Abstract
Amniotic epithelial cells (AECs) represent a useful and noncontroversial source for liver-based regenerative medicine, as they can differentiate into hepatocytes upon transplantation into the liver. However, the possibility that AECs can differentiate into other liver cell types, such as hepatic sinusoidal endothelial cells (HSECs), has never been assessed. In order to test this hypothesis, rat- and human-derived AECs (rAECs and hAECs, respectively) were subjected to endothelial cell tube formation assay in vitro. Moreover, to evaluate differentiation in vivo, the retrorsine (RS) model of liver repopulation was used. Pyrrolizidine alkaloids (including RS) are known to target both hepatocytes and endothelial cells, inducing cell enlargement and inhibition of cell cycle progression. rAECs and hAECs were able to form capillary-like structures when cultured under proangiogenic conditions. For in vivo experiments, rAECs were obtained from dipeptidyl peptidase type IV (DPP-IV, CD26) donors and were transplanted into the liver of recipient CD26 negative animals pretreated with RS. rAEC-derived cells were engrafted in between hepatocytes and resembled HSECs as assessed by morphological analysis and the pattern of expression of CD26. Donor-derived CD26+ cells coexpressed HSEC markers RECA-1 and SE-1, while they lacked expression of typical hepatocyte markers (i.e., cytochrome P450, hepatocyte nuclear factor 4α). As such, these results provide the first evidence that AECs can respond to proangiogenic signals in vitro and differentiate into HSECs in vivo. Furthermore, they support the conclusion that AECs possesses great plasticity and represents a promising tool in the field of regenerative medicine both in the liver and in other organs.
Keywords: amniotic epithelial cells, hepatic sinusoidal endothelial cells, stem cell differentiation, cell transplantation
Introduction
Because of their stem cell characteristics, human-derived amniotic epithelial cells (hAECs) isolated from term placenta have been suggested as a possible source for regenerative medicine strategies based on isolated cell transplantation1–3. We recently reported that hAECs have the ability to differentiate into hepatocytes in vitro and in vivo4 and were able to correct a mouse model of maple syrup urine disease5,6. Moreover, syngeneic rat-derived AECs differentiated into hepatocytes upon transplantation in the retrorsine (RS) model of liver repopulation, with no evidence of cell fusion7. Although hepatocytes are the main cell type in the liver, all other cell types have a role during the regeneration process. After partial hepatectomy, for example, together with the hepatocytes, hepatic sinusoidal endothelial cells (HSECs) and cholangiocytes, have to extensively proliferate in order to form the new vessels and sinusoids, and the bile ducts, respectively, thus reestablishing the functional structure of liver lobules8,9. The possibility that AECs possess the ability to contribute to other cellular compartments of the liver has never been tested. The present study focuses on the differentiation of AECs into HSECs both in vitro and upon transplantation in an animal model of liver repopulation.
Materials and Methods
Animals
All animals were maintained on daily cycles of alternating 12 h light/darkness with food and water available ad libitum. They were fed Purina Rodent Lab Chow diet throughout the experiment and received humane care according to the criteria outlined in the National Institutes of Health Publication 86-23, revised 1985. Animal studies were reviewed and approved by the University of Cagliari Ethical Committee for Animal Experimentation.
Isolation and Maintenance of rAECs, hAECs, and Human Umbilical Vein Endothelial Cells (HUVECs)
rAECs were isolated from the placentae of Fischer 344 wild type (dipeptidyl peptidase type IV, DPP-IV+) pregnant rats at 16–19 d of gestational age as previously described10. A total of 20 placentae from 3 different pregnant rats were used. In order to avoid endothelial cell contamination, the yolk sac (YS) membrane (which is finely vascularized) was carefully peeled and only the inner white avascular membrane (amniotic membrane) was collected (Fig. 1, panels A–C). After a quick wash in phosphate buffered saline (PBS), amniotic membranes were digested in trypsin/ethylenediaminetetraacetic acid [EDTA] 0.05% (Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C for 20–30 min. The cell suspension was passed through a 100 µm strainer, centrifuged, and resuspended in PBS. Cell viability was assessed by Trypan blue dye exclusion and consistently exceeded 98%.
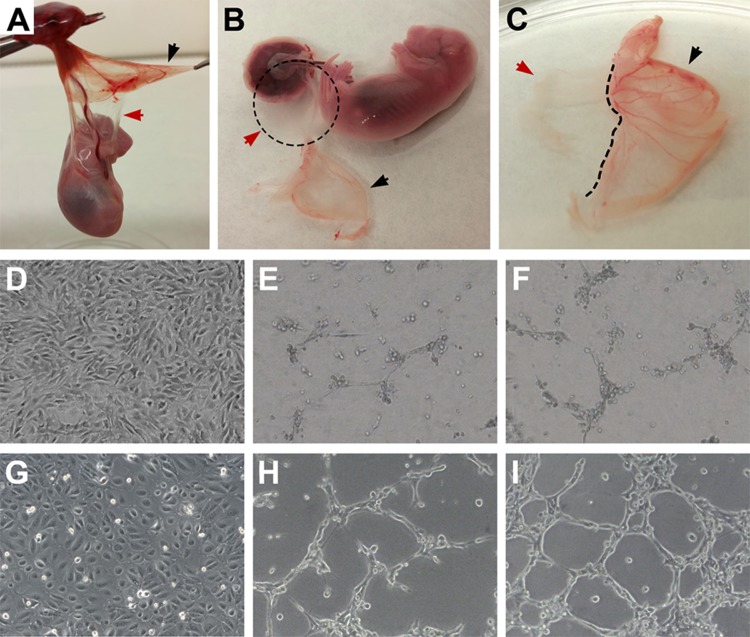
Fig. 1.
(A–C) Isolation of rat-derived amniotic epithelial cells (rAECs). Careful separation of avascular amniotic membrane (red arrow) from the finely vascularized yolk sac (black arrow) is necessary in order to avoid endothelial cell contamination. (D–I) Standard culture of AECs and in vitro tube formation assay (100× magnification). (D) rAEC standard culture conditions on plastic. (E and F) rAEC after 24 h tube formation assay. (G) hAEC standard culture conditions on plastic. (H and I) hAECs after 24 h tube formation assay.
Human term placentae were collected from healthy women undergoing caesarean section after obtaining informed written consent. hAECs were isolated via trypsin/EDTA 0.05% digestion as previously described11. Viability was >90%.
Both rAECs and hAECs were cultured under standard conditions in Dulbecco’s modified Eagle’s medium (DMEM) (high glucose) with 2 mM l-glutamine, 1% nonessential amino acids, 55 µM 2-mercaptoethanol, 1 mM sodium pyruvate, 10% fetal bovine serum (FBS) (all from Thermo Fisher Scientific), and 10 ng/mL epidermal growth factor (EGF, Peprotech, Rocky Hill, NJ, USA).
At the same time as hAEC isolation, HUVECs were isolated from the umbilical cord as previously described12 and used as a positive control. HUVECs were maintained in Medium 200 supplemented with low-serum growth supplement (LSGS, Thermo Fisher Scientific).
Transplantation
Fischer 344 (DPP-IVnull) dipeptidyl peptidase type IV rats were used as recipients (n = 5). Four-week-old female rats were given 2 intraperitoneal injections of 30 mg/kg RS (Sigma-Aldrich, St. Louis, MO, USA), 2 wk apart. One month after RS treatment, recipient animals received 2/3 partial hepatectomy and ∼1.5 × 106 freshly isolated rAECs were injected via a mesenteric vein. Animals were killed at different time points up to 12 mo after cell transplant.
Endothelial Cell Tube Formation Assay
In order to promote capillary-like formation, a well-established angiogenesis assay was used13. Briefly, 5 × 104 rAECs, hAECs, and HUVECs were seeded on chamber slides previously coated with 100 µL of Geltrex (Thermo Fischer Scientific) per cm2. Cells were cultured for 24 h in Medium 200 supplemented with LSGS.
Histochemical and Immunofluorescence Analyses
To follow the fate of transplanted cells, histochemical detection of DPP-IV positive clusters was performed on 5 µm frozen sections as previously described14.
Double immunofluorescence staining of DPP-IV (hereafter referred as CD26) and SE-1, RECA-1, cytochrome P450 (CYP) 2E1, 3A1, or hepatocyte nuclear factor 4α (HNF 4α) was performed on 5 µm thick frozen sections as follows: briefly, slides were fixed in cold acetone for 10′, then blocked for 30′ with goat serum, and incubated 1 h at room temperature (RT) with the primary antibody of interest. Sections were then washed and incubated with anti-mouse or anti-rabbit Dylight 488-conjugated secondary antibodies for 30′ at RT. After wash, slides were blocked for 30′ with goat serum and incubated 1 h at RT with anti-CD26 antibody. Sections were then incubated with Atto 550-conjugated secondary anti-mouse immunoglobulin G for 30′ at RT. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). When 2 mouse antibodies were used in sequential double staining, primary antibody staining was blocked with mouse serum in order to avoid cross-reactivity with the secondary anti-mouse antibody. Control photographs were acquired at this step. Moreover, individual stainings were also performed as a control on separate serial sections (data not shown).
Staining for CD31 was performed on cultured cells after blocking with 4% paraformaldehyde (PFA) for 10′. Cells were permeabilized and epitopes exposed by incubating with 0.1% triton for 10′. After 30′ blocking with goat serum, slides were incubated with anti-CD31 antibody for 2 h at RT. After washing, cells were incubated with secondary anti-rabbit DyLight 488-conjugated antibody for 1 h at RT. Nuclei were counterstained with DAPI.
All slides were examined with an IX71 fluorescence microscope (Olympus, Tokyo, Japan). For a complete list of antibodies, see Table 1.
Table 1.
List of Antibodies.
| Epitope | Host/Type | Dilution | Brand/Cat. No. |
|---|---|---|---|
| CD31/PECAM-1 | Rabbit polyclonal | 1:100 | Novus Biologicals (Littleton, CO, USA) NB100-2284 |
| CD26 | Mouse monoclonal | 1:150 | BD Pharmingen (San Jose, CA, USA) 559639 |
| RECA-1 | Mouse monoclonal | 1:50 | Abcam (Cambridge, United Kingdom) ab9774 |
| SE-1 | Mouse monoclonal | 1:500 | Novus Biologicals NB110-68095 |
| CYP 3A1 | Rabbit polyclonal | 1:500 | Abcam ab22733 |
| CYP 2E1 | Rabbit polyclonal | 1:250 | Abcam ab28146 |
| Hepatocyte nuclear factor 4α | Mouse monoclonal | 1:100 | Abcam ab41898 |
| Anti-Mouse DyLight 488 conj. | Goat polyclonal | 1:250 | Abcam ab96879 |
| Anti-Rabbit DyLight 488 conj. | Goat polyclonal | 1:250 | Abcam ab96899 |
| Anti-Mouse Atto 550 conj. | Goat polyclonal | 1:250 | Sigma (St. Louis, MO, USA) 43394 |
Transmission Electromicroscopy (TEM)
Differentiated hAECs were fixed with 2% glutaraldehyde containing 5–10 M verapamil, 5–10 M EDTA, adjusted to pH 7.4, and then postfixed with aqueous 1% osmium tetroxide. The fixed samples were dehydrated and embedded with epon. The 50 nm ultrathin sections were cut and stained with 2% uranyl acetate and lead. TEM analysis was performed using a JEOL 1210 TEM system (JEOL USA, Peabody, MA, USA).
Results
Rat- and Human-derived AECs Form Capillary-like Structures In Vitro
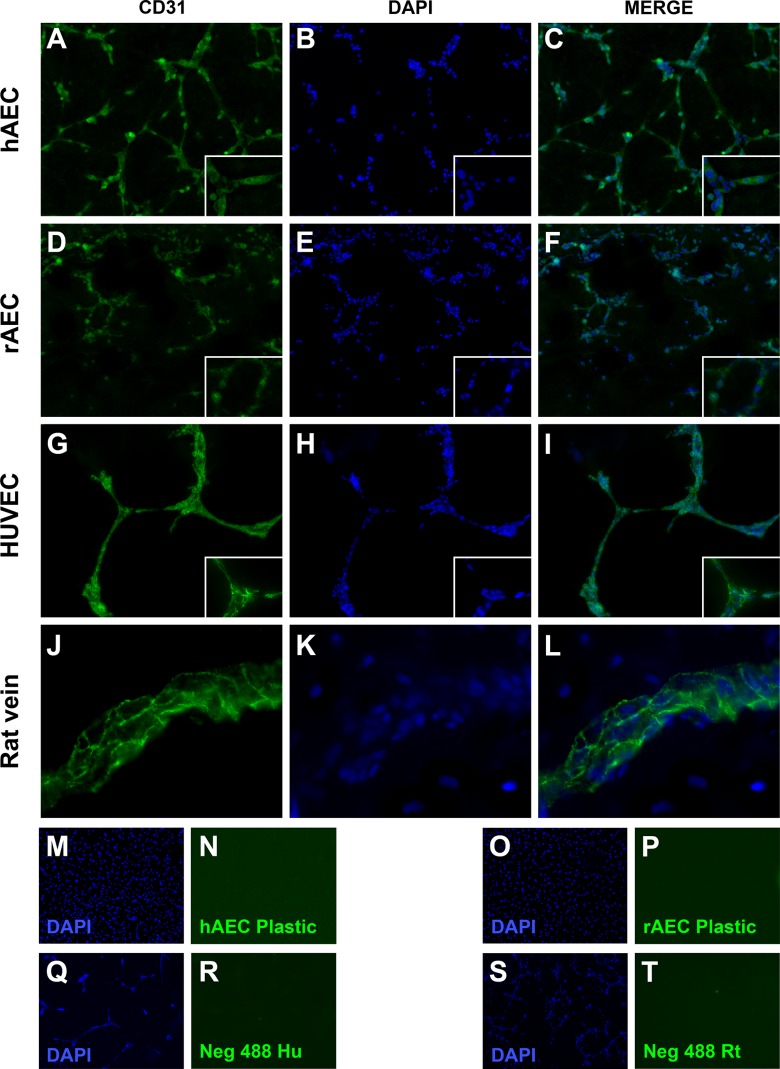
rAECs were isolated by carefully separating the avascular amniotic membrane from the finely vascularized YS (Fig. 1, panels A–C). When cultured on a plastic substrate with standard culture medium, rAECs promptly adhered to the plate and formed a monolayer of cells with homogeneous morphology (Fig. 1, panel D; panel G shows hAECs for comparison). In order to evaluate the ability of both rAECs and hAECs to respond to angiogenic signals, a well-established in vitro assay for endothelial cell tube formation was used13. After 24 h in culture on Geltrex substrate with a proangiogenic medium, both rat and human cells arranged into a capillary-like network of cells (Fig. 1, panels E, F, H, and I). However, rAECs showed a lower capacity to form tubes, which were mostly 1-cell thick (Fig. 1, panels E and F) as compared to hAECs, where thickness ranged from 1 to several cells (Fig. 1, panels H and I). Moreover, the analysis of the ultrastructure of hAEC-derived tubes revealed the presence of a rudimentary lumen (Fig. 2). The expression of endothelial cell marker CD31/platelet endothelial cell adhesion molecule (PECAM-1) was also evaluated on AEC-derived web structures. Both hAECs (Fig. 3, panels A–C) and rAECs (Fig. 3, panels D–F) expressed CD31 in the cytoplasm and at lower levels as compared to controls (HUVECs and rat small vein, Fig. 3, panels G–L), where the expression was mainly localized on the cell membrane. Control samples cultured on plastic did not express the endothelial cell marker (Fig. 3, panels M–P).
Fig. 2.
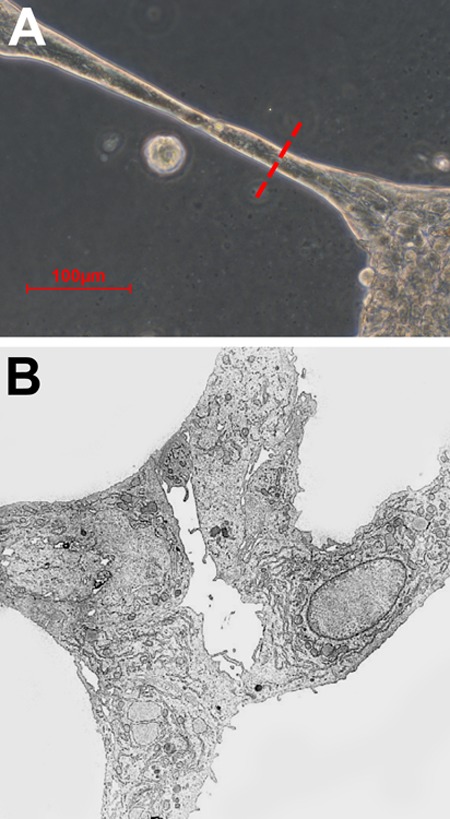
(A) Cross-sectional point for ultrastructural analysis of human-derived amniotic epithelial cell (hAEC)–derived tubular structures. (B) Electron microscopy image of hAEC-derived web with a rudimentary lumen.
Fig. 3.
Immunofluorescence analysis of CD31 expression. (A–C) Human-derived amniotic epithelial cell (hAEC)–derived capillary structures. (D–F) Rat-derived amniotic epithelial cell (rAEC)–derived capillary structures. (G–I) Human umbilical vein endothelial cell–derived capillary structures (human positive antibody control). (J–L) Rat small vein (rat positive antibody control). (M and N) hAECs cultured on plastic, 24 h postisolation. (O and P) rAECs cultured on plastic, 24 h postisolation. (Q and R) Human negative antibody control (no primary antibody). (S and T) Rat negative antibody control (no primary antibody). 100× Magnification. Insets show 200× magnification.
Donor-derived Clusters Have Morphology and Pattern of Expression of CD26 Typical of HSECs
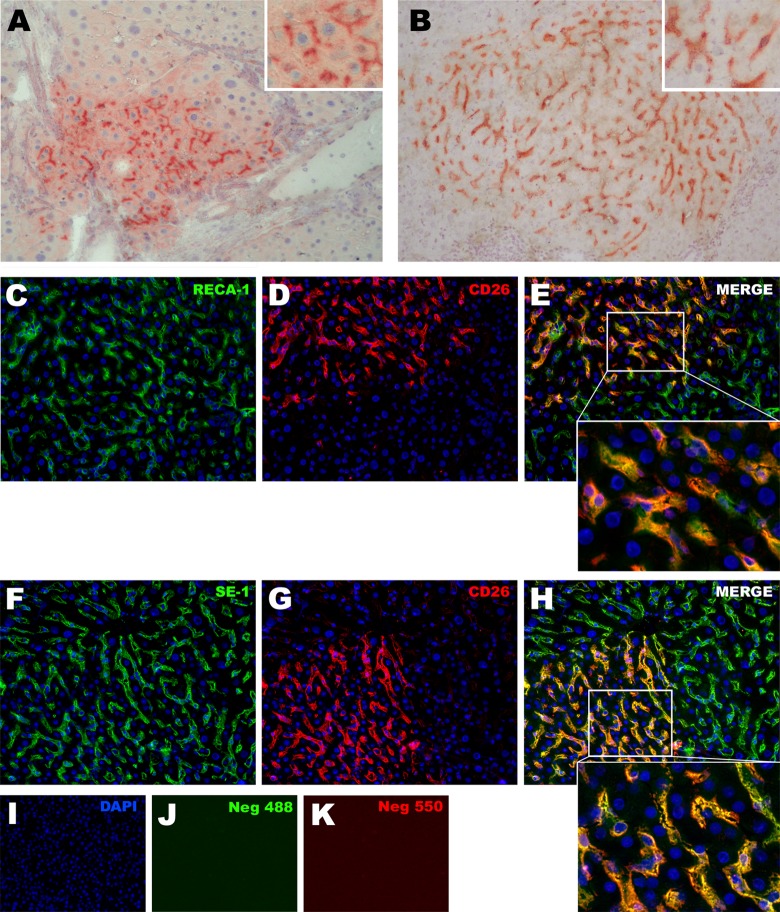
Upon transplantation of rAECs into a rat model for liver repopulation, clusters of donor-derived cells were identified by histochemical detection of CD26 into the liver of recipient animals. As shown in previous studies4,7 and in Fig. 4, panel A, rAECs were able to engraft and differentiate into hepatocytes upon transplantation into the liver of female rats previously treated with 2 doses of 60 mg/kg of RS. As expected, the expression pattern of CD26 in rAEC-derived hepatocytes was polarized and localized at the bile canaliculi between two hepatocytes15. However, when females rats were treated with 2 doses of 30 mg/kg of RS, rAEC-derived clusters consistently showed a different morphology, in which the cells were engrafted in between resident hepatocytes and resembled HSECs as CD26 expression was homogeneously distributed in the cytoplasm (Fig. 4 panel B).
Fig. 4.
(A and B) Histochemical detection of DPP-IV (CD26) positive donor-derived clusters into the liver of recipient animals. (A) Cluster of engrafted rAECs after differentiating into hepatocytes: the expression pattern of CD26 in rAEC-derived cells is polarized and localized at the bile canaliculi between 2 hepatocytes (see inset). (B) rAEC-derived clusters with morphology of hepatic sinusoidal endothelial cells: cells were engrafted in between resident hepatocytes and CD26 expression was homogeneously distributed in the cytoplasm (see inset). (C–H) Double immunofluorescence staining for CD26 and endothelial markers RECA-1 and SE-1 performed on serial liver tissue sections. In all the observed clusters, CD26 positive cells also expressed RECA-1 and SE-1. (C–E) RECA-1 + CD26 staining; (F–H) SE-1 + CD26 staining. 200× Magnification. Insets show 600× magnification.
rAEC-derived Cells Express Markers of HSECs
To confirm the endothelial nature of donor-derived cells, immunofluorescence staining of endothelial markers RECA-1 and SE-1 was performed on serial sections. In all the observed clusters that showed HSEC morphology, CD26 positive cells also expressed RECA-1 (Fig. 4, panels C–E) and SE-1 (Fig. 4, panels F–H).
rAEC-derived Cells Do Not Express Mature Hepatocyte Markers
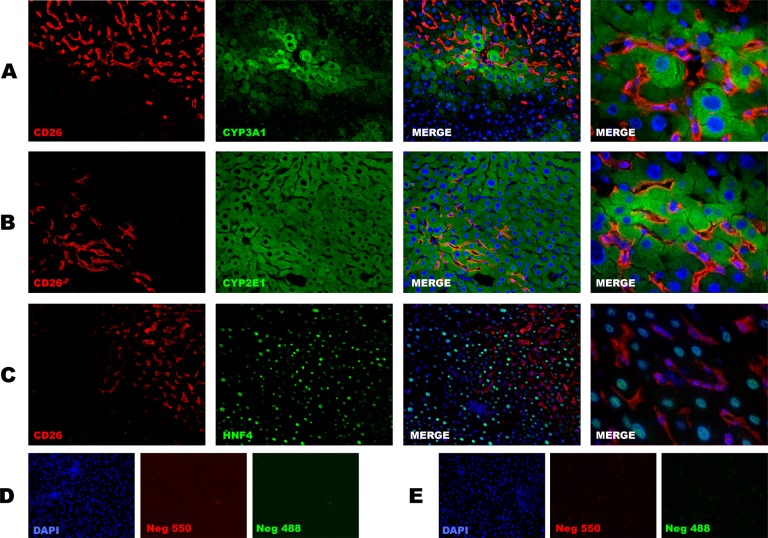
In order to rule out the possibility that donor-derived cells had an intermediate phenotype, immunofluorescence staining for mature hepatocyte markers CYP 3A1, 2E1, and hepatocyte nuclear factor 4α (HNF4α) was performed on serial sections. In all the observed clusters, no colocalization of CD26 with hepatocyte markers was identified, thus confirming the endothelial nature of donor-derived cells (Fig. 5).
Fig. 5.
Double immunofluorescence staining for CD26 and mature hepatocyte markers. In all the observed clusters, no colocalization of CD26 with hepatocyte markers was identified. (A) Cytochrome P450 (CYP) 3A1 + CD26 staining, (B) CYP 2E1 + CD26 staining, and (C) hepatocyte nuclear factor 4α (HNF4α) + CD26 staining. 200× Magnification for all figures except for right column where a 600× field is shown. (D) Negative antimouse control (no primary antibody); (E) Negative antirat control (no primary antibody).
Discussion
In a recent study, Vácz et al. reported transient engraftment of hAECs into the carotid artery of experimental animals after balloon injury without immunosuppressive treatment, although no improvement in vascular function was observed16.
In the present study, we explored the possibility that epithelial cells isolated from the amniotic membrane differentiate into endothelial cells both in vitro and in an animal model of liver repopulation. For the purposes of this study, and considering that placenta is a highly vascularized tissue, it was important to exclude the possible presence of endothelial cells in the rAEC preparation. In mice and rats, the amnion is flanked by the visceral YS17. While the amniotic membrane is thin and avascular, YS is thicker and finely vascularized (Fig. 1, panels A–C). In order to avoid possible endothelial cell contamination, at the time of amnion dissection, YS was carefully removed.
In a first set of experiments, we evaluated the ability of rAECs and hAECs to respond to proangiogenic signals utilizing a well-established protocol for in vitro endothelial cell tube formation13. This assay models the reorganization stage of angiogenesis and is commonly used with putative endothelial cells. When cultured under the appropriate experimental conditions, both rAECs and hAECs reorganized themselves into a network of cells within 24 h. Although rAECs formed fewer and thinner tubular structures as compared to hAECs, it is important to note that the stimuli administered in this assay are optimized for the culture of human rather than rat cells. This suggests that AECs from human and rat origin possess a similar ability to respond to angiogenic signals, although an optimized protocol might be needed for rAECs to respond to the same extent as hAECs. Interestingly, hAEC-derived capillary-like structures were mainly composed of >1 cells, and when analyzed at the ultrastructural levels, they revealed the presence of a rudimentary lumen. Both rAEC- and hAEC-derived tubular structures were positive for the expression of CD31/PECAM-1. However, the localization of this molecule, which is normally expressed at the membrane, was mainly cytoplasmic. This suggests that, although AECs possess the ability to respond to angiogenic signals, they do not express a fully mature endothelial phenotype in vitro.
To test the differentiation of rAECs into endothelial cells in vivo, the RS model of liver repopulation was used. RS is a naturally occurring molecule that is known for its ability to impose a persistent block in the cell cycle of the hepatocytes of treated animals, through the induction of a senescent phenotype18–20. This allows for the growth of transplanted healthy hepatocytes that have the ability to fully repopulate the liver by replacing the impaired resident cells. However, the effect of pyrrolizidine alkaloids (PAs), including RS, is also targeted to endothelial cells21. For example, monocrotaline, a related PA, is able to induce pulmonary hypertension in experimental animals because of its targeted effect on lung endothelial cells22,23.
In the present study, undifferentiated rAECs were isolated from F344 pregnant rats and transplanted into the liver of syngeneic female RS-treated animals. As previously described, when recipients were treated with 2 doses of 60 mg/kg of RSA, rAECs engrafted and formed discrete clusters of mature hepatocytes4,7. However, when the RS dose was reduced to 30 mg/kg, all donor-derived clusters showed a peculiar morphology and a pattern of expression of CD26 that resembled that of HSECs. This suggests that, at lower doses, RS has a more selective toxicity against the endothelium, although further studies are needed to characterize the effects of this molecule on the female rat. Most importantly, clusters of donor-derived cells were stably engrafted into the recipient liver up to 12 months post-transplant (or after transplant). Immunofluorescence analysis of CD26-positive clusters revealed that donor-derived cells expressed RECA-1, a marker specific for the vascular endothelium. Furthermore, they were positive for SE-1, an epitope specifically expressed by HSECs but not extrahepatic vascular endothelial cells. These findings confirmed that transplanted rAECs had differentiated into HSECs. In order to rule out the possibility that, upon transplantation, rAECs acquired an intermediate phenotype, tissue sections were stained with mature hepatocyte markers CYP 3A1, 2E1, and HNF 4α. No signs of colocalization of the above hepatocyte markers with CD26 antigen were discerned, further supporting the conclusion that rAECs fully differentiated into HSECs.
These results represent the first direct evidence that AECs respond to angiogenic signals in vitro and can stably engraft and differentiate into HSECs in vivo. Such findings add to our previous results supporting the conclusion that AECs possess great plasticity. Their ability to differentiate toward the endothelial lineage, particularly in an in vivo setting, makes them an attractive resource for regenerative medicine applications in the liver and possibly other organs.
Acknowledgments
We thank Dr. Franca Marongiu and Dr. Cristian Caria for their valuable scientific support. We also thank A. Saba and R. Marras for their excellent technical and secretarial assistance.
Footnotes
Author Contribution: Monica Serra and Michela Marongiu equally contributed to this work.
Ethical Approval: Animal studies were reviewed and approved by the University of Cagliari Ethical Committee for Animal Experimentation.
Statement of Human and Animal Rights: All animals received humane care according to the criteria outlined in the National Institutes of Health Publication 86–23, revised 1985.
Statement of Informed Consent: Human placentae were collected after obtaining informed written consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Sardinian Regional Government (RAS).
References
- 1. Strom SC, Skvorak K, Gramignoli R, Marongiu F, Miki T. Translation of amnion stem cells to the clinic. Stem Cells Dev. 2013;22(Suppl 1):96–102. [DOI] [PubMed] [Google Scholar]
- 2. Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23(10):1549–1559. [DOI] [PubMed] [Google Scholar]
- 3. Fanti M, Gramignoli R, Serra M, Cadoni E, Strom SC, Marongiu F. Differentiation of amniotic epithelial cells into various liver cell types and potential therapeutic applications. Placenta. 2017. March 30 pii: S0143-4004(17)30220-5. doi: 10.1016/j.placenta.2017.03.020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4. Marongiu F, Gramignoli R, Dorko K, Miki T, Ranade AR, Paola Serra M, Doratiotto S, Sini M, Sharma S, Mitamura K, et al. Hepatic differentiation of amniotic epithelial cells. Hepatology. 2011;53(5):1719–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skvorak KJ, Dorko K, Marongiu F, Tahan V, Hansel MC, Gramignoli R, Arning E, Bottiglieri T, Gibson KM, Strom SC. Improved amino acid, bioenergetic metabolite and neurotransmitter profiles following human amnion epithelial cell transplant in intermediate maple syrup urine disease mice. Mol Genet Metab. 2013;109(2):132–138. [DOI] [PubMed] [Google Scholar]
- 6. Skvorak KJ, Dorko K, Marongiu F, Tahan V, Hansel MC, Gramignoli R, Gibson KM, Strom SC. Placental stem cell correction of murine intermediate maple syrup urine disease. Hepatology. 2013;57(3):1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marongiu M, Serra MP, Contini A, Sini M, Strom SC, Laconi E, Marongiu F. Rat-derived amniotic epithelial cells differentiate into mature hepatocytes in vivo with no evidence of cell fusion. Stem Cells Dev. 2015;24(12):1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol. 2016;65(3):608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3(1):485–513. [DOI] [PubMed] [Google Scholar]
- 10. Nakajima T, Enosawa S, Mitani T, Li XK, Suzuki S, Amemiya H, Koiwai O, Sakuragawa N. Cytological examination of rat amniotic epithelial cells and cell transplantation to the liver. Cell Transplant. 2001;10(4-5):423–427. [PubMed] [Google Scholar]
- 11. Miki T, Marongiu F, Dorko K, Ellis EC, Strom SC: Isolation of amniotic epithelial stem cells Curr Protoc Stem Cell Biol. 2010;Chapter 1: Unit 1E.3. [DOI] [PubMed] [Google Scholar]
- 12. Baudin B, Bruneel A, Bosselut N, Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007;2(3):481–485. [DOI] [PubMed] [Google Scholar]
- 13. Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vivo. Angiogenesis. 2001;4(2):113–121. [DOI] [PubMed] [Google Scholar]
- 14. Thompson NL, Hixson DC, Callanan H, Panzica M, Flanagan D, Faris RA, Hong WJ, Hartel-Schenk S, Doyle D. A Fischer rat substrain deficient in dipeptidyl peptidase IV activity makes normal steady-state RNA levels and an altered protein. Use as a liver-cell transplantation model. Biochem J. 1991;273(Pt 3):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stecca BA, Nardo B, Chieco P, Mazziotti A, Bolondi L, Cavallari A. Aberrant dipeptidyl peptidase IV (DPP IV/CD26) expression in human hepatocellular carcinoma. J Hepatol. 1997;27(2):337–345. [DOI] [PubMed] [Google Scholar]
- 16. Vácz G, Cselenyák A, Cserép Z, Benkő R, Kovács E, Pankotai E, Lindenmair A, Wolbank S, Schwarz CM, Horváthy DB, et al. Effects of amniotic epithelial cell transplantation in endothelial injury. Interv Med Appl Sci. 2016;8(4):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dobreva MP, Pereira PNG, Deprest J, Zwijsen A. On the origin of amniotic stem cells: of mice and men. Int J Dev Biol. 2010;54(5):761–777. [DOI] [PubMed] [Google Scholar]
- 18. Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153(1):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E. Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol. 2001;158(2):771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serra MP, Marongiu F, Sini M, Laconi E. Hepatocyte senescence in vivo following preconditioning for liver repopulation. Hepatology. 2012;56(2):760–768. [DOI] [PubMed] [Google Scholar]
- 21. Edgar JA, Molyneux RJ, Colegate SM. Pyrrolizidine alkaloids: potential role in the etiology of cancers, pulmonary hypertension, congenital anomalies, and liver disease. Chem Res Toxicol. 2015;28(1):4–20. [DOI] [PubMed] [Google Scholar]
- 22. Nogueira-Ferreira R, Vitorino R, Ferreira R, Henriques-Coelho T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: A network approach. Pulm Pharmacol Ther. 2015;35:8–16. [DOI] [PubMed] [Google Scholar]
- 23. Wilson DW, Segall HJ, Pan LC, Lame MW, Estep JE, Morin D. Mechanisms and pathology of monocrotaline pulmonary toxicity. Crit Rev Toxicol. 1992;22(5–6):307–325. [DOI] [PubMed] [Google Scholar]