Abstract
Autophagy, an intracellular bulk degradation process of proteins and organelles, can be induced by myocardial ischemia in the heart. However, the causative role of autophagy in the survival of human cardiac fibroblasts and the underlying mechanisms are incompletely understood. Oxidative stress can induce autophagy in cultured cells upon hydrogen peroxide (H2O2) exposure. Because hydrogen sulfide (H2S) regulates reactive oxygen species (ROS) and apoptosis, we hypothesize that H2S may have a cardioprotective function. To examine our hypothesis, we investigated the regulation of autophagy by the H2S donor sodium hydrosulfide (NaHS), using a cell model of human cardiac fibroblasts from adult ventricles (HCF-av) that suffered from endoplasmic reticulum (ER) stress by H2O2. In the present study, we found that the apoptosis and autophagy were induced along with ER stress by H2O2 in the primary cultured HCF-av cells. In contrast, H2S suppressed HCF-av cell apoptosis and autophagic flux, in part directly by inhibiting ROS production and preserving mitochondrial functions.
Keywords: autophagy, cardiac fibroblasts, hydrogen sulfide, lysosome, endoplasmic reticulum
Introduction
The normal heart is a highly organized structure comprising four major cell types: cardiomyocytes (CMs), cardiac fibroblasts (CFs), endothelial cells (ECs), and vascular smooth muscle cells (VSMCs)1. The proportion of each cell type varies in different species, but overall CFs occupy the myocardium predominantly, accounting for approximately two-thirds of the cell populations. CFs synthesize and organize collagens, fibronectins, and other interstitial components to maintain cardiac integrity during physiologic proliferation and extracellular matrix (ECM) turnover, as well as cardiac remodeling. Because the adult mammalian heart has a negligible regenerative capacity, cardiac injury provides a great challenge for the reparative mechanisms after the loss of CMs, resulting in the formation of a collagen-based scar. Due to their abundance, CFs play an important role during normal and pathologic wound healing following myocardial ischemia, heart failure, and atrial fibrillation2,3. Therefore, fibroblasts represent an attractive therapeutic candidate for heart disease.
Autophagy is a dynamic process of intracellular bulk degradation. The cytosolic proteins and organelles are sequestered into double-membrane vesicles, called autophagosomes, to be fused with lysosomes for degradation4. Primarily, autophagy is a survival mechanism that allows a starving cell or a cell deprived of growth factors to survive. Theoretically, autophagy serves to regulate protein and organelle abundance and quality. Autophagy occurs at basal levels in the normal condition but is substantially increased in several heart diseases, such as acute and chronic ischemia, heart failure, and cardiac hypertrophy5–7. Furthermore, nutritional status, hormonal factors, and other conditions like temperature, oxygen concentration, and cell density are also involved in autophagy regulation8,9.
Although H2S has been considered as a noxious gas with wide-ranging cytotoxic effects, the accumulating scientific evidence shows that H2S plays a pivotal role in cellular signaling and functions, similar to nitric oxide (NO) and carbon monoxide (CO). Our previous preliminary study found that the exogenous H2S donor, sodium hydrosulfide (NaHS), has potent anti-inflammatory effects in a heart that has suffered from acute myocardial infarction in vivo, which may be in part due to the limitation of the recruitment of CD11b+Gr-1+ myeloid cells10,11. Moreover, we also investigated whether NaHS prevented TGF-β1-induced proliferation, migration, regulation of cell growth, transformation to myofibroblasts, and collagen synthesis in human cardiac fibroblasts-to-myofibroblasts assay12. Despite the importance of fibroblasts in cardiac pathologies, the direct effects of exogenous H2S on autophagy in human CFs upon oxidative stress have not been well elucidated. In the present study, we attempted to determine whether the exogenous H2S protected human cardiac fibroblasts-adult ventricular (HCF-av) against hydrogen peroxide (H2O2)-induced endoplasmic reticulum (ER) stress. We used this in vitro model to mimic the ER stress injury to the heart and focused on apoptosis and autophagy. We found that H2S markedly inhibited apoptosis and autophagic flux following ER stress induced by H2O2, supporting that H2S could be used as a new therapeutic reagent for treating oxidative-related diseases.
Materials and Methods
Cell Culture
HCF-av cells were obtained from ScienCell Research Laboratories (Cat# 6310, San Diego, USA) and cultured in fibroblast medium (FM) supplemented with 2% fetal bovine serum (FBS), 1% fibroblast growth supplement (FGS), and 1% penicillin/streptomycin solution (P/S) according to the manufacturer’s protocol. The cells were maintained in a humidified, 37°C incubator with 5% CO2 and 95% air. Cells were subcultured when they became more than 90% confluent. Cells were used for all the in vitro ER stress induction and treatment, measurement of reactive oxygen species (ROS) production, measurement of mitochondrial membrane potential (Δψ), and activity of the lysosomal compartment experiments12.
Animal Study, Transverse Aortic Constriction (TAC) Protocol, and DATS Administration
Male C57BL/6 J mice (10 weeks old) were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Science (SLAC, Shanghai, China). Animal care and experimental procedures were approved by the Ethics Committee on Animal Research of Hubei University of Medicine and the Institutional Animal Care and Use Committee of Cleveland Clinic. The TAC procedure was described previously13. Briefly, the mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg). To create pressure overload of the heart, the chest was opened via minithoracotomy to expose the aortic arch and TAC procedure was performed in 12-week-old mice by placing a 7-0 silk suture around the aortic arch between the brachiocephalic trunk and the left carotid artery. The suture was ligated around a 27-gauge blunt needle and the needle was quickly removed after ligation. Animals that did not survive after the surgeries were excluded from further experiments.
For H2S therapy, the diallyl trisulfide (DATS) was obtained from LKT Laboratories (St. Paul, MN, USA) and stored at −20°C before use. The mice were injected intraperitoneally once per day for 12 weeks after TAC with DATS (200 μg/kg) or vehicle (1% DMSO). The dose of DATS was used for the mice on the basis of previous experience investigating DATS in murine models of cardiac ischemia/reperfusion injury13.
In Vitro ER Stress Induction and Treatment
The HCF-av cells were cultured in serum-free FM for 16 h before treatment and then were challenged with H2O2 (100 μM, Sigma-Aldrich, St. Louis, MO, USA) for 24 h to mimic ER stress injury14–16 in the presence or absence of the exogenous NaHS (100 μM, Sigma-Aldrich). The untreated cells were served as the control group and were used in the following experiments.
Measurement of ROS Production
For measurement of intracellular ROS, the dihydroethidium (DHE, Sigma-Aldrich) was used to monitor ROS production upon different treatments in accordance with the manufacturer’s protocol. Briefly, the subconfluent cells were pretreated with or without NaHS (100 μM) for 30 min and then subjected to H2O2 (100 μM) treatment for 24 h. Cells were incubated with the DHE (5 μM) at 37°C for 30 min and the fluorescence was observed with a Nikon fluorescence microscope (TE-2000U, Nikon, Melville, NY, USA).
Measurement of Mitochondrial Membrane Potential (Δψ)
For measurement of mitochondrial membrane potential (MMP), a mitochondria-specific cationic dye JC-1 (100 nM, Life Technologies, Carlsbad, CA, USA) was used to monitor the MMP under different treatments according to the manufacturer’s protocol. Briefly, the HCF-av cells were treated with or without H2O2 and then were incubated with JC-1 for 10 min in pre-warmed culture medium. Subsequently, the cells were washed three times with pre-warmed culture medium and the MMP was observed with a Nikon fluorescence microscope (TE-2000U, Nikon). Both red and green fluorescence emissions were analyzed after JC-1 staining with Image J software (developed at the National Institutes of Health, Bethesda, MD, USA).
Activity of the Lysosomal Compartment
LysoTracker Deep Red is an ideal fluorescent acidotropic probe that selectively labels vacuoles with low internal pH. Thus, it can be used to label and track functional lysosomes. Briefly, the cells were treated with H2O2 in the presence or absence of NaHS and were then incubated with the LysoTracker Deep Red (70 nM, Life Technologies) in a pre-warmed medium at 37°C for 30 min. Subsequently, the solution was replaced with fresh medium, and the cells were observed using a fluorescence microscope (TE-2000U, Nikon). The activity and intracellular distribution of cathepsin B, a predominant lysosomal protease, was assessed with Magic Red Cathepsin B Detection Kit (Immunochemistry Technologies, LLC, Bloomington, MN, USA). The cells were stained with MagicRed Cathepsin B substrate for 30 min at 37°C and then washed twice with phosphate buffered saline (PBS). Finally, the cells were stained with DAPI (1 μg/ml, Sigma-Aldrich) for 10 min and observed with a fluorescence microscope (TE-2000U, Nikon).
Cell Apoptosis Assay
The cell apoptosis was detected with propidium iodide (PI)/Annexin V assay kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instruction. Briefly, the cells were washed twice with cold PBS and resuspended in 1 × binding buffer at a concentration of 1 × 106 cells/ml. Next, 100 μl cell suspension (1 × 105 cells) was transferred to a 1 ml tube and stained with 5 μl FITC-Annexin V reagents for 30 min and then the cells were stained with 10 μl PI for 10 min at room temperature. Finally, 400 μl 1 × binding buffer was added to each tube. Flow cytometry was performed with the FACScanto II flow cytometer (Becton Dickinson, Mountain View, CA, USA) with excitation at 488 nm. Fluorescent emission of FITC was measured at 515–545 nm and that of DNA–PI complexes at 564–606 nm. Cell debris was excluded from the analysis by an appropriate forward light scatter threshold setting. Compensation was used wherever necessary.
Transmission Electron Microscopy (TEM)
For TEM analysis, the cells were rinsed in PBS and fixed with 2.5% glutaraldehyde in PBS (pH 7.4) for 1 h at 4°C. The cells were washed three times with PBS and then were post-fixed in 1% osmium tetroxide (OsO4) with 1% potassium ferricyanide. Next, the cells were washed with PBS and dehydrated in a gradient of alcohol (30%, 50%, 70%, and 90%) before embedding in epon. TEM was performed with a Philips CM10 (Andover, MA, USA) at 80 kV on ultra-thin sections (100 nm on 200 mesh grids) stained with uranyl acetate and counterstained with lead citrate.
Western Blot Analysis
Western blot analysis was performed as previously described10. Briefly, the cells were washed twice with ice-cold PBS and proteins were extracted using lysis buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 1% Triton X-100, 0.1% SDS, and 100 mM phenylmethylsulfonyl fluoride). The extracted proteins were separated in SDS-polyacrylamide gels and transferred to PVDF membranes (PVDF, Millipore, Burlington Massachusetts, USA ). The membranes were washed three times for 10 min each time with TBST and incubated with primary antibodies at 4°C overnight. The primary antibodies used in this study are listed below: activated caspase 3 p17 (Bioworld; 1:1000 dilution Dublin, OH, USA), BiP (Cell Signaling Technology; 1:1000 dilution Danvers, MA, USA), C/EBP homologous protein (CHOP) (Cell Signaling Technology; 1:1000 dilution), LC3 (Sigma-Aldrich; 1:1000 dilution), Beclin1 (Abcam; 1:1000 dilution, Cambridge, UK), P62/SQSTM1 (Cell Signaling Technology; 1:1000 dilution), Puma (Cell Signaling Technology; 1:1000 dilution), and Ubiquitin (Cell Signaling Technology; 1:1000 dilution). The membranes were washed with TBST followed by incubation with indicated horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA). Detection was performed using enhanced chemiluminescence (ECL) Western blotting detection reagent (G&E) and the data were quantified by densitometry.
Proteasome activity assay
Proteasome activity was measured by aminomethylcoumarin (AMC)-linked synthetic peptide substrates: Ac-Gly-Pro-Leu-Asp-AMC and Suc-Leu-Leu-Val-Tyr-AMC (Proteasome Substrate Pack, Enzo Life Sciences, Farmingdale NY, USA). Proteins were extracted from treated or untreated cells with lysis buffer (50 mM HEPES pH 7.5, 5 mM EDTA, 150 mM NaCl, 1% Triton X-100, and 2 mM ATP). Next, 200 μl of lysate containing equal amounts of protein (5 μg) were incubated for 30 min at 37°C in a dark environment with 2.5 μl of each substrate. The reaction was stopped by stop buffer (ice-cold 96% ethanol). The proteasome activity was detected by Tecan Infinite M200 Plate Reader (380 nm excitation and 460 nm emission, Männedorf, Switzerland).
Statistical analysis
All experiments were carried out in triplicate under identical conditions and data were represented as means ± standard error of the mean (SEM). For animal studies, experiments were performed in duplicate and each group included three mice. Statistical analysis was performed with SPSS software (IBM Corp., Armonk, NY, USA). Different groups were compared by one-way analysis of variance (ANOVA), followed by Tukey’s or Bonferroni post-hoc test when applicable. Comparisons between the two groups were assessed by the t test. A P value less than 0.05 was considered significant.
Results
The Effect of H2O2 on Cell Proliferation of HCF-av Cells
HCF-av cells were treated with H2O2 at different concentrations (0–200 μM) for 24 h. Cell vitality was measured by a Cell Counting Kit (CCK-8, Dojindo, Rockville, MD, USA) according to the manufacturer’s protocol. H2O2 exhibited cytotoxicity in HCF-av cells in a dose-dependent manner. There was no significant loss of vitality with 0 or 25 μM H2O2 in HCF-av cells. In contrast, decreases of nearly 14%, 28%, and 77% cell vitality occurred in HCF-av cells exposed to 50 μM, 100 μM, and 200 μM H2O2 for 24 h, respectively (Figure 1). Therefore, we used 100 μM H2O2 for the next experiments.
Figure 1.

The effect of H2O2 on HCF-av cell vitality. The HCF-av cells were treated with different concentrations of H2O2 for 24 h and then the cell vitality was determined by CCK-8 kit (n = 3). *P < 0.05, **P < 0.01 vs. control.
H2S Reduces ER Stress Induced by H2O2 and TAC
The ER stress was induced by H2O2 in cultured HCF-av cells, which was assessed by the ER stress protein markers immunoglobulin binding protein (BiP) and CHOP. H2O2 challenge provoked a significantly increased Expression of BiP and CHOP compared with the control cells (Figure 2(a–c)). Interestingly, H2S treatment effectively abrogated ER stress by reducing the expression levels of BiP and CHOP induced by H2O2. To further confirm our results, we evaluated the effects of H2S on the ER stress in heart tissues from mice after TAC. As shown in Figure 2(d,e), the ER stress-related markers (BiP, CHOP, and Puma) and caspse-3 were significantly induced by TAC. Strikingly, BiP, CHOP, Puma, and caspase-3 was markedly reduced by H2S after TAC. Altogether, these results demonstrate that H2S protected heart cells against ER stress.
Figure 2.
H2S ameliorates H2O2-induced ER stress in HCF-av cells. (a) Western blot analysis of HCF-av cells upon different treatments was performed to detect BiP and CHOP. β-actin served as the loading control. (b,c) Quantitative analysis of the changes of BiP and CHOP in treated cells. Data represent mean ± SEM (n = 3. *P < 0.05 vs. control; #P < 0.05 vs. NaHS). (d) Representative Western blot analysis for BiP, CHOP, Puma, and caspase 3 expression in hearts from vehicle- and DATS-treated mice. β-actin served as the loading control. (e) Quantitative analysis of the changes of BiP and CHOP in treated cells. Data represent mean ± SEM (n = 3. *P < 0.05 vs. TAC + Vehicle).
H2S Prevents Loss of MMP Induced by H2O2
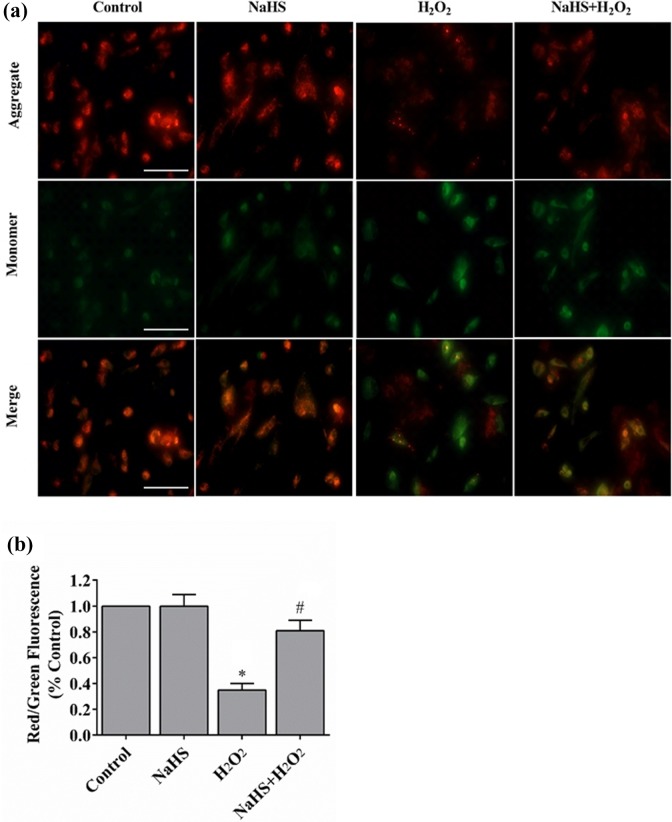
Mitochondrial function is highly susceptible to oxidative damage. Therefore, we investigated whether H2S protected mitochondria from H2O2-induced ER stress. Mitochondria in control cells stained with JC-1 exhibited bright orange fluorescence. However, when cells were exposed to H2O2, they showed fewer and less intense JC-1 fluorescence in mitochondria (Figure 3(a)), which was greatly improved by pretreatment of H2S (Figure 3(b)). These results suggested that H2S could prevent the loss of mitochondrial Δψ upon oxidative stress.
Figure 3.
H2S restores H2O2-induced reduction of Δψ. (a) The Δψ loss was determined by the lipophilic cationic probe JC-1. Red signal indicated JC-1 in mitochondria. Green signal indicated cytosolic JC-1. Magnification, ×400. (b) Quantitative analysis of membrane potential (n = 3). *P < 0.01 vs. control; #P < 0.01 vs. NaHS; #P < 0.01 vs. H2O2.
H2S Suppresses ROS Production Induced by H2O2
To determine the effect of H2S on H2O2-induced ROS production from ER and mitochondria, DHE, a specific fluorescent probe for O2 –, was used to track cellular ROS generation (Figure 4(a)). HCF-av cells were subjected to H2O2 treatment and ROS production was significantly enhanced compared with the control. Conversely, this elevation was markedly suppressed by pretreatment of cells with H2S (Figure 4(a,b)). No significant difference in ROS production was observed with NaHS treatment alone. These results indicated that H2S abrogated ROS production in HCF-av cells.
Figure 4.
H2S suppresses superoxide anion production induced by H2O2. (a) Intracellular superoxide anion production was detected with dihydroethidium and observed by fluorescent microscopy. (b) The fluorescent signal was measured and quantified (n = 6). *P < 0.01 vs. control; #P < 0.01 vs. NaHS; #P < 0.01 vs. H2O2.
H2S Attenuate Cell Apoptosis Induced by H2O2
ROS production is known to promote apoptosis. To evaluate the effect of H2S on ER stress-induced apoptosis, HCF-av cells were subjected to different treatments and the cell death was analyzed by flow cytometry. As shown in Figure 5(a,b), oxidative stress induced by H2O2 resulted in significant cell death (Annexin V+/PI+ cells) compared with the control cells. By contrast, pretreatment of NaHS dramatically reduced cell death induced by H2O2. These results were consistent with the level of activated caspase 3, a cell apoptotic marker (Figure 5(c,d)).
Figure 5.
H2S attenuates cell apoptosis in HCF-av cells induced by H2O2. Cell death analysis of treated cells was performed by flow cytometry with Annexin V/PI double staining (a,b). Representative images and quantitative analysis were shown in (c) and (d), respectively. Data represent mean ± SEM (n = 3; *P < 0.05 vs. control cells, #P < 0.05 vs. indicated cells).
H2S Ameliorates Lysosomal Activity in HCF-av Cells Induced by H2O2
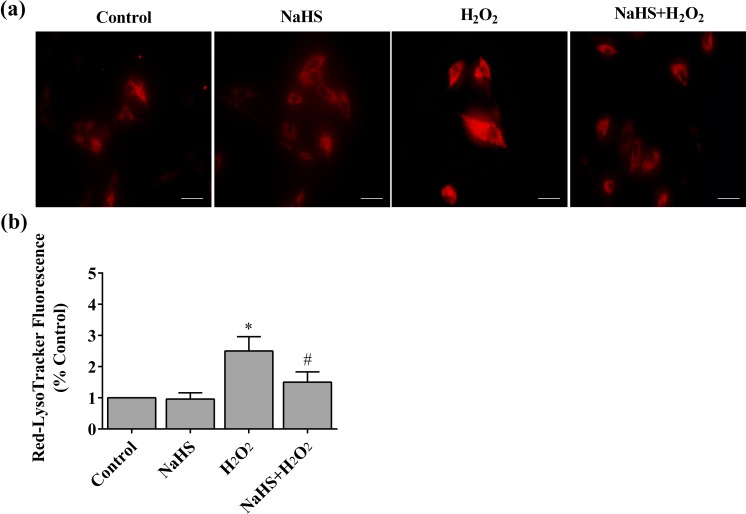
To investigate the role of lysosomal activity on cell apoptosis and damage of ER or mitochondria, HCF-av cells were subjected to different treatments and then incubated with LysoTracker Deep Red, which labeled the highly acidic lysosomal vacuoles and monitored activity of the vacuolar H+-ATPase (v-ATPase). We found that H2O2 exposure markedly increased the LysoTracker red staining. However, this fluorescent signal was significantly decreased by pretreatment of NaHS (Figure 6(a,b)).
Figure 6.
H2S ameliorates lysosomal activity in HCF-av cells induced by H2O2. (a) Cells were subjected to different treatments and then stained with 70 nM LysoTracker® Deep Red (magnification, ×400). (b) The fluorescent signal (red) was measured and quantified (n = 6). *P < 0.01 vs. control; #P < 0.01 vs. NaHS; #P < 0.01 vs. H2O2.
H2S Prevents H2O2-Induced Autophagy
To investigate whether the autophagy was activated in the period following increased lysosomal activity in HCF-av cells undergoing oxidative stress, the expression level of cathepsin B was examined using Magic Red staining. As shown in Figure7(a), H2O2-induced ER stress caused approximately three-fold increase in fluorescence intensity of cathepsin B in HCF-av cells compared to the control cells. Consistent with this finding, cells treated with H2O2 displayed an increased abundance of multilamellar autophagosomes (Figure 7(b)). Strikingly, these phenomena were significantly diminished when the cells were treated with NaHS (Figure 7(c,d)).
Figure 7.
H2S blocks autophagy in HCF-av cells induced by H2O2. (a) Cells were subjected to different treatments and then stained with Magic Red® Cathepsin B Detection Kit (magnification, ×200). (b) Representative TEM micrographs upon H2O2 treatment. (c) The fluorescence (red) intensity of cathepsin B was measured and quantified (n = 6). (d) Quantification of the autophagosome (n = 4). *P < 0.01 vs. control; #P < 0.01 vs. NaHS; #P < 0.05 vs. H2O2.
H2S Regulates the Expression of LC3-II, Beclin1, and P62 During Autophagy
The expression levels of LC3-I/II, Beclin1, and P62 play vital roles for autophagic activity17–19. To further investigate the role of H2S-regulated autophagy induced by H2O2, HCF-av cells were subjected to different treatments and the autophagy-related proteins were detected. As shown in Figure 8(a), the expression levels of Beclin1, LC3-II/LC-I ratio, and P62 were robustly increased when cells were treated with H2O2. Meanwhile, the increases of these key proteins were diminished in cells pretreated with H2S (Figure 8(a–d)). Because p62 plays a key role in both autophagy and the ubiquitin proteasome system, we further investigate the effects of H2S on the ubiquitin proteasome system upon H2O2 treatment in HCF-av cells. As shown in Figure 8(e–g), the proteasome activity and ubiquitin expression did not change when cells were treated with or without H2S plus H2O2. These results suggested that H2S could regulate the autophagic activity but not proteasome activity in HCF-av cells under H2O2 treatment.
Figure 8.
H2S regulates the expression of LC3-II, Beclin1, and P62 during autophagy. (a) Western blot analysis of HCF-av cells upon different treatments was performed to detect LC3I/II, Beclin 1, and P62. (b–d) Quantitative analysis of the changes of LC3I/II, Beclin1, and P62 in treated cells (n = 3). *P < 0.01 vs. control; *P < 0.01 vs. NaHS; #P < 0.05 vs. H2O2. (e) Proteasome activity was measured using AMC-linked substrate peptides under different treatments (n = 3). (f) Representative Western blot analysis for ubiquitin expression. β-actin served as the loading control. (g) Quantitative analysis of the changes of ubiquitin in treated cells (n = 3).
Discussions
Recent studies indicated that H2S was a powerful endogenous second messenger, capable of modulating a variety of physiological or pathophysiological events in mammalian cells and tissues20,21. These results prompted us to investigate the potential role of H2S as a cardioprotective reagent. Previous studies indeed suggest that H2S was a potent cardioprotective signaling molecule reagent for heart disease22,23. Current studies have shown that H2S can regulate the activation of ion channel, and upregulate antioxidant, anti-apoptotic, and anti-inflammatory signaling pathways10,24–26. In the present study, we evaluated effects of NaHS on the in vitro ER stress cell model. H2O2 is one kind of ROS and has been widely used in experiments to mimic the situation of oxidative stress. Different concentrations of H2O2 have been widely used in different cell types, and different cell types have showed different responses to oxidative stress induced by H2O2 27. In the present study, the HCF-av cells were exposed to H2O2 to mimic in vivo ER stress. BiP expression level was robustly increased, which revealed ER stress induced by H2O2 in HCF-av cells (Figure 2). This result is consistent with the accumulation of CHOP (also known as growth-arrest and DNA damage inducible gene 153) in HCF-av cells induced by H2O2, which was a transcription factor and activated at multiple levels during ER stress28–30. Because ER stress is closely related to cell apoptosis, we have found that ER stress significantly elevated the activated caspase 3 level in HCF-av cells induced by H2O2 (Figure 5). Importantly, the activated caspase 3 level is abrogated by H2S treated HCF-av cells induced by H2O2.
Mitochondria played pivotal roles in the two types of cell death: apoptosis and necrosis31. However, autophagy, a cellular stress response, is involved in a variety of diseases and has recently been proposed as a third distinct mode of cell death. Autophagy is a dynamic process involving the rearrangement of subcellular membranes to sequester cytoplasm and organelles which are delivered to the lysosome or vacuole, and then the sequestered cargo is degraded and recycled4. Accumulated evidence indicates that autophagy may constitute an important physiological response to cardiac stresses, ischemia, or pressure overload, which are frequently encountered in patients with coronary artery disease, hypertension, aortic valvular disease, and congestive heart failure. The accumulation of autophagosomes has been noted in cardiac biopsy tissues of patients with these disorders, rodent models of these cardiac diseases, and isolated stressed cardiomyocytes4. Autophagy participated in the constitutive turnover of mitochondria in oxidative tissues, and removal of damaged organelles32,33. One of the conclusions of our study is that the protective mechanism of H2S may be involved in stabilization of the mitochondria in H2O2-induced cell death. Changes of the mitochondrial permeability transition (MPT) and loss of Δψ triggered autophagic scavenging. Our findings suggested that H2O2 exposure reduced JC-1 aggregates in HCF-av cells, indicating mitochondria Δψ was decreased. Conversely, pretreatment of H2S restored the mitochondria Δψ induced by H2O2. Furthermore, autophagic flux was significantly increased following H2O2 exposure, as shown by the multilamellar autophagosomes and the increased LC3-II/LC-I ratio, Beclin1, and p62 protein level. In contrast, exogenous H2S completely abrogates these phenomena.
In eukaryotic cells, the lysosome is a major organelle that contains a lot of enzymes, which can degrade essentially any subcellular component by hydrolases such as proteins, lipids, nucleic acids, and carbohydrates34. Lysosomal enzymes also play a role in the activation of certain types of caspase, which are involved in cell apoptosis. Lysosomes have been referred to as “suicide bags,” as they contribute to autophagic cell death35,36. Moreover, ROS can induce lysosomal permeabilization before mitochondrial dysfunction. Although oxidative stress induces many alterations within the cell, mitochondria may be the first organelle to be demerged by ROS. Lysosomal enzymes have been found to act on mitochondria and promote mitochondrial ROS generation, creating a feedback loop and leading to more lysosomal permeabilization. Our studies determined the effect of H2S on the autophagic activity induced by H2O2. Autophagy was involved in the delivery of autophagosomes and their contents to lysosomes and accomplished the catabolic processes of autophagy. We found that cellular lysosomal activation and the expression level of cathepsin B are both increased in cells with ER stress induced by H2O2, but are diminished by NaHS treatment.
Recent studies have shown that H2S is a strong promoter of angiogenesis37,38 and stimulates cell replication, migration, and tube formation39. Furthermore, H2S also promotes angiogenesis in vivo40 and the proangiogenic effects of H2S on chronic vascular disease have been reported39. However, the protective role of H2S in ER stress and autophagy induced by oxidative stress in the heart is still obscure. A previous study reported that H2S attenuates oxidative stress in the heart through activation of nuclear factor E2-related factor (Nrf2)41, because Nrf2 regulates a large number of gene expressions for enzymes that serve to detoxify pro-oxidative stressors42, such as GPx1 and HO-1, via binding to the antioxidant response element found in the gene’s promoter region40. Maybe that is why H2S can prevent ER stress, autophagy, and heart cell apoptosis induced by H2O2. In the current study we demonstrated that H2S treatment dramatically repressed ER stress marker and autophagy marker expression and cell apoptosis induced by H2O2 using an in vitro model (HCF-av cells). Furthermore, the in vivo model also suggested that ER stress-related markers and heart cell apoptosis were significantly blocked by H2S treatment (Figure 2(d)). Further investigations are needed to determine the precise mechanism by which H2S prevents ER stress, autophagy, and cell apoptosis induced by oxidative stress in the heart.
In summary, H2S pretreatment efficiently protects HCF-av cells from H2O2-induced ER stress, apoptosis, and autophagy, which maintains mitochondria membrane integrity and prevents the activation of caspase 3. Our study suggests that H2S could potentially be a therapeutic reagent for suppressing ER stress in the heart.
Footnotes
Author Contributions: Ao Feng and Chen Ling contributed equally to this work.
Ethical Approval: Animal care and experimental procedures were approved by the Ethics Committee on Animal Research of Hubei University of Medicine and the Institutional Animal Care and Use Committee of Cleveland Clinic.
Statement of Human and Animal Rights: Male C57BL/6 J mice (10 weeks old) were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Science (SLAC, Shanghai, China).
Statement of Informed Consent: Statement of Informed Consent is not applicable for this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Nature Science Foundation of China (No. 81500237) and Special Foundation for Knowledge Innovation of Hubei Province (Nature Science Foundation) (No. 2017CFB563).
References
- 1. Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cunnington RH, Wang B, Ghavami S, Bathe KL, Rattan SG, Dixon IM. Antifibrotic properties of c-Ski and its regulation of cardiac myofibroblast phenotype and contractility. Am J Physiol Cell Physiol. 2011;300(1):C176–C186. [DOI] [PubMed] [Google Scholar]
- 3. Becher PM, Gotzhein F, Klingel K, Escher F, Blankenberg S, Westermann D, Lindner D. Cardiac function remains impaired despite reversible cardiac remodeling after acute experimental viral myocarditis. J Immunol Res. 2017;6590609 doi: 10.1155/2017/6590609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci USA. 2011;108(10):4123–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 2012;125(25):3170–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pistoia V, Pezzolo A. Involvement of HMGB1 in resistance to tumor vessel-targeted, monoclonal antibody-based immunotherapy. J Immunol Res. 2016; 3142365 doi: 10.1155/2016/3142365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8(11):931–937. [DOI] [PubMed] [Google Scholar]
- 9. Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741–752. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Li H, Zhao G, Sun A, Zong NC, Li Z, Zhu H, Zou Y, Yang X, Ge J. Hydrogen sulfide attenuates the recruitment of cd11b(+)gr-1(+) myeloid cells and regulates bax/bcl-2 signaling in myocardial ischemia injury. Sci Rep. 2014;4:4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu T, Li H, Wu B, Zhang L, Wu SW, Wang JN, Zhang YE. Hydrogen sulfide reduces recruitment of cd11b+gr-1+ cells in mice with myocardial infarction. Cell Transplant. 2017;26(5):753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Wang J, Li H, Yuan L, Wang L, Wu B, Ge J. Hydrogen sulfide suppresses transforming growth factor-beta1-induced differentiation of human cardiac fibroblasts into myofibroblasts. Sci China Life Sci. 2015;58(11):1126–1134. [DOI] [PubMed] [Google Scholar]
- 13. Polhemus D, Kondo K, Bhushan S, Bir SC, Kevil CG, Murohara T, Lefer DJ, Calvert JW. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail. 2013;6(5):1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson KJ, Russell AP, Foletta VC. Ndrg2 promotes myoblast proliferation and caspase 3/7 activities during differentiation, and attenuates hydrogen peroxide – but not palmitate-induced toxicity. FEBS Open Bio. 2015;7(5):668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu Y, Zhao KK, Tong Y, Zhou YL, Wang YX, Zhao PQ, Wang ZY. Exogenous NAD(+) decreases oxidative stress and protects H2O2-treated RPE cells against necrotic death through the up-regulation of autophagy. Sci Rep. 2016;5(6):26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Philip L, Shivakumar K. cIAP-2 protects cardiac fibroblasts from oxidative damage: an obligate regulatory role for ERK1/2 MAPK and NK-kappaB. J Mol Cell Cardiol. 2013;9(62):217–226. [DOI] [PubMed] [Google Scholar]
- 17. Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. [DOI] [PubMed] [Google Scholar]
- 18. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanamori H, Takemura G, Goto K, Maruyama R, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S. The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovasc Res. 2011;91(2):330–339. [DOI] [PubMed] [Google Scholar]
- 20. Greabu M, Totan A, Miricescu D, Radulescu R, Virlan J, Calenic B. Hydrogen sulfide, oxidative stress and periodontal diseases: a concise review. Antioxidants. 2016;5(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun J, Aponte AM, Menazza S, Gucek M, Steenbergen C, Murphy E. Additive cardioprotection by pharmacological postconditioning with hydrogen sulfide and nitric oxide donors in mouse heart: S-sulfhydration vs. S-nitrosylation. Cardiovasc Res. 2016;110(1):96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Predmore BL, Lefer DJ. Hydrogen sulfide-mediated myocardial pre- and post-conditioning. Expert Rev Clin Pharmacol. 2011;4(1):83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma SF, Luo Y, Ding YJ, Chen Y, Pu SX, Wu HJ, Wang ZF, Tao BB, Wang WW, Zhu YC. Hydrogen sulfide targets the cys320/cys529 motif in kv4.2 to inhibit the Ito potassium channels in cardiomyocytes and regularizes fatal arrhythmia in myocardial infarction. Antioxid Redox Signal. 2015;23(2):129–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuates ischemia-induced heart failure in mice. Circulation. 2010;122(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105(4):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20(12):2118–2120. [DOI] [PubMed] [Google Scholar]
- 27. Djordjevic VB, Zvezdanovic L, Cosic V. [Oxidative stress in human diseases]. Srp Arh Celok Lek. 2008;136(Suppl 2):158–165. [DOI] [PubMed] [Google Scholar]
- 28. Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu T, Zhou Y, Liu YC, Wang JY, Su Q, Tang ZL, Li L. Coronary microembolization induces cardiomyocyte apoptosis through the LOX-1-dependent endoplasmic reticulum stress pathway involving JNK/P38 MAPK. Can J Cardiol. 2015;31(10):1272–1281. [DOI] [PubMed] [Google Scholar]
- 30. Ryoo HD. Long and short (timeframe) of endoplasmic reticulum stress-induced cell death. FEBS J. 2016;283(20):3718–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361(16):1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu Y, Sun G, Luo Y, Wang M, Chen R, Zhang J, Ai Q, Xing N, Sun X. Cardioprotective effects of notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci Rep. 2016;2(6):21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu X, Pang J, Chen Y, Bucala R, Zhang Y, Ren J. Macrophage migration inhibitory factor (MIF) deficiency exacerbates aging-induced cardiac remodeling and dysfunction despite improved inflammation: role of autophagy regulation. Sci Rep. 2016;3(6):22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klionsky DJAK, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lockshin RA, Zakeri Z. Programmed cell death and apoptosis: origins of the theory. Nat Rev Mol Cell Biol. 2001;2(7):545–550. [DOI] [PubMed] [Google Scholar]
- 36. Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2(8):589–598. [DOI] [PubMed] [Google Scholar]
- 37. Szabo C, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol. 2011;164(3):853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6(11):917–935. [DOI] [PubMed] [Google Scholar]
- 39. Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN, Wang R, Szabo C. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106(51):21972–21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76(1):29–40. [DOI] [PubMed] [Google Scholar]
- 41. Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105(4):365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor e2-related factor 2. Drug Metab Dispos. 2007;35(6):995–1000. [DOI] [PubMed] [Google Scholar]