Abstract
Spreading depolarization (SD) occurs alongside brain injuries and it can lead to neuronal damage. Therefore, pharmacological modulation of SD can constitute a therapeutic approach to reduce its detrimental effects and to improve the clinical outcome of patients. The major objective of this article was to produce a systematic review of all the drugs that have been tested against SD. Of the substances that have been examined, most have been shown to modulate certain SD characteristics. Only a few have succeeded in significantly inhibiting SD. We present a variety of strategies that have been proposed to overcome the notorious harmfulness and pharmacoresistance of SD. Information on clinically used anesthetic, sedative, hypnotic agents, anti-migraine drugs, anticonvulsants and various other substances have been compiled and reviewed with respect to the efficacy against SD, in order to answer the question of whether a drug at safe doses could be of therapeutic use against SD in humans.
Keywords: AMPA receptor, GABA receptor, neurovascular coupling, NMDA receptor, pharmacology, spreading depolarization
Introduction
Spreading depolarization (SD) is a massive depolarization wave of neuronal and glial cells that propagates at a rate of 2–9 mm/min through cerebral gray matter.1 It is characterized by the abruptly developing, near-complete, and sustained breakdown of transmembrane ion gradients, neurotransmitter release, increased energy metabolism, water shifts, and depression of electrical activity. Today, there is enough evidence showing the presence of SD in migraine with aura (MA). SDs also occur in cerebrovascular diseases such as stroke, subarachnoid hemorrhage (SAH), traumatic brain injury (TBI), and intracerebral hemorrhage (ICH). In these conditions, SD occurrence has been associated with neuronal damage, necrosis, degeneration, and poor clinical outcome.2–4 The pathological effects of SD can be in part explained due to its impact on cerebral hemodynamics that produce a cycle of events that have a cumulative effect progressively increasing the degree and spatial extend of ischemia. It is well known that SDs in a healthy, adequately supplied tissue has only slightly damaging, innocuous effects.5–12 In contrast, when neurovascular coupling is impaired or the tissue is inadequately perfused, SD promotes spreading ischemia, excitotoxicity, oxidative stress, worsen hypoxia and neuronal death, therefore, having a negative impact on clinical outcome.13
In the clinical setting, the therapeutic modulation of SD has gained expectations. Pharmacological targeting of SD in the clinic is still in its infancy. Several experimental studies indicate that SD can be modulated by drugs. According to these observations, pharmacological modulation of SD in the clinical setting as a neuroprotective therapy could be feasible. In this article, we focus on the pharmacological agents that have been used against SDs. It is a systematic presentation, classification, and evaluation of drugs that have been tested against SD (Figure 1). After an exhaustive search, we found 114 substances whose therapeutic effect on SD has been investigated, either individually or in direct comparison with each other. We give as part of the introductory segment a brief overview of the relevant aspects of the physiopathology of this phenomenon, followed by the clinical implication in neurological diseases before approaching our main topic. For a more comprehensive and vast description of the cellular and molecular mechanisms and the clinical role of SDs, we refer to the following reviews.2–4,13–16
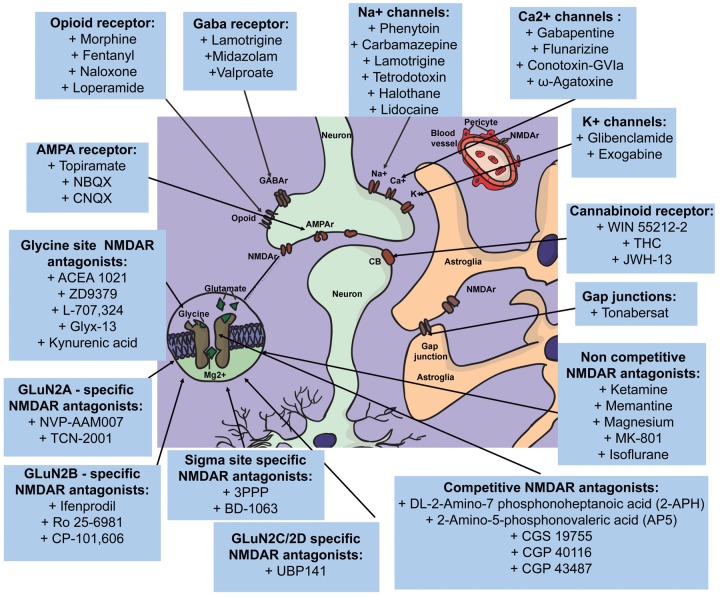
Figure 1.
Various target points play a role in the antagonization of SDs. Among others, NMDA-, GABA-, opioid-, AMPA, and cannabinoid receptors. Moreover, a variety of channels is involved. These channels can be found on neurons, astrocytes, and pericytes. Most substances antagonize SD via a complex machinery that involves multiple of these target points. Substances in the figure are assigned to a target point which they are mostly associated with.
Relevant aspects of the physiopathology of spreading depolarizations
Physiologically, SD corresponds to a self-propagating wave-front of depolarization with neuronal and glial cell implication. It can be accompanied by depression of the electrocorticography (ECoG) activity of a fast negative potential changes, and it usually spreads at a characteristic speed of 2–9 mm/min,1 and resolves after 5–15 min.14,15
Underlying the depolarization, there is a breakdown of ion gradients, such as K+ and H+ increases and Na+, Ca2+, and Cl− decreases. This ionic interchange favors neuronal swelling and dendrite distortion. There are also pH changes – extracellular pH becomes first alkaline and then acidic. The acidosis is associated with the production of CO2 and lactic acid by a pronounced oxygen and glucose consumption related to the increased metabolism, which is necessary to restore the ion homeostasis through the activation of Na+/K+-ATPase and Ca2+ pumps.14–16 SD also induces the release of neurotransmitters into the extracellular space such as glutamate, which activates NMDA, AMPA, or kainate receptors that can lead to excitotoxicity and cellular damage.14–16
SDs can be elicited by a variety of stimulus such as high-frequency electrical pulses, direct current, mechanical stimulation, basicity, hypo-osmolarity, hyperthermia, hypoxia, hyperkalemia, and hypoglycemia, and a variety of chemical agents, such as K+ and glutamate,13,15 hypotonic exposure,17 and edothelin-1.18 The mechanisms of SD induction and propagation in different pathological situations is unclear. The two most important hypotheses are based on extracellular K+ and glutamate diffusion mechanisms versus intracellular propagating agents (including K+ and Ca2+) through gap junctions.15,19
The mechanisms underlying the cerebral hemodynamic responses to SD are not fully understood. SD is associated with increases in energy metabolism that require large increases in regional cerebral blood flow (rCBF). This reaction corresponds to a normal vascular coupling which describes the increase in rCBF supply in response to physiological neuronal activation and the reduction of rCBF with neuronal deactivation. SD has been associated with increments of more than 100% of rCBF, known as spreading hyperemia. However, a brief reduction of rCBF and/or a sustained suppression of rCBF known as spreading oligemia has been detected following the hyperemic response.20,21
In pathological conditions, SD exerts drastic hemodynamic changes. Under hypoxic circumstances, SD can induce an inverse neurovascular coupling, consisting of a prolonged and intense hypoperfusion, also known as spreading ischemia.13–15 The shift from spreading hyperemia to spreading ischemia can be triggered by the decrease in NO availability together with the increase of K+ concentrations. Therefore, during pathological conditions, spreading ischemia can render neural tissue vulnerable to secondary damage up to the development of widespread necrosis.22
Clinical implication of spreading depolarizations
Occurrence of SD in the human brain and its role in the pathophysiological basis of several neurological conditions have been addressed in the clinical sciences. There is sufficient evidence showing that SD has an important role in different neurovascular conditions such as stroke, SAH, TBI, and ICH.2–4 A relation between SDs and MA has also been well documented.23 Different mechanisms for SD development after these conditions have been postulated.
Several studies indicate the association between SD occurrence and functional neuronal damage, neurological degeneration, and poor clinical outcome. The deleterious effects in patients after brain injury have been related with the drastic hemodynamic changes to SD.2,13,24–26 We briefly review the impact of SD on different cerebrovascular diseases and MA.
Subarachnoid hemorrhage
SAH as a consequence of aneurismal rupture is a common condition frequently leading to poor outcome and death. Delayed cerebral ischemia (DCI) constitutes the most important cause of morbidity and mortality after SAH. A link between SD and SAH has been established in a plethora of studies.27–29 In this regard, the incidence of SD in SAH has been reported in more than 70% and has been related to the development of DCI.27,28 It is believed that increases in basal K+ attributable to erythrocytolysis, blood clot hemolytic products and decrease of N+ pump activity (due to vasospasm of cerebral arteries) are triggering factors for SD initiation.13,30
The major morphological and pathological impact that SDs have in patients with SAH is a decrease in the flow of oxygen and nutrients to metabolically active neurons and a dysbalance of vasoconstrictor and vasodilator agents.31 Therefore, when appearing as clusters may lead to delayed neurological deficits and development of new infarcts.27,28 Spreading ischemia has been well detected in patients with aneurysmal SAH and DCI.27,28
In this scenario, factors such as reduction of rCBF, microcirculatory dysfunction, microthrombosis, and hemolytic blood products may provide an important source of SDs leading to spreading ischemia and cortical infarction.13,32 This speaks in favor of SDs as an etiological factor that may contribute to the development of DCI. Therefore, the pharmacological modulation of SDs in SAH may lead to reduction of secondary brain damage and DCI development, resulting in an improvement of patients’ outcome.
Traumatic brain injury
Evidence of the development of SDs after TBI has been well supported in different studies. In TBI patients, SD has been registered between 50 and 60% and seems to increase its incidence with lower levels of mean arterial pressure and cerebral perfusion pressure.26,33 Hypotension, hypoperfusion, and hyperthermia occur commonly in the clinical setting of TBI; they constitute potential triggers of SD.34 Recently, Hinzman et al.35 showed the presence of inverse neurovascular coupling to SD in a group of 24 patients who were subjected to craniotomy after severe TBI. Supporting the association between SD, spreading ischemia and the exacerbation of brain injury after TBI,35 therefore, the major pathological impact that SD has in TBI patients is probably the mismatch of energy supply-demand and a lower perfusion.34 As a result, SDs in TBI contribute to lesion expansion and promote effects of secondary insults that often accompany TBI. In consequence, the control of SDs after TBI might be used to guide the therapeutic decision making in each patient.
Stroke
After an ischemic insult, the presence of SD has been reported in up to 100% of the patients; they arise from the edge of the ischemic core and propagate through the penumbra area.36 The number and duration of SDs after ischemic brain lesions has shown to have a correlation with secondary neuronal damage and further infarct expansion.22 And Also, it has been postulated that SDs are the underlying mechanism of cytotoxic edema in grey matter.13 A plethora of evidence validates the notion of SD as a pathological mechanism leading to secondary damage after stroke.37,38 Ischemia-mediated breakdown of ionic homeostasis is thought to initiate the SD ignition.13 It also has been shown how the supply-demand oxygen-transients mismatch after somatosensory activation of peri-infarct cortex is capable to trigger SDs due to an increase demand or reduced oxygen supply, showing an adverse effect on ischemic tissue outcome.39
The high incidence of SD after stroke and the deleterious consequences points out the relevance of SD therapeutic modulation after an ischemic event in order to reduce the infarct growth. This is in particular challenging, due to the experimental data, indicating that the induced disruption can outweigh the effect of the therapeutic drug (e.g., an NMDA receptor antagonist), and SDs might still occur.40 Nevertheless, the drug might still be very efficacious in the peri-ischemic penumbra. Here, an antagonization of SD could hypothetically unction as a preconditioning and even promote regeneration and plasticity.22,41
Intracerebral hemorrhage
ICH is a severe disease with high ICU mortality and morbidity42 and perihematomal edema progression strongly contributes to neurological deterioration and worse outcome.43 SD has been detected in patients with ICH,24 and it is hypothesized to contribute to the lesion development, although it is not fully clear to which degree.2 Firstly, Fabricius et al. observed SD in two out five patients with ICH,24 and recently, a prospective observational trial by Helbok et al. recorded SD in a cohort of poor grade ICH patients in whom hematoma evacuation was performed.44 Helbok et al. reported the highest SD incidence rate in humans with ICH so far (67%). An increasing hemorrhage volume in ICH is thought to increase the risk of SDs through the extracellular accumulation of K+.44 Since SD facilitates dendritic beading, neuronal swelling, and cytotoxic edema, SD might aggravate or even induce edema formation in the perihematomal brain tissue of ICH patients.44 A therapeutic approach of SD might decrease SD edema expansion.
Migraine with aura
It has been suggested that SDs are responsible for MA in the human visual cortex by showing a retinotopic visual percept induced by SD during aura45 supported by MRI-BOLD studies46 and various animal models.47–49 There is evidence that SD activates the trigeminovascular system, hence provoking headache.50 In patients with MA, episodic dysbalance of excitation and inhibition and a hyperactivity of cortical circuits have been proposed as a trigger of SDs.51 Even though MA is usually injurious and not associated with neuronal damage, spreading ischemia is hypothesized to be the underlying mechanism of migrainous stroke.13 The pharmacological modulation of SDs in MA can serve as a translational therapeutic model to other pathological settings.
Pharmacological targeting of SD
Today, an overwhelming body of evidence supports the concept that prevention of SD or containment of its expansion means less brain damage and is thus of the highest clinical relevance. Treating SD could improve functional outcome. An ideal treatment strategy for SD would have the potential for a pleiotropic effect by positively modulating several of the implicated pathophysiological mechanisms at once. However, energy-depleted tissue complicates the therapeutic targeting that there are still only a few targets that can be successfully addressed by drugs.
Various strategies have been proposed against SD, among them (1) blocking SD initiation, (2) modulating of SD propagation, (3) reduction of SD amplitude, (4) deceleration of SD progression, (5) reduction of SD hemodynamic response, and (6) reversal of the inverse response. All of this can be achieved by addressing various target points, such as NMDA, GABA, AMPA, or opioid receptors and many more. The most effective substances that are applied in humans are ketamine and valproate (Table 1). An antagonism of inverse coupling has been achieved by vasodilators. A partial antagonist effect of adenosine, by shortening of the hypoperfusion, has been observed in SD in rodents.52
Table 1.
Summary of results.
| Most effective agents against SD | Number | Amplitude | Propagation | Threshold | Duration | Frequency | |
|---|---|---|---|---|---|---|---|
| NMDAR antagonist (clinically used) | Ketamine | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ |
| Memantine | ↓ | ↓ | ↓ | ||||
| NMDAR antagonist (animals only) | MK-801 | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ |
| Anesthetic agents | Isoflurane | ↓ | = | ↓ | = | = | ↓ |
| Sevoflurane | = | = | = | = | = | ↓ | |
| Anti-migraine drugs | Valproate | ↓ | ↓ | = | ↑ | = | ↓ |
| Topiramate | ↓ | = | ↓ | ↑ | ↓ | ↓ |
“↓” Means a reductive effect was observed after drug administration, “=” means no effect was noticed, and blank space means the parameter was not tested, “↑” means that the examined parameter was increased after drug administration. Typical parameters under investigation are number, amplitude, propagation, threshold, duration, and frequency. Experimental settings and models are heterogeneous, comprising different animals (chicken, rat, cat, mouse, and swine) and various forms of SD induction (KCl and electrical stimulation). Among the most effective drugs are ketamine, MK-801, and topiramate.
While the present review focuses on pharmacological substances that inhibit SD, there are some additional strategies that have been investigated but will not be addressed in depth. Hyperglycemia has been associated with a lower incidence of SD.53,54 Moreover, in experiments with KCl-induced SD, hypoglycemia was shown to prolong the SD but had no effect on amplitude, incidence, or propagation.55 Hyperoxia has been shown to inhibit SD.56,57 Recently, transcutaneous vagus stimulation has been shown to be efficacious in reducing the susceptibility to KCl and electrically induced SD.58
Methods
Although the treatment of SD is of high clinical importance, it is underreported in medical literature; the present review focused on the following answerable questions: (A) “Which drugs have been tested against SD in vivo and in vitro?” (B) “How efficient are they in reducing incidence and characteristic features of SD?” and (C) “Is a translation into clinical practice feasible or imaginable?” The search for evidence was performed in three databases: PubMed, Science Direct, and Web of Science. The search terms included variations on the condition (“spreading depression, spreading depolarization, cortical depression, anoxic depolarizations, peri-infarct depolarizations”) combined with treatment-related terms (“prevention, treatment, effect, reduction, inhibition, therapy”) and specific target points (“NMDA-, AMPA-, GABA-, opioid, serotonin-receptor, anesthetic, sedative, hypnotic, analgesic agents”). The search was limited to the English language and publications from January 1986 to the present (September 2017). For more details on the strategies used for each database, please contact the authors. The main inclusion and exclusion criteria (language and date) were applied during the screening of the titles and abstracts, whereas the other criteria (experiment type and animal) were addressed in full text review. A total of 138 articles were selected for full text review. Of these, 132 articles were selected for final inclusion. Various strategies have been proposed to target and modulate SD. The properties of SD that have been targeted are the number, amplitude, propagation, threshold, duration, frequency, and hemodynamic response. Only articles that investigated a drug’s effect on number, amplitude, propagation, threshold, duration, frequency, and pial diameter were selected for analysis.
Substances
NMDA receptor antagonists/agonists
NMDA receptor is the target of the most potent inhibitors of SD. We therefore discuss its pathophysiologic role as well as some representative agents.
The NMDA receptor is a heterotetrameric ionotropic glutamate receptor, and expression studies indicate that the functional receptor is composed of at least one NR1 subunit and one or more NR2 subunits.59,60 The highest affinity endogenous ligands of its agonist binding site are L-glutamate and aspartate.61,62 The NMDA receptor controls a non-selective cation channel (with permeability for Na+, K+, and Ca2+ ions) that is gated by Mg2+ in a voltage-dependent manner. In its activated state, this channel can be blocked by various competitive, non-competitive, and glycine site-specific antagonists as well as others. Thus, NMDA receptor antagonists such as ketamine can interfere with SD initiation and expansion by increasing the threshold for K+ and neurotransmitters, and its duration by reducing the influx of Na+ and Ca2+.63 The NMDA receptor has been hypothesized to play a definitive role in neurodegenerative conditions, neuronal death, and various brain disorders.64–68
Failure of NMDA receptor antagonists in clinical trials
The concept of glutamate-induced excitotoxicity served as rationale for the integration of NMDA receptor antagonists into human trials. Although experimental studies have widely shown that the pharmacological blockade of ionotropic glutamate receptors reduces ischemic damage, clinical trials with classical AMPA and NMDA glutamate receptor antagonists have provided negative results.69–71 The main factors that are hypothesized to cause this failure are:72–74
Quality of the molecules (pharmacokinetic deficiencies, inability to reach effective concentrations in the penumbra, shot neuroprotective time window, inappropriate receptor subunit selectivity, high drug toxicity in humans.75
Inequivalent doses compared to rodents75
Development of tolerance,12 for example, upregulation of NMDAr
Side effects, among others, blocking of normal synaptic NMDA activity that promotes neuronal survival76 and blocking of neurogenesis at different stages of recovery.75,77,78
Administration of NMDAr antagonists at a critical period after brain trauma exacerbates brain damage78
Bad design of clinical trials
It is important to recognize that the relationship of the dose to produce inhibition of SD in humans is still unknown for those substances, and the effect on SD was not monitored in those studies. At that time, there was no certain evidence that SDs occurred in humans.
Therapeutic use of NMDA receptor antagonists might be a balancing act. It is known that NMDA receptor play a role in the recovery and neuroplasticity after brain injury.79–84 For example, >40 mg/kg/h s-ketamine inhibited ischemia-induced-neurogenesis,85 but the doses tested in that laboratory study in rodents are approximately 10 times the magnitude of the doses used in humans. Long time therapy and high doses of NMDA receptor blocking may at some point interfere with the recovery of brain functions. For example, NMDA receptor agonist in a late phase after stroke facilitated recovery in rats.79
One possible approach to modulating the NMDA receptor-mediated synaptic transmission in pathological conditions is to do so without altering the physiological excitatory transmission. For instance, Ifenprodil and its analogs block NMDA receptors in a voltage-independent manner without causing a significant reduction in the agonist potency. Ifenprodil’s pharmacological profile includes the ability to increase the potency of ambient protons to block the NMDA receptors.86 Since ischemic tissue is characterized by a reduction of pH (at approximately 6.5),87 it has been hypothesized that because Ifenprodil acts on the proton sensors, it may represent a means of optimizing the design of a new class of neuroprotectants that would target the NMDA receptor only in the pathological condition but not in physiological conditions. This is just one example of potential “loopholes” in the problem of the NMDA receptor.
A total of 42 articles that described tests of NMDA receptor agents were identified. These articles examined 24 NMDA receptor antagonists with respect to their efficacy in modulating or possibly even blocking the initiation, propagation, velocity, threshold, amplitude, and duration of spreading depolarizations (Table 2). The following NMDA receptor antagonists among others have received scrutiny: ketamine, Mk-801, phencyclidin, memantine, Glyx-13, NVP-AAM077, TCN 201, and Ro 25-698. Although most of these substances have been proven to modulate some of the characteristics of SD, only few can inhibit the induction of SD, for instance ketamine and MK-801.
Table 2.
NMDA receptor antagonists.
| Results |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Reference | Species | Type | SD induction | Dosage | Number | Amplitude | Propagation | Threshold | Duration | Frequency |
| Main NMDA receptor antagonists (commonly used, well known substances) | |||||||||||
| Ketamine | Hernándéz-Cáceres et al.40 | Rat | In vivo | CH3CO2K | 6.25/12.5/25/50/100 mg/kg/day ip. | ↓ | = | ↓ | |||
| Ketamine | Gorelova et al.164 | Rat | In vivo | CH3CO2K | 50 mg/kg ip. | ↓ | |||||
| Ketamine | Marrannes et al.63 | Rat | In vivo | Electrical | 10/40/80 mg/kg/day ip. | ↓ | = | ↓ | ↑ | ↓ | |
| Ketamine | Amemori and Bures12 | Rat | In vivo | CH3CO2K | 200 mg/kg ip. + 3 × 100 mg/kg/h | ↓ | ↑ | ||||
| Ketamine | Verhaegen et al.118 | Rat | In vivo | Electrical | 50 mg/kg iv. | ↓ | ↑ | ||||
| Ketamine | Martin et al.88 | Rat | In vivo | Electrical | 50 mg/kg ip. | ↓ | ↓ | ||||
| Ketamine | Rashidy-Pour et al.62 | Rat | In vivo | KCl | 5 × 50 mg/kg ip. | ↓ | ↓ | ↓ | |||
| Ketamine | Krüger et al.89 | Rat | In vitro | KCl | 100 µM | ↓ | ↓ | ↓ | |||
| Ketamine | Sakowitz et al.66 | Humans | Case report | Brain injury | 2–3 mg/kg/h iv. | ↓ | |||||
| Ketamine | Hertle et al.41 | Humans | Retrospective analysis | Brain injury | 200 mg | ↓ | |||||
| Ketamine | Sanchez-Porrás et al.90 | Swine | In vivo | KCl | 2/4 mg/kg/h iv. | ↓ | ↓ | ↓ | ↓ | ||
| Ketamine | Schiefecker et al.68 | Humans | Case report | ICH | 100 mg/h iv. | ↓ | |||||
| Ketamine | Hertle et al.67 | Humans | Retrospective analysis | Brain injury | 100–300 mg/h iv. | ↓ | |||||
| MK-801 | Lauritzen and Hansen91 | Rat | In vivo | Electrical | 3/12 mg/kg | ↓ | = | ↑ | |||
| MK-801 | Nellgard and Wieloch92 | Rat | In vivo | Mechanical | Injection of 0.1 mg/kg ip. | ↓ | |||||
| MK-801 | Gill et al.93 | Rat | In vivo | MCAO | Injection of 3 mg/kg ip. | ↓ | ↓ | ↓ | |||
| MK-801 | Willette et al.94 | Rat | In vivo | KCl | Injection of 0.3/1/3 mg/kg iv. | ↓ | ↓ | ||||
| MK-801 | Rashidy-Pour et al.62 | Rat | In vivo | KCl | Injection of 2.5 mg/kg ip. | ↓ | ↓ | ||||
| MK-801 | Obrenovitch and Zilkha95 | Rat | In vivo | K+ | Injection of 1 mg/kg iv. | ↓ | ↓ | ||||
| MK-801 | Miettinen et al.96 | Rat | In vivo | KCl | Injection of 3 mg/kg ip. | ↓ | ↓ | ||||
| MK-801 | Marrannes et al.97 | Rat | In vivo | Electrical | 3.1 mg/kg | ↓ | = | ↓ | ↑ | ||
| MK-801 | Koroleva et al.98 | Rat | In vivo | MCAO | Injection of 0.5 mg/kg ip. | ↓ | |||||
| MK-801 | van der Hel et al.99 | Rat | In vivo | KCl | Injection of 3 mg/kg iv. | ↓ | = | = | ↓ | ||
| MK-801 | Kunimatsu et al.100 | Rat | In vivo | BCAO | Injection of 2 mg/kg ip. | ↓ | |||||
| MK-801 | Anderson and Andrew101 | Rat | In vitro | KCl | 100 μM | ↓ | |||||
| MK-801 | Peeters et al.102 | Rat | In vivo | KCl | 2 mg/kg ip. | ↓ | ↓ | ||||
| MK-801 | Richter et al.103 | Rat | In vivo | KCl | 3 mg/kg | ↓ | |||||
| MK-801 | Dhir et al.104 | Mice | In vivo | KCl | 0.5/2 mg/kg ip. | ↓ | = | ||||
| MK-801 | Wang et al.105 | Chicken | In vitro | KCl | Local application of 10 μmol/L | ↓ | ↓ | ↓ | |||
| MK-801 | Richter et al.106 | Rat | In vitro | KCl | 3 mg/kg ip. | ↓ | |||||
| MK-801 | Oláh et al.107 | Rat | In vivo | KCl | 200 mg/kg ip. | ↓ | ↓ | ||||
| MK-801 | Shatillo et al.108 | Rat | In vivo | KCl | 10 mg/kg ip. | ↓ | |||||
| MK-801 | Bu et al.109 | Rat | In vivo | K+ | 3/10/30 μmol/L via microdialysis | ↓ | ↓ | ||||
| MK-801 | Srienc et al.110 | Rat | In vitro | Photothrombosis | 3 mg/kg | ↓ | ↓ | ↓ | |||
| Memantine | Peeters et al.102 | Rat | In vivo | KCl | 1/3/10 mg/kg ip. | ↓ | |||||
| Memantine | Santos et al., 2017 (unpublished data) | Swine | In vivo | KCl | 1.5 mg/kg iv. | = | ↓ | ↓ | |||
| Memantine | Srienc et al.110 | Rat | In vivo | Photothrombosis | 10 mg/kg | = | |||||
| Magnesium | van der Hel et al.99 | Rat | In vivo | KCl | 90 mg/kg iv. | ↓ | = | = | ↓ | ||
| Magnesium | Rodrigues et al.111 | Chicken retina | In vitro | Mechanical | 1–4 mM superfusion | ↓ | |||||
| Magnesium | Van Harreveld112 | Chicken retina | In vitro | KCl | 10 mM | ↓ | |||||
| Magnesium | Shibata and Bures113 | Rat | In vivo | KCl | 10% MgCl2 | ↓ | |||||
| Magnesium | Santos et al.114 | Swine | In vivo | KCl | 20 mmol/L local apllic., 40 ml iv. | ↓ | ↓ | ↓ | |||
| Further NMDA receptor antagonists (less known substances) | |||||||||||
| Phencyclidine | Marrannes et al.97 | Rat | In vivo | Electrical | 10 mg/kg/day ip. | = | ↓ | ↑ | |||
| 2-APH | Marrannes et al.97 | Rat | In vivo | Electrical | 10/40 mg/kg/day ip. | ↓ | ↓ | ↑ | |||
| 2-APH | Rashidy-Pour et al.62 | Rat | In vivo | KCl | 2.5 mg/kg ip. | ↓ | |||||
| 2-APH | Lauritzen and Hansen91 | Rat | In vivo | Electrical | 4.5 mg/10 mg/kg | ↓ | |||||
| AP5 | McLachlan165 | Rat | In vivo | KCl | 500 µM | ↓ | |||||
| AP5 | Rashidy-Pour et al.62 | Rat | In vivo | KCl | 10−3 mol/L | . | |||||
| AP5 | Anderson and Andrew101 | Rat | In vitro | KCl | 50/100 μM | ↓ | |||||
| AP5 | Martens-Mantai et al.115 | Rat | In vitro | KCl | 50 μmol/L | ↓ | |||||
| CGS 19755 | Nellgard and Wieloch92 | Rat | In vivo | Mechanical | 0.75 mg/kg ip. | ↓ | |||||
| CGP 40116 | Nellgard and Wieloch92 | Rat | In vivo | Mechanical | 0.25 mg/kg ip. | ↓ | |||||
| CGP 43487 | Nellgard and Wieloch92 | Rat | In vivo | Mechanical | 1.5 mg/kg ip. | ↓ | |||||
| ACEA 1021 | Martin et al.88 | Rat | In vivo | Electrical | 12/40/80/mg/kg/day ip. | = | ↓ | ||||
| ZD9379 | Tatlisumak et al.116 | Rat | In vivo | MCAO | 5 mg/kg bolus + 5 mg/kg/h iv. | ↓ | |||||
| L-707, 324 | Obrenovitch and Zilkha95 | Rat | In vivo | Potassium | 5/10 mg/kg iv. | ↓ | ↓ | ||||
| Glyx-13 | Zhang et al.148 | Rat | In vitro | K+ | Bath application of 1/10/50 μM | = | ↓ | ||||
| KYNA | Oláh et al.107 | Rat | In vivo | KCl | 300 mg/kg ip. | ↓ | = | ||||
| KYNA | Chauvel et al.166 | Chicken retina | In vivo | KCl | 300 mg/kg ip. | = | ↓ | ||||
| KYNA | Chauvel et al.150 | Rat | In vivo | KCl | 300 mg/kg ip. | ↓ | |||||
| KYNA | Anderson and Andrew101 | Chicken retina | In vitro | KCl | 2 mM | ↓ | |||||
| NVP-AAM007 | Wang et al.105 | Chicken retina | In vitro | KCl | Local application of 0.03/0.1/0.3 μmol/L | ↓ | ↓ | ↓ | |||
| NVP-AAM007 | Bu et al.109 | Rat | In vivo | K+ | 0.3/1/3 μmol/L via microdialysis | ↓ | ↓ | ||||
| NVP-AAM007 | Bu et al.109 | Rat | In vitro | K+ | 0.3/1/3 μmol/L | ↓ | ↓ | ||||
| TCN-2001 | Shatillo et al.108 | Rat | In vivo | KCl | 10 mg/kg ip. | = | |||||
| TCN-2001 | Bu et al.109 | Chicken retina | In vitro | K+ | 1/3/9 μmol/L | ↓ | ↓ | ||||
| Ifenprodil | Shatillo et al.108 | Chicken retina | In vivo | KCl | 10 mg/kg ip. | ↓ | |||||
| Ro 25-6981 | Wang et al.105 | Chicken retina | In vitro | KCl | Local application of 1/3/10 μmol/L | ↓ | = | ||||
| Ro 25-6981 | Peeters et al.102 | Adult rat | In vivo | KCl | 1/3/10 mg/kg ip. | ↓ | |||||
| CP-101,606 | Wang et al.105 | Chicken retina | In vitro | KCl | Local application of 1/3/10 μmol/L | = | = | = | |||
| CP-101,606 | Peeters et al.102 | Adult rat | In vivo | KCl | 1/3/10 mg/kg ip. | ↓ | |||||
| CP-101,606 | Menniti et al.117 | Rat | In vivo | Electrical | 1/3.2/10 mg/kg iv. | ↓ | ↓ | ↓ | |||
| UBP141 | Wang et al.105 | Chicken retina | In vitro | KCl | Local application of 1/3/10 μmol/L | = | = | = | |||
| 3PPP | Anderson and Andrew101 | Rat | In vitro | KCl | 100 μM | = | |||||
| BD-1063 | Anderson and Andrew101 | Rat | In vitro | KCl | 100 μM | = | |||||
| Loperamid | Anderson and Andrew101 | Rat | In vitro | KCl | 100 μM | = | |||||
| Spiperone | Anderson and Andrew101 | Rat | In vitro | KCl | 100 μM | ↓ | |||||
| 4-IBP | Anderson and Andrew101 | Rat | In vitro | KCl | 30 μM | ↓ | |||||
Table 2 provides the insight that NMDA receptor is a key contributor to the propagation and initiation of spreading depolarizations and hence a potent target in the treatment of SDs. At the same time, NMDAr antagonism has so far not been successfully translated into clinical neuroprotection. More precisely, the table reveals that the number of SD is the most successful target (as evidenced in 36 articles),12,40,41,62,63,66–68,88–117 whereas an inhibitory effect on amplitude has only been described in nine articles.62,89,90,93,105,107,109,110,114 The threshold has only been under scrutiny in experiments, in which SD is electrically induced and was successfully increased in all of them.63,91,97,118
Ketamine
Resting on the premise that ketamine non-competitively blocks the NMDA receptor and thus restricts the perimembranous cation flow thereby influencing SD, many randomized blinded experiments in vivo and in vitro have been conducted that have successfully demonstrated ketamine’s potency. Effective dosages to affect SD incidence range from 2 mg/kg/h to 200 mg/kg/h.12,66
Marrannes et al. demonstrated that ketamine causes a significant dose-dependent reduction of electrically induced SD in alfentanil-anesthetized adult rats. At a dose of 40 mg/kg, ketamine increased the SD threshold, decreased the propagation velocity, and decreased the duration of the accompanying extracellular DC, K+, and Ca2+ changes. At 80 mg/kg, the elicitation of SD was completely inhibited.63 Amemori and Bures found that ketamine at a dose of 100 mg/kg blocked the occurrence of SD in rats, but the blockade induced by subsequent ketamine injections weakened and finally disappeared.12 Rashidy-Pour et al.62 observed a similar outcome. Ketamine at 50 mg/kg indeed blocked the elicitation of SD. The blockade by the first ketamine injection lasted for 30–45 min. The blocking effect of subsequent injections gradually declined and was not recognizable after a fifth ketamine injection.62 Krüger et al.89 studied the effect of 100 µM ketamine on the characteristics of a KCl-induced SD in parietal cortical slices of adult rats. He ascertained that ketamine significantly reduced the amplitude of the first SD peak and blocked the second SD peak when compared with the controls.89 Hernándéz-Cáceres et al.40 examined the ketamine-induced blockade of SD in pentobarbital-anesthetized rats and presented evidence that ketamine prevented the propagation of SD at 12 mg/kg and at higher doses. The blockade was maximal 20 min after the injection.40 Our group, Sanchéz-Porras et al. used a gyrencephalic swine model to examine ketamine’s effects against SDs. In this swine model, an intensive-medicine setting is recreated in which the animal is monitored for up to 30 h. The major results were that s-ketamine at the human equivalent maximum dose of 2 mg/kg/h decreased the KCl-induced SD spreading and had an effect on the amplitude of SD deflections, as well as on the duration and speed. Moreover, during infusion of this dose of ketamine, there was a sustained decrease in the hemodynamic response following SD. However, only at 4 mg/kg/h of ketamine could the SD induction and expansion be completely inhibited.90 In another experimental setting, we found ketamine’s influence on the vasculature during SD. We observed a decrease of contractility during oligemia but not under hyperemia.119
The experiments and clinical trials involving humans are particularly relevant. Kaube et al.120 assumed that SD is pathophysiologically relevant for the genesis of the auras of migraines and thus investigated the question whether the aura experienced by some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. In 5/11 patients, ketamine reproducibly reduced the severity and duration of the auras.120 Hertle et al.41 documented an association between the relative β-frequency and SD. The relative β-frequency was suppressed up to 2 h prior to SD when compared to periods that were not followed by SD. An inverse correlation of the administration of ketamine with the occurrence of spreading depolarizations has been noted.41 Case reports document the effect of ketamine in two patients with traumatic brain injury and aneurysmal SAH (aSAH),66 as well as a patient with perihematomal edema.68 Another case report described a patient with aSAH who displayed a cluster of SDs under ketamine. The patient subsequently developed severe delayed ischemic strokes and died.27 Most recently, our research group, Santos et al. described a suppressive effect of S-ketamine on SD in patients with aSAH (Santos et al., unpublished data). Sixty-six aSAH patients were prospectively monitored, including ECoG. We retrospectively compared relevant collected variables of patients who received ketamine at any time (n=33) vs. no-ketamine. A multivariable analysis including Poisson, negative binomial, and linear mixed models were performed to show the effect of ketamine on SD incidence and characteristics. On patient level, the mean dose of 2.81 mg/kg/h ketamine started at a mean of 4.6 days after ictus for a mean of 8.1 days was not enough to show significant differences between groups in the total monitoring time of 17 days. But upon analyzing hourly data and considering when ketamine was given or not, we found a clear effect of SD incidence reduction and changes in its electrical characteristics. Doses above the recommended therapeutic range (>2 mg/kg/h) were more effective than therapeutic doses in SAH patients. A reduction of efficacy over the monitoring days in patients was not documented. In order to reach neuroprotection, our results favor a patient individualized ketamine schema with soon start of ketamine and adaptation of the dose to the patient’s conditions, timing after ictus and to the detection of SDs.
Memantine
Memantine is an uncompetitive NMDA receptor antagonist that has been clinically approved for the treatment of Alzheimer’s symptoms.121–123 This drug is already used as a migraine-preventive drug in clinical studies, and the results have been promising.124,125 Currently, positive effects of memantine on cognition in demented patients have been obtained.126 Specifically, it has the potential to improve neuronal plasticity and learning in old animals127 and an ability to enhance learning in rats with learning deficits caused by entorhinal cortex lesions.128 Moreover, it has been observed that memantine reduced the frequency of auras as well as headache in migraneurs, which also suggests an association with SDs.124 Memantine’s pharmacological profile suggests that it has the capacity to block excessive activation of NMDA receptors without affecting normal signaling by the receptor and thus better preserves a critical balance.129 Memantine’s potential to modulate SD has until now only been subject of few experiments (Table 2). Experiments in an in vitro chicken retina model showed a concentration-dependent inhibition of NMDA-evoked SD. A dosage of 12.67 ± 0.99 μM was required to achieve an inhibition of 50% of SD.121 Moreover, memantine showed a significant dose-dependent reduction of the number and amplitude of SD in rats at a dose of 10 mg/kg.102 However, its scientific status is equivocal. Srienc et al. tested memantine in rats in which the retinal vessels had been occluded by photothrombosis and observed no significant effect, but there was a trend towards a reduction of incidence.110 Recently, our group (Santos et al.) tested memantine at a dose of 1.5 mg/kg against KCl-induced SD in a gyrencephalic porcine model. An analysis using ECoG and IOS revealed that memantine applied within the therapeutic range had no suppressive effect on SD. Nevertheless, the amplitude and duration were reduced after the eighth stimulation, at which time the memantine blood concentrations were 200 to 300% of the therapeutic range. A possible reason for these observations might be that the increased potassium concentration of the 11 mM preconditioning reduced the efficacy of NMDA receptor antagonists to suppress SD. In vivo experiments in rats suggest that an increased extracellular K+ concentration reduce the efficacy of NMDA receptor antagonists to suppress SDs.130
MK-801
MK-801 is a well-characterized, potent, and selective NMDA receptor antagonist that has been tested for the suppression of cortical and retinal SD in various in vivo and in vitro experiments on rats, cats, and chickens (Table 2). A complete blockade of the elicitation of SDs is in the range of 2–3 mg/kg,62,63,91,100,131 but experiments such as those conducted by Nellgard and Wieloch showed that even smaller dosages such 0.10 mg/kg inhibited mechanically elicited SD.92 A potency of MK-801 has also been observed by numerous other investigators.93,95,98,108
Although it is a sufficiently potent inhibitor of SD that it is often used as a positive control in experiments, MK-801 has various reported side effects: MK-801 induces marked regional alterations in the local cerebral glucose utilization in rats,132 and a dose of 0.2 mg/kg has been reported to be sufficient to alter object recognition memory.133 Recently, repetitive MK-801 administration has also been documented to induce structural changes that resemble schizophrenia and a dementia-like degeneration in the rat brain.134 At a dose of 0.1 mg/kg, MK-801 also exhibits anxiolytic and antinociceptive effects in primates,135 which raises the question whether it must be considered for further studies.
Magnesium
Magnesium’s multifaceted pharmacological profile includes neuroprotection. Experiments in rodents have shown that the infarct size after MCAO can be reduced by an application of magnesium.136,137 Despite these promising results, clinical randomized controlled trials in which magnesium was used as an intervention in acute stroke demonstrated neither neuroprotection nor reduced death or disability.138 Recently, Yamamoto et al. investigated a potentially preventive effect of continuous cisternal irrigation with MgSO4 on the cerebral vasospasms associated with SAH in a randomized controlled trial but found no protective effect on delayed cerebral ischemia nor on the clinical outcome.139
Neurophysiologically, magnesium has versatile effects including the inhibition of intracellular Ca2+ influx and blocking the NMDA-activated channels.140 Magnesium’s neuroprotective properties and physiological profile provide a rationale for various trials to examine its potential to inhibit SD (Table 2).
Shibata and Bures showed magnesium’s potential to inhibit KCl-induced reverberating SD in rats.113 van der Hel et al.99 similarly observed a significant reduction of the frequency, a delay of the latency and a significant blockade of the generation of KCl-induced SD in rats at 90 mg/kg. Magnesium’s inhibitory potential was also observed in in vitro chicken models.111,112 More recently, our group, Santos et al. investigated magnesium’s effect against SD in the gyrencephalic swine model. A local administration and an intravenous bolus of MgSO4 were tested. Local application of a dose of 10 mmol/LMgSO4 significantly reduced the amplitude of the oligemic response of SD. In contrast, an intravenous application did not alter SD, which indicates that the blood–brain permeability, high renal elimination, and low bioavailability need to be considered when examining magnesium’s therapeutic potential against SD.114 The same principle can be applied for most therapeutic agents.
Further noncompetitive NMDA receptor antagonists: Phencyclidine, 2-APH, AP5, CGS 19755, CGP 40116, CGP 43487, ACEA 1021, ZD9379, L-707, 324, Glyx-13, KYNA, NVP-AAM007, TCN-2001, ifenprodil, Ro 25-6981, CP-101,606, UBP141, 3PPP, BD-1063, loperamid, spiperone, and 4-IBP.
Various non-competitive NMDA receptor antagonists have been tested against SD. Some will be presented in detail in this section. For more details, see Table 2.
Lauritzen and Hansen91 and Marrannes et al.63 observed that DL-2-amino-7-phosphonoheptanoic acid suppressed the incidence of electrically induced SD in rats at a dose of 10 mg/kg, whereas a much higher dose of 160 mg/kg of 2-APH is required to reach complete suppression.
The competitive NMDA-receptor antagonists CGS 19755 (cis-4-phosphonomethyl-2-piperidine carboxylate), CGP 40116 (D-(E)-2-amino-4-methyl-5-phosphono-3-pentenoic acid), and its carboxylester CGP 43487 have been shown to inhibit the elicitation of mechanically induced SD in the rat cortex at doses of 0.75 mg kg−1, 0.25 mg kg−1, and 1.50 mg kg−1, respectively.92
Since the NMDA receptor channel complex contains a glycine recognition site that must be occupied for activation, it can be hypothesized that antagonism of the glycine site might counteract SD. ACEA 1021 (5-nitro-6,7-dichloro-1,4-dihydro-2,3-quinoxalinedione) minimized cerebral infarct volumes.141,142 Martin et al.88 examined ACEA-1021 for its influence on the threshold and propagation rate of electrically induced SD. Although the threshold was unaffected, a dose-dependent deceleration of SD was noted. The elicitation of SD was not inhibited by ACEA 1021 at any dose.88
ZD9379 is a soluble, potent, bioavailable full antagonist at the glycine site.116 It reduced the number of SDs and the infarct size in rats with a permanent MCAO at a dose of 5 mg/kg according to Tatlisumak et al.143
L-707, 324 is a high affinity antagonist at the glycine site of the NMDA receptor that has shown in vivo potency against seizures.144 Its effectiveness against SD has been investigated by Obrenovitch and Zilkha.95 A dose of 10 mg/kg was required to block the induction of SD, whereas 5 mg/kg was sufficient to completely inhibit propagation, but the effect on SD was rather moderate compared to that of a classical inhibitor as MK-801.
Glyx-13 is an NMDA-receptor modulator with glycine-site partial agonist properties that recently has been shown to produce rapid antidepressant responses.145 Its physiological profile permits Glyx-13 to act as an agonist of the NMDA receptor in the absence of saturating D-serine while acting as an antagonist at high concentrations of D-serine. During SD, which elicits the release of high levels of glutamate and D-serine, GLYX-13 is likely to act as an antagonist that would prevent the over-activation of NDMA receptors.146,147 Its interaction with SD has only recently came under the spotlight. Glyx-13 has been observed to increase the refractory period of hippocampal rat SD, to limit the propagation of SD, and to reduce the amplitude of the negative field potential shift and restored the dendritic spines.148 Since Glyx-13 is not an NMDA receptor antagonist, but rather an allosteric modulator, therapeutic application could be facilitated because the side-effects of NMDA receptor channel blockers would be avoided.
Kynurenines, and particularly the endogenous kynurenic acid, exhibit a strong modulatory potential on the neuronal structures in the brainstem, which may play a crucial role in the pathogenesis of migraine.149 Kynurenic acid suppresses SD107,150 and the precursor L-kynurenin also suppresses SD waves and reduced c-fos immunoreactivity and neuronal nitric oxide synthase, which are associated with SD as well as with migraines.150–153 A therapeutic use is improbable because Kynurenic acid facilitates pathological pathways154 and is involved in the development of manic or psychotic symptoms.155
GluN2A, GluN2B, and GluN2C/2D: Specific NMDA antagonists against SD
The GluN2A-selective NMDA receptor antagonist NVP-AAM077 reduced the amplitude and propagation rate of KCl-induced SDs in chicken retina,105,109 30-fold more potent than MK-801. To a slightly lesser extent, the GluN2A-specific antagonist TCN2001 also reduced the amplitude and deaccelerated SD in the chicken retina.109 Contrasting results for TCN-201 in chick retina were found by Shatillo et al., who used BOLD fMRI to examine the drug’s effect against SD but found no inhibitory effect.108
Ro 25-698, a GluN2B-selective receptor antagonist, reduced the amplitude to 51.1% of the initial values at a concentration of 10 µmol/L105 in chicken retina, and similar inhibitory potential was documented in KCl-induced SD in rats.102
CP-101,606, a GluN2B-selective receptor antagonist, prevents the death of rat hippocampal neurons156 and reduces the size of infarcts caused by subdural hematoma in rats.157 Nevertheless, CP-101,606 was ineffective against SD in Wang et al.’s chicken retina model.105 Different results were obtained by Peeteres et al. who described a dose-dependent reduction in the SD numbers and amplitude.102 Similarly, Menniti et al.117 observed that CP-101,606 inhibited SD generation at a dose of 2.25 mg/kg bolus + 2.25 mg/kg/h intravenous infusion. Additionally, the amplitude and propagation velocity were also decreased in a dose-dependent manner.117
Sigma site antagonists/agonists against SD
Sigma receptors can be found throughout the body and CNS,158,159 and the evidence suggests that sigma ligands are associated with neuroprotection.160–162 The exact role of sigma receptors in the pathogenesis of SD is yet to be elucidated.
We reviewed five sigma site-specific NMDA receptor antagonists BD-1063, 3PPP, 4-IBP, carbetapentane, and dextromethorphan.
Anderson and Andrew tested carbetapentane and dextromethorphan against KCl-induced SD in rat brain slices. Both drugs at a dose of 100 µM blocked the generation of SD and prevented the tissue swelling that usually follows SD.101 Moreover, Anderson and Andrew examined 4-IBP, a ςR agonist that has only insignificant cross reactivity at the NMDA receptor sites compared to other sigma agonists.163 4-IBP showed a blocking effect against KCl-induced SD in rodents at a dose of 100 μM, but did not prevent the secondary swelling.101 Another ςR agonist that has an inhibitory effect against SD is SK&F 10047, which showed a dose-dependent inhibition of the incidence of KCl-induced SD in rats.94 In contrast, the ς1 R antagonists BD-1063 and (+)-3-PPP had no inhibitory effects on the KCl-induced SD in rat brain slices.101
Anesthetic, sedative, hypnotic, and analgesic agents
SD susceptibility is modulated by general anesthetics.118,167–171 An anesthetic agent that combines effectiveness against SD and clinical applicability is sevoflurane. To our knowledge, sevoflurane has been tested against KCl-induced SD only by Kitahara et al.168 in rats. A dose-dependent reduction in the frequency and a dose-dependent increase in the DC current have been observed, whereas the number, amplitude, and duration of SD seemed to be unaffected.168
Isoflurane has been examined in six studies with ambiguous results. Similar to most volatile anesthetics, isoflurane acts via various mechanisms and affects different channels and receptors at various levels of the brain. Muscle relaxation is likely induced by isoflurane’s potentiation of glycine receptor activity. Moreover, it antagonizes NMDA receptor and affects the calcium ATPase, ATP synthase, and GABA receptors. Importantly, isoflurane has various adverse effects, such as hypotonia and cardiodepression. Additionally, isoflurane has been associated with neurodegeneration, promotion of apoptosis, and an increase of the amyloid beta protein levels that are associated with Alzheimer’s disease.172 Currently, volatile alternatives as sevoflurane are preferred over isoflurane for clinical use. A protective effect of isoflurane against the initiation of SD has been described in various experiments.168,169,173,174 Specifically, there is evidence for isoflurane’s potential to suppress the SD frequency168,173 and to reduce the propagation speed,173 whereas the amplitude of SD seems to be unaffected by this agent.168
The exact mechanisms by which anesthetics inhibit are yet to be clarified, but may involve their ability to partially antagonize the NMDA receptor. For instance, isoflurane has been associated with a reduction of neuronal depolarization as a reaction to a glutamate and NMDA application175 and can even reduce the mean open time of the NMDA channel.176
Further anesthetic agents that have been tested against SDs but were proven to be either ineffective or only to have a modulatory effect at doses that could never be applied in humans include dexmedetomidine,173,177 benzocaine,181 debucain,178 lidocaine,179–181 midazolam,41 equithesin,182 and thionembutal.167
Table 3 provides the insight that most anesthetic agents exert little or no influence against SD; hence, future investigation should focus on more promising substances. Table 3 shows that none of the potential characteristics of SD (number, amplitude, duration, frequency, and propagation) is a successful target for anesthetic substances. Although three articles show some inhibition against the number of SD,104,169,174 the majority describes no inhibition,41,167–169,171,179,182,183 or even an increased amplitude after drug administration.41 Even more ambiguous results are observed for the effect on frequency. While three articles describe an inhibition,167,168,173 two describe an increase.168,184 Duration168,173,184 has not been affected by any tested anesthetic drug.
Table 3.
Review of the effect of analgesic, sedative, and hypnotic agents against SDs.
| Anesthetic agents |
Results |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Reference | Species | Type | SD induction | Dosage | Number | Amplitude | Propagation | Threshold | Duration | Frequency |
| Isoflurane | Kudo et al.173 | Rat | In vivo | KCl | 0.7 MAC | ↓ | ↓ | ||||
| Isoflurane | Kudo et al.184 | Rat | In vivo | KCl | 1% | = | = | = | |||
| Isoflurane | Kitahara et al.168 | Rat | In vivo | KCl | 0.5/1/2.0 MAC | = | = | = | ↓ | ||
| Isoflurane | Piper and Lambert169 | Cat | In vivo | Mechanical | 15–30% | ↓ | |||||
| Isoflurane | Takagaki et al.174 | Rat | In vivo | Kcl, MCAO | 1 MAC | ↓ | |||||
| Isoflurane | Verhaegen et al.118 | Rat | In vivo | Electrical | 1 MAC | = | = | ||||
| Sevoflurane | Kitahara et al.168 | Rat | In vivo | KCl | 0.5/1/2.0 MAC | = | = | = | ↓ | ||
| Urethane | Kudo et al.185 | Rat | In vivo | KCl | 1.7 ± 0.2 g/kg/h | = | = | ↑ | |||
| Urethane | de Souza et al.184 | Rat | In vivo | KCl | 1.0 g/kg | = | |||||
| Urethane | Guedes and Barreto167 | Rat | In vivo | KCl | 1.0 g/kg | = | ↓ | ||||
| Halothane | Kitahara et al.168 | Rat | In vivo | KCl | 1 MAC | = | = | = | ↑ | ||
| Halothane | Piper and Lambert169 | Cat | In vivo | Mechanical | 60 mg/kg ip. | ↓ | |||||
| Halothane | Verhaegen et al.118 | Rat | In vivo | Electrical | 1 MAC | = | = | ||||
| Halothane | Saito et al.171 | Cat | In vivo | KCl | 60 mg/kg iv. | ↓ | |||||
| α-chloralose | Kudo et al.185 | Rat | In vivo | KCl | 87 ± 31 mg/kg/h | = | = | = | |||
| α-chloralose | Piper and Lambert169 | Cat | In vivo | Mechanical | 60 mg/kg ip. | = | |||||
| α-chloralose | Saito et al.171 | Cat | In vivo | KCl | 60 mg/kg iv. | = | |||||
| α-chloralose | Guedes and Barreto167 | Rat | In vivo | KCl | 40 mg/kg | = | ↓ | ||||
| Pentobarbital | Kudo et al.173 | Rat | In vivo | KCl | 0.7 MAC | = | = | ||||
| Pentobarbital | Kitahara et al.168 | Rat | In vivo | KCl | 1 MAC | = | = | = | ↑ | ||
| Morphine | Hertle et al.41 | Human | Retrospective analysis | Brain injury | 8 mg median drug dose | = | |||||
| Fentanyl | Hertle et al.41 | Human | Retrospective analysis | Brain injury | 0.15 mg median drug dose | = | |||||
| Sufentanil | Hertle et al.41 | Human | Retrospective analysis | Brain injury | 0.06 mg median drug dose | = | |||||
| Propofol | Kudo et al.173 | Cat | In vivo | KCl | 0.7 MAC | = | = | ||||
| Propofol | Dhir et al.104 | Mice | In vivo | KCl | 120/200 mg/kg ip. | ↓ | ↓ | ↓ | |||
| Propofol | Hertle et al.41 | Humans | Retrospective analysis | Brain injury | 150 mg median drug dose | = | |||||
| Propofol | Kudo et al.173 | Cat | In vivo | KCl | 0.7 MAC | = | = | ||||
| Midazolam | Hertle et al.41 | Humans | retrospective analysis | Brain injury | 22.3 mg median drug dose | ↑ | |||||
| Debucain | Risher et al.178 | Human /mice | In vitro/vivo | Photothrombosis | 1 µM | = | ↑ | ||||
| Lidocain | Ayad et al.180 | Rabbit | In vivo | Ischemia | 0.2 mg/kg/min | ↓ | ↓ | ↑ | |||
| Lidocain | Kaube and Goadsby179 | Cat | In vivo | Mechanical | 5 mg/kg iv. | = | = | = | |||
| Equithesin | Sonn and Mayevsky182 | Rat | In vivo | KCl | 0.3 ml/100 g ip. injection | = | = | ↓ | |||
| Thionembutal | Guedes and Barreto167 | Rat | In vivo | KCl | 40 mg/kg | = | ↓ | ||||
| Dexmedetonidine | Kudo et al.173 | Rat | In vivo | KCl | 0.7 MAC | ↓ | ↓ | ||||
“↓” Means a reductive effect was observed after drug administration, “=” means no effect was noticed, and “↑” means that the tested parameter was increased by drug. Typical parameters under investigation are number, amplitude, propagation, threshold, duration, and frequency. Propofol, isoflurane, and lidocaine show strong inhibitory effect while fentanyl and morphine for example exert no effect. Experimental settings and models are heterogeneous, comprising different animals (chicken, rat, rabbit, and swine) and various forms of SD induction (KCl and electrical stimulation).
Anti-migraine drugs
There is evidence that SD plays a causative role in all migraine types, including migraine without aura.185 First, the phenomenological resemblance (e.g., velocity, hyperexcitability and electrocorticogram suppression) between SDs and the scintillating phenomenon that can be observed during migraine supports that SD is the electrophysiological mechanism for the migraine aura.186 Second, chronic administration of anti-migraine drugs has been shown to have an inhibitory effect on SD.50 Third imaging studies of migraine with aura.45,46,187,188 The resulting hypothesis that SD suppression may be a function of anti-migraine drugs has been fueled by new discoveries. In particular, the anti-migraine effect of vagus stimulation (that has been successfully applied against migraine189–191) has recently been tested in the context of SD by Chen et al.58 He observed an inhibitory effect of noninvasive as well as direct stimulation against KCl-induced SD in rats.58
In contrast, SD-blocking substances have come under scrutiny for a potential anti-migraine effect. Ketamine, a proven blocker of SD, has been shown to stop the neurological aura symptoms in some patients but had no effect on the headache.120
Many migraine drugs from different pharmacological classes have been tested against SD so far,50,97,102,110,179,185,192–207 and topiramate and flunarizine were most effective since they exerted an inhibitory effect in all of the revised studies.
Valproate
Valproate was originally used as an anticonvulsant and has a multifaceted action spectrum: it inhibits voltage-dependent sodium channels and T-type calcium currents, augments the action of glutamic acid decarboxylase, and modulates the extracellular signal-regulated kinase pathway.208 Evidence supports its efficacy in migraine prevention and acute migraine therapy.209 Approximately one to three months of valproate or topiramate treatment additionally suppress cortical hyperexcitability in migraineurs.210–212 Currently, valproate is a promising substance for the therapy of SD. A recent study by Ayata et al.50 investigated the efficiency of topiramate, valproate, propranolol, amitriptyline, and methysergide against SD. Chronic daily application of these agents correlated with a dose-dependent deceleration of SD by 40% to 80% and a reduction of susceptibility whereas a single dose was ineffective.50 Further studies support the idea that chronical application is effective,185,202 while some groups report no effect at all.179
Tonabersat
Tonabersat is a novel putative migraine prophylactic agent with a unique stereospecific binding site in the brain. In animal models, tonabersat has shown an inhibitory potential against SD and cerebrovascular responses to trigeminal nerve stimulation.213 With respect to its efficacy in humans, tonabersat failed to significantly reduce the number of headache days in migraineurs when compared to placebo, but it is usually well tolerated.214 With respect to its effect against SD, we found positive preliminary results, showing a potential to reduce the number of SD,192–194 to decelerate SD192,194 and to modulate hemodynamic response.193
Topiramate
Topiramate is generally used as an anticonvulsant for epilepsy. Pharmacologically, topiramate positively modulates the GABAA receptors. GABAA receptors are pentameric ligand-gated ion channels that are involved in neuropathic pain and migraine among other effects and consequentially constitute a therapeutic target.215–218 In regard to efficacy against SD, there are promising results.
Ayata et al.50 showed that 60 and 80 mg/kg/day of topiramate reduced the number of SDs by 30% and 50%, respectively, whereas 40 mg/kg/day was ineffective. Furthermore, an almost complete abolishment of SD was observed after 17 weeks of topiramate treatment, whereas 1 week of treatment even at a high dose (80 mg/kg/day) had little effect, which suggests that a sustained treatment is necessary for a significant suppression.50 Moreover, a suppressive effect on SD frequency and propagation196 and a modulatory effect on hemodynamic response195 have been reported.
Other modulators of the GABAA receptor, such as TPA023, NS11394, and SL651498, have been documented to exert some inhibition against SD in an in vitro chicken model,219 suggesting that GABAA receptors, especially the α2 subtype, might be a responsive therapeutic target.
Flunarizine
Flunarizine is a large hydrophobic fluorinated piperazine derivative that is used in the prophylaxis of migraine.220–224 Flunarizine possesses neuronal calcium channel blocking activity.225 In contrast to other Ca2+-entry blockers, flunarizine does not modify the myogenic activity of vascular smooth muscle.198 This particularity is important because it implies that flunarizine can render cells unresponsive to vasoconstrictive stimuli, without interfering with the normal control of tissue perfusion.198 Evidence also exists for its efficacy against SD. Certain investigators only observed Flunarizine’s effects on hemodynamic response of SDs, but not on the characteristics of SDs,200,226 while other investigators observed Flunarizine’s effect on hemodynamic response as well as SD characteristics.97,199
Flunarizine’s suppressive effect on the number of SDs might originate from a blockade of L-, N-, and P/Q-type voltage-gated Ca2+ channels227 and flunarizine’s shortening effect on duration may be attributed to its inhibitory effect on the cortical hypoperfusion induced by SD.226
Sumatriptan
Sumatriptan is effective in migraine by acting on the serotonin system. Its effects are mediated through vasoconstriction and blockade of neurologic inflammation. Few experiments on sumatriptan’s inhibition of SD have been performed. A dose-dependent reduction of the numbers and amplitude and a deceleration of KCl-induced SDs in isolated chicken retinas at a dosage from 0.05 to 2.00 mM205,228 was observed in the late 1990s, while more recently published studies support these observations.110,204 However, the scientific status of this agent is ambivalent, and some studies observed no reductive effect on SD.194,203,229
Additional anti-migraine drugs that were effective against SDs are lamotrigine,185 riboflavin,185 methylsergide,50,205 amitryptoline,50 and propranolol,50,205,206 but only a few experiments exist. Various anti-migraine agents have shown no effects against SD. Among these are dihydroergotamine,179 ergotamine,205 clonidine,205 lisuride,205 iprazochrome,205 isoprenaline,207 and amylnitrite.207
Table 4 provides the insight that, to date, a variety of in vivo and in vitro models suggest that prophylactic drugs are effective against SD if applied chronically over a long period of time.179,193,205 These substances need to be tested in adequate dosages and in further settings. Table 4 shows that the most successful target of anti-migraine drugs is SD number,50,110,192–195,197–199,204,205 while amplitude and propagation show ambiguous results. Especially, propagation is reported to be increased by certain anti-migraine drugs.192,194
Table 4.
Review of the effect of anti-migraine drugs against SDs.
| Anti-migraine drugs |
Results |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Reference | Species | Type | SD induction | Dosage | Number | Amplitude | Propagation | Threshold | Duration | Frequency |
| Tonabersat | Read et al.192 | Cat | In vivo | KCl | 3–10 mg/kg/day ip. | ↓ | ↑ | ↓ | |||
| Tonabersat | Smith et al.193 | Cat | In vivo | KCl | 3–10 mg/kg/day ip. | ↓ | |||||
| Tonabersat | Bradley et al.194 | Cat | In vivo | KCl | 10 mg/kg/day ip. | ↓ | ↑ | ↓ | |||
| Tonabersat | Read et al.192 | Rat | In vivo | KCl | 10 mg/kg/day ip. | ↓ | |||||
| Topiramate | Akerman and Goadsby195 | Cat, rat | In vivo | Mechanical | 30 mg/kg/day iv. | ↓ | = | ||||
| Topiramate | Unekawa et al.196 | Rat | In vivo | KCl | 50, 100, 200, or 600 mg/kg ip. | ↓ | ↑ | ||||
| Topiramate | Ayata et al.50 | Rat | In vivo | Electrical, KCl | 40–80 mg/kg/day | ↓ | = | ↓ | ↑ | = | |
| Topiramate | Tozzi et al.197 | Rat | In vitro | K+ | 100 μM | ↓ | |||||
| Flunarizine | Marrannes et al.97 | Rat | In vivo | Electrical | 20–40 mg/kg/day ip.; per os 3 × 20 mg/kg/day | = | = | = | ↓ | ||
| Flunarizine | Marrannes et al.97 | Rat | In vivo | Electrical, KCl | 10–20 mg/kg/day ip.; per os 20 mg/kg/day | ||||||
| Flunarizine | Hansen and Lauritzen37 | Rat | In vivo | Mechanical | Per os 20 mg/kg/day | = | = | ||||
| Flunarizine | Wauquier et al.198 | Rat | In vivo | Mechanical | 40 mg/kg/day ip. | ↓ | |||||
| Flunarizine | Li et al.199 | Rat | In vivo | KCl | 3 mg/kg ip. | ↓ | ↓ | ↑ | ↓ | ||
| Flunarizine | Ashton et al.200 | Guinea pig | In vitro | Electrical | 40 mg/kg × 2 per os | ↓ | |||||
| Valproate | Kaube and Goadsby179 | Cat | In vivo | Mechanical | 3.5–7 mg/kg/day iv. | = | = | = | |||
| Valproate | Peeters et al.102 | Rat | In vivo | KCl | 200 mg/kg ip. | = | = | ||||
| Valproate | Tepe et al.201 | Rat | In vivo | KCl | 75 mg/kg ip. | = | = | ||||
| Valproate | Bogdanov et al.185 | Rat | In vivo | KCl | 200 mg/kg/day ip. | ↓ | ↓ | ||||
| Valproate | Hoffmann et al.202 | Rat | In vivo | Electrical, KCl | 200 mg/kg/day ip./iv. | ↑ | |||||
| Valproate | Ayata et al.50 | Rat | In vivo | Electrical, KCl | 25/50/100/200 mg/kg/day ip. | ↓ | = | ↓ | ↑ | = | |
| Sumatriptan | Bradley et al.194 | Cat | In vivo | KCl | 0.3 mg/kg iv. | = | ↑ | = | |||
| Sumatriptan | Read et al.192 | Rat | In vivo | KCl | 0.3 mg/kg iv. | = | |||||
| Sumatriptan | Moskowitz et al.203 | Rat | In vivo | KCl | 0.3 mg/kg iv. | = | |||||
| Sumatriptan | Srienc et al.110 | Rat retina | In vitro | Photothrombosis | 3 mg/kg iv. | ↓ | = | ↓ | |||
| Sumatriptan | Knapp et al.204 | Rat | In vivo | KCl | 0.6 mg/kg ip. | ↓ | |||||
| Sumatriptan | Wiedemann et al.205 | Chicken | In vitro | KCl | 1.5 mM | ↓ | ↓ | ↓ | |||
| Dihydroergotamine | Kaube and Goadsby179 | Cat | In vivo | Mechanical | 15 mg/kg iv. | = | = | ||||
| Ergotamine | Wiedemann et al.205 | Chicken | In vivo | KCl | 10–20 µM | = | = | ||||
| Lamotrigine | Bogdanov et al.185 | Rat | In vivo | KCl | 15 mg/kg/day ip. | ↓ | = | ||||
| Riboflavin | Bogdanov et al.185 | Rat | In vivo | KCl | 20 mg/kg/day ip. | ↓ | = | ||||
| Propranolol | Ayata et al.50 | Rat | In vivo | Electrical, KCl | 20 mg/kg/day ip. | = | = | = | = | = | |
| Propranolol | Ayata et al.50 | Rat | In vivo | Electrical, KCl | 20 mg/kg/day ip. | ↓ | = | ↓ | = | = | |
| Propranolol | Richter et al.206 | Rat | In vivo | Mechanical | Topical application of 250 – l µmol/L to 1 mmol/L | = | = | ↓ | = | ||
| Propranolol | Peeters et al.102 | Adult rat | In vivo | KCl | Ip injection of 20 mg/kg ip. | = | |||||
| Propranolol | Wiedemann et al.205 | Chicken | In vitro | KCl | 500 µmol | ↓ | ↓ | ↓ | |||
| Methylsergide | Ayata et al.50 | Rat | In vivo | Electrical, KCl | Ip injection of 0.1 and 1 mg/kg/day ip. | ↓ | = | = | = | = | |
| Methylsergide | Wiedemann et al.205 | Chicken | In vitro | KCl | 100 µmol | ↓ | ↓ | ↓ | |||
| Amitryptolin | Ayata et al.50 | Rat | In vivo | Electrical, KCl | Ip injection of 10/20 mg/kg/day ip. | ↓ | = | = | = | = | |
| Clonidin | Wiedemann et al.205 | Chicken | In vitro | KCl | 100–500 µM | = | = | = | |||
| Lisuride | Wiedemann et al.205 | Chicken | In vitro | KCl | 100–200 nM | = | = | = | |||
| Iprazochrome | Wiedemann et al.205 | Chicken | In vitro | KCl | 100–200 µM | = | = | = | |||
| Isoprenalin | Kaube et al.207 | Cat | In vivo | Transection | Topical application of 0.1/1% | = | = | ||||
| Amylnitrite | Kaube et al.207 | Cat | In vivo | Transection | Topical application of 0.05% | = | = | ||||
“↓” Means a reductive effect was observed after drug administration, “=” means no effect was noticed, and “↑” means that the tested parameter was increased by drug. Typical parameters under investigation are number, amplitude, propagation, threshold, duration, and frequency. Most substances exert an inhibitory effect on the number of SDs. Only few inhibit more than one variable, for instance, flunarizine, sumatriptan, and propranolol. Experimental settings and models are heterogeneous, comprising different animals (chicken, rat, and cat) and various forms of SD induction (KCl and electrical stimulation).
Further agents tested against SD
In addition to the substances discussed above, we reviewed 54 other articles and identified 60 more substances that had been tested against SDs. Among them are AMPA receptor antagonists, ion channel blockers, cannabinoid receptor agonists, and various other agents such as garlic extract and shrimp carotenoid. The diversity of anti-SD substances underlines the complexity of SD and indicates that more research is necessary.
Table 5 shows that some of the substances that are less known for an inhibitory effect against SD (naloxone,230,231 GYKI 52466,232,233 sulpiride,234 and THC235) show potential to reduce SD number, propagation, and duration and must not be forgotten.
Table 5.
Review of the further substances that have been tested against SDs.
| Results |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Reference | Species | Type | SD induction | Dosage | Number | Amplitude | Propagation | Threshold | Duration | Frequency | IOS area | Pial diameter |
| Further substances that have been tested against SD | |||||||||||||
| Lithium | de Aguiar et al.236 | Rat | In vivo | KCl | 50 mg/kg ip. | ↓ | |||||||
| Quinpirole | Haarmann et al.234 | Rat | In vitro | KCl | 10–200 µmol/l | ↑ | = | ↑ | |||||
| Sulpiride | Haarmann et al.234 | Rat | In vitro | KCl | 0.1–10 µmol/l | ↓ | = | ↓ | |||||
| BIBN4096BS | Tozzi et al.197 | Rat | In vitro | K+ | 0.01–1 μM | ↓ | |||||||
| CGRP 8-37 | Tozzi et al.197 | Rat | In vitro | K+ | 3/10 μM | ↓ | |||||||
| CGRP 8-37 | Colonna et al.237 | Rabbit | In vivo | KCl | Topical 12.8 μM | ↓ | |||||||
| CGRP 8-37 | Wahl et al.238 | Cat | In vivo | KCl | 5 × 10−9−10−6 M | ↓ | |||||||
| CGRP 8-37 | Reuter et al.239 | Rat | In vivo | KCl | (5 × 10−7 M) | ↓ | |||||||
| MK-8825 | Tozzi et al.197 | Rat | In vitro | K+ | 0.1–10 μM | ↓ | |||||||
| SNC80 | Pradhan et al.240 | Mice | In vivo | KCl | Ip. injection of 10 mg/kg | ↓ | |||||||
| Naloxone | Guedes et al.230 | Rat | In vivo | KCl | 10 mg/kg/day | ↓ | |||||||
| Naloxone | Rocha-de-Melo et al.231 | Rat | In vivo | KCl | 10 mg/kg/day sc. | ↓ | |||||||
| SL651498 | Wang et al.219 | Chicken retina | In vitro | KCl | 10 µmol/L local application | ↓ | ↓ | ↓ | |||||
| Tpa023 | Wang et al.219 | Chicken retina | In vitro | KCl | 50 µmol/L local application | ↓ | ↓ | ↓ | |||||
| NS11394 | Wang et al.219 | Chicken retina | In vitro | KCl | 3 µmol L−1 local application | = | = | = | |||||
| Bicuculline | Martens-Mantai et al.115 | Rat | In vitro | KCl | 10 µmol/L local application | ↓ | |||||||
| NBQX | Krüger et al.89 | Rat | In vitro | KCl | 10 µM | = | = | ||||||
| NBQX | Kertész et al.232 | Chicken retina | In vitro | Kainat | Up to 10 µM | ↓ | ↑ | ||||||
| NBQX | Lauritzen and Hansen91 | Rat | In vivo | Electrical | 10/20 mg/kg | = | = | = | |||||
| NBQX | Kunimatsu et al.100 | Rat | In vivo | BCAO | 30 mg/kg ip. | = | |||||||
| NBQX | Nellgard and Wieloch92 | Rat | In vivo | Mechanical | 10 mg/30 mg ip. | = | |||||||
| NBQX | Gressens et al.233 | Chicken retina | In vitro | AMPA, MCAO | 3 × 30 mg/kg ip. | ↑ | |||||||
| CNQX | Anderson and Andrew101 | Rat | In vitro | KCl | 10 μM | = | = | = | |||||
| CNQX | Martens-Mantai et al.115 | Rat | In vitro | KCl | 10 µmol/L local application | ↑ | |||||||
| GYKI 52466 | Kertész et al.232 | Chicken retina | In vitro | Kainat | 20 μM | ↓ | ↑ | ||||||
| GYKI 52466 | Gressens et al.233 | Chicken retina | In vitro | AMPA | 1/3/10 mg/kg ip. | ↑ | |||||||
| GYKI 53655 | Kertész et al.232 | Chicken retina | In vitro | Kainate | 20 μM | ↓ | ↑ | ||||||
| EGIS 8332 | Gressens et al.233 | Chicken retina | In vitro | AMPA, MCAO | 1/3/10 mg/kg ip. | ↑ | |||||||
| EGIS 1068 | Gressens et al.233 | Chicken retina | In vitro | AMPA, MCAO | 1/3/10 mg/kg ip. | ↑ | |||||||
| WIN 55212-2 | Martens-Mantai et al.115 | Rat | In vitro | KCl | 5 µmol/L local application | ↑ | |||||||
| THC | Kazemi et al.235 | Rat | In vitro | KCl | 1–20 μM | ↓ | ↓ | ↓ | |||||
| WIN 55212-2 | Kazemi et al.235 | Rat | In vitro | KCl | 1–10 μM | ↓ | ↓ | ↓ | |||||
| JWH-13 | Kazemi et al.235 | Rat | In vitro | KCl | 1–20 μM | = | = | = | |||||
| 8-OH-DPAT | Krüger et al.89 | Adult rat | In vivo | KCl | 10/100 μM ip. | ↓ | |||||||
| Metoprolol | Kaube and Goadsby179 | Cat | In vivo | Mechanical | 25 mg/kg/day iv. | = | = | = | |||||
| Metoprolol | Alemdar et al.241 | Rat | In vivo | KCl | 5 mg/kg infusion | = | = | ||||||
| Isoprenaline | Kaube et al.207 | Cat | In vivo | Mechanical | 0.1–1% local application | = | = | ||||||
| TTX | Ashton et al.200 | Guinea pig | In vitro | Electrodal | 1.25 × 10−6 M | ↑ | |||||||
| TTX | Sheardown242 | Chicken retina | In vitro | NMDA, kainate | 0.1 μM | = | |||||||
| TTX | Tobiasz and Nicholson243 | Rat | In vivo | KCl | 10–5 M | = | |||||||
| TTX | Aitken et al.244 | Rat | In vitro | Hypoxia | 1 μM | ↓ | ↑ | ||||||
| TTX | Müller and Somjen245 | Rat | In vitro | Hypoxia | 1 μM | ↑ | |||||||
| TTX | Akerman et al.246 | Rat | In vivo | Mechanical | 10 µg/kg | ↓ | |||||||
| TTX | Tozzi et al.197 | Rat | In vitro | K+ | 1 μM | ↓ | |||||||
| ω-Conotoxin-GVIa | Akerman et al.246 | Cat | In vivo | Mechanical | 20 µg/kg ip. | = | = | ||||||
| ω-Conotoxin-GVIa | Richter et al.227 | Rat | In vivo | KCl, mechanical | 10−6 M | ↓ | = | ||||||
| calciseptine | Akerman et al.246 | Cat | In vivo | Mechanical | = | = | |||||||
| Cadmium chloride | Akerman et al.246 | Cat | In vivo | Mechanical | = | = | |||||||
| ω-agatoxin | Richter et al.227 | Rat | In vivo | KCl, mechanical | 10−6 M | = | = | ||||||
| Nimodipine | Richter et al.227 | Rat | In vivo | KCl, mechanical | 10−5 M | = | = | ||||||
| Glibenclamide | Akerman et al.246 | Cat | In vivo | Mechanical | 30 mg/kg ip. | = | = | ||||||
| NG-Nitro-l-Arginine | Wahl et al.238 | Cat | KCl | In vivo | 10−4 M | = | |||||||
| Zaprinast | Wang et al.247 | Rat | Electrical | In vivo | 300 μM | ↓ | |||||||
| Sildenafil | Wang et al.247 | Rat | Electrical | In vivo | 300 μM | = | |||||||
| Amylnitrite | Kaube et al.207 | Cat | Transection | In vivo | 0.05% topical | = | = | ||||||
| Isoprenaline | Kaube et al.207 | Cat | Transection | In vivo | 0.1–1% topical | = | = | ||||||
| shrimp carotinoid | Bezerra Rde et al.248 | Rat | Ethanol | In vivo | 30 µg/kg/day | ↓ | |||||||
| SC-560 | Varga et al.249 | Rat | CAO, KCl | In vivo | 25 µM | = | |||||||
| SC-560 | Gariepy et al.250 | Rat | Mechanical | In vivo | 500 µM | = | |||||||
| NS-398 | Varga et al.249 | Rat | CAO, KCl | In vivo | 100 µM | = | |||||||
| NS-398 | Gariepy et al.250 | Rat | Mechanical | In vivo | 1 mM | = | |||||||
| L161,982 | Varga et al.249 | Rat | CAO, KCl | In vivo | 1 µM | ↓ | |||||||
| Naproxen | Gariepy et al.250 | Rat | Mechanical | In vivo | 100 µM | = | |||||||
| Ozagrel | Gariepy et al.250 | Rat | Mechanical | In vivo | 1 mM | = | |||||||
| PEA | Richter et al.251 | Rat | KCl | In vivo | 20 mg/kg body weight | = | = | = | = | ||||
| Garlic extract | Marschollek et al.252 | Rat | KCl | In vivo; in vitro | 1 ml/L; 500 µL/L | ↓ | = | = | |||||
| Caffeine | de Aguiar et al.253 | Rat | In vivo | KCl | 30 mg/kg ip. | = | |||||||
| Caffeine | de Aguiar et al.253 | Rat | In vivo | KCl | 30 mg/kg ip. | = | |||||||
| Gangliosides | Fernandes de Lima et al.254 | Chicken retina | In vitro | Mechanical | 20 µM | ↓ | ↓ | ||||||
| Yohimbine | Richter et al.206 | Rat | In vivo | Mechanical | 1.75 mmol/L | ↓ | = | ||||||
| Clonidin | Richter et al.206 | Rat | In vivo | Mechanical | 0.56 mmol/L | = | = | ↓ | = | ||||
| Norepinephrine | Richter et al.206 | Rat | In vivo | Mechanical | 1 mmol/L | = | = | ↓ | = | ||||
| TNF | Richter et al.255 | Rat, mouse | In vivo | KCl | 0.05/5 ng | ↓ | ↓ | ↓ | |||||
| Furosemide | Read et al.256 | Cat | In vivo | KCl | 0.2/2/20 g/kg iv. | ↓ | |||||||
| IGF-1 | Grinberg et al.257 | Rat | In vitro | Electrical | 40/100 ng/mL | ↑ | |||||||
| INFγ | Pusic and Kraig258 | Rat | In vitro | KCL | 50,000 U nasally | ↑ | |||||||
| Dimethylsulfoxide | Sun et al.259 | Rat | In vivo | KCl | 0.1/0.4/2/4% iv. | ↓ | |||||||
| Propylthiouracil | Guedes and Pereira-da-Silva260 | Rat | In vivo | KCl | 8 mg/kg ip. | ↓ | |||||||
| Pilocarpine | Guedes and de Vasconcelos261 | Rat | In vivo | KCl | 45/95/190 mg/kg ip. | ↓ | ↓ | ||||||
| Pilocarpine | De Vasconcelos et al.262 | Mouse | In vivo | KCl | 190 mg/kg ip. | ↓ | ↓ | ||||||
| Fluoxetine | Costa Monteiro et al.263 | Rat | In vivo | KCl | 10 mg/kg/day per os | ↓ | |||||||
| Fluoxetine | dos Santos et al.264 | Rat | In vivo | KCl | 5/10/20/40 mg/kg/day | ↓ | |||||||
| Citalopram | Guedes et al.265 | Mouse | In vivo | KCl | 20 mg/kg ip. | ↓ | |||||||
| TPEA | Dietz et al.266 | Mouse | In vitro | Ouabain | 50 µM | ↓ | ↑ | ||||||
| BAPTA | Dietz et al.266 | Mouse | In vitro | Ouabain | 1 mM | ↓ | ↑ | ||||||
“↓” Means a reductive effect was observed after drug administration, “=” means no effect was noticed, and “↑” means that the tested parameter was increased by drug. Typical parameters under investigation are number, amplitude, propagation, threshold, duration, frequency, IOS area, and pial diameter. Most substances show little or no effect. Experimental settings and models are heterogeneous, comprising different animals (chicken, rat, mouse, and cat) and various forms of SD induction (KCl and electrical stimulation).
Conclusions
The most effective group of drugs that are effective to block SD incidence and characteristics are NMDAr antagonists. Still, a refinement of the glutamate receptor antagonist therapy is necessary, including more subtype selectivity or a plural inhibition of glutamate excitotoxicity.
Neuroprotection using SD as a target must be scrutinized in more realistic scenarios, rather than animal models that do not translate to the human gyrencephalic brain, because neither of the strategies tested using them could be translated into the clinic. We have to consider not interfering with neuronal survival and neurogenesis, important factors in the rehabilitation of the patients.
Different from other neuroprotective targets, targeting SD can be finely adjusted and individualized, because we have the possibility to measure SDs in real time using ECoG.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Edgar Santos was supported by the Postdoc Fellowship Program of the Faculty of Medicine Heidelberg.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Woitzik J, Hecht N, Pinczolits A, et al. Propagation of cortical spreading depolarization in the human cortex after malignant stroke. Neurology 2013; 80: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 2.Lauritzen M, Dreier JP, Fabricius M, et al. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab 2011; 31: 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab 2017; 37: 1595–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartings JA, Shuttleworth CW, Kirov SA, et al. The continuum of spreading depolarizations in acute cortical lesion development: examining Leao's legacy. J Cereb Blood Flow Metab 2017; 37: 1571–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horiguchi T, Snipes JA, Kis B, et al. The role of nitric oxide in the development of cortical spreading depression-induced tolerance to transient focal cerebral ischemia in rats. Brain Res 2005; 1039: 84–89. [DOI] [PubMed] [Google Scholar]
- 6.Kawahara N, Ruetzler CA, Klatzo I. Protective effect of spreading depression against neuronal damage following cardiac arrest cerebral ischaemia. Neurol Res 1995; 17: 9–16. [DOI] [PubMed] [Google Scholar]
- 7.Kiss C, Shepard PD, Bari F, et al. Cortical spreading depression augments kynurenate levels and reduces malonate toxicity in the rat cortex. Brain Res 2004; 1002: 129–135. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi S, Harris VA, Welsh FA. Spreading depression induces tolerance of cortical neurons to ischemia in rat brain. J Cereb Blood Flow Metab 1995; 15: 721–727. [DOI] [PubMed] [Google Scholar]
- 9.Matsushima K, Hogan MJ, Hakim AM. Cortical spreading depression protects against subsequent focal cerebral ischemia in rats. J Cereb Blood Flow Metab 1996; 16: 221–226. [DOI] [PubMed] [Google Scholar]
- 10.Otori T, Greenberg JH, Welsh FA. Cortical spreading depression causes a long-lasting decrease in cerebral blood flow and induces tolerance to permanent focal ischemia in rat brain. J Cereb Blood Flow Metab 2003; 23: 43–50. [DOI] [PubMed] [Google Scholar]
- 11.Shen P, Hou S, Zhu M, et al. Cortical spreading depression preconditioning mediates neuroprotection against ischemic stroke by inducing AMP-activated protein kinase-dependent autophagy in a rat cerebral ischemic/reperfusion injury model. J Neurochem 2017; 140: 799–813. [DOI] [PubMed] [Google Scholar]
- 12.Amemori T, Bures J. Ketamine blockade of spreading depression: rapid development of tolerance. Brain Res 1990; 519: 351–354. [DOI] [PubMed] [Google Scholar]
- 13.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011; 17: 439–447. [DOI] [PubMed] [Google Scholar]
- 14.Ayata C, Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev 2015; 95: 953–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev 2001; 81: 1065–1096. [DOI] [PubMed] [Google Scholar]
- 16.Pietrobon D, Moskowitz MA. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci 2014; 15: 379–393. [DOI] [PubMed] [Google Scholar]
- 17.Chebabo SR, Hester MA, Aitken PG, et al. Hypotonic exposure enhances synaptic transmission and triggers spreading depression in rat hippocampal tissue slices. Brain Res 1995; 695: 203–216. [DOI] [PubMed] [Google Scholar]
- 18.Dreier JP, Kleeberg J, Alam M, et al. Endothelin-1-induced spreading depression in rats is associated with a microarea of selective neuronal necrosis. Exp Biol Med (Maywood) 2007; 232: 204–213. [PubMed] [Google Scholar]
- 19.Shapiro BE. Osmotic forces and gap junctions in spreading depression: a computational model. J Comput Neurosci 2001; 10: 99–120. [DOI] [PubMed] [Google Scholar]
- 20.Busija DW, Bari F, Domoki F, et al. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog Neurobiol 2008; 86: 379–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busija DW, Bari F, Domoki F, et al. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res Rev 2007; 56: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura H, Strong AJ, Dohmen C, et al. Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain 2010; 133: 1994–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauritzen M. Cortical spreading depression in migraine. Cephalalgia 2001; 21: 757–760. [DOI] [PubMed] [Google Scholar]
- 24.Fabricius M, Fuhr S, Bhatia R, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain 2006; 129: 778–790. [DOI] [PubMed] [Google Scholar]
- 25.Gorji A. Spreading depression: a review of the clinical relevance. Brain Res Brain Res Rev 2001; 38: 33–60. [DOI] [PubMed] [Google Scholar]
- 26.Hartings JA, Watanabe T, Bullock MR, et al. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain 2011; 134: 1529–1540. [DOI] [PubMed] [Google Scholar]
- 27.Dreier JP, Major S, Manning A, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 2009; 132: 1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain 2006; 129: 3224–3237. [DOI] [PubMed] [Google Scholar]
- 29.Woitzik J, Dreier JP, Hecht N, et al. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2012; 32: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dreier JP, Ebert N, Priller J, et al. Products of hemolysis in the subarachnoid space inducing spreading ischemia in the cortex and focal necrosis in rats: a model for delayed ischemic neurological deficits after subarachnoid hemorrhage? J Neurosurg 2000; 93: 658–666. [DOI] [PubMed] [Google Scholar]
- 31.Koide M, Sukhotinsky I, Ayata C, et al. Subarachnoid hemorrhage, spreading depolarizations and impaired neurovascular coupling. Stroke Res Treat 2013; 2013: 819340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Z, Sanchéz-Porras R, Santos E, et al. Delayed cerebral ischemia after subarachnoid hemorrhage: from vascular spasm to cortical spreading depolarizations. Curr Neurovasc Res 2012; 9: 310–319. [DOI] [PubMed] [Google Scholar]
- 33.Hartings JA, Bullock MR, Okonkwo DO, et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol 2011; 10: 1058–1064. [DOI] [PubMed] [Google Scholar]
- 34.Hartings JA, Strong AJ, Fabricius M, et al. Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma 2009; 26: 1857–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinzman JM, Andaluz N, Shutter LA, et al. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain 2014; 137: 2960–2972. [DOI] [PubMed] [Google Scholar]
- 36.Dohmen C, Sakowitz OW, Fabricius M, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol 2008; 63: 720–728. [DOI] [PubMed] [Google Scholar]
- 37.Hansen AJ, Lauritzen M. The role of spreading depression in acute brain disorders. An Acad Bras Cienc 1984; 56: 457–479. [PubMed] [Google Scholar]
- 38.Strong AJ, Fabricius M, Boutelle MG, et al. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke 2002; 33: 2738–2743. [DOI] [PubMed] [Google Scholar]
- 39.von Bornstadt D, Houben T, Seidel JL, et al. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron 2015; 85: 1117–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernándéz-Cáceres J, Macias-Gonzalez R, Brozek G, et al. Systemic ketamine blocks cortical spreading depression but does not delay the onset of terminal anoxic depolarization in rats. Brain Res 1987; 437: 360–364. [DOI] [PubMed] [Google Scholar]
- 41.Hertle DN, Dreier JP, Woitzik J, et al. Effect of analgesics and sedatives on the occurrence of spreading depolarizations accompanying acute brain injury. Brain 2012; 135: 2390–2398. [DOI] [PubMed] [Google Scholar]
- 42.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014; 85: 660–667. [DOI] [PubMed] [Google Scholar]
- 43.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 44.Helbok R, Schiefecker AJ, Friberg C, et al. Spreading depolarizations in patients with spontaneous intracerebral hemorrhage: association with perihematomal edema progression. J Cereb Blood Flow Metab 2017; 37: 1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 2001; 98: 4687–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Y, Welch KM, Aurora S, et al. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol 1999; 56: 548–554. [DOI] [PubMed] [Google Scholar]
- 47.Eising E, Shyti R, t Hoen PAC, et al. Cortical spreading depression causes unique dysregulation of inflammatory pathways in a transgenic mouse model of migraine. Mol Neurobiol 2017; 54: 2986–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eikermann-Haerter K, Dilekoz E, Kudo C, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest 2009; 119: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eikermann-Haerter K, Baum MJ, Ferrari MD, et al. Androgenic suppression of spreading depression in familial hemiplegic migraine type 1 mutant mice. Ann Neurol 2009; 66: 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayata C, Jin H, Kudo C, et al. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 2006; 59: 652–661. [DOI] [PubMed] [Google Scholar]
- 51.Tottene A, Conti R, Fabbro A, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron 2009; 61: 762–773. [DOI] [PubMed] [Google Scholar]
- 52.Dreier JP, Tille K, Dirnagl U. Partial antagonistic effect of adenosine on inverse coupling between spreading neuronal activation and cerebral blood flow in rats. Neurocrit Care 2004; 1: 85–94. [DOI] [PubMed] [Google Scholar]
- 53.Nedergaard M, Astrup J. Infarct rim: effect of hyperglycemia on direct current potential and [14C]2-deoxyglucose phosphorylation. J Cereb Blood Flow Metab 1986; 6: 607–615. [DOI] [PubMed] [Google Scholar]
- 54.Strong AJ, Smith SE, Whittington DJ, et al. Factors influencing the frequency of fluorescence transients as markers of peri-infarct depolarizations in focal cerebral ischemia. Stroke 2000; 31: 214–222. [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann U, Sukhotinsky I, Eikermann-Haerter K, et al. Glucose modulation of spreading depression susceptibility. J Cereb Blood Flow Metab 2013; 33: 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin HK, Dunn AK, Jones PB, et al. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain 2007; 130: 1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin HK, Oka F, Kim JH, et al. Endothelial dysfunction abrogates the efficacy of normobaric hyperoxia in stroke. J Neurosci 2014; 34: 15200–15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SP, Ay I, de Morais AL, et al. Vagus nerve stimulation inhibits cortical spreading depression. Pain 2016; 157: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chazot PL, Coleman SK, Cik M, et al. Molecular characterization of N-methyl-D-aspartate receptors expressed in mammalian cells yields evidence for the coexistence of three subunit types within a discrete receptor molecule. J Biol Chem 1994; 269: 24403–24409. [PubMed] [Google Scholar]
- 60.Kutsuwada T, Kashiwabuchi N, Mori H, et al. Molecular diversity of the NMDA receptor channel. Nature 1992; 358: 36–41. [DOI] [PubMed] [Google Scholar]
- 61.Monaghan DT, Olverman HJ, Nguyen L, et al. Two classes of N-methyl-D-aspartate recognition sites: differential distribution and differential regulation by glycine. Proc Natl Acad Sci U S A 1988; 85: 9836–9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rashidy-Pour A, Motaghed-Larijani Z, Bures J. Tolerance to ketamine-induced blockade of cortical spreading depression transfers to MK-801 but not to AP5 in rats. Brain Res 1995; 693: 64–69. [DOI] [PubMed] [Google Scholar]
- 63.Marrannes R, Willems R, De Prins E, et al. Evidence for a role of the N-methyl-D-aspartate (NMDA) receptor in cortical spreading depression in the rat. Brain Res 1988; 457: 226–240. [DOI] [PubMed] [Google Scholar]
- 64.Bullock R, Kuroda Y, Teasdale GM, et al. Prevention of post-traumatic excitotoxic brain damage with NMDA antagonist drugs: a new strategy for the nineties. Acta Neurochir Suppl 1992; 55: 49–55. [DOI] [PubMed] [Google Scholar]
- 65.McCulloch J. Glutamate receptor antagonists in cerebral ischaemia. J Neural Transm Suppl 1993; 43: 71–79. [PubMed] [Google Scholar]
- 66.Sakowitz OW, Kiening KL, Krajewski KL, et al. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke 2009; 40: e519–522. [DOI] [PubMed] [Google Scholar]
- 67.Hertle DN, Heer M, Santos E, et al. Changes in electrocorticographic beta frequency components precede spreading depolarization in patients with acute brain injury. Clin Neurophysiol 2016; 127: 2661–2667. [DOI] [PubMed] [Google Scholar]
- 68.Schiefecker AJ, Beer R, Pfausler B, et al. Clusters of cortical spreading depolarizations in a patient with intracerebral hemorrhage: a multimodal neuromonitoring study. Neurocrit Care 2015; 22: 293–298. [DOI] [PubMed] [Google Scholar]
- 69.Plum F. Neuroprotection in acute ischemic stroke. JAMA 2001; 285: 1760–1761. [DOI] [PubMed] [Google Scholar]
- 70.De Keyser J, Sulter G, Luiten PG. Clinical trials with neuroprotective drugs in acute ischaemic stroke: are we doing the right thing? Trends Neurosci 1999; 22: 535–540. [DOI] [PubMed] [Google Scholar]
- 71.Martinez-Vila E, Sieira PI. Current status and perspectives of neuroprotection in ischemic stroke treatment. Cerebrovasc Dis 2001; 11 Suppl 1: 60–70. [DOI] [PubMed] [Google Scholar]
- 72.Lees KR. Cerestat and other NMDA antagonists in ischemic stroke. Neurology 1997; 49: S66–69. [DOI] [PubMed] [Google Scholar]
- 73.Albers GW, Atkinson RP, Kelley RE, et al. Safety, tolerability, and pharmacokinetics of the N-methyl-D-aspartate antagonist dextrorphan in patients with acute stroke. Dextrorphan Study Group. Stroke 1995; 26: 254–258. [DOI] [PubMed] [Google Scholar]
- 74.Sanchez-Porras R, Zheng Z, Sakowitz OW. Pharmacological modulation of spreading depolarizations. Acta Neurochir Suppl 2015; 120: 153–157. [DOI] [PubMed] [Google Scholar]
- 75.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol 2002; 1: 383–386. [DOI] [PubMed] [Google Scholar]
- 76.Jiang X, Tian F, Mearow K, et al. The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem 2005; 94: 713–722. [DOI] [PubMed] [Google Scholar]
- 77.Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999; 283: 70–74. [DOI] [PubMed] [Google Scholar]
- 78.Ikonomidou C, Stefovska V, Turski L. Neuronal death enhanced by N-methyl-D-aspartate antagonists. Proc Natl Acad Sci U S A 2000; 97: 12885–12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhawan J, Benveniste H, Luo Z, et al. A new look at glutamate and ischemia: NMDA agonist improves long-term functional outcome in a rat model of stroke. Future Neurol 2011; 6: 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roman R, Bartkowski H, Simon R. The specific NMDA receptor antagonist AP-7 attenuates focal ischemic brain injury. Neurosci Lett 1989; 104: 19–24. [DOI] [PubMed] [Google Scholar]
- 81.Simon RP, Swan JH, Griffiths T, et al. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science 1984; 226: 850–852. [DOI] [PubMed] [Google Scholar]
- 82.Gotti B, Duverger D, Bertin J, et al. Ifenprodil and SL 82.0715 as cerebral anti-ischemic agents. I. Evidence for efficacy in models of focal cerebral ischemia. J Pharmacol Exp Ther 1988; 247: 1211–1221. [PubMed] [Google Scholar]
- 83.Park CK, Nehls DG, Graham DI, et al. The glutamate antagonist MK-801 reduces focal ischemic brain damage in the rat. Ann Neurol 1988; 24: 543–551. [DOI] [PubMed] [Google Scholar]
- 84.Yu G, Wu F, Wang ES. BQ-869, a novel NMDA receptor antagonist, protects against excitotoxicity and attenuates cerebral ischemic injury in stroke. Int J Clin Exp Pathol 2015; 8: 1213–1225. [PMC free article] [PubMed] [Google Scholar]
- 85.Winkelheide U, Lasarzik I, Kaeppel B, et al. Dose-dependent effect of S(+) ketamine on post-ischemic endogenous neurogenesis in rats. Acta Anaesthesiol Scand 2009; 53: 528–533. [DOI] [PubMed] [Google Scholar]
- 86.Mott DD, Doherty JJ, Zhang S, et al. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci 1998; 1: 659–667. [DOI] [PubMed] [Google Scholar]
- 87.Silver IA, Erecinska M. Intracellular and extracellular changes of [Ca2+] in hypoxia and ischemia in rat brain in vivo. J Gen Physiol 1990; 95: 837–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin H, Warner DS, Todd MM. Effects of glycine receptor antagonism on spreading depression in the rat. Neurosci Lett 1994; 180: 285–289. [DOI] [PubMed] [Google Scholar]
- 89.Krüger H, Heinemann U, Luhmann HJ. Effects of ionotropic glutamate receptor blockade and 5-HT1A receptor activation on spreading depression in rat neocortical slices. Neuroreport 1999; 10: 2651–2656. [DOI] [PubMed] [Google Scholar]
- 90.Sanchez-Porras R, Santos E, Scholl M, et al. The effect of ketamine on optical and electrical characteristics of spreading depolarizations in gyrencephalic swine cortex. Neuropharmacology 2014; 84: 52–61. [DOI] [PubMed] [Google Scholar]
- 91.Lauritzen M, Hansen AJ. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J Cereb Blood Flow Metab 1992; 12: 223–229. [DOI] [PubMed] [Google Scholar]
- 92.Nellgard B, Wieloch T. NMDA-receptor blockers but not NBQX, an AMPA-receptor antagonist, inhibit spreading depression in the rat brain. Acta Physiol Scand 1992; 146: 497–503. [DOI] [PubMed] [Google Scholar]
- 93.Gill R, Andine P, Hillered L, et al. The effect of MK-801 on cortical spreading depression in the penumbral zone following focal ischaemia in the rat. J Cereb Blood Flow Metab 1992; 12: 371–379. [DOI] [PubMed] [Google Scholar]
- 94.Willette RN, Lysko PG, Sauermelch CF. A comparison of (+)SK&F 10047 and MK-801 on cortical spreading depression. Brain Res 1994; 648: 347–351. [DOI] [PubMed] [Google Scholar]
- 95.Obrenovitch TP, Zilkha E. Inhibition of cortical spreading depression by L-701,324, a novel antagonist at the glycine site of the N-methyl-D-aspartate receptor complex. Br J Pharmacol 1996; 117: 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miettinen S, Fusco FR, Yrjanheikki J, et al. Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-D-aspartic acid-receptors and phospholipase A2. Proc Natl Acad Sci U S A 1997; 94: 6500–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marrannes R, Edmonds HL, Jr, Wauquier A, et al. Measurement of ischemic changes in cerebral blood flow by the hydrogen clearance technique and brain cortical temperature. Influence of flunarizine. Arch Int Pharmacodyn Ther 1986; 281: 209–229. [PubMed] [Google Scholar]
- 98.Koroleva VI, Korolev OS, Loseva E, et al. The effect of MK-801 and of brain-derived polypeptides on the development of ischemic lesion induced by photothrombotic occlusion of the distal middle cerebral artery in rats. Brain Res 1998; 786: 104–114. [DOI] [PubMed] [Google Scholar]
- 99.van der Hel WS, van den Bergh WM, Nicolay K, et al. Suppression of cortical spreading depressions after magnesium treatment in the rat. Neuroreport 1998; 9: 2179–2182. [DOI] [PubMed] [Google Scholar]
- 100.Kunimatsu T, Asai S, Kanematsu K, et al. Effects of glutamate receptor agonist on extracellular glutamate dynamics during moderate cerebral ischemia. Brain Res 2001; 923: 178–186. [DOI] [PubMed] [Google Scholar]
- 101.Anderson TR, Andrew RD. Spreading depression: imaging and blockade in the rat neocortical brain slice. J Neurophysiol 2002; 88: 2713–2725. [DOI] [PubMed] [Google Scholar]
- 102.Peeters M, Gunthorpe MJ, Strijbos PJ, et al. Effects of pan- and subtype-selective N-methyl-D-aspartate receptor antagonists on cortical spreading depression in the rat: therapeutic potential for migraine. J Pharmacol Exp Ther 2007; 321: 564–572. [DOI] [PubMed] [Google Scholar]
- 103.Richter F, Bauer R, Lehmenkuhler A, et al. Spreading depression in the brainstem of the adult rat: electrophysiological parameters and influences on regional brainstem blood flow. J Cereb Blood Flow Metab 2008; 28: 984–994. [DOI] [PubMed] [Google Scholar]
- 104.Dhir A, Lossin C, Rogawski MA. Propofol hemisuccinate suppresses cortical spreading depression. Neurosci Lett 2012; 514: 67–70. [DOI] [PubMed] [Google Scholar]
- 105.Wang M, Chazot PL, Ali S, et al. Effects of NMDA receptor antagonists with different subtype selectivities on retinal spreading depression. Br J Pharmacol 2012; 165: 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richter F, Bauer R, Ebersberger A, et al. Enhanced neuronal excitability in adult rat brainstem causes widespread repetitive brainstem depolarizations with cardiovascular consequences. J Cereb Blood Flow Metab 2012; 32: 1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oláh G, Heredi J, Menyhart A, et al. Unexpected effects of peripherally administered kynurenic acid on cortical spreading depression and related blood-brain barrier permeability. Drug Des Devel Ther 2013; 7: 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shatillo A, Salo RA, Giniatullin R, et al. Involvement of NMDA receptor subtypes in cortical spreading depression in rats assessed by fMRI. Neuropharmacology 2015; 93: 164–170. [DOI] [PubMed] [Google Scholar]
- 109.Bu F, Du R, Li Y, et al. NR2A contributes to genesis and propagation of cortical spreading depression in rats. Sci Rep 2016; 6: 23576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Srienc AI, Biesecker KR, Shimoda AM, et al. Ischemia-induced spreading depolarization in the retina. J Cereb Blood Flow Metab 2016; 36: 1579–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodrigues PS, Guimaraes AP, de Azeredo FA, et al. Involvement of GABA and ACh in retinal spreading depression: effects of “low calcium-high magnesium” solutions. Exp Brain Res 1988; 73: 659–664. [DOI] [PubMed] [Google Scholar]
- 112.Van Harreveld A. The nature of the chick's magnesium-sensitive retinal spreading depression. J Neurobiol 1984; 15: 333–343. [DOI] [PubMed] [Google Scholar]
- 113.Shibata M, Bures J. Techniques for termination of reverberating spreading depression in rats. J Neurophysiol 1975; 38: 158–166. [DOI] [PubMed] [Google Scholar]
- 114.Santos E, Leon F, Silos H, et al. Incidence, hemodynamic, and electrical characteristics of spreading depolarization in a swine model are affected by local but not by intravenous application of magnesium. J Cereb Blood Flow Metab 2016; 36: 2051–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martens-Mantai T, Speckmann EJ and Gorji A. Propagation of cortical spreading depression into the hippocampus: The role of the entorhinal cortex. Synapse (New York, NY) 2014 2014/07/23. DOI: 10.1002/syn.21769. [DOI] [PubMed]
- 116.Tatlisumak T, Takano K, Meiler MR, et al. A glycine site antagonist, ZD9379, reduces number of spreading depressions and infarct size in rats with permanent middle cerebral artery occlusion. Stroke 1998; 29: 190–195. [DOI] [PubMed] [Google Scholar]
- 117.Menniti FS, Pagnozzi MJ, Butler P, et al. CP-101,606, an NR2B subunit selective NMDA receptor antagonist, inhibits NMDA and injury induced c-fos expression and cortical spreading depression in rodents. Neuropharmacology 2000; 39: 1147–1155. [DOI] [PubMed] [Google Scholar]
- 118.Verhaegen M, Todd MM, Warner DS. The influence of different concentrations of volatile anesthetics on the threshold for cortical spreading depression in rats. Brain Res 1992; 581: 153–155. [DOI] [PubMed] [Google Scholar]
- 119.Sanchez-Porras R, Santos E, Scholl M, et al. Ketamine modulation of the haemodynamic response to spreading depolarization in the gyrencephalic swine brain. J Cereb Blood Flow Metab 2017; 37: 1720–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaube H, Herzog J, Kaufer T, et al. Aura in some patients with familial hemiplegic migraine can be stopped by intranasal ketamine. Neurology 2000; 55: 139–141. [DOI] [PubMed] [Google Scholar]
- 121.Kertesz S, Kapus G, Gacsalyi I, et al. Deramciclane improves object recognition in rats: potential role of NMDA receptors. Pharmacol Biochem Behav 2010; 94: 570–574. [DOI] [PubMed] [Google Scholar]
- 122.Parsons CG, Gruner R, Rozental J, et al. Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memantine (1-amino-3,5-dimethyladamantan). Neuropharmacology 1993; 32: 1337–1350. [DOI] [PubMed] [Google Scholar]
- 123.Pieta Dias C, Martins de Lima MN, Presti-Torres J, et al. Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience 2007; 146: 1719–1725. [DOI] [PubMed] [Google Scholar]
- 124.Charles A, Flippen C, Romero Reyes M, et al. Memantine for prevention of migraine: a retrospective study of 60 cases. J Headache Pain 2007; 8: 248–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bigal M, Rapoport A, Sheftell F, et al. Memantine in the preventive treatment of refractory migraine. Headache 2008; 48: 1337–1342. [DOI] [PubMed] [Google Scholar]
- 126.Ditzler K. Efficacy and tolerability of memantine in patients with dementia syndrome. A double-blind, placebo controlled trial. Arzneimittelforschung 1991; 41: 773–780. [PubMed] [Google Scholar]
- 127.Barnes CA, Danysz W, Parsons CG. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampal long-term potentiation, short-term exploratory modulation and spatial memory in awake, freely moving rats. Eur J Neurosci 1996; 8: 565–571. [DOI] [PubMed] [Google Scholar]
- 128.Zajaczkowski W, Quack G, Danysz W. Infusion of (+) -MK-801 and memantine – contrasting effects on radial maze learning in rats with entorhinal cortex lesion. Eur J Pharmacol 1996; 296: 239–246. [DOI] [PubMed] [Google Scholar]
- 129.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem 2006; 97: 1611–1626. [DOI] [PubMed] [Google Scholar]
- 130.Petzold GC, Windmuller O, Haack S, et al. Increased extracellular K+ concentration reduces the efficacy of N-methyl-D-aspartate receptor antagonists to block spreading depression-like depolarizations and spreading ischemia. Stroke 2005; 36: 1270–1277. [DOI] [PubMed] [Google Scholar]
- 131.Iijima T, Mies G, Hossmann KA. Repeated negative DC deflections in rat cortex following middle cerebral artery occlusion are abolished by MK-801: effect on volume of ischemic injury. J Cereb Blood Flow Metab 1992; 12: 727–733. [DOI] [PubMed] [Google Scholar]
- 132.Kurumaji A, McCulloch J. Effects of MK-801 upon local cerebral glucose utilisation in conscious rats and in rats anaesthetised with halothane. J Cereb Blood Flow Metab 1989; 9: 786–794. [DOI] [PubMed] [Google Scholar]
- 133.Rogoz Z, Kaminska K. The effect of combined treatment with escitalopram and risperidone on the MK-801-induced changes in the object recognition test in mice. Pharmacol Rep 2016; 68: 116–120. [DOI] [PubMed] [Google Scholar]
- 134.Wu H, Wang X, Gao Y, et al. NMDA receptor antagonism by repetitive MK801 administration induces schizophrenia-like structural changes in the rat brain as revealed by voxel-based morphometry and diffusion tensor imaging. Neuroscience 2016; 322: 221–233. [DOI] [PubMed] [Google Scholar]
- 135.Rupniak NM, Boyce S, Tye S, et al. Anxiolytic-like and antinociceptive effects of MK-801 accompanied by sedation and ataxia in primates. Pharmacol Biochem Behav 1993; 44: 153–156. [DOI] [PubMed] [Google Scholar]
- 136.Izumi Y, Roussel S, Pinard E, et al. Reduction of infarct volume by magnesium after middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab 1991; 11: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 137.Marinov MB, Harbaugh KS, Hoopes PJ, et al. Neuroprotective effects of preischemia intraarterial magnesium sulfate in reversible focal cerebral ischemia. J Neurosurg 1996; 85: 117–124. [DOI] [PubMed] [Google Scholar]
- 138.Muir KW, Lees KR, Ford I, et al. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. Lancet 2004; 363: 439–445. [DOI] [PubMed] [Google Scholar]
- 139.Yamamoto T, Mori K, Esaki T, et al. Preventive effect of continuous cisternal irrigation with magnesium sulfate solution on angiographic cerebral vasospasms associated with aneurysmal subarachnoid hemorrhages: a randomized controlled trial. J Neurosurg 2016; 124: 18–26. [DOI] [PubMed] [Google Scholar]
- 140.Nowak L, Bregestovski P, Ascher P, et al. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984; 307: 462–465. [DOI] [PubMed] [Google Scholar]
- 141.Warner DS, Martin H, Ludwig P, et al. In vivo models of cerebral ischemia: effects of parenterally administered NMDA receptor glycine site antagonists. J Cereb Blood Flow Metab 1995; 15: 188–196. [DOI] [PubMed] [Google Scholar]
- 142.Petty MA, Neumann-Haefelin C, Kalisch J, et al. In vivo neuroprotective effects of ACEA 1021 confirmed by magnetic resonance imaging in ischemic stroke. Eur J Pharmacol 2003; 474: 53–62. [DOI] [PubMed] [Google Scholar]
- 143.Tatlisumak T, Takano K, Meiler MR, et al. A glycine site antagonist ZD9379 reduces number of spreading depressions and infarct size in rats with permanent middle cerebral artery occlusion. Acta Neurochir Suppl 2000; 76: 331–333. [DOI] [PubMed] [Google Scholar]
- 144.Bristow LJ, Hutson PH, Kulagowski JJ, et al. Anticonvulsant and behavioral profile of L-701,324, a potent, orally active antagonist at the glycine modulatory site on the N-methyl-D-aspartate receptor complex. J Pharmacol Exp Ther 1996; 279: 492–501. [PubMed] [Google Scholar]
- 145.Liu RJ, Duman C, Kato T, et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology 2017; 42: 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moskal JR, Burch R, Burgdorf JS, et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs 2014; 23: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moskal JR, Kuo AG, Weiss C, et al. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology 2005; 49: 1077–1087. [DOI] [PubMed] [Google Scholar]
- 148.Zhang XL, Shuttleworth CW, Moskal JR, et al. Suppression of spreading depolarization and stabilization of dendritic spines by GLYX-13, an NMDA receptor glycine-site functional partial agonist. Exp Neurol 2015; 273: 312–321. [DOI] [PubMed] [Google Scholar]
- 149.Tajti J, Szok D, Pardutz A, et al. Where does a migraine attack originate? In the brainstem. J Neural Transm (Vienna) 2012; 119: 557–568. [DOI] [PubMed] [Google Scholar]
- 150.Chauvel V, Vamos E, Pardutz A, et al. Effect of systemic kynurenine on cortical spreading depression and its modulation by sex hormones in rat. Exp Neurol 2012; 236: 207–214. [DOI] [PubMed] [Google Scholar]
- 151.Knyihar-Csillik E, Toldi J, Mihaly A, et al. Kynurenine in combination with probenecid mitigates the stimulation-induced increase of c-fos immunoreactivity of the rat caudal trigeminal nucleus in an experimental migraine model. J Neural Transm (Vienna) 2007; 114: 417–421. [DOI] [PubMed] [Google Scholar]
- 152.Vamos E, Pardutz A, Varga H, et al. l-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 2009; 57: 425–429. [DOI] [PubMed] [Google Scholar]
- 153.Knyihar-Csillik E, Toldi J, Krisztin-Peva B, et al. Prevention of electrical stimulation-induced increase of c-fos immunoreaction in the caudal trigeminal nucleus by kynurenine combined with probenecid. Neurosci Lett 2007; 418: 122–126. [DOI] [PubMed] [Google Scholar]
- 154.Roussel S, Pinard E, Seylaz J. Kynurenate does not reduce infarct size after middle cerebral artery occlusion in spontaneously hypertensive rats. Brain Res 1990; 518: 353–355. [DOI] [PubMed] [Google Scholar]
- 155.Olsson SK, Sellgren C, Engberg G, et al. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipolar Disord 2012; 14: 719–726. [DOI] [PubMed] [Google Scholar]
- 156.Menniti F, Chenard B, Collins M, et al. CP-101,606, a potent neuroprotectant selective for forebrain neurons. Eur J Pharmacol 1997; 331: 117–126. [DOI] [PubMed] [Google Scholar]
- 157.Tsuchida E, Rice M, Bullock R. The neuroprotective effect of the forebrain-selective NMDA antagonist CP101,606 upon focal ischemic brain damage caused by acute subdural hematoma in the rat. J Neurotrauma 1997; 14: 409–417. [DOI] [PubMed] [Google Scholar]
- 158.Quirion R, Bowen WD, Itzhak Y, et al. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci 1992; 13: 85–86. [DOI] [PubMed] [Google Scholar]
- 159.Marrazzo A, Prezzavento O, Pasquinucci L, et al. Synthesis and pharmacological evaluation of potent and enantioselective sigma 1, and sigma 2 ligands. Farmaco 2001; 56: 181–189. [DOI] [PubMed] [Google Scholar]
- 160.Takahashi H, Kirsch JR, Hashimoto K, et al. PPBP [4-phenyl-1-(4-phenylbutyl) piperidine] decreases brain injury after transient focal ischemia in rats. Stroke 1996; 27: 2120–2123. [DOI] [PubMed] [Google Scholar]
- 161.Senda T, Mita S, Kaneda K, et al. Effect of SA4503, a novel sigma1 receptor agonist, against glutamate neurotoxicity in cultured rat retinal neurons. Eur J Pharmacol 1998; 342: 105–111. [DOI] [PubMed] [Google Scholar]
- 162.Nishikawa H, Hashino A, Kume T, et al. Involvement of direct inhibition of NMDA receptors in the effects of sigma-receptor ligands on glutamate neurotoxicity in vitro. Eur J Pharmacol 2000; 404: 41–48. [DOI] [PubMed] [Google Scholar]
- 163.Whittemore ER, Ilyin VI, Woodward RM. Antagonism of N-methyl-D-aspartate receptors by sigma site ligands: potency, subtype-selectivity and mechanisms of inhibition. J Pharmacol Exp Ther 1997; 282: 326–338. [PubMed] [Google Scholar]
- 164.Gorelova NA, Koroleva VI, Amemori T, et al. Ketamine blockade of cortical spreading depression in rats. Electroencephalogr Clin Neurophysiol 1987; 66: 440–447. [DOI] [PubMed] [Google Scholar]
- 165.McLachlan RS. Suppression of spreading depression of Leao in neocortex by an N-methyl-D-aspartate receptor antagonist. Can J Neurol Sci 1992; 19: 487–491. [PubMed] [Google Scholar]
- 166.Chauvel V, Schoenen J, Multon S. Influence of ovarian hormones on cortical spreading depression and its suppression by L-kynurenine in rat. PLoS One 2013; 8: e82279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guedes RC, Barreto JM. Effect of anesthesia on the propagation of cortical spreading depression in rats. Braz J Med Biol Res 1992; 25: 393–397. [PubMed] [Google Scholar]
- 168.Kitahara Y, Taga K, Abe H, et al. The effects of anesthetics on cortical spreading depression elicitation and c-fos expression in rats. J Neurosurg Anesthesiol 2001; 13: 26–32. [DOI] [PubMed] [Google Scholar]
- 169.Piper RD, Lambert GA. Inhalational anesthetics inhibit spreading depression: relevance to migraine. Cephalalgia 1996; 16: 87–92. [DOI] [PubMed] [Google Scholar]
- 170.Saito R, Graf R, Hubel K, et al. Reduction of infarct volume by halothane: effect on cerebral blood flow or perifocal spreading depression-like depolarizations. J Cereb Blood Flow Metab 1997; 17: 857–864. [DOI] [PubMed] [Google Scholar]
- 171.Saito R, Graf R, Hubel K, et al. Halothane, but not alpha-chloralose, blocks potassium-evoked cortical spreading depression in cats. Brain Res 1995; 699: 109–115. [DOI] [PubMed] [Google Scholar]
- 172.Xie Z, Dong Y, Maeda U, et al. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology 2006; 104: 988–994. [DOI] [PubMed] [Google Scholar]
- 173.Kudo C, Toyama M, Boku A, et al. Anesthetic effects on susceptibility to cortical spreading depression. Neuropharmacology 2013; 67: 32–36. [DOI] [PubMed] [Google Scholar]
- 174.Takagaki M, Feuerstein D, Kumagai T, et al. Isoflurane suppresses cortical spreading depolarizations compared to propofol – implications for sedation of neurocritical care patients. Exp Neurol 2014; 252: 12–17. [DOI] [PubMed] [Google Scholar]
- 175.Puil E, el-Beheiry H. Anaesthetic suppression of transmitter actions in neocortex. Br J Pharmacol 1990; 101: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang J, Zorumski CF. Effects of isoflurane on N-methyl-D-aspartate gated ion channels in cultured rat hippocampal neurons. Ann N Y Acad Sci 1991; 625: 287–289. [DOI] [PubMed] [Google Scholar]
- 177.Chiu KM, Lin TY, Lu CW, et al. Inhibitory effect of glutamate release from rat cerebrocortical nerve terminals by alpha2 adrenoceptor agonist dexmedetomidine. Eur J Pharmacol 2011; 670: 137–147. [DOI] [PubMed] [Google Scholar]
- 178.Risher WC, Lee MR, Fomitcheva IV, et al. Dibucaine mitigates spreading depolarization in human neocortical slices and prevents acute dendritic injury in the ischemic rodent neocortex. PLoS One 2011; 6: e22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kaube H, Goadsby PJ. Anti-migraine compounds fail to modulate the propagation of cortical spreading depression in the cat. Eur Neurol 1994; 34: 30–35. [DOI] [PubMed] [Google Scholar]
- 180.Ayad M, Verity MA, Rubinstein EH. Lidocaine delays cortical ischemic depolarization: relationship to electrophysiologic recovery and neuropathology. J Neurosurg Anesthesiol 1994; 6: 98–110. [DOI] [PubMed] [Google Scholar]
- 181.Chebabo SR, do Carmo RJ, Martins-Ferreira H. Effects of local anaesthetics on retinal spreading depression. Exp Brain Res 1993; 96: 363–364. [DOI] [PubMed] [Google Scholar]
- 182.Sonn J, Mayevsky A. Effects of anesthesia on the responses to cortical spreading depression in the rat brain in vivo. Neurol Res 2006; 28: 206–219. [DOI] [PubMed] [Google Scholar]
- 183.de Souza TK, MB ES-G, Rodrigues MC, et al. Anesthetic agents modulate ECoG potentiation after spreading depression, and insulin-induced hypoglycemia does not modify this effect. Neurosci Lett 2015; 592: 6–11. [DOI] [PubMed] [Google Scholar]
- 184.Kudo C, Nozari A, Moskowitz MA, et al. The impact of anesthetics and hyperoxia on cortical spreading depression. Exp Neurol 2008; 212: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bogdanov VB, Multon S, Chauvel V, et al. Migraine preventive drugs differentially affect cortical spreading depression in rat. Neurobiol Dis 2011; 41: 430–435. [DOI] [PubMed] [Google Scholar]
- 186.Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain 1994; 117: 199–210. [DOI] [PubMed] [Google Scholar]
- 187.Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol 1981; 9: 344–352. [DOI] [PubMed] [Google Scholar]
- 188.Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion-weighted imaging defects during spontaneous migrainous aura. Ann Neurol 1998; 43: 25–31. [DOI] [PubMed] [Google Scholar]
- 189.Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia 2002; 22: 482–484. [DOI] [PubMed] [Google Scholar]
- 190.Cecchini AP, Mea E, Tullo V, et al. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci 2009; 30 Suppl 1: S101–104. [DOI] [PubMed] [Google Scholar]
- 191.Basic S, Sporis D, Chudy D, et al. The effect of vagus nerve stimulation on migraine in patient with intractable epilepsy: case report. Neurol Sci 2013; 34: 797–798. [DOI] [PubMed] [Google Scholar]
- 192.Read SJ, Hirst WD, Upton N, et al. Cortical spreading depression produces increased cGMP levels in cortex and brain stem that is inhibited by tonabersat (SB-220453) but not sumatriptan. Brain Res 2001; 891: 69–77. [DOI] [PubMed] [Google Scholar]
- 193.Smith MI, Read SJ, Chan WN, et al. Repetitive cortical spreading depression in a gyrencephalic feline brain: inhibition by the novel benzoylamino-benzopyran SB-220453. Cephalalgia 2000; 20: 546–553. [DOI] [PubMed] [Google Scholar]
- 194.Bradley DP, Smith MI, Netsiri C, et al. Diffusion-weighted MRI used to detect in vivo modulation of cortical spreading depression: comparison of sumatriptan and tonabersat. Exp Neurol 2001; 172: 342–353. [DOI] [PubMed] [Google Scholar]
- 195.Akerman S, Goadsby PJ. Topiramate inhibits cortical spreading depression in rat and cat: impact in migraine aura. Neuroreport 2005; 16: 1383–1387. [DOI] [PubMed] [Google Scholar]
- 196.Unekawa M, Tomita Y, Toriumi H, et al. Suppressive effect of chronic peroral topiramate on potassium-induced cortical spreading depression in rats. Cephalalgia 2012; 32: 518–527. [DOI] [PubMed] [Google Scholar]
- 197.Tozzi A, de Iure A, Di Filippo M, et al. Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci U S A 2012; 109: 18985–18990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wauquier A, Ashton D, Marrannes R. The effects of flunarizine in experimental models related to the pathogenesis of migraine. Cephalalgia 1985; 5 Suppl 2: 119–123. [DOI] [PubMed] [Google Scholar]
- 199.Li F, Qiu E, Dong Z, et al. Protection of flunarizine on cerebral mitochondria injury induced by cortical spreading depression under hypoxic conditions. J Headache Pain 2011; 12: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ashton D, Willems R, Marrannes R, et al. Extracellular ions during veratridine-induced neurotoxicity in hippocampal slices: neuroprotective effects of flunarizine and tetrodotoxin. Brain Res 1990; 528: 212–222. [DOI] [PubMed] [Google Scholar]
- 201.Tepe N, Filiz A, Dilekoz E, et al. The thalamic reticular nucleus is activated by cortical spreading depression in freely moving rats: prevention by acute valproate administration. Eur J Neurosci 2015; 41: 120–128. [DOI] [PubMed] [Google Scholar]
- 202.Hoffmann U, Dilekoz E, Kudo C, et al. Oxcarbazepine does not suppress cortical spreading depression. Cephalalgia 2011; 31: 537–542. [DOI] [PubMed] [Google Scholar]
- 203.Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci 1993; 13: 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Knapp L, Szita B, Kocsis K, et al. Nitroglycerin enhances the propagation of cortical spreading depression: comparative studies with sumatriptan and novel kynurenic acid analogues. Drug Des Devel Ther 2017; 11: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wiedemann M, de Lima VM, Hanke W. Effects of antimigraine drugs on retinal spreading depression. Naunyn Schmiedebergs Arch Pharmacol 1996; 353: 552–556. [DOI] [PubMed] [Google Scholar]
- 206.Richter F, Mikulik O, Ebersberger A, et al. Noradrenergic agonists and antagonists influence migration of cortical spreading depression in rat-a possible mechanism of migraine prophylaxis and prevention of postischemic neuronal damage. J Cereb Blood Flow Metab 2005; 25: 1225–1235. [DOI] [PubMed] [Google Scholar]
- 207.Kaube H, Knight YE, Storer RJ, et al. Vasodilator agents and supracollicular transection fail to inhibit cortical spreading depression in the cat. Cephalalgia 1999; 19: 592–597. [DOI] [PubMed] [Google Scholar]
- 208.Bolay H, Durham P. Pharmacology. Handbook of clinical neurology 2010; 97: 47–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Thomaides T, Karapanayiotides T, Kerezoudi E, et al. Intravenous valproate aborts glyceryl trinitrate-induced migraine attacks: a clinical and quantitative EEG study. Cephalalgia 2008; 28: 250–256. [DOI] [PubMed] [Google Scholar]
- 210.Bowyer SM, Mason KM, Moran JE, et al. Cortical hyperexcitability in migraine patients before and after sodium valproate treatment. J Clin Neurophysiol 2005; 22: 65–67. [DOI] [PubMed] [Google Scholar]
- 211.Mulleners WM, Chronicle EP, Vredeveld JW, et al. Visual cortex excitability in migraine before and after valproate prophylaxis: a pilot study using TMS. Eur J Neurol 2002; 9: 35–40. [DOI] [PubMed] [Google Scholar]
- 212.Aurora SK, Barrodale P, Chronicle EP, et al. Cortical inhibition is reduced in chronic and episodic migraine and demonstrates a spectrum of illness. Headache 2005; 45: 546–552. [DOI] [PubMed] [Google Scholar]
- 213.Goadsby PJ, Ferrari MD, Csanyi A, et al. Randomized, double-blind, placebo-controlled, proof-of-concept study of the cortical spreading depression inhibiting agent tonabersat in migraine prophylaxis. Cephalalgia 2009; 29: 742–750. [DOI] [PubMed] [Google Scholar]
- 214.Cao Y, Zheng OJ. Tonabersat for migraine prophylaxis: a systematic review. Pain Physician 2014; 17: 1–8. [PubMed] [Google Scholar]
- 215.Silberstein SD, Lipton RB, Dodick DW, et al. Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. Headache 2007; 47: 170–180. [DOI] [PubMed] [Google Scholar]
- 216.Diener HC, Tfelt-Hansen P, Dahlof C, et al. Topiramate in migraine prophylaxis – results from a placebo-controlled trial with propranolol as an active control. J Neurol 2004; 251: 943–950. [DOI] [PubMed] [Google Scholar]
- 217.Diener HC, Bussone G, Van Oene JC, et al. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia 2007; 27: 814–823. [DOI] [PubMed] [Google Scholar]
- 218.Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA 2004; 291: 965–973. [DOI] [PubMed] [Google Scholar]
- 219.Wang M, Li Y, Lin Y. GABAA receptor alpha2 subtype activation suppresses retinal spreading depression. Neuroscience 2015; 298: 137–144. [DOI] [PubMed] [Google Scholar]
- 220.Reveiz-Herault L, Cardona AF, Ospina EG, et al. [Effectiveness of flunarizine in the prophylaxis of migraine: a meta-analytical review of the literature]. Rev Neurol 2003; 36: 907–912. [PubMed] [Google Scholar]
- 221.Ludin HP. Flunarizine and propranolol in the treatment of migraine. Headache 1989; 29: 219–224. [DOI] [PubMed] [Google Scholar]
- 222.Lutschg J, Vassella F. [The treatment of juvenile migraine using flunarizine or propranolol]. Schweiz Med Wochenschr 1990; 120: 1731–1736. [PubMed] [Google Scholar]
- 223.Shimell CJ, Fritz VU, Levien SL. A comparative trial of flunarizine and propranolol in the prevention of migraine. S Afr Med J 1990; 77: 75–77. [PubMed] [Google Scholar]
- 224.Grotemeyer KH, Schlake HP, Husstedt IW. [Prevention of migraine with metoprolol and flunarizine. A double-blind crossover study]. Der Nervenarzt 1988; 59: 549–552. [PubMed] [Google Scholar]
- 225.Gulati P, Muthuraman A, Kaur P. Investigation of the role of non-selective calcium channel blocker (flunarizine) on cerebral ischemic-reperfusion associated cognitive dysfunction in aged mice. Pharmacol Biochem Behav 2015; 131: 26–32. [DOI] [PubMed] [Google Scholar]
- 226.Shimazawa M, Hara H, Watano T, et al. Effects of Ca2+ channel blockers on cortical hypoperfusion and expression of c-Fos-like immunoreactivity after cortical spreading depression in rats. Br J Pharmacol 1995; 115: 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Richter F, Ebersberger A, Schaible HG. Blockade of voltage-gated calcium channels in rat inhibits repetitive cortical spreading depression. Neurosci Lett 2002; 334: 123–126. [DOI] [PubMed] [Google Scholar]
- 228.Maranhao-Filho PA, Martins-Ferreira H, Vincent MB, et al. Sumatriptan blocks spreading depression in isolated chick retina. Cephalalgia 1997; 17: 822–825. [DOI] [PubMed] [Google Scholar]
- 229.Green AL, Gu P, De Felice M, et al. Increased susceptibility to cortical spreading depression in an animal model of medication-overuse headache. Cephalalgia 2014; 34: 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Guedes RC, Rocha-de-Melo AP, de Lima KR, et al. Early malnutrition attenuates the impairing action of naloxone on spreading depression in young rats. Nutr Neurosci 2013; 16: 142–146. [DOI] [PubMed] [Google Scholar]
- 231.Rocha-de-Melo AP, de Lima KR, de Albuquerque Jda M, et al. Chronic neonatal exposure of rats to the opioid antagonist naloxone impairs propagation of cortical spreading depression in adulthood. Neurosci Lett 2008; 441: 315–318. [DOI] [PubMed] [Google Scholar]
- 232.Kertész S, Kapus G, Levay G. Interactions of allosteric modulators of AMPA/kainate receptors on spreading depression in the chicken retina. Brain Res 2004; 1025: 123–129. [DOI] [PubMed] [Google Scholar]
- 233.Gressens P, Spedding M, Gigler G, et al. The effects of AMPA receptor antagonists in models of stroke and neurodegeneration. Eur J Pharmacol 2005; 519: 58–67. [DOI] [PubMed] [Google Scholar]
- 234.Haarmann AM, Jafarian M, Karimzadeh F, et al. Modulatory effects of dopamine D2 receptors on spreading depression in rat somatosensory neocortex. Basic Clin Neurosci 2014; 5: 246–252. [PMC free article] [PubMed] [Google Scholar]
- 235.Kazemi H, Rahgozar M, Speckmann EJ, et al. Effect of cannabinoid receptor activation on spreading depression. Iran J Basic Med Sci 2012; 15: 926–936. [PMC free article] [PubMed] [Google Scholar]
- 236.de Aguiar MJ, de Aguiar CR, Guedes RC. Lithium/nutrition interaction in the brain: a single lithium administration impairs spreading depression in malnourished, but not in well-nourished rats. Nutr Neurosci 2011; 14: 159–164. [DOI] [PubMed] [Google Scholar]
- 237.Colonna DM, Meng W, Deal DD, et al. Calcitonin gene-related peptide promotes cerebrovascular dilation during cortical spreading depression in rabbits. Am J Physiol 1994; 266: H1095–1102. [DOI] [PubMed] [Google Scholar]
- 238.Wahl M, Schilling L, Parsons AA, et al. Involvement of calcitonin gene-related peptide (CGRP) and nitric oxide (NO) in the pial artery dilatation elicited by cortical spreading depression. Brain Res 1994; 637: 204–210. [DOI] [PubMed] [Google Scholar]
- 239.Reuter U, Weber JR, Gold L, et al. Perivascular nerves contribute to cortical spreading depression-associated hyperemia in rats. Am J Physiol 1998; 274: H1979–1987. [DOI] [PubMed] [Google Scholar]
- 240.Pradhan AA, Smith ML, Zyuzin J, et al. delta-Opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice. Br J Pharmacol 2014; 171: 2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Alemdar M, Akman O, Selekler H, et al. Does metoprolol inhibit the cortical spreading depression? Acute effects of systematic metropol on CSD in rats. Cephalalgia 2007; 27: 1010–1013. [DOI] [PubMed] [Google Scholar]
- 242.Sheardown MJ. The triggering of spreading depression in the chicken retina: a pharmacological study. Brain Res 1993; 607: 189–194. [DOI] [PubMed] [Google Scholar]
- 243.Tobiasz C, Nicholson C. Tetrodotoxin resistant propagation and extracellular sodium changes during spreading depression in rat cerebellum. Brain Res 1982; 241: 329–333. [DOI] [PubMed] [Google Scholar]
- 244.Aitken PG, Jing J, Young J, et al. Ion channel involvement in hypoxia-induced spreading depression in hippocampal slices. Brain Res 1991; 541: 7–11. [DOI] [PubMed] [Google Scholar]
- 245.Müller M, Somjen GG. Na(+) and K(+) concentrations, extra- and intracellular voltages, and the effect of TTX in hypoxic rat hippocampal slices. J Neurophysiol 2000; 83: 735–745. [DOI] [PubMed] [Google Scholar]
- 246.Akerman S, Holland PR, Goadsby PJ. Mechanically-induced cortical spreading depression associated regional cerebral blood flow changes are blocked by Na+ ion channel blockade. Brain Res 2008; 1229: 27–36. [DOI] [PubMed] [Google Scholar]
- 247.Wang M, Urenjak J, Fedele E, et al. Effects of phosphodiesterase inhibition on cortical spreading depression and associated changes in extracellular cyclic GMP. Biochem Pharmacol 2004; 67: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 248.Bezerra Rde S, Abadie-Guedes R, Melo FR, et al. Shrimp carotenoids protect the developing rat cerebral cortex against the effects of ethanol on cortical spreading depression. Neurosci Lett 2005; 391: 51–55. [DOI] [PubMed] [Google Scholar]
- 249.Varga DP, Puskas T, Menyhart A, et al. Contribution of prostanoid signaling to the evolution of spreading depolarization and the associated cerebral blood flow response. Sci Rep 2016; 6: 31402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gariepy H, Zhao J, Levy D. Differential contribution of COX-1 and COX-2 derived prostanoids to cortical spreading depression-evoked cerebral oligemia. J Cereb Blood Flow Metab 2017; 37: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Richter F, Koulen P, Kaja S. N-Palmitoylethanolamine prevents the run-down of amplitudes in cortical spreading depression possibly implicating proinflammatory cytokine release. Sci Rep 2016; 6: 23481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Marschollek C, Karimzadeh F, Jafarian M, et al. Effects of garlic extract on spreading depression: in vitro and in vivo investigations. Nutr Neurosci 2017; 20: 127–134. [DOI] [PubMed] [Google Scholar]
- 253.de Aguiar MJ, de Aguiar CR, Guedes RC. Caffeine/nutrition interaction in the rat brain: influence on latent inhibition and cortical spreading depression. Eur J Pharmacol 2011; 650: 268–274. [DOI] [PubMed] [Google Scholar]
- 254.Fernandes de Lima VM, Wiedemann M, Klottig H, et al. Exogenous application of gangliosides changes the state of excitability of retinal tissue as demonstrated by retinal spreading depression experiments. Naunyn Schmiedebergs Arch Pharmacol 1997; 355: 507–514. [DOI] [PubMed] [Google Scholar]
- 255.Richter F, Lutz W, Eitner A, et al. Tumor necrosis factor reduces the amplitude of rat cortical spreading depression in vivo. Ann Neurol 2014; 76: 43–53. [DOI] [PubMed] [Google Scholar]
- 256.Read SJ, Smith MI, Benham CD, et al. Furosemide inhibits regenerative cortical spreading depression in anaesthetized cats. Cephalalgia 1997; 17: 826–832. [DOI] [PubMed] [Google Scholar]
- 257.Grinberg YY, van Drongelen W, Kraig RP. Insulin-like growth factor-1 lowers spreading depression susceptibility and reduces oxidative stress. J Neurochem 2012; 122: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pusic AD, Kraig RP. Phasic treatment with interferon gamma stimulates release of exosomes that protect against spreading depression. J Interferon Cytokine Res 2015; 35: 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sun X, Li P, Luo W, et al. Investigating the effects of dimethylsulfoxide on hemodynamics during cortical spreading depression by combining laser speckle imaging with optical intrinsic signal imaging. Lasers Surg Med 2010; 42: 649–655. [DOI] [PubMed] [Google Scholar]
- 260.Guedes RC, Pereira-da-Silva MS. Effect of pre- and postnatal propylthiouracil administration on the propagation of cortical spreading depression of adult rats. Braz J Med Biol Res 1993; 26: 1123–1128. [PubMed] [Google Scholar]
- 261.Guedes RC, de Vasconcelos CA. Sleep-deprivation enhances in adult rats the antagonistic effects of pilocarpine on cortical spreading depression: a dose-response study. Neurosci Lett 2008; 442: 118–122. [DOI] [PubMed] [Google Scholar]
- 262.De Vasconcelos CA, De Oliveira JA, De Oliveira Costat LA, et al. Malnutrition and REM-sleep deprivation modulate in rats the impairment of spreading depression by a single sub-convulsing dose of pilocarpine. Nutr Neurosci 2004; 7: 163–170. [DOI] [PubMed] [Google Scholar]
- 263.Costa Monteiro HM, Lima Barreto-Silva N, Elizabete Dos Santos G, et al. Physical exercise versus fluoxetine: antagonistic effects on cortical spreading depression in Wistar rats. Eur J Pharmacol 2015; 762: 49–54. [DOI] [PubMed] [Google Scholar]
- 264.dos Santos AA, Pinheiro PC, de Lima DS, et al. Fluoxetine inhibits cortical spreading depression in weaned and adult rats suckled under favorable and unfavorable lactation conditions. Exp Neurol 2006; 200: 275–282. [DOI] [PubMed] [Google Scholar]
- 265.Guedes RC, Amancio-Dos-Santos A, Manhaes-De-Castro R, et al. Citalopram has an antagonistic action on cortical spreading depression in well-nourished and early-malnourished adult rats. Nutr Neurosci 2002; 5: 115–123. [DOI] [PubMed] [Google Scholar]
- 266.Dietz RM, Weiss JH, Shuttleworth CW. Zn2+ influx is critical for some forms of spreading depression in brain slices. J Neurosci 2008; 28: 8014–8024. [DOI] [PMC free article] [PubMed] [Google Scholar]