Abstract
Statins have pleiotropic effects that are considered beneficial in preventing cerebral vasospasm and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage (aSAH). Many studies using statins have been performed but failed to show remarkable effects. We hypothesized that a long-acting statin would be more effective, due to a longer half-life and stronger pleiotropic effects. Patients with aSAH were randomly assigned to a pitavastatin group (4 mg daily; n = 54) and a placebo group (n = 54) after repair of a ruptured aneurysm. The primary efficacy end point was vasospasm-related delayed ischemic neurological deficits (DIND), and the secondary end points were cerebral vasospasm evaluated by digital subtraction angiography (DSA), vasospasm-related new cerebral infarctions, and outcome at three months. Severe cerebral vasospasms on DSA were statistically fewer in the pitavastatin group than in the placebo group (14.8% vs. 33.3%; odds ratio, 0.32; 95% confidence interval, 0.11–0.87, p = 0.042); however, the occurrence of DIND and new infarctions and outcome showed no statistically significant differences between the groups. The present study is the first to prove the definite, statin-induced amelioration of cerebral vasospasm on DSA. However, administration of any type of statin at the acute phase of aSAH is not recommended.
Keywords: Subarachnoid hemorrhage, vasospasm, angiography, clinical trials, randomized controlled trials
Introduction
The pathogenesis of delayed cerebral ischemia (DCI) after aneurysmal subarachnoid hemorrhage (aSAH) has not yet been fully clarified. It has been hypothesized that chronic cerebral vasospasm of the major cerebral arteries causes DCI, but it is increasingly recognized that angiographic vasospasm cannot be the sole cause of DCI. For example, vasospasm can be robustly reduced by endothelin antagonists, but this neither reduced DCI nor did it improve patient outcome.1 DCI is associated with worse outcome,2 whereas cerebral vasospasm is not.3 Nimodipine is used as prophylaxis against DCI, albeit with limited efficacy,4 though it had no significant effect on angiographic vasospasm.5–7 The current view is that cerebral vasospasm is likely involved in DCI as a contributor, but there are additional factors with relevance to patient outcome and DCI that seem to be at least as important as or even more important than angiographic vasospasm. These factors include early brain injury,8–11 cortical spreading depolarization,12–15 microcirculatory disturbance,16–18 cerebral autoregulation impairment,19 and impairment in neurovascular coupling.20,21 Angiographic vasospasm and these additional factors may worsen patient outcome in concert with varying degrees of importance for the single players.
Statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors, have pleiotropic effects that are independent of their cholesterol-lowering effect.22 Pleiotropic effects such as an antioxidant effect, up-regulation of endothelial nitric oxide synthase (eNOS), stabilization of endothelial cells, and anti-inflammatory action are considered beneficial for ameliorating the pathogenesis of cerebral vasospasm.22–24 Based on these effects, randomized controlled trials (RCTs) using statins for aSAH were performed for the first time in 2005 and indicated their efficacy in decreasing cerebral vasospasm using transcranial Doppler (TCD), vasospasm-related DIND, and mortality.25,26 Following that, however, most clinical trials using statins on patients with aSAH showed no efficacy for either cerebral vasospasm, DCI, vasospasm-related cerebral infarction, mortality or outcome,27–29 which has been confirmed by several systematic reviews.30–32 As the only exception, pooled analysis in systematic reviews indicated that cerebral vasospasm was lowered by statin-use; however, the result is not conclusive because arterial narrowing was evaluated by TCD in most studies and the criteria for arterial narrowing were not consistent among the studies.25–28
In all of six RCTs and two prospective cohort studies investigating the efficacy of statins for aSAH, simvastatin was used in five RCTs33,34 and in two prospective cohort studies,35,36 and pravastatin was used in one RCT.26 However, the half-life of simvastatin and pravastatin is several hours; therefore, the efficacy might not be adequate to prevent cerebral vasospasm. Atorvastatin, pitavastatin, and rosuvastatin have longer half-lives of approximately 10 to 20 h and, among them, pitavastatin has stronger and more pleiotropic effects,37,38 which led us to expect that pitavastatin could show significant effects on DCI and outcome after aSAH. We have performed a preliminary, experimental study investigating the efficacy of pitavastatin in the rabbit SAH model.39 Arterial narrowing was significantly suppressed by pitavastatin administration, based on its up-regulating effect on NO synthase and suppression of Rho-kinase activity due to inhibition of Rho A. In addition, pitavastatin is lipophilic and has greater permeability in the vascular cell membrane compared to hydrophilic statins.37 Furthermore, pitavastatin has fewer side effects in combined use with multiple drugs, because pitavastatin is not metabolized by cytochrome enzymes in spite of its lipophilicity.38 Therefore, in the present study, we selected pitavastatin as the treatment drug.
We conducted an investigator-initiated, prospective, randomized, double-blind study to determine whether long-acting statin is effective for aSAH in terms of decreasing DCI and improving functional outcome. In addition, its efficacy for arterial narrowing was evaluated by digital subtraction angiography (DSA) in all cases.
Methods
This study was an investigator-initiated, multicenter, double-blind, randomized controlled trial performed at four neurosurgical institutions in the Aomori prefecture, Japan, from December 2014 to June 2016. This study was conducted in compliance with ethics principles originating from the Declaration of Helsinki and ethical guidelines for clinical research. The protocol for this study was assessed and approved by the Committee of Medical Ethics of Hirosaki University Graduate School of Medicine. All participants or their legal representatives provided written, informed consent. This trial is registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000015977).
Participants
Patients with aSAH aged between 20 and 80 years and admitted within 24 h after the onset of SAH and who underwent repair of a ruptured saccular aneurysm by aneurysmal neck clipping or endovascular coiling within 48 h after SAH were eligible for this study. The other inclusion criteria were a diffuse (long axis ≥ 20 mm) or localized (long axis < 20 mm) thick (short axis ≥ 4 mm) subarachnoid clot on CT scan done within 24 h of SAH, and Hunt and Hess grades I–IV evaluated before clipping or coiling. The exclusion criteria were the current use of antiplatelet, anticoagulant agents, and/or any kind of statin, preexisting cerebral damage from past stroke or traumatic brain injury confirmed on CT scan, postoperative neurological deficits due to a clipping or coiling procedure, major neurological deficits due to concomitant intracerebral hematoma induced by aneurysmal rupture, pre-existing severe hepatic, renal, pulmonary or cardiac disease, and pregnancy.
Randomization
After the absence of postoperative intracranial hemorrhage was confirmed on CT done within 6 to 12 h after clipping or coiling, patients were allocated to either the pitavastatin or the placebo group using a randomization list in blocks of four, stratified by site. The administration of placebo or 4 mg pitavastatin orally or through a nasogastric tube once per day was started within 72 h after the ictus and continued for 14 days. All participants and assessors were blinded to group allocation.
Standard of care
All patients in both groups were managed under uniform, standard care in all institutions for 14 days. The circulating blood volume was kept within the normal range, and intravenous administration of 30 mg of fasudil hydrochloride, which has a vasodilatory effect due to Rho-kinase inhibition40 and is recommended under the Japanese stroke guidelines for the management of aSAH, was performed three times per day.
The patients’ conditions were closely monitored in the stroke care unit, and the standard rescue therapy was started when DIND was diagnosed, as described later. After introducing induced hypertension, endovascular infusion of vasodilator was performed within 4 h after the onset of DIND, which was followed by microballoon angioplasty if vasodilation could not be adequately induced by vasodilator infusion.
Clinical assessment
The occurrence of DIND was defined as the development of new, focal neurological deficits, and/or a decreased level of consciousness of at least two points on the Glasgow Coma Scale (GCS) after other possible causes of worsening had been excluded. This was assessed by two independent, blinded site investigators. CT was performed before and after aneurysm repair, and 1, 2, 4, 12 weeks after SAH, and whenever neurological worsening occurred. The type and degree of SAH were evaluated by CT scan on admission within 24 h after the ictus, and a new cerebral infarction attributable to cerebral vasospasm was assessed using follow-up CT scan.
Baseline DSA was performed on admission and follow-up DSA was performed again 9 ± 2 days after SAH and/or within 4 h after the onset of DIND in order to assess angiographic cerebral vasospasm (aVS). The diameters of proximal cerebral arteries, which were bilateral C1 segments of the internal carotid artery, M1 segments of the middle cerebral artery, and A1 segments of the anterior cerebral artery, were measured, and the percent reduction in the arterial diameter was calculated by comparing the baseline DSA and follow-up DSA. The severity of cerebral vasospasm was categorized as none or mild: 0–25% decrease; moderate: 25–50% decrease; and severe: over 50% decrease in arterial diameter on follow-up images compared to the baseline DSA.41,42 All CT scans and DSA images were assessed by two independent, blinded reviewers at the central office.
Blood chemistry, including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and plasma creatinine phosphokinase, was serially examined to confirm biological effects and any adverse events due to pitavastatin.
Primary and secondary end points
The primary efficacy end point was the onset of DIND. The secondary end points were the onset of aVS, new cerebral infarction, clinical outcome, and adverse events. Clinical outcome was assessed by the Glasgow Outcome Scale (GOS). Poor outcome was predefined as severe disability (SD), vegetative state (VS) and dead (D), and favorable outcome was predefined as good recovery (GR) and moderate disability (MD). This was assessed by two, independent, blinded site investigators.
Adverse events
Any adverse events occurring from the start of administration to three days after study treatment discontinuation were monitored, because the effects of pitavastatin have been reported to disappear within 48 h after discontinuing medication. Adverse events of specific interest were myositis or rhabdomyolysis, liver damage, hemorrhagic complications, and interstitial pneumonia. Plasma creatinine phosphokinase and alanine aminotransferase were monitored for clinical signs of hepatitis or myositis. Rhabdomyolysis was defined as plasma creatinine phosphokinase >6000 U/L plus evidence of end-organ damage such as renal failure.
Statistical analysis
Sample size was calculated according to the studies of Lynch et al.25 and Tseng et al.,26 using a suitable statistical procedure. Based on these studies, to detect a difference in the frequency of DIND at the 5% significance level and providing 80% power, we estimated the sample size required was 108 patients (54 per group). An intention-to-treat analysis was conducted. All analyses were carried out using the statistical program JMP Pro, version 11. We used t-tests to compare the averages of continuous variables such as age, and Chi-squared tests to compare the proportions of categorical variables such as gender between the groups. Intergroup differences were measured using the Student t-test, Mann–Whitney U-test, and Kruskal–Wallis-test. A two-sided probability value <0.05 was considered significant. When pitavastatin use significantly affected any end point by univariate analysis, multivariate analyses were performed using logistic regression by including possibly confounding factors.
Results
Patient population
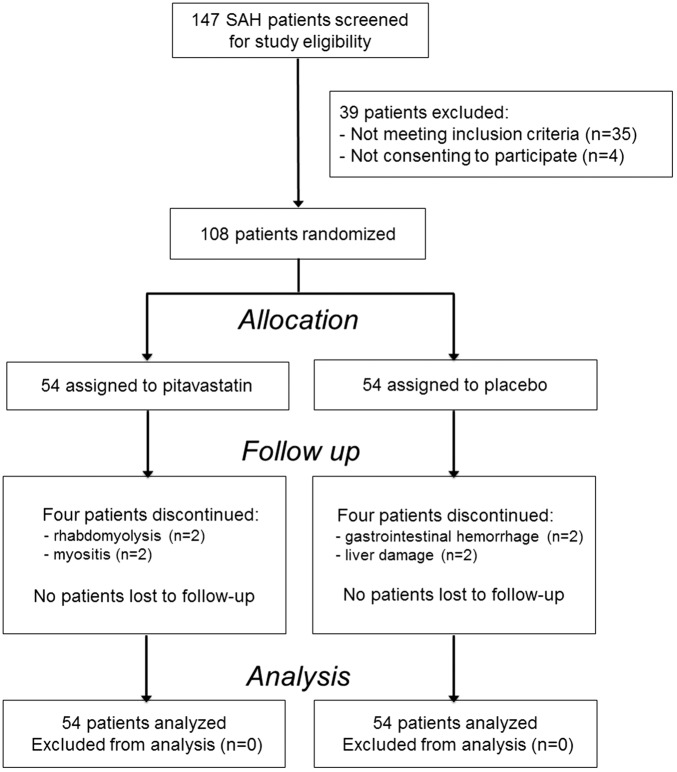
Between December 2014 and June 2016, 147 patients with aneurysmal SAH were admitted within 24 h after the ictus. Of these cases, 108 cases met the inclusion criteria and consented to participate in this study. These 108 cases were randomized to receive either pitavastatin (n = 54) or placebo (n = 54). A flow diagram according to the Consolidated Standards of Reporting Trials guideline is shown in Figure 1.
Figure 1.
Flow diagram of trial patient recruitment according to the consolidated standards of reporting trial guideline.
In the pitavastatin group, pitavastatin was discontinued in four cases; two were due to rhabdomyolysis while the others were due to myositis. In the placebo group, placebo was discontinued in four cases; two were due to liver damage and two were due to gastrointestinal hemorrhage. However, DSA was performed in these eight cases, and all end points analyses could be performed in all cases. Baseline characteristics of the included patients are summarized in Table 1. There were no statistically significant differences in patient profiles between the groups.
Table 1.
Baseline characteristics of included subjects.
| Pitavastatin | Control | p | |
|---|---|---|---|
| No. of subjects | 54 | 54 | |
| Mean age, years (s.d.) | 58 (12) | 55(12) | 0.11 |
| Women, n (%) | 40 (74) | 34 (63) | 0.20 |
| Hypertension, n (%) | 27 (50) | 26 (48) | 0.40 |
| Smoker, n (%) | 24 (44) | 29 (54) | 0.29 |
| Intraventricular hemorrhage, n (%) | 2 (4) | 4 (7) | 0.36 |
| Intracerebral hemorrhage, n (%) | 11 (20) | 6 (12) | 0.29 |
| Coiling, n (%) | 6 (11) | 3 (6) | 0.36 |
| Hunt and Hess grade | 0.31 | ||
| Grade I or II | 41 | 35 | |
| Grade III | 11 | 12 | |
| Grade IV | 2 | 7 | |
| SAH on baseline CT scan | 0.21 | ||
| Thick local | 4 | 3 | |
| Thin diffuse | 1 | 2 | |
| Thick diffuse | 49 | 49 | |
| Aneurysm location | 0.12 | ||
| ACA | 21 | 22 | |
| ICA | 14 | 19 | |
| MCA | 17 | 10 | |
| VA/BA | 2 | 3 | |
| Rescue therapy | |||
| Induced hypertension | 4 | 12 | 0.09 |
| Endovascular infusion of vasodilator | 4 | 12 | 0.09 |
| Balloon PTA | 2 | 2 | — |
SAH: subarachnoid hemorrhage; ACA: anterior cerebral artery; ICA: internal cerebral artery; MCA: middle cerebral artery; VA: vertebral artery; BA: basilar artery; PTA: percutaneous transluminal angioplasty.
Serial changes in TC and LDL-C values are shown in Table 2. The values were expressed as actual values and % reduction compared to the baseline values on Day 0. Not only in the pitavastatin group but also in the control group, both TC and LDL-C decreased significantly 7 and 14 days after SAH compared to the baseline values. The pitavastatin group showed significant reduction of TC on Day 7 and LDL-C on Days 7 and 14 as compared to the control group.
Table 2.
Laboratory data for TC and LDL-C.
| Control group |
Pitavastatin group |
P between groups | |||
|---|---|---|---|---|---|
| Actual value mg/dl (s.d.) | Reduction rate % (s.d.) | Actual value mg/dl (s.d.) | Reduction rate % (s.d.) | ||
| TC | |||||
| Day 0 | 176.5 (27.9) | – | 197.5 (51.3) | – | – |
| Day 7 | 125.7 (17.6)* | 27.9 (9.1) | 116.2 (21.6)* | 38.7 (8.8) | 0.008 |
| Day 14 | 121.4 (31.5)* | 30.5 (25.1) | 115.9 (33.5)* | 39.7 (14.9) | 0.303 |
| LDL-C | |||||
| Day 0 | 104.9 (22.1) | – | 119.4 (43.7) | – | – |
| Day 7 | 72.3 (12.8)* | 25.6 (19.8) | 57.1 (12.9)* | 49.1 (18.3) | 0.001 |
| Day 14 | 67.7 (29.8)* | 29.8 (11.4) | 56.5 (22.8)* | 49.5 (9.1) | 0.034 |
TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol.
p < 0.01 versus the baseline values on Day 0.
Efficacy assessment of study end points
The results of each study end point are shown in Table 3. DIND occurred in seven cases (13.0%) in the pitavastatin group, which was not statistically significantly different in 12 cases (22.2%) in the placebo group. In the pitavastatin group, non-mild aVS, moderate aVS, and severe aVS were, respectively, seen in 28 cases (51.9%), 18 cases (33.3%), and 8 cases (14.8%). In the placebo group, these were respectively seen in 20 cases (37.0%), 16 cases (29.6%), and 18 cases (33.3%). Cases with severe aVS were fewer in the pitavastatin group than in the placebo group considering a statistically significant difference (p = 0.031). The new cerebral infarctions one month after SAH on CT scan were seen in six cases (11.1%) in the pitavastatin group and in 13 cases (24.1%) in the control group, which showed no statistically significant difference. Outcome assessed using GOS at three months after SAH showed that poor outcome consisting of SD, VS, and D were seen in eight cases (14.8%) in the pitavastatin group, which was not statistically significantly different from 10 cases (18.5%) in the placebo group.
Table 3.
Assessments of primary and secondary end points.
| Pitavastatin n = 54 (%) | Control n = 54 (%) | p | |
|---|---|---|---|
| DIND | 7 (13.0) | 12 (22.2) | 0.16 |
| Angiographic vasospasm | 0.03 | ||
| None or mild | 28 (51.9) | 20 (37.0) | |
| Moderate | 18 (33.3) | 16 (29.6) | |
| Severe | 8 (14.8) | 18 (33.3) | |
| Cerebral infarction | 6 (11.1) | 13 (24.1) | 0.12 |
| Poor outcome | 8 (14.8) | 10 (18.5) | 0.39 |
DIND: delayed neurological ischemic deficits.
Therefore, a statistically significant difference between the groups by univariate analyses was seen only in aVS, and multivariate analysis was then performed. The factors reported to have an influence on aVS, such as hypertension, cigarette smoking, poor Hunt and Hess grade, severity of SAH clot on CT scan, intraventricular hemorrhage (IVH), intracerebral hemorrhage, and clipping or coiling, were selected as the confounding factors for multivariate analysis of aVS.43 Multiple logistic regression analysis showed that only pitavastatin use was an independent factor reducing the occurrence of aVS (odds ratio, 0.32; 95% confidence interval, 0.11–0.87, p = 0.042) (Table 4).
Table 4.
Assessment of factors affecting aVS (angiographic vasospasm).
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Risk factor | p | p | 95%CI | OR |
| Pitavastatin use | 0.031 | 0.042 | 0.11–0.87 | 0.323 |
| Age (>65yo) | 0.694 | 0.832 | 0.38–3.64 | 1.124 |
| Hunt & Hess Gr. 3 and 4 | 0.202 | 0.441 | 0.51–1.95 | 1.513 |
| SAH (diffuse, thick) | 0.547 | 0.562 | 0.25–47.8 | 1.987 |
| IVH | 0.189 | 0.439 | 0.23–27.9 | 2.436 |
| Clipping | 0.856 | 0.817 | 0.08–16.6 | 0.747 |
IVH: intraventricular hemorrhage; ICH: intracerebral hemorrhage.
Safety
Adverse events were seen in five cases in the pitavastatin group and in four cases in the placebo group (Table 5). Hemorrhagic complications were seen in one case in the pitavastatin group (one intracerebral hemorrhage) and in two cases in the placebo group (two gastrointestinal hemorrhages). In the intracerebral hemorrhage case, in the pitavastatin group, the hemorrhage was small and required no additional surgery and no discontinuance of pitavastatin. The cases of gastrointestinal hemorrhage in the placebo group required additional treatment and placebo was discontinued in these cases. Myositis or rhabdomyolysis were seen in four cases in the pitavastatin group, which led to discontinuation of pitavastatin but required no additional treatment. Placebo was also stopped in two cases due to an increase in hepatic enzymes, which improved afterward without additional treatment.
Table 5.
Adverse events.
| Pitavastatin n = 54 (%) | Control n = 54 (%) | p | |
|---|---|---|---|
| Hemorrhagic events | |||
| Intracerebral hemorrhage | 1 (1.9) | 0 (0) | 0.32 |
| Gastrointestinal hemorrhage | 0 (0) | 2 (3.7) | 0.12 |
| Hepatic enzymes increase | 0 (0) | 2 (3.7) | 0.12 |
| Rhabdomyolysis | 2 (3.7) | 0 (0) | 0.12 |
| Myositis | 2 (3.7) | 0 (0) | 0.12 |
| Overall events | 5 (9.3) | 4 (7.4) | 0.40 |
Discussion
In the present study, a significant decrease in cerebral vasospasm, as confirmed by cerebral angiography in all cases, was obtained with pitavastatin use. However, pitavastatin could not improve outcome, even when it was long-acting.
Statin began to be used for aSAH due to the expectation that it could increase eNOS expression and the fact that NOS had been shown to decrease after SAH. The first experimental study using the mouse SAH model revealed that cerebral vasospasm was ameliorated and eNOS was upregulated by statin pretreatment.44 Another experimental study indicated that statin induced eNOS upregulation accompanied by ameliorated cerebral vasospasm through activating the phosphatidylinositol 3-kinase/Akt pathway in the rat endovascular perforation SAH model.45 As for the other pleiotropic effects of statin, endothelial stabilization, decrease in vascular inflammation and inhibition of reactive oxygen species (ROS) have been considered beneficial for DCI after SAH, although direct evidence does not exist that statin improves DCI after SAH by means of these other pleiotropic effects, aside from eNOS upregulation.
Amelioration of cerebral vasospasm has been expected in clinical cases based on the results of experimental studies. Among the previous six RCTs and two prospective studies, cerebral vasospasm was evaluated by TCD in four studies25–28 and by cerebral angiography in two studies.33,35 Although only two RCTs showed that the efficacy of statins improved cerebral vasospasm,25,26 the latest systematic review revealed that the overall number of patients with cerebral vasospasm was 29% in the statin-treated group and 39% in the placebo-treated group (pooled RR 0.76 [95% CI, 0.61 to 0.96]; p = 0.02).32 However, this may not be conclusive, because the definitions of cerebral vasospasm on TCD were not uniform among the studies; in the studies by Lynch et al.25 and Garg et al.,28 cerebral vasospasm was defined by the velocity in the middle cerebral artery using TCD: VMCA > 160 cm/s. On the other hand, Tseng et al.26 adopted VMCA >120 cm/s as the definition of cerebral vasospasm, and Chou et al.27 defined it as any peak systolic middle cerebral artery velocity >200 cm/s. In two studies evaluating cerebral vasospasm by DSA, Macedo et al.33 performed DSA only in the presence of changes suggestive of vasospasm; McGirt et al.35 undertook DSA only if symptoms due to vasospasm failed to resolve with hypertensive, hypervolemic, and hemodilutional (HHH) therapy. Neither performed DSA on all patients. In the present study, all cases were evaluated with DSA, and a significant decrease in cerebral vasospasm was obtained by pitavastatin use.
For the end points other than cerebral vasospasm, such as DIND, new cerebral infarction, outcome, and mortality, improvements were shown in only a few of the past studies; Tseng et al. showed a significant decrease in DIND, new cerebral infarction, and mortality, and Macedo et al. indicated a decrease in DIND and mortality. The latest systematic review showed the overall numbers of patients who had DCI, cerebral infarction, favorable outcome, and mortality in a statin-treated group and a placebo-treated group were 19% and 21%, 15% and 18%, 65% and 66%, and 11% and 12%, respectively, which indicated no significant differences between a statin-treated group and a placebo-treated group.32 Also in the present study, in spite of ameliorated cerebral vasospasms obtained by pitavastatin administration, significant reductions in the occurrence of DIND, cerebral infarction, and poor outcome were not achieved.
The results of this study were analogous to the results demonstrated by clazosentan studies, in which improvement in functional outcome and a reduction in mortality rate were not achieved in spite of an improvement in aVS obtained by clazosentan administration.46 Clazosentan studies led us to doubt that cerebral vasospasm is the only factor causing DCI and affecting outcome, and to take other factors into consideration. This was reconfirmed by the results of the present study and the results of the systematic review of the effects of statin in aSAH. As other factors, early brain injury,8–11 cortical spreading depolarization,12–15 microcirculatory disturbance,16–18 cerebral autoregulation impairment,19 and impairment in neurovascular coupling20,21 have been investigated. One of the reasons why pitavastatin showed no significant effects on DIND and functional outcome may be that the pleiotropic effects of pitavastatin might not be associated with an improvement in these other factors, except for cerebral vasospasm.
As another reason, Fasudil, which has been routinely administrated to aSAH patients in Japan according to the Japanese stroke guideline and was used in both the pitavastatin group and the control group in the present study, might make differences in DIND occurrence between the two groups insignificant, since fasudil was proved to have the effect of ameliorating not only cerebral vasospasm but also the occurrence of DIND.40 This is also one of the limitations of the present study.
As the other limitation, we designed the present study with the primary efficacy end point of vasospasm-related DIND according to Lynch et al.25 and Tseng et al.26 However, it has recently been recommended that the primary endpoint should be measured with functional outcome or cerebral infarction on CT or MRI.47 This is because, compared to DIND, cerebral infarction on CT or MRI shows higher sensitivity in detecting DCI.48 These neuroimaging methods are closely associated with functional outcome after aSAH and they can provide higher inter-observer agreement.48 Furthermore, MRI is more informative than CT because MRI is more sensitive and postmortem studies suggest that small cortical lesions have an important effect on outcome after aSAH.49 In the present study, functional outcome and infarction on neuroimaging were secondary endpoints, and CT was mainly used for detecting infarction. These are the limitations of the present study.
As of now, statin monotherapy that includes a long-acting statin is not recommended for the acute phase of aSAH. However, as the efficacy of statins in preventing cerebral vasospasm has been proved in the present study, it might be worthwhile investigating whether combination treatment with other therapies, such as HHH therapy to improve cerebral autoregulation impairment and therapies to improve microcirculatory disturbances, could be useful in the future.18,42,50
Conclusion
This was the first study proving that statin use could induce definite amelioration of cerebral vasospasm on DSA. However, reductions in the occurrences of DIND, new cerebral infarction and poor outcome could not be achieved, even though a long-acting statin was used in the acute phase of SAH. Therefore, the administration of any type of statin in the acute phase of SAH for the purpose of improving a vasospasm-associated clinical manifestation is not recommended.
Acknowledgments
We thank Mark Inglin (University of Basel) for his editorial assistance.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
MN, NM, NS, KAS and HO designed and undertook the study at the principal site. AT, SH, and KAK were the chief investigators at each site. MN and NM collected and analyzed the data. HO provided overall oversight of the research. All authors critically reviewed the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Clinical trial registration
This trial is registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000015977). URL: http://www.umin.ac.jp/. Unique identifier: UMIN000015977.
References
- 1.Vergouwen MDI, Algra A, Rinkel GJE. Endothelin receptor antagonists for aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke 2012; 43: 2671–2676. [DOI] [PubMed] [Google Scholar]
- 2.Vergouwen MDI, Etminan N, Ilodigwe D, et al. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 2011; 31: 1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etminan N, Vergouwen MDI, Ilodigwe D, et al. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2011; 31: 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Gijn J, Kerr RS, Rinkel GJE. Subarachnoid haemorrhage. Lancet 2007; 369: 306–318. [DOI] [PubMed] [Google Scholar]
- 5.Allen GS, Ahn HS, Preziosi TJ, et al. Cerebral arterial spasm – a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med 1983; 308: 619–624. [DOI] [PubMed] [Google Scholar]
- 6.Espinosa F, Weir B, Overton T, et al. A randomized placebo-controlled double-blind trial of nimodipine after SAH in monkeys. Part 1: clinical and radiological findings. J Neurosurg 1984; 60: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 7.Petruk KC, West M, Mohr G, et al. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J Neurosurg 1988; 68: 505–517. [DOI] [PubMed] [Google Scholar]
- 8.Kusaka G, Ishikawa M, Nanda A, et al. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2004; 24: 916–925. [DOI] [PubMed] [Google Scholar]
- 9.Fujii M, Yan J, Rolland WB, et al. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res 2013; 4: 432–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki H. What is early brain injury? Transl Stroke Res 2015; 6: 1–3. [DOI] [PubMed] [Google Scholar]
- 11.Pang J, Chen Y, Kuai L, et al. Inhibition of blood-brain barrier disruption by an apolipoprotein e-mimetic peptide ameliorates early brain injury in experimental subarachnoid hemorrhage. Transl Stroke Res 2017; 8: 257–272. [DOI] [PubMed]
- 12.Dreier JP, Major S, Manning A, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 2009; 132: 1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler MK, Dengler N, Hecht N, et al. Oxygen availability and spreading depolarizations provide complementary prognostic information in neuromonitoring of aneurysmal subarachnoid hemorrhage patients. J Cereb Blood Flow Metab 2017; 37: 1841–1856. [DOI] [PMC free article] [PubMed]
- 14.Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab. Epub ahead of print 1 January 2016. DOI: 10.1177/0271678X16654496. [DOI] [PMC free article] [PubMed]
- 15.Hartings JA, Li C, Hinzman JM, et al. Direct current electrocorticography for clinical neuromonitoring of spreading depolarizations. J Cereb Blood Flow Metab. Epub ahead of print 1 January 2016. DOI: 10.1177/0271678X16653135. [DOI] [PMC free article] [PubMed]
- 16.Ohkuma H, Manabe H, Tanaka M, et al. Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 2000; 31: 1621–1627. [DOI] [PubMed] [Google Scholar]
- 17.Naraoka M, Matsuda N, Shimamura N, et al. The role of arterioles and the microcirculation in the development of vasospasm after aneurysmal SAH. BioMed Res Int 2014; 2014: 253746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McConnell ED, Wei HS, Reitz KM, et al. Cerebral microcirculatory failure after subarachnoid hemorrhage is reversed by hyaluronidase. J Cereb Blood Flow Metab 2016; 36: 1537–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budohoski KP, Czosnyka M, Smielewski P, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke 2012; 43: 3230–3237. [DOI] [PubMed] [Google Scholar]
- 20.Pappas AC, Koide M, Wellman GC. Purinergic signaling triggers endfoot high-amplitude Ca2+ signals and causes inversion of neurovascular coupling after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2016; 36: 1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balbi M, Koide M, Wellman GC, et al. Inversion of neurovascular coupling after subarachnoid hemorrhage in vivo. J Cereb Blood Flow Metab. Epub ahead of print 1 January 2017. DOI: 10.1177/0271678X16686595. [DOI] [PMC free article] [PubMed]
- 22.Endres M. Statins and stroke. J Cereb Blood Flow Metab 2005; 25: 1093–1110. [DOI] [PubMed] [Google Scholar]
- 23.Sabri M, Ai J, Marsden PA, et al. Simvastatin re-couples dysfunctional endothelial nitric oxide synthase in experimental subarachnoid hemorrhage. PLoS One 2011; 6: e17062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reuter B, Rodemer C, Grudzenski S, et al. Effect of Simvastatin on MMPs and TIMPs in human brain endothelial cells and experimental stroke. Transl Stroke Res 2015; 6: 156–159. [DOI] [PubMed] [Google Scholar]
- 25.Lynch JR, Wang H, McGirt MJ, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke 2005; 36: 2024–2026. [DOI] [PubMed] [Google Scholar]
- 26.Tseng MY, Czosnyka M, Richards H, et al. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke 2005; 36: 1627–1632. [DOI] [PubMed] [Google Scholar]
- 27.Chou SHY, Smith EE, Badjatia N, et al. A randomized, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke 2008; 39: 2891–2893. [DOI] [PubMed] [Google Scholar]
- 28.Garg K, Sinha S, Kale SS, et al. Role of simvastatin in prevention of vasospasm and improving functional outcome after aneurysmal sub-arachnoid hemorrhage: a prospective, randomized, double-blind, placebo-controlled pilot trial. Br J Neurosurg 2013; 27: 181–186. [DOI] [PubMed] [Google Scholar]
- 29.Vergouwen MDI, Meijers JCM, Geskus RB, et al. Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled randomized trial. J Cereb Blood Flow Metab 2009; 29: 1444–1453. [DOI] [PubMed] [Google Scholar]
- 30.Sillberg VAH, Wells GA, Perry JJ. Do statins improve outcomes and reduce the incidence of vasospasm after aneurysmal subarachnoid hemorrhage: a meta-analysis. Stroke 2008; 39: 2622–2626. [DOI] [PubMed] [Google Scholar]
- 31.Tseng MY. Summary of evidence on immediate statins therapy following aneurysmal subarachnoid hemorrhage. Neurocrit Care 2011; 15: 298–301. [DOI] [PubMed] [Google Scholar]
- 32.Shen J, Huang K-Y, Zhu Y, et al. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. J Neurosurg 2016; 10: 1–11. [DOI] [PubMed] [Google Scholar]
- 33.Macedo S, Bello Y, Silva A, et al. Effects of simvastatin in prevention of vasospasm in nontraumatic subarachnoid hemorrhage: preliminary data. Crit Care 200913 Suppl): 103.19183430 [Google Scholar]
- 34.Kirkpatrick PJ, Turner CL, Smith C, et al. Simvastatin in aneurysmal subarachnoid haemorrhage (STASH): a multicentre randomised phase 3 trial. Lancet Neurol 2014; 13: 666–675. [DOI] [PubMed] [Google Scholar]
- 35.McGirt MJ, Garces Ambrossi GL, Huang J, et al. Simvastatin for the prevention of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage: a single-institution prospective cohort study. J Neurosurg 2009; 110: 968–974. [DOI] [PubMed] [Google Scholar]
- 36.Wong GKC, Chan DYC, Siu DYW, et al. High-dose simvastatin for aneurysmal subarachnoid hemorrhage: multicenter randomized controlled double-blinded clinical trial. Stroke 2015; 46: 382–388. [DOI] [PubMed] [Google Scholar]
- 37.Davignon J. Pleiotropic effects of pitavastatin. Br J Clin Pharmacol 2012; 73: 518–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito Y. Critical appraisal of the role of pitavastatin in treating dyslipidemias and achieving lipid goals. Vasc Health Risk Manag 2009; 5: 921–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naraoka M, Munakata A, Matsuda N, et al. Suppression of the rho/rho-kinase pathway and prevention of cerebral vasospasm by combination treatment with statin and fasudil after subarachnoid hemorrhage in rabbit. Transl Stroke Res 2013; 4: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibuya M, Suzuki Y, Sugita K, et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg 1992; 76: 571–577. [DOI] [PubMed] [Google Scholar]
- 41.Dankbaar JW, Rijsdijk M, van der Schaaf IC, et al. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology 2009; 51: 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuda N, Naraoka M, Ohkuma H, et al. Effect of cilostazol on cerebral vasospasm and outcome in patients with aneurysmal subarachnoid hemorrhage: a randomized, double-blind, placebo-controlled trial. Cerebrovasc Dis 2016; 42: 97–105. [DOI] [PubMed] [Google Scholar]
- 43.Inagawa T. Risk factors for cerebral vasospasm following aneurysmal subarachnoid hemorrhage: a review of the literature. World Neurosurg 2016; 85: 56–76. [DOI] [PubMed] [Google Scholar]
- 44.Pluta RM, Thompson BG, Dawson TM, et al. Loss of nitric oxide synthase immunoreactivity in cerebral vasospasm. J Neurosurg 1996; 84: 648–654. [DOI] [PubMed] [Google Scholar]
- 45.Sugawara T, Ayer R, Jadhav V, et al. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res 2008; 86: 3635–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macdonald RL, Kassell NF, Mayer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 2008; 39: 3015–3021. [DOI] [PubMed] [Google Scholar]
- 47.Diringer MN, Bleck TP, Hemphill JC, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care society’s multidisciplinary consensus conference. Neurocrit Care 2011; 15: 211–240. [DOI] [PubMed] [Google Scholar]
- 48.Vergouwen MDI, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010; 41: 2391–2395. [DOI] [PubMed] [Google Scholar]
- 49.Dreier JP, Sakowitz OW, Harder A, et al. Focal laminar cortical MR signal abnormalities after subarachnoid hemorrhage. Ann Neurol 2002; 52: 825–829. [DOI] [PubMed] [Google Scholar]
- 50.Terpolilli NA, Feiler S, Dienel A, et al. Nitric oxide inhalation reduces brain damage, prevents mortality, and improves neurological outcome after subarachnoid hemorrhage by resolving early pial microvasospasms. J Cereb Blood Flow Metab 2016; 36: 2096–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]