Abstract
Anaplastic lymphoma kinase (ALK) rearrangement is reported in 3% to 8% of patients with lung adenocarcinoma and can be detected by fluorescent in situ hybridization (FISH) or indirectly by immunohistochemistry. In FISH assay, isolated 5′ signal (loss of 3′ signal) is usually considered negative. We report three young nonsmoking patients with stage IV lung adenocarcinoma. Strong ALK expression in tumor cells detected by immunohistochemistry was observed in all cases, but FISH revealed an isolated 5′ signal pattern. Massive parallel “next-generation” sequencing was performed in two patients and confirmed ALK rearrangement. The three patients were treated and responded to crizotinib after 14, 10, and 31 months.
Introduction
Anaplastic lymphoma kinase (ALK) rearrangements are reported in 3% to 8% of patients with lung adenocarcinoma [1], [2]. ALK rearranged adenocarcinomas often occur in young and nonsmoker patients, with advanced-stage disease and lymph node metastases [3]. Tumors with ALK gene rearrangement have a rapid and pronounced response to the ALK tyrosine kinase inhibitor. Thus, identification of this driver gene alteration is mandatory in routine diagnosis because approved molecular targeted drugs are available [4]. Currently, the most widely established accurate method for identifying ALK rearrangements is fluorescent in situ hybridization (FISH) with an ALK break-apart probe, but immunohistochemistry (IHC) has been validated as an equivalent alternative to FISH for ALK testing according to the newly revised College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology (CAP/IASLC/AMP) guidelines [5]. The FISH criterion for categorizing lung tumors as ALK positive are ≥15% of tumor cells showing a positive signal pattern that includes split signals (at least two signal distances apart) and/or an isolated 3′ signal. Although these two patterns are the most common, other patterns can be observed. Loss of 5′ signal with amplification of 3′ signal is also considered positive for ALK rearrangement. In contrast, loss of 3′ signal (isolated 5′ signal) is usually considered negative [6], [7], [8]. We report here three cases with an isolated 5′ signal pattern by FISH confirmed for two of them as ALK rearrangement by massive parallel “next-generation” sequencing (NGS). All of these three patients had still complete response under crizotinib 14, 10, and 31 months after the diagnosis.
Case Summary
Case 1
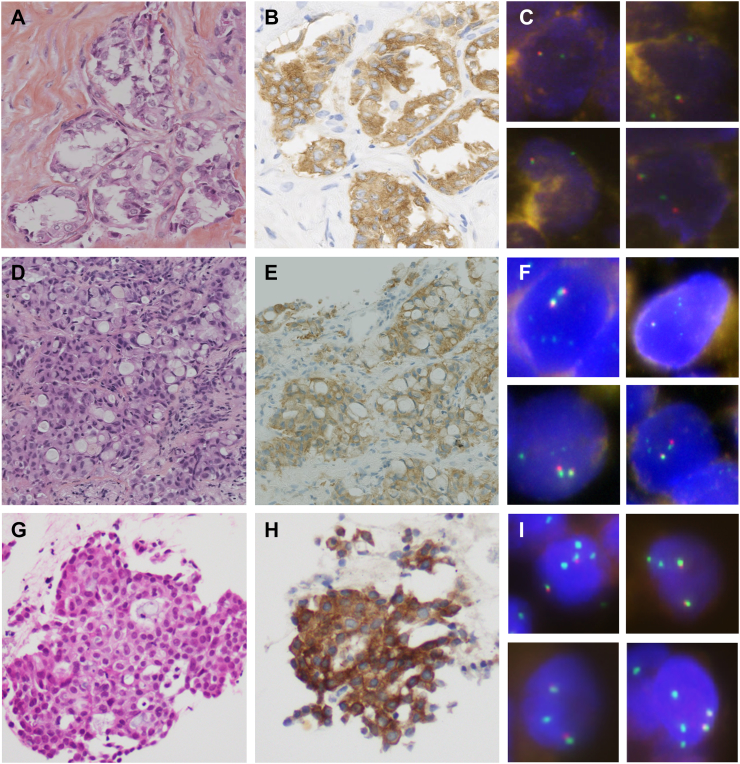
A nonsmoking 46-year-old woman presented with anterior chest pain of several months' duration and asthenia. The patient had no medical history except for an antiphospholipid antibody syndrome. A computed tomography (CT) scan showed a pleural thickening developed on parietal and fissural pleura, without pleural effusion. The CT scan, completed with a positron emission tomography/CT scan, showed neither parenchymal lesion nor lymphadenopathy. Videothoracoscopy revealed a nodular thickened pleura. Microscopically, the pleura was massively infiltrated by an adenocarcinoma of solid, acinar, and cribriform pattern (Figure 1A). In IHC, tumor cells were positive for TTF1 (SPT24, Tebu-Novocastra) and ALK (5A4, Abcam) (Figure 1B), and negative for P40 (BC28, Zytomed). Break-apart FISH (Zytovision) showed isolated 5′ ALK signals with loss of 3′ ALK signals in 60% of tumoral cells, split signals in 10% of tumoral cells, and normal fused signals in 30% of tumoral cells (Figure 1C). The presence of an EML4 (ex6)–ALK (ex20) transcript was confirmed by NGS (Oncomine solid tumor fusion transcript kit, Life Technology). DNA analyses by an NGS 22-gene panel showed no additional mutation (Oncomine solid tumor DNA kit, Life Technologies). The patient received a first-line crizotinib and is in complete response 14 months after the diagnosis.
Figure 1.
Case 1: (A) Adenocarcinoma with solid, acinar, and cribriform pattern (HES) ×40. (B) Strong positivity (3+) of ALK immunohistochemistry (5A4, Abcam). (C) Isolated 5′ ALK signals and rare split signals (Zytovision break-apart ALK FISH).
Case 2: (D) Adenocarcinoma of solid pattern with signet ring cells (HES) ×40. (E) Strong positivity (3+) of ALK immunohistochemistry (5A4, Abcam). (F) Isolated 5′ ALK signals with two normal fusion signal per cell (DAKO break-apart ALK FISH).
Case 3: (G) Adenocarcinoma with solid and cribriform pattern (HES) ×20. (H) Strong positivity (3+) of ALK immunohistochemistry (5A4, Abcam). (I) Isolated 5′ ALK signals (DAKO break-apart ALK FISH).
Case 2
A nonsmoking 45-year-old woman presented with right pleural effusion. A CT scan showed two bilateral pulmonary nodules and several localizations in left adrenal gland and brain. A biopsy of a pulmonary nodule revealed an adenocarcinoma of solid, acinar, and cribriform pattern with a minor mucinous component (Figure 1D). In IHC, tumor cells were positive for TTF1 (SP141, Roche) and ALK (5A4, ByoSystems) (Figure 1E), and negative for P40 (BC28, Roche). Break-apart FISH (DAKO) showed several (3 to 4) very small isolated 5′ ALK signals with loss of 3′ ALK signals in 61% of tumoral cells, associated with split signals in 4% of tumoral cells and fused signals in 35% of tumoral cells (Figure 1F). The presence of an EML4 (ex13)–ALK (ex20) transcript was confirmed by NGS (Oncomine solid tumor fusion transcript kit, Life Technologies). DNA analyses by an NGS 22-gene panel show no additional mutation (Oncomine solid tumor DNA kit, Life Technologies). The patient was treated with crizotinib, which led to a complete regression of pulmonary nodules, right pleural effusion, and adrenal gland and cerebral lesions. The patient is still under crizotinib 10 months after the diagnosis.
Case 3
A nonsmoking 40-year-old man, with no personal history, presented with dyspnea. A CT scan showed a bulky mediastinal mass behind the left main bronchus classified cT4N2M0. The biopsy, made by endobronchial ultrasound-guided transbronchial needle aspiration, showed an adenocarcinoma of cribriform pattern (Figure 1G). In IHC, tumor cells were positive for TTF1 (8G7G3/1, Roche) and ALK (5A4, Clinisciences) (Figure 1H). Break-apart FISH (DAKO) showed two to three 5′ ALK signals with loss of 3′ ALK signals in 87% of tumoral cells and fused signals in 13% of tumoral cells (Figure 1I). DNA analysis showed no mutation on EGFR (HRM PCR screening; fragment analysis, Genescan; Taqman probe; Entrogen kit), KRAS (HRM PCR screening), BRAF (HRM PCR screening), and ERBB2 (HRM PCR screening) genes. Confirmation by RNA NGS could not be performed as tumoral sample was not sufficient for analysis. The evaluation at 6 weeks after the beginning of crizotinib showed a complete response still ongoing 31 months after the diagnosis.
Discussion
To date, FISH is the standard procedure for the detection of ALK rearrangement in non–small cell lung carcinoma (NSCLC). Commonly, ALK break-apart probes were used, including a DNA fragment telomeric to ALK (3′ end) labeled with red fluorophore and a DNA fragment centromeric to ALK (5′ end) labeled green fluorophore. Until now, rearranged ALK is defined by either split signal or isolated 3′ signal (solitary red). Isolated 5′ signal (solitary green) seems to be rare. In our two laboratories, we found only these three cases in 2 years among 367 cases tested. This pattern has usually been considered negative for rearrangement because the ALK tyrosine kinase domain is located in the 3′ region of the gene [8]. In the literature, isolated 5′ signal (loss of 3′ ALK signal) has already been described in six cases (Table 1 [7], [8], [9], [10]), and this pattern has been validated as ALK rearrangement at RT-PCR level in four cases [7], [9], [10]. Interestingly, this atypical FISH pattern was associated to the most common fusion partner, EML4, as in classic cases. Indeed, EML4 was reported in three out of four cases, as in our two cases, and BIRC6 partner was described in one case. Likewise, clinically, patients with 5′ ALK isolated FISH signal pattern adenocarcinoma show similar clinical features as patients with classic ALK FISH pattern tumors: young patients, aged 40 to 46 years and nonsmokers or light smokers. Adenocarcinoma pathological features are also very similar with acinar, solid, or cribriform pattern of growth. The three patients in our series have been successfully treated with crizotinib as reported in one patient in the literature [9].
Table 1.
Isolated 5’ALK Signals ALK FISH Pattern from Literature Reports and Our Cases
| Reference | Sex, Age | ALK IHC | Partner Gene | Crizotinib Response |
|---|---|---|---|---|
| Yoshida [7] | NS | Positive | EML4 | NS |
| Dai [8] | NS | NS | NS | NS |
| Dai [8] | NS | NS | NS | NS |
| Ren [9] | M 44 yo | Positive | EML4 | Yes |
| Li [10] | F 43 yo | Positive | EML4 | NS |
| Li [10] | F 45 yo | Positive | BIRC6 | NS |
| Patient 1 | F 46 yo | Positive | EML4 | Yes |
| Patient 2 | F 45 yo | Positive | EML4 | Yes |
| Patient 3 | M 40 yo | Positive | NS | Yes |
NS, nonspecified; M, male; F, female; yo, years old.
The molecular significance of the loss of the 3′ signal is unclear. According to Li, this atypical FISH pattern is caused by a large deletion of the ALK 3′ region, and the remaining region containing the ALK kinase domain (approximately 30 kb) is too short to be clearly observed by FISH on formalin-fixed, paraffin-embedded specimens [10]. This finding confirms the importance of the first screening by ALK immunohistochemistry as in the new recommendations [5] since immunohistochemistry was positive for all four cases described in literature and also in ours. Nevertheless, we are not aware of any reported cases with negative immunohistochemistry and with this atypical FISH pattern. Moreover, several studies have compared IHC and FISH, and they mainly indicated a high concordance to detect ALK rearrangement [11], [12], [13] as ALK IHC is considered as a screening method to select specimens for ALK FISH testing [4]. Thus, the newly revised CAP/IASLC/AMP guidelines published in March 2018 validated IHC as an equivalent alternative to FISH for ALK testing. Authors claim that treatment decisions can be made when IHC results are clearly positive; however, the specificity of weak staining relative to FISH should be determined in each laboratory during validation [5]. Using these guidelines, all patients of our series with this atypical ALK FISH pattern would have been treated by ALK inhibitor since all showed a strongly positive (3+) ALK IHC even though ALK FISH was considered negative. However, many institutions still perform ALK testing exclusively by FISH or, as in our laboratories, confirm ALK positive staining by FISH; therefore, the report of isolated 5′ signals as positive pattern with efficacy of ALK TKI appeared mandatory. Alternative techniques for the detection of rearrangement, like RNA-based NGS assay, could be used to confirm these discordant cases. Scattone et al. reported seven discordant cases showing ALK FISH-positive cases with complex pattern of rearrangements (deleted, split, and amplified/polysomy patterns) with negative ALK IHC and inefficiency of crizotinib. NGS assay was useful, showing lack of ALK fusion transcripts in these cases, which might explain the absence of the ALK protein [14].
In conclusion, NSCLC with positive ALK IHC and isolated 5′ ALK signals pattern is a rare event that should be considered positive for ALK rearrangement, and patients should benefit from ALK-targeted therapy. This report underlines the importance of using immunohistochemistry as a prescreening method and to confirm discordant or atypical cases by alternative techniques like NGS.
Acknowledgement
We would like to thank Anne Audebourg at the Department of Pathology, Cochin Hospital, Paris, and Maryse Baia at the Department of Pathology, Mondor Hospital, Creteil, for their excellent technical support.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S. Identification of the transforming EML4-ALK fusion gene in non–small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Camidge DR, Kono SA, Lu X, Okuyama S, Barón AE, Oton AB. Anaplastic lymphoma kinase gene rearrangements in non–small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2011;6:774–780. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Liang D, Yao W, Wu W, Li J, Chen M. Immunohistochemical screening and fluorescence in situ hybridization confirmation of ALK translocation in lung adenocarcinoma and its clinicopathological significance: a single-center large-scale investigation of Chinese patients. Hum Pathol. 2014;45:1414–1422. doi: 10.1016/j.humpath.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol. 2015;16:e342–e351. doi: 10.1016/S1470-2045(15)00077-7. [DOI] [PubMed] [Google Scholar]
- 5.Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors; guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2018;13:323–358. doi: 10.1016/j.jtho.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Kwak EL, Bang Y-J, Camidge DR, Shaw AT, Solomon B, Maki RG. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida A, Tsuta K, Nitta H, Hatanaka Y, Asamura H, Sekine I. Bright-field dual-color chromogenic in situ hybridization for diagnosing echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase-positive lung adenocarcinomas. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2011;6:1677–1686. doi: 10.1097/JTO.0b013e3182286d25. [DOI] [PubMed] [Google Scholar]
- 8.Dai Z, Kelly JC, Meloni-Ehrig A, Slovak ML, Boles D, Christacos NC. Incidence and patterns of ALK FISH abnormalities seen in a large unselected series of lung carcinomas. Mol Cytogenet. 2012;5:44. doi: 10.1186/1755-8166-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren S, Hirsch FR, Varella-Garcia M, Aisner DL, Boyle T, Zhou C. Atypical negative ALK break-apart FISH harboring a crizotinib-responsive ALK rearrangement in non–small-cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2014;9:e21–e23. doi: 10.1097/JTO.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Zhang J, Guo L, Chuai S, Shan L, Ying J. Combinational analysis of FISH and immunohistochemistry reveals rare genomic events in ALK fusion patterns in NSCLC that responds to crizotinib treatment. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2017;12:94–101. doi: 10.1016/j.jtho.2016.08.145. [DOI] [PubMed] [Google Scholar]
- 11.Thunnissen E, Bubendorf L, Dietel M, Elmberger G, Kerr K, Lopez-Rios F. EML4-ALK testing in non–small cell carcinomas of the lung: a review with recommendations. Virchows Arch Int J Pathol. 2012;461:245–257. doi: 10.1007/s00428-012-1281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wynes MW, Sholl LM, Dietel M, Schuuring E, Tsao MS, Yatabe Y. An international interpretation study using the ALK IHC antibody D5F3 and a sensitive detection kit demonstrates high concordance between ALK IHC and ALK FISH and between evaluators. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2014;9:631–638. doi: 10.1097/JTO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paik JH, Choe G, Kim H, Choe J-Y, Lee HJ, Lee C-T. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non–small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2011;6:466–472. doi: 10.1097/JTO.0b013e31820b82e8. [DOI] [PubMed] [Google Scholar]
- 14.Scattone A, Catino A, Schirosi L, Caldarola L, Tommasi S, Lacalamita R. Discordance between FISH, IHC, and NGS analysis of ALK status in advanced non–small cell lung cancer (NSCLC): a brief report of 7 cases. Transl Oncol. 2018;12:389–395. doi: 10.1016/j.tranon.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]