Abstract
Lifestyle behaviors in overweight and obese individuals are closely linked to the development, course, and outcomes of type 2 diabetes and multiple comorbid health conditions. Behavior change theory and many randomized controlled studies offer strong support for screening and identifying adults at increased cardiometabolic risk and for providing early intervention to mitigate risk factors to prevent or delay the onset of disease. The current article reviews key lifestyle intervention efficacy and dissemination trials conducted with individuals deemed to be at increased risk for diabetes and describes the rationale for training teams of professionals and community health workers (e.g., promotores [in Spanish]) to implement comprehensive programs, with fidelity, in a variety of medical care and community settings. This evidence-based road map may be used to facilitate the design and implementation of strategies for structured behavioral diabetes risk reduction programs in the public and private healthcare sectors and other relevant community-based platforms serving individuals of Hispanic/Latino origin in the United States and Mexico.
Keywords: Hispanic, Latino, lifestyle, obesity, prevention, type 2 diabetes
INTRODUCTION
Obesity, type 2 diabetes, and associated comorbid conditions are a significant public health burden in North America and globally. In Mexico, there has been a sharp, dramatic acceleration in noncommunicable diseases over the last 2 decades, and the Ministry of Health has responded strongly to increase awareness, education, interventions, healthcare training, and research.1 The main purpose of the current review is to summarize what is known about lifestyle behavior modification research aimed at the primary prevention of diabetes among obese individuals across the risk spectrum, including those with prediabetes and the metabolic syndrome. Most of these investigations have been performed in the United States and relatively few studies include samples comprised solely of Latinos. However, the current review includes several more recent studies of behaviorally based interventions with Latinos residing in urban centers and on the United States/Mexico border (note: in this paper, usage of the terms Hispanic or Latino/Latina is based on terminology used in the published reports). It is suggested that the lessons learned in the field of obesity management and diabetes prevention overall during the past few decades provide an excellent framework for moving forward with diabetes risk reduction efforts aimed at serving communities of Hispanic/Latino origin throughout North America. The current paper also addresses the strengths and limitations of existing community-based lifestyle program adaptations to manage obesity and reduce diabetes risk, identify gaps, and provide suggestions for future research.
Importance of Early Intervention for Obese Individuals with Elevated Risk for Diabetes
There is a long history of obesity theory and research showing that lifestyle self-management programs decrease health risks and provide a broad spectrum of physical and mental health benefits for many. Although individual variability in genetic and cardiometabolic risk, susceptibility to diabetes development, and responsivity to weight loss and weight maintenance interventions remain the focus of multifaceted research studies,2 there are compelling public health reasons not to delay behavioral primary prevention initiatives among obese individuals who are at elevated risk due to race/ethnicity and other factors.3,4 Obesity prevalence is disproportionately higher among persons of Hispanic/Latino origin compared with non-Hispanic whites,5 and the potential for comorbidity and complications is well documented.6–8 Multiple theoretical models support health behavior change efforts9 for obesity, with most current evidence-based lifestyle programs guided by behavioral learning theory and social-cognitive theory.10,11 For example, an investigation of mixed-risk obese women (some with diagnosed diabetes) in a Mexican public hospital system demonstrated that the integration of cognitive behavioral strategies with other diet and weight management approaches enhanced program efficacy for improving health risk factors above and beyond dietary approaches alone.12 Thus, it should be emphasized that educational programs alone, which are not grounded in behavior change theory and intervention, are associated with increases in knowledge and awareness but typically lead to less than optimal weight and physiologic outcomes.
The present review aims to discuss the following: research that highlights key elements of behavior change programs known to reduce cardiometabolic risk; the international efficacy trials that have repeatedly confirmed the importance of lifestyle intervention as a first-line treatment to combat diabetes and cardiovascular disease; and the more recent translation and dissemination studies that have provided guidance and a hopeful message for diabetes prevention in the public health arena. Several prevention-focused studies that have included primarily Hispanic/Latino groups and the implications for future research are also reviewed.
LIFESTYLE INTERVENTIONS FOR OBESITY AND DIABETES PREVENTION: KEY PROGRAM ELEMENTS
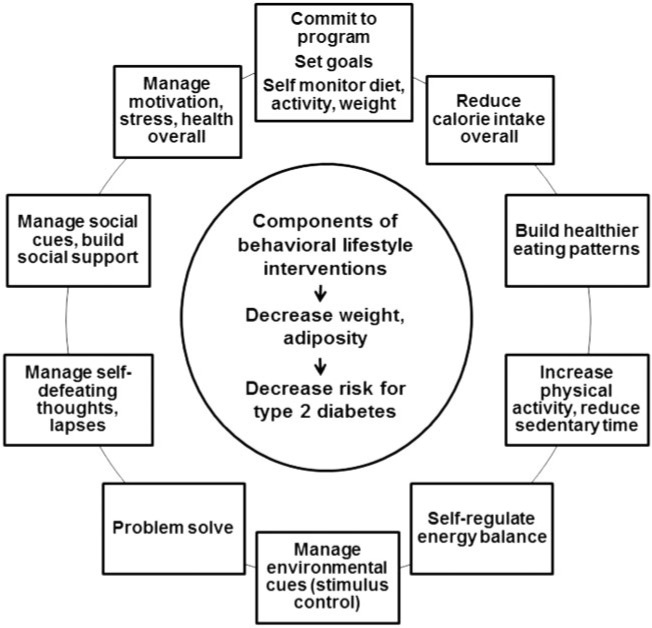
Major efficacy trials, including several international primary prevention studies with 10–20 years of longitudinal follow-up data,13–19 show clear, immediate benefit and a potential carryover effect of structured lifestyle interventions on health risk parameters, when compared with medication or placebo treatment. In these studies, the main intervention goal has been to enable obese persons with prediabetes or impaired glucose tolerance to make feasible changes in eating patterns and physical activity and lose a modest amount of weight in order to, in turn, impact body composition and the longer-term course of dysglycemia. The Group Lifestyle Balance intervention,20–25 the YMCA Diabetes Prevention Program (DPP),26,27 the Special Diabetes Program for Indians Demonstration Project,28 and the US Centers for Disease Control National Diabetes Prevention Program are all current derivations of the efficacious DPP intensive lifestyle intervention that was originally delivered to individuals on a case-by-case basis.29Figure 1 displays the conceptual model and learning sequence that generally characterizes obesity and diabetes prevention interventions. Table 1 presents an example of a recommended delivery schedule for the Group Lifestyle Balance 12-month intervention; this is one of several currently active translation and dissemination programs that have been systematically examined during the past decade.24
Figure 1.
Conceptual model and behavioral learning components of current lifestyle intervention programs for diabetes prevention. Data from Venditti and Kramer (2012).59
Table 1.
Typical model for 12-month, 22-session behavioral lifestyle intervention aimed at diabetes risk reduction
| Typical delivery sequence | Program |
|---|---|
|
Core curriculum (first 6 mo) | |
| Weekly (4/mo) |
|
| Weekly (4/mo) |
|
| Weekly (4/mo) |
|
|
Transition sessions (fade frequency) | |
| Biweekly (2/mo) |
|
| Biweekly or monthly | 15) Balance your thoughts |
| Biweekly or monthly | 16) Strengthen your exercise program |
|
Support sessions (second 6 mo): variable sequence, tailored to community | |
| Monthly | 17) Mindful eating |
| Monthly | 18) Stress and time management |
| Monthly | 19) Standing up for your health |
| Monthly | 20) Heart health |
| Monthly | 21) Stretching: the truth about flexibility |
| Monthly | 22) Looking back and looking forward |
Data from Venditti et al. (2013).24
Many investigators have endeavored to translate the robust DPP behavioral intervention,30 including research groups targeting Latino populations in large primary care systems31 and other community-based clinical settings.32–34 There are many public websites, which include manuals of operation with leaders’ guides, participant behavioral intervention materials in English and Spanish, and other health communication materials derived from evidence-based lifestyle intervention research, including those from the National Institutes of Health-National Institute of Diabetes, Digestive and Kidney Diseases, the National Diabetes Education Program and the Centers for Disease Control and Prevention.
To optimize standard behavioral obesity and diabetes prevention interventions, well trained and/or well-supervised lifestyle interventionists (often referred to as “lifestyle coaches”) provide participants with initial calorie, fat, weight, and activity target goals in ranges (e.g., 1200 kcal–2000 kcal, 150 min of weekly physical activity) known to produce safe, achievable energy balance deficits, modest weight loss, and physiological benefit.35,36 Behavioral interventions generally employ flexible, nonprescriptive dietary guidelines that permit tailoring to personal, familial, and cultural food preferences yet emphasize appropriate calorie reduction to achieve weight, glucose, and cardiometabolic control. Dietary guidelines in Mexico are akin to those provided in the United States, with an emphasis placed on prudent saturated fat reduction, a plant-focused (“whole foods”) eating style, decreases in highly processed and refined grains, salty snacks and sweets, red or processed meats, and sugary beverages. Dietary guidelines have shifted over time, but common behavioral wisdom suggests that helping participants construct affordable, accessible, appealing, and healthy meal and snack patterns that they believe they can incorporate into their daily lifestyle habits, as opposed to emphasizing single problem nutrients, will be more likely to elicit sustainable behavior change, weight management, and improved diabetes risk reduction.37
It is worth noting that the Mexican government (in comparison with the US government) has been remarkably proactive with respect to promoting decreased consumption of sugar-sweetened beverages by instituting a drink tax. Recent data suggest that this policy has influenced consumer behavior in a positive direction.38 This action is wholly consistent with a social learning approach to diabetes prevention, which maintains that environmental change is likely to have the most far-reaching impact on behavior change. Nonetheless, to amplify these benefits, it is also important to examine corollary educational awareness and intervention programs with the potential to influence family and community norms and to increase access to potable water sources, as well as examine impact on cardiometabolic outcomes.
Available cross-sectional data for over 1000 Mexican adults with low socioeconomic status showed that individuals who drank more water indeed consumed fewer caloric beverages.39 One Mexican intervention study found that the provision of drinking water and nutrition counseling to overweight and obese women increased their water intake and partially decreased their sugared beverage consumption; in the obese subgroup, some metabolic syndrome markers were also decreased.40 Certainly, these results are encouraging of further behavioral interventions for individuals with obesity and metabolic syndrome and they have direct implications for health promotion and public health policy. Finally, of equal importance are intervention efforts to increase physical activity (≥150 minutes/week) and reduce the time spent being sedentary. As is the case worldwide, physical inactivity is an acknowledged public health risk factor for diabetes and cardiovascular disease in Mexico. Epidemiological data from the National Health and Nutrition Survey in 2006 and 2012 suggest that the rate of inactivity has increased in Mexicans, particularly older adults in the obese category and those of higher socioeconomic status.41
In contrast, there is a need for more research on the proximal determinants of obesity-related behaviors impacting first- and second-generation Hispanic immigrants to the United States. Available data suggest that a variety of structural factors, including degree of acculturation, family income, household roles and responsibilities, maternal education, literacy, and health literacy, may differentially impact US-born relative to foreign-born immigrants, and the direction of such influence is not always consistent, particularly among Hispanic/Latino immigrants from different countries.42,43 Thus, assessing unique family and community risk factors is also important when adapting diabetes prevention programs. In this regard, qualitative as well as quantitative research is warranted.31,32,44 For example, O’Brien et al.44 conducted focus groups to examine the factors that affect diet and activity in recent Mexican-American immigrants at high risk for diabetes. This study found that lower levels of acculturation and relatively greater economic prosperity and work demands in the United States resulted in less healthy meal planning and food consumption and more sedentary behavior.44
LIFESTYLE INTERVENTION AS A PRIMARY APPROACH TO CHRONIC DISEASE PREVENTION
The science of behavioral lifestyle intervention for diabetes prevention among high-risk persons has advanced considerably over the last several decades. The lifestyle intervention paradigm acknowledges the physiologic, genetic, and metabolic complexity of obesity and diabetes variability in individual response and weight loss maintenance, as well as the need to refine future efforts accordingly.45 Nonetheless, major scientific and professional organizations in the United States and internationally continue to endorse moderate- to high-intensity behavior counseling as a gold standard for treating obesity and prediabetes.46–49 Early programmatic treatment outcome research by Wing et al.50 targeted individuals with diagnosed type 2 diabetes and demonstrated clearly the specific and combined effects of modest calorie and fat restriction, as well as programmed and lifestyle physical activities. At the same time, epidemiological research documented markers of heightened risk and the emergence of complications prior to diabetes diagnosis,51,52 establishing a rationale for primary prevention. Studies examining those with a family history of diabetes or impaired glucose tolerance53 demonstrated the feasibility of behavioral diet and activity interventions and their salutary effects on glucose, insulin, blood pressure, and lipid measures. These studies were followed by several successful, large randomized clinical trials, including the DPP.13–19
The DPP study, with its safe and practical nutrition and activity goals, demonstrated that among participants with impaired glucose tolerance, an intensive lifestyle intervention reduced the cumulative incidence rate of diabetes by 58% compared with placebo treatment, over an average follow-up period of 2.8 years. Metformin, a safe and well-tolerated medication widely used to treat diabetes, was less effective than lifestyle but also reduced the incidence of diabetes by 31% compared with placebo.15 During the subsequent 10 years of follow-up, lifestyle treatment intensity was diminished and behavioral adherence waned, resulting in weight regain, although not back to baseline levels. However, individuals originally assigned to the lifestyle and metformin arms continued to demonstrate decreased incidence of diabetes, with 27% and 17% risk reductions, respectively, compared with placebo. The lifestyle finding, in particular, suggests a legacy or carryover effect for the original behavioral intervention despite diminished treatment intensity.16 Similar patterns of beneficial lifestyle intervention carryover effects extending beyond the most intensive treatment phase have also been shown in China and Finland.14
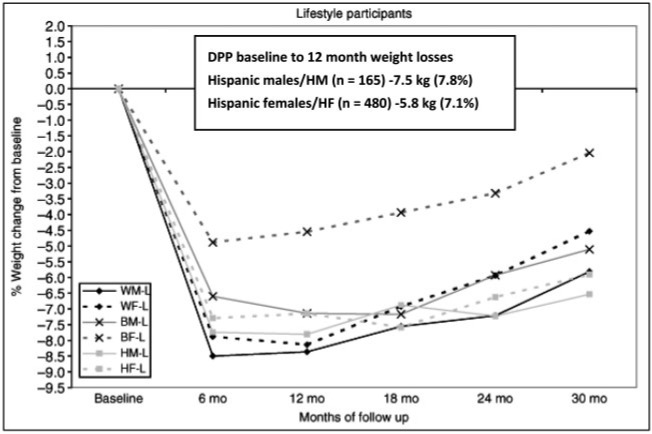
Perhaps one of the most compelling DPP results, from a translational public health perspective, is that the lifestyle group was able to show superior risk reduction compared with metformin or placebo across all racial and ethnic subgroups studied.15 Although the magnitude of weight change, weight loss maintenance, and risk reduction varied among the different high-risk ethnic subgroups studied in the DPP, as reported by West et al.54 (Figure 2), all groups demonstrated modest weight loss and associated risk reduction. Hispanic males and females in the DPP demonstrated an average 7.8% and 7.1% weight loss at 12 months from baseline, respectively, and both groups sustained a loss of at least 6.5% at 30 months. These results are encouraging and should be a catalyst for further dissemination research using DPP culturally tailored programs delivered with high behavioral fidelity.
Figure 2.
Weight change of different DPP race/ethnicity groups over 30 months. Reproduced from West et al. (2008)54 with permission.
DPP TRANSLATION AND DISSEMINATION STUDIES
Since the DPP lifestyle efficacy results were published in 2002,15 numerous translational studies derived from the original DPP protocol have shown that DPP-adapted interventions can produce clinically meaningful outcomes using traditional health professionals and/or well-trained and supervised community health personnel – but there is clearly variability in the outcomes achieved. Documentation for such studies may also be found in other review articles55–60 and meta-analyses.61 Many, but not all, of these translational studies have demonstrated mean percent weight losses in the range of 3%–7% between 0–6 and 0–12 months, with some also documenting corresponding improvements in blood glucose, lipids, insulin measures, waist circumference, and blood pressure. Very few diabetes prevention program dissemination studies describe or assess subjective or objective physical activity outcomes, and this should be targeted in future dissemination work.62 Several DPP translations are pilot studies with small sample sizes, and a majority use nonrandomized pre-post intervention designs, attempting to show the feasibility of implementing DPP-adapted programs in a variety of nonmedical settings.
Although findings to date are encouraging, it has been suggested that stronger randomized controlled or “natural” experiments63 (e.g., cluster designs, time series studies with multiple baselines, comparative effectiveness trials) of diabetes prevention program implementation in large healthcare and other community-based systems are an important next step; such experiments are needed to propel not only the development of behavior change interventions but also public policy for preventive healthcare forward, including standardized evaluation metrics that permit more efficient and reliable cross-experiment comparisons. Comparative effectiveness research studies that leverage the usual care infrastructure (e.g., electronic health record screenings and referrals) or the usual care staff of healthcare and other community services (e.g., public or private primary healthcare systems, worksites, organized societal institutions, or networks, such as schools, neighborhood cultural centers, fitness and recreation centers, senior service agencies, church-based settings) have been put forth as potentially cost-effective and innovative next steps in behavioral diabetes prevention research.
There are additional conceptual and implementation issues to consider in designing lifestyle programs for widespread dissemination. First, the means and costs of preparing, training, certifying, supporting, and evaluating the providers or teams of providers (i.e., the lifestyle coach workforce), either in the community at large or in medical care systems, and how best to create linkage between community-based programs and primary care–based medical providers more efficiently require further study.24 This becomes particularly important when utilizing peer leaders, community health workers (e.g., “lay educators” or “promotores de salud”), and other support staff who have otherwise been shown to enhance healthcare teams delivering evidence-based programs; some of the benefits they provide include their ability to engage and retain community members with common language, as well as their sensitivity to familial, cultural, and economic facilitators and barriers, and/or exposure to diabetes personally or through family members and friends.64 Nevertheless, it is important to also evaluate participant satisfaction in peer-led assessments, as in any other intervention program, because there is some data to suggest that some individuals seeking diabetes prevention and control may prefer or have greater confidence in interventions led or supervised by health professionals compared with peers.65
Second, it is important when tailoring and adapting behavioral programs that the most well-established components (e.g., goal-setting, self-monitoring of diet and activity, social problem-solving, etc.) are not sacrificed, thus reducing behavioral fidelity and potency. This has been the case in some community interventions in which weight loss in the first 6 months was less than 3%. In the original DPP, weight loss was a significant driver of risk reduction, with each kilogram of decreased weight conferring a 16% reduction in diabetes risk.36 Increasing physical activity was also crucial, particularly for weight maintenance over approximately 3 years of follow-up. To this end, research has revealed that self-monitoring, self-awareness, and social support for healthy diet, weight, and activity are central to weight loss intentions, motivation, and behavior change maintenance.66,67 Efforts to enhance the ease of self-monitoring (e.g., mobile prompts, Web-based aids to implementation) or the role played by naturally occurring social support systems (e.g., family, friends, spouses, partners) in the community are important targets. Several of these elements have been the subject of formative research and incorporated into ongoing work by Rosas et al.31 in their DPP-derived intervention studies with Latino adults in California.
EXPANDING THE REACH OF PREDIABETES SCREENING AND PRIMARY PREVENTION PROGRAMS IN COMMUNITY SAMPLES: IMPLICATIONS FOR HISPANIC/LATINO ADAPTATIONS
As indicated previously, most lifestyle behavior modification interventions for obesity and diabetes prevention have been developed and tested primarily with individuals of white European descent, typically in academic medical settings. However, there is a rapidly growing body of community-focused translational chronic disease reduction research in the United States targeting ethnically, racially, and geographically diverse groups with evidence-based programs being implemented outside of traditional academic settings.27,28,33,34,55,57,58,68–78 Given the variability in study design (e.g., single-arm pre-post design vs randomized controlled trials), as well as variability in interventionist training and implementation skill (e.g., health professionals vs community health workers), head-to-head study comparisons are difficult, although many DPP-adapted interventions tend to employ health professionals in a supervisory or a supplemental intervention role.
In addition, there is an emerging research area that aims to document the role, process and outcomes of community health worker (CHW) interventions or promotores de salud. To date, the dynamic contributions of CHW interventions have been examined more carefully for those with diagnosed diabetes than for diabetes prevention efforts.55 The stated goal of most CHW interventions is to reach, screen, and provide health education to medically underserved individuals, including persons with low levels of educational attainment and greater economic challenge than those who tend to be enrolled in randomized clinical trials.79 Typically, these approaches are grounded in social ecological models of public health,9 as well as target screening, identification of early disease markers, and the promotion of health knowledge that will, in turn, dictate referral to other health professionals and prevention/intervention programs.80–83 However, with the advent of both CHW recognition by the US Department of Labor79 and the increased dissemination and training of manualized, evidence-based DPP programs, CHW team-based implementation roles are expanding32,33,64 and there is a need to systematically review these methods and outcomes more closely as well.24
To date, there are limited data on lifestyle interventions for chronic disease prevention being delivered solely by promotores outside of community health centers and hospital clinics and that include relatively large samples of overweight/obese women with very low education and literacy levels on the Texas-Mexico border. This body of research has demonstrated that significantly favorable 3- or 4-month changes in weight and waist circumference, lipids, and glucose measures can be achieved83 and that participants who are high utilizers of community resources (parks, recreational centers) have more significant diet, activity, and weight changes.82 A 6-month lifestyle behavior change intervention facilitated by promotores84 compared 223 overweight and obese, mostly Mexican-American women in Los Angeles who were randomly assigned to either active group intervention or a safety and disaster preparedness class. High retention rates were observed (>80%), and clinically meaningful effects were found for dietary change scores, objectively measured steps, and waist circumference, although not weight changes. Studies of this nature, which integrate CHW as part of a larger community-based participatory research network, show utility and warrant replication and efforts to increase the dose and intensity of the intervention programs in order to achieve more clinically meaningful weight and cardiometabolic outcomes.
ENHANCING SOCIAL SUPPORT NETWORKS FOR HEALTH BEHAVIOR CHANGE
There are significant educational, health literacy, social support, and daily-living barriers and facilitators that need to be addressed when cultivating new self-care behaviors. Research has established previously that obesity is more likely to spread or cluster within social networks (e.g., friends, siblings, couples)85; thus, it stands to reason that it will also be important to examine the ways in which efforts at healthy lifestyle behavior change either do, or do not, ripple through one’s close social networks when embarking upon a weight management or diabetes prevention program. There is emerging qualitative as well as quantitative data on the nature of family or community social support that can have a significant impact on weight and health outcomes, as well as the outward influence of successful behavior change (i.e., role modeling, creating new social norms) within a given individual’s social network because of his or her participation in a structured program. Certainly, in many social and ethnic groups where collectivism, shared values and norms, or centrality of the family unit play an important role, designing studies that exploit these natural tendencies is more likely to be effective and enhance reach.86,87 One example of this is a pilot study that targeted dyads of Mexican-American mothers with type 2 diabetes and their overweight/obese adult daughters within federally qualified health centers to improve dietary intake and weight loss.88 The intervention arm utilized a 16-week program of combined group meetings, home visits, and booster telephone calls from a community health worker and found that, compared with control participants, the intervention group had better weight loss and dietary intake, an increase in social support, and a decrease in undermining behaviors.
CONCLUSION
The research examined in this article indicates that the behavioral programs for obesity and resulting diabetes prevention programs studied over the last several decades provide ample support for the value of structured interventions to promote health behavior change and significantly reduce chronic disease risk in many different communities, including those that are predominantly Hispanic/Latino. The Diabetes Prevention Program study, in particular, and the field of dissemination research that has followed in its wake indicate that all racial, ethnic, and cultural communities stand to benefit from such programs. Given the substantial burden of noncommunicable disease worldwide, stronger research emphasis and support for behavioral lifestyle interventions as a first (and early) line of treatment for individuals and communities at highest risk is essential. For greater public health impact, diabetes prevention research is increasingly being conducted in all the relevant settings in which individuals live, work, study, and play. It is in this capacity, moving from the medical clinic to community settings, that community health workers have the potential to bridge the functions of screening, educational and behavioral prevention programs, and management of chronic diseases, and to make significant contributions, especially in resource-limited healthcare environments.
Considerably more research is needed to extend behavioral lifestyle prevention efforts in the United States and Mexico in order to maximize benefits in Hispanic/Latino communities. Such research should include formal cost-effectiveness analyses demonstrating that proof-of-concept interventions such as the DPP lifestyle intervention can be scaled into population-based approaches and provide a good return on investment. Social contact and accountability and recognition of the powerful influence of the social and cultural environment on lifestyle behaviors are critical, yet repeated in-person group visits are not likely sustainable. Thus, research that examines cost-effective ways to harness the influence of important social networks will help advance the field. In addition, formally recognized lifestyle coach training programs, manualized interventions that are culturally as well as linguistically appropriate, and clearer standards for participant recruitment and implementation dose (such as those currently being mandated by the Centers for Disease Control National Diabetes Prevention Program) are increasingly being utilized by teams of health professionals and community health workers alike. It is anticipated that these advances will accelerate the documentation of quantitative and qualitative health outcomes and propel the lifestyle prevention field forward.
Acknowledgments
The articles in this supplement were presented as part of the Tenth Nestlé Nutrition Conference on Research Perspectives for Prevention of Diabetes: Environment, Lifestyles and Nutrition, held in México City on November 12 and 13, 2014. The conference was organized by the Nestlé Nutrition Fund of the Mexican Health Foundation and the National Institute of Medicine and Nutrition Salvador Zubirán. The supplement coordinators are Ernestina Polo-Oteyza, Mexican Health Foundation, and Héctor Bourges-Rodríguez and Carlos Aguilar-Salinas, National Institute of Medicine and Nutrition Salvador Zubirán, México.
Funding. The conference and this supplement were funded by the Nestlé Nutrition Fund of the Mexican Foundation for Health.
Declaration of interest. The authors have no relevant interests to declare.
REFERENCES
- 1.Barquera S, Campos-Nonato I, Aguilar-Salinas C, et al. Diabetes in Mexico: cost and management of diabetes and its complications and challenges for health policy. Global Health. 2013;9:doi 10.1186/1744-8603-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring). 2015;23:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Schwarz P, Lindstrom J. Long-term benefits from lifestyle interventions for type 2 diabetes prevention: time to expand the efforts. Diabetes Care. 2011;34(Suppl 2):S210–S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips LS, Ratner RE, Buse JB, et al. We can change the natural history of type 2 diabetes. Diabetes Care. 2014;37:2668–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Kruszon-Moran D, Carroll MD, et al. TRends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daviglus ML, Talavera GA, Aviles-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glanz K, Rimer BK, Viswanath K. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco, CA: John Wiley & Sons; 2008. [Google Scholar]
- 10.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44:1175–1184. [DOI] [PubMed] [Google Scholar]
- 11.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Hernandez H, Morales-Amaya UA, Rosales-Valdez R, et al. Adding cognitive behavioural treatment to either low-carbohydrate or low-fat diets: differential short-term effects. Brit J Nutr. 2009;102:1847–1853. [DOI] [PubMed] [Google Scholar]
- 13.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–1789. [DOI] [PubMed] [Google Scholar]
- 15.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. [DOI] [PubMed] [Google Scholar]
- 18.Lindstrom J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran A, Snehalatha C, Mary S, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49:289–297. [DOI] [PubMed] [Google Scholar]
- 20.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37:505–511. [DOI] [PubMed] [Google Scholar]
- 21.Kramer MK, Kriska AM, Venditti EM, et al. A novel approach to diabetes prevention: evaluation of the Group Lifestyle Balance program delivered via DVD. Diabetes Res Clin Pract. 2010;90:e60–e63. [DOI] [PubMed] [Google Scholar]
- 22.Kramer MK, McWilliams JR, Chen HY, et al. A community-based diabetes prevention program: evaluation of the group lifestyle balance program delivered by diabetes educators. Diabetes Educ. 2011;37:659–668. [DOI] [PubMed] [Google Scholar]
- 23.Kramer MK, Miller RG, Siminerio LM. Evaluation of a community Diabetes Prevention Program delivered by diabetes educators in the United States: one-year follow up. Diabetes Res Clin Pract. 2014;106:e49–e52. [DOI] [PubMed] [Google Scholar]
- 24.Venditti EM, Kramer MK. Diabetes Prevention Program community outreach: perspectives on lifestyle training and translation. Am J Prev Med. 2013;44(4 Suppl 4):S339–S345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer MK, Molenaar DM, Arena VC, et al. Improving employee health: evaluation of a worksite lifestyle change program to decrease risk factors for diabetes and cardiovascular disease. J Occup Environ Med. 2015;57:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ackermann RT, Finch EA, Brizendine E, et al. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackermann RT, Liss DT, Finch EA, et al. A randomized comparative effectiveness trial for preventing type 2 diabetes. Am J Public Health. 2015;105:2328–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang L, Manson SM, Beals J, et al. Translating the Diabetes Prevention Program into American Indian and Alaska Native communities: results from the Special Diabetes Program for Indians Diabetes Prevention demonstration project. Diabetes Care. 2013;36:2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoskin MA, Bray GA, Hattaway K, et al. Prevention of diabetes through the lifestyle intervention: lessons learned from the diabetes prevention Program and Outcomes Study and its Translation to Practice. Curr Nutr Rep. 2014;3:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosas LG, Lv N, Xiao L, et al. Evaluation of a culturally-adapted lifestyle intervention to treat elevated cardiometabolic risk of Latino adults in primary care (Vida Sana): a randomized controlled trial. Contemp Clin Trials. 2016;48:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Name MA, Camp AW, Magenheimer EA, et al. Effective translation of an intensive lifestyle intervention for Hispanic women with prediabetes in a community health center setting. Diabetes Care. 2016;39:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien MJ, Perez A, Alos VA, et al. The feasibility, acceptability, and preliminary effectiveness of a Promotora-Led Diabetes Prevention Program (PL-DPP) in Latinas: a pilot study. Diabetes Educ. 2015;41:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ockene IS, Tellez TL, Rosal MC, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ley SH, Hamdy O, Mohan V, et al. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colchero MA, Popkin BM, Rivera JA, et al. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Illescas-Zarate D, Espinosa-Montero J, Flores M, et al. Plain water consumption is associated with lower intake of caloric beverage: cross-sectional study in Mexican adults with low socioeconomic status. BMC Public Health. 2015;15:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez-Cordero S, Barquera S, Rodriguez-Ramirez S, et al. Substituting water for sugar-sweetened beverages reduces circulating triglycerides and the prevalence of metabolic syndrome in obese but not in overweight Mexican women in a randomized controlled trial. J Nutr. 2014;144:1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina C, Janssen I, Campos I, et al. Physical inactivity prevalence and trends among Mexican adults: results from the National Health and Nutrition Survey (ENSANUT) 2006 and 2012. BMC Public Health. 2013;13:1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon-Larsen P, Harris KM, Ward DS, et al. Acculturation and overweight-related behaviors among Hispanic immigrants to the US: the National Longitudinal Study of Adolescent Health. Social Sci Med. 2003;57:2023–2034. [DOI] [PubMed] [Google Scholar]
- 43.Kandula NR, Diez-Roux AV, Chan C, et al. Association of acculturation levels and prevalence of diabetes in the multi-ethnic study of atherosclerosis (MESA). Diabetes Care. 2008;31:1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Brien MJ, Shuman SJ, Barrios DM, et al. A qualitative study of acculturation and diabetes risk among urban immigrant Latinas: implications for diabetes prevention efforts. Diabetes Educ. 2014;40:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bray GA, Wadden TA. Improving long-term weight loss maintenance: can we do it? Obesity (Silver Spring). 2015;23:2–3. [DOI] [PubMed] [Google Scholar]
- 46.American Diabetes A. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American Diabetes A. (5) Prevention or delay of type 2 diabetes. Diabetes Care. 2015;38 (Suppl):S31–S32. [DOI] [PubMed] [Google Scholar]
- 48.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am College Cardiol. 2014;63: 2960–2964 [DOI] [PubMed] [Google Scholar]
- 49.Alberti KGM, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabetic Med. 2007;24:451–463. [DOI] [PubMed] [Google Scholar]
- 50.Wing RR, Shoemaker M, Marcus MD, et al. Variables associated with weight loss and improvements in glycemic control in type II diabetic patients in behavioral weight control programs. Int J Obes. 1990;14:495–503. [PubMed] [Google Scholar]
- 51.Hamman RF. Genetic and environmental determinants of non-insulin-dependent diabetes mellitus (NIDDM). Diabetes/Metabol Rev. 1992;8:287–338. [DOI] [PubMed] [Google Scholar]
- 52.Ratner RE. Type 2 diabetes mellitus: the grand overview. Diabetic Med. 1998;15 (Suppl 4):S4–S7. [DOI] [PubMed] [Google Scholar]
- 53.Wing RR, Venditti E, Jakicic JM, et al. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998;21:350–359. [DOI] [PubMed] [Google Scholar]
- 54.West DS, Elaine Prewitt T, Bursac Z, et al. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring). 2008;16:1413–1420. [DOI] [PubMed] [Google Scholar]
- 55.Hall DL, Lattie EG, McCalla JR, et al. Translation of the Diabetes Prevention Program to ethnic communities in the United States. J Immigrant Minority Health. 2016;18:479–489. [DOI] [PubMed] [Google Scholar]
- 56.Kumanyika SK, Whitt-Glover MC, Haire-Joshu D. What works for obesity prevention and treatment in black Americans? Research directions. Obes Rev. 2014;15(Suppl 4):204–212. [DOI] [PubMed] [Google Scholar]
- 57.Ruggiero L, Oros S, Choi YK. Community-based translation of the diabetes prevention program's lifestyle intervention in an underserved Latino population. Diabetes Educ. 2011;37:564–572. [DOI] [PubMed] [Google Scholar]
- 58.Samuel-Hodge CD, Johnson CM, Braxton DF, et al. Effectiveness of Diabetes Prevention Program translations among African Americans. Obes Rev. 2014;15 (Suppl 4):107–124. [DOI] [PubMed] [Google Scholar]
- 59.Venditti EM, Kramer MK. Necessary components for lifestyle modification interventions to reduce diabetes risk. Curr Diab Rep. 2012;12:138–146. [DOI] [PubMed] [Google Scholar]
- 60.Whittemore R. A systematic review of the translational research on the Diabetes Prevention Program. Transl Behav Med. 2011;1:480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali MK, Echouffo-Tcheugui JB, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Affairs. 2012;31:67–75. [DOI] [PubMed] [Google Scholar]
- 62.Eaglehouse YL, Kramer MK, Rockette-Wagner B, et al. Evaluation of physical activity reporting in community Diabetes Prevention Program lifestyle intervention efforts: a systematic review. Prev Med. 2015;77:191–199. [DOI] [PubMed] [Google Scholar]
- 63.Ackermann RT, Kenrik Duru O, Albu JB, et al. Evaluating diabetes health policies using natural experiments: the natural experiments for translation in diabetes study. Am J Prev Med. 2015;48:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruggiero L, Castillo A, Quinn L, et al. Translation of the diabetes prevention program's lifestyle intervention: role of community health workers. Curr Diab Rep. 2012;12:127–137. [DOI] [PubMed] [Google Scholar]
- 65.Little TV, Wang ML, Castro EM, et al. Community health worker interventions for Latinos with type 2 diabetes: a systematic review of randomized controlled trials. Curr Diab Rep. 2014;14:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wing RR, Tate DF, Gorin AA, et al. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–1571. [DOI] [PubMed] [Google Scholar]
- 67.Venditti EM, Wylie-Rosett J, Delahanty LM, et al. Short and long-term lifestyle coaching approaches used to address diverse participant barriers to weight loss and physical activity adherence. Int J Behav Nutr Phys Act. 2014;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seidel MC, Powell RO, Zgibor JC, et al. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes Care. 2008;31:684–689. [DOI] [PubMed] [Google Scholar]
- 69.Piatt GA, Seidel MC, Chen HY, et al. Two-year results of translating the diabetes prevention program into an urban, underserved community. Diabetes Educ. 2012;38:798–804. [DOI] [PubMed] [Google Scholar]
- 70.Sattin RW, Williams LB, Dias J, et al. Community trial of a faith-based lifestyle intervention to prevent diabetes among African-Americans. J Commun Health. 2016;41:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vanderwood KK, Hall TO, Harwell TS, et al. Implementing a state-based cardiovascular disease and diabetes prevention program. Diabetes Care. 2010;33:2543–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vadheim LM, Brewer KA, Kassner DR, et al. Effectiveness of a lifestyle intervention program among persons at high risk for cardiovascular disease and diabetes in a rural community. J Rural Health. 2010;26:266–272. [DOI] [PubMed] [Google Scholar]
- 73.Kaholokula JK, Wilson RE, Townsend CK, et al. Translating the Diabetes Prevention Program in Native Hawaiian and Pacific Islander communities: the PILI ‘Ohana Project. Transl Behav Med. 2014;4:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katula JA, Vitolins MZ, Morgan TM, et al. The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am J Prev Med. 2013;44(4 Suppl 4):S324–S332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katula JA, Vitolins MZ, Rosenberger EL, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care. 2011;34:1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.West DS, Bursac Z, Cornell CE, et al. Lay health educators translate a weight-loss intervention in senior centers: a randomized controlled trial. Am J Prev Med. 2011;41:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeary KH, Cornell CE, Turner J, et al. Feasibility of an evidence-based weight loss intervention for a faith-based, rural, African American population. Preven Chronic Dis. 2011;8:A146. [PMC free article] [PubMed] [Google Scholar]
- 78.Jaber LA, Pinelli NR, Brown MB, et al. Feasibility of group lifestyle intervention for diabetes prevention in Arab Americans. Diabetes Res Clin Pract. 2011;91:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balcázar HG, de Heer HD. Community health workers as partners in the management of non-communicable diseases. Lancet Global Health. 2015;3:e508–e509. [DOI] [PubMed] [Google Scholar]
- 80.Balcazar H, Perez-Lizaur AB, Izeta EE, et al. Community health workers-promotores de salud in Mexico: history and potential for building effective community actions. J Ambulatory Care Manag. 2016;39:12–22. [DOI] [PubMed] [Google Scholar]
- 81.Balcazar H, Fernandez-Gaxiola AC, Perez-Lizaur AB, et al. Improving heart healthy lifestyles among participants in a Salud para su Corazon promotores model: the Mexican pilot study, 2009-2012. Prev Chronic Dis. 2015;12:E34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Denman CA, Rosales C, Cornejo E, et al. Evaluation of the community-based chronic disease prevention program Meta Salud in Northern Mexico, 2011-2012. Prev Chronic Dis. 2014;11:E154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Heer HD, Balcazar HG, Wise S, et al. Improved cardiovascular risk among Hispanic border participants of the Mi Corazon Mi Comunidad Promotores De Salud Model: the HEART II Cohort Intervention Study 2009-2013. Front Public Health. 2015;3:doi:10.3389/fpubh.2015.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koniak-Griffin D, Brecht M-L, Takayanagi S, et al. A community health worker-led lifestyle behavior intervention for Latina (Hispanic) women: feasibility and outcomes of a randomized controlled trial. Int J Nurs Stud. 2015;52:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leahey TM, LaRose JG, Fava JL, et al. Social influences are associated with BMI and weight loss intentions in young adults. Obesity. 2011;19:1157–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bahr DB, Browning RC, Wyatt HR, et al. Exploiting social networks to mitigate the obesity epidemic. Obesity. 2009;17:723–728. [DOI] [PubMed] [Google Scholar]
- 87.Kim DA, Hwong AR, Stafford D, et al. Social network targeting to maximise population behaviour change: a cluster randomised controlled trial. Lancet. 2015;386:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sorkin DH, Mavandadi S, Rook KS, et al. Dyadic collaboration in shared health behavior change: the effects of a randomized trial to test a lifestyle intervention for high-risk Latinas. Health Psychology. 2014;33:566–575. [DOI] [PubMed] [Google Scholar]