Short abstract
WW domain-containing oxidoreductase (WWOX), an important tumor suppressor, is essential for regulating cell proliferation and apoptosis. Our study demonstrates that low level of WWOX is associated with the triple-negative subtype of breast cancer (TNBC), which has higher stem cell phenotype and chemoresistance. We evaluated the role of WWOX in regulation of breast cancer stem cells (BCSC) phenotype and chemoresistance. Our results showed that knockdown of WWOX increases the stemness of breast cancer cells. Meanwhile, downregulation of WWOX induces the epithelial–mesenchymal transition (EMT) and chemoresistance of breast cancer cell lines. Our findings revealed the role of the WWOX in the regulation of the BCSC population and chemotherapeutic sensitivity and may provide insights for the development of more effective therapies targeting cancer stem cells in breast cancer.
Impact statement
Overcoming resistance to chemotherapy is one of the fundamental issues of clinical treatment and CSCs are responsible for the poor therapeutic effects of chemotherapy. WW domain-containing oxidoreductase (WWOX), an important tumor suppressor, regulates cancer cells’ response to chemotherapy. The major finding of our study is the novel role of WWOX in the chemoresistance of breast cancer through the regulation of cell stemness and EMT. The plasticity may play a crucial role in tumor metastasis, treatment resistance and tumor recurrence. Our findings may shed new light on the alterations of BCSCs and pave the way for the discovery of novel and more effective therapies to treat breast cancer by targeting WWOX.
Keywords: Breast cancer stem cells, chemoresistance, WW domain-containing oxidoreductase
Introduction
Breast cancer is a major health issue for women worldwide. According to the expression levels of ER, PR and HER2, primary breast cancer is classified into four subtypes: luminal A (ER and PR+, HER2–), luminal B (ER, PR, and HER2+), ERBB2 (ER and PR–, HER2+), and triple-negative (ER, PR, and HER2–).1–3 Triple-negative subtype of breast cancer (TNBC) accounts for 10–17% of breast cancer. However, compared to the other breast cancer subtypes, patients with TNBC have the shortest overall survival (OS) and five-year disease free survival (DFS). Specifically, the five-year OS for TNBC is 83%, compared to 98% for the luminal A, 94% for the luminal B, and 94% for HER2 positive breast cancers.1,4,5
The TNBC subtype is enriched in breast cancer stem cells (BCSCs), responsible for tumor aggression, therapy resistance, and poor prognosis.6–11 BCSCs are CD24-negative, CD44-positive cells that might also express other cell surface markers including EpCAM and ALDH.12,13 Specifically, the expression levels of CD24, CD44, EpCAM, and ALDH allow the identification of distinct BCSCs. CD24-negative, CD44-positive, and EpCAM-positive BCSCs are involved in the EMT, while ALDH-positive BCSCs are associated with the mesenchymal–epithelial transition (MET).14 CD24-negative, CD44-positive BCSCs are relatively quiescent cells and resistant to chemotherapy, while ALDH-positive (MET-type) BCSCs are highly proliferative.14 BCSCs can transition between the EMT and MET states and such plasticity may play a crucial role in treatment resistance, tumor metastasis, and tumor recurrence.
WWOX, an important tumor suppressor, is significantly associated with breast cancer progression and unfavorable prognosis. The full-length WWOX has two N-terminal WW domains, which can bind the PPXY motif.15 The WW–PPXY interaction mediates WWOX functions. WWOX regulates the cell cycle and apoptosis by interacting with proteins that have PPXY motifs, such as p73 (a p53 homologue), erythroblastic leukemia viral oncogene homolog 4 (ErbB4), activator protein 2γ (AP-2γ), c-Jun, and RUNX2: specifically, WWOX suppresses their transcriptional function by sequestering them in the cytoplasm.16–20 Interestingly, the WWOX-ΔNp63 interaction leads to increased chemosensitivity to cisplatin and increased cell death rate. On the other hand, CSCs are generally recognized as the cause of chemoresistance. Therefore, the aim of this study was to investigate WWOX function in the regulation of BCSCs.
Materials and methods
Cell culture and transfection
Transformed breast epithelial cell line MCF-10A was obtained from ATCC (VA, USA) and cultured in DMEM/F12 supplemented with 20 ng/ml recombinant human EGF (Peprotech, 100–15), 0.5 µg/ml hydrocortisone (Sigma, H-0888), 10 µg/ml insulin (Sigma, I-1882), 1% penicillin/streptomycin, and 5% horse serum (Hyclone). Breast cancer cell lines MCF-7, T47D, SKBR3, MDA-MB-453, and MDA-MB-231 were obtained from ATCC (VA, USA) and cultured in DMEM supplemented with 10% FBS (Hyclone). BT549 was cultured in DMEM medium (Hyclone) supplemented with 10% FBS (Hyclone) and 1 µg/ml insulin (Sigma, I-1882). All cells lines were characterized by Genetic Testing Biotechnology Corporation (Suzhou, China). MCF-7 cells were transfected with two shRNA-WWOX lentivirus (GenePharma, Shanghai, China) to generate WWOX-silenced MCF-7 (MCF7-shWWOX-1 and shWWOX-2). The sequences of WWOX-specific shRNAs were:
shWWOX-1: AAGACTCAGTGGGAACATC
shWWOX-2: GCAGTGCATCCTGGAAATATG
Western blot analysis
The cells were lysed by RIPA buffer with phosphatase inhibitors and protease inhibitors (Roche). After quantification with BCA reagent, individual cell lysates (40 µg/lane) were separated by 10% SDS-PAGE gel electrophoresis. The proteins were transferred onto PVDF membranes (Millipore). The PVDF membranes were treated with 5% fat-free dry milk in TBST and incubated with anti-E-cadherin (1:1000, ab1416, Abcam, MA), anti-ZO-1 (1:1000, 339100, Thermo Fisher, MA), anti-Vimentin (1:200, 10366, Proteintech, Wuhan, China), anti-WWOX (1:200, #374449, Santa Cruz, Texas, CA), anti-Oct-4 (1:1000, WL1005a, Wanleibio, Shenyang, China), anti-Nanog (1:1000, #4903, Cell Signaling Technology, MA), anti-C-Myc (1:200, #4903, Santa Cruz, Texas, CA), anti-Sox2 (1:1000, #3579, Cell Signaling Technology, MA), and anti-GAPDH (1:10000, HRP-60004, Proteintech, Wuhan, China) overnight at 4°C. After being washed three times with TBST, the membranes were treated with HRP conjugated with secondary antibodies and visualized using chemiluminescent (ECL) Plus reagent (Millipore).
Cell viability assay
MCF-7 shCon, MCF-7 shWWOX-1, and MCF-7 shWWOX-2 cells were cultured in 96-well plates and treated in triplicate with cisplatin (CDDP, 5/10 µg/ml), doxorubicin (ADM, 2.5/5 µg/ml), and paclitaxel (PTX, 2.5/5 nM) for 24, 48, and 72 h. During the last 4 h of culture, all cells were exposed to 5 mg/ml of the MTT reagent. Individual wells were dissolved in 200 µl DMSO and the absorbance was measured at 570 nm in a microreader.
Immunofluorescence
MCF-7 shCon, MCF-7 shWWOX-1, and MCF-7 shWWOX-2 cells were fixed with a 4% paraformaldehyde for 10 min and permeabilized in 0.1% Triton X-100 for 10 min. After being blocked with 5% bovine serum albumin (BSA) and 10% horse sera in PBS for 1 h at room temperature, the cells were incubated with anti-E-cadherin (1:200, ab1416, Abcam, MA) and anti-ZO-1 (1:200, 339100, Thermo Fisher, MA) at 4°C overnight. The cells were incubated with Alexa Fluor 488-conjugated secondary antibodies (Invitrogen) and co-stained with DAPI. Fluorescent signals were captured under a confocal laser scanning microscope (Leica SP5II).
Flow cytometry analysis
According to the manufacturer’s instruction, MCF-7 shCon, MCF-7 shWWOX-1, and MCF-7 shWWOX-2 cells were harvested and stained with APC-anti-CD44 and PE-anti-CD24 (Biolegend). The cells were analyzed by flow cytometry (BD FACSCanto II) and analyzed by FlowJ software, as described previously.21
Mammosphere formation assay
1 × 104 cells/well of MCF-7 shCon, MCF-7 shWWOX-1, and MCF-7 shWWOX-2 cells were cultured in ultralow attachment six-well plates (Corning). Then, FBS-free DMEM:F12 media (Gibco) supplemented with 20 µl/ml B27 (Invitrogen) and 20 ng/ml EGF (Peprotech) was added to the well. The cells were exposed to half volume of fresh medium (500 µl) every three days. On day 10 post incubation, the formed mammospheres in individual wells were captured under a light microscope.
Statistical analysis
The experimental data are expressed as the mean ± standard deviation (SD). The difference groups were analyzed by Student’s t test using GraphPad Prism Version 7.00. P-value of <0.05 was considered statistically significant.
Results
WWOX expression in the different breast cancer subtypes
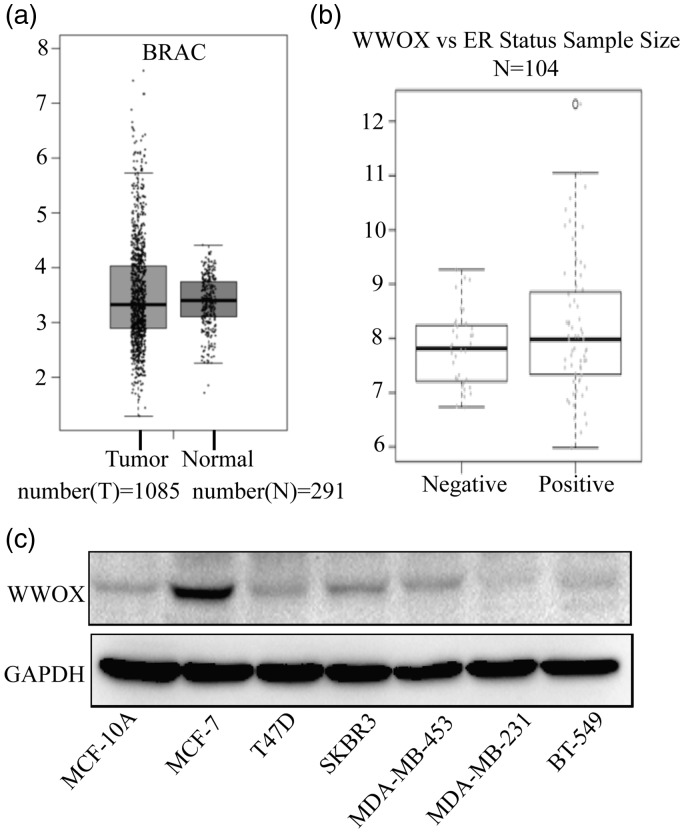
To study the role of WWOX in breast cancer, we used the GEPIA database (http://gepia.cancer-pku.cn/detail.php) of the Peking university (China) to analyze the mRNA expression profiling of WWOX in normal breast tissues (n = 291) and breast cancer tissues (n = 1085). Compared with normal breast tissues, breast cancer tissues had reduced levels of WWOX (P < 0.05; Figure 1(a)). Furthermore, a significant correlation was found between ER status and WWOX expression: the expression of WWOX in ER-positive breast cancers was higher than that in ER-negative cancers (P < 0.05; Figure 1(b)).
Figure 1.
The expression of WWOX in breast cancer and normal cell lines. (a) GEPIA mRNA expression profiling data relative to WWOX expression in breast cancer tissues (n = 1085) and normal breast tissues (n = 291). (b) Relationship between WWOX expression and ER status. (c) WWOX expression in normal breast cells (MCF-10A), luminal A (MCF-7 and T47D), HER2+ (SKBR3 and MDA-MB-453) and TNBC (MDA-MB-231 and BT-549) breast cancer cells.
WWOX: WW domain-containing oxidoreductase.
To further determine the relationship between WWOX expression and the progression of breast cancer, we assessed the expression level of WWOX in the breast epithelial cell line MCF-10A and in different breast cancer cell lines, including two luminal A subtype (MCF-7 and T47D), two HER2-positive subtype (SKBR3 and MDA-MB-453), and two TNBC subtype (MDA-MB-231 and BT549) cell lines. We found that WWOX protein expression in MCF-7 cells was higher than that in the other cell lines. On the other hand, WWOX levels were the lowest in the TNBC cell lines (Figure 1(c)). This expression pattern strongly suggests that WWOX might be involved in progression of breast cancer, particularly of TNBC. TNBC cell lines have a higher stem cell phenotype and chemoresistance compared to other breast cancer cell lines. Therefore, we decided to investigate the role of WWOX in the stemness of the cells.
WWOX downregulation induces stem cell-like properties in breast cancer cells
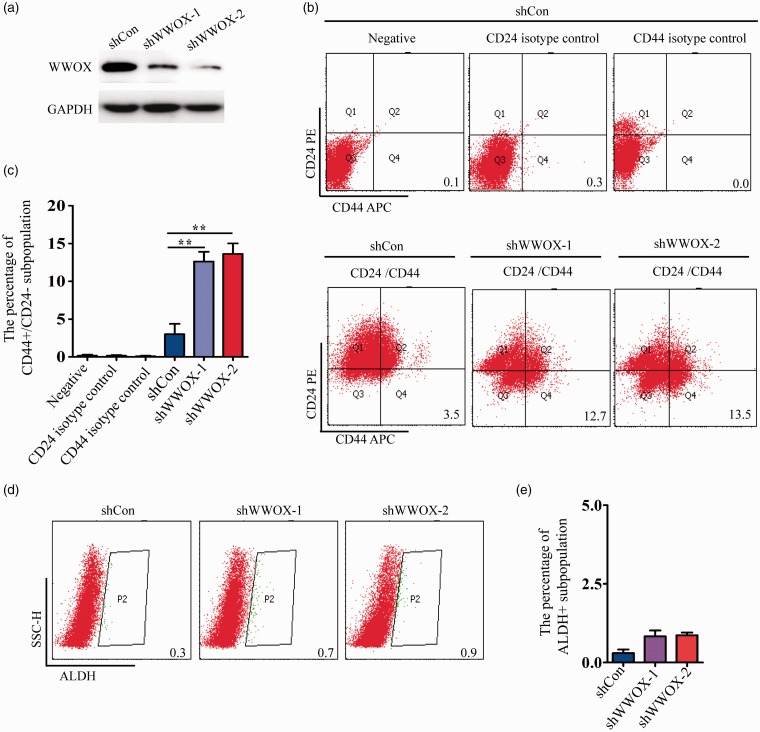
To understand the association between WWOX and stemness in breast cancer, we first used FACS assays to determine the proportion of CD44high/CD24low and ALDHhigh cells in MCF-7 cells in which WWOX had been knocked down; these cell surface markers are often used for BCSCs separation. We found that the CD44high/CD24low subpopulation significantly increased (4–5 folds) upon WWOX knockdown (Figure 2(b) and (c)). However, the ALDHhigh subpopulation did not significantly change after WWOX silencing (Figure 2(d) and (e)).
Figure 2.
Knockdown of WWOX augments the CD44high/CD24low subpopulation of MCF-7 cells. (a) WWOX protein level of shCon and shWWOX-1/2 transfected MCF-7 cells was detected by Western blot. (b) FACS profiles of the CD44high/CD24low subpopulation upon WWOX knockdown. (c) Quantification of the CD44high/CD24low subpopulation upon WWOX knockdown. (d) FACS profiles of the ALDHhigh subpopulation upon WWOX knockdown. (e) Quantification of the ALDHhigh subpopulation upon WWOX knockdown.
WWOX: WW domain-containing oxidoreductase.
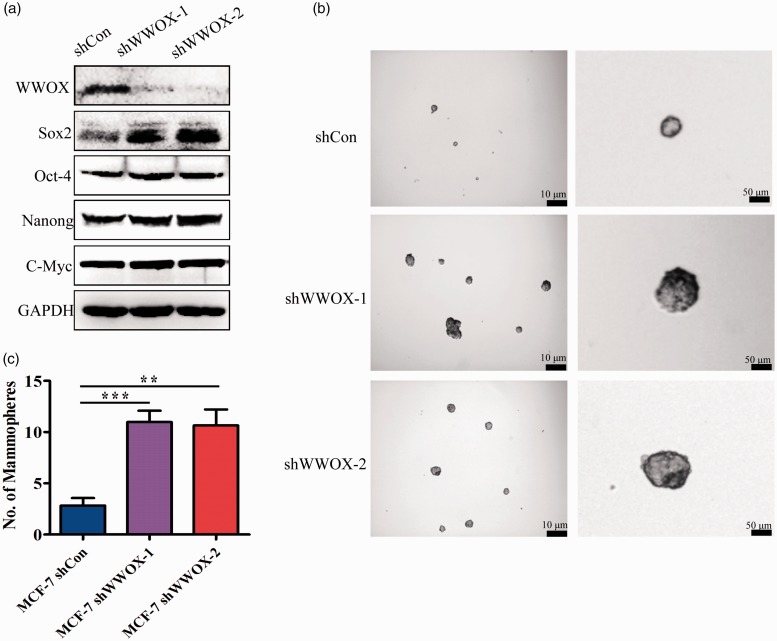
To further examine the role of WWOX on cancer stem cell (CSC) properties, we examined the expression of the stem cell markers Sox2, Oct-4, Nanong, and c-Myc. Our results showed that in WWOX-knockdown cells, the expression of Sox2 was significantly upregulated, and the expression of Oct-4, Nanong and c-Myc increased slightly (Figure 3(a)). The mammosphere formation in an EGF-supplemented serum-free medium is an assay for CSC self-renewal assessment.22 WWOX-silenced MCF-7 cells formed more and bigger mammospheres than control MCF-7 cells (Figure 3(b) and (c)). These results demonstrate that WWOX knockdown increases the stemness of breast cancer cells.
Figure 3.
Knockdown of WWOX I induces the stemness of MCF-7 cells. (a) Western blot analysis of Sox2, Oct-4, Nanog and c-Myc in WWOX-knockdown cells. (b) Representative images of mammospheres formed by control and WWOX-knockdown cells. (c) Quantification of mammospheres formed by control and WWOX-knockdown cells.
WWOX: WW domain-containing oxidoreductase.
Knockdown of WWOX regulates EMT in breast cancer cells
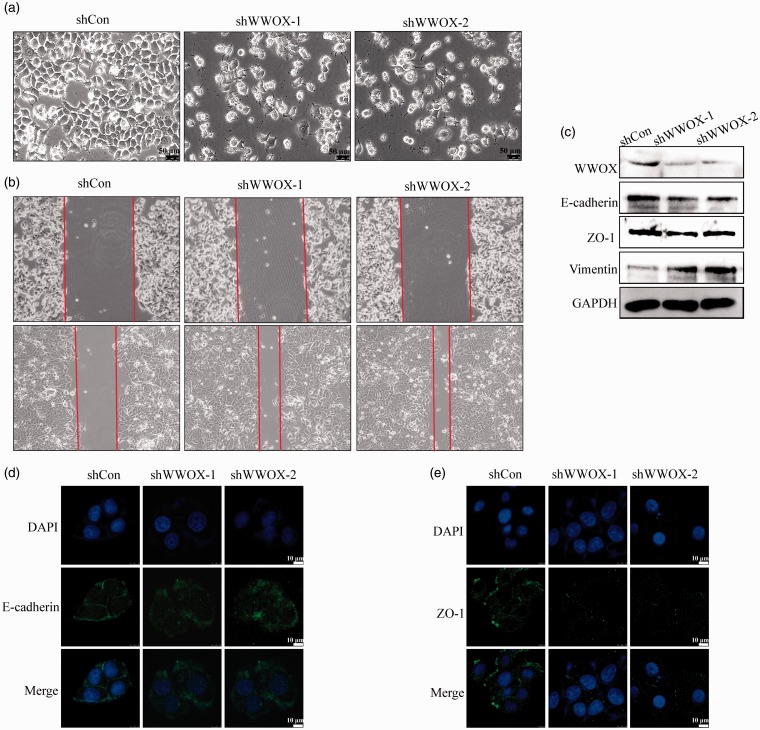
EMT is usually coupled with cancer progression. Because the CD44high/CD24low BCSCs subpopulation is associated with EMT, we investigated whether WWOX knockdown affects EMT. CSCs can transition between the EMT and MET states and this transition may contribute to the formation of metastases and resistance to treatment.23–25 We first observed the cell morphology after WWOX knockdown. Slender pseudopodia accompanied the irregular appearance in WWOX-silenced cells, whereas the control cells appeared oval or round in shape (Figure 4(a)). The migration of WWOX knockdown cells was assessed with monolayer wound healing assay. As shown in Figure 4(b), WWOX knockdown significantly induced the migration of MCF-7 cells. We then examined the expression of EMT markers upon WWOX knockdown. We found that the epithelial markers E-cadherin and ZO-1 were downregulated, while the mesenchymal marker vimentin was upregulated (Figure 4(c)). We further examined the location of E-cadherin and ZO-1 in WWOX knockdown cells. Immunofluorescence staining showed that E-cadherin and ZO-1 re-localized from the cell membrane to the cytoplasm after WWOX knockdown (Figure 4(d) and (e)). Therefore, downregulation of WWOX induced the EMT and WWOX-knockdown cells have mesenchymal-like features.
Figure 4.
Knockdown of WWOX increases the mesenchymal features of breast cancer cells. (a) Morphology of WWOX-knockdown and control cells. (b) Knockdown of WWOX increases cell migration in wound healing assay. (c) The expression of cell polarity-related proteins in control and WWOX-knockdown cells. (d) Cellular localization of E-cadherin in control and WWOX-knockdown cells. (e) Cellular localization of ZO-1 in control and WWOX-knockdown cells.
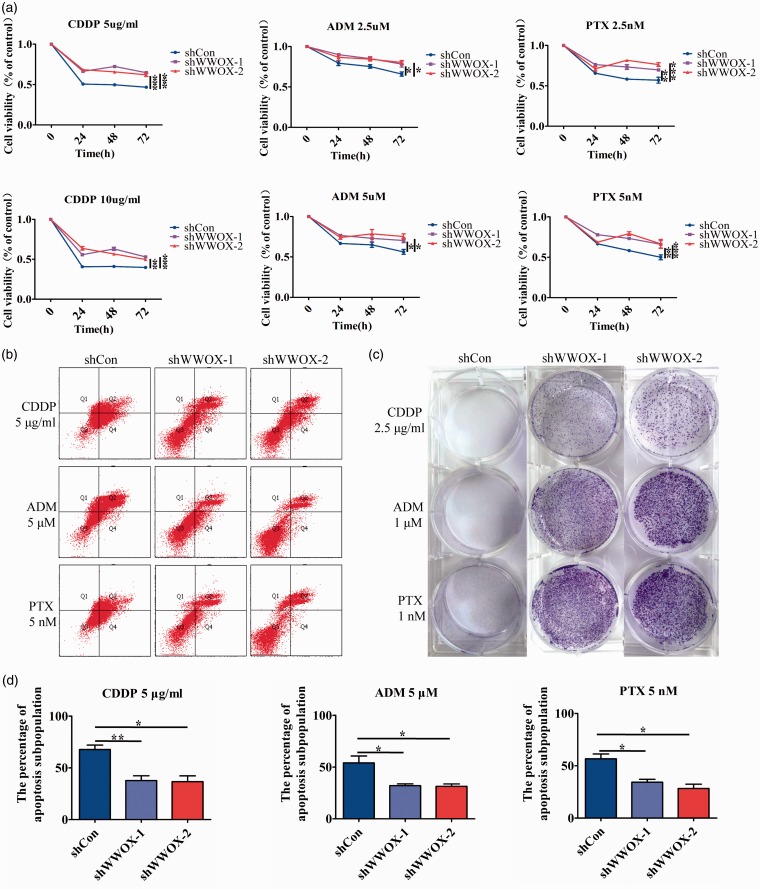
Knockdown of WWOX increases the resistance of breast cancer cells to chemotherapeutics
Overcoming resistance to chemotherapy is one of the fundamental issues of clinical treatment and CSCs are responsible for the poor therapeutic effects of chemotherapy. Therefore, we examined the effect of WWOX on chemoresistance. We choose cisplatin (CDDP, platinum chemotherapy drug), doxorubicin (ADM, anthracycline chemotherapy drug), and paclitaxel (PTX, taxus chemotherapy drug). The chemoresistance of breast cancer cell lines was assayed by MTT, apoptosis, and clone formation assays. Compared with control cells, WWOX-silenced cells showed higher cell viability under CDDP, ADM, or PTX treatment (Figure 5(a)). Annexin-FITC flow cytometry results showed that the percentage of apoptotic cells upon CDDP, PTX, and ADM treatment was lower in WWOX-silenced MCF-7 cells than in control cells (Figure 5(b) and (d)). Finally, in clone formation assays, WWOX-knockdown cells were more capable than control cells to form colonies upon CDDP, ADM, and PTX treatment (Figure 5(c)). These results showed that knockdown of WWOX increased the chemoresistance of breast cancer cells.
Figure 5.
Knockdown of WWOX induces chemotherapy chemoresistance. (a) The cell survival rates of control or WWOX-knockdown cells after treatment with CDDP (5 and 10 µg/ml), ADM (2.5 and 5 µM) and PTX (2.5 and 5 nM) for 24, 48 or 72 h. (b) Control or WWOX-silenced cells were treated with CDDP (5 µg/ml), ADM (5 µM), and PTX (5 nM) for 48 h, incubated with 7-AAD and PE, and then examined by FACS. (c) Clone formation in control or WWOX-silenced cells after treatment with CDDP (2.5 μg/ml), ADM (1 µM), and PTX (1 nM) for 14 days. (d) The percentage of apoptosis cells of control or WWOX-silenced cells upon CDDP, PTX, and ADM treatment.
Discussion
Breast cancer is a major health issue for women worldwide. Adjuvant systemic chemotherapy, anti-HER2 therapy, and endocrine therapy are conventional treatments for breast cancer. Unfortunately, chemoresistance, attributed to increased stemness of breast cancer cells, remains a serious threat to patients with breast cancer. WWOX, an important tumor suppressor, is associated with the progression of carcinomas. Studies have shown that the expression of WWOX is reduced and lost in 55–63.2% and 29% of breast cancer tissues, respectively. Additionally, WWOX expression is positively correlated with the ER/PR status and negatively associated with the clinical stages of breast cancer.26,27 These findings indicate that WWOX deregulation is associated with carcinogenesis and progression of breast cancer.
Previous studies have shown that WWOX regulates cancer cells’ response to chemotherapy by three mechanisms. First, WWOX interacts with p53 and the p53 homologue protein ΔNP63 to regulate cell death.28,29 Second, knockdown of WWOX expression leads to radiation and cisplatin resistance through the dysregulation of DSB repair and the enhanced efficiency of likely mutagenic repair processes.30 Third, the absence of WWOX induces chemotherapeutic drug resistance by regulating autophagy responses and ER stress in cancer cells.31,32 The major finding of our study is the novel role of WWOX in the chemoresistance of breast cancer through the regulation of cell stemness. Low WWOX expression is associated with TNBC, which has stem cell-like features and poor prognosis. The fragile site where WWOX is located is a cellular sensor of extrinsic or intrinsic DNA replication stress. Therefore, loss or reduction of WWOX expression may contribute to the resistance of such stresses.
In our studies, we observed that WWOX knockdown induced stemness in breast cancer cells. The expression of Sox2 and the ability to form mammospheres significantly increased upon WWOX knockdown. FACS analysis showed that downregulation of WWOX was associated with the increase in the CD44high/CD24low subpopulation: this subpopulation, labeled as EMT-CSCs, has low expression of the epithelial marker E-cadherin and high expression the mesenchymal marker vimentin.14 Previous studies have suggested that the CD44high/CD24low subpopulation is relatively quiescent and resistant to cytotoxic chemotherapy and radiation therapy.33 In line with these studies, our data confirmed that knockdown of WWOX enhances EMT and chemoresistance. Immunofluorescence and Western-blot analyses showed that WWOX-knockdown cells have a mesenchymal phenotype and increased resistance to chemotherapy drugs, such as CDDP, ADM, and PTX.
The mechanism of WWOX-mediated regulation of stemness in breast cancer remains to be elucidated. For this purpose, we used the STRING database (https://string-db.org/cgi/input.pl?sessionId=Vs0Ha29yPWOy&input_page_active_form=examples) to analyze the regulatory network of WWOX (Supplement Figure 1). The network analysis predicted 10 top functional partners of WWOX (Supplement Table 1): YAP1, MAPK8, and SMAD4 are important members of Hippo, MAPK, and TGFβ signaling pathways, respectively. These pathways play an important role in regulating cell differentiation, proliferation, and apoptosis in response to a wide range of intracellular and extracellular signals.21,34–38 Therefore, WWOX may regulate the stemness of BCSCs through such pathways. Our findings may shed new light on the alterations of BCSCs and pave the way for the discovery of novel and more effective therapies to treat breast cancer by targeting WWOX.
Authors’ contributions
Juan Li performed experiments, analyzed the data including statistical analysis, and revised the manuscript. Liu Jie and Pingping Li provided technical support and revised the manuscript. Peijun Liu and Can Zhou were involved in the design, interpretation of the studies. All authors read and approved the final version of the manuscript.
Supplemental Material
Supplemental material for The downregulation of WWOX induces epithelial–mesenchymal transition and enhances stemness and chemoresistance in breast cancer by Juan Li, Jie Liu, Pingping Li, Can Zhou and Peijun Liu in Experimental Biology and Medicine
Acknowledgments
The authors thank Yan Zhang for proofreading of the manuscript.
Declaration Of Conflicting Interests
The author(s) declare no conflict of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by Grant from the National Natural Science Foundation of China (Nos. 81502620, 81502413).
References
- 1.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jefferey SS, Rees CA. Molecular portraits of human breast tumors. Nature 2000; 406:747–5 [DOI] [PubMed] [Google Scholar]
- 2.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004; 10:5367–74 [DOI] [PubMed] [Google Scholar]
- 3.Sorlie T, Tibshirani R, Parker J, Hastit T, Marron JS, Nobel A. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 2003; 100:8418–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA 2006; 295:2492–502 [DOI] [PubMed] [Google Scholar]
- 5.Montagna E, Maisonneuve P, Rotmensz N. Heterogeneity of triple-negative breast cancer: histologic subtyping to inform the outcome. Clin Breast Cancer 2013; 13:31–9 [DOI] [PubMed] [Google Scholar]
- 6.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010; 29:4741–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallini P, Lennard T, Kirby J, Meeson A. Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Cancer Treat Rev 2014; 40:341–8 [DOI] [PubMed] [Google Scholar]
- 8.Phillips TM, McBride WH, Pajonk F. The response of CD24(–/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 2006; 98:1777–85 [DOI] [PubMed] [Google Scholar]
- 9.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. LET-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007; 131:1109–23 [DOI] [PubMed] [Google Scholar]
- 10.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001; 7:1028–34 [DOI] [PubMed] [Google Scholar]
- 11.Sims-Mourtada J, Niamat RA, Samuel S, Eskridge C, Kmiec EB. Enrichment of breast cancer stem-like cells by growth on electrospun polycaprolactone-chitosan nanofiber scaffolds. Int J Nanomedicine 2014; 9:995–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100:3983–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginestier C, Hur MH, Charafe-Jauffret E. ALDHI is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007; 1:555–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Cong Y, Wang D. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports 2014; 2:78–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 2007; 13:12–22 [DOI] [PubMed] [Google Scholar]
- 16.Aqeilan RI, Donati V, Gaudio E, Nicoloso MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H, Croce CM, Elenius K. Association of WWOX with ErbB4 in breast cancer. Cancer Res 2007; 67:9330–6 [DOI] [PubMed] [Google Scholar]
- 17.Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the WWOX tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res 2004; 64:8256–61 [DOI] [PubMed] [Google Scholar]
- 18.Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G, Huebner K, Croce CM. Functional association between WWOX tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci USA 2004; 101:4401–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM, Aqeilan RI. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res 2006; 66:11585–9 [DOI] [PubMed] [Google Scholar]
- 20.Kurek KC, Del Mare S, Salah Z, Abdeen S, Sadiq H, Lee SH, Gaudio E, Zanesi N, Jones KB, DeYoung B, Amir G, Gebhardt M, Warman M, Stein GS, Stein JL, Lian JB, Aqeilan RI. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res 2010; 70:5577–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li P, Wang Y, Mao X. CRB3 downregulation confers breast cancer stem cell traits through TAZ/beta-catenin. Oncogenesis 2017; 6:e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006; 9:391–403 [DOI] [PubMed] [Google Scholar]
- 23.Raimondi C, Gradilone A, Naso G. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treatment 2011; 130:449–55 [DOI] [PubMed] [Google Scholar]
- 24.Brabletz T. To differentiate or not – routes towards metastasis. Nat Rev Cancer 2012; 12:425–36 [DOI] [PubMed] [Google Scholar]
- 25.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Płuciennik E, Kusińska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX – the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur J Surg Oncol 2006; 32:153–7 [DOI] [PubMed] [Google Scholar]
- 27.Nune MI, Ludes-Meyers J, Abba MC, Kil H, Abbey NW, Page RE, Sahin A, Klein-Szanto AJ, Aldaz CM . Frequent loss of WWOX expression in breast cancer: correlation with estrogen receptor status. Breast Cancer Res Treat 2005; 89:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang MF, Chou PY, Wang WJ, Sze CI, Chang NS. Tumor suppressor WWOX and p53 alterations and drug resistance in glioblastomas. Front Oncol 2013; 3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salah Z, Bar-Mag T, Kohn Y, Pichiorri F, Palumbo T, Melino G. Tumor suppressor WWOX binds to DeltaNp63alpha and sensitizes cancer cells to chemotherapy. Cell Death Disease 2013; 4:e48031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrock MS, Batar B, Lee J, Druck T, Ferguson B, Cho JH, Akakpo K, Hagrass H, Heerema NA, Xia F, Parvin JD, Aldaz CM, Huebner K. WWOX–Brca1 interaction: role in DNA repair pathway choice. Oncogene 2017; 36:2215–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai CW, Lai FJ, Sheu HM, Lin YS, Chang TH, Jan MS. WWOX suppresses autophagy for inducing apoptosis in methotrexate-treated human squamous cell carcinoma. Cell Death Dis 2013; 4:e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janczar S, Nautiyal J, Xiao Y, Curry E, Sun M, Zanini E, Paige AJW, Gabra H. WWOX sensitises ovarian cancer cells to paclitaxel via modulation of the ER stress response. Cell Death Dis 2017; 8:e2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Lewis MT, Huang J. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Institute 2008; 100:672–9 [DOI] [PubMed] [Google Scholar]
- 34.Lu T, Li Z, Yang Y, Ji W, Yu Y, Niu X, Zeng Q, Xia W, Lu S. The Hippo/YAP1 pathway interacts with FGFR1 signaling to maintain stemness in lung cancer. Cancer Lett 2018; 423:36–46 [DOI] [PubMed] [Google Scholar]
- 35.Roy S, Roy S, Kar M, Padhi S, Saha A, Anuja K, Banerjee B. Role of p38 MAPK in disease relapse and therapeutic resistance by maintenance of cancer stem cells in head and neck squamous cell carcinoma. J Oral Pathol Med 2018; 47:492–501 [DOI] [PubMed] [Google Scholar]
- 36.Kali A, Ostapchuk YO, Belyaev NN. TNFα and TGFβ-1 synergistically increase the cancer stem cell properties of MiaPaCa-2 cells. Oncol Lett 2017; 14:4647–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song S, Xie M, Scott AW, Jin J, Ma L, Dong X, Skinner HD, Johnson RL, Ding S, Ajani JA. A novel YAP1 inhibitor targets CSC-enriched radiation-resistant cells and exerts strong antitumor activity in esophageal adenocarcinoma. Mol Cancer Ther 2018; 17:443–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu N, Mei L, Fan X, Tang C, Ji X, Hu X, Shi W, Qian Y, Hussain M, Wu J, Wang C, Lin S, Wu X. Phosphodiesterase 5/protein kinase G signal governs stemness of prostate cancer stem cells through Hippo pathway. Cancer Lett 2016; 378:38–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for The downregulation of WWOX induces epithelial–mesenchymal transition and enhances stemness and chemoresistance in breast cancer by Juan Li, Jie Liu, Pingping Li, Can Zhou and Peijun Liu in Experimental Biology and Medicine