Abstract
Background:
Lead is considered a neurotoxic agent. We aimed to evaluate the blood lead level (BLL) in young population and determine probable risk factors of lead exposure in Iran.
Methods:
In a cross-sectional study, a total of 100 children were entered and their BLLs were checked.
Results:
In all, 25 and 8 patients had BLLs above 5 and 10 µg/dL, respectively. There was a significant univariate correlation between BLL and place of living, water pipe type, using dairy products, and stature in both cut-offs of 5 and 10 µg/dL. Binary regression analysis showed that pipe type was associated with high BLLs at cut-offs of 5 and 10 µg/dL, respectively. Also, there was an association between 50th percentile of stature for age and cut-off of 5 µg/dL.
Conclusions:
Higher BLLs may be seen in short stature pediatric population. Polyvinyl chloride (PVC) and polypropylene water pipes may even cause more release of lead and result in higher absorption of this metal in the pediatric population.
Keywords: Lead, exposure, screening, children
Background
In 2009, it was estimated that almost 310 000 lead-poisoned children younger than 5 years of age did exist in the United States. Although the prevalence and severity of childhood lead exposure have significantly reduced since the removal of lead from paint and gasoline in 1970s, the exact prevalence of this exposure is not clear in many countries of the world including Iran.1
In Middle East, although limited, there are few studies that have reported the prevalence of lead toxicity in children. In 2006, Safi et al mentioned that mean blood lead level (BLL) of children living in Gaza, Israel, and Jordan was 2 µg/dL showing a significant decrease in comparison with the previous studies in this region mainly due to phasing out of the leaded gasoline as other studies from the same region in 1980s had shown a mean BLL ranging between 8 and 15 µg/dL in this age range.2–4 In Saudi Arabia, this measure has been reported to be 5 µg/dL.5 However, studies from other countries in this region are lacking.
In developed countries, several screening programs are being run to determine high BLLs from the early stages of conception in pregnant women. In fact, US Preventive Service Task Force recommends screening for BLLs at the age of 12 months for all children with identifiable risk factors and all children who live in societies with high or unknown BLLs.6 However, in developing countries where the prevalence of lead toxicity and exposure is still high including Iran, such screening programs do not exist. This puts the young population of these countries at the risk of lead poisoning and its long-lasting complications.
The main aim of such screening programs is to reduce the severe and life-time complications of lead toxicity in young people. Signs and symptoms of lead poisoning include constipation, abdominal colic, anemia, renal failure, weakened immune system, impaired function of the central nervous system, low birth weight, still birth and miscarriage, and premature birth.7,8 In children, impaired growth is another major complication of lead poisoning.
In Iran, there is no screening system in pregnant women and young children. This is while different routes of lead exposure have been identified in this country.9,10 Lead-contaminated opium has been determined as a route of lead exposure in Iran although its exact source remains unclear. It has been claimed that exposure to lead-contaminated opium through inhalation or ingestion may result in lead poisoning.11 Therefore, inhalational exposure to lead-contaminated opium may be a route of poisoning in the children who are exposed to their parents’ opium smoke. Recent data show high prevalence of lead toxicity in children, even those who were not exposed to opioids, suggesting polluted environment as the cause of lead exposure.12 Pollution in the city centers due to leaded gasoline is not nowadays a major problem in Iran as leaded gasoline is currently out of the Iranian market. Mean lead level was reported to be quite high in Iranian children in previous studies.13
Objectives
The main goals of this study were to determine the mean BLL in population of the children referring to our pediatric clinic for routine control of growth and development, after exclusion of those with history of lead toxicity in past, and determine factors accompanying lead exposure in this age population in our area.
Methods
Study design and setting and selection of participants
This single-center, cross-sectional study was conducted using a convenience sampling of all pediatric patients visited in pediatric clinic of Loghman Hakim Hospital from May to July 2017. Although this institution is the only tertiary hospital for pediatric poisoned patients in the capital city and the largest in the country,14 children were visited in general pediatric clinics for routine control of growth and development. Patients younger than 14 years of age who had referred to the outpatient clinic for routine checkups or causes other than signs and symptoms of lead poisoning were included. Subjects who disinclined to participate in the study, those older than 14 years of age, and those with a history of lead poisoning were excluded. Those with severe conditions and in need for hospitalization were also excluded.
Using a self-made questionnaire, basic and clinical characteristics of the patients including their sex, age, weight, height, their parents’ education and occupation, history of lead toxicity in the family, family history of opium abuse, living and playing in the industrial areas, age of the residential building and water pipe type, history of pica, type of the toys the children played with, using calcium and iron supplements as well as type of the cooking pots and possible signs and symptoms of lead poisoning were documented for each single patient. Weight (kilogram) and height (centimeter) were evaluated considering the national normal values using digital weighting scales wearing light clothing and a wall-mounted stadiometer without shoes and socks. A 1 mL venous blood sample was taken from the child after taking consent from his/her parents/guardians.
Hemoglobin and mean corpuscular volume (MCV) was documented. Blood lead level was measured using Lead Care II (ESA Biosciences, Inc. 22 Alpha Road, Chelmsford, MA, USA) with ability to check the whole BLL between 3.3 and 65 µg/dL (range of detection). Lead Care II relies on electrochemistry and a sensor to detect lead in the whole blood where the kit is specific for quantitative measurement of lead in fresh whole blood specimens. The device was calibrated for each 48-batch run using standard quality control detectors of lead set at levels of 27.4 ± 4 and 9.8 ± 3 µg/dL. Results obtained on quality control samples were within the expected range. Any value below the detection limit of 3.3 µg/dL was assigned a value of 1.6 µg/dL (midpoint between 0 and 3.2 µg/dL) for statistical analysis. Possible levels more than 65 µg/dL were considered to be sent to reference laboratory to be checked by atomic absorption technique.
Study outcome
Blood specimens were collected only once for each child during visit in the clinic. Adverse outcomes were defined as BLL ⩾ 5 µg/dL and BLL ⩾ 10 µg/dL according to recent and previous Centers for Disease Control and Prevention (CDC) levels of concern for adverse health outcomes in children, respectively.15
Normal values
Anemia was defined based on normal mean and lower normal limits of hemoglobin (Hgb), hematocrit (Hct), and MCV for each age and sex.16 National standard measures of height and weight for age were used for the classification of the patients as short stature/underweight (<3rd percentile), 3rd to 14th percentile, 15th to 49th percentile, 50th to 84th percentile, 85th to 97th percentile, and tall/overweight (>97th percentile).17 We dichotomized height growth (ie, below or more than 50th percentile of stature for age) for binary regression to see whether it showed significant correlation in univariate analysis.
Power calculation
Assuming a = 0.05, an error of 0.02, and a standard deviation of 0.1,18 using n = (A2 × s2)/d2 formula, 100 pediatric patients were recruited. All children who referred to our pediatric clinic were included.
Data analysis
The variables were entered into statistical package for social software (SPSS) version 21 and analyzed using descriptive and analytical analyses by chi-squared test, Fisher Exact test, and Mann-Whitney U test. “Enter” binary logistic model was performed to determine independent variables that had an association with lead level of more than limit of detection (LOD; <3.3), 5, or 10 µg/dL. Linear regression model was used to evaluate the relationship between BLL and independent variables. Odds ratio was calculated using Exp(B), that is, the exponentiation of the B coefficient. One-sample Kolmogorov-Smirnov Test was used to determine whether the variables were normally distributed. Median (interquartile range [IQR]) and mean (SD) were used to express non-normal and normal continuous variables, respectively. A P value less than .05 was considered to be statistically significant.
Ethics approval
The study was approved by our local ethics committee in Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1396.74).
Results
Of the 100 children evaluated, 53 were male. Their median (IQR) age was 60 months [19, 96] with minimum age of 4 days and maximum of 12 years. The parents had mainly low socioeconomic status with 56% of the fathers being low-income workers (less than poverty line, <US$1190 per month in Iran) and 91% of the mothers being housewives. Of the possible signs and symptoms of lead poisoning, pallor (anemia), abdominal pain, constipation, failure to thrive, and bone and muscle pain were detected in 19%, 18%, 15%, 9%, 4%, and 4% of the children, respectively; 8% were reported to be difficult children and 1% had attention deficit hyperactivity disorder (ADHD).
A total of 46 children (46%) had a BLL less than LOD. Median BLL was 3.4 (1.6, 5.1) (1.6, 41.7) µg/dL. We used BLL as a continuous variable in linear regression model and it showed that pipe type and residential area (P < .001 and P = .023, respectively) were variables that could significantly predict BLL. Figure 1 shows non-significant correlation of the age and BLL in metal vs plastic pipe users; 25 and 8 patients had BLLs above 5 and 10 µg/dL, respectively. Considering 5 and 10 µg/dL as critical BLLs in 2 different analyses, there were no statistically significant differences between the groups at these cut-offs regarding their age, sex, exposure to opium smoke or oral opium, age of their residential building, and pica habits. However, other significantly different factors were detected in groups at 3.2 (LOD), 5, and 10 µg/dL cut-offs, which are shown in Table 1. Mean corpuscular volume and hemoglobin were also similar in groups at cut-offs of 5 and 10 µg/dL. There was a significant univariate association between place of living (industrial vs non-industrial area), water pipe type (metal vs polyvinyl chloride [PVC] and polypropylene pipes), using dairy products more than a liter per day (yes vs no), and stature in both blood lead cut-off levels of 5 and 10 µg/dL. “Enter” binary regression analysis showed that 50th percentile of stature for age (odds ratio of 0.196 [0.047, 0.810] associated with high blood levels at cut-off of 5 µg/dL). Pipe type was the only predictor of high BLL at cut-offs of 3.2, 5, and 10 µg/dL (Table 2). Median (IQR) and mean (±SD) BLL in metal pipe users were 1.6 (1.6, 3.7) and 3.2 ± 2.5 (range: 1.6-17.3 µg/dL) which was significantly less than PVC and polypropylene pipe users with 6.6 (2.9, 12.4) and 8.6 ± 9.3 (range: 1.6-41.37 µg/dL; P < .001; Figure 2). Pallor (indicator of anemia) was related to using iron-calcium supplements with an odds ratio (95% confidence interval [CI]) of 4.5 (1.2, 16.7; P = .018).
Figure 1.

Correlation of the age and BLL in metal vs plastic pipe users. BLL indicates blood lead level.
Table 1.
Comparison of different variables between the children with BLLs below and above 5 and 10 µg/dL.
| Independent variable | BLL < LOD n = 46 |
BLL ⩾ LOD n = 54 |
Significance | BLL ⩽ 5 n = 75 |
BLL > 5 n = 25 |
Significance | BLL ⩽ 10 n = 92 |
BLL > 10 n = 8 |
Significance | Total n = 100 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) age (month) (min, max) | 45 (9, 84) (0.1, 144) |
60 (27, 96) (0.1, 144) |
.910a | 60 (16, 96) (0.1, 144) |
60 (26, 96) (0.1, 144) |
.808a | 60 (19, 96) (0.1, 144) |
30 (15, 65) (9, 96) |
.269a | 60 (19, 96) (0.1, 144) |
|
| Factory in residential area | 0 | 11 (21.2) | .001 b | 5 (6.1) | 6 (26.1) | .02 b | 8 (9) | 3 (37.5) | .045 c | 11 (11) | |
| Water pipe | New | 9 (19.6) | 20 (37) | .055b | 19 (25.3) | 10 (40) | .162b | 24 (26.1) | 5 (62.5) | .043 c | 29 (29) |
| Old | 37 (80.4) | 34 (63) | 56 (74.7) | 15 (60) | 68 (73.9) | 3 (37.5) | 71 (71) | ||||
| Water pipe type | Metal | 42 (91.3) | 38 (70.4) | .028b | 67 (89.3) | 13 (52) | .001 b | 79 (85.9) | 1 (12.5) | .001 b | 80 (80) |
| Plastic | 4 (8.7) | 14 (25.9) | 8 (10.7) | 10 (40) | 13 (14.1) | 5 (62.5) | 18 (18) | ||||
| Unknown | 0 | 2 (3.7) | 0 | 2 (8) | 0 | 2 (25) | 2 (2) | ||||
| Toy types | Plastic | 36 (78.3) | 41 (75.9) | .440b | 58 (77.3) | 19 (76) | .268b | 70 (76.1) | 7 (87.5) | .004 b | 77 (77) |
| No plastic | 0 | 1 (1.9) | 0 | 1 (4) | 0 | 1 (12.5) | 1 (1) | ||||
| Both | 0 | 2 (3.7) | 1 (1.3) | 1 (1.3) | 2 (2.2) | 0 | 2(2) | ||||
| No toy | 10 (21.7) | 10 (18.5) | 16 (21.3) | 4 (16) | 20 (21.7) | 0 | 20 (20) | ||||
| Positive history of lead toxicity in family* | 0 | 4 (7.4) | .122c | 2 (2.7) | 2 (8) | .260c | 2 (2.2) | 2 (25) | .031 c | 4 (4) | |
| Iron/calcium supplement use >1 g/day | 4 (8.7) | 7 (13) | .541c | 7 (9.3) | 4 (16) | .460c | 8 (8.7) | 3 (37.5) | .041 c | 11 (11) | |
| Using dairy products >1 L/day | No | 45 (97.8) | 48 (88.9) | .120c | 73 (97.3) | 20 (80) | .01 c | 89 (96.7) | 4 (50) | .001 c | 93 (93) |
| Yes | 1 (2.2) | 6 (11.1) | 2 (2.7) | 5 (20) | 3 (3.3) | 4 (50) | 7 (7) | ||||
| Stature growth curve | <3% | 1 (2.2) | 2 (3.7) | .301b | 1 (1.3) | 3 (12) | .002 b | 1 (1.1) | 3 (37.5) | .001 b | 4 (4) |
| 3%-14% | 7 (15.2) | 18 (33.3) | 7 (9.3) | 9 (36) | 15 (16.3) | 1 (12.5) | 16 (16) | ||||
| 15%-49% | 16 (34.8) | 17 (31.5) | 33 (44) | 6 (24) | 37 (40.2) | 2 (25) | 39 (39) | ||||
| 50%-84% | 13 (28.3) | 11 (20.4) | 19 (25.3) | 6 (24) | 23 (25) | 2 (25) | 25 (25) | ||||
| 85%-97% | 4 (8.7) | 4 (7.4) | 9 (12) | 1 (4) | 10 (10.9) | 0 | 10 (10) | ||||
| >97% | 5 (10.9) | 2 (3.7) | 6 (8) | 0 | 6 (6.5) | 0 | 6 (6) |
Abbreviation: BLL, blood lead level; IQR, interquartile range; LOD: limit of detection. Bold values are significant at p < 0.05
Using Mann-Whitney U-test.
Using Pearson chi-squared test.
Using Fisher Exact test
Dad was dead in 2 cases.
Table 2.
“Enter” regression analysis of the variables with significant association to BLL.
| Dependent variable | Independent variable | Beta | SE of beta | OR (95% CI)1 | Model significance and Nagelkerke R2 |
|---|---|---|---|---|---|
| BLL >10 µg/dLa | Pipe type (metal vs plastic) | 3.665 | 1.531 | 39.049 (1.943, 784.687) | 0.001, 0.580 |
| BLL >5 µg/dLb | Stature growth curve (<50% vs ⩾50%) | −1.630 | 0.724 | 0.196 (0.047, 0.810) | 0.001, 0.402 |
| Pipe type (metal vs plastic) | 2.077 | 0.636 | 7.979 (2.292, 27.774) | ||
| BLL ⩾detection limit (3.2 µg/dL)c | Pipe type (metal vs plastic) | 1.278 | 0.590 | 3.591 (1.130, 11.415) | 0.001, 0.257 |
Abbreviations: BLL: blood lead level; CI, confidence interval; OR, odds ratio; SE, standard error.
Using all binary variables with a significant univariate test including:
15% stature growth curve, using dairy products, positive history of lead toxicity in family, using iron-calcium supplements, water pipe type, muscle pain complaint, and factory in residential area.
50% stature growth curve, using dairy products, water pipe type, muscle pain complaint, and factory in residential area.
Water pipe type and factory in residential area.
Figure 2.
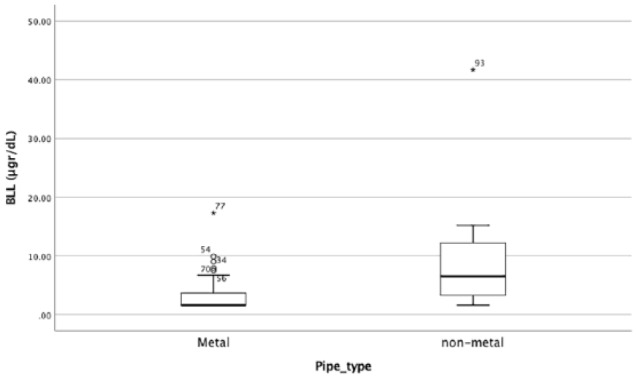
Correlation of the pipe type and BLL. BLL indicates blood lead level.
Discussion
Lead can affect Iranian children through air, water, soil, food (particularly milk, fish, flour, vegetables, tea, lemon juice, tomato paste, and rice), toys, dried herbs/herbal medicine, and makeup products. Many of these sources of exposure are also a lead source in the other countries based on the geographical area and nutritional habits.7,8 Central nervous system is the most vulnerable part of the body to lead poisoning in young children. Although a BLL of 30 µg/dL has been mentioned as the normal BLL,7 far less levels are acceptable in young children and fetus.19 Blood lead levels of 5 μg/dL or less have been mentioned as acceptable BLLs in children as they are probably safe and do not cause lead-related complications.15 Our results showed that lead-polluted atmosphere in industrial areas might increase the BLL in children living in there. The possible role of living in polluted areas and increasing BLL has already been mentioned in pregnant women.20 Most of these children lived in areas where upholstery or paint industry was active, a finding compatible with previous studies.21 Many of toxic effects of lead are reversible if lead exposure is identified and discontinued early but high BLL and delay in treatment may lead to irreversible symptoms. Chronic low-level lead exposure may cause hearing loss and decreased intelligence quotient (IQ).22–24
One interesting finding of this study is that despite routine beliefs, use of plastic PVC and polypropylene pipes accompanied a higher BLL in the children, while it is generally assumed that old pipes accompany a higher BLL. This may be due to the poor quality of the PVC pipes and leakage from them. The high-quality PVC pipes should contain a mean of 85% of PVC resin, while in the poor-quality pipes, this measure is almost 55%.25 Role of PVC toys in development of lead toxicity has already been declared in Iran.26 On the other hand, the mean lead concentration in old and new PVC and polypropylene pipes is higher than metallic pipes and exceeds Environmental Protection Agency (EPA) standards or World Health Organization (WHO) guidelines.27 Therefore, it seems that the current attempt for changing the type of the pipes from metal to plastic polypropylene and PVC ones in the water distribution systems is not successful enough to prevent plumbism and care should be given to use high-quality pipes for such purpose.28–30
The other finding of this study was the risk of short stature in the children with high BLLs. In fact, risk of being less than 50th percentile of stature for age was almost 5 times more in the children with BLLs ⩾5 µg/dL. Although lead toxicity has been mentioned as 1 out of 4 remediable conditions resulting in short stature,31 Mahram et al32 could not find any significant difference in growth parameters of the affected children. Blood lead level may also represent a mixed marker for unidentified genetic, ethnic, environmental and sociocultural variables, race, sex, and nutrition that affect growth.33 However, the significant correlation between stature and BLL (in the range of 5-41.7 µg/dL) in regression analysis is strong enough to deserve investigation in future studies with consideration of the multiple biologic mechanisms particularly parents’ body mass indices, by which low-level lead exposure could affect the children’s stature.
Positive history of lead poisoning in the family, exposure to opium smoke, and ingestion of at least 1 L of dairy products per day were accompanied by higher BLLs. It is assumed that using 1 L dairy products or 1 g calcium intake per day is considered a healthy diet to prevent lead absorption as there is a competition between lead and minerals.34 Family exposure to lead, such as using lead-contaminated opium in the house, my increase risk of lead absorption in children. However, we have no explanation why using more dairy products and calcium-iron supplements increased the risk of higher BLL except for the background diseases including anemia, which might increase lead absorption, per se.
Limitations
It is recommended to re-check BLL by atomic absorption method when the blood sample lead level is shown to be high using Lead Care II. Although there were fairly few patients with BLLs greater than 5 and 10, these cases with high BLLs were not confirmed by atomic absorption method, which is definitely a limitation of this study. Also, the new manual notebook of the device recommends capillary samples for the best results as the results may be underestimated using venous samples; however, we checked venous samples because we had started recruiting cases before this warning. Maternal cigarette smoking data were not available. All of our cases were taken from the children who had referred to the pediatric clinic of one center in Tehran and more sample collections from different parts of the city and even country were warranted to determine the exposure pattern in our children. Using the very small sample size from a single referral center in Tehran and obtaining such high levels of lead in our patients shows the importance and great need for screening programs in the Iranian children to prevent future complications of lead exposure in them.
Conclusions
Higher BLLs may be seen in shorter pediatric population. Polyvinyl chloride and polypropylene water pipes may even cause more release of lead and result in higher absorption of lead in the pediatric population. Having more than 25% of the children in the group with BLLs ⩾5 µg/dL definitely shows a high rate of lead exposure in Iranian children compared with studies from other parts of the world.35 It should prompt a public health response. Blood lead level screening is recommended in high-risk pediatric population in national scale. Also, evaluation of the actual water lead content in PVC pipes is a public health concern and warranted in future studies.
Acknowledgments
The study was approved by our local ethics committee in Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1396.74).
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: HHM and LG designed the study. NG and FF collected data. HHM and LG analyzed and interpreted the data. NZ was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
ORCID iDs: Nasim Zamani  https://orcid.org/0000-0002-2091-0197
https://orcid.org/0000-0002-2091-0197
Hossein Hassanian-Moghaddam  https://orcid.org/0000-0003-4370-0544
https://orcid.org/0000-0003-4370-0544
References
- 1. Warniment C, Tsang K, Galazka SS. Lead poisoning in children. Am Fam Physician. 2010;81:751–757. [PubMed] [Google Scholar]
- 2. Safi J, Fischbein A, El Haj S, et al. Childhood lead exposure in the Palestinian authority, Israel, and Jordan: results from the Middle Eastern regional cooperation project, 1996–2000. Environ Health Perspect. 2006;114:917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friberg L, Vahter M. Assessment of exposure to lead and cadmium through biological monitoring: results of a UNEP/WHO global study. Environ Res. 1983;30:95–128. [DOI] [PubMed] [Google Scholar]
- 4. Richter ED, Berant M, Grauer F, Tamir A, Berkowitz A, Oppenheim D. Zinc protoporphyrin levels in children in Haifa—a pilot study. Sci Total Environ. 1986;48:109–121. [DOI] [PubMed] [Google Scholar]
- 5. Shaik AP, Sultana SA, Alsaeed AH. Lead exposure: a summary of global studies and the need for new studies from Saudi Arabia. Dis Markers. 2014;2014:415160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rischitelli G, Nygren P, Bougatsos C, et al. Screening for elevated lead levels in childhood and pregnancy: an updated summary of evidence for the US Preventive Services Task Force. Pediatrics. 2006;118:e1867–e1895. [DOI] [PubMed] [Google Scholar]
- 7. Karrari P, Mehrpour O, Abdollahi M. A systematic review on status of lead pollution and toxicity in Iran; guidance for preventive measures. Daru. 2012;20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maleknejad S, Heidarzadeh A, Rahbar M, et al. Evaluation of serum lead levels in children with constipation and normal controls in northern Iran. Iran J Pediatr. 2013;23:417–422. [PMC free article] [PubMed] [Google Scholar]
- 9. Zamani N, Jamshidi F. Abuse of lead-contaminated opium in addicts. Singapore Med J. 2012;53:698. [PubMed] [Google Scholar]
- 10. Zamani N, Hassanian-Moghaddam H, Latifi M. Abdominopelvic CT in a patient with seizure, anemia, and hypocalcemia. Gastroenterology. 2017;152:27–28. [DOI] [PubMed] [Google Scholar]
- 11. Alinejad S, Aaseth J, Abdollahi M, et al. Clinical aspects of opium adulterated with lead in Iran: a review. Basic Clin Pharmacol Toxicol. 2018;122:56–64. [DOI] [PubMed] [Google Scholar]
- 12. Ghasemi A, Nakhaei AA, Ghamsari AA, Salehi M. Anemia, iron deficiency anemia and lead poisoning in children with opioid toxicity: a study in North East of Iran. Iran J Ped Hematol Oncol. 2017;7:90–97. [Google Scholar]
- 13. Zaman T, Hossein Zadeh H. Lead poisoning in a highly polluted district of Tehran in high school children. Iranian J Pediatr. 1999;9:207–212. [Google Scholar]
- 14. Hassanian-Moghaddam H, Hakiminejhad M, Farnaghi F, Mirafzal A, Zamani N, Kabir A. Eleven years of children methadone poisoning in a referral center: a review of 453 cases. J Opioid Manag. 2017;13:27–36. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention (CDC). CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention. Atlanta, GA: CDC; 2012. http://www.cdc.gov/nceh/lead/acclpp/final_document_010412.pdf. Accessed Jan 2019. [Google Scholar]
- 16. Brue of Family Health & Population, Office of Children’s Health, Iranian Ministry of Health and Medical Education. Combined care for healthy children (Special for physicians). 2nd ed.; 2004. http://fhc.sums.ac.ir/files/khanevade/form/KUDAKAN/wccbukletDr.pdf.
- 17. Lerner NB. The anemias. In: Kliegman RM, Stanton BF, St Geme JW, Schor NF, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia PA: Elsevier; 2016. [Google Scholar]
- 18. McMichael A, Vimpani G, Robertson E, et al. The Port Pirie cohort study: maternal blood lead and pregnancy outcome. J Epidemiol Community Health. 1986;40:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shefa ST, Héroux P. Both physiology and epidemiology support zero tolerable blood lead levels. Toxicol Lett. 2017;280:232–237. [DOI] [PubMed] [Google Scholar]
- 20. Hassanian-Moghaddam H, Zamani N, Hamidi F, et al. Blood lead levels in pregnant women referring to midwifery clinic in a referral center in Tehran. J Res Med Sci. 2018;23:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kianoush S, Balali-Mood M, Mousavi SR, et al. Comparison of therapeutic effects of garlic and d-Penicillamine in patients with chronic occupational lead poisoning. Basic Clin Pharmacol Toxicol. 2012;110:476–481. [DOI] [PubMed] [Google Scholar]
- 22. Mahram M. Intelligence quotient level of the children living high lead areas in Zanjan province. Behbood. 2004;7:36–42. [Google Scholar]
- 23. Roth JA, Salvi R. Ototoxicity of divalent metals. Neurotox Res. 2016;30:268–282. [DOI] [PubMed] [Google Scholar]
- 24. Liu J, Li L, Wang Y, et al. Impact of low blood lead concentrations on IQ and school performance in Chinese children. PLoS ONE. 2013;8:e65230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. PVC versus polypropylene sewer pipes. http://www.uni-bell.org/communication/images/pvc_vs_polypropylene_pp_sewer_pipe.pdf. Accessed Jan 2019.
- 26. Pourmoghaddas H, Pishkar AR, Kavehzade FM. Percentage of toxic trace elements; Pb, Cr, and Cd in certain plastic toys, Isfahan city. J Shahid Sadoughi Univ Med Sci Health Serv. 2006;14:59–64. [Google Scholar]
- 27. Tashauoei HR, Hajian Nejad M, Amin MM, et al. A study on leakage of heavy metals from the PVC and polypropylene pipes used in the water distribution system in Isfahan. Health Syst Res. 2010;6:373–382. [Google Scholar]
- 28. Zhang Y, Griffin A, Edwards M. Nitrification in premise plumbing: role of phosphate, pH and pipe corrosion. Environ Sci Technol. 2008;42:4280–4284. [DOI] [PubMed] [Google Scholar]
- 29. Karr C. Reducing childhood lead exposure: translating new understanding into clinic-based practice. Pediatr Ann. 2008;37:748–756. [DOI] [PubMed] [Google Scholar]
- 30. Levin R, Brown MJ, Kashtock ME, et al. Lead exposures in U.S. children, 2008: implications for prevention. Environ Health Perspect. 2008;116:1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haymond M, Kappelgaard AM, Czernichow P, et al. Early recognition of growth abnormalities permitting early intervention. Acta Paediatr. 2013;102:787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahram M, Mousavinasab N, Dinmohammadi H, et al. Effect of living in lead mining area on growth. Indian J Pediatr. 2007;74:555–559. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz J, Angle C, Pitcher H. Relationship between childhood blood lead levels and stature. Pediatrics. 1986;77:281–288. [PubMed] [Google Scholar]
- 34. Markowitz M. Lead poisoning. In: Kliegman RM, Stanton BF, St Geme JW, Schor NF, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 35. Gawarammana IB, Dargan PI, Woodcock S, et al. Should all patients with unexplained anaemia be screened for chronic lead poisoning? Hum Exp Toxicol. 2006;25:645–649. [DOI] [PubMed] [Google Scholar]


