Abstract
Background
Chronic thromboembolic pulmonary hypertension (CTEPH) is classified as group IV pulmonary hypertension. This study aimed to report our institutional experience in managing CTEPH.
Methods
We prospectively collected the data of 23 patients diagnosed with CTEPH between August 2001 and August 2017 in Linkou Chang Gung Memorial Hospital. Baseline characteristics including functional class (FC), 6-minute walk distance (6MWD), comorbidities, hematological and biochemical data, echocardiography, cardiac catheterization, and selective pulmonary angiography were recorded at diagnosis. All patients were referred to a cardiac surgeon for pulmonary endarterectomy (PEA) assessment.
Results
The mean age at diagnosis was 48.4 ± 16.1 years. Nineteen patients (83%) underwent PEA with mean postoperative follow-up of 37.7 ± 42.8 months. The in-hospital mortality rate of PEA was 11%. The 1-, 2-, 3- and 5-year overall survival rates were 89%, 89%, 81%, and 50%, respectively. After 3 months of PEA, all patients had improvements in FC, 6MWD (from 326 ± 62 to 420 ± 63 m), B-type natriuretic peptide level (from 602 ± 599 to 268 ± 565 pg/mL), and systolic pulmonary artery pressure (from 79 ± 19 to 48 ± 19 mmHg). The patients with proximal disease (Jamieson type 1 or 2) had better survival than those with distal disease (Jamieson type 3 or 4), but there was no significant difference in mortality between FC III and IV. All of the four patients who did not undergo PEA survived for more than 3 years.
Conclusions
Significant improvements in symptoms, functional capacity, and hemodynamics were achieved in the CTEPH patients after PEA. However, the overall survival was still unsatisfactory.
Keywords: Functional class, Jamieson classification, Pulmonary endarterectomy, Pulmonary hypertension, Pulmonary vascular disease
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is classified as group IV pulmonary hypertension (PH) and defined as precapillary PH with mean pulmonary artery pressure (MPAP) ≥ 25 mmHg and pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg and at least one detected segmental pulmonary perfusion defect despite at least 3 months of effective anticoagulant therapy.1 Although the exact prevalence and annual incidence rates of CTEPH are still unknown, several studies have reported that it may occur in at least 5 per million individuals annually.2,3 A history of pulmonary embolism (PE) has been reported in almost three-quarters of patients diagnosed with CTEPH.4 The pathophysiology of CTEPH begins with obstruction of large or middle-sized pulmonary arteries by unresolved thrombi in most cases.5 In contrast, small vessel remodeling occurs in non-obstructed vessels in the presence of high flow-related shear stress, inflammation, or cytokine release.2 Both major vessel lesions and microvascular disease can lead to the progression of CTEPH and impaired hemodynamics.5 Patients generally develop symptoms of increased pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR), as well as a consequent reduction in exercise capacity.6-8 Although CTEPH is life-threatening, it is potentially curable. Pulmonary endarterectomy (PEA) is the standard therapeutic approach for CTEPH.1,9,10 The majority of CTEPH patients who undergo PEA have been reported to have significant symptomatic relief and functional improvement.6,11,12 However, the diagnosis of CTEPH is challenging with its nonspecific symptoms or signs.2,13 In addition, PEA has been performed in only a few centers in very few cases. The aim of this study was to report our institutional experience in managing CTEPH.
MATERIALS AND METHODS
Study population
We prospectively collected data of patients diagnosed with CTEPH between August 2001 and August 2017 in Chang Gung Memorial Hospital (CGMH). Laboratory data including hematology, biochemistry, and immunology were collected at the time of diagnosis. All of the patients completed a lung perfusion scan, 2D echocardiography, chest computed tomography (CT), cardiac catheterization, and selective pulmonary angiography before enrollment. In addition, medical records were reviewed for the presence of risk factors such as venous thromboembolism (VTE), thyroid or estrogen replacement therapy, antiphospholipid syndrome (APS), malignancy, splenectomy, non-O blood group, and hypercoagulation. Baseline functional capacity was assessed using the World Health Organization functional classification (FC) system and 6-minute walk distance (6MWD). All patients were referred to a cardiac surgeon to assess their suitability for surgery.
The follow-up period of the survivors ended on October 1, 2017. Patients who underwent PEA were evaluated for functional capacity, B-type natriuretic peptide (BNP), and echocardiography 3 months, 12 months and yearly after surgery. This study was reviewed and approved by the CGMH Institutional Review Board (IRB No.: 201701372B0).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) while categorical variables were presented as numbers and percentages. Nonparametric statistical analyses were carried out using IBM Statistical Product and Service Solutions (SPSS) (version 22). Differences in 6MWD and oxyhemoglobin saturation by pulse oximetry (SpO2) between baseline and 3 months after PEA were compared using the nonparametric Wilcoxon signed-rank test. Differences in FC before surgery and at 3 months after surgery were assessed using McNemar’s test. Differences in echocardiographic data between baseline, 3 months, 12 months and last evaluation in the PEA patients were compared using repeat-measures ANOVA.
Overall survival analysis was performed based on a prospective approach. We used the Kaplan-Meier method for the survival analysis, and the differences between survival curves were assessed using the log-rank test. A p-value < 0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
We recruited 23 patients, including 19 females (83%) and 4 males (17%), with a mean age of 48.4 ± 16.1 years at the time of diagnosis (Table 1). PEA was performed in 19 patients including 15 females and 4 males, while four patients did not undergo PEA. One patient refused surgery, one patient was not indicated for surgery because of surgical inaccessibility of vascular occlusions, and one patient had distal occlusion and comorbid hemolytic anemia. The other patient was deemed unsuitable for surgery by the cardiac surgeon because of the high surgical risk of comorbidities. All of the four patients received pulmonary arterial hypertension (PAH)-specific drug therapy, and all survived for more than 3 years. Two of them expired due to right heart failure (Table 2).
Table 1. Baseline demographic characteristics of patients with chronic thromboembolic pulmonary hypertension.
| Variables | Mean ± SD or number (%) | Range |
| Female, n (%) | 19 (83%) | |
| Age at diagnosis, years | 48.4 ± 16.1 | 9-71 |
| BMI, kg/m2 | 24.4 ± 7.0 | 14.2-46.7 |
| SBP, mmHg | 125 ± 18 | 96-160 |
| DBP, mmHg | 81 ± 13 | 58-106 |
| HR, beats/min | 89 ± 12 | 54-122 |
| SpO2, % | 90 ± 8 | 77-100 |
| FC II/III/IV, n (%) | 1 (4%)/14 (61%)/8 (35%) | |
| 6MWD, m | 338 ± 86 | 180-474 |
| Non-O blood group, n (%) | 21 (91%) | |
| Symptoms to diagnosis, months | 27.7 ± 61.5 | 2-275 |
| VTE, n (%) | 19 (83%) | |
| Acute PE, n (%) | 17 (74%) | |
| DVT, n (%) | 8 (35%) | |
| Smoking, n (%) | 5 (22%) | |
| Estrogen replacement therapy, n (%) | 4 (17%) | |
| Comorbidities | ||
| Hypertension, n (%) | 7 (30%) | |
| Malignancy*, n (%) | 5 (22%) | |
| APS, n (%) | 4 (17%) | |
| AF, n (%) | 4 (17%) | |
| Asthma, n (%) | 4 (17%) | |
| CAD, n (%) | 3 (13%) | |
| Others#, n (%) | 7 (30%) | |
| Laboratory profiles | ||
| Hemoglobin, g/dL | 13.6 ± 2.4 | 9.4-18.5 |
| WBC, 103/μL | 9.0 ± 3.9 | 3.8-18.8 |
| Platelet, 103/μL | 243 ± 109 | 68-518 |
| D-dimer, ng/mL | 2853 ± 2869 | 170-10000 |
| Fibrinogen, mg/dL | 368 ± 117 | 186-640 |
| Protein C, % | 78.5 ± 22.7 | 43.7-124.1 |
| Protein S, % | 100.5 ± 36.3 | 22.9-185.3 |
| AST, U/L | 39 ± 26 | 16-120 |
| ALT, U/L | 40 ± 43 | 5-181 |
| Creatinine, mg/dL | 0.9 ± 0.3 | 0.4-1.6 |
| Uric acid, mg/dL | 7.2 ± 2.6 | 3.5-12.5 |
| Total cholesterol, mg/dL | 168 ± 46 | 108-280 |
| Fasting glucose, mg/dL | 106 ± 30 | 74-198 |
| BNP, pg/mL | 429 ± 500 | 19-1911 |
| 2D echocardiography | ||
| TRV, m/s | 4.2 ± 0.5 | 2.7-4.8 |
| SPAP, mmHg | 76 ± 18 | 32-102 |
| MPAP | 48 ± 11 | 22-64 |
| Tei index | 0.58 ± 0.24 | 0.06-1.00 |
| TRV/VTIRVOT ratio | 0.45 ± 0.18 | 0.14-0.78 |
| TAPSE, cm | 1.61 ± 0.41 | 1.10-2.43 |
| RA area, cm2 | 24 ± 8 | 10-43 |
| Systolic LVeI | 1.60 ± 0.53 | 1.10-3.18 |
| Diastolic LVeI | 1.42 ± 0.26 | 1.02-2.08 |
| RVFAC, % | 25 ± 8 | 17-43 |
| Pericardial effusion, n (%) | 8 (35%) | |
| Cardiac catheterization | ||
| RAP, mmHg | 12 ± 7 | 3-30 |
| SPAP, mmHg | 84 ± 20 | 51-129 |
| MPAP, mmHg | 51 ± 10 | 32-74 |
| PAWP, mmHg | 11 ± 5 | 3-21 |
| CO, L/min | 3.0 ± 1.0 | 1.6-4.8 |
| CI, L/min·m2 | 1.9 ± 0.6 | 1.2-3.1 |
| PVR, WU | 12.4 ± 6.8 | 4.5-28.1 |
| MvO2, % | 58 ± 13 | 37-76 |
| SaO2, % | 92 ± 6 | 75-100 |
| Treatment | ||
| PAH-specific drugs, n (%) | 8 (35%) | |
| PDE5i, n (%) | 7 (30%) | |
| ERA, n (%) | 2 (9%) | |
| sCGS, n (%) | 6 (26%) | |
| PEA, n (%) | 19 (83%) | |
| Bridging therapy, n (%) | 3 (16%) | |
| IVC filter implantation, n (%) | 5 (22%) | |
| BPA, n (%) | 4 (17%) |
Data are expressed as mean ± SD for continuous variables and as number and percentage for categorical variables.
* Malignancy included 2 colorectal cancer, 1 breast cancer, 1 ovarian with endometrial cancer, and 1 cervical cancer; # Others included 2 diabetes mellitus, 2 systemic lupus erythematosus, 1 embolic stroke, 1 chronic obstructive pulmonary disease, and 1 hemolytic anemia with splenectomy.
AF, atrial fibrillation; ALT, alanine aminotransferase; APS, antiphospholipid syndrome; AST, aspartate aminotransferase; BMI, body mass index; BNP, B-type natriuretic peptide; BPA, balloon pulmonary angioplasty; CAD, coronary artery disease; CI, cardiac index; CO, cardiac output; DBP, diastolic blood pressure; DVT, deep venous thrombosis; ERA, endothelin receptor antagonist; FC, functional class; HR, heart rate; IVC, inferior vena cava; LVeI, left ventricular eccentricity index; MPAP, mean pulmonary artery pressure; MvO2, mixed venous oxygen saturation; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PDE5i, phosphodiesterase-5 inhibitor; PE, pulmonary embolism; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance; RA, right atrial; RAP, right atrial pressure; RVFAC, right ventricular fractional area change; SaO2, arterial oxygen saturation; SBP, systolic blood pressure; sCGS, soluble guanylate cyclase stimulator; SPAP, systolic pulmonary artery pressure; SpO2, oxyhemoglobin saturation by pulse oximetry; TAPSE, tricuspid annular plane systolic excursion; TRV, peak tricuspid regurgitation velocity; VTIRVOT, velocity time integral at the right ventricular outflow tract; VTE, venous thromboembolism; WBC, white blood cell; 6MWD, 6-minute walk distance.
Table 2. Individual data of the 4 patients who did not undergo pulmonary endarterectomy.
| Variables | Case 1 | Case 2 | Case 3 | Case 4* |
| Sex | Female | Female | Female | Female |
| Age, years | 66 | 71 | 40 | 36 |
| Follow-up period, months | 69 | 64 | 119 | 44 |
| Functional class | ||||
| At diagnosis | III | III | III | IV |
| Last evaluation | IV | II | II | III |
| 6MWD, m | ||||
| At diagnosis | 338 | 416 | 387 | 221 |
| Last evaluation | 286 | 390 | 429 | 390 |
| Level of pulmonary artery stenosis by selective angiography# | Distal | Distal | Distal | Proximal (by CTA) |
| Cardiac catheterization | ||||
| RAP, mmHg | 15 | 8 | 10 | 8 |
| SPAP, mmHg | 123 | 68 | 65 | 96 |
| MPAP, mmHg | 74 | 43 | 46 | 61 |
| PAWP, mmHg | 12 | 14 | ||
| CO, L/min | 2.5 | 3.8 | 4 | |
| CI, L/min·m2 | 1.9 | 2.7 | 2.7 | |
| PVR, WU | 29.2 | 5.3 | 8.2 | 8.0 |
| MvO2, % | 51 | 72 | 73 | |
| SaO2, % | 88 | 92 | 95 | 77 (SpO2) |
| Reasons of no PEA | Patient’s refusal | Surgical inaccessible | Surgical inaccessible | Co-morbidities of SLE and APS |
| PAH-specific drugs | Sildenafil | Riociguat | Riociguat | Bosentan + sildenafil |
| Survival outcome | Expired | Alive | Alive | Expired |
* Hemodynamic data by echocardiography; # Proximal lesions indicate main or lobal pulmonary artery stenosis, whilst distal lesions indicate segmental or subsegmental stenosis.
APS, antiphospholipid syndrome; CI, cardiac index; CO, cardiac output; CTA, CT angiography of chest; MPAP, mean pulmonary artery pressure; MvO2, mixed venous oxygen saturation; PAH, pulmonary arterial hypertension; PAWP, pulmonary artery wedge pressure; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SaO2, arterial oxygen saturation; SLE, systemic lupus erythematosus; SPAP, systolic pulmonary artery pressure; SpO2, oxyhemoglobin saturation by pulse oximetry; 6MWD, 6-minute walk distance.
Table 1 shows the demographic characteristics and laboratory data of the CTEPH patients. The FC during the first presentation was as follows: class II in 1 patient (4%), class III in 14 patients (61%), and class IV in 8 patients (35%). A history of VTE was found in 83% of the patients overall, of whom 74% had PE and 35% had deep venous thrombosis (DVT). The non-O blood group accounted for 91% of the patients. Five (22%) patients were smokers. One patient had undergone splenectomy. Three patients took oral contraceptive pills and one took a selective estrogen receptor modulator. With regards to comorbidities, 7 patients had hypertension, 5 had malignancy (2 were found prior to a diagnosis of CTEPH, and 3 were discovered after a diagnosis of CTEPH), 4 had APS, 4 had atrial fibrillation, 4 had asthma, 3 had coronary artery disease, 2 had diabetes mellitus, 2 had systemic lupus erythematosus, 1 had embolic stroke, 1 had chronic obstructive pulmonary disease, and 1 had hemolytic anemia.
The 6MWD was 338 ± 86 m. 2D echocardiography showed pericardial effusion in 35% of the patients. The estimated systolic pulmonary artery pressure (SPAP) was 76 ± 18 mmHg, which was calculated by the peak tricuspid regurgitation velocity (TRV) with a simplified Bernoulli equation.14-16 The MPAP was 48 ± 11 mmHg, which was obtained from the formula MPAP = 0.61 × SPAP + 2.17,18 For the patients who had no tricuspid regurgitation, MPAP was obtained by pulmonary regurgitation velocity.19 The ratio of TRV and velocity time integral at the right ventricular outflow tract (VTIRVOT) was 0.45 ± 0.18, and right ventricular fractional area change (RVFAC) and tricuspid annular plane systolic excursion (TAPSE) were 25 ± 8% and 1.64 ± 0.41 cm, respectively. Right heart catheterization showed SPAP of 84 ± 20 mmHg, MPAP of 51 ± 10 mmHg, and PAWP of 11 ± 5 mmHg. PVR was 12.4 ± 6.8 WU. The cardiac output (CO) and cardiac index (CI) were 3.0 ± 1.0 L/min and 1.9 ± 0.6 L/min·m2, respectively.
Eight patients (35%) received PAH-specific medication, including 4 patients who did not undergo PEA. Riociguat was administered in 6 patients. Three patients (16%) received medication before PEA. Inferior vena cava (IVC) filter implantation was performed in 5 patients (22%) who had DVT.
Operation and postoperative care
Table 3 shows the perioperative and postoperative characteristics of the 19 patients who underwent PEA 22.0 ± 35.5 months after the diagnosis. Twelve patients (63%) had FC III and 7 patients (37%) had FC IV. The patients were categorized according to Jamieson classification based on the location of thromboemboli during PEA.20 Nine patients (47%) had Jamieson type I disease (J1), 6 (32%) had type II disease (J2), 3 (16%) had type III disease (J3), and 1 (5%) had type IV disease (J4). Other combined operations or procedures were performed in 5 patients (26%) simultaneously, including coronary artery bypass surgery in 2, closure of patent fossa ovale in 2, and surgery for atrial septal defect in 1 patient.
Table 3. Perioperative and postoperative characteristics of the patients undergoing pulmonary endarterectomy.
| Variables | Mean ± SD or number (%) | Range |
| Number of patients, n (%) | 19 (100%) | |
| Pre-PEA FC | ||
| III, n (%) | 12 (63%) | |
| IV, n (%) | 7 (37%) | |
| Diagnosis to PEA, months | 22.0 ± 35.5 | 0-128 |
| Cardiac catheterization | ||
| RAP, mmHg | 13 ± 7 | 3-30 |
| SPAP, mmHg | 86 ± 20 | 51-129 |
| MPAP, mmHg | 51 ± 11 | 32-74 |
| PAWP, mmHg | 11 ± 5 | 3-21 |
| CO, L/min | 3.0 ± 1.0 | 1.6-4.8 |
| CI, L/min·m2 | 1.9 ± 0.6 | 1.2-3.1 |
| PVR, WU | 13.1 ± 6.9 | 4.5-28.1 |
| MvO2, % | 56 ± 12 | 37-76 |
| SaO2, % | 92 ± 6 | 75-100 |
| Jamieson classification of PTE | ||
| J1, n (%) | 9 (47%) | |
| J2, n (%) | 6 (32%) | |
| J3, n (%) | 3 (16%) | |
| J4, n (%) | 1 (5%) | |
| Operation time | ||
| CPB time, min | 226 ± 83 | 90-413 |
| TCA time, min | 60 ± 31 | 20-125 |
| Combined operation*, n (%) | 5 (26%) | |
| Postoperative care | ||
| ICU stay, days | 8 ± 13 | 1-55 |
| Hospital stay, days | 19 ± 26 | 6-119 |
| Endotracheal intubation, days | 5 ± 6 | 1-20 |
| ECMO#, days | 10 ± 7 | 3-20 |
| PAH-specific drug | ||
| sCGS, n (%) | 4 (21%) | |
| Post-PEA follow-up, months | 37.7 ± 42.8 | 0.2-150 |
Data are expressed as mean ± SD for continuous variables and as number and percentage for categorical variables.
* Combined operation included 2 coronary artery bypass graft surgery, 2 patent fossa ovale closure, and 1 atrial septal defect closure. # ECMO was done in 4 patients.
CI, cardiac index; CO, cardiac output; CPB, cardiopulmonary bypass; ECMO, extracorporeal membrane oxygenation; FC, functional class; ICU, intensive care unit; J1, Jamieson type 1 disease; J2, Jamieson type 2 disease; J3, Jamieson type 3 disease; J4, Jamieson type 4 disease; MPAP, mean pulmonary artery pressure; MvO2; mixed venous oxygen saturation; PAWP, pulmonary artery wedge pressure; PEA, pulmonary endarterectomy; PTE, pulmonary thromboembolism; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SaO2, arterial oxygen saturation; sCGS, soluble guanylate cyclase stimulator; SPAP, systolic pulmonary artery pressure; TCA, total circulatory arrest.
The mean duration of stay of the patients in the intensive care unit (ICU) was 8 ± 13 days. Reperfusion lung injury or edema after PEA was closely monitored by SpO2, arterial blood gas and chest X-ray. Of the 19 PEA patients, 13 (68%) suffered from lung edema, and one patient had bronchial hemorrhage. The mean duration of reperfusion injury was 9 ± 9 days (ranging from 1 to 33 days). Most of them were recovered by conservative management such as maintaining adequate oxygenation, diuretics and fluid restriction. However, 4 unstable patients (21%) underwent extracorporeal membrane oxygenation (ECMO) therapy, one of whom received an intra-aortic balloon pump. Among them, early mortality was found in 2 patients (both on the 7th day after PEA). The causes of death were progressive deterioration of right heart failure and brainstem compression related to diffuse brain swelling, respectively. Three patients had neurological sequelae due to cerebral infarction or intracranial hemorrhage.
Postoperative follow-up
The mean follow-up period was 37.7 ± 42.8 months. There were significant improvements in FC 3 months after PEA (Figure 1). All 17 patients had improved FC. Of note, 8 (47%) of them improved by more than 1 class. The 6MWD significantly increased from 326 ± 62 to 420 ± 63 m (Figure 2A). The BNP level was significantly reduced after PEA (602 ± 599 vs. 268 ± 565 pg/mL, p = 0.017). Additionally, SpO2 was significantly increased after surgery (88 ± 5 vs. 93 ± 4%, p = 0.048) (Figure 2B), and even further improved at the last evaluation of the follow-up period (93 ± 4 vs. 95 ± 4%, p = 0.042).
Figure 1.
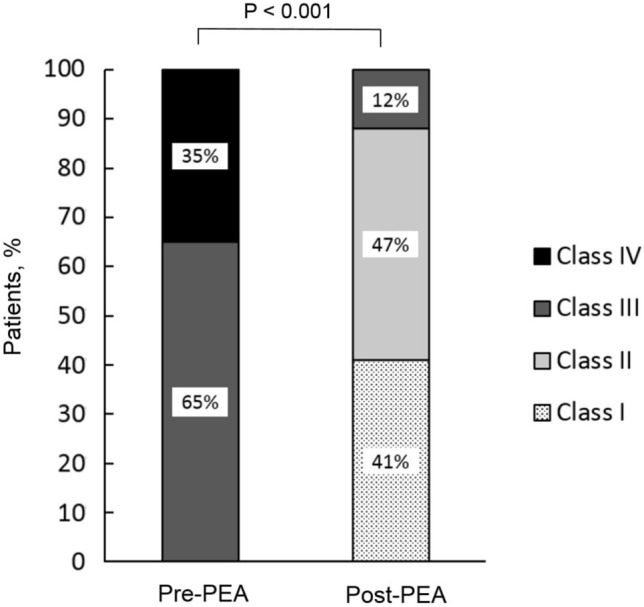
Functional class in patients with chronic thromboembolic pulmonary hypertension at baseline and 3 months after pulmonary endarterectomy. PEA, pulmonary endarterectomy.
Figure 2.
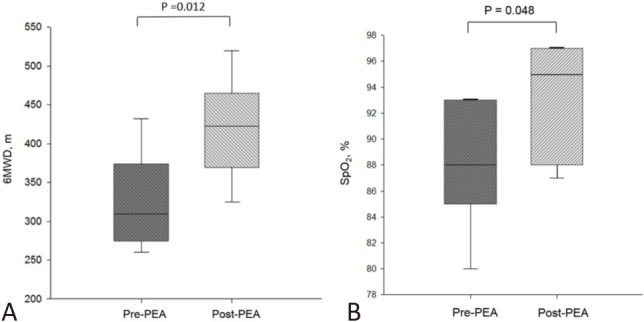
Significant improvement in (A) 6-minute walk distance (326 ± 62 m vs. 420 ± 63 m, p = 0.012) and (B) oxyhemoglobin saturation by pulse oximetry (88 ± 5% vs. 93 ± 4%, p = 0.048) before and 3 months after pulmonary endarterectomy. PEA, pulmonary endarterectomy.
Table 4 shows the echocardiographic data before and after long-term follow-up after PEA. Tricuspid regurgitation improved after the procedure. There were significant reductions in SPAP (79 ± 19 vs. 48 ± 19 mmHg), and TRV/VTIRVOT ratio (0.44 ± 0.17 vs. 0.24 ± 0.55) 3 months after PEA. Moreover, significant reductions were found in the right atrial (RA) area (23 ± 6 vs. 17 ± 4 cm2), right ventricular end-systolic area (RVESA) (18 ± 6 vs. 13 ± 4), and left ventricular eccentricity index (LVeI) both in systole (1.55 ± 0.32 vs. 1.14 ± 0.17) and diastole (1.36 ± 0.20 vs. 1.01 ± 0.18). The significant changes in these parameters remained until the last evaluation. Although insignificant changes were noted during the first 3 months, right ventricular end-diastolic area (RVEDA) (23 ± 5 vs. 17 ± 6 cm2), RVFAC (25 ± 8% vs. 34 ± 14%) and caval index (32 ± 25% vs. 57 ± 21%) significantly improved at the last evaluation during follow-up.
Table 4. Preoperative and postoperative echocardiographic data of patients receiving pulmonary endarterectomy.
| Variables | Pre-PEA | 3 months | 12 months | Last evaluation* |
| Patients, n | 17 | 17 | 16 | 17 |
| TR grading 0/1/2/3#, n | 1/9/6/1 | 6/9/2/0‡ | 6/7/3/0‡ | 11/5/1/0‡ |
| SPAP, mmHg | 79 ± 19 | 48 ± 19‡ | 56 ± 21‡ | 54 ± 19‡ |
| Tei index | 0.55 ± 0.29 | 0.43 ± 0.37 | 0.38 ± 0.26 | 0.37 ± 0.29 |
| TRV/VTIRVOT ratio | 0.44 ± 0.17 | 0.24 ± 0.55‡ | 0.30 ± 0.13‡ | 0.31 ± 0.09‡ |
| TAPSE, cm | 1.58 ± 0.42 | 1.49 ± 0.48 | 1.53 ± 0.27 | 1.59 ± 0.44 |
| RA area, cm2 | 23 ± 6 | 17 ± 4‡ | 19 ± 6‡ | 16 ± 7‡ |
| Systolic LVeI | 1.55 ± 0.32 | 1.14 ± 0.17‡ | 1.17 ± 0.29‡ | 1.19 ± 0.20‡ |
| Diastolic LVeI | 1.36 ± 0.20 | 1.01 ± 0.18‡ | 1.06 ± 0.09‡ | 1.14 ± 0.23‡ |
| RVEDA, cm2 | 23 ± 5 | 19 ± 7 | 19 ± 7 | 17 ± 6‡ |
| RVESA, cm2 | 18 ± 6 | 13 ± 4‡ | 13 ± 7‡ | 12 ± 6‡ |
| RVFAC, % | 25 ± 8 | 32 ± 13 | 35 ± 13‡ | 34 ± 14‡ |
| Caval index†, % | 32 ± 25 | 52 ± 22 | 49 ± 20 | 57 ± 21‡ |
| Pericardial effusion, n (%) | 5 (29) | 0 (0) ‡ | 0 (0) ‡ | 0 (0) ‡ |
| BNP, pg/mL | 602 ± 599 | 286 ± 565‡ | 163 ± 194‡ | 159 ± 182‡ |
Data are expressed as mean ± SD for continuous variables and as a number with percentage for categorical variables.
* Last evaluation performed 30[19,46] months after PEA (range 6-150 months); # Tricuspid regurgitation (TR) grading: absent/trivial = 0, mild = 1, moderate = 2, severe = 3; † Caval index represents inferior vena cava collapse ratio between expiration and inspiration; ‡ p < 0.05 vs pre-PEA.
No significant difference among the data in 3 months vs. 12 months and last evaluation.
BNP, B-type natriuretic peptide; LVeI, left ventricular eccentricity index; PEA, pulmonary endarterectomy; RA, right atrial; RVEDA, right ventricular end-diastolic area; RVESA, right ventricular end-systolic area; RVFAC, right ventricular fractional area change; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TRV, peak tricuspid regurgitation velocity; VTIRVOT, velocity time integral at the right ventricular outflow tract.
Overall survival in the patients receiving PEA is shown in Figure 3. No patient was lost to follow-up or underwent lung transplant during this period. Both 1-year and 2-year survival rates were 89%, and the 3-year and 5-year survival rates were 81% and 50%, respectively. Six patients died, and the causes of death were as follows: 2 patients had right heart failure, 2 had sepsis, 1 had hypoxic encephalopathy, and 1 had end-stage colon cancer.
Figure 3.
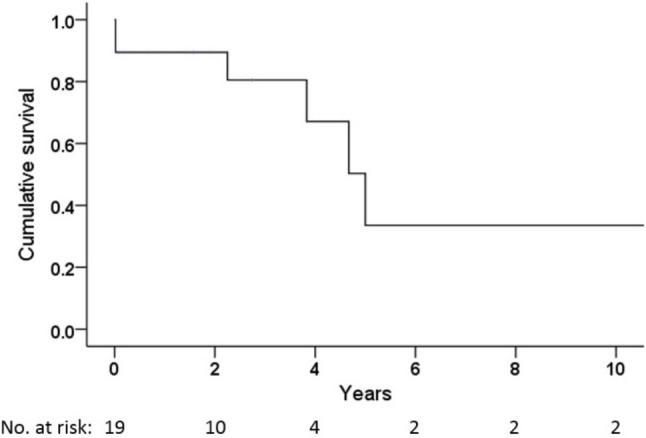
Kaplan-Meier estimates of the overall survival in patients with chronic thromboembolic pulmonary hypertension undergoing pulmonary endarterectomy. The 1-, 2-, 3-, and 5-year survival rates were 89%, 89%, 81%, and 50%, respectively.
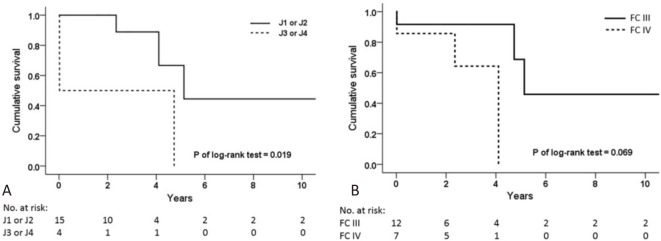
The patients with proximal thromboembolic diseases (J1 or J2) had better overall survival outcomes than those with distal diseases (J3 or J4) (Figure 4A). However, there was no significant difference in survival between the FC III and FC IV patients (Figure 4B).
Figure 4.
Kaplan-Meier analysis of the overall survival in different locations of pulmonary thromboembolism and functional class of patients undergoing pulmonary endarterectomy. (A) The survival rate of proximal diseases (J1 or J2) was better than that of distal thromboembolic diseases (J3 or J4), p = 0.019. (B) No significant difference was noted in the survival rate between functional class III and IV, p = 0.069. J1, Jamieson type 1 disease; J2, Jamieson type 2 disease; J3, Jamieson type 3 disease; J4, Jamieson type 4 disease.
DISCUSSION
PEA was performed in 19 of 23 CTEPH patients (83%). Significant improvements in the FC, 6MWD, and BNP levels were found after the procedure. Moreover, follow-up echocardiography revealed improvements in right ventricular (RV) function and hemodynamics. The postoperative 1-, 2-, 3-, and 5-year overall survival rates were 89%, 89%, 81%, and 50%, respectively. Although the first 2-year survival rates are comparable with those of international studies, the outcomes after 2 years were inferior to other studies.12,21 Moreover, a higher in-hospital mortality rate and more neurological sequelae were found in this study.
CTEPH is a rare complication of PE, and the prognosis is generally poor.1 It can potentially be cured by PEA.1,8,9 The in-hospital mortality rate of PEA is below 5% in experienced centers,6,12,22 and therefore PEA is a standard therapeutic approach for CTEPH. However, there are difficulties in standardizing the operability of patients due to patient suitability, resource availability, and expertise of the surgical team.1 In our case series, there were 3 major considerations for verifying the CTEPH patients as PEA candidates: (1) surgical accessibility of organized thrombi; (2) surgical risk evaluation, especially for comorbidities; and (3) patients’ willingness to undergo the operation. A high PVR over 1500 dyn.s.cm-5 (19 WU) has been regarded as one of the criteria for inoperability in previous studies.4,6,21 Notably, 3 of the patients who received the procedure had similarly high PVR in this study, and both of the patients with in-hospital early mortality had high PVR. However, Kim and colleagues reported that excellent post-PEA outcomes without increased risks of morbidity and mortality could still be achieved even in patients with high PVR.23 Therefore, the surgical techniques and postoperative care are crucial.
The same surgeon performed the procedure in 17 (89%) of the 19 patients who underwent PEA. For the 17 patients who survived to hospital discharge, the cardiopulmonary bypass (CPB) time and total circulatory arrest (TCA) time were 211 ± 71 and 54 ± 26 minutes, respectively. However, the 2 patients with in-hospital early mortality had a prolonged CPB (413 and 289 min) and TCA (125 and 89 min). Both of them required ECMO after PEA due to unstable hemodynamics. One patient was categorized as J4 and received combination surgery with coronary artery bypass graft, and the other was J3 with a history of breast cancer diagnosed 5 years before PEA. In spite of the fact that the surgical risk of PEA is determined predominantly by the location and the extent of organized thrombi, there are several other critical determinants including the degree of hemodynamic compromise and prolonged CPB and TCA times.9 This could be the main reason why a higher in-hospital mortality rate (10.5%) was observed in this series than in other cohort studies (2.2-4.4%).12,22
A recent international prospective registry reported a 3-year survival rate of 89% in patients undergoing PEA, compared to only 70% in those who did not undergo PEA. Mortality was significantly associated with preoperative FC IV, increased RA pressure, and malignancy in both operated and non-operated groups. Among the operated patients, the survivors tended to have a higher 6MWD and CI, as well as lower RA pressure and PVR.21 Although FC III/IV accounted for the majority of PEA patients, Delcroix and colleagues reported that 19% of their patients had FC I/II.21 At the University of California San Diego Medical Center (UCSD), 11.2% and 6.6% of FC II CTEPH patients underwent surgery between March 1999~October 2006 and October 2006~December 2010, respectively.12 Better survival outcomes have been reported in patients with FC I/II than in those with FC III/IV.9 However, all of the PEA patients in our case series had FC III/IV, and none of them had FC I/II. No significant differences in survival were found between the patients with FC III and FC IV (Figure 4B), which may be due to the small sample size. Additionally, we found more advanced hemodynamic compromise at the time of diagnosis in our patients. The MPAP and PVR were 51 ± 11 mmHg and 13.1 ± 6.9 WU, respectively, which were higher than those in previous studies (MPAP 43-47 mmHg and PVR 7-11 WU).4,13,21 This also indicates the late diagnosis of our patients. Hence, we observed poorer long-term outcomes than those in the other studies.12,21,24 This highlights that an early diagnosis and treatment are important determinants of outcomes. In recent years, we have referred our patients for PEA as soon as possible. Among the 19 PEA patients, the duration from a diagnosis of CTEPH to PEA was longer for those diagnosed from 2001 to 2009 (50.3 ± 40.9 months, n = 8) than those diagnosed from 2010 to 2017 (1.5 ± 2.3 months, n = 11). The 2 early mortality patients belonged to the former group.
There was a high frequency of malignancy (22%) in our case series. Two patients had breast and intraepithelial cervical cancer prior to the diagnosis of CTEPH. Of the 3 patients who were diagnosed after PEA, 2 had colorectal adenocarcinoma and 1 had endometrial cancer. Poor outcomes have been reported in CTEPH associated with malignancy.21
Surgical accessibility of organized thrombi in this study was evaluated according to Jamieson classification during PEA.20 Nine patients (47%) were classified as J1 with visible fresh thrombi localized within major vessels. Six patients (32%) were classified as J2 with thickened intima in lobar branches and no visible major vessel thrombus, and another 3 patients (16%) were classified as J3 with distal lesions that were confined to segmental and subsegmental vessels. Only 1 patient was categorized as J4 with no evident intravascular lesion despite thorough surgical exploration. Thistlethwaite et al. reported that the location of thromboembolic diseases was a predictor for short-term mortality and postoperative hospitality duration, with the J4 patients having the poorest outcomes.20 The 2 patients with in-hospital mortality in this study were categorized as J3 and J4. The patients with proximal thromboembolic diseases (J1 or J2) had better overall survival outcome than those with distal diseases (J3 or J4) (Figure 4A), which is consistent with previous studies.22,25 Moreover, the patients with proximal diseases had a significantly greater extent of hemodynamic improvement than those with distal diseases after PEA.20 However, despite the fact that the incidence of J3 seems to have increased in the past 2 decades, a continual decline in mortality can still be obtained by making an accurate diagnosis, improving surgical techniques, and appropriate postoperative care.12,26
Improvement in RV function is regarded to be a favorable determinant of PEA efficiency.14 In this study, improved RV function and hemodynamics were observed after PEA. Normalized RVFAC and LVeI indicates improved RV function and interventricular interaction after PEA, however the postoperative reduction in TAPSE was insignificant. This is in line with previous studies which reported that TAPSE could be influenced by changes in heart motion after cardiac surgery.27-29 An enlarged RA area (> 18 cm2) suggests increased RV pressure,30 and reduced RA area, RVEDA and PAP after PEA imply reduced RV pressure. In addition, the preoperative TRV/VTIRVOT ratio is in accordance with high PVR.31 A ratio over 0.38 has been reported to indicate a PVR of > 8 WU.32 The significant decline in TRV/VTIRVOT ratio in our case series indicates the positive effect of PVR reduction after PEA.
The European international prospective registry reported that 29% of operated patients received bridging therapy with PAH-specific drugs, and mainly sildenafil and bosentan. However, the patients who received bridging therapy had an increased risk of mortality.21 The major reason may have been that medication therapy was used for those who were originally more hemodynamically compromised.21 In addition, delayed PEA due to medication therapy was also suggested in another retrospective analysis.33 Among the PEA candidates in our study, 3 patients (16%) received PAH-specific drugs before PEA due to severe clinical symptoms and limited exercise capacity. All of them were categorized as FC IV and had high preoperative PVR, with 1 in-hospital early mortality after PEA.
The distribution of ABO blood group varies among countries and ethnic groups, and a high non-O blood group percentage has been widely observed in CTEPH patients.4,21,34,35 Ninety-one percent of our patients belonged to the non-O blood group, which is in line with previous reports. Non-O carriers generally have a higher risk of VTE because of susceptibility of the ABO locus for VTE.36 However, abnormal structures in fibrinogen or fibrin molecules affect thrombus resolution, regardless of the intact plasma fibrinolytic system in patients with CTEPH.5,37-40 A history of acute PE has been observed in most patients with CTEPH,5,35 and was reported in 74.8% of patients in an international prospective registry;4 a similar proportion of patients was observed in our series (74%).
This study is limited by the small sample size that was collected from a single center. Therefore, possible selection bias might have affected our results. In addition, we did not evaluate the prognostic factors for PEA in this study due to the small study population. Of note, most of the CTEPH patients were diagnosed in the advanced stage, and all of the PEA patients had FC III/IV in this case series. Disease awareness and early diagnosis of CTEPH remain challenging. Furthermore, surgical techniques and postoperative care are vital. Moreover, we did not discuss balloon pulmonary angioplasty (BPA) or PAH-specific therapy in the management of CTEPH due to the limited number of cases.
CONCLUSIONS
PEA is an important therapeutic approach for CTEPH. Significant improvements in symptoms, functional capacity, and hemodynamics were achieved after the procedure. However, the overall survival was still unsatisfactory. Disease awareness, early diagnosis, surgical techniques, and postoperative care remain challenging. Further, the roles of BPA and PAH-specific medications in patients who do not receive surgery are evolving. All of these issues need to be clarified; therefore, a nationwide multicenter registration study is necessary for this uncommon disease.
Acknowledgments
This study was supported by grant from the National Science Council (NMRPG3G0471), Taiwan.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation. 2014;130:508–518. doi: 10.1161/CIRCULATIONAHA.114.009309. [DOI] [PubMed] [Google Scholar]
- 3.Pepke-Zaba J, Jansa P, Kim NH, et al. Chronic thromboembolic pulmonary hypertension: role of medical therapy. Eur Respir J. 2013;41:985–990. doi: 10.1183/09031936.00201612. [DOI] [PubMed] [Google Scholar]
- 4.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Torbicki A, Dorfmüller P, et al. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26:160112. doi: 10.1183/16000617.0112-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Lang I. Chronic thromboembolic pulmonary hypertension: a distinct disease entity. Eur Respir Rev. 2015;24:246–252. doi: 10.1183/16000617.00001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang IM, Pesavento R, Bonderman D, Yuan JX. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: a current understanding. Eur Respir J. 2013;41:462–468. doi: 10.1183/09031936.00049312. [DOI] [PubMed] [Google Scholar]
- 9.Madani M, Mayer E, Fadel E, Jenkins DP. Pulmonary endarterectomy. Patient selection, technical challenges, and outcomes. Ann Am Thorac Soc. 2016;13:S240–S247. doi: 10.1513/AnnalsATS.201601-014AS. [DOI] [PubMed] [Google Scholar]
- 10.Guth S, Wiedenroth CB, Kramm T, Mayer E. Pulmonary endarterectomy for the treatment of chronic thromboembolic pulmonary hypertension. Expert Rev Respir Med. 2016;10:673–684. doi: 10.1080/17476348.2016.1176915. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins D, Mayer E, Screaton N, Madani M. State-of-the-art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur Respir Rev. 2012;21:32–39. doi: 10.1183/09059180.00009211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution’s experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97–103. doi: 10.1016/j.athoracsur.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Lankeit M, Krieg V, Hobohm L, et al. Pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2018;37:205–208. doi: 10.1016/j.healun.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Ho WJ, Lin CP, Wang CL, et al. Improvement of right ventricular function in pulmonary arterial hypertension with disease-specific therapy – a clinical observational study. Acta Cardiol Sin. 2014;30:236–244. [PMC free article] [PubMed] [Google Scholar]
- 15.Pussadhamma B, Suwannakrua W, Toparkngarm P, et al. Relationship between peripheral arterial stiffness and estimated pulmonary pressure by echocardiography in systemic sclerosis. Acta Cardiol Sin. 2017;33:514–522. doi: 10.6515/ACS20170220A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang LY, Lee KT, Lin CP, et al. Long-term survival of patients with pulmonary arterial hypertension at a single center in Taiwan. Acta Cardiol Sin. 2017;33:498–509. doi: 10.6515/ACS20170612A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steckelberg RC, Tseng AS, Nishimura R, et al. Derivation of mean pulmonary artery pressure from noninvasive parameters. J Am Soc Echocardiogr. 2013;26:464–468. doi: 10.1016/j.echo.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Chemla D, Castelain V, Humbert M, et al. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313–1317. doi: 10.1378/chest.126.4.1313. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CH, Ho WJ, Huang WC, et al. 2014 Guidelines of Taiwan Society of Cardiology (TSOC) for the management of pulmonary arterial hypertension. Acta Cardiol Sin. 2014;30:401–444. [PMC free article] [PubMed] [Google Scholar]
- 20.Thistlethwaite PA, Mo M, Madani MM, et al. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J Thorac Cardiovasc Surg. 2002;124:203–211. doi: 10.1067/mtc.2002.127313. [DOI] [PubMed] [Google Scholar]
- 21.Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation. 2016;133:859–871. doi: 10.1161/CIRCULATIONAHA.115.016522. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457–1464. doi: 10.1016/s0003-4975(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim NH, Madani MM, Pretorius V, et al. Pulmonary thromboendarterectomy outcome in patients with high pre-operative PVR: UCSD single center experience. Am J Respir Crit Care Med:Am Thoracic Soc. 2013:A3342. [Google Scholar]
- 24.Wang KY. The changing landscape of pulmonary arterial hypertension in 21st century. Acta Cardiol Sin. 2017;33:510–513. doi: 10.6515/ACS20170810A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamieson S, Auger W, Fedullo P, et al. Experience and results with 150 pulmonary thromboendarterectomy operations over a 29-month period. J Thorac Cardiovasc Surg. 1993;106:116–127. [PubMed] [Google Scholar]
- 26.D'Armini AM, Morsolini M, Mattiucci G, et al. Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2014;148:1005–1012. doi: 10.1016/j.jtcvs.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 27.Unsworth B, Casula RP, Kyriacou AA, et al. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J. 2010;159:314–322. doi: 10.1016/j.ahj.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanobini M, Saccocci M, Tamborini G, et al. Postoperative echocardiographic reduction of right ventricular function: is pericardial opening modality the main culprit? Biomed Res Int. 2017;2017:4808757. doi: 10.1155/2017/4808757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamborini G, Muratori M, Brusoni D, et al. Is right ventricular systolic function reduced after cardiac surgery? A two-and three-dimensional echocardiographic study. Eur J Echocardiogr. 2009;10:630–634. doi: 10.1093/ejechocard/jep015. [DOI] [PubMed] [Google Scholar]
- 30.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Opotowsky AR, Clair M, Afilalo J, et al. A simple echocardiographic method to estimate pulmonary vascular resistance. Am J Cardiol. 2013;112:873–882. doi: 10.1016/j.amjcard.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlahos AP, Feinstein JA, Schiller NB, Silverman NH. Extension of Doppler-derived echocardiographic measures of pulmonary vascular resistance to patients with moderate or severe pulmonary vascular disease. J Am Soc Echocardiogr. 2008;21:711–714. doi: 10.1016/j.echo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Jensen KW, Kerr KM, Fedullo PF, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation. 2009;120:1248–1254. doi: 10.1161/CIRCULATIONAHA.109.865881. [DOI] [PubMed] [Google Scholar]
- 34.Bonderman D, Wilkens H, Wakounig S, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33:325–331. doi: 10.1183/09031936.00087608. [DOI] [PubMed] [Google Scholar]
- 35.Bonderman D, Turecek PL, Jakowitsch J, et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2003;90:372–376. doi: 10.1160/TH03-02-0067. [DOI] [PubMed] [Google Scholar]
- 36.Wu O, Bayoumi N, Vickers M, et al. ABO (H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:62–69. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 37.Olman MA, Marsh JJ, Lang IM, et al. Endogenous fibrinolytic system in chronic large-vessel thromboembolic pulmonary hypertension. Circulation. 1992;86:1241–1248. doi: 10.1161/01.cir.86.4.1241. [DOI] [PubMed] [Google Scholar]
- 38.Morris TA, Marsh JJ, Chiles PG, et al. Fibrin derived from patients with chronic thromboembolic pulmonary hypertension is resistant to lysis. Am J Respir Crit Care Med. 2006;173:1270–1275. doi: 10.1164/rccm.200506-916OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh JJ, Chiles PG, Liang NC, Morris TA. Chronic thromboembolic pulmonary hypertension-associated dysfibrinogenemias exhibit disorganized fibrin structure. Thromb Res. 2013;132:729–734. doi: 10.1016/j.thromres.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 40.Lang IM, Dorfmuller P, Vonk Noordegraaf A. The pathobiology of chronic thromboembolic pulmonary hypertension. Ann Am Thorac Soc. 2016;13 Suppl 3:S215–S221. doi: 10.1513/AnnalsATS.201509-620AS. [DOI] [PubMed] [Google Scholar]