Abstract
Positron emission tomography (PET) can, when used with appropriate radioligands, non-invasively capture temporal and spatial information about acute changes in brain neurotransmitter systems. We here evaluate the 5-HT1B receptor partial agonist PET radioligand, [11C]AZ10419369, for its sensitivity to detect changes in endogenous cerebral serotonin levels, as induced by different pharmacological challenges. To enable a direct translation of PET imaging data to changes in brain serotonin levels, we compared the [11C]AZ10419369 PET signal in the pig brain to simultaneous measurements of extracellular serotonin levels with microdialysis after various acute interventions (saline, escitalopram, fenfluramine). The interventions increased the cerebral extracellular serotonin levels to two to six times baseline, with fenfluramine being the most potent pharmacological enhancer of serotonin release. The interventions induced a varying degree of decline in [11C]AZ10419369 binding in the brain, consistent with the occupancy competition model. The observed correlation between changes in the extracellular serotonin level in the pig brain and the 5-HT1B receptor occupancy indicates that [11C]AZ10419369 binding is sensitive to changes in endogenous serotonin levels to a degree equivalent to that reported of [11C]raclopride to dopamine, a much used approach to detect in vivo change in cerebral dopamine.
Keywords: Positron emission tomography, serotonin, brain imaging, kinetic modelling, neurosurgery
Introduction
Several studies have investigated PET radioligands for their ability to be displaced by endogenously released serotonin. Such PET radioligands would be an important tool to investigate disorders associated to specific serotonin subtypes and serotonergic dysfunction as well as pharmacological treatments targeting these receptors. Unfortunately, the outcomes in humans have not convincingly shown the expected effect of pharmacological interventions supposed to increase brain interstitial serotonin.1,2
The 5-HT1B receptor is one of 14 mammalian serotonin receptor subtypes; each receptor is associated with specific physiological impact and has a distinct regional distribution in the brain.3,4 The 5-HT1B receptor is located on the terminals both presynaptically, where it acts as a 5-HT autoreceptor regulating serotonin release, and postsynaptically as a heteroreceptor, where it interacts with other neurotransmitter systems such as acetylcholine, glutamate, GABA and dopamine.3 The receptor is involved in regulation of several physiological functions such as increased secretion of corticosterone and prolactin, sexual function, appetite, thermoregulation and locomotion.3 Further, the 5-HT1B receptor has been associated to many neuropsychiatric disorders such as depression, anxiety and OCD.5 From a clinical perspective, modulation of serotonin at the 5-HT1B receptor has a high therapeutic potential. Hence, the ability to image changes in serotonin would constitute an important contribution to unveil disease mechanisms and to identify therapeutic options.
PET studies with the 5-HT1B receptor radioligands [11C]AZ104193696,7 or [11C]P9438 in humans and non-human primates have investigated the extent of radioligand displacement from the receptor by an increase in endogenous serotonin elicited by a serotonergic challenge. In non-human primates, studies6,7 demonstrate a dose-dependent decrease in radioligand binding across brain regions following intervention with fenfluramine (a strong serotonin releaser) or a high dose of the serotonin reuptake inhibitor (SSRI) escitalopram. In humans, however, an increased radioligand binding was observed following a clinical relevant dose of escitalopram.7 Similarly, in another study in humans, the cerebral binding of the 5-HT1A receptor PET radioligand [11C]CUMI-101 was also found to increase following a citalopram9 challenge although no change was found in another [11C]CUMI-101 study.10 This unexpected finding in humans was interpreted in both studies as being caused by autoreceptor function, with 5-HT1B or 5-HT1A autoreceptor stimulation inhibiting serotonin release.
Such paradoxical SSRI-induced effects on interstitial brain serotonin level have not been observed in microdialysis studies, which is the gold standard procedure to investigate changes in neurotransmitter levels. Microdialysis studies demonstrate that 5-HT1B autoreceptor function can dampen or even cancel out the anticipated SSRI-induced increase in cerebral serotonin level in a region-dependent way, but it does not decrease the serotonin level compared to baseline.11–13 However, microdialysis studies to our knowledge have so far only investigated SSRI doses manifold above clinically relevant doses. Therefore, the conflicting outcomes of the human and the non-human primate PET studies may be explained by species differences or differences in the doses applied since a high SSRI dose would be expected to lead to a larger increase in brain interstitial serotonin levels.
The aim of the present study was to measure the effect of pharmacological challenges – also in clinically relevant doses – on the serotonin level by cerebral microdialysis and to correlate this to the change in the non-displaceable binding potential (BPND) of the 5-HT1B receptor partial agonist radioligand [11C]AZ10419369 in the pig brain.
We hypothesized that pharmacologically induced acute increases in extracellular brain serotonin level were associated with a decline in [11C]AZ10419369 5-HT1B receptor binding, in consistency with the competition model.1
Material and methods
Animals
We used 10 9-10-week old Danish Landrace female pigs weighing 22 ± 1.3 kg (mean ± SD) in this study. All animal experiments were performed in accordance with the European Communities Council Resolves on 22 September 2010 (2010/63/EU) and approved by the Danish Veterinary and Food Administration's Council for Animal Experimentation (Journal No. 2012-15-2934-00156), and is in compliance with the ARRIVE guidelines (www.nc3rs.org.uk/arrive-guidelines). We performed tranquilization, anesthesia, intubation, installation of arterial and venous intravenous lines in the morning, monitoring and sacrifice of animals late afternoon was done as previously described.14 On the day of investigation, we performed surgery with implantation of microdialysis guide cannulas and CMA 12 metal-free microdialysis probe (CMA Microdialysis) bilaterally into the medial prefrontal cortex (mPFC) using a well-validated procedure to allow full embedment of the active part of the microdialysis probe in only the grey matter of cortex. The procedural details are given in our previous study15 and a schematic diagram of the events on the investigation day in Figure 1.
Figure 1.
Microdialysis measurements were obtained with a time resolution of 15 min at baseline (B1–B4) prior to intervention with saline (S1–S4) or escitalopram/fenfluramine (I1–I4).
Study design
Both PET scans were conducted on the same day as within scan challenge with saline (scan 1) and escitalopram or fenfluramine (scan 2). Upon insertion of microdialysis probes bilaterally in the mPFC, we placed the pig in the PET scanner and allowed a 2 h-washout period to obtain steady serotonin baseline level. Two PET scans, each lasting 120 min, were conducted 30 min apart. We administered the pharmacological interventions intravenously 56.5 min after injection of the radioligand with the aim to acutely increase the extracellular serotonin levels. The interventions consisted of either saline (controls, n = 10), escitalopram 0.28 mg/kg (selective serotonin reuptake inhibitor (SSRI), n = 5) or fenfluramine 0.5 mg/kg (serotonin releaser, n = 5). The investigators were not blinded to the interventions but the pigs were randomized.
In pilot experiments prior to a previous study,15 we tested the effect of three different doses of fenfluramine; 0.05 mg/kg, 0.5 mg/kg and 2.0 mg/kg, but when 2 mg/kg was given, the pig started to shiver and showed a large increase in vital signs, so the injection was interrupted after ¾ had been given. Since the dose of 0.05 mg/kg did not induce any significant alterations in microdialysate 5-HT levels, we chose to give a dose of 0.5 mg/kg. This dose has shown to elicit a 9–11-fold increase in serotonin level consistent with the spectrum that allows comparison of changes in BPND at an 8-fold increase in neurotransmitter level such as reported for [11C]raclopride to dopamine.16
Microdialysis and serotonin measurements
The probes had a membrane length of 4 mm and a molecular weight cut-off value of 20 kDa. They were perfused with a standard Ringer solution (147 mM NaCl, 4 mM KCl and 2.3 mM CaCl2, adjusted to pH 6.5) at a flow rate of 1.0 uL/min. From both probes, we collected 15-min time-series of samples of extracellular fluid to be analyzed off-line for monoamines by HPLC. After a 2 h-washout period, we collected 20 samples with 15 min interval. The microdialysate samples were quickly frozen on dry ice and stored in a −80 ℃ freezer until further analyzed.
We determined the relative changes in serotonin concentrations in the dialysate samples by HPLC with electrochemical detection. The column was a Prodigy 3 µ ODS (3) C18 (DA 2 mm × 100 mm, particle size 3 µm, Phenomenex, Torrance, California, USA). The mobile phase consisted of 55 mM sodium acetate, 1 mM octanesulfonic acid, 0.1 mM Na2EDTA and 8% acetonitrile, adjusted to pH 3.2 with 0.1 M acetic acid, and was degassed with an on-line degasser. Ten microliters of the dialysate sample were injected, and the flow rate was 0.15 mL/min. The electrochemical detection was done with an amperometric detector (Antec Decade, Antec, Leiden, the Netherlands) with a glassy carbon electrode set at 0.8 V, with an Ag/AgCl reference electrode.
The output of the HPLC was recorded by the program “LC solution” (Shimadzu, Columbia, Maryland, USA), which also was used to calculate the peak areas. We determined the baseline level on the basis of the mean peak area obtained by the HPLC from the four samples preceding the pharmacological intervention of each PET scan. We used the baseline level to calculate the relative change in the serotonin levels of the four samples following the pharmacological intervention during the remaining period of each PET scan. In 19 of the performed 20 pig scans, stable baseline serotonin levels were achieved on one or both sides. In one pig, stable baseline level was not reached at the saline intervention and therefore this microdialysis data were excluded from the saline intervention analyses.
| (1) |
According to the competition model,1 there are three factors, which determine the ability of a tracer to detect changes in synaptic neurotransmission: affinity of the neurotransmitter for its receptor, KNT, the basal neurotransmitter concentration in the interstitial fluid, FNT, and the challenge-induced change in neurotransmitter concentration, ΔFNT.
PET scanning protocol
PET scans were obtained in list mode with a high resolution research tomograph (HRRT) scanner, with the pig in the prone position. [11C]AZ10419369 was given as an intravenous bolus injection and data acquisition began at the time of injection. The synthesis and radiochemical labeling of [11C]AZ10419369 has previously been described.17
The average injected radioactivity of [11C]AZ10419369 was 466 MBq (range, 399–510 MBq; n = 20) and the average injected mass was 1.75 μg (range 0.17–9.58 μg; n = 20) with no significant difference between scans (paired t-test). To ensure that the pharmacological challenges did not induce changes in blood [11C]AZ10419369 time activity curves (TAC), we measured arterial whole blood radioactivity, in the beginning continuously for 20 min using an ABSS autosampler (Allogg Technology, Mariefred, Sweden) counting coincidences in a lead-shielded detector, and later blood samples were manually drawn at 2.5, 5, 10, 20, 30, 50, 70 and 90 min after injection and the radioactivity in whole blood and plasma samples was measured in a well counter (Cobra 5003, Packard Instruments, PerkinElmer, Skovlunde, Denmark) that was cross-calibrated to the HRRT scanner and autosampler. Radiolabeled parent compound and metabolites were measured in plasma using HPLC with online radioactivity detection as previously described.18
Quantification of PET data
The [11C]AZ10419369 HRRT PET data were acquired in 3D list mode and reconstructed using a 3D–OSEM–PSF algorithm19 with MAP-TR attenuation correction20 into a dynamic image dataset consisting of 44 frames of increasing length (6 × 10, 6 × 20, 4 × 30, 9 × 60, 6 × 120, 4 × 180, 2 × 240, 1 × 300, 1 × 360, 1 × 420, 1 × 540, 1 × 780, 1 × 1380, 1 × 660 s). Images consisted of 207 planes of 256 × 256 voxels of 1.22 × 1.22 × 1.22 mm3. For each pig, a time-weighted average image was calculated including all frames. This image was used for automatic atlas labeling of the individual brain PET images.
Our in-house pig atlas21 was made from transferring atlas labels from a recently published MRI-based pig atlas with 178 segmented regions22 to an average PET template made of PET and MR images in pigs obtained from a recently published study with the PET radioligand 5-HT2A receptor agonist [11C]Cimbi-36.15
Further processing of the [11C]AZ10419369 PET scans in this study was done by generating an average PET scan, which was cropped and co-registered to our in-house pig atlas using the Flirt algorithm from the FSL software package (FSL 5.0.8, FMRIB Software Library, Release 5.0 (c) 2012, The University of Oxford, UK). The estimated 12 parameter affine transformation matrix was then used for transforming the atlas labels into individual pig spaces.
Each coregistration was verified by visual inspection before extraction of TACs from the following volumes of interest (VOIs) combined from the atlas labels: neocortex (28.0 cc) (frontal cortex (4.9 cc), somatosensorimotor cortex (5.9 cc), occipital cortex (5.6 cc), insula (2.8 cc) and temporal cortex (3.8 cc)), thalamus (1.4 cc), striatum (2.0 cc) and hippocampus (0.8 cc). We used cerebellum (4.4 cc) as the reference region. TACs for these VOIs were extracted from the dynamic PET dataset.
We estimated the 5-HT1B receptor binding of [11C]AZ10419369 using the extended simplified reference tissue model (ESRTM)23 before and after the serotonergic challenge given at 56.5 min. This would allow a 30-s administration interval until splitting of the dataset at 57 min, which we in pilot studies found gave stable BPND0/1 estimates before and after challenge. Frames acquired at time interval 0–57 min formed the basis for calculation of BPND0 and frames acquired at time interval 57–120 min for BPND1.
The relative decrease in BPND in the VOI was calculated as
| (2) |
To reject outliers, the standard coefficient of variance (COV) was calculated for all regional BPNDs quantified by the ESRTM model. Data with COV larger than 15% were excluded, and regions with excluded data (n < 5) were not included in further analysis.
Statistical analysis
Similar to our previous study in pigs with [11C]Cimbi-36,15 we correlated the occupancy as measured with PET with the highest peak increase (of the left or right microdialysis probe) in extracellular 5-HT levels. Unless performed with very time-consuming methods24 incompatible with the use of short-lived radioisotopes and the need to conduct two PET studies, microdialysis experiments do not generate absolute serotonin concentrations. Instead, we assumed a fairly stable baseline concentration of the pig cerebral interstitial fluid serotonin of about 1.7 nM, equal to what has been found in mice.25–29 ΔFNT was then computed as 1.7 nM times the relative peak increase in serotonin level minus 1.7 nM. The data were fitted to the competition model given in equation (1) with a non-linear regression analysis as previously described.15 We used the Wald Runs-Test for randomness to test if the curve fitted by non-linear regression to the occupancy model deviated from the data.
The mean increase in interstitial serotonin level (5-HT), as measured by microdialysis and the mean percentual (%) change in BPND of the PET scans, calculated by equation (2), was estimated in the three conditions: saline (n = 10), escitalopram (n = 5) and fenfluramine (n = 5). The change in BPND (PET) and 5-HT (microdialysis) was analyzed post hoc for significance between saline and either two interventions (Wilcoxon signed rank test) for differences in each region of interest.
Our study design with a within-scan saline challenge in the first scan, followed by a second scan where the intervention was given 56.5 min after the radioligand injection allowed for an assessment of the reproducibility of [11C]AZ10419369 binding, both within the individual scan and between the first part of the two scans. We performed test-retest analysis of BPND0 (PET 1 and 2) and BPND0/1 (PET 1) in neocortex. First, we tested for significant order effect (Wilcoxon signed-rank test). Then, we determined the intraclass correlation coefficients (ICC) modelled as a two-way mixed ANOVA with absolute agreement and average measurement in a within-subject design. ICC above 0.80 is considered good and ICC above 0.90 is considered excellent. Lastly, we calculated the required sample size for each intervention of escitalopram and fenfluramine to reach significance level of 0.05 with a power of 0.8 given the effect size determined by group parameters of mean ± SD (BPND0 and BPND1) and ICC for the neocortex region.
Results
In vivo microdialysis
Fenfluramine produced a powerful increase in extracellular serotonin level to a peak 580 ± 286% (n = 5) relative to baseline level (100%) following the within-scan pharmacological challenge. Escitalopram produced a peak increase in serotonin to 233 ± 120% (n = 5) and saline intervention did not change the serotonin level in the pig brain (n = 9) relative to baseline level (Figure 2). The time course of the challenge-induced increase in extracellular serotonin level is given in Figure 3.
Figure 2.
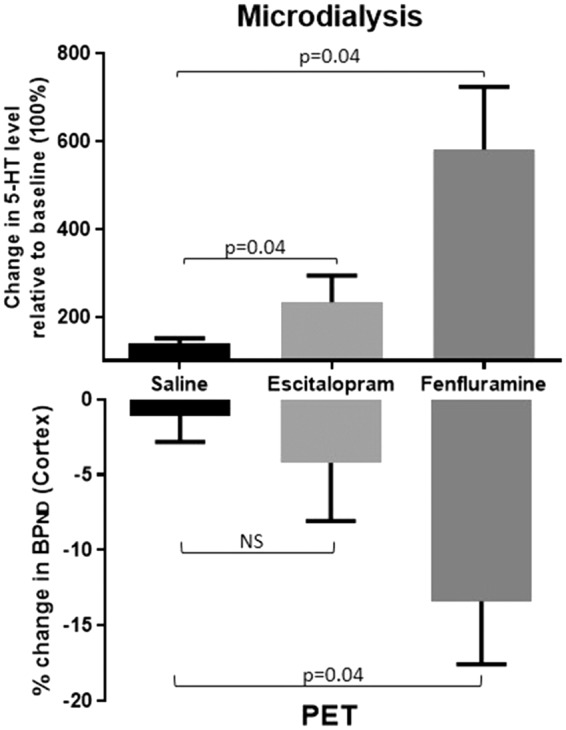
Changes in brain interstitial 5-HT levels (upper panel) and BPND (lower panel) following a within-scan challenge of saline (n = 10), escitalopram (n = 5), or fenfluramine (n = 5). The upper panel shows the changes in the peak interstitial cerebral 5-HT level relative to baseline (100 %) ± SEM as measured by microdialysis in the mPFC. The lower panel shows the relative decrease in BPND as measured by PET in the pig brain neocortex. Changes were statistically evaluated by comparing the escitalopram and fenfluramine interventions to saline with Wilcoxon signed-rank test.
Figure 3.
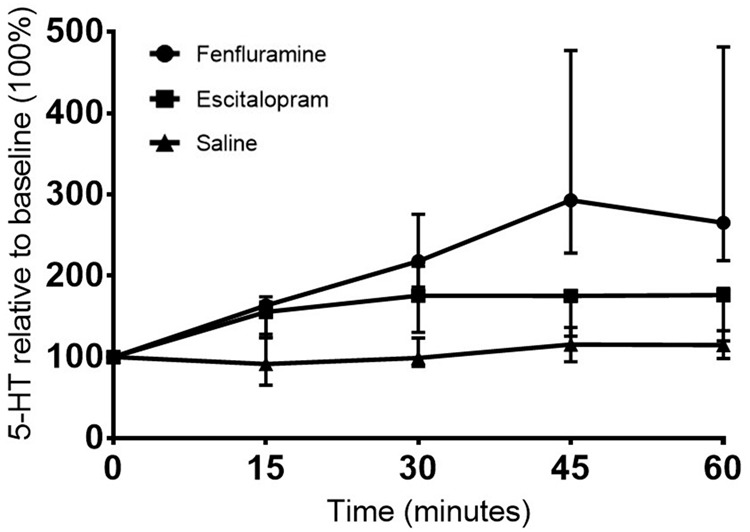
The median time course of the increase in extracellular cerebral 5-HT level after a pharmacological challenge of saline, escitalopram or fenfluramine administered at time 0 min. The increase in 5-HT level is presented relative to baseline (100 %) with median ± quartiles.
In vivo PET imaging
With PET, we measured a significant decrease in BPND of [11C]AZ10419369, corresponding to an increase in interstitial serotonin level induced by escitalopram and fenfluramine (Figure 2). Saline interventions did not induce a change in BPND measures. The BPND0, BPND1 and the relative change (%) in BPND of [11C]AZ10419369 in the neocortex after saline, escitalopram or fenfluramine interventions are given for all pigs individually in the supplementary material (Table 1). The average binding potential of all VOIs is listed in Table 1 in the three conditions. A significant change in binding between groups was observed in the occipital cortex (p = 0.01) and neocortex (p = 0.02) (Kruskal–Wallis test).
Table 1.
Non-displaceable BPNDs from the two PET scans, and % change in BPND0 and BPND1, as calculated by equation (2).
| Volumes of interest | BPND0 (mean ± SD) | BPND1 (mean ± SD) | ΔBPND (%) (mean ± SD) | BPND 0/1 | BPND 0/0 |
|---|---|---|---|---|---|
| Saline (n = 10) |
ICC | ||||
| Cortex (total) | 0.66 ± 0.10 | 0.66 ± 0.13 | −1 ± 5 | 0.98 | 0.94 |
| Frontal | 0.57 ± 0.11 | 0.54 ± 0.13 | −5 ± 7 | 0.97 | 0.92 |
| Somatosensory | 0.60 ± 0.10 | 0.58 ± 0.14 | −4 ± 8 | 0.96 | 0.95 |
| Occipital | 0.78 ± 0.11 | 0.74 ± 0.12 | −5 ± 3 | 0.97 | 0.84 |
| Insula | 0.73 ± 0.10 | 0.76 ± 0.14 | 5 ± 8 | 0.91 | 0.87 |
| Temporal | 0.71 ± 0.12 | 0.72 ± 0.14 | 1 ± 6 | 0.97 | 0.95 |
| Thalamus | 0.90 ± 0.14 | 0.95 ± 0.22 | 4 ± 10 | 0.90 | 0.92 |
| Dorsal striatrum | 1.49 ± 0.20 | 1.59 ± 0.20 | 7 ± 5 | 0.85 | 0.80 |
| Escitalopram
(n = 5) |
Required sample size | ||||
| Cortex (total) | 0.71 ± 0.10 | 0.68 ± 0.12 | −4 ± 8 | 11 | 19 |
| Frontal | 0.64 ± 0.12 | 0.60 ± 0.13 | −6 ± 12 | 8 | 16 |
| Somatosensory | 0.65 ± 0.11 | 0.60 ± 0.13 | −8 ± 9 | 8 | 9 |
| Occipital | 0.79 ± 0.11 | 0.73 ± 0.10 | −7 ± 6 | 5 | 11 |
| Insula | 0.81 ± 0.09 | 0.83 ± 0.15 | 2 ± 9 | 126 | 149 |
| Temporal | 0.76 ± 0.10 | 0.72 ± 0.12 | −5 ± 7 | 8 | 11 |
| Thalamus | 0.98 ± 0.11 | 1.03 ± 0.23 | 4 ± 13 | 67 | 63 |
| Dorsal striatrum | 1.64 ± 0.23 | 1.76 ± 0.30 | 8 ± 12 | 17 | 21 |
| Fenfluramine
(n = 5) |
|||||
| Cortex (total) | 0.64 ± 0.09 | 0.56 ± 0.12 | −13 ± 8 | 5 | 6 |
| Frontal | 0.56 ± 0.11 | 0.47 ± 0.14 | −18 ± 10 | 5 | 6 |
| Somatosensory | 0.58 ± 0.11 | 0.50 ± 0.14 | −15 ± 10 | 6 | 6 |
| Occipital | 0.73 ± 0.07 | 0.61 ± 0.11 | −16 ± 8 | 4 | 5 |
| Insula | 0.70 ± 0.11 | 0.64 ± 0.12 | −8 ± 9 | 8 | 11 |
| Temporal | 0.66 ± 0.11 | 0.57 ± 0.15 | −13 ± 8 | 5 | 6 |
| Thalamus | 0.95 ± 0.20 | 0.89 ± 0.22 | −7 ± 8 | 24 | 20 |
| Dorsal striatrum | 1.51 ± 0.12 | 1.48 ± 0.19 | −2 ± 6 | 110 | 131 |
Note: The intercorrelation coefficient (ICC) is given for both within-PET scan (BPND 0/1) and between-PET scans (BPND 0/0) along with the required sample size given a power of 0.8, significance level of 0.05 and the effect size determined by the mean BPND ± SD and the ICC.
5-HT versus 5-HT1B receptor occupancy
The fenfluramine-induced changes in 5-HT level were significantly increased compared to saline, as measured by both PET and microdialysis, while the escitalopram-induced changes in 5-HT level was only significantly higher when measured by microdialysis (Figure 2).
The corresponding measures of the PET occupancy and the FNT were fitted to equation (1) and the observed correlation did not deviate significantly from the occupancy model (Figure 4). The affinity of the neurotransmitter for its receptor, KNT, was computed to 74 ± 27 nM, about 43 times higher than the baseline brain interstitial serotonin concentration. Figure 4 shows that when brain interstitial serotonin level increases 8-fold, [11C]AZ10419369 decreases by 15% (CI7, 24%).
Figure 4.
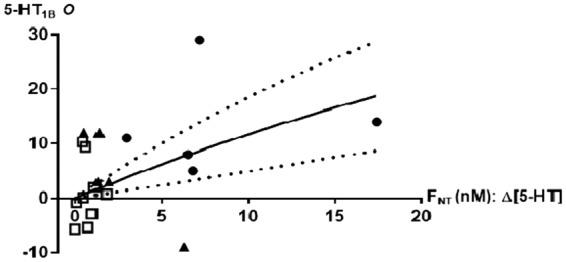
Relationship between relative increase in brain interstitial 5-HT levels (ΔFNT) and neocortical 5-HT1B receptor occupancy O (%) with 95% confidence intervals. Interventions include saline (square), escitalopram (filled triangle), and fenfluramine (filled circle). Data are based on 10 pigs with two PET scans and are fitted with nonlinear regression analysis according to equation (1), resulting in a KNT of 74 nM.
When we compute the data to the model with AUC instead of peak, we find that KNT is 27 nM (instead of 74 nM), and the data still conform to the model. For example, a 8-fold increase in serotonin level would be associated with a 32% (rather than 15%) decline in BPND.
Test-retest analyses of [11C]AZ10419369 PET measures
In neocortex, the [11C]AZ10419369 had a very small test-retest variability of −1 ± 5% (within scan) in BPND0/1 (PET scan 1) and 2 ± 9% (between scans) in BPND0 (PET scan 1/2) and the corresponding ICC values were 0.98 and 0.94. We did not observe a consistent order effect (Wilcoxon signed-rank test, matched pairs). The required sample size to statistically identify a fenfluramine associated 13% decline in BPND in neocortex is n = 5 and to find an escitalopram associated 4% decline in BPND is n = 11 (Table 1).
Discussion
We here show first, that intravenous injections of escitalopram and fenfluramine both induce an acute increase in the cerebral interstitial fluid concentration of serotonin in the brain. Secondly, we show that the interventions induce a varying degree of decline in [11C]AZ10419369 binding in the brain, consistent with the occupancy competition model. The observed correlation between changes in the extracellular serotonin level in the pig brain and the 5-HT1B receptor occupancy indicates that [11C]AZ10419369 binding is sensitive to changes in endogenous serotonin levels.
The pharmacological interventions induced an average 2-fold (escitalopram 0.28 mg/kg) and 6-fold (fenfluramine 0.5 mg/kg) increase in cerebral serotonin release compared to baseline level in the mPFC in the pig brain. In order to compare the outcome of our study with previously reported clinical studies,7,9,10 we deliberately chose to use a clinically relevant dose of escitalopram. However, somewhat unexpectedly, compared to the high dose of escitalopram (2 mg/kg) given in our previous pig study, it does not seem that using a clinically relevant dose of 0.28 mg/kg is associated with substantially less serotonin release.15 The fenfluramine-induced changes in cerebral interstitial serotonin were in line with our previous combined microdialysis and PET study in pigs with the 5-HT2A receptor agonist [11C]-Cimbi3615 as well as microdialysis studies in rats30 and non-human primates.31
Depending on the pharmacological intervention, the change in 5-HT1B receptor binding as measured by the PET radioligand [11C]AZ10419369 ranged between an average 4% (escitalopram) and 16% (fenfluramine). A statistically significant decrease in BPND was only found in brain areas with high binding (the occipital cortex) or when a large brain volume was encountered (entire neocortex), suggesting that good count statistics is important to reveal smaller changes in BPND. The paired data on microdialysis and [11C]AZ10419369 (Figure 4) comply with the competition model supporting that [11C]AZ10419369 PET is sensitive to changes in endogenous serotonin levels when the serotonin release is sufficiently high.
As speculated by Finnema et al.,32 regional differences in SERT, 5-HT1B auto- and heteroreceptor densities may impact the BPND by separate mechanisms. High-density SERT regions may express a higher SSRI-induced increase in synaptic serotonin level as compared to low-density SERT regions, which may produce a less or no decrease in BPND. Given that the regional relationship between 5-HT1B auto- and heteroreceptor densities is largely unknown, it is difficult to predict in which regions one can expect to see the highest sensitivity to changes in synaptic serotonin.
KNT varies across brain regions and species, but in order to relate to equation (1), we assumed a fixed value for interstitial fluid serotonin. KNT would change accordingly, but an 8-fold increase in serotonin level would still result in a 15% decrease in BPND. Based on the data shown in Figure 4, we estimated KNT (the affinity of serotonin to the 5-HT1B receptor) to be 74 nM. For comparison, KNT of [11C]-Cimbi36 is 14.3 nM15 and the concentration of 3H-5-HT that labels 50% of cloned human 5-HT1B receptors is 0.6–15.8 nM (PDSP Ki database).We find it relevant to compare the sensitivity of [11C]AZ10419369 to serotonin with that of [11C]-raclopride to dopamine, since the latter is an often used approach to detect in-vivo change in cerebral dopamine, but also to the sensitivity of [11C]-Cimbi36 and [18F]altanserin to detect changes in serotonin. From microdialysis studies in non-human primates, it is known that when amphetamine 0.3 mg/kg is given intravenously, the interstitial dopamine concentration increases approximately 8-fold.33–36 In non-human primates, an 8-fold increase in dopamine results in a decrease in [11C]-raclopride BPND of 13%.16 This is consistent with findings in humans, where intravenous amphetamine 0.2–0.3 mg/kg results in a decrease in striatal BPND of 15.5%16 or 13%.37–39 With the 5-HT2A agonist receptor [11C]-Cimbi36 in pigs,15 we have recently demonstrated that a pharmacological challenge of fenfluramine and escitalopram induces a serotonergic response compatible with the competition mode l,1 and an 8-fold increase in the cerebral interstitial serotonin level corresponds to a 46% change in occupancy. Here we demonstrate (Figure 4) that an 8-fold increase in interstitial serotonin results in a decrease in [11C]AZ10419369 BPND of 15% (CI 7, 25%), equivalent to the sensitivity of [11C]-raclopride to dopamine, but three times less sensitive than [11C]-Cimbi36 is to changes in the serotonin level. We here show that [11C]AZ10419369 is twice as sensitive to competition from serotonin as [11F]altanserin was in a human study,40 following a single dose of dexfenfluramine (40 mg). Moreover, whereas [11C]-raclopride can only be used to reliably assess dopamine changes in striatum, both [11C]AZ1041936 and [11C]-Cimbi36 can – due to the widespread and relatively uniform distribution of the 5-HT1BR and 5-HT2AR – theoretically be used to determine regional serotonin changes in the entire brain. It is an important feature of a PET radioligand to be able to identify changes also in smaller brain areas, since changes in the cerebral neurotransmitter level could occur in confined brain regions, which potentially could allow for functional imaging of serotonin release. Further, it has been demonstrated in microdialysis studies in rats11,12 that the serotonergic response is attenuated by autoregulation from negative feedback of the somatodendritic 5-HT1A autoreceptors. Blocking the autoregulation by pindolol, a 5-HT1A autoreceptor antagonist, thus potentiates the serotonergic response to SSRIs.13 In pigs, we have also previously observed a higher serotonergic response to escitalopram in combination with pindolol than in mono-intervention with escitalopram.15
Although in a different species, our data do not lend support for an acute 5-HT1B autoreceptor-induced decrease in cerebral interstitial serotonin levels upon a single and clinical relevant dose of escitalopram or citalopram as speculated in two human PET studies with [11C]AZ104193697 and [11C]CUMI-101.9 The apparently conflicting outcome in the human studies with the non-human primate studies6,7,32 and to some extent our pig study may be explained by species differences or differences in study design. Also, it cannot be excluded that the much higher doses of escitalopram used in the non-human primates could induce a larger serotonin release that was more easily detected by PET.
In order to assess the stability of our design, we also investigated the test-retest variability of BPND in the pig. We demonstrated an excellent reproducibility of [11C]AZ10419369 binding to cortical 5-HT1B receptors with a test-retest variability and ICC comparable to the outcome reported in human studies of [11C]Cimbi-36 binding to 5-HT2A receptors41 and a little better than that of [11C]-SB207145 binding to 5-HT4 receptors42 and [11C]P943 to 5-HT1B receptors.43
A limitation of our study is that microdialysis allows only for measurements in a few selected brain regions, while PET, on the other hand, can generate information about changes in the serotonin level of the entire brain. The low number of pigs in the citalopram and fenfluramine groups also limits the conclusions that can be made for smaller brain regions.
In conclusion, we here show that the [11C]AZ10419369 PET signal correlates to the pharmacologically induced changes in interstitial serotonin brain level compatible with the occupancy model. The observed correlation indicates that [11C]AZ10419369 is sensitive to changes in endogenous serotonin levels, but that is only detectable with PET when the serotonin release is sufficiently high. The reproducibility of [11C]AZ10419369 is excellent. The sensitivity of [11C]AZ10419369 to detect changes in extracellular serotonin level is comparable to that of [11C]raclopride to detect changes in dopamine level.
Differences in earlier studies may thus be ascribed to the efficacy of the pharmacological interventions to change interstitial brain serotonin levels. Verifying the direct correlation between pharmacologically induced changes in serotonin and [11C]AZ10419369 PET occupancy is an important step prior to conduction of clinical trials and the calibration allows for estimating the regional relative change in interstitial serotonin in patients in future studies.
Supplementary Material
Acknowledgements
The authors gratefully thank Jytte Rasmussen, Bente Dall and Szabolcs Lehel for excellent technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study received grant funding from the Lundbeck Foundation (R170-2014-994 and R183-2014-3836) for running costs and for PhD salary (LMJ).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
All authors revised and gave final approval of the manuscript. Following authors contributed to the conception (LMJ and GMK), drafting of the article (LMJ, PW, CS and GMK), design (LMJ, PW, SK, LF and GMK), acquisition of data (LMJ, SK, PW) and analysis and interpretation of data (LMJ, PW, GMK).
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Paterson LM, Tyacke RJ, Nutt DJ, et al. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J Cereb Blood Flow Metab 2010; 30: 1682–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finnema SJ, Scheinin M, Shahid M, et al. Application of cross-species PET imaging to assess neurotransmitter release in brain. Psychopharmacology 2015; 232: 4129–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology 1999; 38: 1083–1152. [DOI] [PubMed] [Google Scholar]
- 4.Beliveau V, Ganz M, Feng L, et al. A High-resolution in vivo atlas of the human brain's serotonin system. J Neurosci 2017; 37: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moret C, Briley M. The possible role of 5-HT1B/D receptors in psychiatric disorders and their potential as a target for therapy. Eur J Pharmacol 2000; 404: 1–12. [DOI] [PubMed] [Google Scholar]
- 6.Finnema SJ, Varrone A, Hwang TJ, et al. Fenfluramine-induced serotonin release decreases [11C]AZ10419369 binding to 5-HT1B-receptors in the primate brain. Synapse 2010; 64: 573–577. [DOI] [PubMed] [Google Scholar]
- 7.Nord M, Finnema SJ, Halldin C, et al. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int J Neuropsychopharmacol 2013; 16: 1577–1586. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove KP, Kloczynski T, Nabulsi N, et al. Assessing the sensitivity of [11C]p943, a novel 5-HTIB radioligand, to endogenous serotonin release. Synapse 2011; 65: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvaraj S, Turkheimer F, Rosso L, et al. Measuring endogenous changes in serotonergic neurotransmission in humans: a [11C]CUMI-101 PET challenge study. Mol Psychiatry 2012; 17: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 10.Pinborg LH, Feng L, Haahr ME, et al. No change in [11C]CUMI-101 binding to 5-HT1A receptors after intravenous citalopram in human. Synapse 2012; 66: 880–884. [DOI] [PubMed] [Google Scholar]
- 11.Gobert A, Rivet J-M, Cistarelli L, et al. Potentiation of the fluoxetine-induced increase in dialysate levels of serotonin (5-HT) in the frontal cortex of freely moving rats by combined blockade of 5-HT1A and 5-HT1B receptors with WAY 100,635 and GR 127,935. J Neurochem 1997; 68: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 12.Hjorth S. Serotonin 5-HT1A Autoreceptor blockade potentiates the ability of the 5-HT reuptake inhibitor citalopram to increase nerve terminal output of 5-HT in vivo: a microdialysis study. J Neurochem 1993; 60: 776–779. [DOI] [PubMed] [Google Scholar]
- 13.Artigas F, Romero L, de Montigny C, et al. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 1996; 19: 378–383. [DOI] [PubMed] [Google Scholar]
- 14.Andersen VL, Hansen HD, Herth MM, et al. 11C-labeling and preliminary evaluation of pimavanserin as a 5-HT2A receptor PET-radioligand. Bioorg Med Chem Lett 2015; 25: 1053–1056. [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen LM, Weikop P, Villadsen J, et al. Cerebral 5-HT release correlates with [11C]Cimbi36 PET measures of 5-HT2A receptor occupancy in the pig brain. J Cereb Blood Flow Metab 2017; 37: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breier A, Su T-P, Saunders R, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 1997; 94: 2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Cunha-Bang S, Hjordt LV, Perfalk E, et al. Serotonin 1B receptor binding is associated with trait anger and level of psychopathy in violent offenders. Biol Psychiatry. Epub ahead of print 7 March 2016. [DOI] [PubMed] [Google Scholar]
- 18.Gillings N. A restricted access material for rapid analysis of [11C]-labeled radiopharmaceuticals and their metabolites in plasma. Nucl Med Biol 2009; 36: 961–965. [DOI] [PubMed] [Google Scholar]
- 19.Hong IK, Chung ST, Kim HK, et al. Ultra fast symmetry and SIMD-based projection-backprojection (SSP) algorithm for 3-D PET image reconstruction. IEEE Trans Med Imaging 2007; 26: 789–803. [DOI] [PubMed] [Google Scholar]
- 20.Keller SH, Svarer C, Sibomana M. Attenuation correction for the HRRT PET-scanner using transmission scatter correction and total variation regularization. IEEE Trans Med Imaging 2013; 32: 1611–1621. [DOI] [PubMed] [Google Scholar]
- 21.Villadsen J, Hansen HD, Jørgensen LM, et al. Automatic delineation of brain regions on MRI and PET images from the pig. J Cereb Blood Flow Metab 2017; 37(1S): 145. [Google Scholar]
- 22.Saikali S, Meurice P, Sauleau P, et al. A three-dimensional digital segmented and deformable brain atlas of the domestic pig. J Neurosci Meth 2010; 192: 102–109. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Chen M-K, Endres CJ, et al. An extended simplified reference tissue model for the quantification of dynamic PET with amphetamine challenge. Neuroimage 2006; 33: 550–563. [DOI] [PubMed] [Google Scholar]
- 24.Olson RJ, Justice JB. Quantitative microdialysis under transient conditions. Anal Chem 1993; 65: 1017–1022. [DOI] [PubMed] [Google Scholar]
- 25.Gardier AM, David DJ, Jego G, et al. Effects of chronic paroxetine treatment on dialysate serotonin in 5-HT1B receptor knockout mice. J Neurochem 2003; 86: 13–24. [DOI] [PubMed] [Google Scholar]
- 26.Tao R, Ma Z, Auerbach SB. Differential effect of local infusion of serotonin reuptake inhibitors in the raphe versus forebrain and the role of depolarization-induced release in increased extracellular serotonin. J Pharmacol Exp Ther 2000; 294: 571–579. [PubMed] [Google Scholar]
- 27.Guiard BP, David DJP, Deltheil T, et al. Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int J Neuropsychopharmacol 2008; 11: 79–92. [DOI] [PubMed] [Google Scholar]
- 28.Calcagno E, Canetta A, Guzzetti S, et al. Strain differences in basal and post-citalopram extracellular 5-HT in the mouse medial prefrontal cortex and dorsal hippocampus: relation with tryptophan hydroxylase-2 activity. J Neurochem 2007; 103: 1111–1120. [DOI] [PubMed] [Google Scholar]
- 29.Deltheil T, Guiard BP, Cerdan J, et al. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology 2008; 55: 1006–1014. [DOI] [PubMed] [Google Scholar]
- 30.Udo de Haes JI, Cremers TIFH, Bosker F-J, et al. Effect of increased serotonin levels on [18F]MPPF binding in rat brain: fenfluramine vs the combination of citalopram and ketanserin. Neuropsychopharmacology 2005; 30: 1624–1631. [DOI] [PubMed] [Google Scholar]
- 31.Udo de Haes JI, Harada N, Elsinga PH, et al. Effect of fenfluramine-induced increases in serotonin release on [18F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse 2006; 59: 18–26. [DOI] [PubMed] [Google Scholar]
- 32.Finnema SJ, Varrone A, Hwang T-J, et al. Confirmation of fenfluramine effect on 5-HT1B receptor binding of [11C]AZ10419369 using an equilibrium approach. J Cereb Blood Flow Metab 2012; 32: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000; 20: 423–451. [DOI] [PubMed] [Google Scholar]
- 34.Laruelle M, Iyer RN, Al-Tikriti MS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse 1997; 25: 1–14. [DOI] [PubMed] [Google Scholar]
- 35.Tsukada H, Nishiyama S, Kakiuchi T, et al. Is synaptic dopamine concentration the exclusive factor which alters the in vivo binding of [11C]raclopride? PET studies combined with microdialysis in conscious monkeys. Brain Res 1999; 841: 160–169. [DOI] [PubMed] [Google Scholar]
- 36.Narendran R, Jedema HP, Lopresti BJ, et al. Imaging dopamine transmission in the frontal cortex: a simultaneous microdialysis and [11C]FLB 457 PET study. Mol Psychiatry 2014; 19: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez D, Gil R, Slifstein M, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 2005; 58: 779–786. [DOI] [PubMed] [Google Scholar]
- 38.Martinez D, Narendran R, Foltin RW, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 2007; 164: 622–629. [DOI] [PubMed] [Google Scholar]
- 39.Schneier FR, Abi-Dargham A, Martinez D, et al. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depress Anxiety 2009; 26: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quednow BB, Treyer V, Hasler F, et al. Assessment of serotonin release capacity in the human brain using dexfenfluramine challenge and [18F]altanserin positron emission tomography. Neuroimage 2012; 59: 3922–3932. [DOI] [PubMed] [Google Scholar]
- 41.Ettrup A, Svarer C, McMahon B, et al. Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36: test–retest reproducibility and head-to-head comparison with the antagonist [18F]altanserin. Neuroimage 2016; 130: 167–174. [DOI] [PubMed] [Google Scholar]
- 42.Marner L, Gillings N, Comley RA, et al. Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J Nucl Med 2009; 50: 900–908. [DOI] [PubMed] [Google Scholar]
- 43.Saricicek A, Chen J, Planeta B, et al. Test-retest reliability of the novel 5-HT receptor PET radioligand [C]P943. Eur J Nucl Med Mol Imaging 2015; 42: 468–477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



