Abstract
Pigs share many anatomical and physiological features with humans, offering a unique and viable model for biomedical research. Although porcine female germline stem cells (FGSCs) were identified in the juvenile ovary, no reports described the isolation and purification of FGSCs from the pig at sexual maturity. Here, we isolated, purified, and cultured FGSCs from porcine ovaries at sexual maturity. Furthermore, we established and characterized the porcine FGSC (pFGSC) lines. In addition, we found that pFGSC lines could differentiate into oocytes when injection into tissue grafts, including human ovarian tissues. The results show that FGSCs exist in ovaries of Banna mini-pigs at juvenile and sexually maturity. These findings have implications in animal biotechnology applications and regeneration medicine.
Keywords: pig, ovary, female germline stem cell, sexual maturity
Introduction
The pig is an important model organism for biomedical applications and other biological investigations, with characteristics including diversity, valuable products, and a relatively short gestation period. Furthermore, pigs have many similarities to humans, such as in organ sizes, physiology, and immunology1. Therefore, porcine germ cell research has great potential for developing disease models or disease-free and genetically engineered porcine products for agricultural purposes, as well as for biomedical applications such as modeling human disease mechanisms and developing patient-specific therapies for tissue or organ transplantation.
Germline stem cells (GSCs) generate haploid gametes, that is, sperm or oocytes, to transmit genetic information from one generation to the next. In our previous studies, neonatal and adult mouse female germline stem cells (FGSCs) were isolated and purified from ovarian tissues2,3. Based on our protocol, White et al. isolated and purified oogonial stem cells (OSCs, also termed FGSCs) from adult mouse ovaries and the ovarian cortical tissue of healthy reproductive-age women using an antibody against DEAD box polypeptide 4 (DDX4)4. Ding et al. developed a strategy to guide FGSC differentiation into germinal vesicle oocytes in vitro and investigated the associated developmental mechanisms5. Moreover, FGSCs were also isolated and purified from the lower vertebrate species medaka fish6 and zebrafish7. Zhang et al. reported the successful generation of transgenic or gene knockdown mice using mouse FGSCs8, and Zhou et al. produced fat-1 transgenic rats utilizing a postnatal FGSC line9. Although porcine FGSCs were identified in the juvenile ovary10, no reports described the isolation and purification of FGSCs from the pig at sexual maturity.
Banna mini-pig is obtained from a Chinese pig after more than 20 generations of inbreeding. Highly homozygous inbred experimental animals are used for biomedical and life sciences research to provide high sensitivity, specificity, and good reproducibility of experiments. Interspecific animal genes are highly homozygous and have a clear genetic background, which can reduce the errors caused by individual genetic differences in experiments. Banna mini-pig is a unique and precious resource in China, and its inbred lines are close to purebred9. With successful isolation, purification, and culture of FGSCs from Banna mini-pig, we can carry out reproductive protection of this species or expand its population culture.
In this study, we have isolated, purified, and cultured FGSCs from ovaries of 8- and 32-week-old Banna mini-pigs for the first time. Moreover, we have established and characterized the pig FGSC lines. The results show that FGSCs exist in ovaries of juvenile and sexually mature Banna mini-pigs. These findings have implications in animal biotechnology applications and regeneration medicine.
Materials and Methods
Animals
Banna mini-pigs, aged 8 and 32 weeks, were obtained from the School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai. Female nude mice, 6 weeks old, were from SLAC Laboratory Animal Co., Shanghai, China. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Shanghai, and were conducted in accordance with the National Research Council Guide for Care and Use of Laboratory Animals.
Porcine Female Germline Stem Cell Isolation and Purification
Porcine FGSCs (pFGSCs) were isolated using a two-step enzymatic digestion method, as described previously. Briefly, ovaries collected from 8- and 32-week-old Banna mini-pigs were enzymatically dissociated in a buffer containing collagenase (1 mg/ml, Type IV; Sigma-Aldrich, MO, USA) for 30 min with gentle agitation in a 37°C water bath, centrifuged and then washed once with phosphate-buffered saline (PBS). The ovarian tissues were further digested in 1 mM EDTA and 0.01% trypsin at 37°C for 15–20 min. The dissociated ovarian cell suspensions were filtered through a sterile cell strainer (70 μm nylon mesh). pFGSCs were enriched by immunomagnetic isolation of Fraglis+ cells. Goat anti-rabbit IgG microbeads (MiltenyiBiotec, Germany) were precoated with an anti-Fragilis primary antibody (Abcam, ab15592, UK). Fraglis+ cells were isolated by magnetic separation, according to the manufacturer’s instructions.
Porcine Female Germline Stem Cell Culture
STO cells were cultured in Dulbecco’s modified Eagle’s medium with high glucose (Life Technologies, MA, USA), 1% nonessential amino acids (Life Technologies, MA, USA), 10% fetal bovine serum (FBS; Life Technologies, MA, USA), glutamine (2 mM, Sigma-Aldrich, MO, USA), penicillin (30 mg/L, Sigma-Aldrich, MO, USA), and streptomycin (75 mg/L, Sigma-Aldrich, MO, USA). The STO cells were first treated with mitomycin C (10 μg/ml, Sigma-Aldrich, MO, USA) for 3 h, then washed with PBS and plated on a 0.2% (w/w) gelatin-coated well of a 48-well plate. The culture medium for pFGSCs consisted of minimum essential medium α (MEM-α), 10% FBS, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 2 mM L-glutamine, 0.1 mM β-mercaptoethanol (Sigma-Aldrich, MO, USA), 10 ng/ml LIF (leukemia inhibitory factor; Santa Cruz Biotechnology, CA, USA), 25 μg/ml vitamin C (Sigma-Aldrich, MO, USA), 10 ng/ml EGF (mouse epidermal growth factor; Sigma-Aldrich, MO, USA), 40 ng/ml human GDNF (glial cell line-derived neurotrophic factor; R&D Systems, MN, USA), 10 ng/ml human bFGF (basic fibroblast growth factor; BD Biosciences Clontech, Japan), and 15 mg/L penicillin. pFGSCs were cultured on STO feeder cells in FGSC culture medium (400 μl per well). The medium was changed every other day. All cultures were maintained at 37°C in a 95% air, 5% CO2 incubator.
Total RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted using Trizol reagent (Qiagen, Germany) according to the manufacturer’s instructions. Approximately 2 μg RNA from cells or ovarian tissue was reverse-transcribed using M-MuLV reverse transcriptase following the manufacturer’s recommended protocol (MMLV First-Strand cDNA Synthesis Kit; Qiagen, Germany). The resulting cDNA samples were used for gene expression analysis. Polymerase chain reaction (PCR) was performed using 2 × Es Taq MasterMix (CWBIO, Beijing, China), with primers shown in Table 1.
Table 1.
Primers for RT-PCR Analysis.
| Gene | Sequence(5′-3′) | Accession No. |
|---|---|---|
| Ddx4 | F:ttgcaggacgagatttgatg | AY626785.1 |
| R:ccaattctcgagttggtgt | ||
| Dazl | F:cctccaaccatgatgaatcc | EU430405.1 |
| R:gggcaaaatatcagctcctg | ||
| Fragilis | F:catgtcgtctggtccctgt | XR_093659.2 |
| R:gtggaggcataggcctgg | ||
| Oct4 | F:cacctcaggtcggagtgg | NM_001113060.1 |
| R:agcttggcaaattgttcga | ||
| Nanog | F:atccagcttgtccccaaag | NM_001129971.1 |
| R:atttcattcgctggttctgg | ||
| Sox2 | F:gcctgggcgccgagtgga | NM_001123197 |
| R:ggcgagccgttcatgtaggtctg | ||
| Rex-1 | F:tttctgagtacgtgccaggcaa | XM_003359865.2 |
| R:gaacggagagatgctttctcagag |
Immunoanalysis
FGSC cultures were fixed with 4% paraformaldehyde (PFA; 20 min, room temperature) and washed twice with PBS. The cells were incubated in blocking solution (10% normal goat serum in PBS) for 30 min at room temperature, then incubated with the appropriate primary antibody (rabbit polyclonal anti-Vasa [1:200 dilution; Abcam, UK], rabbit polyclonal anti-Oct4 [1:250 dilution; Chemicon, UK]) or mouse monoclonal anti-GFP (1:100 dilution; CST, MA, USA) at 4°C overnight. After washing, cells were incubated with fluorescein isothiocyanate conjugated secondary antibody (goat anti-rabbit IgG, 1:400 dilution) in the dark, washed with PBS, and then stained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride for 10 min. Appropriate negative controls, omitting the primary antibody, were also prepared. Images were obtained and analyzed using an Olympus fluorescence microscope (Japan).
Alkaline Phosphatase Staining
Cells were fixed with 4% PFA in PBS for 2 min at room temperature, washed three times with PBS, stained with naphthol AS-MX phosphate and Fast Red TR salt in 100 mM Tris buffer (pH 8.2–8.4) for 10–30 min at room temperature, and then washed with PBS to terminate staining.
Teratoma Assay
pFGSCs (1×106 cells) or porcine-induced pluripotent stem cells (piPS cells) were subcutaneously injected into nude mice, in triplicate. Teratomas were formed in the piPSs injected group after 1 month. In the pFGSC-injected group, there was no tumor formation. The teratomas were removed, fixed in 4% PFA and processed for paraffin sectioning.
EGFP Lentivirus Infection and Transplantation of Porcine Female Germline Stem Cells
With written informed consent, ovarian cortical tissues were surgically removed from reproductive-age women. The human study was approved by the Investigation Review Committee of The First People’s Hospital of Chenzhou, Hunan Province, and all patients provided written informed consent. All experiments were carried out in accordance with the approved protocols.
EGFP lentivirus was purchased from a commercial service (GM100101-1; Genomeditech, Shanghai China). After culturing pFGSCs for 3–5 days, lentivirus infection was performed according to the manufacturer’s instructions. Next, pFGSCs were washed three times with PBS, collected, and resuspended in PBS. pFGSC suspensions (40 μl, containing 1.3×105 cells) were injected into porcine or human ovarian cortical tissue and then xenografted into nude mice as described previously. Xenografts were removed after 14 days. The in-vivo differentiation of pFGSCs was assessed by immunofluorescence.
Result
Isolation, Purification, Long-Term Culture, and Characterization of pFGSCs
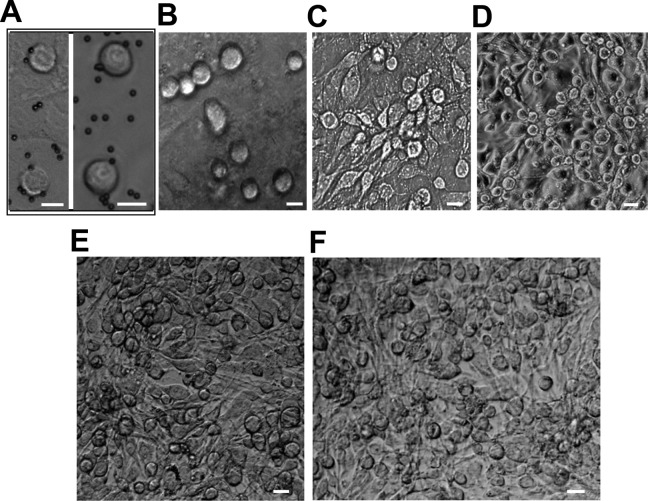
Based on our previous findings, two-step enzymatic digestion and Fragilis-based immunomagnetic sorting were used to isolate and purify pFGSCs, including those from 32-week-old (sexually mature) (mpFGSC) and 8-week-old (juvenile) (jpFGSC) pigs. The isolated cells were round, with high nucleus/cytoplasm ratios (Fig. 1A). For each experiment, Fragilis-positive cells were obtained from an 8-week-old (200–280 cells) or 32-week-old (100–150 cells) pig. The experiments were repeated more than six times. The association between female germ cells or FGSCs and immunomagnetic beads is illustrated in Fig. 1A. Freshly isolated Fragilis-positive cells were seeded on mitomycin C-treated STO feeder cells (derived from mouse SIM embryonic fibroblasts, strain SIM, 5 × 104 cells/cm2, ATCC) (Fig. 1A), then cultured for one (Fig. 1B), four (Fig. 1C), or eight weeks (Fig. 1D) (5–8 days per passage). During this time period, FGSC numbers increased and their morphology remained similar to that of newly prepared FGSCs (Fig. 1A). Furthermore, jpFGSC (Fig. 1E) and mpFGSC (Fig. 1F) lines were established after passaging the cells over 30 times.
Fig. 1.
Isolation, purification, and culture of porcine FGSCs. (A). An example of the morphology of FGSCs purified by MACS (magnetic-activated cell sorting), based on an anti-Fragilis antibody (left, pFGSCs from 32-week ovaries; right, pFGSCs from 8-week ovaries). Scale bar, 10 µm. (B). pFGSCs from 8-week ovaries cultured on mitomycin C-treated STO cells for 1 week. Scale bar, 10 µm. (C). pFGSCs from 32-week ovaries cultured on mitomycin C-treated STO cells for 4 weeks. Scale bar, 20 µm. (D). pFGSCs from 8-week ovaries cultured on mitomycin C-treated STO cells for 4 weeks. Scale bar, 20 µm. (E–F). Representative morphologies of pFGSC lines: (E). pFGSCs from 8-week ovaries; (F). pFGSCs from 32-week ovaries. Scale bars, 20µm.
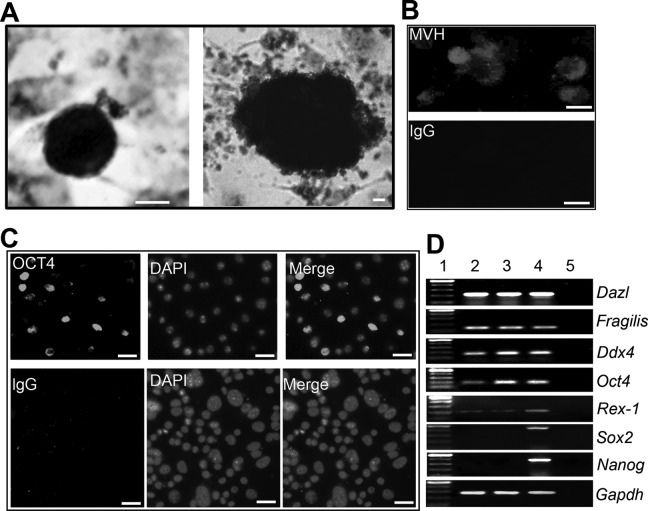
Although pFGSCs were positive for alkaline phosphatase (AP), the staining intensity seemed weaker than that of pig iPS cells (Fig. 2A). To further characterize the pFGSC lines, we used immunofluorescence and RT-PCR to examine expression of Dazl (a germ cell-specific RNA-binding protein)11, Fragilis (Ifitm3, interferon-induced transmembrane protein 3)12, DDX4 (exclusively expressed in germ cells), Oct4 (a germ cell-specific transcriptional factor)13, Rex-1 (zfp42, zinc-finger protein 42)14, Sox2 (a key transcription factor in regulating stemness related to pluripotency),15 and Nanog (a pluripotency sustaining factor)16, using immunofluorescence and RT-PCR analysis (Fig. 2B–D). Both cell lines expressed Dazl, Fragilis, DDX4, Oct4, and Rex-1, indicating stemness and germ cell characteristics. Moreover, pFGSCs did not form teratomas when injected into nude mice. In contrast, teratomas were developed from pig iPS cells within 6–8 weeks of transplantation (Fig. 3). Therefore, the pFGSCs were adult stem cells, distinct from pluripotent stem cells.
Fig. 2.
Characterization of porcine FGSCs. (A). Alkaline phosphatase (AP) staining of pFGSCs (left, pFGSCs; right, pESCs). Scale bar, 10 µm. (B). Immunostaining of pFGSCs for Mvh. IgG was used as a negative control. Scale bar, 10 µm. (C). Oct4 expression in pFGSCs with DAPI counterstaining. IgG was used as a negative control. Scale bar, 20 µm. (D). Gene expression profiles of jpFGSC and mpFGSC lines. Lane 1, 100-bp DNA marker; lane 2, pFGSCs from 8-week ovaries; lane 3, pFGSCs from 32-week ovaries; lane 4, PCR of RNA sample without reverse transcription.
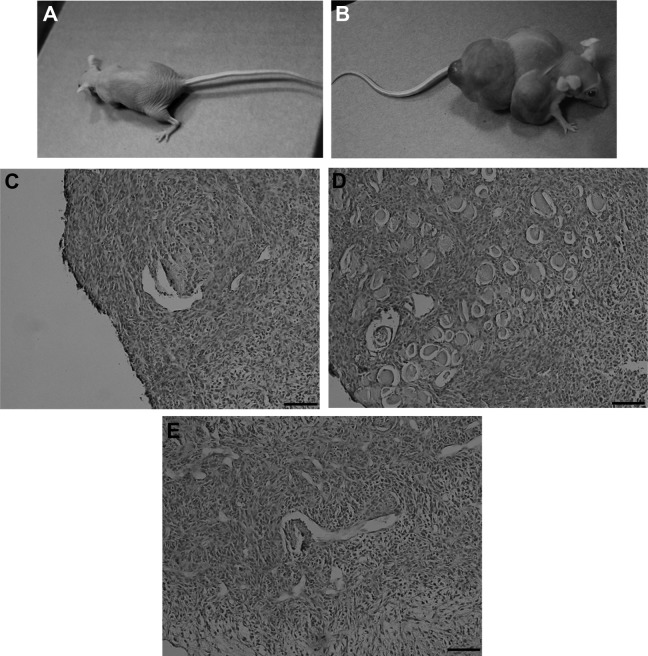
Fig. 3.
Teratoma formation assay for pFGSCs injected into nude mice. (A). No teratoma was formed after pFGSC transplantation. (B). Teratomas were formed after piPS cell transplantation. (C–E). Histological analysis of teratomas showed that they contained the three embryonic germ layers. Scale bar, 40 µm.
In-Vivo Differentiation of pFGSCs
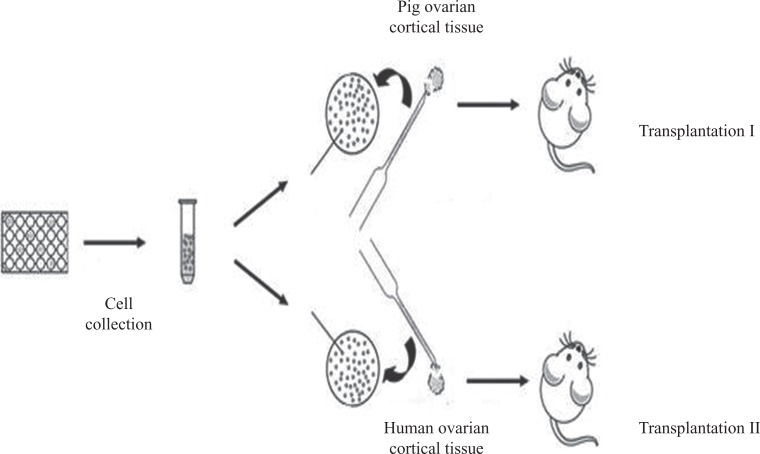
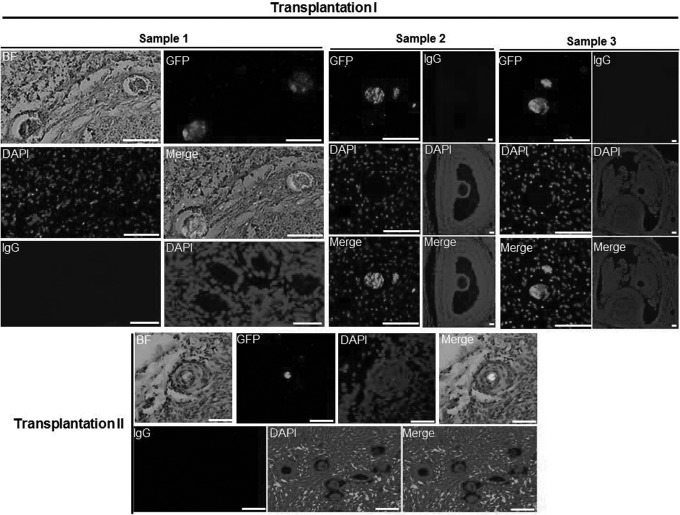
To test the differentiation potential of pFGSCs, the experiments of pFGSC transplantation, summarized schematically in Fig. 4, were performed. After stable labeling with GFP, pFGSCs were injected into a small piece (2–3 mm wide × 2–3 mm thick) of outer cortex obtained from ovaries of a reproductive adult pig or human. The cortical samples injected with pFGSCs were then transplanted subcutaneously into nude mice. At 2 weeks after transplantation, GFP-positive oocytes were detectable in tissue from the recipients (Fig. 5). This suggested that the pFGSCs had differentiated into oocytes in the tissue grafts. It was especially interesting that the pFGSCs differentiated into oocytes in the human tissue.
Fig. 4.
Scheme of pFGSC injection into porcine or human ovarian cortical tissue and xenograft transplantation into subcutaneous tissues of nude mice.
Fig. 5.
pFGSCs generated oocytes when transplanted into recipient ovarian tissue. Transplantation I: representative immunohistochemical staining of EGFP+ cells, surrounded by smaller EGFP− cells, in porcine ovarian cortical tissues injected with EGFP+ pFGSCs and xenografted into nude female mice. Nuclei were counterstained with DAPI. IgG was used as a negative control (NC). Scale bar, 40 µm. Transplantation II: representative immunohistochemical staining of EGFP+ cells, surrounded by smaller EGFP− cells, in human ovarian cortical tissues injected with EGFP+ pFGSCs and xenografted into nude female mice. Nuclei were counterstained with DAPI. IgG was used as an NC. Scale bar, 40 µm. BF: bright field.
Discussion
Pigs have many similarities to humans, so it is an important animal model for human disease research. Banna mini-pig is a special and rare species, and its inbred lines are close to purebred. It is not only the animal model of human disease research, but also an important sample for genetic function development and research. Therefore, the successful establishment of FGSC lines is important in the establishment of the human disease model and the reproductive protection of Banna mini-pigs.
In pig reproductive physiology, the estrus cycle spans a period of 18–24 days. It consists of a follicular phase, lasting 5–7 days, and a luteal phase, lasting 13–15 days. During the follicular phase, small antral follicles develop into large, pre-ovulatory follicles, and may be ovulated from the follicles. Most pigs are inseminated during the second or third estrus. The reproductive stages are controlled by a system of positive and negative feedback through reproductive hormones, including gonadotrophin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), progesterone (P4), and 17β-estradiol (E2). Therefore, sexual maturity is characterized by the appearance of a reproductive cycle and production and metabolism of sex hormones. Lian et al. reported that Banna mini-pigs were sexually mature at 4–6 months of age17, and we have done a proteomics analysis of the 32-week-old Banna mini-pigs and confirmed that they were sexually mature18.
Based on this, we isolated and purified FGSCs from ovaries of 8- and 32-week-old Banna mini-pigs, establishing these FGSC lines for the first time. To further characterize the FGSC lines, we examined expression of germ cell marker genes, AP staining, and teratoma formation. The results showed that the FGSC lines had stem cell and germ cell characteristics and they did not form teratomas when injected into nude mice, suggesting they are similar to other FGSC lines, such as those of humans, mice, and rats2,5,9. The findings indicated that both lines had characteristics of GSCs and adult stem cells. Moreover, both pFGSC lines differentiated into oocytes when injected into tissue grafts, particularly in human ovarian tissues. This suggested that pFGSC can develop in the human ovarian micro-environment. It is possible that the ovaries of humans and pigs have similar micro-environments. This finding has implications for applications of regeneration medicine.
In conclusion, we isolated, purified, and cultured FGSCs from ovaries of Banna mini-pig, a special and rare species, at the juvenile and sexually mature stages. Furthermore, we established the pig FGSC lines and characterized them. In addition, pFGSC lines differentiated into oocytes when injected into tissue grafts, including human ovarian tissues. These findings have implications for animal biotechnology applications and for biomedical research.
Acknowledgments
The authors thank Lei Xiao for his generous gift of piPS cells.
Footnotes
Ethical Approval: All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Shanghai, and were conducted in accordance with the National Research Council Guide for Care and Use of Laboratory Animals.
Statement of Human and Animal Rights: All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Shanghai, and were conducted in accordance with the National Research Council Guide for Care and Use of Laboratory Animals.
Statement of Informed Consent: The human subjects used in this article have informed and obtained informed consent.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Basic Research Program of China (2017YFA0504201); National Natural Science Foundation of China (81720108017).
References
- 1. Brandl U, Michel S, Erhardt M, Brenner P, Burdorf L, Jöckle H, Bittmann I, Rössle M, Mordstein V, Baschnegger H. Transgenic animals in experimental xenotransplantation models: orthotopic heart transplantation in the pig-to-baboon model. Transplant Proc. 2007;39(2):577–578. [DOI] [PubMed] [Google Scholar]
- 2. Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, Wu J. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nature Cell Biology. 2009;11(5):631–636. [DOI] [PubMed] [Google Scholar]
- 3. Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using Fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20(12):2197–2204. [DOI] [PubMed] [Google Scholar]
- 4. White YAR, Woods DC, Yasushi T, Osamu I, Hiroyuki S, Tilly JL. Oocyte formation by mitotically-active germ cells purified from ovaries of reproductive age women. Nat Med. 2012;18(3):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding X, Liu G, Xu B, Wu C, Ning H, Ni X, Wang J, Du M, Teng X, Wu J. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep. 2016;6:28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328(5985):1561–1563. [DOI] [PubMed] [Google Scholar]
- 7. Wong TT, Tesfamichael A, Collodi P. Production of zebrafish offspring from cultured female germline stem cells. PLoS One. 2013;8(5):e62660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, Sun K, Zou K, Wang L, Xiong J, Xiang J, Wu J. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3(2):132–141. [DOI] [PubMed] [Google Scholar]
- 9. Zhou L, Wang L, Kang JX, Xie W, Li X, Wu C, Xu B, Wu J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod. 2014;20(3):271–281. [DOI] [PubMed] [Google Scholar]
- 10. Bai Y, Yu M, Hu Y, Qiu P, Liu W, Zheng W, Peng S, Hua J. Location and characterization of female germline stem cells (FGSCs) in juvenile porcine ovary. Cell Prolif. 2013;46(5):516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruggiu M, Speed R, Taggart M, Mckay SJ, Kilanowski F, Saunders P, Dorin J, Cooke HJ. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389(6646):73–77. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka SS, Yamaguchi YL, Tsoi B, Lickert H, Tam PP. IFITM/Mil/Fragilis family proteins IFITM1 and IFITM3 play distinct roles in mouse primordial germ cell homing and repulsion. Dev Cell. 2005;9(6):745–756. [DOI] [PubMed] [Google Scholar]
- 13. Scholer HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344(6265):435–439. [DOI] [PubMed] [Google Scholar]
- 14. Xu J, Sylvester R, Tighe AP, Chen S, Gudas LJ. Transcriptional activation of the suppressor of cytokine signaling-3 (SOCS-3) gene via STAT3 is increased in F9 REX1 (ZFP-42) knockout teratocarcinoma stem cells relative to wild-type cells. J Mol Biol. 2008;377(1):28–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, Ko MS, Niwa H. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9(6):625–635. [DOI] [PubMed] [Google Scholar]
- 16. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113(5):643–655. [DOI] [PubMed] [Google Scholar]
- 17. Lian L, Li J, Wang H, Hu W, Li G, Zhao Y. Banna miniature pig. Chin J Animal Sci. 1994;30(5):15–17. [Google Scholar]
- 18. Hou L, Wang J, Wang Y, Hua X, Wu J. Compared proteomic analysis of 8-and 32-week-old postnatal porcine ovaries. Cell Biochem Funct. 2018; 36(1): 34–42. [DOI] [PubMed] [Google Scholar]