Abstract
Since the cryoballoon (CB) was introduced into clinical practice, more than 400,000 patients have undergone a pulmonary vein (PV) isolation with a CB throughout the world. Although the efficacy of the first-generation CB was limited, the recently introduced second-generation CB has achieved a greater uniformity in cooling, which has facilitated a shorter time to PV isolation, shorter procedural times, higher rates of freedom from atrial fibrillation and low rates of PV reconnections. Currently, a single short freeze strategy with a single 28 mm balloon has become the standard technique based on the balance of procedural efficacy and safety. However, enhanced cooling characteristics may also result in a greater potential for collateral damage to non-cardiac structures. Knowledge about the potential complications is essential when performing the procedure. In this article, we describe the important complications that should be noted during a CB procedure, and how to minimise the risk of complications based on our experience.
Keywords: Cryoballoon, pulmonary vein isolation, complications, catheter ablation, atrial fibrillation
Pulmonary vein isolation (PVI) has become an accepted therapeutic strategy for AF.[1] The cryoballoon (CB) ablation system has been introduced into clinical practice as a tool for a single-shot anatomical based-PVI, and a comparable efficacy of the CB ablation to radiofrequency (RF) ablation has been demonstrated in a prospective randomised study.[2–4]
The recently introduced second-generation CB (Arctic Front Advance, Medtronic) has become the standard tool owing to the greater cooling effect and higher efficacy when compared with the first-generation CB.[5,6] However, this also raises concern of collateral damage to non-cardiac structures. Several recent studies have shown that the enhanced cooling effect successfully reduced the freezing interval to 180 seconds (single freeze) or time-to-isolation of the guided-strategy, and eliminated the bonus freeze without a reduction in long-term efficacy.[7–10] This article focuses on the representative complications in second-generation CB ablation procedures.
Phrenic Nerve Injury
CB ablation is associated with a significant risk of phrenic nerve injury (PNI) due to the limited balloon size, and right PNI is the most common complication in the CB ablation procedure.[3–6,11] The reported incidence of PNI varies owing to different definitions (of PNI), balloon generations (first or second), balloon size, freezing regimen and protective manoeuvres. Currently, continuous monitoring of the diaphragmatic compound motor action potentials (CMAPs) has become an accepted technique in clinical practice, which involves freezing being immediately terminated with a double-stop technique, active deflation, when the CMAP significantly decreases.[12–15]
We looked at the incidence and characteristics of PNI in 550 AF patients who underwent PVI using one 28 mm second-generation CB and a single 3-minute freeze strategy under CMAP monitoring.[16] A total of 34 (6.2%) patients experienced PNI during the right superior pulmonary vein (RSPV; n=30) and inferior PV ablation (n=4). However, no patients experienced left PNI. Applications were interrupted using double-stop techniques after median 136-second (25–75th percentile: 104–158) applications, and a PVI was already achieved in all but one case. Persistent AF, larger RSPV ostia, and deeper balloon positions on fluoroscopy were associated with higher incidences of PNI. The incidence of PNI during the procedure, and 1 day and 1 month afterwards was 6.2%, 2.4%, and 1.6%, respectively. All PNI was asymptomatic and reversible during the follow-up period. The CMAP amplitude during the emergent deflation predicted the delay in the PNI recovery, and all incidents of PNI recovered by the next day in patients with a remaining CMAP amplitude of >0.2 mV.
These data suggest that early recognition of CMAP amplitude reductions and immediate active deflations appear to be essential for early recovery from PNI. The key to minimising the risk of PNI is to ensure the balloon position is as antral as possible, and for this purpose, a proximal-seal technique (Figure 1) is recommended to avoid any deep CB positioning.[6] If no leak is visible on venography, withdraw the CB slightly and allow a leak around the PV—balloon interface to better define the PV ostium and ensure a proximal ablation. Then, reapply only the minimal amount of pressure needed to regain the occlusion before the ablation. Since the CB size becomes slightly larger when the freezing starts, a small leakage is generally sealed by the CB applications. However, it should be noted that PNI could occur even when the balloon position is proximal, presumably because the phrenic nerve course varies among patients. Therefore, careful CMAP monitoring is mandatory during applications regardless of the RSPV size.
Figure 1: Proximal Seal Technique.
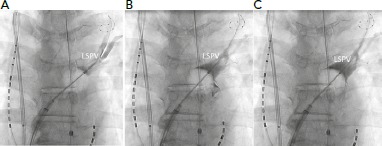
Instead of initiating ablation after the initial venogram despite contrast retention (A), the CB is gently pulled back to reveal the real PV ostium by noting contrast leak (B). Then, the CB is slightly pushed to obtain a complete occlusion at the real PV ostium (C). CB = cryoballoon; LSPV = left superior PVl; PV = pulmonary vein.
Obtaining a stable position of the pacing catheter is important given that catheter dislodgement also results in a decrease in the CMAP amplitude. Pacing should be continued during the initial thawing time because PNI could occur during that period.[16] Since this complication is usually asymptomatic, a chest X-ray is recommended before the procedure and on the next day. PNI is almost always asymptomatic and reversible during the follow-up period if the procedure was carefully performed, thus PNI is the most common, but is not a serious complication in the CB ablation procedure.
PV Stenosis
PV stenosis has been a well-recognised complication of AF ablation regardless of the use of the energy sources.[1,17] Moreover, there are data showing a progression of stenosis during the 3 months after RF ablation.[17] The reported incidence of PV stenosis could differ due to different ablation techniques, definitions of PV stenosis, and intensity of the screening for this complication. Generally, it is believed that cryoablation has a lower risk of PV stenosis due to tissue shrinkage when compared with RF ablation because of the preservation of the basic underlying tissue architecture with preserved endocardial contours and minimal cartilage formation after the ablation.[18] Since PV stenosis has not been evaluated with adequate modalities in the vast majority of centres, the reported data is limited. A few have revealed that first-generation CB ablation could result in PV stenosis and that a longer application time and use of a 23 mm CB increased the risk of this complication.[3]
Severe PV stenosis has also been reported after the introduction of the second-generation CB.[19,20] We investigated 276 patients who underwent CB PVI using one 28 mm balloon with a single 3-minute freeze strategy.[21] If the balloon temperatures reached –60°C or PNI was suspected, freezing was terminated. Enhanced cardiac CT was obtained before and >3 months after the procedure. Follow-up CT obtained at a median of 5.0 months post-procedure revealed no PVs with moderate (50–75%) or severe (>75%) stenosis. Asymptomatic mild stenosis (25–50%) was documented in 16/1,101 (1.4%) PVs, but did not progress during the follow-up period.
These results are presumably because the applications were terminated when the balloon temperature reached −60°C and the maximal application duration was 180 seconds. Also, the proximal seal technique was applied to avoid the balloon being vigorously wedged inside the PVs. The use of a 23 mm CB should to avoided because almost all PVs could be isolated by 28 mm CB and a small balloon could become wedged inside the vein.[22] Based on the present study data, PV stenosis might not be an issue with the current second-generation CB ablation strategy if the procedure is carefully performed, and routine evaluation of PV stenosis seems not to be necessary.
Cardiac Tamponade
Cardiac tamponade is the most common potentially life-threatening complication associated with AF ablation. In a dedicated worldwide survey, cardiac tamponade was reported to be the most frequent cause of peri-procedural death, accounting for 25% of the deaths, of which 3% occurred later than 30 days post-procedure.[1,23,24]
We analysed the incidence and characteristics of cardiac tamponade in 5,222 AF ablation procedures in 3,483 patients.[25] Cardiac tamponade occurred in 51 procedures/patients, and the incidence was 0.98% per procedure and 1.46% per patient. While there was no significant predictor of this complication, the use of a CB was associated with a lower incidence. The results are in accordance with a randomised prospective study and retrospective registry showing a lower risk of tamponade in CB ablation compared with RF ablation.[4,26] The RF ablation requires multiple catheters including mapping catheters and an ablation catheter for the PVI, and the complexity could explain the higher incidence of tamponade. RF ablation of tissue resulted in a reduction in the forces required to perforate the atrial wall; however, treatment with cryoablation did not significantly alter the forces required to induce a perforation, which may explain the lower incidence of tamponade.[27] Although the incidence is low in CB ablation procedures, careful manipulation of the CB with a guidewire (Achieve catheter, Medtronic) and careful manoeuvring of the FlexCath sheath (Medtronic) to avoid scratching the atrium are essential to avoid this complication.
Oesophageal Injury and Atrio-oesophageal Fistulae
Atrio-oesophageal fistulae are a rare complication of PVI using not only RF but also CB.[1] This is a direct result of the proximity of the oesophagus and the posterior wall of the LA.[28,29] In RF ablation, strategies such as modifying energy delivery at the posterior LA close to the oesophagus can minimise the risk. However, in the CB ablation, the posterior LA lesion size cannot specifically be controlled. In the CB ablation, a total of 11 cases of atrio-oesophageal fistulae were reported from more than 120,000 cases worldwide, which is considerably lower than that in RF ablation.[30] The balloon inflation time was significantly longer in the patients with atrio-oesophageal fistulae than in those without, and all cases of atrio-oesophageal fistulae occurred in relation to the left PVs.
Since the occurrence of oesophageal fistulae is rare, studies evaluating the impact of oesophageal protection measures have considered the occurrence of endoscopy-detected oesophageal lesions (EDOLs) as the yardstick of the comparison. Despite some limitations of monitoring the luminal oesophageal temperature (LOT), it is suggested as one method of possibly minimising the risk of EDOLs based on the data that EDOLs were more frequently observed in patients with a lower oesophageal temperature during CB ablation.[31–33] However, the use of an oesophageal probe for LOT monitoring to avoid oesophageal injury during AF ablation remains unproven in the current guidelines.[1]
We investigated 104 patients with paroxysmal AF undergoing second-generation cryoballoon ablation with a single 3-minute freeze strategy followed by endoscopy.[34] Temperature probes were used in the first 40 patients, but not in the other 64 patients. The incidence of ODELs was significantly higher to monitor LOT in the former rather than the latter group (8/40 versus 1/64; p<0.0001). The use of oesophageal probes was the sole predictor of EDOLs. These data suggest that oesophageal temperature probes themselves may contribute to the thermal injury of the oesophagus. Ahmed et al. reported a 17% incidence of EDOLs after first-generation CB ablation with the use of oesophageal probes, while Guiot et al. reported a 0% incidence without the use of oesophageal probes.[35,36] Our results were in accordance with these data. We speculate that, during cryoablation of the left inferior pulmonary vein (LIPV), the oesophagus gets wedged between the balloon anteriorly and thoracic spinal column or aorta posteriorly, increasing the likelihood of exposure to injury (Figure 2).[34] We recommend that:
Figure 2: In Type-A, the Oesophagus is Located Between the Right and Left Inferior Pulmonary Vein Ostia.
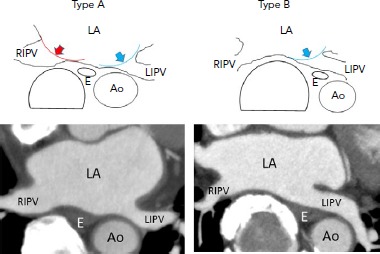
Vagal nerve networks may be widely impaired by CB applications applied at the RIPV (red curve) and LIPV (blue curve) ostia, respectively. In type-B, the oesophagus is located close to the LIPV ostium, but apart from the RIPV ostium. The vagal nerve networks may be partly impaired by CB applications applied at the LIPV (blue curve) ostia, but not all are impaired. On the contrary, the oesophagus gets wedged between the balloon anteriorly and thoracic spinal column or aorta posteriorly, increasing the likelihood of exposure to oesophageal injury. Ao: aorta; CB = cryoballoon; E = oesophagus; LA = left atrium; LIPV = left inferior pulmonary vein; RIPV = right inferior pulmonary vein.
Freezing at the LIPV should be short.
Proton-pump inhibiters should be prescribed for 1 month after the procedure to facilitate the healing of oesophageal injury.
Deep sedation should be avoided.
It has been reported that the use of general anaesthesia increases the risk of oesophageal damage in RF ablation presumably due to reduced motility and reduced deglutination of the oesophagus.[37] In the current short-freezing strategy, regardless of the oesophageal temperature monitoring, the risk of oesophageal fistulae seems to be extremely low with CB ablation.
Gastric Hypomotility
The vagal nerve fibres innervating the pyloric sphincter and stomach travel in the left vagal trunk along the anterior aspect of the oesophagus close to the posterior LA and PVs.[28] It is well known that injury to the vagal nerve can result in gastric hypomotility characterised by delayed gastric emptying in the absence of an obstructing structural lesion in the stomach manifested as abdominal bloating.[38] The incidence is likely underestimated because most asymptomatic patients have not been systemically screened.
As mentioned above, we investigated the incidence of silent gastric hypomotility in 104 patients with paroxysmal AF undergoing second-generation cryoballoon with a single 3-minute freeze strategy followed by endoscopy.[33,34] Temperature probes were used in the first 40 patients to monitor the oesophageal temperature, but not in the other 64 patients. The presence of food in the stomach after overnight fasting without obstruction was defined as gastroparesis. The incidence of silent gastric hypomotility was similar between the groups (7/40 versus 11/64; p=0.967), and it was resolved in all patients on repeat endoscopy performed 1–3 months later. The oesophageal temperature was similar in patients with and without silent gastric hypomotility. In multivariate analyses, a shorter distance between the oesophagus and the right inferior PV ostium was the sole predictor of gastric hypomotility. The study clarified that second-generation CB ablation carried a significant risk of silent gastric hypomotility, and the anatomical location of the oesophagus — rather than oesophageal temperature — helped to identify high-risk populations for gastric hypomotility. It is likely that gastric hypomotility frequently occurs immediately after CB ablation but only a few patients become symptomatic.
Another study showed that 3% (n=3) of patients exhibited symptomatic gastric hypomotility despite cryoapplication being terminated when the LOT reached 25°C.[39] The symptoms (abdominal bloating and repeated vomiting) manifested 2–5 days post-procedure, after the stomach had time to be filled with food, and abdominal imaging demonstrated marked gastric dilatation with retained food. After fasting for 4–5 days and treatment with panthenol, metoclopramide, and erythromycin, the symptoms were gone and imaging findings showed a complete recovery 7–11 days post-procedure.[40] We defined a type-A oesophageal location when the oesophagus was located between the inferior PVs (apart from the LIPV and relatively close to the RIPV) at the inferior PV level, and type-B oesophageal location when the oesophagus was surrounded by the descending aorta, spine, and LIPV (close to the LIPV and apart from the RIPV; Figure 2). The incidence was significantly higher in patients with a type-A rather than a type-B oesophageal location (11.1% versus 1.2%; p=0.083), which was in accordance with the reported incidence of asymptomatic gastric hypomotility (33.3% versus 10.7%).[34]
The higher incidence of gastric hypomotility with CBs compared with RF ablation may be explained by the differences in the lesion configuration or greater transmural penetration by the CB ablation. We assume that the complex network of nerves located at the anterior aspect of the oesophagus may be widely damaged during ablation of the LIPV and the RIPV in patients whose oesophagus is located between these veins (type-A), increasing the risk of gastric hypomotility (Figure 2). It does not make sense to use LOT monitoring to anticipate this complication. We recommend that:
The freezing time should be short for the lower PVs, especially in the high-risk population evaluated on pre-procedural imaging.
Excessive drinking and eating should be avoided post-procedure given the high incidence of asymptomatic gastric hypomotility.
Stroke
Since the incidence of a stroke is low, protective manoeuvres have been considered for silent strokes that were detected on diffusion-weighted MRI on the day after the procedure.[41] Possible embolic materials are thrombi, gas bubbles and particulate debris produced during an LA ablation, and silent strokes have been produced experimentally by injecting small-size solid particles or gaseous microbubbles into the brain in animal models.[42–44] In RF ablation, the most important step toward reducing symptomatic stroke and transient ischaemic attack rates is to implement uninterrupted anticoagulation into the management of patients undergoing ablation.[1] According to guidelines, all AF ablation procedures should be performed under uninterrupted warfarin or dabigatran (class 1, level A).[1] However, cryoablation is generally regarded as tissue-friendly and is associated with a significantly lower incidence of thrombus formation compared with RF ablation.[18]
We investigated the factors associated with the incidence of silent strokes during second-generation CB ablation.[45,46] We gave 256 AF patients a brain MRI 1 day after the PVI using second-generation cryoballoons with a single 28 mm balloon and a short-freeze strategy. Silent strokes were detected in 26.5% (n=68) of the patients, and none of the patients reported any neurological symptoms. Reinsertion of a once withdrawn cryoballoon and additional LA mapping with a multielectrode catheter significantly increased the incidence of silent strokes. Transient coronary air embolisms were significantly associated with the incidence of silent strokes.
On the contrary, an uninterrupted anticoagulation regimen, cryoballoon air removal with extracorporeal balloon inflations, strength of the MRI magnet, internal electrical cardioversion, and touch-up ablation were not associated with the incidence of silent strokes. These results suggest that air embolisms are the main mechanism of silent strokes, and the injected air volume might determine the type of lesion.
These findings underscore the importance of careful de-airing management during procedures using large bore transseptal sheaths in the LA. As well as the strict anticoagulation protocol according to the guidelines[1], we recommend:
Careful sheath management;
Submerged loading of the catheter into the introducer before sheath insertion to minimise the ingression of air;
Slow catheter insertion and withdrawal from the sheath because air can be introduced into the transseptal sheath with suction when catheters are removed.
Reinsertion of a used CB and exchanging catheters with complex geometries via the FlexCath sheath, should be avoided.
Clinical Perspective
Cryoballoon ablation is associated with several procedural complications and that knowledge is essential for physicians who are involved in the procedure.
Balloon positioning and freeze dosing are the key to minimise the risk of complication while maintaining the procedure’s efficacy.
Acknowledgments
The authors acknowledge John Martin’s assistance in the preparation of this manuscript.
References
- 1.Calkins H, Kuck KH, Cappato R et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632–96. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Kojodjojo P, O’Neill MD, Lim PB et al. Pulmonary venous isolation by antral ablation with a large cryoballoon for treatment of paroxysmal and persistent atrial fibrillation: medium-term outcomes and non-randomised comparison with pulmonary venous isolation by radiofrequency ablation. Heart. 2010;96:1379–84. doi: 10.1136/hrt.2009.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packer DL, Kowal RC, Wheelan KR et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 4.Kuck KH, Brugada J, Fürnkranz A et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–45. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 5.Martins RP, Hamon D, Césari O et al. Safety and efficacy of a second-generation cryoballoon in the ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2014;11:386–93. doi: 10.1016/j.hrthm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Su W, Kowal R, Kowalski M et al. Best practice guide for cryoballoon ablation in atrial fibrillation: the compilation experience of more than 3,000 procedures. Heart Rhythm. 2015;12:1658–66. doi: 10.1016/j.hrthm.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Ciconte G, de Asmundis C, Sieira J et al. Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm. 2015;12:673–80. doi: 10.1016/j.hrthm.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki S, Hachiya H, Nakamura H et al. Pulmonary vein isolation using a second-generation cryoballoon in patients with paroxysmal atrial fibrillation: one-year outcome using a single big-balloon 3-minute freeze technique. J Cardiovasc Electrophysiol. 2016;27:1375–80. doi: 10.1111/jce.13078. [DOI] [PubMed] [Google Scholar]
- 9.Chun KR, Stich M, Fürnkranz A et al. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm. 2017;14:495–500. doi: 10.1016/j.hrthm.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Aryana A, Kenigsberg DN, Kowalski M et al. Verification of a novel atrial fibrillation cryoablation dosing algorithm guided by time-to-pulmonary vein isolation: Results from the Cryo-DOSING Study (Cryoballoon-ablation DOSING Based on the Assessment of Time-to-Effect and Pulmonary Vein Isolation Guidance). Heart Rhythm. 2017;14:1319–25. doi: 10.1016/j.hrthm.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Metzner A, Rausch P, Lemes C et al. The incidence of phrenic nerve injury during pulmonary vein isolation using the second-generation 28 mm cryoballoon. J Cardiovasc Electrophysiol. 2014;25:466–70. doi: 10.1111/jce.12358. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi F, Dubuc M, Guerra PG, Khairy P. Phrenic nerve monitoring with diaphragmatic electromyography during cryoballoon ablation for atrial fibrillation: the first human application. Heart Rhythm. 2011;8:1068–71. doi: 10.1016/j.hrthm.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski M, Ellenbogen KA, Koneru JN. Prevention of phrenic nerve injury during interventional electrophysiologic procedures. Heart Rhythm. 2014;11:1839–44. doi: 10.1016/j.hrthm.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh J, Sepahpour A, Chan KH et al. Immediate balloon deflation for prevention of persistent phrenic nerve palsy during pulmonary vein isolation by balloon cryoablation. Heart Rhythm. 2013;10:646–52. doi: 10.1016/j.hrthm.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni N, Su W, Wu R. How to prevent, detect and manage complications caused by cryoballoon ablation of atrial fibrillation. Arrhythm Electrophysiol Rev. 2018;7:18–23. doi: 10.15420/aer.2017.32.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki S, Kajiyama T, Watanabe T et al. Characteristics of phrenic nerve injury during pulmonary vein isolation using a 28-mm second-generation cryoballoon and short freeze strategy. piiJ Am Heart Assoc. 2018;7:e008249. doi: 10.1161/JAHA.117.008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes DR, Monahan KH, Packer D. Pulmonary vein stenosis complicating ablation for atrial fibrillation: clinical spectrum and interventional considerations. JACC Cardiovasc Interv. 2009. pp. 267–76. [DOI] [PubMed]
- 18.Khairy P, Chauvet P, Lehmann J et al. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. 2003;107:2045–50. doi: 10.1161/01.CIR.0000058706.82623.A1. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K, Nitta J, Sato A et al. Hemoptysis after five months of cryoballoon ablation: what is the relationship? HeartRhythm Case Rep. 2017;3:357–9. doi: 10.1016/j.hrcr.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narui R, Tokuda M, Matsushima M et al. Incidence and factors associated with the occurrence of pulmonary vein narrowing after cryoballoon ablation. Circ Arrhythm Electrophysiol. 2017;10:e004588. doi: 10.1161/CIRCEP.116.004588. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki S, Kajiyama T, Hada M et al. Does second-generation cryoballoon ablation using the current single short freeze strategy produce pulmonary vein stenosis? Int J Cardiol. 2018;272:175–8. doi: 10.1016/j.ijcard.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Schmidt B, Bordignon S et al. Practical techniques in cryoballoon ablation: how to isolate inferior pulmonary veins. Arrhythm Electrophysiol Rev. 2018;7:11–7. doi: 10.15420/aer.2018;1;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappato R, Calkins H, Chen SA et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 24.Cappato R, Calkins H, Chen SA et al. Delayed cardiac tamponade after radiofrequency catheter ablation of atrial fibrillation: a worldwide report. J Am Coll Cardiol. 2011;58:2696–7. doi: 10.1016/j.jacc.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Hamaya R, Miyazaki S, Taniguchi H et al. Management of cardiac tamponade in catheter ablation of atrial fibrillation: single-centre 15 year experience on 5222 procedures. Europace. 2018;20:1776–82. doi: 10.1093/europace/eux307. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M, Dorwarth U, Andresen D et al. German ablation registry: Cryoballoon vs. radiofrequency ablation in paroxysmal atrial fibrillation – one-year outcome data. Heart Rhythm. 2016;13:836–44. doi: 10.1016/j.hrthm.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Quallich SG, Van Heel M, Iaizzo PA. Optimal contact forces to minimize cardiac perforations before, during, and/or after radiofrequency or cryothermal ablations. Heart Rhythm. 2015;12:291–6. doi: 10.1016/j.hrthm.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Quintana D, Cabrera JA, Climent V et al. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation. 2005;112:1400–5. doi: 10.1161/CIRCULATIONAHA.105.551291. [DOI] [PubMed] [Google Scholar]
- 29.Romero J, Avendano R, Grushko M et al. Oesophageal injury during af ablation: techniques for prevention. Arrhythm Electrophysiol Rev. 2018;7:24–31. doi: 10.15420/aer.2017.46.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.John RM, Kapur S, Ellenbogen KA, Koneru JN. Atrioesophageal fistula formation with cryoballoon ablation is most commonly related to the left inferior pulmonary vein. Heart Rhythm. 2017;14:184–9. doi: 10.1016/j.hrthm.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Metzner A, Burchard A, Wohlmuth P et al. Increased incidence of esophageal thermal lesions using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol. 2013;6:769–75. doi: 10.1161/CIRCEP.113.000228. [DOI] [PubMed] [Google Scholar]
- 32.Fürnkranz A, Bordignon S, Böhmig M et al. Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm. 2015;12:268–74. doi: 10.1016/j.hrthm.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 33.Miyazaki S, Nakamura H, Taniguchi H et al. Esophagus related complications during second-generation cryoballoon ablation insight from simultaneous esophageal temperature monitoring from two esophageal probes. J Cardiovasc Electrophysiol. 2016;27:1038–44. doi: 10.1111/jce.13015. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki S, Nakamura H, Taniguchi H et al. Gastric hypomotility after second-generation cryoballoon ablation-unrecognized silent nerve injury after cryoballoon ablation. Heart Rhythm. 2017;14:670–7. doi: 10.1016/j.hrthm.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed H, Neuzil P, d’Avila A et al. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm. 2009;6:962–9. doi: 10.1016/j.hrthm.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 36.Guiot A, Savouré A, Godin B, Anselme F. Collateral nervous damages after cryoballoon pulmonary vein isolation. J Cardiovasc Electrophysiol. 2012;23:346–51. doi: 10.1111/j.1540-8167.2011.02219.x. [DOI] [PubMed] [Google Scholar]
- 37.Di Biase L, Saenz LC, Burkhardt DJ et al. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol. 2009;2:108–12. doi: 10.1161/CIRCEP.108.815266. [DOI] [PubMed] [Google Scholar]
- 38.Shah D, Dumonceau JM, Burri H et al. Acute pyloric spasm and gastric hypomotility: an extracardiac adverse effect of percutaneous radiofrequency ablation for atrial fibrillation. J Am Coll Cardiol. 2005;46:327–30. doi: 10.1016/j.jacc.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa K, Miyazaki S, Hisazaki K et al. Gastric hypomotility after luminal esophageal temperature guided second-generation cryoballoon pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2018;11:e006691. doi: 10.1161/CIRCEP.118.006691. [DOI] [PubMed] [Google Scholar]
- 40.Kuwahara T, Takahashi A, Takahashi Y et al. Clinical characteristics and management of periesophageal vagal nerve injury complicating left atrial ablation of atrial fibrillation: lessons from eleven cases. J Cardiovasc Electrophysiol. 2013;24:847–51. doi: 10.1111/jce.12130. [DOI] [PubMed] [Google Scholar]
- 41.Deneke T, Jais P, Scaglione M et al. Silent cerebral events/lesions related to atrial fibrillation ablation: a clinical review. J Cardiovasc Electrophysiol. 2015;26:455–63. doi: 10.1111/jce.12608. [DOI] [PubMed] [Google Scholar]
- 42.Haines DE, Stewart MT, Dahlberg S et al. Microembolism and catheter ablation I: a comparison of irrigated radiofrequency and multielectrode-phased radiofrequency catheter ablation of pulmonary vein ostia. Circ Arrhythm Electrophysiol. 2013;6:16–22. doi: 10.1161/CIRCEP.111.973453. [DOI] [PubMed] [Google Scholar]
- 43.Takami M, Lehmann HI, Parker KD et al. Effect of left atrial ablation process and strategy on microemboli formation during irrigated radiofrequency catheter ablation in an in vivo model. Circ Arrhythm Electrophysiol. 2016;9:e003226. doi: 10.1161/CIRCEP.115.003226. [DOI] [PubMed] [Google Scholar]
- 44.Haines DE, Stewart MT, Barka ND et al. Microembolism and catheter ablation II: effects of cerebral microemboli injection in a canine model. Circ Arrhythm Electrophysiol. 2013;6:23–30. doi: 10.1161/CIRCEP.112.973461. [DOI] [PubMed] [Google Scholar]
- 45.Miyazaki S, Watanabe T, Kajiyama T et al. Thromboembolic risks of the procedural process in second-generation cryoballoon ablation procedures: analysis from real-time transcranial doppler monitoring. piiCirc Arrhythm Electrophysiol. 2017;10:e005612. doi: 10.1161/CIRCEP.117.005612. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki S, Kajiyama T, Yamao K et al. Silent cerebral events/lesions after second-generation cryoballoon ablation: How can we reduce the risk of silent strokes? Heart Rhythm. 2018;1:41–8. doi: 10.1016/j.hrthm.2018.07.011. [DOI] [PubMed] [Google Scholar]


