⩾18 years) SSc subjects with a baseline visit in the CSRG cohort between January 2005 and July 2017, and in the ASCS between January 2007 and June 2017.
Ethics committee approval for this study was obtained at McGill University (Montreal, Quebec, Canada) and at all participating CSRG and ASCS study sites. All subjects provided informed written consent to participate in the data collection protocols.
Primary outcomes
Severe GI disease was defined using a previously published definition [8]. According to this definition, severe GI disease was considered present if a physician reported the presence of malabsorption, the need for hyperalimentation, one or more episodes of pseudo-obstruction and/or a ⩾10% weight loss in association with the use of antibiotics for small intestinal bacterial overgrowth within the last year or oesophageal stricture. Malabsorption was defined in the CSRG registry by physician reports that the patient answered yes to ‘Do you pass stools that are difficult to flush, particularly foul smelling or associated with a ring of grease in the toilet bowl’ and/or low ferritin with no blood loss, elevated international normalized ratio, low vitamin B12 (in the absence of pernicious anaemia), low carotene, or low magnesium or calcium otherwise unexplained. Malabsorption was defined in the ASCS registry as being actively treated with cyclic antibiotics and presence of chronic diarrhoea within the last year. Hyperalimentation was defined as nutritional supplementation either through a regular feeding tube (nasogastric or percutaneous endoscopic gastrostomy) or i.v. total parenteral nutrition. Episodes of pseudo-obstruction were identified by physician reports. Oesophageal strictures in the CSRG were identified by physician reports of subjects requiring oesophageal dilatation, whereas the ASCS defined oesophageal stricture as those having definite evidence on either endoscopy or barium swallow.
Health-related quality of life (HRQoL) was measured using version 2 of the Medical Outcomes Study Short Form-36 (SF-36) [13]. The SF-36 is a self-administered questionnaire covering eight domains: physical functioning, social functioning, role limitations related to physical problems, role limitations related to emotional problems, mental health, vitality, bodily pain and general health perceptions. Each domain can be scored separately, with scores ranging from 0, indicating the worst health state, to 100, indicating the best health state. Domain scores can also be summarized into a Physical Component Summary (PCS) score and a Mental Component Summary (MCS) score [14]. The PCS and MCS are scored using norm-based scoring based on a general population sample to produce T scores for each patient (mean of 50 and s.d. of 10). Thus, for the two summary scores, HRQoL is worse than average if it is below 50 and better than average if it is above 50, and each point is one-tenth of an s.d. The SF-36 has been previously validated in rheumatic diseases including SSc [15–17].
Mortality was assessed at any point during the study period based on information provided to study physicians, or on information provided to a research assistant at the time of scheduling a yearly visit. A standardized death case report form was completed by the treating doctor for all deaths in every centre to identify the cause of death according to SSc-related and non-SSc-related causes.
Clinical covariates
Demographic and lifestyle characteristics (age, sex, ethnicity, education and smoking status) were collected through patient self-report. Disease duration was recorded by study physicians. Full-time and part-time workers were considered as employed whereas unemployed subjects, retired subjects and full-time students were considered as not employed. Subjects on temporary or permanent disability were identified as disabled.
Skin involvement was assessed using the modified Rodnan skin score (mRSS), a validated measure of skin thickening in SSc [18]. This score ranges from 0 (no involvement) to 3 (severe thickening) in 17 areas (total score range 0–51) [19]. Subjects were classified into lcSSc (skin involvement distal to the elbows and knees with or without facial involvement) and dcSSc (skin involvement proximal to the elbows and knees, with or without truncal involvement) subsets. Those with a clinical diagnosis of SSc but no skin involvement were included in the lcSSc subset [20].
Study physicians performed a standardized history and physical examination. The presence of digital ulcers (DU) on any aspect of the finger and distal to the PIP joint were recorded. The presence of telangiectasias, defined by visible dilatation of superficial cutaneous blood vessels that collapse upon pressure (excluding normal sun exposure-related telangiectasia) at any time prior to the baseline study visit, were also recorded. Any history of swollen joints or synovitis, excluding degenerative arthritides, were defined as an inflammatory polyarthritis. Inflammatory myositis was also recorded according to physician’s report. Faecal incontinence was recorded if patients self-reported faecal soilage prior to the baseline study visit.
The presence of interstitial lung disease (ILD) was determined using a published clinical decision tool [21]. According to this algorithm, ILD was considered present if a high resolution CT scan of the lung was interpreted by an experienced radiologist as showing ILD. Alternatively, in cases where no high resolution CT was available, ILD was considered present if a chest X-ray was reported as showing either increased interstitial markings (not thought to be due to congestive heart failure) or fibrosis, and/or if a study physician reported the presence of typical velcro-like crackles on physical examination. Pulmonary function tests were performed in local laboratories working in accordance with American Thoracic Society standards. The percent predicted value for forced vital capacity (FVC) was extracted from laboratory reports.
Pulmonary hypertension was defined as an estimated systolic pulmonary artery pressure ⩾45 mmHg measured using the Doppler flow measurement of the flow measurement of the tricuspid regurgitant jet on echocardiography (an estimate that correlates strongly with the values reported in right-sided heart catheter studies [22]).
Serology
Autoantibody analysis of the CSRG cohort was performed in a central laboratory at the University of Calgary where samples were aliquotted and stored at −80°C until needed. ACA (CENP-A and CENP-B), anti-topoisomerase I antibodies and anti-RNA polymerase III antibodies (RP11 and RP155) were detected by Euroline SSc profile line immunoassay (Euroimmun, Luebeck, Germany) according to manufacturer’s instructions. The analyses of the ASCS cohort were performed at local laboratories according to local assay and protocol. With the intent of optimizing specificity, antibodies were reported as absent (negative, equivocal and low titres) and present (moderate and high titres).
Statistical analysis
Descriptive statistics were used to summarize baseline demographic and clinical characteristics of the patients. Continuous variables are presented as mean ± s.d. and categorical variables are presented as counts and percentages. Student’s t-test and Wilcoxon-Mann–Whitney U test were used to compare continuous variables. χ2 test and Fisher’s exact test were used for categorical variables. A multivariate logistic regression model was used to identify independent predictors of severe GI disease. Sociodemographic and clinical variables associated with severe GI disease with P-values <0.1 in univariate analyses were included in the multivariate model.
Cumulative incidence of severe GI disease was calculated using a Kaplan–Meier plot. Mortality rates between subjects with and without severe GI disease were compared using a Cox proportional hazards analysis, with severe GI disease modelled as a time-dependent variable and adjusted for age and sex. Subjects were censored at death or last study visit if they were still alive and had not developed severe GI disease.
The impact of severe GI involvement on HRQoL was assessed using a longitudinal mixed effect model with SF-36 PCS and MCS as outcome variables and severe GI disease and time as exposure variables. The longitudinal dataset was only available for subjects enrolled in the CSRG registry and all consecutive outcome and exposure variables were included. In order to improve model fit, different models were compared using a likelihood ratio test (anova function in R) [23]. The final crude model was generated for the fixed effects of severe GI disease and both the fixed and the random effects of time. An adjusted multivariate model was also generated with additional covariates likely to impact HRQoL (i.e. fixed effects of age, sex, mRSS, FVC, DU, inflammatory myositis and inflammatory arthritis). All the mixed models were generated using the lmer and lme function under the lme4 and lmerTest package in R [24, 25].
In multivariate analyses, P-values ⩽0.05 were considered statistically significant. Longitudinal mixed effect model were generated with R version 3.2.0 for Windows (http://r-project.org) and all other statistical analysis were performed with SAS v.9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of the study sample
The study cohort consisted of 556 SSc subjects (311 Canadian and 245 Australian subjects) with disease duration <2 years (Table 1). Of these, the mean age was 54.0 ± 13.0 years, 449 (80.8%) were women, 460 (88.5%) were white, the mean disease duration was 1.1 ± 0.5 years and 259 (46.6%) had dcSSc. ACA were present in 149 (31.8%) of the subjects, whereas anti-topoisomerase I and anti-RNA polymerase III antibodies were found in 100 (21.5%) and 94 (23.2%), respectively.
Table 1.
Baseline characteristics of study subjects according to presence of GI disease (N = 556)
| Severe GI disease (n = 72) | No GI disease (n = 484) | ||||
|---|---|---|---|---|---|
| n (%) or mean (s.d.) | No. missing | n (%) or mean (s.d.) | No. missing | P-value | |
| Age, years | 53.2 (14.0) | 0 | 54.2 (12.8) | 1 | 0.533 |
| Female, % | 58 (80.6) | 0 | 391 (80.8) | 0 | 0.963 |
| White, % | 61 (89.7) | 4 | 399 (88.3) | 32 | 0.731 |
| Aboriginal, % | 2 (2.9) | 4 | 8 (1.8) | 32 | 0.627 |
| Post-secondary education, % | 28 (41.8) | 5 | 165 (39.4) | 65 | 0.708 |
| Employment status, % | 4 | 27 | 0.775 | ||
| Employed | 34 (50.0) | 237 (51.9) | |||
| Not employed | 15 (22.1) | 102 (22.3) | |||
| Disabled | 19 (27.9) | 118 (25.8) | |||
| Current smoker, % | 6 (8.8) | 4 | 52 (11.4) | 26 | 0.534 |
| Disease duration, years | 1.1 (0.5) | 0 | 1.1 (0.5) | 0 | 0.470 |
| BMI, kg/m2 | 25.3 (5.1) | 2 | 26.3 (5.8) | 29 | 0.226 |
| Diffuse disease, % | 47 (65.3) | 0 | 212 (43.8) | 0 | < 0.001 |
| mRSS (0–51) | 17.8 (12.3) | 0 | 12.3 (11.1) | 3 | < 0.001 |
| ILD, % | 22 (30.6) | 0 | 148 (31.3) | 11 | 0.900 |
| FVC, % predicted | 90.0 (21.5) | 4 | 93.2 (20.3) | 52 | 0.236 |
| Pulmonary hypertension, % | 5 (7.7) | 7 | 44 (11.8) | 111 | 0.333 |
| Digital ulcers, % | 25 (34.7) | 0 | 179 (37.1) | 2 | 0.692 |
| Telangiectasias, % | 45 (63.4) | 1 | 243 (51.2) | 9 | 0.054 |
| Inflammatory myositis, % | 10 (14.5) | 3 | 20 (4.5) | 42 | 0.003 |
| Inflammatory arthritis, % | 25 (39.7) | 9 | 119 (27.8) | 56 | 0.053 |
| Faecal incontinence, % | 9 (13.4) | 5 | 30 (6.9) | 47 | 0.061 |
| Autoantibodies, % | |||||
| ACA | 12 (21.1) | 15 | 137 (33.3) | 73 | 0.062 |
| Anti-topoisomerase I | 13 (22.8) | 15 | 87 (21.3) | 75 | 0.791 |
| Anti-RNA polymerase III | 13 (25.5) | 21 | 81 (22.9) | 130 | 0.680 |
| Severe GI disease, % | |||||
| Malabsorption | 42 (58.3) | 0 | |||
| Hyperalimentation | 14 (19.4) | 0 | |||
| Pseudo-obstruction | 20 (27.8) | 0 | |||
| SIBO or ES with ≥10% weight loss | 7 (9.7) | 0 | |||
| Death during follow-up | |||||
| Mortality, % | 14 (19.4) | 0 | 70 (14.5) | 0 | 0.271 |
| Cause of death | |||||
| Scleroderma-related | |||||
| PAH | 3 | 16 | |||
| ILD | 1 | 12 | |||
| Combined severe PAH and ILD | 1 | 4 | |||
| Scleroderma myocardial involvement | 1 | 2 | |||
| Renal failure due to scleroderma renal crisis | 2 | 7 | |||
| Scleroderma gut involvement | 3 | 6 | |||
| Not scleroderma-related | |||||
| Ischaemic heart disease | 0 | 2 | |||
| Cerebrovascular disease | 0 | 1 | |||
| Renal failure not due to scleroderma renal crisis | 0 | 1 | |||
| Malignancy | 2 | 10 | |||
| Other | 0 | 3 | |||
| Unknown | 1 | 6 | |||
GI: gastrointestinal; mRSS: modified Rodnan skin score; FVC: forced vital capacity; ILD: interstitial lung disease; SIBO: small intestinal bacterial overgrowth; ES: oesophageal stricture; PAH: pulmonary arterial hypertension.
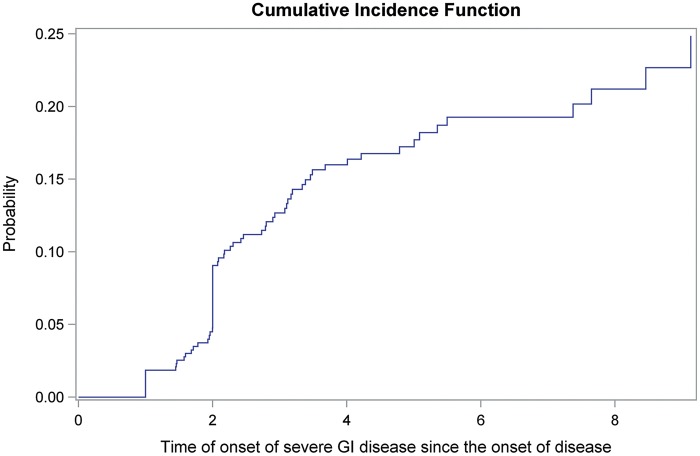
Incidence of severe GI disease
In this inception SSc cohort, 26 (4.7%) subjects had severe GI disease at the baseline study visit and 46 (8.3%) developed it over a mean follow-up time of 4.3 ± 3.0 years, including malabsorption in 42 (58.3%), hyperalimentation in 14 (19.4%), pseudo-obstruction in 20 (27.8%) and a ⩾10% weight loss associated with the use of antibiotics for small intestinal bacterial overgrowth or oesophageal stricture in 7 (9.7%). The probability of developing severe GI disease early in the course of disease was high, estimated at 9.1% at 2 years and 16.0% at 4 years (Fig. 1). The cumulative incidence continued to rise thereafter, reaching 19.3% at 6 years and 21.3% at 8 years.
Fig. 1.
Cumulative incidence curve to show time to onset of severe GI disease since the onset of disease (N=556)
Baseline predictors of severe GI disease
Subjects who developed severe GI disease were more likely to have diffuse cutaneous disease (65.3 vs 43.8%, P < 0.001), higher skin scores (mRSS 17.8 ± 12.3 vs 12.3 ± 11.1, P < 0.001) and inflammatory myositis (14.5 vs 4.5%, P = 0.003) compared with those who did not (Table 1). In addition, the presence of telangiectasia (68.0 vs 52.0%, P = 0.054), inflammatory arthritis (39.7 vs 27.8%, P = 0.053) and faecal incontinence (13.4 vs 6.9%, P = 0.061) tended to be associated with severe GI disease. There were trends for subjects who developed severe GI disease to have less ACA positivity (21.1 vs 33.3%, P = 0.062). There were no significant differences between both groups in age, ethnicity, disease duration, education or smoking status. No differences were noted in other clinical variables, including ILD, DU and pulmonary hypertension.
In multivariate logistic regression analysis (Table 2), three variables were independently associated with severe GI disease: inflammatory myositis (odds ratio 4.68, 95% CI 1.65, 13.24, P = 0.004), telangiectasias (odds ratio 2.45, 95% CI 1.65, 13.24, P = 0.015) and mRSS (odds ratio 1.03, 95% CI 1.01, 1.07, P = 0.046).
Table 2.
Multivariate logistic regression to identify independent predictors of severe GI disease in SSc
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| mRSS | 1.03 | 1.01, 1.07 | 0.046 |
| Telangiectasias | 2.45 | 1.19, 5.04 | 0.015 |
| Inflammatory myositis | 4.68 | 1.65, 13.24 | 0.004 |
| Inflammatory arthritis | 1.66 | 0.84, 3.28 | 0.148 |
| Faecal incontinence | 1.71 | 0.59, 4.96 | 0.327 |
| ACA | 0.81 | 0.33, 2.00 | 0.643 |
Variables included in the model are those with P < 0.1 in univariate analyses. GI: gastrointestinal; mRSS: modified Rodnan skin score.
Mortality
A total of 14 (19.4%) and 70 (14.5%) subjects with and without severe GI disease, respectively, died during the follow-up period. After adjusting for age and sex, the hazard ratio for death in those with severe GI disease compared with those without was 2.27 (95% CI 1.27, 4.09, P = 0.006; Table 3). Age was also found to be independently associated with the risk of death (hazard ratio 1.06, 95% CI 1.04, 1.08, P < 0.001), whereas female sex was protective (hazard ratio 0.54, 95% CI 0.33, 0.88, P = 0.14).
Table 3.
Cox proportional hazard model showing the association between severe GI disease and risk of death starting from disease onset (N = 556)
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| Model 1 (crude) | |||
| Severe GI disease over time | 1.91 | 1.06, 3.45 | 0.032 |
| Model 2 (adjusted) | |||
| Severe GI disease over time | 2.27 | 1.27, 4.09 | 0.006 |
| Female | 0.54 | 0.33, 0.88 | 0.014 |
| Age at disease onset | 1.06 | 1.04, 1.08 | <0.001 |
GI: gastrointestinal.
HRQoL
A total of 1132 visits with completed SF-36 data were available for analysis on 311 Canadian patients (Table 4). After adjusting for sex, mRSS, FVC, DU, inflammatory myositis and inflammatory arthritis, severe GI disease was associated with worse physical (SF-36 PCS β = −2.37, s.e. 1.04, P = 0.023) and mental (SF-36 MCS β = −2.86, s.e. 1.16, P = 0.014) HRQoL. Our model also showed that follow-up time and higher FVC had a positive impact on the MCS, whereas older age, worse skin score, lower FVC and inflammatory myositis were negatively associated with the PCS.
Table 4.
Longitudinal mixed effects model to assess the relationship between severe GI disease and health-related quality of life (n = 311)
| SF-36 PCS | SF-36 MCS | |||||
|---|---|---|---|---|---|---|
| β | s.e. | P-value | β | s.e. | P-value | |
| Model 1 (crude) | ||||||
| Intercept | 37.58 | 0.63 | <0.001 | 47.03 | 0.71 | <0.001 |
| Severe GI disease | −1.73 | 1.01 | 0.087 | −3.30 | 1.12 | 0.003 |
| Time, in years | 0.03 | 0.14 | 0.801 | 0.37 | 0.16 | 0.024 |
| Model 2 (adjusted) | ||||||
| Intercept | 37.20 | 3.53 | <.001 | 35.85 | 3.69 | <0.001 |
| Severe GI disease | −2.37 | 1.04 | 0.023 | −2.86 | 1.16 | 0.014 |
| Time, in years | 0.03 | 0.14 | 0.808 | 0.49 | 0.17 | 0.005 |
| Female | −0.52 | 1.51 | 0.730 | −1.77 | 1.55 | 0.255 |
| Age, in years | −0.10 | 0.05 | 0.037 | 0.06 | 0.05 | 0.195 |
| mRSS | −0.21 | 0.04 | <0.001 | 0.05 | 0.04 | 0.214 |
| FVC % predicted | 0.10 | 0.02 | <0.001 | 0.10 | 0.02 | <0.001 |
| Digital ulcers | −0.14 | 0.57 | 0.801 | −0.14 | 0.72 | 0.841 |
| Inflammatory myositis | −3.53 | 1.06 | <0.001 | 0.42 | 1.30 | 0.745 |
| Inflammatory arthritis | −0.72 | 0.75 | 0.341 | 0.01 | 0.92 | 0.993 |
SF-36: Short Form 36; GI: gastrointestinal; MCS: Mental Component Summary; PCS: Physical Component Summary; mRSS: modified Rodnan skin score; FVC: forced vital capacity.
Discussion
Severe GI disease was common in this inception cohort, affecting >15% of subjects by the fourth year of the disease. The presence of inflammatory myositis, telangiectasias and higher skin scores were independently associated with the onset of severe GI involvement. As per our hypothesis, severe GI disease was associated with a striking increase in the risk of mortality and impairment in HRQoL.
In the past, severe GI disease has been reported to occur in 5–8% of SSc subjects [8, 26]. However, most studies included subjects with both incident and prevalent disease, raising the spectre of survival bias. Our study of subjects with only incident disease indicates that severe GI disease is several times more common than previously thought. Moreover, our results showed an approximately 2-fold higher risk of mortality in those with severe GI disease in a large, unselected, multicentre cohort of early SSc subjects, again mitigating the risk of bias.
The pathogenesis of SSc is poorly understood but has been hypothesized to be mediated by vasculopathy, immunologic dysregulation and fibrosis [27]. Although large gaps persist in our understanding of the underlying mechanisms of GI involvement in SSc, it seems that myogenic abnormalities may have a major contributory role [28–31]. We found a strong association between severe GI disease and inflammatory myositis, suggesting a common link in the pathogenesis of these two manifestations of SSc. Nishimagi et al. [32] previously proposed that injury to both skeletal muscle and intestinal smooth muscle could occur simultaneously in early SSc subjects with severe GI involvement. In addition, we found a higher rate of faecal incontinence in subjects with severe GI involvement, which seems to further support the pathogenic role of smooth muscle injury in SSc [31, 33]. Additional studies are needed to explore the pathogenic role of myopathy in severe GI disease related to SSc.
Independent associations between severe GI disease, higher skin scores and telangiectasia are intriguing. Previous papers have suggested that telangiectasia represent underlying vasculopathy [34, 35], whereas higher skin scores are associated with more active fibrosis [36, 37]. These associations support the possibility that multiple mechanisms including fibrosis and vasculopathy contribute to the pathogenesis of GI disease in SSc [38–40], in addition to muscle involvement.
Complications from GI dysmotility have been reported to be associated with ACA positivity [41, 42]. Interestingly, in our cohort, subjects with severe GI involvement had a lower rate of ACA positivity compared with those without severe GI involvement. In a large Japanese cohort of SSc subjects with disease duration <2 years, ACA were absent in all subjects who were admitted to the hospital for severe GI disease [32]. This highlights that predictors for severe GI involvement differ from those with mild or moderate GI involvement.
Our results not only show an increased risk of mortality, but also an association with significant impairment in HRQoL. Other studies have also shown an influence of GI involvement on HRQoL in SSc [40, 43–45] and our study confirms these findings for subjects with severe GI involvement using a generic measure of health status. Using robust methodology accounting for bias due to missing data [46, 47], we were able to assess the independent relationship between the presence of severe GI disease and both physical and mental health status impairment. We believe that further study assessing the impact of severe GI disease on HRQoL using a GI-specific measure tool would be warranted as well. To our knowledge, this is the first study of the relationship between GI involvement in SSc and HRQoL using a longitudinal rather than cross-sectional database.
This study is not without limitations. Most importantly, the presence of severe GI disease relied largely on physicians’ reports and was not objectively verified. However, the fact that all study physicians were rheumatologists with experience in the care of patients with SSc provides support for the validity of these diagnoses. Second, our study does not include data on overlap with other autoimmune disease. Even though this would have provided more insight on SSc severity, diseases with direct impact on GI symptoms (inflammatory bowel disease, coeliac disease) were unlikely to be present in our cohort [48–50]. Third, one could argue that some subjects with severe GI disease died before they entered the cohort. Indeed, data from a large multinational inception cohort (including CSRG and ASCS subjects) show that mortality in early SSc is significant [3]. However, the latter study included patients within 4 years of disease onset and very few deaths were recorded before 2 years of disease. Our study included subjects with even shorter disease duration and therefore we believe that bias from left-truncation is limited. If in fact we did miss some deaths from severe GI disease before 2 years of disease, then our results are a conservative estimate of the actual impact of severe GI involvement in SSc. Finally, even though the ASCS data collection protocol collected information regarding gastric antral vascular ectasia, this marker of GI disease severity was not included in this study since it was not part of the CSRG collection protocol. Additionally, our data were collected in two separate longitudinal cohorts that used different definitions for some of the outcomes of interest, including the definition of malabsorption and oesophageal strictures. We acknowledge that there could be some heterogeneity in the definition of severe GI disease; nevertheless, the combination of the two cohorts allowed us to sample a large multicentre cohort of subjects with very early disease and prospectively collected data. The limitations of our analysis are counter-balanced by its strengths and we believe that our study provides the most robust estimate of severe GI disease in SSc to date.
Conclusions
In conclusion, we found that severe GI disease is common in early SSc and is associated with an increased risk of mortality and a significant impairment in HRQoL. Subjects at high risk for developing severe GI involvement were those with markers of muscle inflammation, skin fibrosis and vasculopathy. Myositis was found to be the strongest independent predictor for severe GI disease and suggests that a common pathway might be targeted in order to address both the skeletal muscle and intestinal smooth muscle involvement. Further research should focus on understanding and identifying preventive strategies for this serious manifestation of SSc.
Acknowledgements
We would like to acknowledge Dr Michelle Wilson and Ms Candice Rabusa for their substantial contribution with data extraction. We would also like to thank Dr Mireille Schnitzer for her assistance with data analysis.
Canadian Scleroderma Research Group (CSRG) investigators:
M. Baron, Montreal, Quebec; M. Hudson, Montreal, Quebec; G. Gyger, Montreal, Quebec; J. Pope, London, Ontario; M. Larché, Hamilton, Ontario; N. Khalidi, Hamilton, Ontario; A. Masetto, Sherbrooke, Quebec; E. Sutton, Halifax, Nova Scotia; D. Robinson, Winnipeg, Manitoba; T.S. Rodriguez-Reyna, Mexico City, Mexico; D. Smith, Ottawa, Ontario; C. Thorne, Newmarket, Ontario; P.R. Fortin, Quebec, Quebec; M. Fritzler, Mitogen Advanced Diagnostics Laboratory, Cumming School of Medicine, Calgary, Alberta.
Australian Scleroderma Interest Group (ASIG) investigators:
L. Croyle, Melbourne, Victoria; J. de Jager, Gold Coast, Queensland; N. Ferdowsi, Melbourne, Victoria; C. Hill, Adelaide, South Australia; R. Laurent, Sydney, New South Wales; S. Lester, Adelaide, South Australia; G. Major, Newcastle, New South Wales; K. Morrisroe, Melbourne, Victoria; P. Nash, Sunshine Coast, Queensland; G. Ngian, Melbourne, Victoria; M. Nikpour, Melbourne, Victoria; S. Proudman, Adelaide, South Australia; M. Rischmueller, Adelaide, South Australia; J. Roddy, Perth, Western Australia; J. Sahhar, Melbourne, Victoria; L. Schrieber, Sydney, New South Wales; W. Stevens, Melbourne, Victoria; G. Strickland, Geelong, Victoria; A. Sturgess, Sydney, New South Wales; V. Thakkar, Liverpool, New South Wales; K. Tymms, Canberra, Australian Capital Territory; J. Walker, Adelaide, South Australia; P. Youseff, Sydney, New South Wales; J. Zochling, Hobart, Tasmania.
Funding: The Canadian Scleroderma Research Group (CSRG) is funded by the Canadian Institutes of Health Research (CIHR) (grant #FRN 83518), the Scleroderma Society of Canada and its provincial Chapters, Scleroderma Society of Ontario, Scleroderma Society of Saskatchewan, Sclérodermie Québec, Cure Scleroderma Foundation, INOVA Diagnostics Inc. (San Diego, CA, USA), Dr Fooke Laboratorien GmbH (Neuss, Germany), Euroimmun (Lubeck, Germany), Mikrogen GmbH (Neuried, Germany), Fonds de la recherche du Québec - Santé (FRQS), the Canadian Arthritis Network (CAN) and the Lady Davis Institute of Medical Research of the Jewish General Hospital, Montreal, QC. The CSRG has also received educational grants from Pfizer and Actelion pharmaceuticals. M.H. is funded by the Fonds de la recherche du Québec – Santé (FRQS). The Australian Scleroderma Interest Group (ASIG) is supported by Scleroderma Australia, Scleroderma Victoria, Arthritis Australia, Actelion Australia, MOVE, The Australian Rheumatology Association, The Scleroderma Clinical Trials Consortium, St Vincent’s Hospital Research Endowment Fund, Bayer, CSL Biotherapies, GlaxoSmithKline Australia, Roche and Pfizer. N.R. is funded by the Hôpital Maisonneuve Rosemont Department of Medicine Foundation Fund. M.H. is funded by the Fonds de la recherche du Québec – Santé (FRQS). M.N. holds a National Health and Medical Research Council of Australia Career Development Fellowship (APP 1126370).
Disclosure statement: All authors have no conflicts of interest to declare. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Contributor Information
Canadian Scleroderma Research Group (CSRG):
M Baron, M Hudson, G Gyger, J Pope, M Larché, N Khalidi, A Masetto, E Sutton, D Robinson, T S Rodriguez-Reyna, D Smith, C Thorne, P R Fortin, and M Fritzler
Australian Scleroderma Interest Group (ASIG):
L Croyle, J de Jager, N Ferdowsi, C Hill, R Laurent, S Lester, G Major, K Morrisroe, P Nash, G Ngian, M Nikpour, S Proudman, M Rischmueller, J Roddy, J Sahhar, L Schrieber, W Stevens, G Strickland, A Sturgess, V Thakkar, K Tymms, J Walker, P Youseff, and J Zochling
References
- 1. Sjogren RW. Gastrointestinal motility disorders in scleroderma. Arthritis Rheum 1994;37:1265–82. [DOI] [PubMed] [Google Scholar]
- 2. Rubio-Rivas M, Royo C, Simeón CP, Corbella X, Fonollosa V.. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:208–19. [DOI] [PubMed] [Google Scholar]
- 3. Hao Y, Hudson M, Baron M. et al. Early mortality in a multinational systemic sclerosis inception cohort. Arthritis Rheumatol 2017;69:1067–77. [DOI] [PubMed] [Google Scholar]
- 4. Tyndall AJ, Bannert B, Vonk M. et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 5. Domsic R, Fasanella K, Bielefeldt K.. Gastrointestinal manifestations of systemic sclerosis. Dig Dis Sci 2008;53:1163–74. [DOI] [PubMed] [Google Scholar]
- 6. Wielosz E, Borys O, Zychowska I, Majdan M.. Gastrointestinal involvement in patients with systemic sclerosis. Pol Arch Med Wewn 2010;120:132–6. [PubMed] [Google Scholar]
- 7. Forbes A, Marie I.. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford) 2009;48:iii36–9. [DOI] [PubMed] [Google Scholar]
- 8. Steen VD, Medsger TA Jr.. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000;43:2437–44. [DOI] [PubMed] [Google Scholar]
- 9. Alhajeri H, Hudson M, Fritzler M. et al. 2013 American College of Rheumatology/European League against rheumatism classification criteria for systemic sclerosis outperform the 1980 criteria: data from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken) 2015;67:582–7. [DOI] [PubMed] [Google Scholar]
- 10. Masi AT, Subcommittee For Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 1980;23:581–90. [DOI] [PubMed] [Google Scholar]
- 11. LeRoy EC, Medsger TA Jr.. Criteria for the classification of early systemic sclerosis. J Rheumatol 2001;28:1573–6. [PubMed] [Google Scholar]
- 12. van den Hoogen F, Khanna D, Fransen J. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. [DOI] [PubMed] [Google Scholar]
- 13. Ware JE Jr, Sherbourne CD.. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 14. Tucker G, Adams R, Wilson D.. New Australian population scoring coefficients for the old version of the SF-36 and SF-12 health status questionnaires. Qual Life Res 2010;19:1069–76. [DOI] [PubMed] [Google Scholar]
- 15. Johnson SR, Glaman DD, Schentag CT, Lee P.. Quality of life and functional status in systemic sclerosis compared to other rheumatic diseases. J Rheumatol 2006;33:1117–22. [PubMed] [Google Scholar]
- 16. Georges C, Chassany O, Mouthon L. et al. [Quality of life assessment with the MOS-SF36 in patients with systemic sclerosis]. Rev Med Interne 2004;25:16–21. [DOI] [PubMed] [Google Scholar]
- 17. Khanna D, Furst DE, Clements PJ. et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol 2005;32:832–40. [PubMed] [Google Scholar]
- 18. Furst DE, Clements PJ, Steen VD. et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol 1998;25:84–8. [PubMed] [Google Scholar]
- 19. Clements PJ, Lachenbruch PA, Seibold JR. et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol 1993;20:1892–6. [PubMed] [Google Scholar]
- 20. Diab S, Dostrovsky N, Hudson M. et al. Systemic sclerosis sine scleroderma: a multicenter study of 1417 subjects. J Rheumatol 2014;41:2179–85. [DOI] [PubMed] [Google Scholar]
- 21. Steele R, Hudson M, Lo E, Baron M.. Clinical decision rule to predict the presence of interstitial lung disease in systemic sclerosis. Arthritis Care Res (Hoboken) 2012;64:519–24. [DOI] [PubMed] [Google Scholar]
- 22. Hsu VM, Moreyra AE, Wilson AC. et al. Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol 2008;35:458–65. [PubMed] [Google Scholar]
- 23. Winter B. Linear Models and Linear Mixed Effects Models in R with Linguistic Applications. arXiv: 1308.5499. 2013. http://arxiv.org/pdf/1308.5499.pdf (31 May 2018, date last accessed).
- 24. Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. arXiv: 1406.5823. 2014. https://arxiv.org/pdf/1406.5823.pdf (31 May 2018, date last accessed).
- 25. Kuznetsova A, Brockhoff PB, Christensen RH.. lmerTest package: tests in linear mixed effects models. J Stat Softw 2017;82:1–26. [Google Scholar]
- 26. Muangchan C, Markland J, Robinson D. et al. The 15% rule in scleroderma: the frequency of severe organ complications in systemic sclerosis. A systematic review. J Rheumatol 2013;40:1545–56. [DOI] [PubMed] [Google Scholar]
- 27. Furue M, Mitoma C, Mitoma H. et al. Pathogenesis of systemic sclerosis-current concept and emerging treatments. Immunol Res 2017;65:790–7. [DOI] [PubMed] [Google Scholar]
- 28. Kumar S, Singh J, Kedika R. et al. Role of muscarinic-3 receptor antibody in systemic sclerosis: correlation with disease duration and effects of IVIG. Am J Physiol Gastrointest Liver Physiol 2016;310:G1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar S, Singh J, Rattan S. et al. Review article: pathogenesis and clinical manifestations of gastrointestinal involvement in systemic sclerosis. Aliment Pharmacol Ther 2017;45:883–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greydanus MP, Camilleri M.. Abnormal postcibal antral and small bowel motility due to neuropathy or myopathy in systemic sclerosis. Gastroenterology 1989;96:110–5. [DOI] [PubMed] [Google Scholar]
- 31. Lepri G, Guiducci S, Bellando-Randone S. et al. Evidence for oesophageal and anorectal involvement in very early systemic sclerosis (VEDOSS): report from a single VEDOSS/EUSTAR centre. Ann Rheum Dis 2015;74:124–8. [DOI] [PubMed] [Google Scholar]
- 32. Nishimagi E, Tochimoto A, Kawaguchi Y. et al. Characteristics of patients with early systemic sclerosis and severe gastrointestinal tract involvement. J Rheumatol 2007;34:2050–5. [PubMed] [Google Scholar]
- 33. Fynne L, Worsøe J, Laurberg S, Krogh K.. Faecal incontinence in patients with systemic sclerosis: is an impaired internal anal sphincter the only cause? Scand J Rheumatol 2011;40:462–6. [DOI] [PubMed] [Google Scholar]
- 34. Hurabielle C, Avouac J, Lepri G. et al. Skin telangiectasia and the identification of a subset of systemic sclerosis patients with severe vascular disease. Arthritis Care Res (Hoboken) 2016;68:1021–7. [DOI] [PubMed] [Google Scholar]
- 35. Shah AA, Wigley FM, Hummers LK.. Telangiectases in scleroderma: a potential clinical marker of pulmonary arterial hypertension. J Rheumatol 2010;37:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krieg T, Takehara K.. Skin disease: a cardinal feature of systemic sclerosis. Rheumatology (Oxford) 2006;48:iii14–8. [DOI] [PubMed] [Google Scholar]
- 37. Becker MO, Riemekasten G.. Risk factors for severity and manifestations in systemic sclerosis and prediction of disease course. Expert Rev Clin Immunol 2016;12:115–35. [DOI] [PubMed] [Google Scholar]
- 38. Gyger G, Baron M.. Gastrointestinal manifestations of scleroderma: recent progress in evaluation, pathogenesis, and management. Curr Rheumatol Rep 2012;14:22–9. [DOI] [PubMed] [Google Scholar]
- 39. Sjogren RW. Gastrointestinal features of scleroderma. Curr Opin Rheumatol 1996;8:569–75. [DOI] [PubMed] [Google Scholar]
- 40. Frech TM, Mar D.. Gastrointestinal and hepatic disease in systemic sclerosis. Rheum Dis Clin North Am 2018;44:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Srivastava N, Hudson M, Tatibouet S. et al. Thinking outside the box—the associations with cutaneous involvement and autoantibody status in systemic sclerosis are not always what we expect. Semin Arthritis Rheum 2015;45:184–9. [DOI] [PubMed] [Google Scholar]
- 42. Gonzalez R, Storr M, Bloching H. et al. Autoantibody profile in progressive systemic sclerosis as markers for esophageal involvement. J Clin Gastroenterol 2001;32:123–7. [DOI] [PubMed] [Google Scholar]
- 43. Omair MA, Lee P.. Effect of gastrointestinal manifestations on quality of life in 87 consecutive patients with systemic sclerosis. J Rheumatol 2012;39:992–6. [DOI] [PubMed] [Google Scholar]
- 44. Frantz C, Avouac J, Distler O. et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: a large international survey. Semin Arthritis Rheum 2016;46:115–23. [DOI] [PubMed] [Google Scholar]
- 45. Strickland G, Pauling J, Cavill C, McHugh N.. Predictors of health-related quality of life and fatigue in systemic sclerosis: evaluation of the EuroQol-5D and FACIT-F assessment tools. Clin Rheumatol 2012;31:1215–22. [DOI] [PubMed] [Google Scholar]
- 46. Gosho M, Maruo K, Ishii R, Hirakawa A.. Analysis of an incomplete longitudinal composite variable using a marginalized random effects model and multiple imputation. Stat Methods Med Res 2018;27:2200–15. [DOI] [PubMed] [Google Scholar]
- 47. Anota A, Barbieri A, Savina M. et al. Comparison of three longitudinal analysis models for the health-related quality of life in oncology: a simulation study. Health Qual Life Outcomes 2014;12:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Forbess LJ, Gordon JK, Doobay K. et al. Low prevalence of coeliac disease in patients with systemic sclerosis: a cross-sectional study of a registry cohort. Rheumatology (Oxford) 2013;52:939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nisihara R, Utiyama SR, Azevedo PM, Skare TL.. Celiac disease screening in patients with scleroderma. Arg Gastroenterol 2011;48:163–4. [DOI] [PubMed] [Google Scholar]
- 50. Tseng CC, Yen JH, Tsai WC. et al. Reduced incidence of Crohn’s disease in systemic sclerosis: a nationwide population study. BMC Musculosket Disord 2015;16:251. [DOI] [PMC free article] [PubMed] [Google Scholar]