Abstract
Objectives
To characterize the 10 year relationship between anti-transcriptional intermediary factor 1 antibody (anti-TIF1-Ab) positivity and cancer onset in a large UK-based adult DM cohort.
Methods
Data from anti-TIF1-Ab-positive/-negative adults with verified diagnoses of DM from the UK Myositis Network register were analysed. Each patient was followed up until they developed cancer. Kaplan–Meier methods and Cox proportional hazard modelling were employed to estimate the cumulative cancer incidence.
Results
Data from 263 DM cases were analysed, with a total of 3252 person-years and a median 11 years of follow-up; 55 (21%) DM cases were anti-TIF1-Ab positive. After 10 years of follow-up, a higher proportion of anti-TIF1-Ab-positive cases developed cancer compared with anti-TIF1-Ab-negative cases: 38% vs 15% [hazard ratio 3.4 (95% CI 2.2, 5.4)]. All the detected malignancy cases in the anti-TIF1-Ab-positive cohort occurred between 3 years prior to and 2.5 years after DM onset. No cancer cases were detected within the following 7.5 years in this group, whereas cancers were detected during this period in the anti-TIF1-Ab-negative cases. Ovarian cancer was more common in the anti-TIF1-Ab-positive vs -negative cohort: 19% vs 2%, respectively (P < 0.05). No anti-TIF1-Ab-positive case <39 years of age developed cancer, compared with 21 (53%) of those ≥39 years of age.
Conclusion
Anti-TIF1-Ab-positive-associated malignancy occurs exclusively within the 3 year period on either side of DM onset, the risk being highest in those ≥39 years of age. Cancer types differ according to anti-TIF1-Ab status, and this may warrant specific cancer screening approaches.
Keywords: dermatomyositis, myopathies, autoantibodies, epidemiology, cancer
Rheumatology key messages
Anti-TIF1-Ab-positive dermatomyositis is associated with an increased risk of malignancy, particularly ovarian cancer.
This increased risk is only present in the 3 years on either side of dermatomyositis onset.
This significant association only exists for those ≥39 years of age.
Introduction
The idiopathic inflammatory myopathies (IIMs) are a group of diseases characterized by skeletal muscle inflammation, usually symmetrical proximal muscle weakness, elevated skeletal muscle enzymes and the presence of circulating myositis-specific/associated autoantibodies (MSA/MAAs) [1, 2]. A strong association with cancer development has been noted in the IIMs, especially in DM [3, 4]. A meta-analysis reported that the risk of cancer development in DM is around five times higher than that in the general population. The DM cancer risk is twice that compared with the cancer risk in polymyositis, and it is well recognized that cancer risk is highest in the years prior to and following IIM onset [3].
The presence of anti-transcriptional intermediary factor 1 antibody (anti-TIF1-Ab) is DM specific and confers an even greater risk of cancer development, with the risk of cancer-associated myositis (CAM; cancer occurring within 3 years prior to or following an IIM onset) ranging from 38 to 71% [5–9]. It remains unclear whether the cancer site distribution differs according to anti-TIF1-Ab status.
Anti-TIF1-Ab is one of the most common antibodies to be detected in juvenile DM, yet here it is not associated with malignancy [10]. Fujimoto et al. [10] indicated a potential age cut-off for cancer development in adults with DM, whereby anti-TIF1-Ab positivity appears to confer no cancer risk for those DM patients <40 years of age. Further confirmation of this observation would help to inform clinical cancer screening. Prior studies have only followed study participants up to a maximum of 3 years following IIM onset, due in part to the arbitrary definition of CAM. Therefore the long-term risk of cancer associated with anti-TIF1-Ab positivity remains unclear.
In 2007, using data from the UK Myositis Network (UKMyoNet), Chinoy et al. [6] investigated the prevalence of anti-TIF1-Ab in IIM cases with and without cancer and reported that 50% of anti-TIF1-Ab-positive cases suffered from CAM. This same cohort has now been followed up for a further 10 years and additional anti-TIF1-Ab-positive/-negative participants have been recruited. Further knowledge of the longer-term risk of cancer, common sites of malignancy and age association would usefully inform cancer screening strategies and optimize patient management. The current study aimed to compare the 10 year cancer risk of anti-TIF1-Ab-positive vs -negative DM cases using UKMyoNet data.
Methods
As anti-TIF1-Ab is DM specific, only DM cases were analysed. Adult DM participants (≥18 years of age at DM diagnosis) were recruited from 74 participating centres across the UK (listed in the Acknowledgements). All had confirmed probable or definite DM according to the Bohan and Peter criteria [11, 12]. The International Myositis Classification Criteria [13] score was also calculated for each case and only those with a score of at least 55% (‘probable IIM’) were included. A standardized, single-page clinical pro forma was used to collect baseline data regarding demographics and clinical features. Date of DM onset was defined as the time of symptom onset, according to the recruiting consultant.
All patients were tested for the presence of MSA/MAAs. Two 10 ml whole blood samples were taken into EDTA to enable separation of plasma by centrifugation, permitting autoantibody testing for determination of the presence of MSA anti-synthetases (anti-Jo-1, -PL7, -PL12, -EJ, -OJ, -KS, -Zo), other MSAs (anti-TIF1, -Mi-2, -SAE, -SRP, -MDA5, -NXP2, -HMGCR) and MAA (anti-PM-Scl, -Ku, -U1-RNP, -U3-RNP). All autoantibodies were detected using radio-immunoprecipitation, except anti-HMGCR, which was detected by ELISA.
Patients’ written consent to participate was obtained according to the Declaration of Helsinki, ethical approval having been gained locally at each participating centre (North West Research Multicentre Research Ethics Committee 98/8/86). Consent included permission to allow individual patient details to be registered with the UK Health and Social Care Information Centre (HSCIC) and from which future cancer diagnosis information could be obtained. UKMyoNet recruitment began in 1999 and cancer occurrence linkage was carried out until December 2017.
Analysis
Baseline clinical variables of the anti-TIF1-Ab-positive and -negative cohorts were compared. Categorical variables were compared through chi-squared testing and non-normally distributed continuous variables through Mann–Whitney U testing.
Where a cancer diagnosis was made, the time between DM onset and cancer diagnosis was calculated and employed as the follow-up time. Where no cancer diagnosis was made, the time between DM onset and the follow-up cut-off date (December 2017) was calculated. Cases were censored at the end of their follow-up period if no cancer diagnosis was made. The proportion of each cohort that had developed a cancer within the 3 years preceding DM onset was calculated. Subsequently, using this proportion as a baseline, the cumulative incidence of cancer occurrence after DM onset was estimated according to Kaplan–Meier methods. Hazard ratios (HRs) for the time to cancer diagnosis were calculated using a Cox proportional hazard model adjusted for age, gender and smoking status and then according to anti-TIF1-Ab status.
Results
Cohort characteristics
Data from 263 (69% female) verified DM cases with a total of 3252 person-years follow-up were analysed (Table 1). The median follow-up time was 10 years [interquartile range (IQR) 6–15] for anti-TIF1-Ab-positive cases and 12 years (IQR 8–18) for anti-TIF1-Ab-negative cases. Fifty-five (21%) of the studied cases were anti-TIF1-Ab-positive. Of the 208 anti-TIF1-Ab-negative cases, 40 (19%) were Jo-1 positive, 15 (7%) were positive for Mi2 and 8 (4%) were positive for NXP2. A higher proportion of the anti-TIF1-Ab-positive cases were female compared with anti-TIF1-Ab-negative cases. Equal proportions of each cohort smoked. The age of DM onset was significantly older for those that subsequently developed cancer compared with those that did not (P < 0.05). The median age of DM onset and cancer onset were similar in the anti-TIF1-Ab-positive and -negative cohorts.
Table 1.
Baseline demographics and time to cancer occurrence for the anti-TIF1-Ab-positive and negative cohorts
| Total cohort (n = 263) | Anti-TIF1-Ab positive (n = 55) | Anti-TIF1-Ab negative (n = 208) | ||
|---|---|---|---|---|
| Female, n (%) | 181 (69) | 44 (80) | 137 (66)* | |
| Smokers, n (%) | 82 (31) | 17 (31) | 65 (31) | |
| Follow-up, years, median (IQR) | 10 (7–17) | 10 (6–15) | 12 (8–18) | |
| Age of DM onset, years, median (IQR) | No subsequent cancer diagnosis | 47 (35–60) | 45 (34–58) | 47 (37–59) |
| Subsequent cancer diagnosis | 59 (48–66) | 59 (50–65) | 58 (49–69) | |
| Age at cancer diagnosis, years, median (IQR) | 61 (52–68) | 60 (52–67) | 62 (52–70) | |
| Time from DM to cancer onset, years, median (IQR) | 4.3 (1.5–9.4) | 1.4 (0.7–2.5) | 5.0 (2.5–10.4)* | |
| Cancer cases during total follow-up period, n (%) | 53 (20) | 21 (38) | 32 (15)* | |
| Cases classified as CAMa, n (%) | 42 (16) | 21 (38) | 21 (10)* | |
| Patients with cancer within 3 years preceding DM onset, n (%) | 16 (6) | 7 (13) | 9 (4)* | |
| Patients with cancer within 3 years following DM onset, n (%) | 26 (10) | 14 (26) | 12 (6)* |
aCAM: cancer occurring within 3 years either side of DM onset.
Variables of the anti-TIF1-Ab-positive cohort were compared against those of the anti-TIF1-Ab-negative cohort. *P < 0.01 (categorical variables were compared with the chi-squared test, continuous variables were compared using the Mann–Whitney U test). Anti-TIF1-Ab: anti-transcriptional intermediary factor 1 antibody.
Cancer diagnoses during follow-up
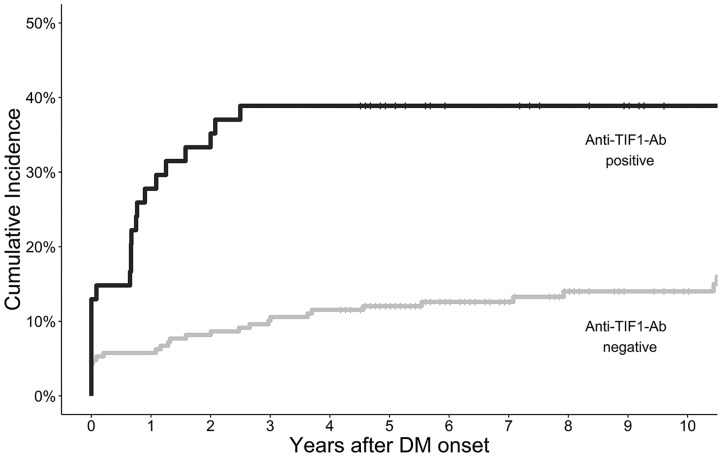
Fifty-three (20%) of the overall cohort were diagnosed with cancer during the entire follow-up period. An increased proportion of anti-TIF1-Ab-positive cases developed cancer throughout the study period compared with anti-TIF1-Ab-negative cases [21 (38%) vs 32 (15%)] (Fig. 1). The median time from DM onset to subsequent cancer diagnosis throughout the entire follow-up period was shorter for anti-TIF1-Ab-positive compared with anti-TIF1-Ab-negative cases [1.4 years (IQR 0.7–2.5) vs 5.0 (2.5–10.4)]. All detected cancers in the anti-TIF1-Ab-positive cases occurred within 2.5 years following DM onset; no further cases of cancer were detected within the remaining follow-up period. In contrast, new cases of cancer were detected in anti-TIF1-Ab-negative cases throughout the 3–10 year period following DM onset, with 9.8% (95% CI 7.5, 12.0) at 5 years, 11.7% (95% CI 9.2, 14.2) at 7.5 years and 14.1% (95% CI 11.2, 16.8) at 10 years.
Fig. 1.
Kaplan-Meier plot of cumulative incidence of cancer onset following dermatomyositis diagnosis stratified according to anti-TIF1-Ab status
Anti-TIF1-Ab: anti-transcriptional intermediary factor 1 antibody.
Cox proportional hazard modelling, adjusted for age, gender and smoking status, revealed that anti-TIF1-Ab positivity was significantly associated with a shorter time between DM and cancer onset (during the entire follow-up period) [HR 3.2 (95% CI 1.8, 5.5)].
Malignancy was more frequent in older compared with younger anti-TIF1-Ab-positive cases. Fifteen anti-TIF1-Ab-positive cases were <39 years of age at DM onset and none developed cancer during the follow-up period, whereas 21 (53%) of the 40 anti-TIF1-Ab-positive cases ≥39 years of age at DM onset developed cancer during the follow-up period. Older age was significantly associated with cancer onset [HR 1.04 (95% CI 1.02, 1.07)]; the significant association between anti-TIF1-Ab positivity and early cancer onset only existed for those ≥39 years of age [HR 4.1 (95% CI 2.5, 6.7) vs HR 0.8 (95% CI 0.1, 6.5) for patients ≥39 and <39 years of age, respectively].
Site of cancer
Within the anti-TIF1-Ab-positive cohort, breast cancer was the most common malignancy (33%), followed by ovarian cancer (19%) and lymphoma (14%). Breast cancer (25%), lymphoma (13%) and bowel cancer (13%) were the three most common cancers in the anti-TIF1-Ab-negative cohort. Ovarian cancer constituted a significantly higher proportion of cancers in the anti-TIF1-Ab-positive vs -negative cases: 19% vs 2%, respectively (chi-squared P-values <0.05). Five of the 53 (9%) cancers in the whole cohort were ovarian and 4 (80%) of these occurred in anti-TIF1-Ab-positive cases. Four of the 16 (25%) documented cancers in the anti-TIF1-Ab-positive female cohort were ovarian, compared with 1 of 26 cancers (4%) in the female anti-TIF1-Ab-negative cohort.
Discussion
This is the first study to investigate the temporal relationship between anti-TIF1-Ab positivity and cancer onset in a large DM cohort over a 10 year follow-up period after DM onset. Anti-TIF1-Ab-positive-associated malignancy occurred exclusively within 3 years either side of the DM onset, and no incident cancer cases were detected in this group within the subsequent 7.5 years. This finding is not likely to be due to a disparity in follow-up time between anti-TIF1-Ab-positive and -negative cases, as the median follow-up times were similar for both groups: 10 years and 12 years, respectively. Further, this finding is unlikely to be due to differences in cancer detection methods, as both cohorts’ cancer diagnoses were identified through HSCIC data, ensuring capture of all incident cancers during the follow-up period. Although a number of studies have now confirmed the strong association between anti-TIF1-Ab positivity and increased malignancy risk within 3 years of IIM onset [5–8], no previous study has investigated the longer-term risks.
We have also shown that the distribution of cancer sites appears to vary according to anti-TIF1-Ab status, with female anti-TIF1-Ab-positive patients at increased risk of ovarian cancer [14, 15]. A number of studies have highlighted the increased risk of ovarian cancer associated with DM, and the current study has confirmed this finding over a 10 year longitudinal follow-up. However, this is the first large study to identify that ovarian cancer is overrepresented in anti-TIF1-Ab-positive individuals, suggesting that the true association between DM and ovarian cancer may be through possession of anti-TIF1-Abs. Clinicians should therefore apply increased vigilance for potential ovarian pathology in anti-TIF1-Ab-positive cases.
This study has identified that the significant association between anti-TIF1-Ab positivity and early cancer diagnosis exists only for those ≥39 years of age. This finding is consistent with the finding by Fujimoto et al. [10], who found that anti-TIF1-Ab-positive adults <40 years of age did not suffer from co-existing malignancy, whereas those >40 years of age had a high risk of cancer (75%). Our findings add strength to the hypothesis that there exists a subset of young adult anti-TIF1-Ab-positive cases who do not have a discernible increased risk of cancer, similar to that observed in TIF1-Ab-positive juvenile DM [16]. Given that older age of IIM onset is associated with CAM regardless of autoantibody positivity [15], and anti-TIF1-Ab positivity confers an increased risk, detailed screening for cancer in anti-TIF1-Ab-positive patients ≥39 years of age is advocated.
The strengths of this study include the large anti-TIF1-Ab-positive and -negative cohorts and the long-term detection of cancers through the HSCIC. Survival bias is a limitation, as patients would have to have survived sufficiently long to be recruited into UKMyoNet. Potential inaccuracy of identification of DM onset may have led to under- or overestimation of the cumulative incidence of cancer; however, this is unlikely to have impacted the substantial difference in cancer incidence between the anti-TIF1-Ab-positive and -negative cohorts. Further, the lack of data on titres of anti-TIF1-Ab and lack of serial samples have limited the ability to investigate the utility of repeat sampling. Finally, variation in sampling times throughout each patient’s disease stage and treatment course could potentially have impacted upon positivity for anti-TIF1-Ab.
A distinct approach to cancer screening according to anti-TIF1-Ab status can be advocated. It may be that a focus on screening for cancer within the first 3 years after DM onset and particularly screening for ovarian cancer in anti-TIF1-Ab-positive female patients may be required. Our findings also strengthen the hypothesis that inflammatory myopathies represent a paraneoplastic reaction initiated by attempted immune-mediated clearance of a cancer [17].
Conclusions
This study has characterized the long-term relationship between anti-TIF1-Ab status and cancer onset. Testing for anti-TIF1-Ab in newly diagnosed DM cases can help guide cancer screening strategy. The findings that earlier cancer onset is associated with anti-TIF1-Ab positivity, particularly in those ≥39 years of age, and that more cancers are ovarian are of important clinical significance.
Acknowledgements
Members of the UK Adult Onset Myositis Immunogenetic Collaboration who recruited and enrolled subjects are as follows: Drs Yasmeen Ahmed (Llandudno General Hospital), Raymond Armstrong (Southampton General Hospital), Robert Bernstein (Manchester Royal Infirmary), Carol Black (Royal Free Hospital, London), Simon Bowman (University Hospital, Birmingham), Ian Bruce (Manchester Royal Infirmary), Robin Butler (Robert Jones & Agnes Hunt Orthopaedic Hospital, Oswestry), John Carty (Lincoln County Hospital), Chandra Chattopadhyay (Wrightington Hospital), Easwaradhas Chelliah (Wrightington Hospital), Fiona Clarke (James Cook University Hospital, Middlesbrough), Peter Dawes (Staffordshire Rheumatology Centre, Stoke on Trent), Joseph Devlin (Pinderfields General Hospital, Wakefield), Christopher Edwards (Southampton General Hospital), Paul Emery (Academic Unit of Musculoskeletal Disease, Leeds), John Fordham (South Cleveland Hospital, Middlesbrough), Alexander Fraser (Academic Unit of Musculoskeletal Disease, Leeds), Hill Gaston (Addenbrooke’s Hospital, Cambridge), Patrick Gordon (King’s College Hospital, London), Bridget Griffiths (Freeman Hospital, Newcastle), Harsha Gunawardena (Frenchay Hospital, Bristol), Frances Hall (Addenbrooke’s Hospital, Cambridge), Beverley Harrison (North Manchester General Hospital), Elaine Hay (Staffordshire Rheumatology Centre, Stoke on Trent), Lesley Horden (Dewsbury District General Hospital), John Isaacs (Freeman Hospital, Newcastle), Adrian Jones (Nottingham University Hospital), Sanjeet Kamath (Staffordshire Rheumatology Centre, Stoke on Trent), Thomas Kennedy (Royal Liverpool Hospital), George Kitas (Dudley Group Hospitals Trust, Birmingham), Peter Klimiuk (Royal Oldham Hospital), Sally Knights (Yeovil District Hospital, Somerset), John Lambert (Doncaster Royal Infirmary), Peter Lanyon (Queen’s Medical Centre, Nottingham), Ramasharan Laxminarayan (Queen’s Hospital, Burton Upon Trent), Bryan Lecky (Walton Neuroscience Centre, Liverpool), Raashid Luqmani (Nuffield Orthopaedic Centre, Oxford), Jeffrey Marks (Steeping Hill Hospital, Stockport), Michael Martin (St. James University Hospital, Leeds), Dennis McGonagle (Academic Unit of Musculoskeletal Disease, Leeds), Neil McHugh (Royal National Hospital for Rheumatic Diseases, Bath), Francis McKenna (Trafford General Hospital, Manchester), John McLaren (Cameron Hospital, Fife), Michael McMahon (Dumfries & Galloway Royal Infirmary, Dumfries), Euan McRorie (Western General Hospital, Edinburgh), Peter Merry (Norfolk & Norwich University Hospital, Norwich), Sarah Miles (Dewsbury & District General Hospital, Dewsbury), James Miller (Royal Victoria Hospital, Newcastle), Anne Nicholls (West Suffolk Hospital, Bury St. Edmunds), Jennifer Nixon (Countess of Chester Hospital, Chester), Voon Ong (Royal Free Hospital, London), John Packham (Staffordshire Rheumatology Centre, Stoke on Trent), Nicolo Pipitone (King’s College Hospital, London), Michael Plant (South Cleveland Hospital, Middlesbrough), Gillian Pountain (Hinchingbrooke Hospital, Huntington), Thomas Pullar (Ninewells Hospital, Dundee), Mark Roberts (Salford Royal Foundation Trust), Paul Sanders (Wythenshawe Hospital, Manchester), David Scott (King’s College Hospital, London), David Scott (Norfolk & Norwich University Hospital, Norwich), Michael Shadforth (Staffordshire Rheumatology Centre, Stoke on Trent), Thomas Sheeran (Cannock Chase Hospital, Cannock, Staffordshire), Arul Srinivasan (Broomfield Hospital, Chelmsford), David Swinson (Wrightington Hospital), Lee-Suan Teh (Royal Blackburn Hospital, Blackburn), Michael Webley (Stoke Mandeville Hospital, Aylesbury), Brian Williams (University Hospital of Wales, Cardiff), and Jonathan Winer (Queen Elizabeth Hospital, Birmingham).
Funding: This study was jointly supported by Arthritis Research UK (18474), Myositis UK, the European Science Foundation for EuMyoNet support, Association Francaise Contre Les Myopathies and the Medical Research Council (MR/N003322/1). UKMyoNet (Adult Onset Myositis Immunogenetic Collaboration until renamed in 2008) is supported by the Manchester Academic Health Science Centre.
This report includes independent research supported by the NIHR Biomedical Research Centre Funding Scheme. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health.
Disclosure statement: The authors have no conflicts of interest to disclose.
Contributor Information
UKMyoNet:
Drs Yasmeen Ahmed, Raymond Armstrong, Robert Bernstein, Carol Black, Simon Bowman, Ian Bruce, Robin Butler, John Carty, Chandra Chattopadhyay, Easwaradhas Chelliah, Fiona Clarke, Peter Dawes, Joseph Devlin, Christopher Edwards, Paul Emery, John Fordham, Alexander Fraser, Hill Gaston, Patrick Gordon, Bridget Griffiths, Harsha Gunawardena, Frances Hall, Beverley Harrison, Elaine Hay, Lesley Horden, John Isaacs, Adrian Jones, Sanjeet Kamath, Thomas Kennedy, George Kitas, Peter Klimiuk, Sally Knights, John Lambert, Peter Lanyon, Ramasharan Laxminarayan, Bryan Lecky, Raashid Luqmani, Jeffrey Marks, Michael Martin, Dennis McGonagle, Neil McHugh, Francis McKenna, John McLaren, Michael McMahon, Euan McRorie, Peter Merry, Sarah Miles, James Miller, Anne Nicholls, Jennifer Nixon, Voon Ong, John Packham, Nicolo Pipitone, Michael Plant, Gillian Pountain, Thomas Pullar, Mark Roberts, Paul Sanders, David Scott, David Scott, Michael Shadforth, Thomas Sheeran, Arul Srinivasan, David Swinson, Lee-Suan Teh, Michael Webley, Brian Williams, and Jonathan Winer
References
- 1. Betteridge Z, McHugh N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med 2016;280:8–23. [DOI] [PubMed] [Google Scholar]
- 2. Merlo G, Clapasson A, Cozzani E et al. Specific autoantibodies in dermatomyositis: a helpful tool to classify different clinical subsets. Arch Dermatol Res 2017;309:87–95. [DOI] [PubMed] [Google Scholar]
- 3. Qiang JK, Kim WB, Baibergenova A, Alhusayen R. Risk of malignancy in dermatomyositis and polymyositis. J Cutan Med Surg 2017;21:131–6. [DOI] [PubMed] [Google Scholar]
- 4. Dobloug GC, Garen T, Brunborg C, Gran JT, Molberg Ø. Survival and cancer risk in an unselected and complete Norwegian idiopathic inflammatory myopathy cohort. Semin Arthritis Rheum 2015;45:301–8. [DOI] [PubMed] [Google Scholar]
- 5. Targoff IN, Mamyrova G, Trieu EP et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum 2006;54:3682–9. [DOI] [PubMed] [Google Scholar]
- 6. Chinoy H, Fertig N, Oddis CV, Ollier WER, Cooper RG. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann Rheum Dis 2007;66:1345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trallero-Araguás E, Labrador-Horrillo M, Selva-O’Callaghan A et al. Cancer-associated myositis and anti-p155 autoantibody in a series of 85 patients with idiopathic inflammatory myopathy. Medicine (Baltimore) 2010;89:47–52. [DOI] [PubMed] [Google Scholar]
- 8. Kaji K, Fujimoto M, Hasegawa M et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology 2007;46:25–8. [DOI] [PubMed] [Google Scholar]
- 9. Hida A, Yamashita T, Hosono Y et al. Anti-TIF1-γ antibody and cancer-associated myositis: a clinicohistopathologic study. Neurology 2016;87:299–308. [DOI] [PubMed] [Google Scholar]
- 10. Fujimoto M, Hamaguchi Y, Kaji K et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum 2012;64:513–22. [DOI] [PubMed] [Google Scholar]
- 11. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. [DOI] [PubMed] [Google Scholar]
- 12. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–7. [DOI] [PubMed] [Google Scholar]
- 13. Lundberg IE, Miller FW, Tjärnlund A, Bottai M. Diagnosis and classification of idiopathic inflammatory myopathies. J Intern Med 2016;280:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hill CL, Zhang Y, Sigurgeirsson B et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 2001;357:96–100. [DOI] [PubMed] [Google Scholar]
- 15. Kang EH, Lee SJ, Ascherman DP et al. Temporal relationship between cancer and myositis identifies two distinctive subgroups of cancers: impact on cancer risk and survival in patients with myositis. Rheumatology (Oxford) 2016;55:1631–41. [DOI] [PubMed] [Google Scholar]
- 16. Tansley S, Wedderburn LR. Comparing and contrasting clinical and serological features of juvenile and adult-onset myositis. Curr Opin Rheumatol 2015;27:601–7. [DOI] [PubMed] [Google Scholar]
- 17. Shah AA, Casciola-Rosen L, Rosen A. Review: cancer-induced autoimmunity in the rheumatic diseases. Arthritis Rheumatol 2015;67:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]