Abstract
Hemophilia A (HA) is an X-linked recessive disorder caused by mutations in the factor VIII (FVIII) gene leading to deficient blood coagulation. The current standard of care is frequent infusions of plasma-derived FVIII or recombinant B-domain-deleted FVIII (BDD-FVIII). While this treatment is effective, many patients eventually develop FVIII inhibitors that limit the effectiveness of the infused FVIII. As a monogenic disorder, HA is an ideal target for gene or cell-based therapy. Several studies have investigated allogeneic stem cell therapy targeting in utero or postnatal treatment of HA but have not been successful in completely correcting HA. Autologous in utero transplantation of mesenchymal stem cells is promising for treatment of HA due to the naive immune status of the fetal environment as well as its potential to prevent transplant rejection and long-term FVIII inhibitor formation. HA can be diagnosed by chorionic villus sampling performed during the first trimester (10 to 13 wk) of gestation. In this study, we used an established protocol and isolated placenta-derived mesenchymal stromal cells (PMSCs) from first trimester chorionic villus tissue and transduced them with lentiviral vector encoding the BDD-FVIII gene. We show that gene-modified PMSCs maintain their immunophenotype and multipotency, express, and secrete high levels of active FVIII. PMSCs were then transplanted at embryonic day 14.5 (E14.5) into wild-type fetuses from time-mated pregnant mice. Four days after birth, pups were checked for engraftment, and varying levels of expression of human green fluorescent protein were found in the organs tested. This study shows feasibility of the approach to obtain PMSCs from first trimester chorionic villus tissue, genetically modify them with the FVIII gene, and transplant them in utero for cell-mediated gene therapy of HA. Future studies will involve evaluation of long-term engraftment, phenotypic correction in HA mice, and prevention of FVIII inhibitor development by this approach.
Keywords: hemophilia A, factor VIII, placenta-derived mesenchymal stromal cells (PMSCs), chorionic villus sampling, in utero transplantation (IUT)
Introduction
Hemophilia A (HA) is an X-linked recessive bleeding disorder caused by mutations in the gene encoding factor VIII (FVIII), a protein essential for blood clotting. HA occurs in ∼1 in 5,000 male births1. Patients with severe HA, defined as FVIII activity less than 1%, suffer from debilitating hemarthroses (bleeds in the joints), life-threatening internal bleeding, and potentially fatal intracranial hemorrhages2. The current standard of care for HA is FVIII protein substitution therapy (PST), which offers reliable prophylactic and therapeutic relief from bleeding episodes and decreases the incidence of childhood hemophilic arthropathy3. However, PST is not a cure, is prohibitively expensive, and is unavailable to ∼75% of the world’s HA patients. In addition, ∼30% of HA patients undergoing PST develop inhibitory antibodies to FVIII, increasing morbidity and mortality and drastically increasing the cost of treatment4–6. Novel therapies to achieve sustained FVIII expression in HA patients are needed to overcome the serious limitations of current treatments.
As a monogenic disorder with a broad therapeutic window, HA is considered a highly attractive target for gene therapy7, and even a small increase in FVIII levels substantially decreases complications. A recent landmark clinical trial in hemophilia B (in which clotting factor IX [FIX] is deficient) showed that gene therapy achieved FIX expression in two-thirds of the patients8. However, such success has not been achieved for HA because of the distinct molecular complexity of FVIII9,10. Moreover, issues surrounding the durability of gene expression by gene therapy and host immune responses against gene transfer vectors remain11.
Cell-based gene therapy has been actively investigated to treat HA because if engrafted successfully, the transplanted cells could provide sustained production of FVIII and may represent a curative treatment. So far, mesenchymal stromal cells (MSCs), endothelial cells, endothelial progenitor cells (EPCs), and other types of cells have been investigated for HA treatment, some of which have shown promising phenotypic correction in animal models12–23. Postnatal expression of FVIII could provoke an immune response that precludes long-term and sustained FVIII expression24. In contrast to the postnatal environment, the fetal environment contains numerous characteristics that may allow improvement in stem cell–based therapies. The fetal environment is beneficial to stem cell engraftment because it is naturally receptive to remodeling and regeneration of fetal tissues by stem cells and it is highly conducive to expansion of stem cell compartments12,25–28. Indeed, large-scale migration of stem cells occurs naturally only in fetal life, allowing the systemic distribution of the transplanted stem cells. If stem cells are transplanted in utero, the unique regenerative gestational environment may promote engraftment into developing tissues, supporting long-term survival and functionality of the transplanted cells29. The immunological naïveté of the early gestation fetus is thought to be predisposed to achieving tolerance to foreign antigens if presented before a critical window30 and may enable tolerance induction, preventing later inhibitory FVIII-antibody formation31. Additionally, the fetus is much smaller in size allowing the delivery of proportionately more cells than can be given postnatally27.
Chorionic villus sampling (CVS) performed during 10 to 13 wk of gestation allows genetic disorders such as HA to be diagnosed early in pregnancy. Cells obtained from this tissue could be a potential source of autologous therapy for HA by in utero transplantation (IUT) of FVIII gene-modified cells prior to the development of the immune regulatory system in the fetus. We have previously established a tissue explant culture protocol to isolate placenta-derived MSCs (PMSCs) from the chorionic villus of placenta tissue that possesses all the properties of MSCs32. Full-length FVIII is very complex and challenging to use for the purpose of cell transduction. B-domain-deleted FVIII (BDD-FVIII) is a shorter form of FVIII where the heavily glycosylated B domain is deleted and has been shown to be as functionally active as the full-length FVIII33. In this study, we test the feasibility of isolating PMSCs from first trimester chorionic villus tissue and successfully transducing them with the BDD-FVIII gene to express functional FVIII. We then evaluated the potential of the BDD-FVIII expressing PMSCs for IUT in wild-type mice.
Materials and Methods
Isolation and Expansion of PMSCs from Human Early Gestation Placenta
Discarded deidentified first trimester gestation placental tissue (11 to 12 wk) was collected at the University of California, Davis (UCD) Medical Center. The study was submitted to the UCD Institutional Review Board (IRB) and determined to be exempt from review.
PMSCs were isolated from dissected chorionic villus tissue by using our well-established explant culture method developed in our laboratory32,34. Isolated cells were cultured in complete culture media for PMSCs consisting of high-glucose Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS; Hyclone, Thermo Fisher Scientific, Logan, UT, USA) and 100 U/mL penicillin and 100 µg/mL streptomycin. Passages 4 to 7 were used in all our studies presented here.
Lentiviral Vector Transduction of PMSCs
All lentiviral constructs were synthesized at the UCD Institute for Regenerative Cures (IRC) vector core. For creating BDD-FVIII-expressing lentiviral vector, plasmid containing BDD-FVIII complementary DNA (cDNA) was purchased from Addgene (plasmid #46775), and cDNA was inserted into the lentiviral vector pCCLc (Takara Bio USA Inc, Mountain View, CA, USA) that confers neomycin resistance: pCCLc-MNDU3-BDD-FVIII-PGK-NEO-WPRE. The control vector did not have the BDD-FVIII sequence: pCCLc-MNDU3-PGK-NEO-WPRE. Luciferase (LUC) and enhanced green fluorescent protein (EGFP) containing lentiviral vector (pCCLc-MNDU3-LUC-PGK-EGFP-WPRE) was created for tracking analysis. For transduction of lentiviral vectors, 1 × 106 cells were seeded in T150 flasks and allowed to adhere overnight. Cells were double transduced with either the BDD-FVIII vector or the control vector and the LUC/GFP vector in transduction media consisting of high-glucose DMEM, 10% FBS, and 8 μg/mL protamine sulfate (MP Biomedicals, LLC, USA). All vectors were transduced at a multiplicity of infection of 10 for 6 h. Cells were then washed twice with 1× phosphate-buffered saline (PBS) and cultured in complete media for 72 h. After 72 h, media containing 200 µg/mL of G418 (EMD, Billerica, MA, USA) was added to the cells and screened for neomycin resistance for 7 d. This screening will eliminate cells that were transduced with LUC/GFP vector only and retain the cells that had either FVIII gene only or both FVIII and LUC/GFP genes. Cells were then cultured and expanded in complete medium.
PMSC Characterization by Flow Cytometry and Trilineage Differentiation
Cells were analyzed by flow cytometry as previously described32,34. They were stained with FITC-CD44 (560977), PE-CD44 (51-9007656), PE-CD73 (561014), APC-CD45 (560973), PE-CD31 (560983), APC-CD29 (561794), PE-CD90 (561970), PE-CD34 (550761), APC-CD105 (562408), AF647-HLA-DR (563591), and FITC-HLA-DR (555560), all from BD Biosciences, San Jose, CA, USA and APC-HLA-G (BioLegend #335909) or appropriate isotype controls (BioLegend #400221, B.D. #51-9007655, 556650, 550854, and 556655). BD™ anti-mouse Ig, κ CompBeads were used to generate compensation controls. Transduction efficiency was assessed by GFP flow cytometry analysis. Flow cytometry was performed using FACSCanto cytometer (BD Biosciences) for cell immunophenotyping and an Attune NxT cytometer (Thermo Fisher Scientific, Waltham, MA, USA) for GFP analysis. All data were further analyzed using FlowJo software (version 10) (FlowJo LLC, Ashland, OR, USA). Trilineage differentiation of PMSCs to osteogenic, adipogenic, and chondrogenic lineages before and after transduction was performed as described earlier32,34.
Western Blot Analysis
Total lysates were obtained by incubating cells in radioimmunoprecipitation assay buffer (RIPA) lysis buffer (Thermo Fisher Scientific) supplemented with 1% Protease Inhibitor Cocktail (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 4 °C. The lysates were loaded onto 4% to 12% Bis-Tris SDS-NuPAGE gels (Thermo Fisher Scientific), and the proteins were transferred to nitrocellulose membrane (BioRad, Hercules, CA, USA). The membrane was blocked for 1 h in 1% casein. The blocked membrane was incubated overnight with 1:5,000 dilution of primary antibody (Hematologic Technologies, Essex Junction, VT, USA) in 1% casein, then washed with Tris-buffered saline with 0.5% Tween20 and incubated with secondary antibody conjugated with horseradish peroxidase (Thermo Fisher Scientific). After further washing, the membrane was incubated with chemiluminescent substrate (WestDura; Thermo Fisher Scientific) and exposed to a ChemiDoc MP Imaging system (Bio-Rad).
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was obtained from cells using the RNeasyPlus Mini kit (Qiagen, Germantown, MD, USA), and cDNA synthesized from 1 µg of RNA using Superscript II Reverse transcriptase (Thermo Fisher Scientific) was used in a polymerase chain reaction (PCR) using the following primers FVIII: forward-5′ cagtcttgaaacgccatcaa 3′, reverse-5′ aatcccagagcctctccact 3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH): forward-5′ gccagcatcgccccacttga 3′; reverse-5′ cggtcgtagcggggtgaact 3′. Genes were amplified using AccuPower PCR premix (Bioneer, Alameda, CA, USA) at an annealing temperature of 60 °C for 35 cycles and products were analyzed using a 2% agarose gel.
Quantitative Polymerase Chain Reaction (qPCR)
Tissues were homogenized using a motorized tissue grinder (Thermo Fisher Scientific). Total RNA was obtained using the RNeasyPlus Mini kit (Qiagen) and cDNA was synthesized from 1 µg of RNA using Superscript II Reverse transcriptase (Thermo Fisher Scientific). For quantitative analysis, RT-PCR was performed on a MX3005P thermocycler (Stratagene, La Jolla, CA, USA) using SYBR green PCR master mix (Applied Biosystems, Foster City, CA, USA). The primers used were GAPDH: forward-5′ attcaacggcacagtcaagg 3′, reverse-5′ tggatgcagggatgatgttc 3′; EGFP primer: forward-5′ agtccgccctgagcaaaga 3′, reverse-5′ tccagcaggaccatgtgatc 3′. Genes were amplified for 40 cycles with an annealing temperature of 58 °C. delta threshold cycle (▵Ct) values of GFP expression were calculated using GAPDH as control.
Enzyme-Linked Immunosorbent Assay (ELISA)
Cells were seeded (5 × 105 cells/well of a 6-well culture dish) and cultured in 2 mL of complete media. After 72 h, conditioned media was collected and centrifuged at 1,877 × g for 10 min to remove cell debris and the cleared media was stored at −80 °C. FVIII protein present in undiluted conditioned media was quantified using FVIII ELISA kit (Affinity Biologicals, Ancaster, ON, Canada) per the manufacturer’s instructions. Calibrator Plasma (Affinity Biologicals) was used as the standard.
Chromogenic Assay
FVIII activity in undiluted conditioned media prepared as described above was assessed using a Coamatic FVIII kit (Chromogenix, West Chester, OH, USA) per the manufacturer’s instructions. Coagulation reference calibration plasma (Technoclone, Wien, Austria) was used as the standard.
Animals and Surgery
All animal procedures were approved by the UCD Institutional Animal Care and Use Committee. All facilities used during the study period were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Time-mated C57BL/6 “wild-type” mice were bred in-house at UCD. At E14.5, the mouse was placed under isoflurane anesthesia (5% induction, 2% maintenance) and was positioned supine on a heat pad and maintained at 37 °C, monitored by a rectal temperature probe. The abdomen was clipped of fur and prepped for surgery with alternating rounds of 70% alcohol and beta iodine. A 2-cm laparotomy was performed and the uterus was carefully exteriorized with cotton applicators. The uterus was examined for the presence of fetuses and the number of normal and nonviable fetuses was noted. One of the viable fetuses was gently restrained through the uterus at the cranial and caudal ends in order to immobilize the fetus, so that the fetal abdomen was exposed to the surgeon. Care was taken to avoid manipulation of the placenta. A glass pipette that had been pulled to a pore size of 70 μm was loaded with 5 μL of 2.5 × 105 PMSCs that were resuspended in PBS mixed with blue food coloring at a 1:10 ratio. Adding the blue food coloring did not affect the viability of the cells tested by trypan blue exclusion. With the aid of a 10× operating microscope, the glass pipette was inserted through the uterus and into the fetal intraperitoneal (IP) cavity, and the cell suspension was injected into the IP space. The blue food coloring allowed for visual confirmation that the cell suspension was injected correctly into the IP space and not into the adjacent organs (intestine and liver). Each subsequent viable fetus was injected in the same manner with the same PMSC dose. The uterus was then returned to its original location in the abdomen and the maternal laparotomy was closed in 2 layers with absorbable 4-0 vicryl suture. The mouse then received a subcutaneous injection of 1 mL PBS for fluid resuscitation and a 0.05 mg/kg dose of buprenorphine for pain regulation. The animal was allowed to recover from anesthesia and was given subsequent doses of buprenorphine every 12 h for the first 2 postoperative days.
The mouse was allowed to carry her pregnancy to term and gave birth to her pups via standard vaginal delivery at E20.5. At postnatal day 4, each pup received an IP injection of 10 mL/kg luciferin (Gold Biotechnology, Olivette, MO, USA) with a 30-gauge needle. At 10 min postinjection, the pups were euthanized by CO2 inhalation and the heart, lungs, liver, kidneys, and spleen were dissected free from the pups. The organs were then placed on a plastic petri dish and were imaged within 30 min of collection by an in vivo imaging system (Kodak, Rochester, NY, USA) for expression of LUC and GFP. Following imaging, organs were stored in RNA later (Qiagen) for PCR analysis. Mean intensities and area of tissues were calculated using Carestream Molecular Imaging software, version 5 (Woodbridge, CT, USA). Since heart tissue did not express any GFP, mean GFP intensity of heart tissue was considered as the background. The total GFP intensity of each tissue was calculated using the equation:
Statistics
Data were reported as mean ± SD (for flow cytometry analysis) and mean ± standard error of mean (SEM) for ELISA and chromogenic assays, respectively. Statistical analysis was performed by unpaired Student’s t test using PRISM 7 (GraphPad Software Inc., San Diego, CA, USA), and differences were considered significant if P < 0.05.
Results
Derivation of PMSCs and FVIII Transduction
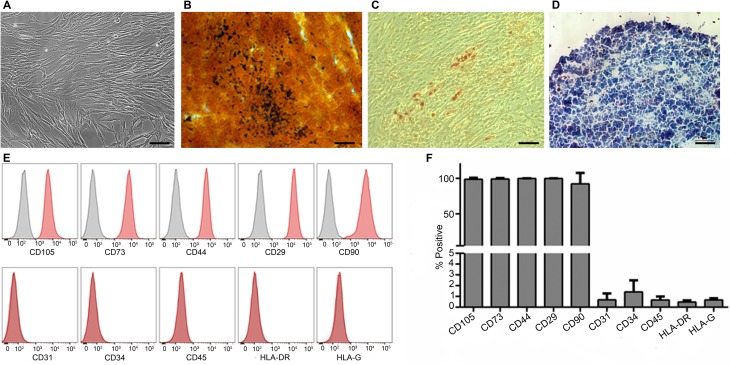
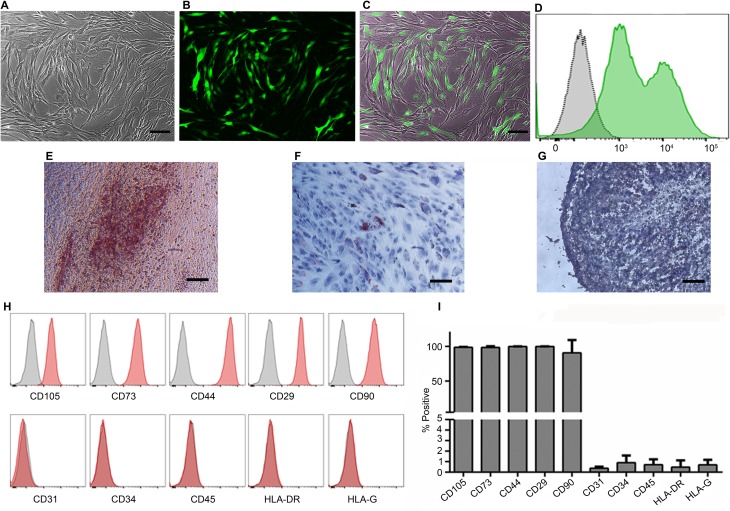
PMSCs were isolated from first trimester chorionic villus tissue using our previously established explant culture method32,34. The isolated cells showed the typical spindle-shaped morphology of MSCs and were capable of differentiation into osteogenic, adipogenic, and chondrogenic lineages (Fig. 1A to D). They also possessed the mesenchymal surface immunophenotype and were positive for surface markers CD105 (98.7% ± 2.2%), CD73 (99.15% ± 1.32%), CD44 (99.85% ± 0.17%), CD29 (99.95% ± 0.1%), and CD90 (92.25% ± 15.3%) and negative for CD31 (0.65% ± 0.6%), CD34 (1.42% ± 1.07%), CD45 (0.68% ± 0.32%), HLA-DR (0.48% ± 0.15%), and HLA-G (0.67% ± 0.16%; Fig. 1E and F). To derive FVIII-expressing PMSCs, we constructed a lentiviral vector pCCLc-MNDU3-BDD-FVIII-PGK-NEO-WPRE that encoded the BDD-FVIII. PMSCs were then transduced with this vector and screened for neomycin resistance to ensure that all cells expressed the FVIII protein. For tracking analysis after IUT, we transduced the cells with a lentiviral vector that expressed LUC and GFP with a transduction efficiency of 61.5% ± 21% (Fig. 2A to D). FVIII/LUC-GFP double transduced cells maintained their trilineage differentiation potential (Fig. 2E to G). They also retained their mesenchymal immunophenotype and expressed the positive surface markers CD105 (98.63% ± 0.73%), CD73 (98.43% ± 1.79%), CD44 (99.85% ± 0.3%), CD29 (99.95% ± 0.06%), and CD90 (90.63% ± 18.5%) and were negative for CD31 (0.38% ± 0.15%), CD34 (0.91% ± 0.68%), CD45 (0.72% ± 0.5%), HLA-DR (0.48% ± 0.64%), and HLA-G (0.7% ± 0.47%; Fig. 2H and I). Cells that were transduced with lentiviral vector without the BDD-FVIII served as control for all our in vitro analysis.
Figure 1.
Characterization of first trimester PMSCs. Representative phase contrast image showing first trimester PMSCs spindle-shaped morphology (A) and differentiation into osteogenic (B), adipogenic (C), and chondrogenic (D) lineages. Flow cytometric analysis of MSC immunophenotype for expression of surface markers (E) and quantitative analysis (F) are shown. n = 4 cell lines. 10× magnification, scale bar = 100 µm. PMSC, placenta-derived mesenchymal stromal cell; MSC, mesenchymal stromal cell.
Figure 2.
Characterization of PMSCs transduced with lentiviral vectors. Representative images of phase contrast (A), GFP (B), and overlay of phase contrast and GFP (C). GFP flow cytometry analysis of PMSCs transduced with GFP-LUC vector for in vivo cell tracking (D). Trilineage differentiation of transduced PMSCs into osteogenic (E), adipogenic (F), and chondrogenic lineages (G). Flow cytometric analysis of MSC immunophenotype for expression of surface markers after transduction with both BDD-FVIII and GFP-LUC vectors (H) and quantitative analysis (I) are shown. n = 4 transduced PMSC cell lines. 10× magnification, scale bar = 100 µm. PMSC, placenta-derived mesenchymal stromal cell; MSC, mesenchymal stromal cell; GFP, green fluorescent protein; LUC, luciferase; BDD-FVIII, B-domain-deleted factor VIII.
Analysis of BDD-FVIII Expression by PMSCs
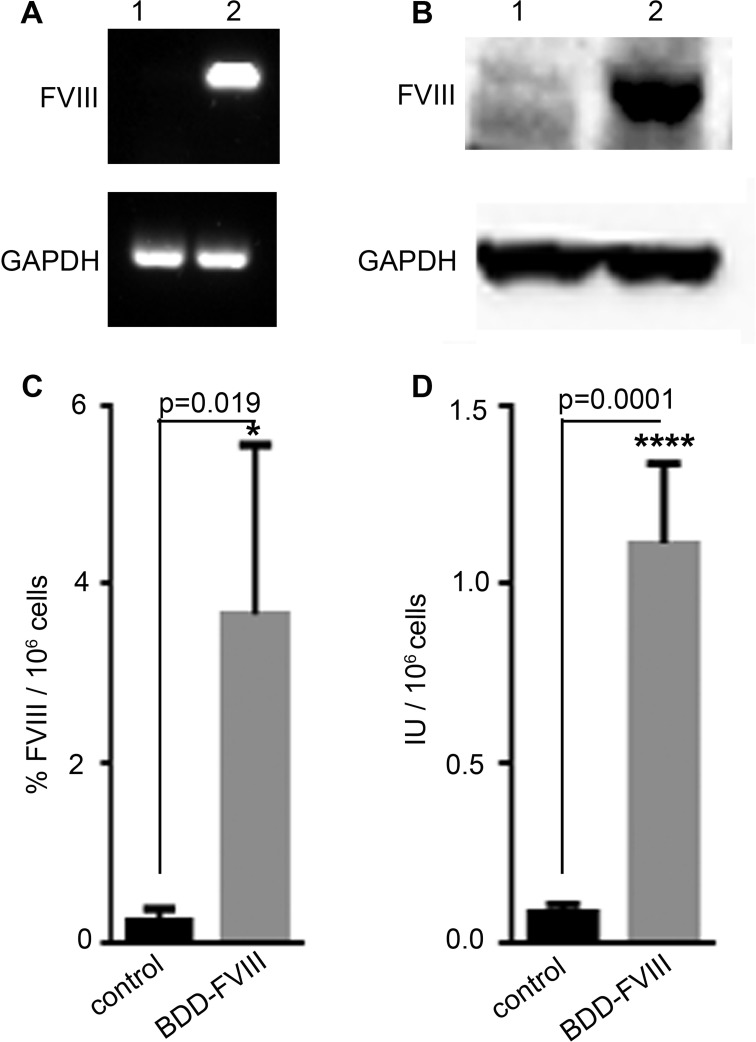
The expression of BDD-FVIII by transduced PMSCs was first analyzed by RT-PCR. FVIII was expressed only in PMSCs transduced with the BDD-FVIII vectors (Fig. 3A, lane 2). No expression was observed in PMSCs transduced with the control vectors (Fig. 3A, lane 1). Western blot analysis using a full-length FVIII antibody also showed high expression of a 160 kDa band only in the BDD-FVIII transduced cells (Fig. 3B, lane 2) and not in the control cells (Fig. 3B, lane 1). The protein size corresponds to the full-length BDD-FVIII that has not undergone posttranslational processing.
Figure 3.
Assessment of FVIII expression by transduced PMSCs. RT-PCR (A) and Western blot (B) analysis of expression of BDD-FVIII by transduced PMSCs (A: lane 2 and B: lane 2, respectively), compared to PMSCs transduced with control vector (A: lane 1 and B: lane 1, respectively). Comparison of human FVIII secretion by ELISA (C) and activity by chromogenic assay (D) between PMSCs transduced with BDD-FVIII vector and PMSCs transduced with control vector. n = 4 pairs of PMSC cell lines. PMSC, placenta-derived mesenchymal stromal cell; BDD-FVIII, B-domain-deleted factor VIII; FVIII, factor VIII; RT-PCR, reverse transcription PCR; ELISA, enzyme-linked immunosorbent assay.
To evaluate the secretion of BDD-FVIII by transduced PMSCs, human FVIII enzyme-linked immunosorbent assay (ELISA) was used to measure the secreted amount of FVIII into the medium using human calibrator plasma as reference standard. FVIII was secreted at significantly high levels (3.67% ± 0.94%/106 cells) by BDD-FVIII transduced cells compared to control cells (0.23% ± 0.08%/106 cells; P = 0.0109; n = 4; Fig. 3C). To assess whether the secreted FVIII protein was functionally active, a coagulation chromogenic assay was used to test their activity using human plasma as standard. FVIII activity was significantly higher in BDD-FVIII transduced cells (1.11 ± 0.11 International Units (IU)/106 cells) compared to control cells (0.08 ± 0.01 IU/106 cells; P = 0.0001; n = 4; Fig. 3D).
IUT of Transduced PMSCs
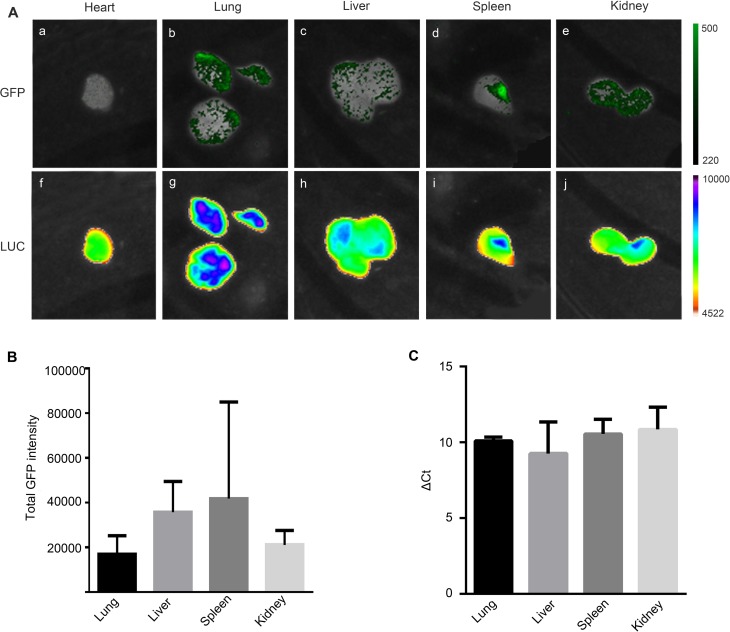
BDD-FVIII-transduced MSCs (2.5 × 105 cells) were transplanted in utero intraperitoneally into wild-type mice at 14 d GA. Four days after birth, the organs (heart, lungs, liver, spleen, and kidney) were dissected and subjected to GFP (Fig. 4A, a to e, respectively) and LUC imaging analysis (Fig. 4A, f to j). Varied levels of expression were observed in lungs, liver, spleen, and kidney. Heart tissue that showed no expression (Fig. 4A, a) was used as our background control for GFP quantitation (Fig. 4B). GFP expression in the tissues was also correlated by quantitative PCR analysis (Fig. 4C). Together the data confirmed that PMSCs persisted 10 d after IUT in the immunocompetent xenogeneic environment and engrafted in multiple organs in the transplanted animals.
Figure 4.
IUT of BDD-FVIII expressing PMSCs. Representative images of GFP (A, a to e) and luciferase (A, f to j) signaling in heart, lung, liver, spleen, and kidney tissues, respectively, in pups 4 d after birth which was 10 d after IUT of BDD-FVIII-transduced PMSCs and quantitation of GFP signaling as described in Methods (B). Quantitative real-time PCR analysis of EGFP expression in the tissues (C). n = 4 pups. PMSC, placenta-derived mesenchymal stromal cell; BDD-FVIII, B-domain-deleted factor VIII; FVIII, factor VIII; IUT, in utero transplantation; GFP, green fluorescent protein.
Discussion
HA caused by mutations in the FVIII gene that is involved in blood coagulation serves as a good candidate for cell-based therapy. One major advantage of cell-based therapy is the sustained production of the protein by transduced cells once they are engrafted. With respect to HA, even a sustained 1% to 2% of clotting activity of FVIII protein will alleviate many of the complications faced by HA patients35–37. MSCs can be obtained from several tissue sources and have numerous benefits for regenerative applications as a delivery vehicle. MSCs can be isolated and expanded in large numbers in vitro and can be transduced by FVIII-encoding lentiviral vectors. Due to the big size of the FVIII gene, it was technically challenging to package all 3 genes (FVIII, GFP, and LUC) in the same vector and achieve satisfactory transduction efficiency. Therefore, we designed the dual-transduction approach used in this study to facilitate both therapeutic function (FVIII production) and tracking (GFP and LUC expression).
The neomycin screening eliminated all non-FVIII producing cells, but the remaining FVIII-producing cells might not be uniformly coexpressing the GFP/LUC vector. However, this did not limit our ability to detect the signal of GFP/LUC after IUT as a sufficient percentage of the transplanted cells expressed both vectors as shown in Fig. 4. MSCs may play a role in immune modulation aiding in engraftment after transplantation, but tumorigenicity of MSCs after transduction with FVIII lentiviral vectors needs to be considered for future long-term studies.
To circumvent the issue of immune rejection of transplanted cells, an autologous stem cell source is required25. HA can be diagnosed in the first trimester of pregnancy by CVS, and hence, the excess tissue obtained by this procedure could serve as a source of autologous PMSCs29 for potential therapeutic treatment. We have already established that PMSCs can be derived from second trimester chorionic villus tissue32,34. In this study, we show that PMSCs could be obtained from first trimester chorionic villus that possesses all the characteristics of MSC phenotype. We were then able to transduce the PMSCs with a lentiviral vector that encodes the BDD-FVIII gene and demonstrated that these transduced PMSCs can express active FVIII protein. Full-length FVIII and BDD-FVIII have been reported to undergo posttranslational processing by furin cleavage into heavy and light chain products38. In our case, we did not see any posttranslational processing and instead we observed only the unprocessed 160 kDa protein. Nevertheless, based on our chromogenic assay, the activity of the secreted protein was maintained. Inhibition of furin has been shown to increase secretion and decrease intracellular retention of BDD-FVIII in mammalian cells and may be advantageous for HA therapy39.
One of the potential problems of any postnatal autologous cell-based therapy of HA is the humoral response resulting in the production of anti-FVIII antibodies leading to eventual decreased efficiency of FVIII activity and eventual relapse of the HA symptoms12. Hence, an in utero approach prior to immune induction by the fetus to FVIII is required for this therapy to be successful. In our study, we introduced the FVIII transduced PMSCs in utero in wild-type mice at E14.5 and have shown the persistence of these cells at least for 10 d after transplantation in the immunocompetent xenogeneic environment. We have also tested IUT at other GAs; however, the small size of the fetuses in mice makes IUT earlier than E14 technically challenging. Compared to humans where the first mature α/β T cells are seen at 10 to 12 wk of gestation, in mice it appears only in the final days of fetal gestation40. Hence, in this mouse model, we are probably performing our cell transplantation prior to appearance of T cells, which may allow for the observed immune tolerance of the introduced xenogeneic human PMSCs. We observed varied levels of engraftment of PMSCs in the tissues tested. Long-term engraftment of transplanted cells plays a key role in the success of any cell-based therapy, and for successful HA therapy, MSCs need to engraft in organs close to the vasculature to secrete the synthesized FVIII into circulation. To date several existing reports state that MSCs show poor engraftment efficiency in spite of their immunomodulatory characteristics, but those studies were conducted in adults where the immune regulatory system is completely different than the fetus41,42. Several studies utilize MSCs for IUT in both animal models and human patients with promising results27,43–47. Specifically for HA, because of the nature of the disease low engraftment of transplanted cells might be enough to alleviate many of the symptoms of HA35–37. Future studies will involve long-term engraftment of first trimester PMSCs and secretion of human FVIII after IUT and subsequent tests in an HA mouse model for phenotypic correction of HA and immune tolerance by assessment of anti-FVIII antibodies. Long-term safety studies will also be performed to evaluate the tumorigenicity of these FVIII-gene-modified transplanted cells. This study is a stepping-stone for utilizing CVS-derived early gestational PMSCs for cell-based therapy of HA.
Acknowledgments
We would like to acknowledge Josephine Tsang, Andrea Kulinich, and Ali Khavari for their help in the analysis of trilineage differentiation in nontransduced cells.
Footnotes
Ethical Approval: Discarded deidentified placental tissues were collected at the University of California, Davis (UCD) Medical Center. This study was submitted to the UCD Institutional Review Board (IRB) and it was determined that the study did not meet the criteria for human subjects research as defined by the DHHS and was thus exempt from review. All animal procedures were approved by the UCD Institutional Animal Care and Use Committee (IACUC #20073).
Statement of Human and Animal Rights: This article does not contain any studies with human subjects. All animal procedures were approved by the UCD IACUC. All facilities used during the study period were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by departmental startup funds to Dr. Aijun Wang provided by the Department of Surgery, UC Davis.
References
- 1. Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361(9371):1801–1809. [DOI] [PubMed] [Google Scholar]
- 2. Kulkarni R, Soucie JM, Lusher J, Presley R, Shapiro A, Gill J, Manco-Johnson M, Koerper M, Mathew P, Abshire T, et al. Sites of initial bleeding episodes, mode of delivery and age of diagnosis in babies with haemophilia diagnosed before the age of 2 years: a report from The Centers for Disease Control and Prevention’s (CDC) Universal Data Collection (UDC) project. Haemophilia. 2009;15(6):1281–1290. [DOI] [PubMed] [Google Scholar]
- 3. Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, Ingram JD, Manco- Johnson ML, Funk S, Jacobson L, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. [DOI] [PubMed] [Google Scholar]
- 4. Chalmers EA, Brown SA, Keeling D, Liesner R, Richards M, Stirling D, Thomas A, Vidler V, Williams MD, Young D, et al. Early factor VIII exposure and subsequent inhibitor development in children with severe haemophilia A. Haemophilia. 2007;13(2):149–155. [DOI] [PubMed] [Google Scholar]
- 5. Gouw SC, van der Bom JG, Ljung R, Escuriola C, Cid AR, Claeyssens-Donadel S, van Geet C, Kenet G, Makipernaa A, Molinari AC, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 2013;368(3):231–239. [DOI] [PubMed] [Google Scholar]
- 6. Calvez T, Chambost H, Claeyssens-Donadel S, d’Oiron R, Goulet V, Guillet B, Heritier V, Milien V, Rothschild C, Roussel-Robert V, et al. Recombinant factor VIII products and inhibitor development in previously untreated boys with severe hemophilia A. Blood. 2014;124(23):3398–3408. [DOI] [PubMed] [Google Scholar]
- 7. Roybal JL, Santore MT, Flake AW. Stem cell and genetic therapies for the fetus. Semin Fetal Neonatal Med. 2010;15(1):46–51. [DOI] [PubMed] [Google Scholar]
- 8. Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miao HZ, Sirachainan N, Palmer L, Kucab P, Cunningham MA, Kaufman RJ, Pipe SW. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103(9):3412–3419. [DOI] [PubMed] [Google Scholar]
- 10. Powell JS, Ragni MV, White GC, 2nd, Lusher JM, Hillman-Wiseman C, Moon TE, Cole V, Ramanathan-Girish S, Roehl H, Sajjadi N, et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood. 2003;102(6):2038–2045. [DOI] [PubMed] [Google Scholar]
- 11. Sokal EM, Lombard C, Mazza G. Mesenchymal stem cell treatment for hemophilia: a review of current knowledge. J Thromb Haemost. 2015;13(suppl 1): S161–S166. [DOI] [PubMed] [Google Scholar]
- 12. Porada CD, Sanada C, Kuo CJ, Colletti E, Mandeville W, Hasenau J, Zanjani ED, Moot R, Doering C, Spencer HT, et al. Phenotypic correction of hemophilia A in sheep by postnatal intraperitoneal transplantation of FVIII-expressing MSC. Exp Hematol. 2011;39(12):1124–1135 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Q, Gong X, Gong Z, Ren X, Ren Z, Huang S, Zeng Y. The mesenchymal stem cells derived from transgenic mice carrying human coagulation factor VIII can correct phenotype in hemophilia A mice. J Genet Genomics. 2013;40(12):617–628. [DOI] [PubMed] [Google Scholar]
- 14. Fomin ME, Zhou Y, Beyer AI, Publicover J, Baron JL, Muench MO. Production of factor VIII by human liver sinusoidal endothelial cells transplanted in immunodeficient uPA mice. PLoS One. 2013;8(10): e77255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin Y, Chang L, Solovey A, Healey JF, Lollar P, Hebbel RP. Use of blood outgrowth endothelial cells for gene therapy for hemophilia A. Blood. 2002;99(2):457–462. [DOI] [PubMed] [Google Scholar]
- 16. Kumaran V, Benten D, Follenzi A, Joseph B, Sarkar R, Gupta S. Transplantation of endothelial cells corrects the phenotype in hemophilia A mice. J Thromb Haemost. 2005;3(9):2022–2031. [DOI] [PubMed] [Google Scholar]
- 17. Follenzi A, Benten D, Novikoff P, Faulkner L, Raut S, Gupta S. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J Clin Invest. 2008;118(3):935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Q, Fahs SA, Kuether EL, Cooley BC, Weiler H, Montgomery RR. Targeting FVIII expression to endothelial cells regenerates a releasable pool of FVIII and restores hemostasis in a mouse model of hemophilia A. Blood. 2010;116(16):3049–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu L, Mah C, Fletcher BS. Sustained FVIII expression and phenotypic correction of hemophilia A in neonatal mice using an endothelial-targeted sleeping beauty transposon. Mol Ther. 2006;13(5):1006–1015. [DOI] [PubMed] [Google Scholar]
- 20. Matsui H, Shibata M, Brown B, Labelle A, Hegadorn C, Andrews C, Hebbel RP, Galipeau J, Hough C, Lillicrap D. Ex vivo gene therapy for hemophilia A that enhances safe delivery and sustained in vivo factor VIII expression from lentivirally engineered endothelial progenitors. Stem Cells. 2007;25(10):2660–2669. [DOI] [PubMed] [Google Scholar]
- 21. Matsui H. Endothelial progenitor cell-based therapy for hemophilia A. Int J Hematol. 2012;95(2):119–124. [DOI] [PubMed] [Google Scholar]
- 22. Xu D, Alipio Z, Fink LM, Adcock DM, Yang J, Ward DC, Ma Y. Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci USA. 2009;106(3):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park CY, Kim DH, Son JS, Sung JJ, Lee J, Bae S, Kim JH, Kim DW, Kim JS. Functional correction of large factor VIII gene chromosomal inversions in hemophilia a patient-derived iPSCs using CRISPR-Cas9. Cell Stem Cell. 2015;17(2):213–220. [DOI] [PubMed] [Google Scholar]
- 24. Wells KE, Maule J, Kingston R, Foster K, McMahon J, Damien E, Poole A, Wells DJ. Immune responses, not promoter inactivation, are responsible for decreased long-term expression following plasmid gene transfer into skeletal muscle. FEBS Lett. 1997;407(2):164–168. [DOI] [PubMed] [Google Scholar]
- 25. Flake AW. In utero stem cell transplantation. Best Pract Res Clin Obstet Gynaecol. 2004;18(6):941–958. [DOI] [PubMed] [Google Scholar]
- 26. Tiblad E, Westgren M. Fetal stem-cell transplantation. Best Pract Res Clin Obstet Gynaecol. 2008;22(1):189–201. [DOI] [PubMed] [Google Scholar]
- 27. Chan J, Waddington SN, O’Donoghue K, Kurata H, Guillot PV, Gotherstrom C, Themis M, Morgan JE, Fisk NM. Widespread distribution and muscle differentiation of human fetal mesenchymal stem cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells. 2007;25(4):875–884. [DOI] [PubMed] [Google Scholar]
- 28. Porada CD, Rodman C, Ignacio G, Atala A, Almeida-Porada G. Hemophilia A: an ideal disease to correct in utero. Front Pharmacol. 2014;5:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pixley JS, Zanjani ED. In utero transplantation: disparate ramifications. World J Stem Cells. 2013;5(2):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shields LE, Lindton B, Andrews RG, Westgren M. Fetal hematopoietic stem cell transplantation: a challenge for the twenty-first century. J Hematother Stem Cell Res. 2002;11(4):617–631. [DOI] [PubMed] [Google Scholar]
- 31. Nijagal A, Le T, Wegorzewska M, Mackenzie TC. A mouse model of in utero transplantation. J Vis Exp. 2011;(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lankford L, Selby T, Becker J, Ryzhuk V, Long C, Farmer D, Wang A. Early gestation chorionic villi-derived stromal cells for fetal tissue engineering. World J Stem Cells. 2015;7(1):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pittman DD, Alderman EM, Tomkinson KN, Wang JH, Giles AR, Kaufman RJ. Biochemical, immunological, and in vivo functional characterization of B-domain-deleted factor VIII. Blood. 1993;81(11):2925–2935. [PubMed] [Google Scholar]
- 34. Wang A, Brown EG, Lankford L, Keller BA, Pivetti CD, Sitkin NA, Beattie MS, Bresnahan JC, Farmer DL. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med. 2015;4(6):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. High KA. Gene transfer as an approach to treating hemophilia. Circ Res. 2001;88(2):137–144. [DOI] [PubMed] [Google Scholar]
- 36. Saenko EL, Ananyeva NM, Moayeri M, Ramezani A, Hawley RG. Development of improved factor VIII molecules and new gene transfer approaches for hemophilia A. Curr Gene Ther. 2003;3(1):27–41. [DOI] [PubMed] [Google Scholar]
- 37. Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232(1):25–32. [DOI] [PubMed] [Google Scholar]
- 38. Johnston JM, Denning G, Doering CB, Spencer HT. Generation of an optimized lentiviral vector encoding a high-expression factor VIII transgene for gene therapy of hemophilia A. Gene Ther. 2013;20(6):607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siner JI, Samelson-Jones BJ, Crudele JM, French RA, Lee BJ, Zhou S, Merricks E, Raymer R, Nichols TC, Camire RM, et al. Circumventing furin enhances factor VIII biological activity and ameliorates bleeding phenotypes in hemophilia models. JCI Insight. 2016;1(16): e89371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv Immunol. 2012;115:73–111. [DOI] [PubMed] [Google Scholar]
- 41. Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354(3):700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1): F31–F42. [DOI] [PubMed] [Google Scholar]
- 43. Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282–1286. [DOI] [PubMed] [Google Scholar]
- 44. Chen CP, Liu SH, Huang JP, Aplin JD, Wu YH, Chen PC, Hu CS, Ko CC, Lee MY, Chen CY. Engraftment potential of human placenta-derived mesenchymal stem cells after in utero transplantation in rats. Hum Reprod. 2009;24(1):154–65. [DOI] [PubMed] [Google Scholar]
- 45. Le Blanc K, Gotherstrom C, Ringden O, Hassan M, McMahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79(11):1607–1614. [DOI] [PubMed] [Google Scholar]
- 46. Chan JK, Gotherstrom C. Prenatal transplantation of mesenchymal stem cells to treat osteogenesis imperfecta. Front Pharmacol. 2014;5:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chou SH, Kuo TK, Liu M, Lee OK. In utero transplantation of human bone marrow-derived multipotent mesenchymal stem cells in mice. J Orthop Res. 2006;24(3):301–312. [DOI] [PubMed] [Google Scholar]