Abstract
Central nervous system (CNS) injuries, such as stroke, traumatic brain injury (TBI) and spinal cord injury (SCI), are important causes of death and long-term disability worldwide. MicroRNA (miRNA), small non-coding RNA molecules that negatively regulate gene expression, can serve as diagnostic biomarkers and are emerging as novel therapeutic targets for CNS injuries. MiRNA-based therapeutics include miRNA mimics and inhibitors (antagomiRs) to respectively decrease and increase the expression of target genes. In this review, we summarize current miRNA-based therapeutic applications in stroke, TBI and SCI. Administration methods, time windows and dosage for effective delivery of miRNA-based drugs into CNS are discussed. The underlying mechanisms of miRNA-based therapeutics are reviewed including oxidative stress, inflammation, apoptosis, blood–brain barrier protection, angiogenesis and neurogenesis. Pharmacological agents that protect against CNS injuries by targeting specific miRNAs are presented along with the challenges and therapeutic potential of miRNA-based therapies.
Keywords: MicroRNA mimics, microRNA inhibitors, stroke, traumatic brain injury, spinal cord injury
MiRNAs in central nervous system injuries
Overview of miRNAs
MicroRNAs (miRNAs or miRs) are small RNA that do not code for proteins.1,2 The miRNA biogenesis and function have previously been reviewed in detail.3 In brief, a miRNA gene is transcribed by RNA polymerase II (Pol II), generating the primary miRNA (pri-miRNA). In the nucleus, the RNase III endonuclease Drosha and the double-stranded RNA-binding domain (dsRBD) protein DGCR8/Pasha cleave the pri-miRNA to produce a 2-nt 3′ overhang containing the ∼70-nt precursor miRNA (pre-miRNA). Exportin-5 transports the pre-miRNA into the cytoplasm. In the cytoplasm, the pre-miRNA is cleaved by another RNase III endonuclease, Dicer, together with the dsRBD protein TRBP/Loquacious, releasing the 2-nt 3′ overhang containing a ∼21-nt miRNA:miRNA duplex. Each miRNA stand is incorporated into an Argonaute-containing RNA-induced silencing complex (RISC). The RISC-loaded miRNA contains seed region that binds to the complementary sequences in the 3′ untranslated regions (3′UTRs) of its target genes (mRNAs), resulting in negative regulation, such as transcript degradation or post-translational suppression.
Generally, each miRNA can regulate hundreds of target genes,4 with greater than one-third of all human genes being predicted to be regulated by miRNAs.5 MiRNAs are implicated in all cellular processes, including cell proliferation, cell differentiation and death, cellular metabolism, and immune responses in physiological as well as pathological conditions.6–9 Since their discovery in the 1990s,1,2 they are being investigated as biomarkers for a variety of diseases including cancer, stroke, traumatic brain injury (TBI) and spinal cord injury (SCI).10–34
MiRNAs have also generated interest as drug targets,35 because they have several desirable features for drug development including: (1) a single miRNA down-regulates hundreds of targets by binding to the 3′UTR of its target genes;36–42 (2) miRNAs are short ∼22 nucleotides in length for which miRNA drugs can easily be designed; (3) miRNAs are often conserved between species;43 (4) miRNA drugs can be delivered in vivo via several drug delivery systems that have been approved for human use.44,45 Several pharmaceutical companies have been pursuing miRNA therapeutics over the last decade, with several miRNA drugs advanced to human trials, such as miravirsen, RG-101, RG-125/AZD4076, MRX34, and TagomiRs.35,45–48 These studies support the feasibility of miRNA therapies for humans. Although most miRNA drugs in current clinical trials are focused on cancer, increasing numbers of miRNA-based drugs (e.g. anti-miR-497, anti-Let-7f, anti-miR-181, anti-miR-15a/16-1, anti-miR-23a, miR-424 mimic, miR-124 mimic, miR-122 mimic, miR-21 mimic, and others) have been tested in experimental stroke, TBI and SCI models.49–64 We will discuss these miRNA-based therapeutic applications and the underlying mechanisms for non-CNS diseases and CNS injuries in detail in the following sections.
Altered miRNA profiles in CNS injuries
MiRNAs expression studies have demonstrated many miRNAs increase or decrease in brain, blood, CSF, and/or saliva after CNS injuries.18–34 Targeting several miRNAs (e.g., miR-497, Let-7f, miR-181, miR-15a/16-1, miR-23a, miR-424, miR-124, miR-122, miR-21, others) that are altered after CNS injuries, we and others have examined the therapeutic efficacy of miRNA drugs (miRNA inhibitor or miRNA mimic in relation to one miRNA and one type of CNS injuries) to improve outcomes after experimental stroke, TBI or SCI.49–64 Moreover, these miRNA studies are also beginning to broaden our understanding of the pathogenesis of these injuries.65
As shown in Figure 1, increases of miRNAs (miR-497, Let-7f, miR-181, miR-15a/16, miR-23a, miR-424) down-regulate their target genes (i.e. Bcl-2, IGF-1, SHPA5, FGF2, FGFR1, VEGFR2),49–60 whereas decreases of miRNAs (miR-124, miR-122, miR-21) up-regulate their target genes (i.e. Src, ROCK, Pla2g2a, Rhdbf1, Nos2, PTEN).61–64 These complex miRNA-target interactions can affect many processes including neuronal death, neurogenesis, angiogenesis, platelet aggregation, leukocyte infiltration, blood–brain barrier (BBB) disruption, and tissue infarction that affect neurological deficits after CNS injuries (Figure 1).
Figure 1.
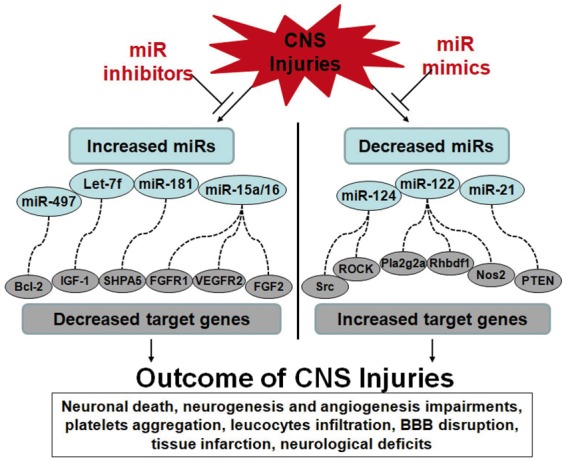
Altered miRNAs regulate target genes resulting in multiple outcomes after CNS injuries. Dysfunctioned miRNAs during CNS injuries causes multiple pathological outcomes, including neuronal death, neurogenesis and angiogenesis impairments, platelets aggregation, leucocytes infiltration, BBB disruption, tissue infarction, neurological deficits, and others. Left panel: Increased miRNAs lead to down-regulation of their target genes, resulting in deteriorated outcomes after CNS injuries. The miRNA-target gene pairs include miR-497::Bcl-2, Let-7f::IGF-1, miR-181::SHPA5, miR-15a/16::FGFR1/VEGFR2/FGF2, and others. Right panel: Decreased miRNAs lead to up-regulation of their target genes, resulting in detrimental outcomes after CNS injuries. The miRNA-target gene pairs include miR-124::ROCK/Src, miR-122::Pla2g2a/Rhbdf1/NOS2, miR-21::PTEN, and others. MiRNA inhibitors or mimics can either suppress increased miRNAs or elevate decreased miRNAs, respectively, to improve multiple outcomes after CNS injuries.
Circulating miRNAs as diagnostic and prognostic biomarkers for CNS injuries
MiRNA sequencing in cancer tissue has demonstrated the potential of miRNAs for diagnostics in clinical applications.10–13 Because sampling brain tissue from living humans is not practical for most neurological diseases, investigators have used blood, plasma, CSF, and saliva as accessible sources of RNA to perform miRNA expression profiling studies in CNS diseases.66 MiRNAs in various biofluids are very stable, often found in association with Argonaut protein, microvesicles, or exosomes which protect them from RNAases, and represent potentially informative biomarkers for a range of diseases including CNS injuries.12,67
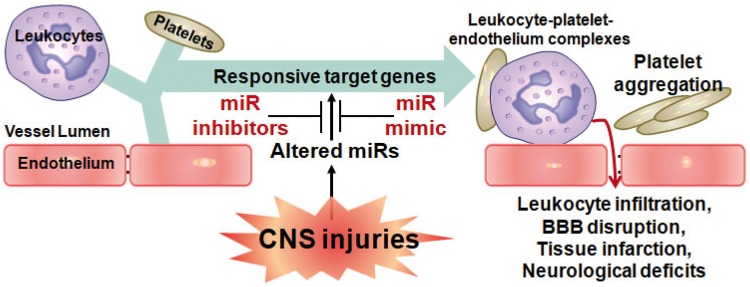
Blood is often used when studying immune response and blood coagulation in CNS injuries.68 This is because a large number of blood cells (leukocytes, platelets, erythrocytes) contain abundant miRNAs and play critical roles in CNS injuries, including inflammatory response, platelet aggregation, platelet-monocyte complexes, leukocytes adhesion to endothelium and subsequent leukocytes infiltration that result in BBB disruption and parenchymal CNS injuries (Figure 2).69–77 Recent interest has focused on the potential diagnostic and prognostic application of plasma, serum and saliva miRNAs. It has been suggested that the changes of miRNAs in plasma or saliva may be derived from injured CNS cells and recruited blood cells, as microvesicles and exosomes containing miRNAs are released from these cells into plasma and saliva.78,79 However, the origin and release mechanisms of miRNAs found in plasma and saliva are still not thoroughly understood.
Figure 2.
Function of circulating microRNAs in CNS injuries. During CNS injuries, decreased miRNAs in blood lead to upregulation of their target inflammatory genes and blood clotting genes, and thus results in multiple outcomes after CNS injuries, including leukocyte-platelet-endothelium complexes, platelet aggregation, leukocyte infiltration, BBB disruption, tissue infarction, neurological deficits, and others. MiRNA mimics replace the decreased miRNAs in blood and accordingly attenuate these detrimental outcomes after CNS injuries.
MiRNAs as biomarkers in animal models
Jeyaseelan et al.18 first examined miRNA expression profiles in whole blood after transient ischemic stroke. They found: (1) miR-19b, miR-290, and miR-292-5p increased and miR-103 and miR-107 decreased after 24-h reperfusion; and (2) miR-150, miR-195, miR-352, miR-26b, miR-103, miR-107, miR-26a, let-7c and others changed expression after 48-h reperfusion.18 Using Taqman rodent miRNA arrays, we examined miRNA expression profiles in whole blood and brain 24 h after ischemic stroke, hemorrhagic stroke, and kainic acid induced status epilepticus.19 These data showed: (1) the blood miRNA response profiles were different for each condition; (2) many miRNAs changed more than 1.5 fold in blood and brain after each experimental manipulation, and several miRNAs were up- or down-regulated in both brain and blood after a given injury; (3) a few miRNAs (e.g. miR-298, miR-155, miR-362-3p) were up- or down-regulated more than 2-fold in both brain and blood after several different injuries.19 These two studies confirmed that blood miRNAs could be of utility as biomarkers for CNS injuries.18,19
Subsequent studies have shown miR-124 increases ∼150-fold in plasma after ischemic stroke.20 Let-7i, miR-122, miR-340-5p, miR-200b, and miR-874 are modulated in serum after post-blast TBI in rats.21 MiR-9, miR-219 and MiR-384-5p increased in the serum of mice 12 h after SCI.33 MiR-133a-5p, miR-378, miR-378b-3p, miR-365-3p, miR-133b, miR-10b, miR-885-5p, miR-130a, miR-100, miR-208b and others were altered in serum at 1 and 3 days after SCI in pigs, and these strongly correlated with outcome measures at 12 weeks post SCI.34
MiRNAs as biomarkers in patients
In patients with stroke and TBI, differences in miRNA levels have been reported for extracellular miRNA in plasma (circulating miRNA), and for intracellular miRNA from blood cells.80 In the subacute and chronic phase of ischemic stroke, circulating plasma levels of miR-21, miR-221,81 and miR-14582 are increased, whereas miR-210 is decreased.83 In acute stroke, circulating miR-143-3p, miR125a-5p, miR-125b-5p are reported to be increased in both derivation and validation cohorts.84 A decrease in miR-150-5p measured within 72 h is a predictor of 90-day mortality.85 A number of other extracellular miRNAs have been associated with stroke as reviewed by Dewdney et al.86 In TBI, a number of circulating miRNAs are reported to change, with an increase in miR-765, miR-16, miR-125-5b, miR-1515, miR-199a-3p, miR-20a, miR-21, miR-27a, miR-27b, miR-30d, miR-328, miR-335, miR-362-3p, miR-92a, miR-486, miR-505*, miR-451 and decreases in miR-142-3p, miR-423-3p, miR-425-5p, and miR-502.23–25,87 Several miRNAs changed in stroke overlap with those in TBI including miR-125-5b, miR-16 and miR-27a.
There has been substantial variability in the extracellular miRNA reported to be different between these studies. Most have been small and had differences in time of sample collection post-stroke, patient characteristics, methods of sample collection, methods of RNA isolation and miRNA measurement, and methods to assess for cell lysis contribution to extracellular miRNA. Factors such as type of tube used for blood collection, volume of blood collected, time from collection to processing, centrifugation speed and time and freeze thaw cycles all are reported to affect microparticle generation and circulating miRNA.88 As our understanding of circulating miRNA improves, it is clear that standardized protocols for circulating miRNA are needed for future studies in stroke. The source of miRNA in plasma also remains to be established. The differential contribution of platelets and peripheral blood cells as well as other tissues to the circulating miRNA pool may account for some of the variability observed in studies.88 Levels of miRNA in plasma are very low, thus small contributions from other tissues or lysed cells could significantly impact results.
At the intracellular level, miRNAs in circulating leukocytes and other blood cells in patients with stroke have been studied. In a study of chronic stroke 6–18 months post event, 157 miRNAs were differentially expressed in young stroke compared to healthy controls.89 In acute stroke, we reported miR-122, miR-148a, let-7i, miR-19a, miR-320d, miR-4429 were decreased and miR-363, miR-487b were increased compared to vascular risk factor controls.22,90 Several of the identified miRNAs in acute cerebral ischemia overlap with those reported in subacute and chronic stroke including miR-19a, miR-320d, miR-363, and miR-487b. In patients with stroke, let-7i microRNA has been shown to regulate important aspects of the immune response in cerebral ischemia.90 Further study is needed to determine the course of miRNA changes over time after ischemic stroke as well as how intracellular miRNA relates to extracellular plasma cellular miRNA.
Development and therapeutics of miRNA-based drugs for non-CNS diseases
MiRNA-based therapeutics can presently be divided into two categories: miRNA mimics and miRNA inhibitors. If specific miRNAs are downregulated and correlate with disease progression, miRNA mimics could compensate for the functional activity of the lost miRNAs. In contrast, if specific miRNAs are upregulated and appear to contribute to disease pathogenesis, it might be beneficial to suppress the over-expressed miRNAs using miRNA inhibitors (or antimiRs).
Overview of miRNA mimics and inhibitors
MiRNA mimics are synthetic short double-stranded oligonucleotides imitating miRNA precursors. Once introduced into cells, these oligonucleotides can be recognized by miRNA biogenesis machinery and processed accordingly.91,92 Since the strand of interest (guide strand) needs to be identical to the native mature miRNA, miRNA mimics are constructed with one “guide strand” and one fully or partially complementary “passenger strand.”91
MiRNA inhibitors inhibit the interaction between miRNA and the microRNA-induced silencing complex (miRISC) proteins or between the miRISC and its target mRNAs.93 AntimiRs were originally designed as single-stranded antisense oligonucleotides (ASOs), which traditionally target a specific mRNA to block its translation into protein or trigger its destruction.94 AntimiRs now refer to the modified ASOs having the full or partial complementary reverse sequence of a mature miRNA.45,95
Chemical modifications of miRNA mimics and inhibitors
For miRNA mimics, the “guide strand” must be identical to mature miRNA, and position-specific chemical modifications are made to the “passenger strand” to ensure that only the “guide strand” is loaded onto the RISC.96 To enhance the stability but not interfere with the recognition by the RISC, only limited chemical modifications can be made to the “guide strand.”97 The 2′-sugar modification, such as 2′-O-methyl and 2′-fluoro (2′-F), helps to protect against nucleases, which improve the potency and stability of the guide strand without interfering with RISC loading.98,99 Strategies to improve the cellular uptake, such as cholesterol conjugation, can also result in off-target effects.95 Currently, commercially available miRNA mimics are normally modified by methylation of the “passenger strand” for increased stability,45 though vendors often do not disclose their chemical modifications.100
Several chemical modifications have improved the stability, permeability and specificity of miRNA inhibitors.95,101 Current modifications include phosphorothioate containing oligonucleotides,102,103 addition of 2′-O-methyl (2′-O-methyl) to phosphorothioate nucleotides, 2′-O-methoxyethyl-Oligonucleotides (2′-O-MOE, which are also called antagomirs),104 locked nucleic acid (LNA) modified antimiRs,105 fluorine derivatives (2′deoxy-2′-fluoro-RNA),106 peptide nucleic acids modified antimiRs107,108 or mixed modifications among these approaches. As a traditional and non-specific modification, cholesterol conjugation at the 3′ end of the strand can improve tissue distribution and cellular uptake.109 Novel chemical modifications are continuing to be developed like a pH low insertion peptide-modified antimiR to inhibit miR-155 in lymphoma.110
MiRNA-based therapeutics for non-CNS diseases in animal models
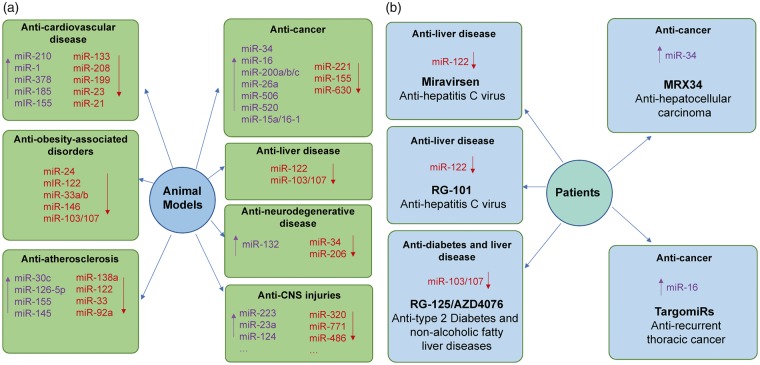
There has been an enormous increase in studies of the role of miRNA in human diseases using a variety of animal disease models.32,45,111–119 MiRNA-based therapeutic studies in animals (Figure 3(a)) have provided new avenues for drug development for different human diseases.32,45,111–119 Though this review focuses on CNS injuries, miR treatments are being tested in variety of pathological conditions in animal models.
Figure 3.
MicroRNA-based therapeutics in experimental diseased animal models and human patients. (a) MiRNA-based therapeutic applications in animal models of human diseases. In animal models, various miRNA-based therapeutics possess promising treatment potential against different human diseases, including cardiovascular diseases, obesity-associated disorders, atherosclerosis, cancers, liver diseases, neurodegenerative diseases and central nervous system (CNS) injuries. (b) MiRNA-based therapeutic applications in patients. To our knowledge, five miRNA-based drugs have been tested in clinical trials. Miravirsen and RG-101 are miR-122 inhibitors and are designed for the treatment of HCV-induced liver disease. RG-125/AZD4076 is miR-103/107 inhibitor and is designed for the treatment of Type 2 Diabetes and non-alcoholic fatty liver diseases. MRX34 is miR-34 mimic and is designed for the treatment of advanced hepatocellular carcinoma. TargomiRs is miR-16 mimic and is designed for the treatment of recurrent thoracic cancer. “↑”, upregulate miRNA levels by various miRNA mimics; “↓”, downregulate miRNA levels by various miRNA inhibitors.
Examples include heart failure,120 where miR-17-92, miR-126, miR-24, miR-214 and miR-34 show anti-angiogenic effects, while miR-210 exhibits pro-angiogenic functions.112,121 Cardiac hypertrophy, which can precede heart failure,122 can be abrogated with miR-1 mimic,123 or overexpression of miR-133,124 miR-378,125 miR-185 and miR-155.112 Inhibition of the miR-208 family, miR-212/132 family, miR-199b, miR-23, miR-21 and miR-15 family can improve cardiac outcomes.126 Cardiomyocyte death, which can accompany heart failure,127 can be decreased by carvedilol, a β-adrenergic blocker, which increases miR-133 expression.128
Intravenous (IV), chemically modified, cholesterol-conjugated miR-122 antagomir reduced miR-122 levels in liver and decreased hepatitis C virus (HCV) replication.111,129 IV LNA-modified miR-122 antagomir in chimpanzees chronically suppressed HCV viremia.130 In hepatocellular carcinoma, miR-122 silencing in liver reduced tumor cell proliferation, increased apoptosis and cell-cycle arrest, and increased mouse survival.131
A miR-24 antagomir in an obesity mouse model alleviated hyperlipidemia and fatty liver.132 An LNA-antimiR to miR-122 in African green monkeys increased hepatic fatty acid oxidation and reduced plasma cholesterol and cholesterol synthesis.133 Antagomirs of miR-33a and miR-33b in African green monkeys increased plasma HDL-cholesterol.134 LNA-miR-146b antagomir significantly reduced body weight and fat volume in mice fed a high-fat diet.135 MiR-103/107 antagomir improved glucose homeostasis and insulin sensitivity, suggesting a treatment for type 2 diabetes and obesity.136
In cancer research, IV miR-34 mimic reduced tumor growth and enhanced survival rates in mouse models of hepatocellular carcinoma (HCC).137 MiR-34 mimics also improve outcomes in animal cancer models of liver,138 prostate,139 lung,138 and pancreas.140 MiR-200 family members improve outcomes for ovarian and lung cancers,141,142 miR-26a for hepatocellular cancer,143 miR-506 and miR-520 for ovarian cancer,144,145 and miR-15/16 cluster for leukemia.146 Anti-miR-10b improved breast cancer outcomes,147 anti-miR-221 treatment improved HCC,131 anti-miR-155 treatment improved lymphoma110,148 and anti-miR-630 improved ovarian cancer.149
MiRNA-based therapeutics for non-CNS diseases in patients
Several miRNA-based drugs tested in preclinical animal models have been advanced to human clinical trials including miravirsen, RG-101, RG-125/AZD4076, MRX34, and TagomiRs (Figure 3(b)). Although none of these miRNA-based drugs in human clinical trials are related to the brain, these drugs still indicate their great potential in the therapeutic application for CNS injuries.
Miravirsen, a miR-122 inhibitor, entered phase IIa clinical trials.46 Subcutaneous injections of miravirsen in patients with chronic HCV genotype 1 infection showed prolonged dose-dependent reductions in HCV RNA levels without evidence of viral resistance or side effects.46 RG-101, another miR-122 inhibitor, has completed a phase I trial targeting HCV-infected patients as well.45 A single subcutaneous dose of RG-101 produced a sustained viral load reduction with a favorable safety profile.
RG-125/AZD4076, an N-acetylgalactosamine (GalNAc)-conjugated anti-miR-103/107 oligonucleotide, was proceeded to a phase 1/IIa clinical trial since July 2016 by AstraZeneca. Preliminary data indicated that RG-125/AZD4076 can improve insulin sensitivity in Type 2 diabetes and non-alcoholic fatty liver diseases. However, the development of RG-125/AZD4076 has been halted as the clinical programs were scuttled in June 2017.150
MRX34, a liposome-based miR-34 mimic, has entered a Phase I clinical trial in patients with advanced HCC.151 MRX34 appears to act as a tumor suppressor by inhibiting multiple oncogenic pathways and stimulating anti-tumor immune responses. However, due to multiple immune-related severe adverse events (SAEs) observed in study patients, the clinical Phase I study for MRX34 was halted on 20 September 2016.152
TargomiRs, a miR-16-based miRNA mimic, is going into phase I clinical trials to treat patients with recurrent thoracic cancer.153 The miR-16 family has been implicated as a tumor suppressor in a range of cancer types, and now TargomiRs is being tested for safety and effectiveness against malignant pleural mesothelioma (MPM) and non-small cell lung cancer (NSCLC).153
Moreover, many miRNA-base drugs are currently in the development pipeline to initiate clinical trials for different human diseases. For example, MGN-1374, targeting miR-15/195, is under the preclinical stage for the treatment of post-myocardial infarction. MGN-9103, an LNA-modified ASOs targeting miR-208, is in the pipeline for the treatment of chronic heart failure.154
MiRNA-based therapeutics for CNS injuries in experimental animal models
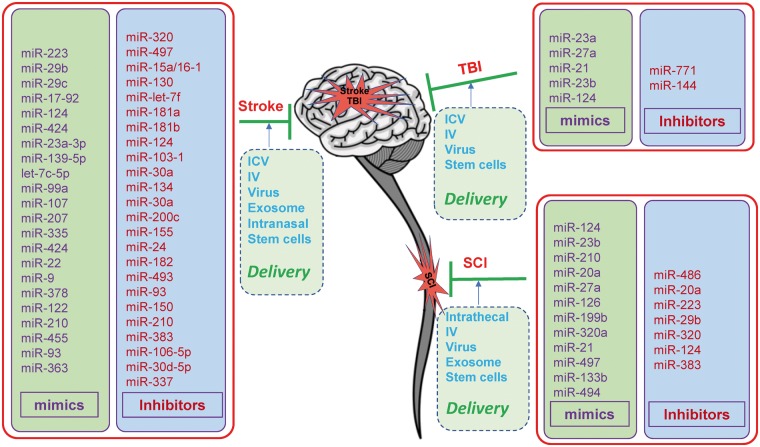
MiRNA-based drugs have been tested in various experimental animal models for their protective effects and mechanisms of action in stroke, TBI and SCI (Figure 4).32,117,155–157
Figure 4.
MicroRNA-based therapeutics for CNS injuries in experimental animal models. In the brain or spinal cord, downregulating miRNA expression by specific miRNA inhibitors, or upregulating miRNA expression by specific miRNA mimics in experimental animal models of stroke, traumatic brain injury (TBI) or spinal cord injury (SCI) revealed potential therapeutic effects against these CNS injuries. Currently, the delivery methods for the miRNA mimics or inhibitors mainly include intracerebroventricular (ICV) injection, continuous ICV infusion with osmotic minipumps, intravenous (IV) administration, intranasal administration, intrathecal administration, virus-mediated delivery, exosome-mediated delivery and stem cell-mediated delivery. In addition, a number of miRNA-based drugs have been administrated to experimental animals by combined methods to achieve the maximal delivery efficacy.
MiRNA-based therapeutics for stroke
Accumulating studies have been performed to investigate the therapeutic values of miRNA mimics against stroke. A number of miRNA mimics protect against ischemic injury in animal stroke models including miR-223,158 miR-29b,159,160 miR-29c,161 miR-17-92,162,163 miR-122, miR-124,61,164–167 miR-210,168,169 miR-424,59,170 miR-23a-3p,56 miR-139-5p,171 miR-let-7c-5p,172 miR-99a,173 miR-107,174 miR-207,175 miR-335,176 miR-22,177 miR-9,178 miR-378,179 miR-122,180 miR-210,169 miR-455,181 miR-93182 and miR-363.183 These miR-mimics usually reduce infarct volume and edema, and improve acute neurological outcomes and long-term functional recovery. For example, lentiviral overexpression of miR-29b or intracerebroventricular (ICV) administration of miR-29c mimic before MCAO attenuated stroke-induced infarction, edema, BBB disruption, and improved functional recovery.159–161 Overexpression of miR-124 by mimic, viral vector, modified liposomes and constructed exosomes before or after MCAO significantly reduced infarction volume and functional impairment in mice, and enhanced neuronal survival and neurovascular remodeling.61,164–167 Pre- and post-stroke upregulation of miR-424 with lentivirus and miR-424 mimic decreased cerebral infarction size and brain edema after MCAO, by inhibiting neuronal apoptosis, microglial activation, and oxidative stress.59,170 Moreover, ICV injection of miR-139-5p mimic attenuated the infarct volume and inhibited neuronal apoptosis in a rat neonatal hypoxia-ischemia model.171 ICV injection of let-7c-5p mimic decreased infarct volume and attenuated neurological deficits by suppressing microglial and caspase-3 activation.172 Furthermore, IV miR-122 mimic decreased neurological deficits and brain infarction volume, maintained vessel integrity and attenuated pro-inflammatory cytokines.180 IV administration of miR-17-92 cluster-enriched exosomes in rats after MCAO significantly improved neurological function and enhanced oligodendrogenesis, neurogenesis and neurite remodeling/neuronal dendrite plasticity in the ischemic boundary zone.163 IV administration of miR-363 mimic after MCAO reduced infarct volume, preserved forebrain microvessels and improved sensory motor performance in middle-aged female rats.183
On the other hand, a number of miRNA inhibitors have been tested in rodent stroke models as well. Inhibition of miRNAs that improve outcomes in rodent stroke models include miR-145,184 miR-320a,185 miR-497,49 miR-let-7f,50 miR-181a,51,186,187 miR-181b,55 miR-103-1,188 miR-30a,52,189 miR-124,190 miR-134,57 miR-200c,58 miR-155,191–193 miR-335,176 miR-24,194 miR-182,195 miR-493,196 miR-93,197 miR-150,193 miR-210,198 miR-383,199 miR-106b-5p,200 miR-15a/16-1,60 miR-30d-5p,201 miR-337202 and miR-337-3p.203 Inhibition of these miRNAs reduces infarct volumes, edema, inflammation and neuronal loss, and improves neurological outcomes. ICV administration of anti-miR-320a immediately after MCAO effectively reduced brain infarct volume and brain edema in a rat model of cerebral ischemia that correlated with upregulation of aquaporins 1 and 4 mRNA and protein.185 We reported that ICV infusion of an miR-497 antagomir knocked down cerebral miR-497, enhanced anti-apoptotic proteins in the ischemic region, decreased infarction volumes and improved neurological outcomes in mice after focal cerebral ischemia.49 Similarly, an antagomir of miR-let-7f reduced cortical and striatal infarcts and preserved sensorimotor function in intact female rats but not in males or ovariectomized females after stroke.50 MiR-181a inhibition alleviated stroke-induced brain injury by increasing GRP78 protein and anti-apoptotic proteins expression, and reducing apoptosis and inflammation.51,186,187 MiR-155 and miR-493 inhibitors improved blood flow, angiogenesis, and neurological function after stroke.191–193,196 Moreover, pretreatment with miR-200c antagomir protected against post-stroke neurological deficits by increasing Reelin, a regulator of neuronal migration and synaptogenesis.58 Pretreatment with miR-150 antagomir protected BBB-associated proteins to preserve BBB integrity during stroke.204 Systematic administration of miR-106b-5p antagomir decreased neurological deficits and infarct volumes that correlated with inhibiting apoptosis and oxidative stress after cerebral ischemia.200 We also showed that IV miR-15a/16-1 antagomir reduced cerebral infarct volume and brain water content, and improved neurological outcomes following stroke that correlated with upregulation of anti-apoptotic proteins and decreasing proinflammatory molecules.60
MiRNA-based therapeutics for TBI
Several miRNA mimics have been demonstrated to exert neuroprotection against TBI in experimental animal models. MiR-23a and miR-27a mimics injected into the brain ventricles significantly attenuated cortical lesion volume and neuronal cell loss in hippocampus of a mouse TBI model.205,206 Similarly, miR-21 mimic alleviated brain edema, decreased BBB damage and loss of tight-junction proteins, lessened lesion volume and improved long-term neurological function after TBI.63,207 Lentiviral overexpression of miR-23b decreased lesion volume, brain edema, and neurological deficits, and improved cognition.208 IV injection of exosomes wrapped miR-124-3p mimics inhibited neuronal inflammation and promoted neurite outgrowth in mice after experimental TBI.209
To our knowledge, two miRNA inhibitors have also been found to exhibit protective effects against TBI. Central administration of a miR-711 hairpin inhibitor reduced cortical lesion volume, neuronal cell loss in cortex and hippocampus, and alleviated long-term neurological dysfunction in a mouse TBI model.210 Similarly, a miR-144 antagomir reduced lesion volume, alleviated brain edema and improved cognitive functions in a rat TBI model.211
MiRNA-based therapeutics for SCI
MiRNA mimics that have neuroprotective role in experimental SCI models include miR-124,212–216 miR-23b,217 miR-210,218 miR-20a,219 miR-27a,220 miR-126,221 miR-199,222 miR-320a,223 miR-21,224 miR-497,225 miR-133b226 and miR-494.227,228 Upregulation of miR-124 by transplantation of lentiviral-miR-124 infected neural stem cells (NSCs) or bone marrow-derived mesenchymal stem cells (BMSCs) into the injured rat spinal cord reduced lesion cavity volume and improved function.212–214,216 The miR-210 mimic promoted angiogenesis and astrogliosis and improved functional recovery in a mouse SCI model.218 MiR-27a ameliorated inflammatory damage at the blood–spinal cord barrier (BSCB) after spinal cord ischemia/reperfusion,220 and miR-21 mimic exerted neuroprotective effects in spinal cord against ischemia/reperfusion injury.224
Inhibition of specific dysregulated miRNAs by miRNA inhibitors can also effectively reduce the detrimental outcomes after SCI. A miR-20a inhibitor injected into the injured spinal cord promoted motor neuron survival and neurogenesis.229 Intrathecal administration of a miR-486 inhibitor to the injured site ameliorated spinal cord damage and improved motor function.230 Similarly, a miR-223 antagomir protected injured spinal cord and promoted functional recovery of the hindlimbs.231 The lentiviral-antagomir-320 when administered before SCI improved hind-limb motor function and increased the number of intact motor neurons in the lumbar spinal cord after SCI.232 Intraspinal injection of adeno-associated virus (AAV)-miR-383 infected BMSCs increased intact tissue, decreased cavity volume, and enhanced recovery of locomotor activity in rats following SCI.233
Delivery methods of miRNA-based drugs to the CNS
The BBB limits the direct access of most compounds to the brain or spinal cord. Several methods have been developed for delivery of miRNA-based drugs to the CNS for the treatment of stroke, TBI and SCI (Figure 4).
ICV injection
ICV injection, which bypasses the BBB, is often used to deliver miRNA-based drugs to the brain in experimental animal models.234 MiRNA mimics delivered via ICV injection include: miR-29c,161 miR-210,168 miR-23a-3p,56 miR-139-5p,171 miR-let-7c-5p,172 miR-99a,173 miR-107,174 miR-207,175 miR-335176 and miR-424,59 and miR-378.179 MiRNA inhibitors delivered by ICV injection include: miR-145,184 miR-497,49 miR-181b,55 miR-103-1,188 miR-24,194 miR-320,185 miR-let-7f,50 miR-181a,51 miR-124,164,190 miR-200c,58 miR-181,187 miR-155,193 miR-182,195 miR-493,196 miR-93,197 miR-150,204 miR-210198 and miR-30-5p.201 ICV injection of mimics of miR-23a/27a205 and miR-21,63,207 and inhibitors of miR-711210 and miR-144211 improve neurological outcomes following TBI. Though these types of studies are convenient, and provide proof of principle, they probably will not translate to the clinic.
Intrathecal administration
Intrathecal administration into the subarachnoid space is often used to deliver miRNA-based drugs to injured spinal cord or into the cisterna magna at the base of the brain. MiRNA injected intrathecally after SCI have included: miR-486,230 miR-23b,217 miR-223,231 miR-27a,220 miR-126,221 miR-199b,222 miR-320a,223 miR-21,224 miR-497,225 miR-124,235 miR-494,227,228 and miR-383.233 As an example, miR-126 mimic injected via subdural catheters seven days after SCI increased levels of miR-126, promoted angiogenesis and inhibited leukocyte extravasation into injured spinal cord.221
IV injection
Recent methods have been developed to facilitate miRNA crossing the BBB including modifications of mimics and inhibitors, delivery via PEG lysosomes and other methods. IV injection has many advantages including ease, large administration volumes,236 potential for reaching all injured tissues, and most importantly, it is clinically applicable with little risk.212 An IV miR-155 inhibitor decreased miR-155, promoted brain angiogenesis, reduced tissue damage and improved functional recovery in a mouse stroke model.191 IV administration of other inhibitors including miR-181,187 miR-383,199 miR-106-5p,200 miR-15a/16-160 and miR-337-3p,203 and IV mimics including miR-124,61 miR-9,178 miR-122,180 miR-93182 and miR-363183 decreased damage and improved function in rodent stroke models.
Intranasal administration
Intranasal administration is also a non-invasive, and potentially clinically relevant approach for drug delivery to bypass the BBB and allow access to the CNS.237 It can avoid fast systemic clearance and potentially decrease side-effects.198 Peptides, proteins, vectors and even stem cells have been delivered intranasally mostly in rodents to treat CNS injuries including stroke.237,238 Intranasal and ICV administration of the LNA-miR-210 inhibitor 4 h post-hypoxia ischemia in neonatal rats produced similar decreases of tissue damage and improvements in neurological function later in life.198
Virus-mediated delivery
Modified AAV and lentiviruses can deliver siRNA/shRNA into targeted genomes.239 Similar viral overexpression of miR-29b,159,160 miR-17-92,162 miR-124,165 miR-424,59 miR-22177 and miR-210,169 and knockdown of miR-30a52 and miR-13457 have improved stroke outcomes. Lentivirus overexpression of miR-23b208 and miR-27a206 improved outcomes following rodent TBI. Lentiviral inhibition of miR-320232 and miR-124235 protected spinal cords against ischemia-reperfusion injury and improved hindlimb motor function. Though viral studies can provide proof-of-concept, they would have little clinical application if they have to be delivered prior to injury.
Exosome-mediated delivery
Exosomes are endogenous, cell-secreted nano-scale diameter vesicles. Exosomes appear to cross the BBB and carry cargoes such as proteins, lipids and nucleotides (mRNAs and miRNAs) to mediate brain remodeling after stroke.167,240–242 Several miRNAs were shown to be delivered to the brain by exosomes based on their ability to carry miRNAs.240 IV miR-124-loaded RVG-Lamp2b-modified exosomes injected after brain ischemia promoted cortical neural progenitor cell differentiation, cortical neurogenesis, and protected against ischemic brain injury.167 IV miR-17-92 cluster-enriched exosomes improved neurological function following a stroke and enhanced oligodendrogenesis, neurogenesis, and neurite remodeling in the ischemic penumbra.163
Stem cell-mediated delivery
Various stem cells, including bone marrow-derived mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs), have been used to treat experimental stroke, TBI and SCI,243–252 and some of these are in clinical trials.253–261 However, stems cells do have limitations, mainly in terms of differentiation potential, tumorigenic activity and others.262–264 Since miRNAs are key regulators of stem cell renewal and differentiation, miRNAs have been used for bioengineering stem cells to overcome these hurdles.265–267 Although no reports of the miR-engineered stem cells are being tested in treatment of patients with CNS injuries yet, recent studies have demonstrated that miR-engineered stem cells (i.e. miR-705, miR–381, miR-17-92, miR-124 over-expressed bone MSCs or NSCs) versus non-engineered ones can improve functional outcomes following various experimental CNS injuries.163,212,268–270 MiRNA often are contained in exosomes, and can be used as a method to shuttle miRNA from one cell to another. This may have potential to modulate stem cells in a manner that is beneficial following stroke or TBI. In a rat MCAO stroke model, exosomes enriched in miR-17-92 cluster administers by IV injection resulted in improved neurological function and enhanced oligodendrogenesis, neurogenesis and neurite remodeling in the ischemic boundary zone.163 Authors promoted this may be mediated through miRNA-mediated downregulation of PTEN, Akt, mTOR and BSK-3B activity.163 With further study, potentially specific microRNA delivery with exosomes could be a novel approach to treat neurological disease and improve outcomes.
Time windows and dosage of miRNA-based drugs
Depending on the question being studied, miRNA-based drugs have been administered at a variety of time points from 6 days before to 10 days after MCAO. If a miRNA drug has a short half-life, multiple injections or continuous infusions may be required. Since IV injections are a clinically relevant method, most groups deliver miRNA-based drugs intravenously from 5 min to 4.5 h after MCAO, the therapeutic window for tPA in humans and during a time there is salvageable penumbra. Drugs administered one day and later after MCAO usually do not affect infarct volumes, but can sometimes improve behavioral outcomes during the recovery phases following MCAO. Viruses take time to express miRNA mimics or inhibitors and thus may injected 1 day to 14 days prior to the MCAO. The optimal time windows for administration of miRNA-based drugs for TBI and SCI must be determined empirically and likely depend on the question being studied and the model being used.
Doses of miRNA-based drugs should produce significant effects on predicted target genes, with mimics usually decreasing expression, and antagonists increasing expression of predicted targets. Half-life must be assessed to determine whether multiple injections or infusions are needed. Practically, dosing depends on delivery methods and animals used. For intraventricular injections in mice, concentrations ranged from 3 pmol/µl188 to 100 pmol/µl,181 with a single ICV injection of < 10 µl, followed by continuous infusion at 1 µl/h for 72–120 h. For ICV injections in rats, concentrations range from 5 pmol/µl to 25 nmol/µl with similar volumes as mice. For IV injections of 50–100 µl in mice post MCAO, concentrations varied from 30 pmol/g60,187 to 10–25 µg/g.191,203 For rats, similar volumes of miRNA-based drugs with concentrations of 0.6–7 mg/kg have been used. Doses of viruses carrying miRNA mimics or inhibitors are determined empirically.162,165,169,189
Mechanisms of miRNA-based therapeutics for CNS injuries
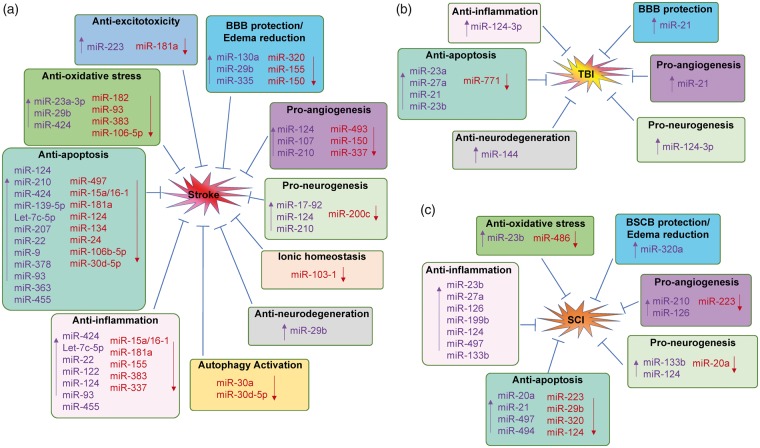
Since miRNAs can regulate so much of the genome, they can have multiple mechanisms of action against CNS injuries even from a single miRNA. These include anti-excitotoxicity, anti-oxidative stress, anti-endoplasmic reticulum stress, anti-inflammation, anti-apoptosis, anti-neurodegeneration, autophagy regulation, BBB protection and edema reduction, pro-angiogenesis, and neuronal and axonal regeneration. Some of these are discussed here (Figure 5).
Figure 5.
Mechanisms of microRNA-based therapeutics for CNS injuries. Therapeutic mechanisms of microRNA-based drugs for stroke (A), traumatic brain injury (TBI, B) and spinal cord injury (SCI, C). (a) Upregulation or downregulation specific miRNAs in the brain exhibits protective effects against stroke via different mechanisms, including anti-excitotoxicity, anti-oxidative stress, anti-inflammation, anti-apoptosis, BBB protection and edema reduction, pro-angiogenesis, pro-neurogenesis, anti-neurodegeneration, maintain ionic homeostasis and activation of autophagy. (b) Manipulation of several miRNAs exerts neuroprotection again TBI through mechanisms involving anti-inflammation, anti-apoptosis, BBB protection, pro-angiogenesis, pro-neurogenesis and anti-neurodegeneration. (c) A number of miRNAs are also involved for the potential therapy of SCI through various mechanisms, including anti-oxidative stress, anti-inflammation, anti-apoptosis, anti-neurodegeneration, BSCB protection and edema reduction, pro-angiogenesis and pro-neurogenesis. Moreover, many miRNAs possess the ability to regulate the pathophysiological events against CNS injuries via several different mechanisms. “↑”, upregulate miRNA levels by miRNA mimics; “↓”, downregulate miRNA levels by miRNA inhibitors.
Excitotoxicity
Several miRNA therapeutics attenuate excitotoxicity following ischemic stroke. For example, AAV-mediated overexpression of miR-223 in the hippocampus lowered the levels of glutamate receptor 2 (GluR2) and NMDAR subunit 2B (NR2B), inhibited NMDA-induced calcium influx in hippocampal neurons, and protected the brain from neuronal cell death following transient global ischemia.158 MiR-181a antagomir prevented the decrease of glutamate transporter 1 and reduced astrocyte dysfunction, resulting in increased neuronal survival in the hippocampal CA1 region.186
Oxidative stress
Upregulation of miR-23a-3p, miR-99a and miR-424 or inhibition of miR-93 and miR-182 protected ischemic brain by decreasing oxidative stress.56,59,173,195,197,271–273 For example, miR-23a-3p mimic reduced production of nitric oxide (NO) and 3-nitrotyrosine (3-NT), and increased expression of manganese SOD (MnSOD) to decrease oxidative stress in a mouse MCAO model.56 MiR-93 antagomir decreased infarction volume and improved function,197 which correlated with increased expression level of erythroid 2-related factor (Nrf2) and its downstream gene hemeoxygenase-1 (HO-1).274,275 In addition, miR-486 inhibition induced the expression of NeuroD6, which ameliorated SCI-induced ROS via upregulation of thioredoxin-like 1 (TXNL1) and glutathione peroxidase 3 (GPX3).230
Inflammation
Many miRNA-based drugs are anti-inflammatory. Specifically, most of the anti-inflammatory mechanisms involve the suppression of astrocytes activation, cytokines secretion and leukocyte extravasation. For example, overexpression of miR-424 and miR-let-7c-5p reduces infarct volume and improves neurological function, partially by inhibition of astrocyte activation after stroke.170,172 Overexpression of miR-22 and miR-122 or inhibition of miR-15a/16-1 reduces inflammatory molecules TNF-α, IL-6, MCP-1, COX-2, iNOS, and VCAM-1 in ischemic brain.60,177,180 Exosome-mediated delivery of miR-124-3p promotes M2 microglia polarization and inhibits tissue inflammation following TBI.209 MiR-27a, miR-199b, miR-124, miR-497 or miR-133b mimics inhibit SCI-induced inflammatory responses by reducing astrocyte/macrophage activation, and inhibiting NF-κB/IL-1β or IKKβ-NF-κB pathways.215,220,222,226
Apoptosis
Some miRNA-based drugs decrease apoptosis by decreasing pro-apoptotic proteins (e.g. Bax) and/or increasing anti-apoptotic proteins (e.g. Bcl-2, Bcl-w, Bcl-xl).276–279 MiR-497, miR-181a, miR-24, miR-15a/16-1 and miR-106b-5p antagomirs or miR-124 and miR-210 mimics enhance Bcl-2/-w/-xl protein levels in ischemic brain, attenuate infarction, and improve functional outcomes after stroke.49,60,61,168,186,194,200 A miR-124 inhibitor reduced infarction in a mouse MCAO model by inhibiting members of the apoptosis-stimulating proteins of p53 family (iASPP).164 MiR-23a, miR-27a, miR-21 and miR-23b decrease several pro-apoptotic proteins (e.g. Puma, Noxa, Bax, cleaved-caspase-3) in TBI.63,205,208 Inhibiting miR-29b or activating miR-20a, miR-21 and miR-494 mitigates apoptosis by inhibiting phosphatase and tensin homolog (PTEN) expression and activating AKT/mTOR signaling pathway in SCI.228
BBB/BSCB protection
Damage to the BBB following stroke and TBI, or the BSCB following SCI promotes entry of cytokines, chemokines, other inflammatory molecules and leukocytes that can promote tissue injury and edema.280–284 This entire cascade can be mitigated by some miRNAs. Downregulating miR-150 stabilizes TJ protein ZO-1,191 and increases claudin-5 and angiopoietin receptor Tie-2204 to improve BBB function after stroke. Anti-miR-320 or anti-mir-130 treatment upregulates the expression of aquaporins following stroke (AQP) 4, which have been implicated in cerebral edema clearance.185,285 MiR-320a mimic improved spinal cord ischemia/reperfusion-induced lower limb motor function, alleviated BBB disruption and decreased spinal water content by suppressing AQP1 expression.223
Angiogenesis
Angiogenesis, which may contribute to recovery following stroke, TBI and SCI,286–288 is modulated by several miRNAs. MiR-107 mimic reduced ischemic brain infarction and increased the number of capillaries in the penumbra by enhancing endothelial VEGF165/164 levels.174 MiR-21 mimic improvements in neurological outcome following TBI correlated in part with upregulation of VEGF, Angiopoietin-1 (Ang-1) and Tie-2 (receptor of Ang-1).63 MiR-210 promoted angiogenesis in injured spinal cord via inhibition of protein-tyrosine phosphate 1B and ephrin-A3 in rats.218 The mir-223 antagomir also promoted angiogenesis after SCI.231
Neurogenesis
Neurogenesis, which occurs in many areas of brain including the subventricular zone (SVZ), the subgranular layer (SGL) of the dentate gyrus (DG) in the hippocampus,289,290 cortex, and spinal cord, is modulated by stroke, TBI and SCI in part via miRNA.291–294 Overexpression of the miR-17-92 cluster in the SVZ significantly increased the proliferation of NSCs and promoted neurogenesis after stroke,162 by inhibiting its target gene PTEN, and consequently increasing the phosphorylation of protein kinase B (Akt), mechanistic target of rapamycin (mTOR), and glycogen synthase kinase 3β (GSK-3β).163 A miR-20a inhibitor improved motor neuron survival, increased neurogenesis and improved function in mice following SCI by rescuing expression of the miR-20a target gene neurogenin 1 (Ngn1).229
MiRNAs as therapeutic targets of pharmacological agents in CNS injuries
In addition to miRNA mimics and inhibitors, miRNA-based therapeutics for CNS injuries may also include the pharmacological agents that exert neuroprotection via the regulation of functional miRNAs. As shown in Table 1, several pharmacological agents that protect against stroke, TBI and SCI regulate specific miRNA that may partially mediate these effects.295–309 VELCADE upregulates miR-146a to inactivate the ischemia and tissue plasminogen activator (tPA) potentiated toll-like receptor (TLR) signaling pathway and thus helps protects against acute ischemic stroke.295 Arctigenin can upregulate the levels of miR-16 and miR-199a to reduce IKKα and IKKβ expression and inhibit NF-κB signaling pathway and promote cholinergic signaling;302 hydrogen gas303 regulates oxidative stress via upregulating miR-21. Several agents that are neuroprotective in SCI also regulate several miRNAs (Table 1), including ferulic acid which helped improve functional recovery in SCI rats by inhibiting miR-590 and increasing VEGF.304
Table 1.
MicroRNAs as therapeutic targets of pharmacological agents for CNS injuries.
| CNS injuries | Agents | miRNAs regulation | Mechanisms | References |
|---|---|---|---|---|
| Stroke | VELCADE | ↑ miR-146a | Inactivating ischemia and tPA potentiated toll-like receptor (TLR) signaling pathway | Zhang et al.295 |
| Acetylbritannilactone | ↓ miR-155 | Blocking the pro-inflammatory action of miR-155 in ischemic brain | Wen et al.296 | |
| Trimetazidine (TMZ) | ↑ miR-21 | Increasing PI3K pathway signaling and finally counteracted the apoptotic effects | Yang et al.297 | |
| Atorvastatin | ↓ miR-199a-5p | Increasing Glycogen synthase kinase-3β (GSK-3β) | Zuo et al.298 | |
| 6-[3-adamantyl-4-hydroxyphenyl]-2-napthalene carboxylic acid (AHPN) | ↓ miR-182 | Increasing global conjugation of small ubiquitin-like modifier (SUMO) | Bernstock et al.299 | |
| Nicorandil | ↑ miR-7 | Depressing the endoplasmic reticulum (ER) stress and attenuated ischemia-induced inflammatory responses and astrocytic damage | Dong et al.300 | |
| TBI | Arctigenin (ARC) | ↑ miR-16 and miR-199a | Reduce IKKα and IKKβ expression and inhibit NF-κB signaling pathway | Song et al.302 |
| Hydrogen gas (H2) | ↑ miR-21 | Alleviating brain edema and infarction after TBI, and upregulating endogenous antioxidant enzymes and downregulated oxidative products | Wang et al.303 | |
| SCI | Hydrogen sulfide (H2S) | ↓ miR-30c | Upregulating Beclin-1 and LC3II expression in spinal cord | Li et al.305 |
| ↑ miR-485-5p | Suppressing TNF receptor type 1-associated DEATH domain protein (TRADD) expression | Chen et al.306 | ||
| Naringenin (NR) | ↓ miR-223 | Inhibiting SCI-induced activation of neutrophils | Shi et al.307 | |
| Ferulic acid | ↓ miR-590 | Elevating the level of VEGF | Li et al.304 | |
| Tetramethylpyrazine (TMP) | ↑ miR-21 | Anti-apoptotic effects | Huang et al.308 | |
| ↓ miR-214-3p | Increasing the expression of anti-apoptotic protein Bcl212 | Fan et al.309 |
Note: Various pharmacological agents showing protective effects against stroke, TBI and SCI via regulating different miRNAs. “↑”, upregulate or “↓”, downregulate miRNA levels by pharmacological agents.
CNS: central nervous system; SCI: spinal cord injury.
The therapeutic effects of several pharmacological agents, including Acetylbritannilactone (ABL),296 Trimetazidine(TMZ),297 Nicorandil,300 arctigenin,302 hydrogen gas (H2),303 hydrogen sulfide (H2S),305,306 ferulic acid304 are depended on the modulation of specific miRNAs, since blocking the modulation of these miRNAs can completely abolish the protective effects of these agents against CNS injuries. For example, H2S pretreatment reduced spinal cord infarct zone and improved hindlimb motor function by downregulating miR-30c expression in a rat ischemia/reperfusion (I/R) model. MiR-30c mimic pretreatment abrogated the spinal cord protective effect of H2S.305
Challenges, perspectives and future goals
One general challenge in miRNA-based therapeutics is to avoid rapid degradation by the abundant RNases in the circulation or in the endocytic compartment of cells. Although chemical modifications have helped solve this problem, the half-life of some constructs can be very short which may require repeated injections or infusions.
A major problem in the miRNA therapeutics field is potential off-target effects. Some of these occur because commercially available miRNA mimics can produce unexpected off-target effects induced by the “passenger-strand” of the mimics.100 Another explanation for “off-target” effects is that though a given miRNA may regulate one or several target genes to improve functional outcomes following stroke, TBI and SCI, the miRNA may act on other target genes to produce unwanted side effects or even activate pathways that counteract the protective effects. Ways of increasing specificity of miRNA effects to selected target genes and blocking off-target effects are needed. Such differential effects on different target genes might explain studies where a miR-20a inhibitor improved neural cell survival following SCI229 in one study, whereas miR-20a mimics improved neuronal survival via an anti-apoptotic pathway219 in another study. The time course of miRNA effects on their targets must be carefully considered. For example, early administration of miR-124 mimic to ischemic brain significantly increased neuronal survival, whereas later administration did not.166
MiRNA delivery for CNS injuries, like all pharmacological agents, is complicated by the need to circumvent or penetrate the BBB or BSCB. This explains why nearly 2/3 of published studies have used ICV administration or intrathecal injection to bypass the BBB or BSCB. Though such studies demonstrate proof-of-principle, they usually would not translate to humans. Viral-mediated knockdown or overexpression of miRNAs usually requires time, so that this method would not be useful in acute CNS injuries, but could be considered during recovery if any immune reactions are carefully assessed and controlled. Nasal administration appears to allow some compounds to gain access to brain, though most of these studies have been performed in rodents, and it is not clear that such methods can be translated to humans with a different anatomy. Nasal administration is also complicated potentially by inconsistent delivery in patients with upper respiratory infections or sinus infections, but has the theoretical advantage of avoiding systemic side effects. IV injection is the accepted method, but requires chemical modifications of miRNA mimics and antagomiRs to obtain cell and BBB penetration. Systemic off-target effects can become problematic. Despite these challenges, tissue-specific and cellular receptors targeted AAV has provided an exciting way to deliver miRNA to the places of interest, and is currently in use in a number of preclinical models and clinical trials for gene therapy in cancer.95 It is possible exosomes, with or without stem cells, or engineered nanoparticles-miRNA-based drugs may improve the ability to cross the BBB and to avoid delivery to unwanted sites.
In stroke therapies, numerous neuroprotective agents have been proven effective in various preclinical animal studies.310 Unfortunately, none of them yield translational efficacy in clinical trials. Most large vessel ischemic stroke patients have permanent occlusion and could not restore the blood flow to allow effective concentration of neuroprotectants in the affected brain areas might account for this mismatch.311,312 Currently, none of miR-based drugs have been advanced to the development of clinical stroke therapies. If following the traditional way for previously tested neuroprotectants to evaluate miR-based drugs for stroke therapy, there may be a long way to go for the success in clinical trials. Fortunately, by using salvageable penumbral tissue criteria, recent DAWN trials revealed that thrombectomy has brought about significant benefits for acute ischemic stroke patients up to 24 h since symptom onset.313–315 In this context, combination therapy of selected miR-based drugs and recanalization via endovascular thrombectomy might provide new insight for the treatment of ischemic stroke in the future.
Many previous studies have not considered that miRNAs are differentially expressed in neurons, astrocytes, oligodendrocytes, and microglia in the CNS,316 and there is a specific complement of endothelial cell miRNAs that are likely to be important in stroke, TBI and SCI.317 Understanding the functions of miRNAs in specific cell types under normal and pathological conditions could improve specificity and efficacy of miRNA strategies. More attention should also be paid to elucidate the downstream therapeutic targets of specific miRNA in specific cell types following CNS injuries, and to identify target genes that produce unwanted side effects.
Although numerous studies demonstrated the possible signaling pathways and miRNA target genes, the functional significance of many of the target genes in mediating therapeutic effects of miRNA-base drugs were not further verified by means of overexpression, knockdown, or functional inhibition. Additional mechanistic investigations of miRNA target genes are needed to elucidate the underlying mechanisms of miRNA-based therapeutics towards CNS injuries.
Male animals have been mostly utilized in miRNA-based therapeutic applications in vivo. However, brain miRNA responses differ following focal cerebral ischemia between male and female mice,318,319 and sex can affect stroke outcomes.320–322 Thus, more effort should be devoted to include female animals in the experimental design. Indeed, there have been significant differences regarding miRNA-based therapeutics between male and female animals. For example, miR-363-3p mimics reduced the caspase-3 activity and brain infarct volumes in middle-aged female mice, but had no effects on stroke outcomes or caspase activities in young males.183
Despite the challenges, miRNA-based therapeutics have become promising strategies for CNS injuries. Much remains to be done to understand how miRNA exert their effects via hundreds of targets, developing approaches to identify candidate miRNAs, designing chemical formulations and delivery methods to target brain and cross the BBB, and methods to decrease off target effects. Successful clinical trials and clinically effective miRNA drugs will certainly enhance the future of this nascent field.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health Grants: NS091175, NS094930, NS086820 (KJ Yin); NS075035, NS079153, AG042292, NS101718 (FR Sharp, GC Jickling); NS089901 (DZ Liu).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75: 843–854. [DOI] [PubMed] [Google Scholar]
- 2.Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000; 403: 901–906. [DOI] [PubMed] [Google Scholar]
- 3.Winter J, Jung S, Keller S, et al. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 2009; 11: 228–234. [DOI] [PubMed] [Google Scholar]
- 4.Guarnieri DJ, DiLeone RJ. MicroRNAs: a new class of gene regulators. Ann Med 2008; 40: 197–208. [DOI] [PubMed] [Google Scholar]
- 5.Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer 2006; 6: 259–269. [DOI] [PubMed] [Google Scholar]
- 6.Matsubara H, Takeuchi T, Nishikawa E, et al. Apoptosis induction by antisense oligonucleotides against miR-17-5p and miR-20a in lung cancers overexpressing miR-17-92. Oncogene 2007; 26: 6099–6105. [DOI] [PubMed] [Google Scholar]
- 7.Hatfield S, Ruohola-Baker H. microRNA and stem cell function. Cell Tissue Res 2008; 331: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi Y, Liu G, Yang R. MicroRNAs: novel regulators during the immune response. J Cell Physiol 2009; 218: 467–472. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier BR, Wollheim CB. MicroRNAs: ‘ribo-regulators’ of glucose homeostasis. Nat Med 2006; 12: 36–38. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Xu Q, Wu F, et al. Using peripheral blood mRNA signature to distinguish between breast cancer and benign breast disease in non-conclusive mammography patients. Cancer Biol Ther 2011; 10: 1235–1239. [DOI] [PubMed] [Google Scholar]
- 11.Olmos D, Brewer D, Clark J, et al. Prognostic value of blood mRNA expression signatures in castration-resistant prostate cancer: a prospective, two-stage study. Lancet Oncol 2012; 13: 1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105: 10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allegra A, Alonci A, Campo S, et al. Circulating microRNAs: new biomarkers in diagnosis, prognosis and treatment of cancer (review). Int J Oncol 2012; 41: 1897–1912. [DOI] [PubMed] [Google Scholar]
- 14.Hydbring P, Badalian-Very G. Clinical applications of microRNAs. F1000Res 2013; 2: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 2007; 8: 93–103. [DOI] [PubMed] [Google Scholar]
- 16.Filip A. [MiRNA – new mechanisms of gene expression control]. Postepy Biochem 2007; 53: 413–419. [PubMed] [Google Scholar]
- 17.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 2014; 20: 460–469. [DOI] [PubMed] [Google Scholar]
- 18.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 2008; 39: 959–966. [DOI] [PubMed] [Google Scholar]
- 19.Liu DZ, Tian Y, Ander BP, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab 2010; 30: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laterza OF, Lim L, Garrett-Engele PW, et al. Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem 2009; 55: 1977–1983. [DOI] [PubMed] [Google Scholar]
- 21.Balakathiresan N, Bhomia M, Chandran R, et al. MicroRNA let-7i is a promising serum biomarker for blast-induced traumatic brain injury. J Neurotrauma 2012; 29: 1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jickling GC, Ander BP, Zhan X, et al. microRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS One 2014; 9: e99283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitra B, Rau TF, Surendran N, et al. Plasma micro-RNA biomarkers for diagnosis and prognosis after traumatic brain injury: a pilot study. J Clin Neurosci 2017; 38: 37–42. [DOI] [PubMed] [Google Scholar]
- 24.Bhomia M, Balakathiresan NS, Wang KK, et al. A panel of serum MiRNA biomarkers for the diagnosis of severe to mild traumatic brain injury in humans. Sci Rep 2016; 6: 28148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Pietro V, Ragusa M, Davies D, et al. MicroRNAs as novel biomarkers for the diagnosis and prognosis of mild and severe traumatic brain injury. J Neurotrauma 2017; 34: 1948–1956. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Chandran R, Barry ES, et al. Identification of serum microRNA signatures for diagnosis of mild traumatic brain injury in a closed head injury model. PLoS One 2014; 9: e112019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hicks SD, Johnson J, Carney MC, et al. Overlapping microRNA expression in saliva and cerebrospinal fluid accurately identifies pediatric traumatic brain injury. J Neurotrauma 2018; 35: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redell JB, Liu Y, Dash PK. Traumatic brain injury alters expression of hippocampal microRNAs: potential regulators of multiple pathophysiological processes. J Neurosci Res 2009; 87: 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JJ, Loeffert AC, Stokes J, et al. Association of salivary microRNA changes with prolonged concussion symptoms. JAMA Pediat 2018; 172: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandran R, Sharma A, Bhomia M, et al. Differential expression of microRNAs in the brains of mice subjected to increasing grade of mild traumatic brain injury. Brain Injury 2017; 31: 106–119. [DOI] [PubMed] [Google Scholar]
- 31.Liu NK, Wang XF, Lu QB, et al. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol 2009; 219: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ning B, Gao L, Liu RH, et al. microRNAs in spinal cord injury: potential roles and therapeutic implications. Int J Biol Sci 2014; 10: 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hachisuka S, Kamei N, Ujigo S, et al. Circulating microRNAs as biomarkers for evaluating the severity of acute spinal cord injury. Spinal Cord 2014; 52: 596–600. [DOI] [PubMed] [Google Scholar]
- 34.Tigchelaar S, Streijger F, Sinha S, et al. Serum microRNAs reflect injury severity in a large animal model of thoracic spinal cord injury. Sci Rep 2017; 7: 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt MF. Drug target miRNAs: chances and challenges. Trends Biotechnol 2014; 32: 578–585. [DOI] [PubMed] [Google Scholar]
- 36.Betel D, Koppal A, Agius P, et al. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 2010; 11: R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zhang Z. Computational biology in microRNA. Wiley Interdiscip Rev RNA 2015; 6: 435–452. [DOI] [PubMed] [Google Scholar]
- 38.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 39.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakymiw A, Pauley KM, Li S, et al. The role of GW/P-bodies in RNA processing and silencing. J Cell Sci 2007; 120: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 41.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A 2006; 103: 4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005; 433: 769–773. [DOI] [PubMed] [Google Scholar]
- 43.Christopher AF, Kaur RP, Kaur G, et al. MicroRNA therapeutics: discovering novel targets and developing specific therapy. Perspect Clin Res 2016; 7: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomed 2012; 7: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017; 16: 203–222. [DOI] [PubMed] [Google Scholar]
- 46.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013; 368: 1685–1694. [DOI] [PubMed] [Google Scholar]
- 47.Mirna Therapeutics I, www.mirnarx.com/pipeline/mirna-MRX34.html (2014, accessed 14 April 2018).
- 48.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov 2010; 9: 775–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin KJ, Deng Z, Huang H, et al. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis 2010; 38: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selvamani A, Sathyan P, Miranda RC, et al. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One 2012; 7: e32662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouyang YB, Lu Y, Yue S, et al. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis 2012; 45: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang P, Liang J, Li Y, et al. Down-regulation of miRNA-30a alleviates cerebral ischemic injury through enhancing beclin 1-mediated autophagy. Neurochem Res 2014; 39: 1279–1291. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, Ouyang Y, Xiong X, et al. Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Exp Neurol 2014; 264C: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi H, Sun BL, Zhang J, et al. miR-15b suppression of Bcl-2 contributes to cerebral ischemic injury and is reversed by sevoflurane preconditioning. CNS Neurol Disord Drug Targets 2013; 12: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng Z, Li J, Li Y, et al. Downregulation of miR-181b in mouse brain following ischemic stroke induces neuroprotection against ischemic injury through targeting heat shock protein A5 and ubiquitin carboxyl-terminal hydrolase isozyme L1. J Neurosci Res 2013; 91: 1349–1362. [DOI] [PubMed] [Google Scholar]
- 56.Zhao H, Tao Z, Wang R, et al. MicroRNA-23a-3p attenuates oxidative stress injury in a mouse model of focal cerebral ischemia-reperfusion. Brain Res 2014; 1592: 65–72. [DOI] [PubMed] [Google Scholar]
- 57.Chi W, Meng F, Li Y, et al. Impact of microRNA-134 on neural cell survival against ischemic injury in primary cultured neuronal cells and mouse brain with ischemic stroke by targeting HSPA12B. Brain Res 2014; 1592: 22–33. [DOI] [PubMed] [Google Scholar]
- 58.Stary CM, Xu L, Sun X, et al. MicroRNA-200c contributes to injury from transient focal cerebral ischemia by targeting reelin. Stroke 2015; 46: 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu P, Zhao H, Wang R, et al. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke 2015; 46: 513–519. [DOI] [PubMed] [Google Scholar]
- 60.Yang X, Tang X, Sun P, et al. MicroRNA-15a/16-1 antagomir ameliorates ischemic brain injury in experimental stroke. Stroke 2017; 48: 1941–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Y, Gui H, Li Q, et al. MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther 2013; 19: 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu DZ, Jickling GC, Ander BP, et al. Elevating microRNA-122 in blood improves outcomes after temporary middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab 2016; 36: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ge XT, Lei P, Wang HC, et al. miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep 2014; 4: 6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu JZ, Huang JH, Zeng L, et al. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma 2013; 30: 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol 2013; 9: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharp FR, Jickling GC, Stamova B, et al. Molecular markers and mechanisms of stroke: RNA studies of blood in animals and humans. J Cereb Blood Flow Metab 2011; 31: 1513–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A 2011; 108: 5003–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, Lu Y, Han J. Peripheral blood microRNAs: a novel tool for diagnosing disease? Intractable Rare Dis Res 2012; 1: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 2003; 62: 127–136. [DOI] [PubMed] [Google Scholar]
- 70.Hallenbeck JM, Hansson GK, Becker KJ. Immunology of ischemic vascular disease: plaque to attack. Trends Immunol 2005; 26: 550–556. [DOI] [PubMed] [Google Scholar]
- 71.Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol 2011; 10: 471–480. [DOI] [PubMed] [Google Scholar]
- 72.Eltzschig HK, Eckle T. Ischemia and reperfusion – from mechanism to translation. Nat Med 2011; 17: 1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gronberg NV, Johansen FF, Kristiansen U, et al. Leukocyte infiltration in experimental stroke. J Neuroinflammation 2013; 10: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi Y, Zhang L, Pu H, et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun 2016; 7: 10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87: 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jickling GC, Liu D, Ander BP, et al. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 2015; 35: 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsang JC, Lo YM. Circulating nucleic acids in plasma/serum. Pathology 2007; 39: 197–207. [DOI] [PubMed] [Google Scholar]
- 79.Gahan PB, Swaminathan R. Circulating nucleic acids in plasma and serum. Recent developments. Ann N Y Acad Sci 2008; 1137: 1–6. [DOI] [PubMed] [Google Scholar]
- 80.Koutsis G, Siasos G, Spengos K. The emerging role of microRNA in stroke. Curr Top Med Chem 2013; 13: 1573–1588. [DOI] [PubMed] [Google Scholar]
- 81.Tsai PC, Liao YC, Wang YS, et al. Serum microRNA-21 and microRNA-221 as potential biomarkers for cerebrovascular disease. J Vasc Res 2013; 50: 346–354. [DOI] [PubMed] [Google Scholar]
- 82.Gan CS, Wang CW, Tan KS. Circulatory microRNA-145 expression is increased in cerebral ischemia. Genet Mol Res 2012; 11: 147–152. [DOI] [PubMed] [Google Scholar]
- 83.Zeng L, Liu J, Wang Y, et al. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci 2011; 3: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 84.Tiedt S, Prestel M, Malik R, et al. RNA-Seq identifies circulating miR-125a-5p, miR-125b-5p, and miR-143-3p as potential biomarkers for acute ischemic stroke. Circ Res 2017; 121: 970–980. [DOI] [PubMed] [Google Scholar]
- 85.Scherrer N, Fays F, Mueller B, et al. MicroRNA 150-5p improves risk classification for mortality within 90 days after acute ischemic stroke. J Stroke 2017; 19: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dewdney B, Trollope A, Moxon J, et al. Circulating MicroRNAs as biomarkers for acute ischemic stroke: a systematic review. J Stroke Cerebrovasc Dis 2018; 27: 522–530. [DOI] [PubMed] [Google Scholar]
- 87.Redell JB, Moore AN, Ward NH, 3rd, et al. Human traumatic brain injury alters plasma microRNA levels. J Neurotrauma 2010; 27: 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchell AJ, Gray WD, Hayek SS, et al. Platelets confound the measurement of extracellular miRNA in archived plasma. Sci Rep 2016; 6: 32651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan KS, Armugam A, Sepramaniam S, et al. Expression profile of MicroRNAs in young stroke patients. PLoS One 2009; 4: e7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jickling GC, Ander BP, Shroff N, et al. Leukocyte response is regulated by microRNA let7i in patients with acute ischemic stroke. Neurology 2016; 87: 2198–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circ Res 2008; 103: 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Latronico MV, Condorelli G. Therapeutic use of microRNAs in myocardial diseases. Curr Heart Fail Rep 2011; 8: 193–197. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release 2013; 172: 962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Broderick JA, Zamore PD. MicroRNA therapeutics. Gene Ther 2011; 18: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res 2012; 110: 496–507. [DOI] [PubMed] [Google Scholar]
- 96.Chen PY, Weinmann L, Gaidatzis D, et al. Strand-specific 5’-O-methylation of siRNA duplexes controls guide strand selection and targeting specificity. RNA 2008; 14: 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med 2014; 6: 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiu YL, Rana TM. siRNA function in RNAi: a chemical modification analysis. RNA 2003; 9: 1034–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allerson CR, Sioufi N, Jarres R, et al. Fully 2’-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J Med Chem 2005; 48: 901–904. [DOI] [PubMed] [Google Scholar]
- 100.Sokilde R, Newie I, Persson H, et al. Passenger strand loading in overexpression experiments using microRNA mimics. RNA Biol 2015; 12: 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Port JD, Sucharov C. Role of microRNAs in cardiovascular disease: therapeutic challenges and potentials. J Cardiovasc Pharmacol 2010; 56: 444–453. [DOI] [PubMed] [Google Scholar]
- 102.Campbell JM, Bacon TA, Wickstrom E. Oligodeoxynucleoside phosphorothioate stability in subcellular extracts, culture media, sera and cerebrospinal fluid. J Biochem Biophys Methods 1990; 20: 259–267. [DOI] [PubMed] [Google Scholar]
- 103.van Rooij E. The art of microRNA research. Circ Res 2011; 108: 219–234. [DOI] [PubMed] [Google Scholar]
- 104.Geary RS, Watanabe TA, Truong L, et al. Pharmacokinetic properties of 2’-O-(2-methoxyethyl)-modified oligonucleotide analogs in rats. J Pharmacol Exp Ther 2001; 296: 890–897. [PubMed] [Google Scholar]
- 105.Wahlestedt C, Salmi P, Good L, et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc Natl Acad Sci U S A 2000; 97: 5633–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pallan PS, Greene EM, Jicman PA, et al. Unexpected origins of the enhanced pairing affinity of 2’-fluoro-modified RNA. Nucl Acids Res 2011; 39: 3482–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davis S, Lollo B, Freier S, et al. Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res 2006; 34: 2294–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oh SY, Ju Y, Kim S, et al. PNA-based antisense oligonucleotides for micrornas inhibition in the absence of a transfection reagent. Oligonucleotides 2010; 20: 225–230. [DOI] [PubMed] [Google Scholar]
- 109.Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004; 432: 173–178. [DOI] [PubMed] [Google Scholar]
- 110.Cheng CJ, Bahal R, Babar IA, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015; 518: 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Otsuka M, Kishikawa T, Yoshikawa T, et al. MicroRNAs and liver disease. J Hum Genet 2017; 62: 75–80. [DOI] [PubMed] [Google Scholar]
- 112.Wang H, Cai J. The role of microRNAs in heart failure. Biochim Biophys Acta 2017; 1863: 2019–2030. [DOI] [PubMed] [Google Scholar]
- 113.Abente EJ, Subramanian M, Ramachandran V, et al. MicroRNAs in obesity-associated disorders. Arch Biochem Biophys 2016; 589: 108–119. [DOI] [PubMed] [Google Scholar]
- 114.Quinlan S, Kenny A, Medina M, et al. MicroRNAs in neurodegenerative diseases. Int Rev Cell Mol Biol 2017; 334: 309–343. [DOI] [PubMed] [Google Scholar]
- 115.Pereira P, Queiroz JA, Figueiras A, et al. Current progress on microRNAs-based therapeutics in neurodegenerative diseases. Wiley Interdiscip Rev RNA 2017; 8: e1409. [DOI] [PubMed] [Google Scholar]
- 116.Laffont B, Rayner KJ. MicroRNAs in the pathobiology and therapy of atherosclerosis. Can J Cardiol 2017; 33: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li G, Morris-Blanco KC, Lopez MS, et al. Impact of microRNAs on ischemic stroke: from pre- to post-disease. Prog Neurobiol 2017; 10.1016/j.pneurobio.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 118.Chandran R, Mehta SL, Vemuganti R. Non-coding RNAs and neuroprotection after acute CNS injuries. Neurochem Int 2017; 111: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh A, Sen D. MicroRNAs in Parkinson’s disease. Exp Brain Res 2017; 235: 2359–2374. [DOI] [PubMed] [Google Scholar]
- 120.Mandic L, Traxler D, Gugerell A, et al. Molecular imaging of angiogenesis in cardiac regeneration. Curr Cardiovasc Imaging Rep 2016; 9: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu S, Huang M, Li Z, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 2010; 122: S124–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu P, Zhang Y, Li C, et al. Class III PI3K-mediated prolonged activation of autophagy plays a critical role in the transition of cardiac hypertrophy to heart failure. J Cell Mol Med 2015; 19: 1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karakikes I, Chaanine AH, Kang S, et al. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc 2013; 2: e000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Care A, Catalucci D, Felicetti F, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med 2007; 13: 613–618. [DOI] [PubMed] [Google Scholar]
- 125.Ganesan J, Ramanujam D, Sassi Y, et al. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation 2013; 127: 2097–2106. [DOI] [PubMed] [Google Scholar]
- 126.Montgomery RL, Hullinger TG, Semus HM, et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation 2011; 124: 1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chiong M, Wang ZV, Pedrozo Z, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis 2011; 2: e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu C, Hu Y, Hou L, et al. beta-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J Mol Cell Cardiol 2014; 75: 111–121. [DOI] [PubMed] [Google Scholar]
- 129.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005; 438: 685–689. [DOI] [PubMed] [Google Scholar]
- 130.Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 2010; 327: 198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park JK, Kogure T, Nuovo GJ, et al. miR-221 silencing blocks hepatocellular carcinoma and promotes survival. Cancer Res 2011; 71: 7608–7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ng R, Wu H, Xiao H, et al. Inhibition of microRNA-24 expression in liver prevents hepatic lipid accumulation and hyperlipidemia. Hepatology 2014; 60: 554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Elmen J, Lindow M, Schutz S, et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008; 452: 896–899. [DOI] [PubMed] [Google Scholar]
- 134.Rayner KJ, Esau CC, Hussain FN, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011; 478: 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahn J, Lee H, Jung CH, et al. MicroRNA-146b promotes adipogenesis by suppressing the SIRT1-FOXO1 cascade. EMBO Mol Med 2013; 5: 1602–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Trajkovski M, Hausser J, Soutschek J, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011; 474: 649–653. [DOI] [PubMed] [Google Scholar]
- 137.Bader AG. miR-34 – a microRNA replacement therapy is headed to the clinic. Front Genet 2012; 3: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wiggins JF, Ruffino L, Kelnar K, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res 2010; 70: 5923–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011; 17: 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pramanik D, Campbell NR, Karikari C, et al. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther 2011; 10: 1470–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pecot CV, Rupaimoole R, Yang D, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun 2013; 4: 2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cortez MA, Valdecanas D, Zhang X, et al. Therapeutic delivery of miR-200c enhances radiosensitivity in lung cancer. Mol Ther 2014; 22: 1494–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kota J, Chivukula RR, O’Donnell KA, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 2009; 137: 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang D, Sun Y, Hu L, et al. Integrated analyses identify a master microRNA regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell 2013; 23: 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nishimura M, Jung E-J, Shah MY, et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov 2013; 3: 1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Calin GA, Cimmino A, Fabbri M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A 2008; 105: 5166–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gabriely G, Yi M, Narayan RS, et al. Human glioma growth is controlled by microRNA-10b. Cancer Res 2011; 71: 3563–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Babar IA, Cheng CJ, Booth CJ, et al. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci U S A 2012; 109: E1695–E1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rupaimoole R, Ivan C, Yang D, et al. Hypoxia-upregulated microRNA-630 targets Dicer, leading to increased tumor progression. Oncogene 2016; 35: 4312–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.AstraZeneca halts development of regulus NASH candidate. GEN News Highlights 12. June 2017; 81254486. [Google Scholar]
- 151.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov 2013; 12: 847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mirna therapeutics halts phase 1 clinical study of MRX34. Business Wire 20 September 2016: 20160920006814.
- 153.Smith B, Agarwal P, Bhowmick NA. MicroRNA applications for prostate, ovarian and breast cancer in the era of precision medicine. Endocr Relat Cancer 2017; 24: R157–R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chakraborty C, Sharma AR, Sharma G, et al. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucl Acids 2017; 8: 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhalala OG. The emerging impact of microRNAs in neurotrauma pathophysiology and therapy. In: Kobeissy FH (ed.) Brain neurotrauma: Molecular, neuropsychological, and rehabilitation aspects. Boca Raton, FL: CRC Press/Taylor & Francis, 2015, Chapter 26. [PubMed]
- 156.Saugstad JA. Non-coding RNAs in stroke and neuroprotection. Front Neurol 2015; 6: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab 2010; 30: 1564–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Harraz MM, Eacker SM, Wang X, et al. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc Natl Acad Sci U S A 2012; 109: 18962–18967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khanna S, Rink C, Ghoorkhanian R, et al. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J Cereb Blood Flow Metab 2013; 33: 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Y, Huang J, Ma Y, et al. MicroRNA-29b is a therapeutic target in cerebral ischemia associated with aquaporin 4. J Cereb Blood Flow Metab 2015; 35: 1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pandi G, Nakka VP, Dharap A, et al. MicroRNA miR-29c down-regulation leading to de-repression of its target DNA methyltransferase 3a promotes ischemic brain damage. PLoS One 2013; 8: e58039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu XS, Chopp M, Wang XL, et al. MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem 2013; 288: 12478–12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xin H, Katakowski M, Wang F, et al. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 2017; 48: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu X, Li F, Zhao S, et al. MicroRNA-124-mediated regulation of inhibitory member of apoptosis-stimulating protein of p53 family in experimental stroke. Stroke 2013; 44: 1973–1980. [DOI] [PubMed] [Google Scholar]
- 165.Doeppner TR, Doehring M, Bretschneider E, et al. MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathol 2013; 126: 251–265. [DOI] [PubMed] [Google Scholar]
- 166.Hamzei Taj S, Kho W, Riou A, et al. MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 2016; 91: 151–165. [DOI] [PubMed] [Google Scholar]
- 167.Yang J, Zhang X, Chen X, et al. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids 2017; 7: 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qiu J, Zhou XY, Zhou XG, et al. Neuroprotective effects of microRNA-210 on hypoxic-ischemic encephalopathy. Biomed Res Int 2013; 2013: 350419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zeng LL, He XS, Liu JR, et al. Lentivirus-Mediated overexpression of microRNA-210 improves long-term outcomes after focal cerebral ischemia in mice. CNS Neurosci Ther 2016; 22: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhao H, Wang J, Gao L, et al. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke 2013; 44: 1706–1713. [DOI] [PubMed] [Google Scholar]
- 171.Qu Y, Wu J, Chen D, et al. MiR-139-5p inhibits HGTD-P and regulates neuronal apoptosis induced by hypoxia-ischemia in neonatal rats. Neurobiol Dis 2014; 63: 184–193. [DOI] [PubMed] [Google Scholar]
- 172.Ni J, Wang X, Chen S, et al. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behav Immun 2015; 49: 75–85. [DOI] [PubMed] [Google Scholar]
- 173.Tao Z, Zhao H, Wang R, et al. Neuroprotective effect of microRNA-99a against focal cerebral ischemia-reperfusion injury in mice. J Neurol Sci 2015; 355: 113–119. [DOI] [PubMed] [Google Scholar]
- 174.Li Y, Mao L, Gao Y, et al. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci Rep 2015; 5: 13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tao J, Liu W, Shang G, et al. MiR-207/352 regulate lysosomal-associated membrane proteins and enzymes following ischemic stroke. Neuroscience 2015; 305: 1–14. [DOI] [PubMed] [Google Scholar]
- 176.Liu FJ, Kaur P, Karolina DS, et al. MiR-335 regulates hif-1alpha to reduce cell death in both mouse cell line and rat ischemic models. PLoS One 2015; 10: e0128432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yu H, Wu M, Zhao P, et al. Neuroprotective effects of viral overexpression of microRNA-22 in rat and cell models of cerebral ischemia-reperfusion injury. J Cell Biochem 2015; 116: 233–241. [DOI] [PubMed] [Google Scholar]
- 178.Wei N, Xiao L, Xue R, et al. MicroRNA-9 mediates the cell apoptosis by targeting Bcl2l11 in ischemic stroke. Mol Neurobiol 2016; 53: 6809–6817. [DOI] [PubMed] [Google Scholar]
- 179.Zhang N, Zhong J, Han S, et al. MicroRNA-378 alleviates cerebral ischemic injury by negatively regulating apoptosis executioner caspase-3. Int J Mol Sci 2016; 17: 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu da Z, Jickling GC, Ander BP, et al. Elevating microRNA-122 in blood improves outcomes after temporary middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab 2016; 36: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yao S, Tang B, Li G, et al. miR-455 inhibits neuronal cell death by targeting TRAF3 in cerebral ischemic stroke. Neuropsychiatr Dis Treat 2016; 12: 3083–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tian F, Yuan C, Hu L, et al. MicroRNA-93 inhibits inflammatory responses and cell apoptosis after cerebral ischemia reperfusion by targeting interleukin-1 receptor-associated kinase 4. Exp Ther Med 2017; 14: 2903–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Selvamani A, Sohrabji F. Mir363-3p improves ischemic stroke outcomes in female but not male rats. Neurochem Int 2017; 107: 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dharap A, Bowen K, Place R, et al. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 2009; 29: 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sepramaniam S, Armugam A, Lim KY, et al. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J Biol Chem 2010; 285: 29223–29230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Moon JM, Xu L, Giffard RG. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J Cereb Blood Flow Metab 2013; 33: 1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xu LJ, Ouyang YB, Xiong X, et al. Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Exp Neurol 2015; 264: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vinciguerra A, Formisano L, Cerullo P, et al. MicroRNA-103-1 selectively downregulates brain NCX1 and its inhibition by anti-miRNA ameliorates stroke damage and neurological deficits. Mol Ther 2014; 22: 1829–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang P, Zhang N, Liang J, et al. Micro-RNA-30a regulates ischemia-induced cell death by targeting heat shock protein HSPA5 in primary cultured cortical neurons and mouse brain after stroke. J Neurosci Res 2015; 93: 1756–1768. [DOI] [PubMed] [Google Scholar]
- 190.Zhu F, Liu JL, Li JP, et al. MicroRNA-124 (miR-124) regulates Ku70 expression and is correlated with neuronal death induced by ischemia/reperfusion. J Mol Neurosci 2014; 52: 148–155. [DOI] [PubMed] [Google Scholar]
- 191.Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T, et al. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J Neurosci 2015; 35: 12446–12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pena-Philippides JC, Caballero-Garrido E, Lordkipanidze T, et al. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J Neuroinflammation 2016; 13: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xing G, Luo Z, Zhong C, et al. Influence of miR-155 on cell apoptosis in rats with ischemic stroke: role of the ras homolog enriched in brain (Rheb)/mTOR pathway. Med Sci Monit 2016; 22: 5141–5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu W, Chen X, Zhang Y. Effects of microRNA-21 and microRNA-24 inhibitors on neuronal apoptosis in ischemic stroke. Am J Transl Res 2016; 8: 3179–3187. [PMC free article] [PubMed] [Google Scholar]
- 195.Yi H, Huang Y, Yang F, et al. MicroRNA-182 aggravates cerebral ischemia injury by targeting inhibitory member of the ASPP family (iASPP). Arch Biochem Biophys 2017; 620: 52–58. [DOI] [PubMed] [Google Scholar]
- 196.Li Q, He Q, Baral S, et al. MicroRNA-493 regulates angiogenesis in a rat model of ischemic stroke by targeting MIF. FEBS J 2016; 283: 1720–1733. [DOI] [PubMed] [Google Scholar]
- 197.Wang P, Liang X, Lu Y, et al. MicroRNA-93 downregulation ameliorates cerebral ischemic injury through the Nrf2/HO-1 defense pathway. Neurochem Res 2016; 41: 2627–2635. [DOI] [PubMed] [Google Scholar]
- 198.Ma Q, Dasgupta C, Li Y, et al. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol Dis 2016; 89: 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pei L, Meng S, Yu W, et al. Inhibition of microRNA-383 ameliorates injury after focal cerebral ischemia via targeting PPARgamma. Cell Physiol Biochem 2016; 39: 1339–1346. [DOI] [PubMed] [Google Scholar]
- 200.Li P, Shen M, Gao F, et al. An antagomir to microRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol 2017; 54: 2901–2921. [DOI] [PubMed] [Google Scholar]
- 201.Zhao F, Qu Y, Zhu J, et al. miR-30d-5p plays an important role in autophagy and apoptosis in developing rat brains after hypoxic-ischemic injury. J Neuropathol Exp Neurol 2017; 76: 709–719. [DOI] [PubMed] [Google Scholar]
- 202.Fan Y, Ding S, Sun Y, et al. MiR-377 regulates inflammation and angiogenesis in rats after cerebral ischemic injury. J Cell Biochem 2018; 119: 327–337. [DOI] [PubMed] [Google Scholar]
- 203.Wang X, Suofu Y, Akpinar B, et al. Systemic antimiR-337-3p delivery inhibits cerebral ischemia-mediated injury. Neurobiol Dis 2017; 105: 156–163. [DOI] [PubMed] [Google Scholar]
- 204.Fang Z, He QW, Li Q, et al. MicroRNA-150 regulates blood-brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J 2016; 30: 2097–2107. [DOI] [PubMed] [Google Scholar]
- 205.Sabirzhanov B, Zhao Z, Stoica BA, et al. Downregulation of miR-23a and miR-27a following experimental traumatic brain injury induces neuronal cell death through activation of proapoptotic Bcl-2 proteins. J Neurosci 2014; 34: 10055–10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sun L, Zhao M, Wang Y, et al. Neuroprotective effects of miR-27a against traumatic brain injury via suppressing FoxO3a-mediated neuronal autophagy. Biochem Biophys Res Commun 2017; 482: 1141–1147. [DOI] [PubMed] [Google Scholar]
- 207.Ge X, Han Z, Chen F, et al. MiR-21 alleviates secondary blood-brain barrier damage after traumatic brain injury in rats. Brain Res 2015; 1603: 150–157. [DOI] [PubMed] [Google Scholar]
- 208.Sun L, Liu A, Zhang J, et al. miR-23b improves cognitive impairments in traumatic brain injury by targeting ATG12-mediated neuronal autophagy. Behav Brain Res 2017; 34: 3–14. [DOI] [PubMed] [Google Scholar]
- 209.Huang S, Ge X, Yu J, et al. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 2018; 32: 512–528. [DOI] [PubMed] [Google Scholar]
- 210.Sabirzhanov B, Stoica BA, Zhao Z, et al. miR-711 upregulation induces neuronal cell death after traumatic brain injury. Cell Death Differ 2016; 23: 654–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sun L, Zhao M, Zhang J, et al. MiR-144 promotes beta-amyloid accumulation-induced cognitive impairments by targeting ADAM10 following traumatic brain injury. Oncotarget 2017; 8: 59181–59203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xu W, Li P, Qin K, et al. miR-124 regulates neural stem cells in the treatment of spinal cord injury. Neurosci Lett 2012; 529: 12–17. [DOI] [PubMed] [Google Scholar]
- 213.Zou D, Chen Y, Han Y, et al. Overexpression of microRNA-124 promotes the neuronal differentiation of bone marrow-derived mesenchymal stem cells. Neural Regen Res 2014; 9: 1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhao Y, Jiang H, Liu XW, et al. MiR-124 promotes bone marrow mesenchymal stem cells differentiation into neurogenic cells for accelerating recovery in the spinal cord injury. Tissue Cell 2015; 47: 140–146. [DOI] [PubMed] [Google Scholar]
- 215.Louw AM, Kolar MK, Novikova LN, et al. Chitosan polyplex mediated delivery of miRNA-124 reduces activation of microglial cells in vitro and in rat models of spinal cord injury. Nanomedicine 2016; 12: 643–653. [DOI] [PubMed] [Google Scholar]
- 216.Song JL, Zheng W, Chen W, et al. Lentivirus-mediated microRNA-124 gene-modified bone marrow mesenchymal stem cell transplantation promotes the repair of spinal cord injury in rats. Exp Mol Med 2017; 49: e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Im YB, Jee MK, Choi JI, et al. Molecular targeting of NOX4 for neuropathic pain after traumatic injury of the spinal cord. Cell Death Dis 2012; 3: e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ujigo S, Kamei N, Hadoush H, et al. Administration of microRNA-210 promotes spinal cord regeneration in mice. Spine 2014; 39: 1099–1107. [DOI] [PubMed] [Google Scholar]
- 219.Liu XJ, Zheng XP, Zhang R, et al. Combinatorial effects of miR-20a and miR-29b on neuronal apoptosis induced by spinal cord injury. Int J Clin Exp Pathol 2015; 8: 3811–3818. [PMC free article] [PubMed] [Google Scholar]
- 220.Li XQ, Lv HW, Wang ZL, et al. MiR-27a ameliorates inflammatory damage to the blood-spinal cord barrier after spinal cord ischemia: reperfusion injury in rats by downregulating TICAM-2 of the TLR4 signaling pathway. J Neuroinflammation 2015; 12: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hu J, Zeng L, Huang J, et al. miR-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res 2015; 1608: 191–202. [DOI] [PubMed] [Google Scholar]
- 222.Zhou HJ, Wang LQ, Xu QS, et al. Downregulation of miR-199b promotes the acute spinal cord injury through IKKbeta-NF-kappaB signaling pathway activating microglial cells. Exp Cell Res 2016; 349: 60–67. [DOI] [PubMed] [Google Scholar]
- 223.Li XQ, Fang B, Tan WF, et al. miR-320a affects spinal cord edema through negatively regulating aquaporin-1 of blood-spinal cord barrier during bimodal stage after ischemia reperfusion injury in rats. BMC Neurosci 2016; 17: 10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.He F, Ren Y, Shi E, et al. Overexpression of microRNA-21 protects spinal cords against transient ischemia. J Thorac Cardiovasc Surg 2016; 152: 1602–1608. [DOI] [PubMed] [Google Scholar]
- 225.Xu M, Wang HF, Zhang YY, et al. Protection of rats spinal cord ischemia-reperfusion injury by inhibition of MiR-497 on inflammation and apoptosis: possible role in pediatrics. Biomed Pharmacother 2016; 81: 337–344. [DOI] [PubMed] [Google Scholar]
- 226.Theis T, Yoo M, Park CS, et al. Lentiviral delivery of miR-133b improves functional recovery after spinal cord injury in mice. Mol Neurobiol 2017; 54: 4659–4671. [DOI] [PubMed] [Google Scholar]
- 227.Gu S, Xie R, Liu X, et al. Long coding RNA XIST contributes to neuronal apoptosis through the downregulation of akt phosphorylation and is negatively regulated by miR-494 in rat spinal cord injury. Int J Mol Sci 2017; 18: 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhu H, Xie R, Liu X, et al. MicroRNA-494 improves functional recovery and inhibits apoptosis by modulating PTEN/AKT/mTOR pathway in rats after spinal cord injury. Biomed Pharmacother 2017; 92: 879–887. [DOI] [PubMed] [Google Scholar]
- 229.Jee MK, Jung JS, Im YB, et al. Silencing of miR20a is crucial for Ngn1-mediated neuroprotection in injured spinal cord. Hum Gene Ther 2012; 23: 508–520. [DOI] [PubMed] [Google Scholar]
- 230.Jee MK, Jung JS, Choi JI, et al. MicroRNA 486 is a potentially novel target for the treatment of spinal cord injury. Brain 2012; 135: 1237–1252. [DOI] [PubMed] [Google Scholar]
- 231.Liu D, Huang Y, Jia C, et al. Administration of antagomir-223 inhibits apoptosis, promotes angiogenesis and functional recovery in rats with spinal cord injury. Cell Mol Neurobiol 2015; 35: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.He F, Shi E, Yan L, et al. Inhibition of micro-ribonucleic acid-320 attenuates neurologic injuries after spinal cord ischemia. J Thorac Cardiovasc Surg 2015; 150: 398–406. [DOI] [PubMed] [Google Scholar]
- 233.Wei GJ, An G, Shi ZW, et al. Suppression of microRNA-383 enhances therapeutic potential of human bone-marrow-derived mesenchymal stem cells in treating spinal cord injury via GDNF. Cell Physiol Biochem 2017; 41: 1435–1444. [DOI] [PubMed] [Google Scholar]
- 234.Gibaldi M. Gibaldi’s drug delivery systems in pharmaceutical care, Bethesda, MD, USA: ASHP, 2007. [Google Scholar]
- 235.Liu K, Yan L, Jiang X, et al. Acquired inhibition of microRNA-124 protects against spinal cord ischemia-reperfusion injury partially through a mitophagy-dependent pathway. J Thorac Cardiovasc Surg 2017; 154: 1498–1508. [DOI] [PubMed] [Google Scholar]
- 236.Glascock JJ, Osman EY, Coady TH, et al. Delivery of therapeutic agents through intracerebroventricular (ICV) and intravenous (IV) injection in mice. J Vis Exp 2011; 56: e2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev 2012; 64: 614–628. [DOI] [PubMed] [Google Scholar]
- 238.Tayebati SK, Nwankwo IE, Amenta F. Intranasal drug delivery to the central nervous system: present status and future outlook. Curr Pharm Des 2013; 19: 510–526. [PubMed] [Google Scholar]
- 239.Fasanaro P, Greco S, Ivan M, et al. microRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther 2010; 125: 92–104. [DOI] [PubMed] [Google Scholar]
- 240.Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659. [DOI] [PubMed] [Google Scholar]
- 241.Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci 2014; 8: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Record M, Subra C, Silvente-Poirot S, et al. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 2011; 81: 1171–1182. [DOI] [PubMed] [Google Scholar]
- 243.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 2015; 17: 11–22. [DOI] [PubMed] [Google Scholar]
- 244.Tatarishvili J, Oki K, Monni E, et al. Human induced pluripotent stem cells improve recovery in stroke-injured aged rats. Restor Neurol Neurosci 2014; 32: 547–558. [DOI] [PubMed] [Google Scholar]
- 245.Cordeiro MF, Horn AP. Stem cell therapy in intracerebral hemorrhage rat model. World J Stem Cells 2015; 7: 618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest 2004; 113: 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hoehn M, Kustermann E, Blunk J, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci U S A 2002; 99: 16267–16272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ramos-Cabrer P, Justicia C, Wiedermann D, et al. Stem cell mediation of functional recovery after stroke in the rat. PLoS One 2010; 5: e12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chopp M, Zhang XH, Li Y, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport 2000; 11: 3001–3005. [DOI] [PubMed] [Google Scholar]
- 250.Tsuji O, Miura K, Okada Y, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A 2010; 107: 12704–12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 2005; 25: 4694–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Harting MT, Jimenez F, Xue H, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg 2009; 110: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Steinberg GK, Kondziolka D, Wechsler LR, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke 2016; 47: 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.De Keyser J. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005; 58: 653–654; author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 255.Lee JS, Hong JM, Moon GJ, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010; 28: 1099–1106. [DOI] [PubMed] [Google Scholar]
- 256.Bhasin A, Kumaran SS, Bhatia R, et al. Safety and feasibility of autologous mesenchymal stem cell transplantation in chronic stroke in indian patients. A four-year follow up. J Stem Cells Regen Med 2017; 13: 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med 2006; 355: 2376–2378. [DOI] [PubMed] [Google Scholar]
- 258.Cox CS, Jr, Hetz RA, Liao GP, et al. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells 2017; 35: 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yoon SH, Shim YS, Park YH, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: phase I/II clinical trial. Stem Cells 2007; 25: 2066–2073. [DOI] [PubMed] [Google Scholar]
- 260.Lima C, Pratas-Vital J, Escada P, et al. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med 2006; 29: 191–203; discussion 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kang KS, Kim SW, Oh YH, et al. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy 2005; 7: 368–373. [DOI] [PubMed] [Google Scholar]
- 262.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature 2006; 441: 1094–1096. [DOI] [PubMed] [Google Scholar]
- 263.Bang OY, Kim EH, Cha JM, et al. Adult stem cell therapy for stroke: challenges and progress. J Stroke 2016; 18: 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lee KH, Nam H, Jeong da E, et al. Sensitive tumorigenic potential evaluation of adult human multipotent neural cells immortalized by hTERT gene transduction. PLoS One 2016; 11: e0158639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol 2009; 10: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.De Los Angeles A, Ferrari F, Xi R, et al. Hallmarks of pluripotency. Nature 2015; 525: 469–478. [DOI] [PubMed] [Google Scholar]
- 267.Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell 2015; 16: 119–134. [DOI] [PubMed] [Google Scholar]
- 268.Ji M, Wang W, Li S, et al. Implantation of bone mesenchymal stem cells overexpressing miRNA705 mitigated ischemic brain injury. Mol Med Rep 2017; 16: 8323–8328. [DOI] [PubMed] [Google Scholar]
- 269.Shi X, Yan C, Liu B, et al. miR-381 Regulates neural stem cell proliferation and differentiation via regulating Hes1 expression. PLoS One 2015; 10: e0138973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 270.Mao S, Li X, Wang J, et al. miR-17-92 facilitates neuronal differentiation of transplanted neural stem/precursor cells under neuroinflammatory conditions. J Neuroinflammation 2016; 13: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology 2008; 55: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res 2008; 30: 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med 2005; 38: 1433–1444. [DOI] [PubMed] [Google Scholar]
- 274.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 2009; 284: 13291–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Keum YS, Choi BY. Molecular and chemical regulation of the Keap1-Nrf2 signaling pathway. Molecules 2014; 19: 10074–10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zipfel GJ, Babcock DJ, Lee JM, et al. Neuronal apoptosis after CNS injury: the roles of glutamate and calcium. J Neurotrauma 2000; 17: 857–869. [DOI] [PubMed] [Google Scholar]
- 277.Zhang N, Yin Y, Xu SJ, et al. Inflammation & apoptosis in spinal cord injury. Ind J Med Res 2012; 135: 287–296. [PMC free article] [PubMed] [Google Scholar]
- 278.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol 2004; 14: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke 2009; 40: e331–e339. [DOI] [PubMed] [Google Scholar]
- 280.Sharma HS. Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr Pharm Des 2005; 11: 1353–1389. [DOI] [PubMed] [Google Scholar]
- 281.Cohen DM, Patel CB, Ahobila-Vajjula P, et al. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed 2009; 22: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Thal SC, Neuhaus W. The blood–brain barrier as a target in traumatic brain injury treatment. Arch Med Res 2014; 45: 698–710. [DOI] [PubMed] [Google Scholar]
- 283.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013; 19: 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shlosberg D, Benifla M, Kaufer D, et al. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol 2010; 6: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sepramaniam S, Ying LK, Armugam A, et al. MicroRNA-130a represses transcriptional activity of aquaporin 4 M1 promoter. J Biol Chem 2012; 287: 12006–12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yin K-J, Hamblin M, Eugene Chen Y. Angiogenesis-regulating microRNAs and ischemic stroke. Curr Vasc Pharmacol 2015; 13: 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 2010; 11: 298–308. [PMC free article] [PubMed] [Google Scholar]
- 288.Rauch MF, Hynes SR, Bertram J, et al. Engineering angiogenesis following spinal cord injury: a coculture of neural progenitor and endothelial cells in a degradable polymer implant leads to an increase in vessel density and formation of the blood-spinal cord barrier. Eur J Neurosci 2009; 29: 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tobin MK, Bonds JA, Minshall RD, et al. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab 2014; 34: 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zheng W, ZhuGe Q, Zhong M, et al. Neurogenesis in adult human brain after traumatic brain injury. J Neurotrauma 2013; 30: 1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002; 8: 963–970. [DOI] [PubMed] [Google Scholar]
- 292.Jin K, Sun Y, Xie L, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci 2003; 24: 171–189. [DOI] [PubMed] [Google Scholar]
- 293.Teramoto T, Qiu J, Plumier JC, et al. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest 2003; 111: 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist 2009; 15: 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhang L, Chopp M, Liu X, et al. Combination therapy with VELCADE and tissue plasminogen activator is neuroprotective in aged rats after stroke and targets microRNA-146a and the toll-like receptor signaling pathway. Arterioscler Thromb Vasc Biol 2012; 32: 1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wen Y, Zhang X, Dong L, et al. Acetylbritannilactone modulates microRNA-155-mediated inflammatory response in ischemic cerebral tissues. Mol Med 2015; 21: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yang Q, Yang K, Li AY. Trimetazidine protects against hypoxia-reperfusion-induced cardiomyocyte apoptosis by increasing microRNA-21 expression. Int J Clin Exp Pathol 2015; 8: 3735–3741. [PMC free article] [PubMed] [Google Scholar]
- 298.Zuo Y, Wang Y, Hu H, et al. Atorvastatin protects myocardium against ischemia-reperfusion injury through inhibiting miR-199a-5p. Cell Physiol Biochem 2016; 39: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 299.Bernstock JD, Lee YJ, Peruzzotti-Jametti L, et al. A novel quantitative high-throughput screen identifies drugs that both activate SUMO conjugation via the inhibition of microRNAs 182 and 183 and facilitate neuroprotection in a model of oxygen and glucose deprivation. J Cereb Blood Flow Metab 2016; 36: 426–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Dong YF, Chen ZZ, Zhao Z, et al. Potential role of microRNA-7 in the anti-neuroinflammation effects of nicorandil in astrocytes induced by oxygen-glucose deprivation. J Neuroinflammation 2016; 13: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yuen CM, Yeh KH, Wallace CG, et al. EPO-cyclosporine combination therapy reduced brain infarct area in rat after acute ischemic stroke: role of innate immune-inflammatory response, micro-RNAs and MAPK family signaling pathway. Am J Transl Res 2017; 9: 1651–1666. [PMC free article] [PubMed] [Google Scholar]
- 302.Song J, Li N, Xia Y, et al. Arctigenin confers neuroprotection against mechanical trauma injury in human neuroblastoma SH-SY5Y cells by regulating miRNA-16 and miRNA-199a expression to alleviate inflammation. J Mol Neurosci 2016; 60: 115–129. [DOI] [PubMed] [Google Scholar]
- 303.Wang L, Zhao C, Wu S, et al. Hydrogen gas treatment improves the neurological outcome after traumatic brain injury via increasing Mir-21 expression. Shock 2017: 10.1097/SHK.0000000000001018. [DOI] [PubMed]
- 304.Li Z, Wang S, Li W, et al. Ferulic acid improves functional recovery after acute spinal cord injury in rats by inducing hypoxia to inhibit microRNA-590 and elevate vascular endothelial growth factor expressions. Front Mol Neurosci 2017; 10: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Li L, Jiang HK, Li YP, et al. Hydrogen sulfide protects spinal cord and induces autophagy via miR-30c in a rat model of spinal cord ischemia-reperfusion injury. J Biomed Sci 2015; 22: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Chen Z, Zhang Z, Zhang D, et al. Hydrogen sulfide protects against TNF-α induced neuronal cell apoptosis through miR-485-5p/TRADD signaling. Biochem Biophys Res Commun 2016; 478: 1304–1309. [DOI] [PubMed] [Google Scholar]
- 307.Shi LB, Tang PF, Zhang W, et al. Naringenin inhibits spinal cord injury-induced activation of neutrophils through miR-223. Gene 2016; 592: 128–133. [DOI] [PubMed] [Google Scholar]
- 308.Huang JH, Cao Y, Zeng L, et al. Tetramethylpyrazine enhances functional recovery after contusion spinal cord injury by modulation of MicroRNA-21, FasL, PDCD4 and PTEN expression. Brain Res 2016; 1648: 35–45. [DOI] [PubMed] [Google Scholar]
- 309.Fan Y, Wu Y. Tetramethylpyrazine alleviates neural apoptosis in injured spinal cord via the downregulation of miR-214-3p. Biomed Pharmacother 2017; 94: 827–833. [DOI] [PubMed] [Google Scholar]
- 310.Chen X, Wang K. The fate of medications evaluated for ischemic stroke pharmacotherapy over the period 1995-2015. Acta Pharm Sin B 2016; 6: 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx 2004; 1: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Xu SY, Pan SY. The failure of animal models of neuroprotection in acute ischemic stroke to translate to clinical efficacy. Med Sci Monit Basic Res 2013; 19: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 314.Hacke W. A New DAWN for imaging-based selection in the treatment of acute stroke. N Engl J Med 2018; 378: 81–83. [DOI] [PubMed] [Google Scholar]
- 315.Bhaskar S, Stanwell P, Cordato D, et al. Reperfusion therapy in acute ischemic stroke: dawn of a new era? BMC Neurol 2018; 18: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jovicic A, Roshan R, Moisoi N, et al. Comprehensive expression analyses of neural cell-type-specific miRNAs identify new determinants of the specification and maintenance of neuronal phenotypes. J Neurosci 2013; 33: 5127–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yin KJ, Hamblin M, Chen YE. Non-coding RNAs in cerebral endothelial pathophysiology: emerging roles in stroke. Neurochem Int 2014; 77: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lusardi TA, Murphy SJ, Phillips JI, et al. MicroRNA responses to focal cerebral ischemia in male and female mouse brain. Front Mol Neurosci 2014; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Saugstad JA. MicroRNAs as effectors of brain function. Stroke 2013; 44: S17–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Alkayed NJ, Harukuni I, Kimes AS, et al. Gender-linked brain injury in experimental stroke. Stroke 1998; 29: 159–165; discussion 66. [DOI] [PubMed] [Google Scholar]
- 321.Sohrabji F, Park MJ, Mahnke AH. Sex differences in stroke therapies. J Neurosci Res 2017; 95: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Girijala RL, Sohrabji F, Bush RL. Sex differences in stroke: review of current knowledge and evidence. Vasc Med 2017; 22: 135–145. [DOI] [PubMed] [Google Scholar]