Abstract
Osteopontin (OPN), expressed by various immune cells, plays a critical role in leukocyte migration. Although OPN was found to selectively induce the expression of proinflammatory chemokines, the molecular mechanisms that control OPN gene expression and its underlying mechanism for migration and recruitment of inflammatory cells remain largely unknown. In this study, real-time polymerase chain reaction and enzyme-linked immunosorbent assay were used to determine OPN and monocyte chemoattractant protein 1 (MCP-1) expression. Signaling and molecular events between OPN and MCP-1 were analyzed by Western blot. Leukocyte migration in the presence of OPN was measured by chemotaxis assay. Our data indicated that phosphoinositide 3-kinase (PI3K), c-Jun NH2-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK) that are activated upon stimulation with lipopolysaccharide were shown to upregulate OPN expression. Endogenous production of OPN was attributable to increased production of MCP-1, and this effect could be blocked by an anti-β1 integrin antibody and JNK and p38 kinase inhibitors. Furthermore, we found that the effect of OPN on inflammatory cell migration was mediated through inducing the expression of MCP-1 in monocytes. These results support a role of OPN in monocyte migration via MCP-1, which may represent an additional mechanism for innate and adaptive immune responses.
Keywords: osteopontin, MCP-1, PI3K, migration, monocyte
Introduction
Osteopontin (OPN), a multifunctional phosphorylated glycoprotein, is involved in many physiological and pathological processes including tumor growth and metastasis, bone remodeling, immune responses, and inflammation1–3. Various types of cancers express high levels of OPN4. For example, OPN can be an important player in the occurrence, reoccurrence, and metastasis of lung cancer5 while downregulated to reduce the malignant potential6. In the immune system, OPN is secreted by monocytes, macrophages, dendritic cells, and activated T lymphocytes and is present in the extracellular fluid and at sites of inflammation as well as in the extracellular matrix of mineralized tissue1,7 with a critical role in many inflammatory autoimmune diseases2,8. It is widely accepted that OPN acts as an important proinflammatory cytokine with pleiotropic functions including activities related to Type 1 T helper properties, adhesion, and migration9,10.
Our previous study on disease-specific biomarkers of acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients by integrating clinical informatics with inflammatory mediators indicated that OPN played an important role in the occurrence of AECOPD and was associated with the disease severities11. OPN has several functional domains such as the thrombin cleavage site and the Gly-Arg-Gly-Asp-Ser sequence, through which it interacts with receptors, integrins, and CD44 variants, to activate phosphoinositide 3-kinase (PI3K)/protein kinase B and nuclear factor kappa-light-chain-enhancer of activated B cells signal pathways that induce distinct patterns of cytokine/chemokine expression and specific immune responses12–14. Monocyte chemoattractant protein 1 (MCP-1), a ligand of C-C motif chemokine receptor 2, is a key chemokine involved in adhesion and migration of monocyte/macrophages and activated T cells15,16. The concentration of OPN was significantly elevated and appeared to correlate with the serum levels of inflammation markers and increased expression of MCP-117,18. Additionally, OPN could regulate the alternative activation of monocytes via MCP-1 production19.
In this study, we hypothesized that the role of OPN in inflammatory microenvironment could potentially be linked to its involvement in the induction of MCP-1. In particular, we addressed the molecular mechanisms that control OPN gene expression and the potential association and interaction mechanisms between OPN and MCP-1 in monocytes using various experimental systems. The present study furthermore investigated the role of OPN in the regulation of monocyte migration. Our results have important implications in the understanding of the role of OPN in the inflammatory microenvironment, perhaps, in other autoimmune conditions.
Materials and Methods
Cell lines and reagents
Human monocyte U937 cells were obtained from Shanghai Institute for Biological Science. Cells were cultured in RPMI 1640 (Hyclone, Logan, UT, USA), supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (FBS; Bio International, Auckland, New Zealand). All cells were maintained at 37 °C in a humidified incubator with 5% carbon dioxide. Human recombinant OPN; ELISA kits for MCP-1; and antihuman MCP-1; and β1 and β3 integrin neutralizing antibody were purchased from R&D Systems China Co. Ltd (Shanghai, China). PI3K-specific inhibitor LY294002, extracellular signal-regulated kinase (ERK)-specific inhibitor PD98059, c-Jun NH2-terminal kinase (JNK)-specific inhibitor SP600125, and p38 kinase–specific inhibitor SB203580 were purchased from BioVision Inc. (Milpitas, CA, USA). Antibodies specific to OPN and horseradish peroxidase–coupled secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Measurement of gene expression
Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out using RT-PCR with SYBRGreen. Cell cultures were washed in phosphate-buffered saline and total RNA was isolated using a guanidinium isothiocyanate–/chloroform-based technique (Trizol, Invitrogen, Carlsbad, CA, USA). RNA was subsequently reverse transcribed to cDNA with the SuperScript First-Strand Synthesis System (Invitrogen, USA). Primer (Invitrogen) concentrations (10 nM) were optimized before use. SYBRGreen PCR master kit was used with the appropriate concentrations (10 nM) of forward and reverse primers in a total volume of 20 µL. Optimization was carried out for each gene-specific primer prior to the experiment to confirm that 10 nmol/L primer concentrations did not produce nonspecific primer-dimer amplification signals in no-template control wells. qRT--PCR was carried out using an ABI 7000 PCR instrument (Eppendorf, Hamburg, Germany) with the 2-stage program parameters provided by the manufacturer as follows: 1 min at 95 °C, and then 40 cycles of 5 s at 95 °C and 30 s at 60 °C. The sequences of the primer sets used for this analysis are listed in Supplemental Table 1. Specificity of the produced amplification product was confirmed by the examination of dissociation reaction plots. A distinct single peak indicated that a single DNA sequence was amplified during RT-PCR. Each sample was tested in triplicate with qRT-PCR, and the samples obtained from 3 independent experiments were used for analysis of relative gene expression. Data were normalized to housekeeping gene in each sample.
Measurement of protein expression
To measure the expression of OPN and the signal pathway induced by lipopolysaccharide (LPS), U937 cells were cultured in 6-well plate (1 × 105 cells/well) for 24 h and treated with LY294002, SP600125, SB203580, and PD98059 at 10, 20, 30 μM for another 2 h. Then, the cells were stimulated with or without LPS (Escherichia coli, 055: B5, Sigma-Aldrich, St. Louis, MO, USA) at 1 µg/mL for 24 h. Intracellular protein was extracted by radioimmunoprecipitation assay lysis immediately. Protein samples (40 μg) were mixed with an equal volume of 5 × sodium dodecyl sulfate (SDS) sample buffer, boiled for 5 min, and then separated through 10% SDS-polyacrylamide gel electrophoresis gels. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes by electrophoretic transfer. Membranes were blocked in 5% dry milk (2 h), rinsed, and incubated with primary antibodies (diluted at 1:500) in tris-buffered saline tween-20 (TBST) at 4 °C overnight. Primary antibody was then removed by washing in TBST and labeled by incubating with 0.1 mg/mL peroxidase-labeled secondary antibodies (against mouse) for 2 h. Following 3 washes in TBST, bands were visualized by enhanced chemiluminescence and exposed to X-ray film. All results were calculated by Phoretix 1D pro software.
Production of MCP-1
The U937 cells were cultured in 24-well cell culture microplates at 1×105 cells/well for 24 h and then treated with LPS at concentrations of 0.01, 0.1, and 1 µg/mL for an additional 24 h, respectively, to study LPS-induced MCP-1 production. Cells were preincubated with OPN small interfering RNA (siRNA) at concentrations of 10, 20, and 40 pmol before LPS stimulation to explore the role of OPN in LPS-induced MCP-1 production. Cells were treated with OPN at 500 ng/mL or vehicle and pretreated with antihuman β1 and β3 integrin antibody at concentrations of 1, 10, and 20 μg/mL or LY294002, SP600125, SB203580, and PD98059 inhibitors at 10, 20, 30 uM, respectively, for 24 h to investigate the involvement of various signal pathways. Each experiment was done in 6 replicate wells for each drug concentration and each time point. Levels of MCP-1 in supernatant were measured by ELISA at the absorbance of 450 nm.
Cell migration assay
Cell migration assays were performed by using transwell chambers (5 μm, 24-well insert; Corning, Lowell, MA, USA). Cell were resuspended in serum-free medium with 0.1% bovine serum albumin. A total of 5 × 104 cells in 200 μl media were plated in the upper chambers. Medium containing 10% FBS and OPN or antihuman MCP-1 neutralizing antibody were added to the lower chamber, the IgG as nonspecific control. After incubation of 24 h, cells migrated from the upper chamber to the lower one. We then counted the cells under inverted microscope and photographed them with 100× magnification. Parallel experiments were performed in triplicate for each group.
RNA interference (siRNA) and transfection
OPN siRNA and nonspecific control siRNA were purchased from Shanghai GenePharma Co. Ltd. (Shanghai, China). Transfection of siRNA was performed per commercial protocol coming along with each product. Briefly, for the transient transfection of siRNA, U937 cells were placed in 6- or 12-well plates, optimal confluent condition for transfection was determined, and 10- to 40-pmol siRNA was used for each transfection. And each experiment included controls containing the transfection reagent with control siRNA. After 24 h, OPN were detected by RT-PCR and Western blot analysis. In addition, we selected the sequence with successful transfection for further study.
Statistical analysis
Data were represented as mean ± standard error of the mean of more than 3 separate experiments performed in triplicate. After the analyses of variance, statistical significance was compared between groups by the Student t test. P values less than 0.05 were considered to be significant.
Results
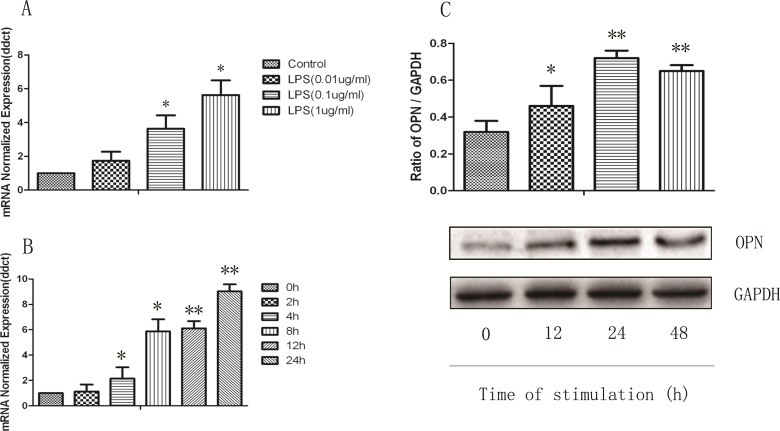
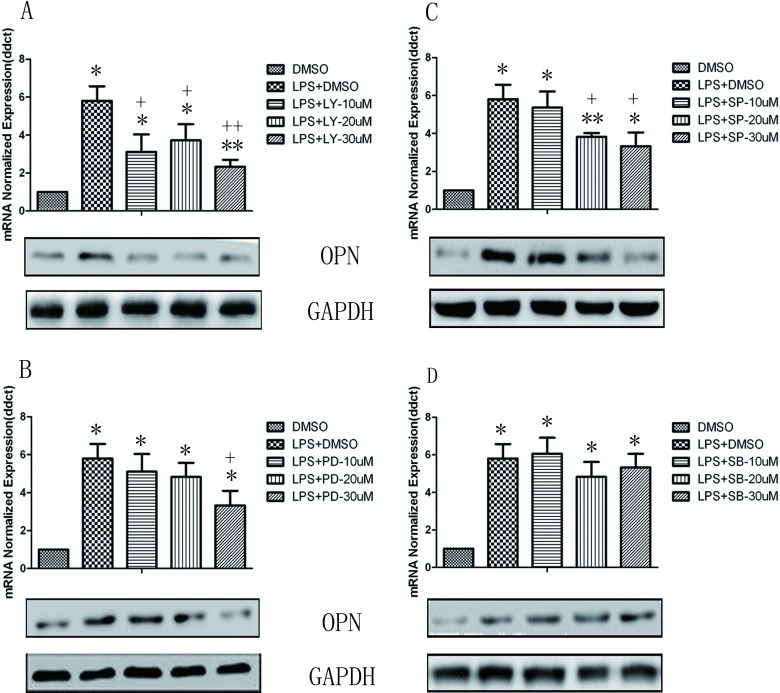
We first examined OPN gene expression in U937 monocytes stimulated with LPS for various concentrations and times by RT-PCR. As shown in Fig. 1, the messenger RNA (mRNA) expression of OPN in monocytes significantly increased at the stimulation of LPS at 1 μg/mL from 4 h and on, which is maintained till 24 h (P < 0.05 or 0.01, respectively). In a similar fashion, constitutive expression of OPN protein was found in unstimulated control U937 monocytes, and the OPN protein level was greatly increased and reached its peak after being stimulated with LPS for 24 h (P < 0.05 or 0.01, respectively). The stimulation of toll-like receptor 4 (TLR4) by LPS induces the activation of PI3K and mitogen-activated protein kinases (MAPK) including JNK, ERK, and p38 kinase. To investigate the role of these signal pathways in LPS-induced OPN expression, the PI3K-specific inhibitor LY294002, JNK-specific inhibitor SP60012, ERK-specific inhibitor PD98089, and p38 inhibitor SB203580 were used in the setting of LPS-induced OPN expression. As illustrated in Fig. 2, the gene and protein expression of OPN after LPS stimulation was significantly decreased by LY294002 (Fig. 2A), PD98089 (Fig. 2B), and SP60012 (Fig. 2C; P < 0.05 or 0.01, respectively), but not by SB203580 (Fig. 2D) treatment. These results demonstrate that LPS-induced PI3K, ERK, and JNK activation is associated with significantly increased OPN expression in monocytes.
Fig. 1.
Lipopolysaccharide (LPS) induces increased production of osteopontin (OPN) in monocytes U937. (A) Messenger RNA expression of OPN from U937 cells in response to LPS (0.01, 0.1, and 1 µg/mL) at 8 h. (B) U937 cells were stimulated with LPS (1 μg/mL) for indicated times. (C) LPS induced OPN protein expression for indicated times. Intracellular protein was extracted by radioimmunoprecipitation assay lysis and detected by Western blot as described in Material and Methods section. Each data point represents mean ± standard error of the mean of 3 experiments. * and ** stand for P values less than 0.05 and 0.01, as compared to control.
Fig. 2.
Involvement of phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase pathways in the regulation of osteopontin (OPN) expression. The U937 cells were pretreated with dimethyl sulfoxide (DMSO), PI3K-specific inhibitor LY294002 (LY, A), extracellular signal-regulated kinase–specific inhibitor PD98089 (PD, B), c-Jun NH2-terminal kinase-specific inhibitor SP60012 (SP, C), and p38 inhibitor SB203580 (SB, D) at various concentrations for 2 h and then stimulated with 1 μg/mL lipopolysaccharide (LPS). Expression level of OPN was examined by real-time polymerase chain reaction at 8 h and Western blot at 24 h. Each data point represents mean ± standard error of the mean of 3 experiments. * and ** stand for P values less than 0.05 and 0.01, as compared with DMSO alone; + and ++ stand for P values less than 0.05 and 0.01, as compared to LPS (1 µg/mL) and DMSO, respectively.
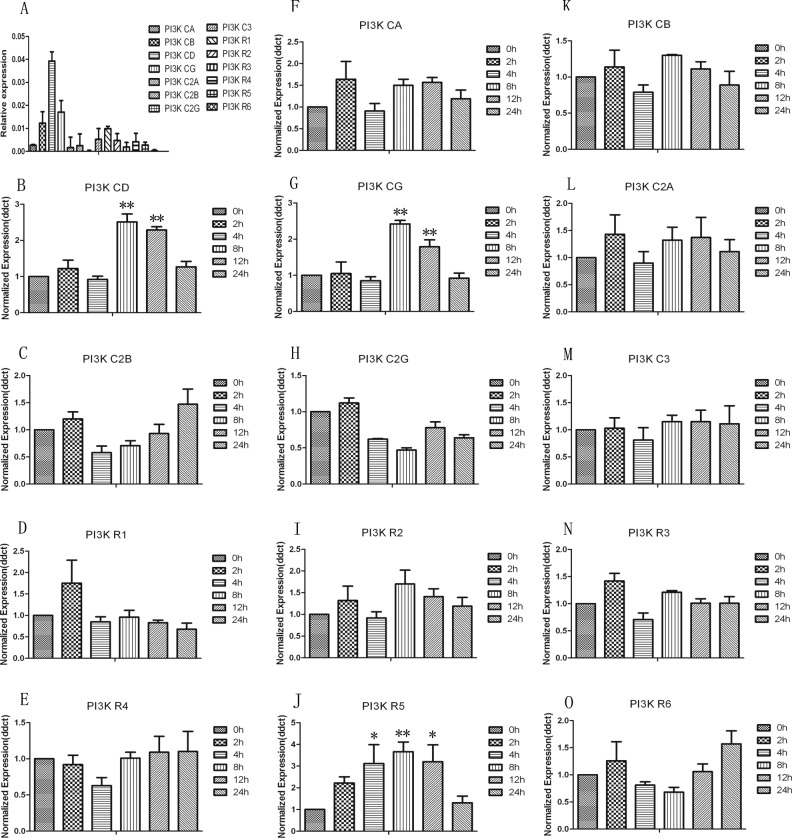
Of those signal pathways, PI3K plays a critical role in LPS-induced OPN production, as indicated by the most significant inhibition in Fig. 2A. To further explore the isoforms of PI3K involved in this process, 14 PI3K isoforms were detected by RT-PCR after LPS stimulation for an indicated time. Only the expression of PI3KCA, PI3KCG, and PI3KR5 was significantly increased at various times (P < 0.05 or 0.01, respectively), Fig. 3, and the others remained unchanged and showed the same expression as that of unstimulated monocytes during an 8-h period of LPS stimulation.
Fig. 3.
Involvement of phosphoinositide 3-kinase (PI3K) isoforms in response to lipopolysaccharide (LPS). (A) Messenger RNA expression of 14 PI3K isoforms of U937 was examined by real-time polymerase chain reaction (RT-PCR). (B–O) To further explore the isoforms of PI3K involved in this process, 14 PI3K isoforms were detected by RT-PCR after LPS stimulation for an indicated time. * and ** stand for –P values less than 0.05 and 0.01, as compared to 0 h.
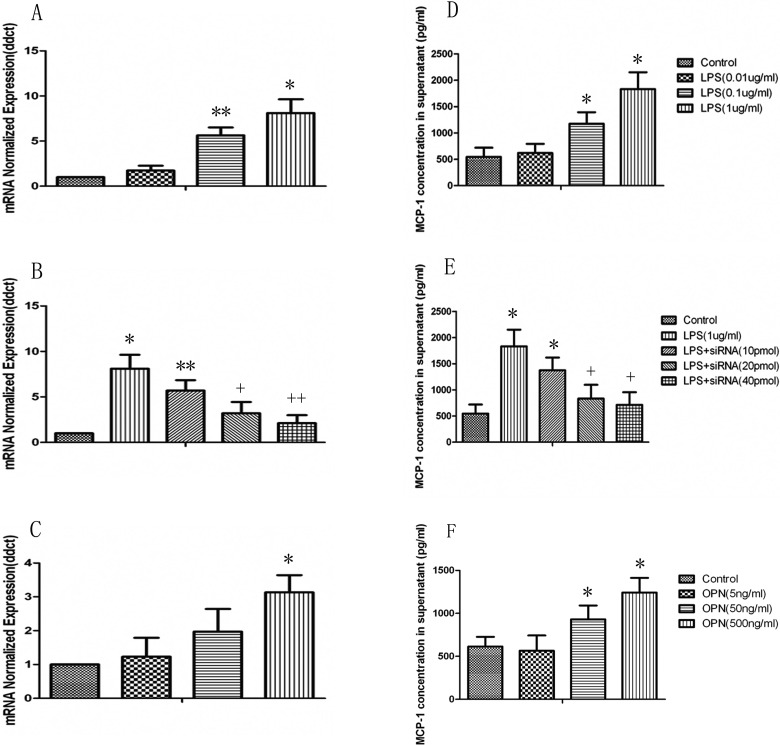
Consistent with the LPS-induced expression of OPN, the mRNA expression and protein production of MCP-1 in U937 monocytes significantly increased at the stimulation of LPS at both 0.1 and 1 μg/mL with a dose-dependent pattern as shown in Fig. 4A and D (P < 0.05 or 0.01, respectively). A positive correlation of OPN and MCP-1 expression in monocyte was observed. Cells were pretreated with OPN siRNA to investigate the potential role of endogenous OPN in LPS-induced overexpression and overproduction of MCP-1 mRNA and proteins. Pretreatment with OPN siRNA at concentrations of 20 and 40 pmol could significantly prevent from LPS-induced overexpression of MCP-1 mRNA and overproduction of MCP-1 proteins, as compared with those pretreated with vehicle and challenged with LPS (P < 0.05 or 0.01 in Fig. 4B and E, respectively). Furthermore, recombinant human OPN from the dose of 50 ng/mL and on significantly increased the expression and production of MCP-1 mRNA and proteins (P < 0.05 in Fig. 4C and F), as compared with those stimulated with vehicle.
Fig. 4.
Lipopolysaccharide (LPS)-induced monocyte chemoattractant protein 1 (MCP-1) production is osteopontin (OPN)-dependent in U937. (A) Messenger RNA (mRNA) and protein (D) expression of MCP-1 from U937 cells in response to LPS (0.01, 0.1, and 1 µg/mL) were detected by real-time polymerase chain reaction (RT-PCR) at 8 h and ELISA at 24 h. The U937 cells were stimulated with LPS (1 µg/mL) after pretreatment with OPN siRNA. Total RNA was extracted and subjected to reverse transcription followed by RT-PCR to detect MCP-1 mRNA at 8 h (B). MCP-1 in cell-free supernatants after 24 h stimulated by LPS was assayed using ELISA (E). The U937 cells were stimulated with OPN (5, 50, and 500 ng/mL) for 8 h to detect MCP-1 mRNA (C) or 24 h to detect its secretion of MCP-1 in supernatants (F). Each data point represents mean ± standard error of the mean of 3 experiments. * and ** stand for P values less than 0.05 and 0.01, as compared to the control; + and ++ stand for P values less than 0.05 and 0.01, as compared to LPS (1 µg/mL), respectively.
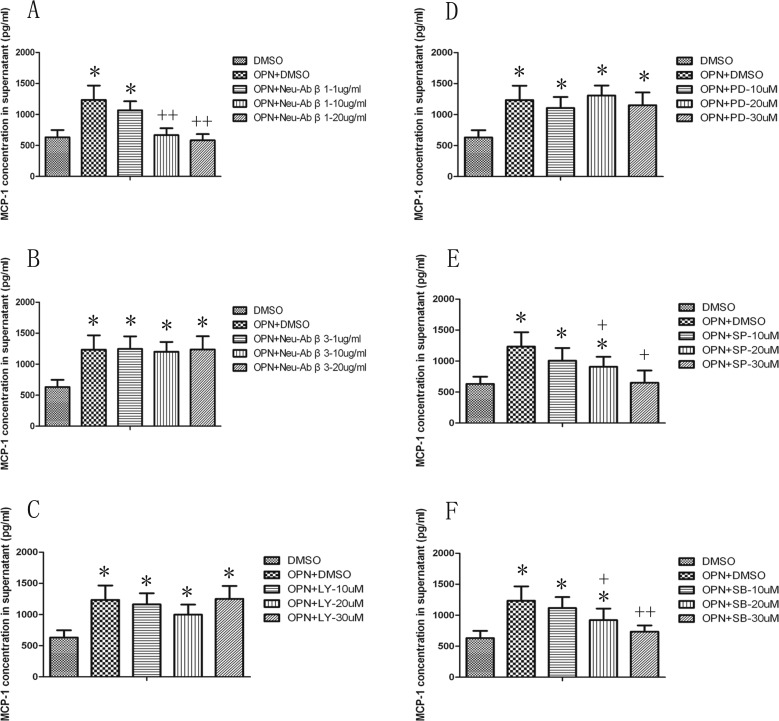
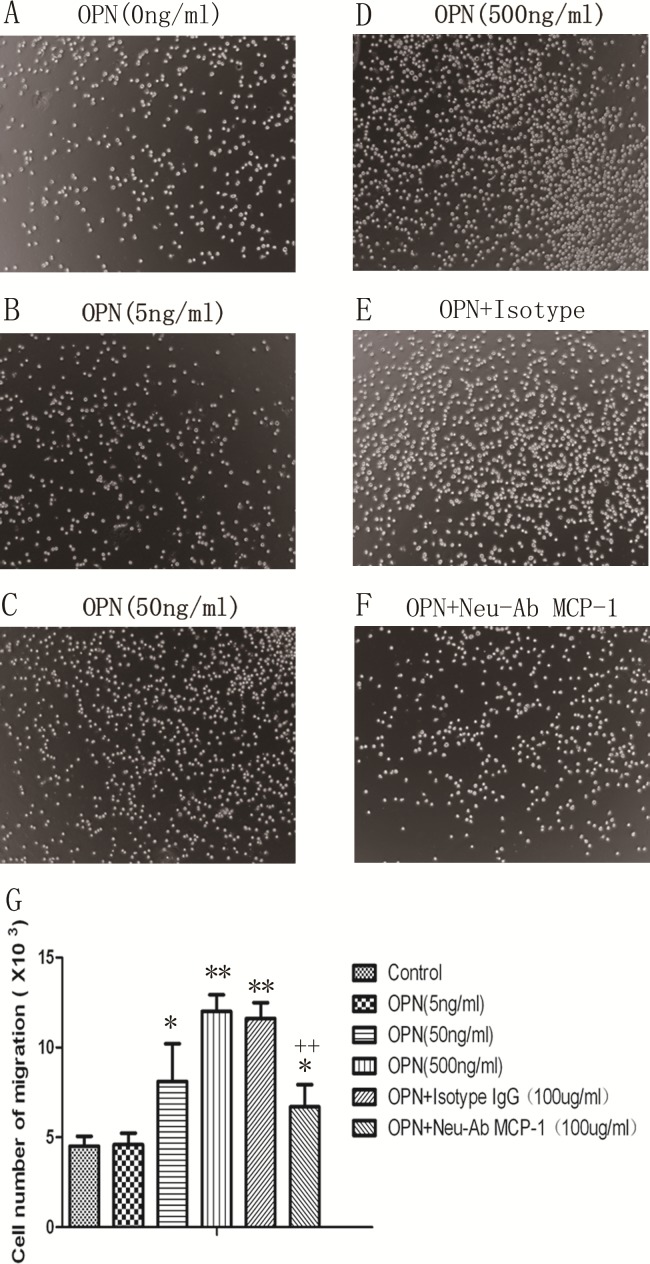
To address which receptors and signal pathways were responsible for the observed effect of OPN in the induction MCP-1. Figure 5 demonstrated that the integrin β1, not integrin β3 neutralizing antibodies, at the concentration of 10 μg/mL or 20 μg/mL could block LPS-induced MCP-1 production (P < 0.01), as compared with those pretreated with vehicle and challenged with OPN. The activity of OPN in the induction of MCP-1 was selectively inhibited by antagonists specific for JNK (SP600125) and p38 kinase (SB203580; P < 0.05 or 0.01, respectively), but not those specific for PI3K (LY294002) or ERK (PD98059), supporting the role of both the JNK and p38 kinase pathways in this process. We hypothesized that OPN might play an active role in promoting the migration of proinflammatory cells into inflamed lung through induction of MCP-1. As illustrated in Fig. 6, OPN promoted monocytes migration in a dose-dependent manner, and this observed effect was significantly inhibited when MCP-1 was blocked by its neutralizing antibody, confirming a partial role of MCP-1 in OPN-induced monocyte migration.
Fig. 5.
Effects of integrin β1 and β3 neutralizing antibodies, phosphoinositide 3-kinase (PI3K), extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and P38 inhibitors on osteopontin (OPN)-induced monocyte chemoattractant protein 1 (MCP-1) production. MCP-1 production from cells was measured 24 h after the culture with dimethyl sulfoxide (DMSO) alone, OPN at 500 ng/mL plus DMSO, integrin β1 neutralizing antibody (Neu-Ab β1; A), and integrin β3 neutralizing antibody (Neu-Ab β3; B) at doses of 1, 10, or 20 µg/mL, or PI3K-specific inhibitor LY294002 (LY, C), ERK-specific inhibitor PD98089 (PD, D), JNK-specific inhibitor SP60012 (SP, E), and p38 inhibitor SB203580 (SB, F) at doses of 10, 20, or 30 µM. * and ** stand for –P values less than 0.05 and 0.01, as compared to cells only with DMSO; + and ++ stand for –P values less than 0.05 and 0.01, as compared to OPN and DMSO, respectively. Data are presented as mean ± standard error of the mean, and each group has 3 measurements.
Fig. 6.
Role of monocyte chemoattractant protein 1 (MCP-1) on osteopontin (OPN)-induced migration of U937 cells. U937 cell migration significantly increased after stimulation with OPN, as compared to control (A–D), while cells treated with MCP-1 isotype (E) or neutralizing antibody (F) at doses of 100 μg/mL. Data were presented as mean ± standard error of the mean, and each group has 3 measurements (G). * and ** stand for –P values less than 0.05 and 0.01, as compared to control; ++ stand for P values less than 0.01, as compared to OPN (500 ng/mL) and isotype, respectively.
Discussion
OPN expressed by various immune cells, induces the expression of many proinflammatory chemokines, further to modulate both innate and adaptive immune responses. Its expression is tissue-specific and subject to regulation by various transcription factors20. The evidence from previous studies indicated that OPN played a critical role in tumor metastasis and adhesion, potentially through the regulation of different receptors, such as integrins21, epidermal growth factor receptor22, and hepatocyte growth factor receptor23. There is evidence suggesting that OPN is associated with some inflammatory diseases9. However, its mechanism of action is unknown. Our data indicated that monocytes per se may act as a primary receptor to be stimulated by a stimuli like LPS to promote OPN expression and as the secondary reactor to induce the MCP-1 production to promote the inflammation and immune responses. These findings and our observations from the previous study24 prompted us to hypothesize that OPN is critically involved in monocyte migration and further to accelerate the development of the local inflammatory microenvironment.
TLR agonists potentially through direct binding to TLR and inducing various signaling pathways, including PI3K and MAPK, resulting in the production of various proinflammatory mediators and type I interferon25. There is evidence suggesting that the mechanism of control OPN transcription in LPS-stimulated macrophages is a nitric oxide (NO)-dependent pattern26. While pretreatment with L-NAME to inhibit NO synthesis can’t ablate LPS-induced OPN expression, which demonstrated that an NO-independent mechanism may be present to regulate LPS-induced OPN expression in macrophages. The present study evidenced that the potential mechanism by which monocytes are regulated to produce OPN could be that OPN overproduced directly by monocytes per se in the inflammatory condition and/or stimuli like LPS through PI3K, ERK, and JNK signaling pathways. This notion was supported by detection of that treatment with the PI3K-specific inhibitor LY294002, ERK-specific inhibitor PD98089, and JNK-specific inhibitor SP60012 significantly decreased OPN expression to a level equivalent to that of unstimulated controls. The PI3K activation has been recently found to play an important role in the development of acute and chronic lung inflammation and injury27. Additionally, PI3K involvement in OPN expression has been shown in some tumor cells28,29. Consistently, PI3K also plays a crucial role in LPS-induced OPN production in monocytes, as indicated by the most significant inhibition in our study. Another novel aspect of the study is that the PI3KCA, PI3KCG, and PI3KR5 were significantly increased after LPS stimulation. These results shed new light on the understanding of the role of PI3K isoforms in OPN production in monocytes. Thus, selective blocking of these proinflammatory functions of OPN through specific PI3K isoforms inhibitors is likely to be therapeutically advantageous.
Many regulatory factors may contribute to the molecular mechanism by which LPS can stimulate monocytes to produce MCP30. It was reported that the level of MCP-1 was markedly decreased in the arthritic lesions of treated mice, which were treated with anti-OPN antibody through promotion of apoptosis of both murine and human-activated T cells31. Similar efficacy of OPN antibody treatment was demonstrated in a study of rheumatoid arthritis that correlated with reduced levels of MCP-1 and infiltration of inflammatory cells18. It is conceivable that OPN has a broad spectrum of proinflammatory properties that are all related to the key processes of the inflammatory cascade. Consistent with this results, the present study further demonstrates that both exogenous and endogenous OPN could induce the overproduction of MCP-1 in monocytes. Apart from OPN, the levels of MCP-1 may be partially influenced by other factors, as was evidenced by the incomplete inhibition effect when an OPN siRNA was used and challenged with LPS, as compared with those without LPS. OPN has been shown to interact with integrins and CD44 to regulate cell migration32 and proinflammatory cytokine production10,33. In this study, we found that the signaling pathway of OPN–integrin β1–JNK/p38 axis may play a critical and dependent role in the mechanism of MCP-1 production of monocytes, evidenced by the finding that the overproduction of MCP-1 by OPN was prevented by integrin β1 neutralizing antibody and JNK and p38 kinase inhibitors. It implies that the OPN–integrin β1–JNK/p38 chain can be the potential new anti-inflammatory therapeutic target in acute or chronic lung inflammatory diseases.
Inflammatory cells’ migration to the site of inflammation plays an important role in the initiation and perpetuation of organ-specific inflammation and immune responses. A number of transcription factors have been found to regulate these cells’ migration. There are some proinflammatory molecules, such as matrix metalloproteinase and OPN, which could act in synergy with chemokines to promote leukocyte migration24,34. However, the potential mechanism of interaction of these molecules in an inflammatory milieu, which helps perpetuate local inflammation, is not understood. The present study suggested that monocyte migration was correlated with the levels of OPN, and this observed effect was significantly inhibited by neutralizing antibody to MCP-1, confirming a partial role of MCP-1 in OPN-induced monocyte migration. Injection of MCP-1 into rabbit joints was found to induce marked macrophage infiltration in the affected joint35. While inhibition of MCP-1 with its antagonist resulted in decreasing macrophage infiltration to ameliorate disease severity in adjuvant-induced arthritis36. The present study delineated a novel mechanism, whereby OPN aberrantly produced by monocytes promotes migration of inflammatory cells through the induction of MCP-1, this feedback regulation may be very important for further inflammation exacerbation.
Conclusions
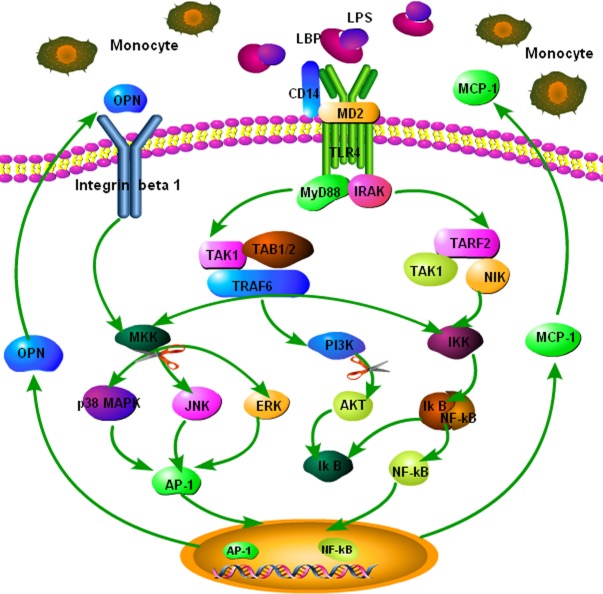
In summary, the present study demonstrated that LPS increased the overproduction of OPN and MCP-1 from monocytes, which could be blocked by anti-OPN neutralizing antibody. The signaling pathways of PI3K, ERK, and JNK are involved in LPS-induced OPN expression. Additionally, our results provide new evidence that both endogenous and exogenous OPN could increase the overproduction MCP-1 through OPN–integrin β1–JNK/p38 pathway activation. As summarized in Fig. 7, these new findings related to the signaling mechanism of OPN provide critical clues in our attempt to design an antibody or small-molecule therapeutic intervention for the treatment of inflammation diseases, such as acute lung injury or AECOPD, using OPN as the target.
Fig. 7.
Proposed mechanism of osteopontin (OPN) stimulated monocyte chemoattractant protein 1 (MCP-1) production and migration of monocytes. OPN could directly stimulate MCP-1 production and migration of monocytes through the activation of integrin β1-c-Jun NH2-terminal kinase/p38 pathway, then lead to the recruitment of inflammatory cells and the formation of the inflammatory microenvironment. This has been highlighted as an important factor responsible for the sensitivity of inflammatory diseases to therapies and prognosis of patients.
Supplemental Material
Supplemental Material, Supplement_Table_1-170808 for Regulatory Roles of Osteopontin in Production of Monocyte-Origin MCP-1 by Liying Shi, Lin Shi, Xiangdong Wang, and Jiantai He in Cell Transplantation
Footnotes
Author Contributions: Liying Shi and Lin Shi contributed equally to perform the biological experiments, carry out the statistical analysis, and write the article. Jiantai He and Xiangdong Wang conceived and designed the study. All authors read and proofed the final manuscript.
Ethical Approval: Ethical Approval is not applicable.
Statement of Human and Animal Rights: Statement of Human and Animal Rights is not applicable.
Statement of Informed Consent: Statement of Informed Consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by The National Nature Science Foundation of China (91230204, 81270099, 81320108001, and 81270131) and The Shanghai Committee of Science and Technology (12JC1402200, 12431900207, and 11410708600).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27(1):103–118. [DOI] [PubMed] [Google Scholar]
- 2. Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19(5–6):333–345. [DOI] [PubMed] [Google Scholar]
- 3. Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107(9):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68(1):152–161. [DOI] [PubMed] [Google Scholar]
- 5. Wang XM, Li J, Yan MX, Liu L, Jia DS, Geng Q, Lin HC, He XH, Li JJ, Yao M. Integrative analyses identify osteopontin, LAMB3 and ITGB1 as critical pro-metastatic genes for lung cancer. PLoS One. 2013;8(2):e55714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardner HA, Berse B, Senger DR. Specific reduction in osteopontin synthesis by antisense RNA inhibits the tumorigenicity of transformed Rat1 fibroblasts. Oncogene. 1994;9(8):2321–2326. [PubMed] [Google Scholar]
- 7. Denhardt DT, Giachelli CM, Rittling SR. Role of osteopontin in cellular signaling and toxicant injury. Annu Rev Pharmacol Toxicol. 2001;41:723–749. [DOI] [PubMed] [Google Scholar]
- 8. Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–864. [DOI] [PubMed] [Google Scholar]
- 9. Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294(5547):1731–1735. [DOI] [PubMed] [Google Scholar]
- 10. Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, Martin SF, Simon JC, Weiss JM. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106(3):946–955. [DOI] [PubMed] [Google Scholar]
- 11. Chen H, Song Z, Qian M, Bai C, Wang X. Selection of disease-specific biomarkers by integrating inflammatory mediators with clinical informatics in AECOPD patients: a preliminary study. J Cell Mol Med. 2012;16(6):1286–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Philip S, Kundu GC. Osteopontin induces nuclear factor kappa B-mediated promatrix metalloproteinase-2 activation through I kappa B alpha/IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J Biol Chem. 2003;278(16):14487–14497. [DOI] [PubMed] [Google Scholar]
- 13. Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2001;276(49):46024–46030. [DOI] [PubMed] [Google Scholar]
- 14. Xie Y, Li Y, Kong Y. OPN induces FoxM1 expression and localization through ERK 1/2, AKT, and p38 signaling pathway in HEC-1A cells. Int J Mol Sci. 2014;15(12):23345–23358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Szekanecz Z, Kim J, Koch AE. Chemokines and chemokine receptors in rheumatoid arthritis. Semin Immunol. 2003;15(1):15–21. [DOI] [PubMed] [Google Scholar]
- 16. Maghazachi AA, al-Aoukaty A, Schall TJ. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J Immunol. 1994;153(11):4969–4977. [PubMed] [Google Scholar]
- 17. Lazar M, Sullivan J, Chipitsyna G, Aziz T, Salem AF, Gong Q, Witkiewicz A, Denhardt DT, Yeo CJ, Arafat HA. Induction of monocyte chemoattractant protein-1 by nicotine in pancreatic ductal adenocarcinoma cells: role of osteopontin. Surgery. 2010;148(2):298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zheng W, Li R, Pan H, He D, Xu R, Guo TB, Guo Y, Zhang JZ. Role of osteopontin in induction of monocyte chemoattractant protein 1 and macrophage inflammatory protein 1beta through the NF-kappa B and MAPK pathways in rheumatoid arthritis. Arthritis Rheum. 2009;60(7):1957–1965. [DOI] [PubMed] [Google Scholar]
- 19. Sun J, Feng A, Chen S, Zhang Y, Xie Q, Yang M, Shao Q, Liu J, Yang Q, Kong B, Qu X. Osteopontin splice variants expressed by breast tumors regulate monocyte activation via MCP-1 and TGF-beta1. Cell Mol Immunol. 2013;10(2):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res. 2004;121(2):228–241. [DOI] [PubMed] [Google Scholar]
- 21. Fong YC, Liu SC, Huang CY, Li TM, Hsu SF, Kao ST, Tsai FJ, Chen WC, Chen CY, Tang CH. Osteopontin increases lung cancer cells migration via activation of the alphavbeta3 integrin/FAK/Akt and NF-kappa B-dependent pathway. Lung Cancer. 2009;64(3):263–270. [DOI] [PubMed] [Google Scholar]
- 22. Tuck AB, Hota C, Wilson SM, Chambers AF. Osteopontin-induced migration of human mammary epithelial cells involves activation of EGF receptor and multiple signal transduction pathways. Oncogene. 2003;22(8):1198–1205. [DOI] [PubMed] [Google Scholar]
- 23. Tuck AB, Elliott BE, Hota C, Tremblay E, Chambers AF. Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (Met). J Cell Biochem. 2000;78(3):465–475. [DOI] [PubMed] [Google Scholar]
- 24. Xu G, Nie H, Li N, Zheng W, Zhang D, Feng G, Ni L, Xu R, Hong J, Zhang JZ. Role of osteopontin in amplification and perpetuation of rheumatoid synovitis. J Clin Invest. 2005;115(4):1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19(1):24–32. [DOI] [PubMed] [Google Scholar]
- 26. Gao C, Guo H, Mi Z, Wai PY, Kuo PC. Transcriptional regulatory functions of heterogeneous nuclear ribonucleoprotein-U and -A/B in endotoxin-mediated macrophage expression of osteopontin. J Immunol. 2005;175(1):523–530. [DOI] [PubMed] [Google Scholar]
- 27. Gustafson AM, Soldi R, Anderlind C, Scholand MB, Qian J, Zhang X, Cooper K, Walker D, McWilliams A, Liu G, Szabo E, Brody J, Massion PP, Lenburg ME, Lam S, Bild AH, Spira A. Airway PI3 K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med. 2010;2(26):26ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mi Z, Guo H, Wai PY, Gao C, Wei J, Kuo PC. Differential osteopontin expression in phenotypically distinct subclones of murine breast cancer cells mediates metastatic behavior. J Biol Chem. 2004;279(45):46659–46667. [DOI] [PubMed] [Google Scholar]
- 29. Packer L, Pavey S, Parker A, Stark M, Johansson P, Clarke B, Pollock P, Ringner M, Hayward N. Osteopontin is a downstream effector of the PI3-kinase pathway in melanomas that is inversely correlated with functional PTEN. Carcinog. 2006;27(9):1778–1786. [DOI] [PubMed] [Google Scholar]
- 30. Lu JB, Yao XX, Xiu JC, Hu YW. MicroRNA-125b-5p attenuates lipopolysaccharide-induced monocyte chemoattractant protein-1 production by targeting inhibiting LACTB in THP-1 macrophages. Arch Biochem Biophys. 2016;590:64–71. [DOI] [PubMed] [Google Scholar]
- 31. Fan K, Dai J, Wang H, Wei H, Cao Z, Hou S, Qian W, Wang H, Li B, Zhao J, Xu H, Yang C, Guo Y. Treatment of collagen-induced arthritis with an anti-osteopontin monoclonal antibody through promotion of apoptosis of both murine and human activated T cells. Arthritis Rheum. 2008;58(7):2041–2052. [DOI] [PubMed] [Google Scholar]
- 32. Weber GF, Zawaideh S, Hikita S, Kumar VA, Cantor H, Ashkar S. Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J Leukoc Biol. 2002;72(4):752–761. [PubMed] [Google Scholar]
- 33. Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. 2008;181(11):7480–7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Girolamo N, Indoh I, Jackson N, Wakefield D, McNeil HP, Yan W, Geczy C, Arm JP, Tedla N. Human mast cell-derived gelatinase B (matrix metalloproteinase-9) is regulated by inflammatory cytokines: role in cell migration. J Immunol. 2006;177(4):2638–2650. [DOI] [PubMed] [Google Scholar]
- 35. Akahoshi T, Wada C, Endo H, Hirota K, Hosaka S, Takagishi K, Kondo H, Kashiwazaki S, Matsushima K. Expression of monocyte chemotactic and activating factor in rheumatoid arthritis. Regulation of its production in synovial cells by interleukin-1 and tumor necrosis factor. Arthritis Rheum. 1993;36(6):762–771. [DOI] [PubMed] [Google Scholar]
- 36. Shahrara S, Proudfoot AE, Park CC, Volin MV, Haines GK, Woods JM, Aikens CH, Handel TM, Pope RM. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J Immunol. 2008;180(5):3447–3456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplement_Table_1-170808 for Regulatory Roles of Osteopontin in Production of Monocyte-Origin MCP-1 by Liying Shi, Lin Shi, Xiangdong Wang, and Jiantai He in Cell Transplantation