Abstract
Background
This National Cancer Database (NCDB) analysis evaluates the clinical outcomes of postoperative chemotherapy followed by concurrent chemoradiation (C + CRT) compared to concurrent chemoradiation (CRT) alone or adjuvant chemotherapy alone (C) for resected pancreatic cancer.
Methods
The NCDB was queried for primary stage I‐II, cT1‐3N0‐1M0, resected pancreatic adenocarcinoma treated with adjuvant C, CRT, or C + CRT (2004‐2015). Patients treated with C + CRT were compared with those treated with C (cohort C) and CRT (cohort CRT). Baseline patient, tumor, and treatment characteristics were examined. Kaplan‐Meier analysis, multivariable Cox proportional hazards method, forest plot, and propensity score matching were used.
Results
Among 5667 patients, median follow‐up was 34.7, 45.2, and 39.7 months for the C, CRT, and C + CRT cohorts, respectively. By multivariable analysis for all patients, C and CRT had worse OS compared to C + CRT. Treatment interactions were seen among pathologically node‐positive disease. C + CRT was favored in 1‐3 and 4+ positive lymph node diseases when compared to C or CRT alone, but none of the treatment options were significantly favored in node negative disease. Using propensity score matching, 2152 patients for cohort C and 1774 patients for cohort CRT were matched. C + CRT remained significant for improved OS for both cohort C (median OS 23.3 vs 20.0 months) and cohort CRT (median OS 23.4 vs 20.8 months).
Conclusion
This NCDB study using propensity score matched analysis suggests an OS benefit for C + CRT compared to C or CRT alone following surgical resection of pancreatic cancer, particularly for patients with pathologically positive lymph nodes.
Keywords: adjuvant chemoradiation, adjuvant chemotherapy, adjuvant radiation, adjuvant therapy, National Cancer Database, resectable pancreatic cancer
1. INTRODUCTION
Pancreatic adenocarcinoma, the fourth leading cause of cancer death in the United States, is a treatment challenge with a dismal median survival of 12.4 months.1 Surgical resection is considered the only potentially curative approach, though survival rates are modest, with a 5‐year overall survival (OS) of 7%‐17%.2, 3, 4 With local failure rates as high as 73% after surgery,2, 3, 4 various adjuvant therapies, including chemoradiation (CRT), have been investigated in clinical trials and institutional studies as a means to address the poor clinical outcomes in patients with pancreatic adenocarcinoma. Several reports have demonstrated improved OS with the use of adjuvant chemoradiation, with median OS times ranging from 19.5 to 25.2 months.5, 6, 7, 8, 9 Several National Cancer Database (NCDB) studies have similarly shown improved OS with adjuvant CRT.10, 11, 12
Literature for the role of chemotherapy (C) before CRT for resected pancreatic adenocarcinoma is limited. A phase III study of adjuvant fluorouracil vs gemcitabine, given for 3 weeks followed by CRT, and then an additional 3 months of C, found no difference in OS with either agent.13 A NCDB analysis, did however, report a survival benefit with chemotherapy prior to CRT for locally advanced pancreatic cancer.14 Due to a lack of comparative studies, the value of C prior to CRT specifically for early‐stage pancreatic cancer remains unclear.
This study compares the outcomes of patients who received C + CRT vs those who received C or CRT alone for stage I‐II, resected pancreatic cancer.
2. METHODS
2.1. Patient population
The NCDB registry was used to identify patients with pancreatic adenocarcinoma diagnosed between 2004 and 2015 (the most recent dataset available at the time of this study). The NCDB is a nationwide cancer database that captures approximately 70% of newly diagnosed cancer cases in the United States and includes 34 million historical records.15 It provides access to de‐identified datasets from Commission on Cancer‐accredited programs through online application. This study was exempt from institutional review board review.
Our patient selection criteria are shown in Figure 1. We selected from our initial query of patients with stage I‐II, clinical T1‐3N0‐1M0 pancreatic adenocarcinoma who had been treated with curative‐intent resection followed by adjuvant chemotherapy and conventionally fractionated radiation therapy. American Joint Committee on Cancer (AJCC) 6th and 7th editions were used to determine stage I‐II disease in 2004‐2015.
Figure 1.
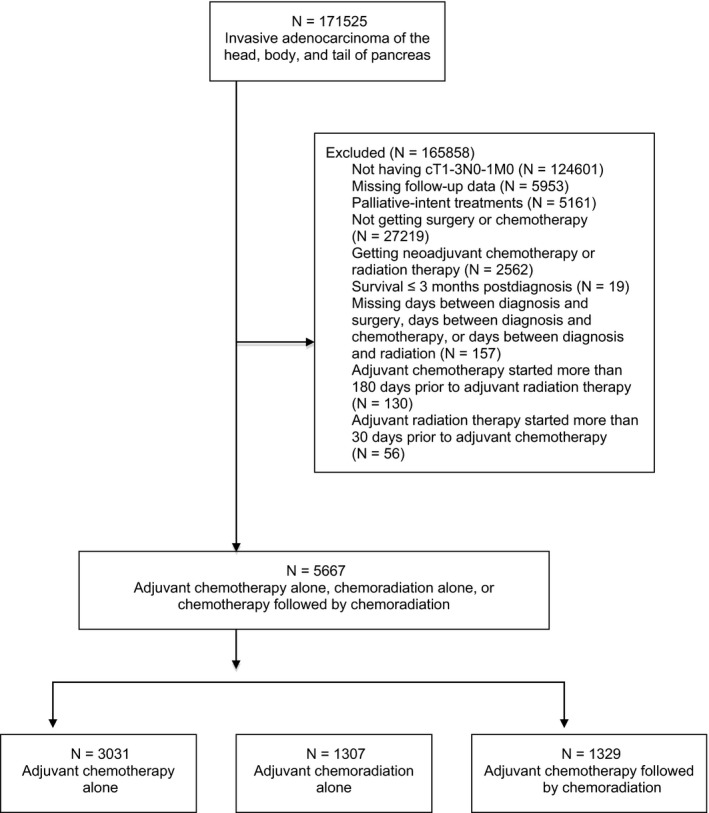
CONSORT diagram for patient selection criteria
Whipple surgery was defined as local or partial pancreatectomy and duodenectomy with partial gastrectomy. Whipple‐variant surgery was characterized as partial pancreatectomy with duodenectomy, total pancreatectomy alone, or total pancreatectomy with subtotal gastrectomy or duodenectomy.16 Patients treated with chemotherapy or radiation therapy within 30 days of each other were considered to have received adjuvant CRT alone. Those who were treated with adjuvant chemotherapy within 31‐180 days prior to the radiation therapy were defined as having received C + CRT.14 Patients who received adjuvant chemotherapy more than 180 days prior to adjuvant radiation therapy were excluded.
Patients were excluded if they had incomplete follow‐up data, missing radiation dose or fractionation information, incomplete data on the number of days between diagnosis and treatments, or missing information regarding surgical margins. Patients treated with palliative‐intent or with neoadjuvant chemotherapy or radiation were also excluded. To address immortal time bias, those with postdiagnosis survival duration of <3 months were not included.17
Baseline patient, tumor, and treatment characteristics for analysis included the following: facility type, age, gender, race, insurance type, income level, residential setting, Charlson‐Deyo comorbidity score (CDS), year of diagnosis, primary tumor location within pancreas, tumor grade, tumor size, clinical T and N stages, pathologic T and N stages, number of biopsy‐positive lymph nodes, surgery type, surgical margin, total radiation dose, and chemotherapy use. Surgical margin was categorized as either negative (R0) or positive (R1, R2, positive margin not otherwise specified). Patients were stratified by age ≥66 or <66 years, and tumor size <3.1 or ≥3.1 cm based on their median values. The household income level of each patient's residential area was based on the 2012 American Community Survey data adjusted for inflation (the most recent data at the time of this study) and was stratified above or below the median value of $48 000. CA 19‐9 factor was coded by the NCDB with a cut off of <98 or ≥98 Units/mL, although CA 19‐9 was not used for propensity score matching due to missing data in 3064 (54.1%) of patients. Local and distant failure/progression information is also unable to be analyzed based on data from the NCDB. Important prognostic variables such as the patient's initial performance status, type and duration of chemotherapy received, and toxicity outcomes are unavailable in the NCDB. The primary endpoint was overall survival (OS), time between the diagnosis and the last follow‐up or death.
2.2. Statistical analysis
OS was evaluated using the Kaplan‐Meier method and log‐rank tests. Fisher's exact test and Mann‐Whitney U test were used to compare categorical and continuous variables between two treatment cohorts, respectively. Logistic regression univariable (UVA) and multivariable analyses (MVA) were used to determine potential factors that predicted the use of postoperative chemotherapy and were reported as odds ratio (OR). Cox proportional hazard UVA and MVA were used to determine factors that predict the OS and were reported as hazards ratio (HR). MVA was initially constructed using all statistically significant variables from UVA and was finalized using a backward stepwise elimination. Only patients with complete information on such variables were included. Potential interactions between the treatment and other covariates were examined using Cox MVA by adding interaction terms.18 When the interaction terms were statistically significant, the final Cox MVA model was re‐analyzed for each subgroup of covariates, and a forest plot was constructed to illustrate the direction and magnitude of treatment effects.18
To minimize selection bias, propensity score matching was used. Match‐pairs were constructed by matching baseline patient, tumor, and treatment characteristics. Variables of interest include facility type, year of diagnosis, age, CDS, tumor grade, tumor size, surgery type, chemotherapy use, total radiation dose, pathologic T and N stages, and additional variables that were statistically significant in Cox proportional hazard MVA results for each cohort. All matching was performed in a 1:1 ratio without any replacement and was based on nearest neighbor method with a caliper distance of 0.2 of the standard deviation of the logit of the propensity score.19 Matching was performed using MatchIt package (version 3.0.1). R software (version 3.5.0, R Foundation for Statistical Computing, Vienna, Austria) was used for all aforementioned analyses. All P values were two‐sided. A P value <0.05 was considered statistically significant.
3. RESULTS
A total of 5667 patients with resected clinical stage I‐II, T1‐3N0‐1M0 pancreatic adenocarcinoma were identified for analysis. Of those, adjuvant C, CRT, and C + CRT were delivered to 3031, 1307, and 1329 patients, respectively. Overall follow‐up was 37.4 months (IQR [interquartile range] 24.5‐59.3). The majority of patients had pathologic T3N1 adenocarcinoma of the pancreatic head with negative surgical margins (Tables 1 and 2). Of the 2636 (1307 + 1329) patients who received RT, 2050 (1001 for CRT, 1049 for C + CRT) patients received RT to the pancreas, and 420 (221 for CRT, 199 for C + CRT) patients received RT to the abdomen (not otherwise specified); therefore, 93.7% (2470/2636) of patients received RT to the pancreas or abdomen. A total of 107 (107/1307 = 8.2%) and 106 (106/1329 = 8.0%) patients received <45 Gy in cohort CRT and C + CRT, respectively.
Table 1.
Baseline characteristics for cohort C
| Before matching | After matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | C + CRT | P | C | C + CRT | P | |||||
| N | % | N | % | N | % | N | % | |||
| Facility | ||||||||||
| Nonacademic | 1454 | 48 | 789 | 59 | <0.001 | 622 | 58 | 631 | 59 | 0.73 |
| Academic | 1557 | 51 | 521 | 39 | 454 | 42 | 445 | 41 | ||
| NA | 20 | 1 | 19 | 1 | 0 | 0 | 0 | 0 | ||
| Age | ||||||||||
| <66 | 1297 | 43 | 762 | 57 | <0.001 | 620 | 58 | 589 | 55 | 0.19 |
| ≥66 | 1734 | 57 | 567 | 43 | 456 | 42 | 487 | 45 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Gender | ||||||||||
| Female | 1497 | 49 | 663 | 50 | 0.77 | |||||
| Male | 1534 | 51 | 666 | 50 | ||||||
| NA | 0 | 0 | 0 | 0 | ||||||
| Race | ||||||||||
| White | 2645 | 87 | 1148 | 86 | 0.60 | |||||
| Black | 270 | 9 | 121 | 9 | ||||||
| Other | 95 | 3 | 49 | 4 | ||||||
| NA | 21 | 1 | 11 | 1 | ||||||
| Insurance | ||||||||||
| None | 70 | 2 | 34 | 3 | <0.001 | |||||
| Nonprivate | 1846 | 61 | 684 | 51 | ||||||
| Private | 1096 | 36 | 601 | 45 | ||||||
| NA | 19 | 1 | 10 | 1 | ||||||
| Income | ||||||||||
| Above median | 1947 | 64 | 870 | 65 | 0.37 | 704 | 65 | 707 | 66 | 0.93 |
| Below median | 1053 | 35 | 441 | 33 | 372 | 35 | 369 | 34 | ||
| NA | 31 | 1 | 18 | 1 | 0 | 0 | 0 | 0 | ||
| Residential setting | ||||||||||
| Metro | 2470 | 81 | 1086 | 82 | 0.84 | |||||
| Urban | 410 | 14 | 181 | 14 | ||||||
| Rural | 49 | 2 | 18 | 1 | ||||||
| NA | 102 | 3 | 44 | 3 | ||||||
| Charlson‐Deyo Score | ||||||||||
| 0‐1 | 2805 | 93 | 1248 | 94 | 0.11 | 1011 | 94 | 1008 | 94 | 0.86 |
| ≥2 | 226 | 7 | 81 | 6 | 65 | 6 | 68 | 6 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Year of diagnosis | ||||||||||
| 2004‐2007 | 195 | 6 | 74 | 6 | <0.001 | 62 | 6 | 46 | 4 | 0.13 |
| 2008‐2011 | 1376 | 45 | 696 | 52 | 506 | 47 | 543 | 50 | ||
| 2012‐2015 | 1460 | 48 | 559 | 42 | 508 | 47 | 487 | 45 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Primary tumor site | ||||||||||
| Head | 2391 | 79 | 1105 | 83 | <0.001 | |||||
| Body | 237 | 8 | 105 | 8 | ||||||
| Tail | 403 | 13 | 119 | 9 | ||||||
| NA | 0 | 0 | 0 | 0 | ||||||
| Tumor grade | ||||||||||
| Well diff | 202 | 7 | 112 | 8 | 0.024 | 78 | 7 | 91 | 8 | 0.083 |
| Mod diff | 1450 | 48 | 652 | 49 | 559 | 52 | 566 | 53 | ||
| Poor diff | 1126 | 37 | 448 | 34 | 431 | 40 | 400 | 37 | ||
| Other | 30 | 1 | 20 | 2 | 8 | 1 | 19 | 2 | ||
| NA | 223 | 7 | 97 | 7 | 0 | 0 | 0 | 0 | ||
| Tumor size (cm) | ||||||||||
| <3.1 | 1466 | 48 | 657 | 49 | 0.35 | 526 | 49 | 526 | 49 | 1 |
| ≥3.1 | 1511 | 50 | 636 | 48 | 550 | 51 | 550 | 51 | ||
| NA | 54 | 2 | 36 | 3 | 0 | 0 | 0 | 0 | ||
| Clinical T stage | ||||||||||
| 1 | 505 | 17 | 195 | 15 | 0.15 | |||||
| 2 | 1180 | 39 | 509 | 38 | ||||||
| 3 | 1346 | 44 | 625 | 47 | ||||||
| NA | 0 | 0 | 0 | 0 | ||||||
| Clinical N stage | ||||||||||
| 0 | 2122 | 70 | 871 | 66 | 0.0036 | |||||
| 1 | 909 | 30 | 458 | 34 | ||||||
| NA | 0 | 0 | 0 | 0 | ||||||
| Pathologic T stage | ||||||||||
| 0 | 1 | 0 | 1 | 0 | 0.013 | 0 | 0 | 1 | 0 | 0.81 |
| 1 | 158 | 5 | 45 | 3 | 41 | 4 | 37 | 3 | ||
| 2 | 404 | 13 | 153 | 12 | 110 | 10 | 123 | 11 | ||
| 3 | 2299 | 76 | 1056 | 79 | 908 | 84 | 898 | 83 | ||
| 4 | 38 | 1 | 22 | 2 | 17 | 2 | 17 | 2 | ||
| Other | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| NA | 130 | 4 | 52 | 4 | 0 | 0 | 0 | 0 | ||
| Pathologic N stage | ||||||||||
| 0 | 900 | 30 | 293 | 22 | <0.001 | 257 | 24 | 256 | 24 | 1 |
| 1 | 1988 | 66 | 971 | 73 | 819 | 76 | 820 | 76 | ||
| NA | 143 | 5 | 65 | 5 | 0 | 0 | 0 | 0 | ||
| Number of positive lymph nodes | ||||||||||
| 0 | 920 | 30 | 297 | 22 | <0.001 | |||||
| 1‐3 | 1173 | 39 | 585 | 44 | ||||||
| 4+ | 848 | 28 | 415 | 31 | ||||||
| NA | 90 | 3 | 32 | 2 | ||||||
| Surgery | ||||||||||
| Whipple variant | 941 | 31 | 397 | 30 | 0.33 | 364 | 34 | 325 | 30 | 0.079 |
| Whipple | 1409 | 46 | 650 | 49 | 477 | 44 | 528 | 49 | ||
| Other | 681 | 22 | 282 | 21 | 235 | 22 | 223 | 21 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Surgical margin | ||||||||||
| Negative | 2429 | 80 | 968 | 73 | <0.001 | 826 | 77 | 807 | 75 | 0.36 |
| Positive | 510 | 17 | 336 | 25 | 250 | 23 | 269 | 25 | ||
| NA | 92 | 3 | 25 | 2 | 0 | 0 | 0 | 0 | ||
| Chemotherapy | ||||||||||
| Single agent | 2376 | 78 | 766 | 58 | <0.001 | 650 | 60 | 634 | 59 | 0.51 |
| Multi agent | 655 | 22 | 563 | 42 | 426 | 40 | 442 | 41 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Radiation dose (Gy) | ||||||||||
| Median | — | 50.4 | NA | |||||||
| IQR | — | 50.0‐50.4 | ||||||||
C, chemotherapy; CRT, chemoradiation; diff, differentiated; IQR, interquartile range; mod, moderately; NA, not available; poor, poorly.
Table 2.
Baseline characteristics for cohort CRT
| Before matching | After matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CRT | C + CRT | P | CRT | C + CRT | P | |||||
| N | % | N | % | N | % | N | % | |||
| Facility | ||||||||||
| Nonacademic | 848 | 65 | 789 | 59 | 0.0085 | 548 | 62 | 539 | 61 | 0.70 |
| Academic | 452 | 35 | 521 | 39 | 339 | 38 | 348 | 39 | ||
| NA | 7 | 1 | 19 | 1 | 0 | 0 | 0 | 0 | ||
| Age | ||||||||||
| <66 | 674 | 52 | 762 | 57 | 0.0030 | 472 | 53 | 498 | 56 | 0.23 |
| ≥66 | 633 | 48 | 567 | 43 | 415 | 47 | 389 | 44 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Gender | ||||||||||
| Female | 645 | 49 | 663 | 50 | 0.79 | |||||
| Male | 662 | 51 | 666 | 50 | ||||||
| NA | 0 | 0 | 0 | 0 | ||||||
| Race | ||||||||||
| White | 1141 | 87 | 1148 | 86 | 0.19 | |||||
| Black | 119 | 9 | 121 | 9 | ||||||
| Other | 32 | 2 | 49 | 4 | ||||||
| NA | 15 | 1 | 11 | 1 | ||||||
| Insurance | ||||||||||
| None | 31 | 2 | 34 | 3 | 0.071 | |||||
| Nonprivate | 728 | 56 | 684 | 51 | ||||||
| Private | 533 | 41 | 601 | 45 | ||||||
| NA | 15 | 1 | 10 | 1 | ||||||
| Income | ||||||||||
| Above median | 733 | 56 | 870 | 65 | <0.001 | 547 | 62 | 563 | 63 | 0.46 |
| Below median | 544 | 42 | 441 | 33 | 340 | 38 | 324 | 37 | ||
| NA | 30 | 2 | 18 | 1 | 0 | 0 | 0 | 0 | ||
| Residential setting | ||||||||||
| Metro | 1023 | 78 | 1086 | 82 | 0.049 | |||||
| Urban | 201 | 15 | 181 | 14 | ||||||
| Rural | 31 | 2 | 18 | 1 | ||||||
| NA | 52 | 4 | 44 | 3 | ||||||
| Charlson‐Deyo Score | ||||||||||
| 0‐1 | 1235 | 94 | 1248 | 94 | 0.56 | 836 | 94 | 841 | 95 | 0.68 |
| ≥2 | 72 | 6 | 81 | 6 | 51 | 6 | 46 | 5 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Year of diagnosis | ||||||||||
| 2004‐2007 | 210 | 16 | 74 | 6 | <0.001 | 69 | 8 | 47 | 5 | 0.11 |
| 2008‐2011 | 680 | 52 | 696 | 52 | 458 | 52 | 467 | 53 | ||
| 2012‐2015 | 417 | 32 | 559 | 42 | 360 | 41 | 373 | 42 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Primary tumor site | ||||||||||
| Head | 1076 | 82 | 1105 | 83 | 0.015 | |||||
| Body | 78 | 6 | 105 | 8 | ||||||
| Tail | 153 | 12 | 119 | 9 | ||||||
| NA | 0 | 0 | 0 | 0 | ||||||
| Tumor grade | ||||||||||
| Well diff | 108 | 8 | 112 | 8 | 0.64 | 75 | 8 | 79 | 9 | 0.13 |
| Mod diff | 629 | 48 | 652 | 49 | 460 | 52 | 478 | 54 | ||
| Poor diff | 455 | 35 | 448 | 34 | 346 | 39 | 315 | 36 | ||
| Other | 13 | 1 | 20 | 2 | 6 | 1 | 15 | 2 | ||
| NA | 102 | 8 | 97 | 7 | 0 | 0 | 0 | 0 | ||
| Tumor size (cm) | ||||||||||
| <3.1 | 575 | 44 | 657 | 49 | 0.0050 | 410 | 46 | 410 | 46 | 1 |
| ≥3.1 | 697 | 53 | 636 | 48 | 477 | 54 | 477 | 54 | ||
| NA | 35 | 3 | 36 | 3 | 0 | 0 | 0 | 0 | ||
| Clinical T stage | ||||||||||
| 1 | 158 | 12 | 195 | 15 | 0.15 | |||||
| 2 | 517 | 40 | 509 | 38 | ||||||
| 3 | 632 | 48 | 625 | 47 | ||||||
| NA | 0 | 0 | 0 | 0 | ||||||
| Clinical N stage | ||||||||||
| 0 | 874 | 67 | 871 | 66 | 0.48 | |||||
| 1 | 433 | 33 | 458 | 34 | ||||||
| NA | 0 | 0 | 0 | 0 | ||||||
| Pathologic T stage | ||||||||||
| 0 | 1 | 0 | 1 | 0 | 0.61 | 1 | 0 | 1 | 0 | 0.95 |
| 1 | 49 | 4 | 45 | 3 | 30 | 3 | 36 | 4 | ||
| 2 | 169 | 13 | 153 | 12 | 108 | 12 | 105 | 12 | ||
| 3 | 980 | 75 | 1056 | 79 | 731 | 82 | 729 | 82 | ||
| 4 | 22 | 2 | 22 | 2 | 17 | 2 | 16 | 2 | ||
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| NA | 86 | 7 | 52 | 4 | 0 | 0 | 0 | 0 | ||
| Pathologic N stage | ||||||||||
| 0 | 338 | 26 | 293 | 22 | 0.0075 | 228 | 26 | 226 | 25 | 0.96 |
| 1 | 874 | 67 | 971 | 73 | 659 | 74 | 661 | 75 | ||
| NA | 95 | 7 | 65 | 5 | 0 | 0 | 0 | 0 | ||
| Number of positive lymph nodes | ||||||||||
| 0 | 363 | 28 | 297 | 22 | <0.001 | |||||
| 1‐3 | 585 | 45 | 585 | 44 | ||||||
| 4+ | 324 | 25 | 415 | 31 | ||||||
| NA | 35 | 3 | 32 | 2 | ||||||
| Surgery | ||||||||||
| Whipple variant | 342 | 26 | 397 | 30 | 0.10 | 253 | 29 | 256 | 29 | 0.98 |
| Whipple | 668 | 51 | 650 | 49 | 437 | 49 | 436 | 49 | ||
| Other | 297 | 23 | 282 | 21 | 197 | 22 | 195 | 22 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Surgical margin | ||||||||||
| Negative | 891 | 68 | 968 | 73 | 0.012 | 643 | 72 | 637 | 72 | 0.79 |
| Positive | 386 | 30 | 336 | 25 | 244 | 28 | 250 | 28 | ||
| NA | 30 | 2 | 25 | 2 | 0 | 0 | 0 | 0 | ||
| Chemotherapy | ||||||||||
| Single agent | 824 | 63 | 766 | 58 | 0.0047 | 536 | 60 | 515 | 58 | 0.33 |
| Multi agent | 483 | 37 | 563 | 42 | 351 | 40 | 372 | 42 | ||
| NA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Radiation dose (Gy) | ||||||||||
| Median | 50.4 | 50.4 | 0.18 | 50.4 | 50.4 | 0.17 | ||||
| IQR | 50.0‐54.0 | 50.0‐50.4 | 50.0‐54.0 | 50.0‐50.4 | ||||||
C, chemotherapy; CRT, chemoradiation; diff, differentiated; IQR, interquartile range; mod, moderately; NA, not available; poor, poorly.
On logistic MVA for all patients, patients with diagnosis between 2008 and 2011 (OR 2.17, P < 0.001) and 2012 and 2015 (OR 1.99, P < 0.001), pathologic nodal diseases (OR 1.37, P < 0.001 for 1‐3 positive nodes; OR 1.32, P = 0.0034 for 4+ positive nodes), positive surgical margin (OR 1.25, P = 0.0062), and receipt of multiagent chemotherapy (OR 2.01, P < 0.001) were more likely to receive C + CRT compared to C or CRT alone. Patients treated at academic facilities (OR 0.73, P < 0.001), older than 66 years old (OR 0.66, P < 0.001), from low‐income regions (OR 0.83, P = 0.0083), with pancreatic tail disease (OR 0.68, P = 0.0011), and poorly differentiated histology (OR 0.74, P = 0.019) were less likely to undergo C + CRT.
On Cox MVA for all patients (Table 3), those older than 66 years old (HR 1.14, P < 0.001), from low‐income regions (HR 1.10, P = 0.0082), with higher CDS (HR 1.23, P = 0.0017), moderately (HR 1.18, P = 0.018) or poorly differentiated (HR 1.51, P < 0.001) disease, tumors larger than 3.1 cm (HR 1.26, P < 0.001), pathologic positive nodal diseases (HR 1.46, P < 0.001 for 1‐3 positive nodes; HR 1.79, P < 0.001 for 4+ positive nodes), high CA 19‐9 (≥98 U/mL) (HR 1.30, P < 0.001), and positive surgical margins (HR 1.47, P < 0.001) were associated with worse mortality. When compared to C + CRT, those treated with C (HR 1.31, P < 0.001) or CRT alone (HR 1.24, P < 0.001) had worse survival outcomes. Improved overall survival was observed in those treated at academic facilities (HR 0.83, P < 0.001) and pathologic T1‐2 diseases (HR 0.87, P = 0.0051).
Table 3.
Cox UVA and MVA for all cohorts
| Variable | Cox UVA | Cox MVA | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Facility | ||||||
| Nonacademic | 1 | Ref | 1 | Ref | ||
| Academic | 0.88 | 0.82‐0.93 | <0.001 | 0.83 | 0.78‐0.89 | <0.001 |
| Age | ||||||
| <66 | 1 | Ref | 1 | Ref | ||
| ≥66 | 1.16 | 1.09‐1.23 | <0.001 | 1.14 | 1.06‐1.22 | <0.001 |
| Gender | ||||||
| Female | 1 | Ref | ||||
| Male | 1.01 | 0.95‐1.08 | 0.64 | |||
| Race | ||||||
| White | 1 | Ref | ||||
| Black | 0.98 | 0.88‐1.10 | 0.76 | |||
| Other | 0.95 | 0.79‐1.14 | 0.55 | |||
| Insurance | ||||||
| None | 1 | Ref | ||||
| Nonprivate | 1.15 | 0.94‐1.42 | 0.18 | |||
| Private | 0.95 | 0.77‐1.17 | 0.64 | |||
| Income | ||||||
| Above median | 1 | Ref | 1 | Ref | ||
| Below median | 1.14 | 1.07‐1.21 | <0.001 | 1.10 | 1.02‐1.18 | 0.0082 |
| Residential setting | ||||||
| Metro | 1 | Ref | 1 | Ref | ||
| Urban | 1.08 | 0.99‐1.18 | 0.086 | |||
| Rural | 1.37 | 1.10‐1.71 | 0.0052 | 1.20 | 0.94‐1.53 | 0.15 |
| Charlson‐Deyo score | ||||||
| 0‐1 | 1 | Ref | 1 | Ref | ||
| ≥2 | 1.2 | 1.06‐1.35 | 0.0033 | 1.23 | 1.08‐1.40 | 0.0017 |
| Year of diagnosis | ||||||
| 2004‐2007 | 1 | Ref | ||||
| 2008‐2011 | 0.97 | 0.88‐1.08 | 0.63 | |||
| 2012‐2015 | 0.90 | 0.80‐1.00 | 0.051 | |||
| Primary tumor site | ||||||
| Head | 1 | Ref | ||||
| Body | 0.92 | 0.81‐1.03 | 0.15 | |||
| Tail | 0.95 | 0.87‐1.05 | 0.33 | |||
| Tumor grade | ||||||
| Well diff | 1 | Ref | 1 | Ref | ||
| Mod diff | 1.22 | 1.07‐1.38 | 0.0022 | 1.18 | 1.03‐1.35 | 0.018 |
| Poor diff | 1.60 | 1.41‐1.82 | <0.001 | 1.51 | 1.32‐1.73 | <0.001 |
| Other | 1.19 | 0.86‐1.66 | 0.30 | |||
| Tumor size (cm) | ||||||
| <3.1 | 1 | Ref | 1 | Ref | ||
| ≥3.1 | 1.42 | 1.34‐1.52 | <0.001 | 1.26 | 1.18‐1.35 | <0.001 |
| Pathologic T stage | ||||||
| 0‐2 | 1 | Ref | 1 | Ref | ||
| 3‐4 | 0.72 | 0.66‐0.79 | <0.001 | 0.87 | 0.79‐0.96 | 0.0051 |
| Number of positive lymph nodes | ||||||
| 0 | 1 | Ref | 1 | Ref | ||
| 1‐3 | 1.61 | 1.48‐1.74 | <0.001 | 1.46 | 1.34‐1.59 | <0.001 |
| 4+ | 2.08 | 1.91‐2.27 | <0.001 | 1.79 | 1.63‐1.97 | <0.001 |
| Surgery | ||||||
| Whipple variant | 1 | Ref | ||||
| Whipple | 1.04 | 0.97‐1.12 | 0.30 | |||
| Other | 1.03 | 0.94‐1.12 | 0.51 | |||
| Surgical margin | ||||||
| Negative | 1 | Ref | 1 | Ref | ||
| Positive | 1.64 | 1.53‐1.76 | <0.001 | 1.47 | 1.36‐1.59 | <0.001 |
| Chemotherapy | ||||||
| Single agent | 1 | Ref | ||||
| Multi agent | 1.03 | 0.96‐1.10 | 0.47 | |||
| Radiation dose (Gy) | ||||||
| 1 Gy increase | 1.00 | 1.00‐1.00 | 1 | |||
| Treatment | ||||||
| C + CRT | 1 | Ref | 1 | Ref | ||
| CRT | 1.19 | 1.09‐1.30 | <0.001 | 1.24 | 1.12‐1.37 | <0.001 |
| C | 1.15 | 1.07‐1.24 | <0.001 | 1.31 | 1.20‐1.43 | <0.001 |
CI, confidence interval; diff, differentiated; HR, hazard ratio; mod, moderately; MVA, multivariable analysis; poor, poorly; Ref, reference; UVA, univariate analysis.
After Cox MVA, treatment interactions were observed in positive nodal disease subgroups (1‐3 positive nodes: HR 0.78, P = 0.020; 4+ positive nodes: HR 0.79, P = 0.041). No other treatment interactions were seen in age (HR 0.94, P = 0.46), CDS (HR 0.98, P = 0.88), years of diagnosis (2008‐2011: HR 1.05, P = 0.76; 2012‐2015: HR 1.05, P = 0.79), tumor size (HR 0.93, P = 0.40), surgical margin (HR 0.89, P = 0.23), or pathologic T stages (HR 1.10, P = 0.40). On subgroup analysis (Figure 2), nodal disease favored C + CRT when compared to C or CRT alone (0 positive node: HR 0.96, P = 0.67; 1‐3 positive nodes: HR 0.74, P < 0.001; 4+ positive nodes: HR 0.75, P < 0.001).
Figure 2.
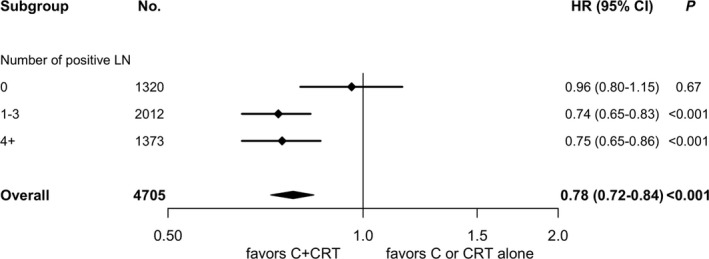
Forest plot for subgroup analysis. C, adjuvant chemotherapy; C + CRT, adjuvant chemotherapy followed by chemoradiation; CI, confidence interval; CRT, chemoradiation; HR, hazards ratio; LN, lymph node; No., number of patients
3.1. Cohort C
The C group had a median follow‐up of 34.7 months (IQR 22.9‐54.6), and the C + CRT group had that of 39.7 months (IQR 26.7‐59.5). The median OS was 21.1 months (IQR 12.0‐34.7) for the C group and 23.4 months (IQR 15.6‐39.3) for the C + CRT group (log‐rank P < 0.001). OS at 2 years was 48.8% for the C group and 53.1% for the C + CRT group.
A total of 2152 patients were matched. All variables were well balanced between these two groups (Table 1). The overall median follow‐up for the matched patients was 36.7 months (IQR 24.7‐54.5). The median OS was 20.0 months (IQR 11.5‐33.6) for the C group and 23.3 months (IQR 15.6‐39.2) for the C + CRT group (Figure 3; log‐rank P < 0.001). OS at 2 years was 45.2% for the C group and 52.3% for the C + CRT group.
Figure 3.
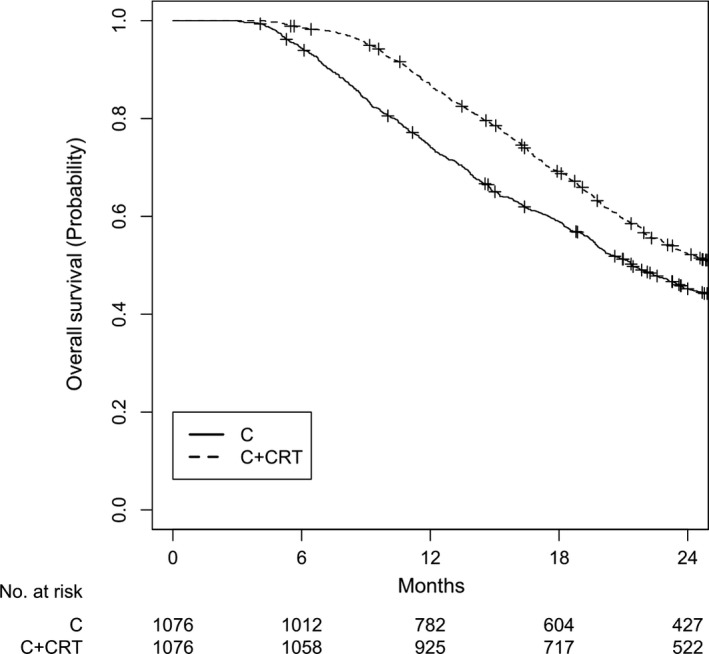
Overall survival for cohort C after matching. P < 0.001. C, adjuvant chemotherapy; C + CRT, adjuvant chemotherapy followed by chemoradiation
3.2. Cohort CRT
The CRT and C + CRT groups had a median follow‐up of 45.2 and 39.7 months, respectively. The median OS was 21.1 months (IQR 12.5‐36.0) for the CRT group and 23.4 months (15.6‐39.3) for the C + CRT group (log‐rank P < 0.001). OS at 2 years was 46.2% and 53.1% for the CRT and C + CRT groups, respectively.
A total of 1774 patients were matched. All variables were well balanced (Table 2). The overall follow‐up was 40.2 months (IQR 26.0‐58.3). The CRT group had a median OS of 20.8 months (IQR 12.5‐34.7) and the C + CRT group had that of 23.4 months (IQR 16.0‐40.0). OS at 2 years was 46.6% for the CRT group and 52.5% for the C + CRT group (Figure 4; log‐rank P < 0.001).
Figure 4.
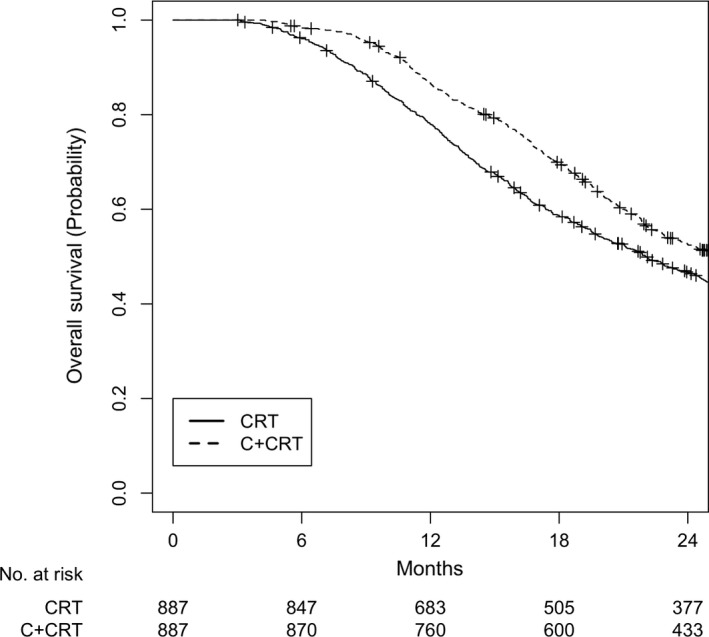
Overall survival for cohort CRT after matching. P < 0.001. CRT, adjuvant chemoradiation; C + CRT, adjuvant chemotherapy followed by chemoradiation
4. DISCUSSION
To our knowledge, this is the first study to compare adjuvant C + CRT vs C or CRT alone for stage I‐II resected pancreatic cancer. This study suggests a survival benefit favoring the use of C + CRT for resected pancreatic cancer, specifically in cases of pathologically node‐positive disease.
The C + CRT cohort included over 70% patients with pathologically staged T3 and N1 disease, which are notably associated with worse prognosis.20, 21, 22 Despite this, the C + CRT still had better OS compared to the CRT alone cohort. The median OS was 23 months, which is comparable to or better than previously reported survival outcomes for adjuvant CRT alone.5, 6, 7, 8, 9, 10
The use of adjuvant C in addition to CRT has only been investigated in a few studies.6, 23, 24, 25 RTOG 9704 delivered C before and after adjuvant CRT for resected pancreatic cancer. A large number of included patients had T3‐4N1 disease and positive surgical margins. Median OS was 17.1 months for fluorouracil and 20.5 months for gemcitabine.25 Likely due to the initial publication of RTOG 9704 in 2008, our logistic MVA results demonstrated that those diagnosed between 2008 and 2015 were more likely to receive C + CRT compared to those diagnosed between 2004 and 2007. A prior institutional study showed that delaying CRT until after >1 cycle of adjuvant C is not associated with worse mortality when compared to adjuvant CRT only.24 Our study is the first report showing that delaying CRT until after 30‐180 days of adjuvant C may have survival benefits.
Our Cox MVA results (Table 3) showed that moderately or poorly differentiated tumors, larger tumor size, and pathologic N1 disease were adverse prognostic factors for mortality. This association is consistent with prior studies.26, 27, 28, 29, 30, 31, 32, 33, 34, 35 Older age, more medical comorbidities, low income, and positive surgical margins were also shown to be associated with worse mortality in our study, and this finding is also consistent with other reports.36, 37, 38, 39 In this study, treatment at academic facilities was an independent favorable prognostic factor for OS. This finding is consistent with prior analyses showing improved outcomes at high volume centers related to better surgical outcomes, which may explain why living in a rural area was associated with worse mortality.40, 41, 42 Although additional factors contributing to improved OS at academic facility may include patient self‐selection, higher socioeconomic status and/or performance status.
From our logistic MVA results, patients with pathologic T3‐4N1 diseases and positive surgical margins were more likely to receive C + CRT. A prior study has also shown that patients with a higher disease burden were more likely to receive adjuvant therapies.43
In our study, the use of multiagent chemotherapy was not a favorable prognostic factor for survival. This finding is in contrast to theEuropean Study Group for Pancreatic Cancer‐4 (ESPAC‐4) trial. Despite including 61% of patients with positive surgical margins and 79% with N1 disease in the ESPAC‐4 trial, adjuvant gemcitabine combined with capecitabine significantly improved survival.28 In NCDB, multiagent chemotherapy was recorded as the first course, and it is possible that some chemotherapy regimens were changed during the course of treatments. This change in chemotherapy regimens is not recorded in NCDB, which may explain this discrepancy.
4.1. Limitations
This study has a number of limitations, many of which are inherent to performing a retrospective review. Various potential prognostic factors, such as smoking and alcohol history, performance status, molecular tests, and the type and duration of chemotherapy, are not recorded by the NCDB. Outcomes such as local or distant recurrences, toxicity, and cancer‐specific survival were also unavailable. More than half of the CA 19‐9 values, an important prognostic factor for resectability and survival, were missing from this dataset and could not be included for propensity score matched analysis.27, 44, 45, 46, 47 The NCDB also does not include information on disease progression; therefore, this study cannot address the possibility of a patient received RT for progression of disease on chemotherapy. This limitation is inherent to all NCDB analyses and limit interpretation of our findings. Further, RT may have been palliative‐intent for a minority of the included patients based on site and dose of RT, but since both CRT and C‐CRT had similar numbers of patients receiving <45 Gy, it is unlikely these patients would change the conclusions of this study. Since the NCDB is not a population‐based database, our findings may not be generalized to other patient populations.
Up to 79% of patients in RTOG 9704 experienced chemotherapy‐related toxicity. A meta‐analysis of adjuvant treatments for resected pancreatic adenocarcinoma also showed significant toxicity with the addition of chemotherapy to chemoradiation.48 It is possible that those patients who received C + CRT may have had a better initial performance status in order to tolerate the additional toxicity of chemotherapy, thus leading to better survival outcomes compared to those receiving C or CRT alone.13 This potential confounder may also explain the improved survival seen in other institutional studies.6, 23 However, it is unlikely that improved performance status was the only factor contributing to this overall survival benefit, since patients with node negative diseases would have also favored C + CRT in our study. In addition, no treatment interaction was seen with CDS or with age on Cox MVA in this study, nor were CDS or age predictors on logistic MVA for the receipt of C + CRT. Since all patients in our study underwent adjuvant therapies, the difference in performance status between the C + CRT and other regimens is unlikely to independently explain the survival benefits.
5. CONCLUSION
In summary, this analysis suggests improved survival for adjuvant C + CRT following resected pancreatic cancer with node‐positive disease. More studies may be warranted to investigate the benefit of adding adjuvant chemotherapy to CRT and the ideal sequencing of these regimens.
CONFLICT OF INTEREST
All authors declare that they have no competing interests.
Ma SJ, Hermann GM, Prezzano KM, Serra LM, Iovoli AJ, Singh AK. Adjuvant chemotherapy followed by concurrent chemoradiation is associated with improved survival for resected stage I‐II pancreatic cancer. Cancer Med. 2019;8:939–952. 10.1002/cam4.1967
REFERENCES
- 1. Simons JP, Ng SC, McDade TP, Zhou Z, Earle CC, Tseng JF. Progress for resectable pancreatic [corrected] cancer?: a population‐based assessment of US practices. Cancer. 2010;116(7):1681‐1690. [DOI] [PubMed] [Google Scholar]
- 2. Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37(3):1519‐1524. [DOI] [PubMed] [Google Scholar]
- 3. Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long‐term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221(1):59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66(1):56‐61. [DOI] [PubMed] [Google Scholar]
- 5. Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5‐fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230(6):776‐782; discussion 782‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975‐2005). J Clin Oncol. 2008;26(21):3511‐3516. [DOI] [PubMed] [Google Scholar]
- 7. Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil‐based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26(21):3503‐3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899‐903. [DOI] [PubMed] [Google Scholar]
- 9. Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single‐institution experience. Ann Surg. 1997;225(5):621‐633; discussion 633‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rutter CE, Park HS, Corso CD, et al. Addition of radiotherapy to adjuvant chemotherapy is associated with improved overall survival in resected pancreatic adenocarcinoma: An analysis of the National Cancer Data Base. Cancer. 2015;121(23):4141‐4149. [DOI] [PubMed] [Google Scholar]
- 11. Krishnan M, Ahmed A, Walters RW, Silberstein PT. Factors affecting adjuvant therapy in stage III pancreatic cancer‐analysis of the national cancer database. Clin Med Insights Oncol. 2017;11:1179554917728040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kooby DA, Gillespie TW, Liu Y, et al. Impact of adjuvant radiotherapy on survival after pancreatic cancer resection: an appraisal of data from the national cancer data base. Ann Surg Oncol. 2013;20(11):3634‐3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil‐based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019‐1026. [DOI] [PubMed] [Google Scholar]
- 14. Torgeson A, Lloyd S, Boothe D, et al. Multiagent induction chemotherapy followed by chemoradiation is associated with improved survival in locally advanced pancreatic cancer. Cancer. 2017;123(19):3816‐3824. [DOI] [PubMed] [Google Scholar]
- 15. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhong J, Patel K, Switchenko J, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123(18):3486‐3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park HS, Gross CP, Makarov DV, Yu JB. Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(5):1365‐1373. [DOI] [PubMed] [Google Scholar]
- 18. Barraclough H, Govindan R. Biostatistics primer: what a clinician ought to know: subgroup analyses. J Thorac Oncol. 2010;5(5):741‐746. [DOI] [PubMed] [Google Scholar]
- 19. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer R, Breidert M, Keck T, Makowiec F, Lohrmann C, Harder J. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol. 2012;18(2):118‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Massucco P, Ribero D, Sgotto E, Mellano A, Muratore A, Capussotti L. Prognostic significance of lymph node metastases in pancreatic head cancer treated with extended lymphadenectomy: not just a matter of numbers. Ann Surg Oncol. 2009;16(12):3323‐3332. [DOI] [PubMed] [Google Scholar]
- 22. Tarantino I, Warschkow R, Hackert T, et al. Staging of pancreatic cancer based on the number of positive lymph nodes. Br J Surg. 2017;104(5):608‐618. [DOI] [PubMed] [Google Scholar]
- 23. Wilkowski R, Thoma M, Duhmke E, Rau HG, Heinemann V. Concurrent chemoradiotherapy with gemcitabine and cisplatin after incomplete (R1) resection of locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2004;58(3):768‐772. [DOI] [PubMed] [Google Scholar]
- 24. Wo JY, Childs SK, Szymonifka J, et al. Delaying chemoradiation until after completion of adjuvant chemotherapy for pancreatic cancer may not impact local control. Pract Radiat Oncol. 2014;4(2):e117‐e123. [DOI] [PubMed] [Google Scholar]
- 25. Regine WF, Winter KA, Abrams R, et al. Fluorouracil‐based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5‐year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18(5):1319‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single‐institution experience. J Gastrointest Surg. 2006;10(9):1199‐1210; discussion 1210‐1191. [DOI] [PubMed] [Google Scholar]
- 27. Yovino S, Maidment BW 3rd, Herman JM, et al. Analysis of local control in patients receiving IMRT for resected pancreatic cancers. Int J Radiat Oncol Biol Phys. 2012;83(3):916‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC‐4): a multicentre, open‐label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011‐1024. [DOI] [PubMed] [Google Scholar]
- 29. Dusch N, Weiss C, Strobel P, Kienle P, Post S, Niedergethmann M. Factors predicting long‐term survival following pancreatic resection for ductal adenocarcinoma of the pancreas: 40 years of experience. J Gastrointest Surg. 2014;18(4):674‐681. [DOI] [PubMed] [Google Scholar]
- 30. Helm J, Centeno BA, Coppola D, et al. Histologic characteristics enhance predictive value of American Joint Committee on Cancer staging in resectable pancreas cancer. Cancer. 2009;115(18):4080‐4089. [DOI] [PubMed] [Google Scholar]
- 31. Wasif N, Ko CY, Farrell J, et al. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol. 2010;17(9):2312‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crippa S, Partelli S, Zamboni G, et al. Poorly differentiated resectable pancreatic cancer: is upfront resection worthwhile? Surgery. 2012;152(3 Suppl 1):S112‐119. [DOI] [PubMed] [Google Scholar]
- 33. Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32(1):37‐43. [DOI] [PubMed] [Google Scholar]
- 34. John BJ, Naik P, Ironside A, et al. Redefining the R1 resection for pancreatic ductal adenocarcinoma: tumour lymph nodal burden and lymph node ratio are the only prognostic factors associated with survival. HPB (Oxford). 2013;15(9):674‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kimbrough CW, St Hill CR, Martin RC, McMasters KM, Scoggins CR. Tumor‐positive resection margins reflect an aggressive tumor biology in pancreatic cancer. J Surg Oncol. 2013;107(6):602‐607. [DOI] [PubMed] [Google Scholar]
- 36. Canyilmaz E, Serdar L, Uslu GH, Soydemir G, Bahat Z, Yoney A. Evaluation of prognostic factors and survival results in pancreatic carcinomas in Turkey. Asian Pac J Cancer Prev. 2014;14(11):6573‐6578. [DOI] [PubMed] [Google Scholar]
- 37. Kim KS, Kwon J, Kim K, Chie EK. Impact of resection margin distance on survival of pancreatic cancer: a systematic review and meta‐analysis. Cancer Res Treat. 2017;49(3):824‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population‐based, linked database analysis of 396 patients. Ann Surg. 2003;237(1):74‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim YJ, Seo DW, Pack KM, et al. The prognostic factors of pancreatic cancer can be different according to clinical stages. Korean J Gastroenterol. 2008;51(3):181‐189. [PubMed] [Google Scholar]
- 40. Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high‐risk surgery. N Engl J Med. 2011;364(22):2128‐2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gooiker GA, Lemmens VE, Besselink MG, et al. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg. 2014;101(8):1000‐1005. [DOI] [PubMed] [Google Scholar]
- 42. Haj Mohammad N, Bernards N, Besselink MG, et al. Volume matters in the systemic treatment of metastatic pancreatic cancer: a population‐based study in the Netherlands. J Cancer Res Clin Oncol. 2016;142(6):1353‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bakens MJ, van der Geest LG, van Putten M, et al. The use of adjuvant chemotherapy for pancreatic cancer varies widely between hospitals: a nationwide population‐based analysis. Cancer Med. 2016;5(10):2825‐2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim YC, Kim HJ, Park JH, et al. Can preoperative CA19‐9 and CEA levels predict the resectability of patients with pancreatic adenocarcinoma? J Gastroenterol Hepatol. 2009;24(12):1869‐1875. [DOI] [PubMed] [Google Scholar]
- 45. Kinsella TJ, Seo Y, Willis J, et al. The impact of resection margin status and postoperative CA19‐9 levels on survival and patterns of recurrence after postoperative high‐dose radiotherapy with 5‐FU‐based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol. 2008;31(5):446‐453. [DOI] [PubMed] [Google Scholar]
- 46. Smith RA, Bosonnet L, Ghaneh P, et al. Preoperative CA19‐9 levels and lymph node ratio are independent predictors of survival in patients with resected pancreatic ductal adenocarcinoma. Dig Surg. 2008;25(3):226‐232. [DOI] [PubMed] [Google Scholar]
- 47. Fujioka S, Misawa T, Okamoto T, et al. Preoperative serum carcinoembryonic antigen and carbohydrate antigen 19‐9 levels for the evaluation of curability and resectability in patients with pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14(6):539‐544. [DOI] [PubMed] [Google Scholar]
- 48. Liao WC, Chien KL, Lin YL, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta‐analysis. Lancet Oncol. 2013;14(11):1095‐1103. [DOI] [PubMed] [Google Scholar]


