Abstract
Objective
To identify transcription factors that could contribute to direct reprogramming of fibroblasts towards smooth muscle cell fate.
Approach and Results
We screened various combinations of transcription factors, including Myocd, Mef2C, Mef2B, Mkl1, Gata4, Gata5, Gata6, Ets1, and their corresponding carboxyterminal fusions to the transactivation domain of MyoD –indicated by *-, for their effects on reprogramming mouse embryonic fibroblasts (MEFs) and human adult dermal fibroblasts (hFBs) to the smooth muscle cell fate as determined by the expression of specific markers. The combination of three transcription factors, Myocd (or Myocd*) with Mef2C (or Mef2C*) and Gata6, was the most efficient in enhancing expression of smooth muscle marker genes and decreasing fibroblast gene expression. Additionally, the derived induced smooth muscle-like cells, iSMCs, showed a contractile phenotype in response to carbachol.
Conclusions
Combination of Myocd and Gata6 with Mef2C* (MG2*) could sufficiently and efficiently direct differentiation of mouse embryonic and human dermal fibroblasts into iSMCs, thus opening new opportunities for disease modeling, tissue engineering and personalized medicine.
Keywords: Smooth muscle cells, Reprogramming, Differentiation
Graphical Abstract
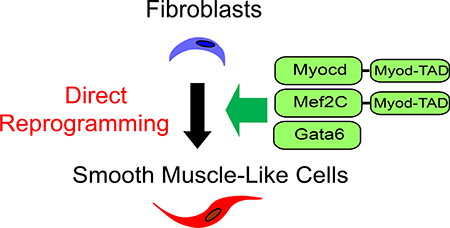
Smooth muscle is non-striated and involuntary muscle tissue within the walls of blood vessels including aorta, arteries, and veins, consisting of non-striated, spindle-shaped cells. Directly deriving smooth muscle cells (SMCs) from fibroblasts in vitro would provide new opportunities to study various diseases through modeling including aortic aneurysms, atherosclerosis and stroke, as well as facilitate tissue engineering and drug screening in personalized medicine2. Myocardin (Myocd) is a master regulator of smooth muscle differentiation3–6. Myocardin was reported as inefficient for induction of smooth muscle differentiation from multipotent embryonic cells7 although Myocd overexpression is sufficient to confer structural and functional attributes of the smooth muscle contractile phenotype8. A cardiac myocardin isoform containing a unique N-terminal Mef2-binding motif is able to interact with Mef2 and activate transcription through a subset of Mef2-dependent regulatory elements in the heart9. Recently, it was reported that Myocd overexpression could directly convert human endothelial progenitor cells to induced smooth muscle cells10. For clinical purposes, identifying transcription factors that could directly reprogram smooth muscle cells from fibroblast is highly significant. For instance, in the aorta, endothelial dysfunction and inflammation induce dysregulated expansion of the myofibroblasts (activated fibroblasts) population. Although the results here may not directly translate to leverage resident myofibroblasts for treatment, the present studies on direct reprogramming of fibroblasts into SMC is an encouraging step towards that next frontier11, 12. In this study, we used mouse embryonic fibroblasts (MEFs) to screen various combinations of transcription factors such as Myocd, Mef2C, Mef2B, Mkl1, Gata4, Gata5, Gata6, Ets1, and the carboxy-terminus fusion genes of Myocd, Mef2C, Mef2B and Mkl1 with the transactivation domain of MyoD, to facilitate epigenetic remodeling of chromatin1, 13 (Myocd*, Mef2C*, Mef2B* and Mkl1*). After systematic testing of groupings, we successfully identified multiple combinations of transcription factors able to promote fibroblast-to-induced Smooth Muscle-like Cells (iSMC) differentiation. We demonstrated that the combination of transcription factors Myocd*, Gata6 and Mef2C* (M*G2*) could sufficiently and efficiently activate smooth muscle specific myosin heavy chain (Myh11) and downregulate fibroblast markers. The main findings were replicated in human dermal fibroblasts, with Myocd, Gata6 and Mef2C* (MG2*) efficiently rendering hiSMCs in this system.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Preparation of mouse fibroblast cells and vascular smooth muscle cells
Mouse embryonic fibroblasts (MEF) were prepared from E13.5 embryos of the C57BL/6 mice as previously reported1. Vascular smooth muscle cells were isolated from 15-week C57BL/6 mice as previously reported14.
Generation of iSMCs
Mouse iSMC
The mouse MyoD M3 domain (amino acids 1–62) was fused to full-length cDNAs encoding mouse Myocd (kind gift of Dr. Olson)9, Mef2B, Mef2C, and Mkl1 at the carboxy-terminus to create the fusion genes Myocd*, Mef2B*, Mef2C*, and Mkl1*. pMXs-IP (kind gift of Dr. Kitamura)15, 16 vectors encoding the fusion genes and wild-type genes Myocd, Mef2B, Mef2C, Mkl1, Ets1, Gata4 and Gata6, as well as the “empty vector” virus control, were separately transfected into Plat-E cells (kind gift of Dr. Kitamura)17 with Lipofectamine 2000 (Thermo Fisher Scientific). The supernatants containing retroviruses encoding these genes were harvested after 48hrs (called day −1) as described previously18, filtered through a 0.45-μm syringe filter. Titration of retrovirus was performed using the Retro-X qRT-PCR Titration Kit (Takara Bio USA) according to the manufacturer’s protocol. The differentiation protocol is summarized in Figure 1A. On day –2, MEFs were seeded at a density of 2.5 × 103 cells/cm2 in each well of 48-well, or 24-well plates in fibroblast medium containing Dulbecco’s Modified Eagle Medium (DMEM), 10% fetal bovine serum (FBS), and Penicillin-Streptomycin (Thermo Fisher Scientific) for the virus transduction. Various combinations of fresh 0.3mL of virus supernatant (include 2.5×109 ± 0.2 copies) were added to 1.0 × 104 MEF on day −1 with 10 μg/mL polybrene. The virus supernatant was changed to fibroblast medium on day 0. On day 1, the mouse fibroblast medium was changed to SMC medium containing Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12), 10% KnockOut Serum Replacement (KSR), 2 ng/mL Recombinant Human TGF-β1 (PeproTech), 10 ng/mL Human PDGF-BB (Shenandoah Biotechnology), and 1% Penicillin-Streptomycin, which was replaced every other day thereafter (Figure 1 A).
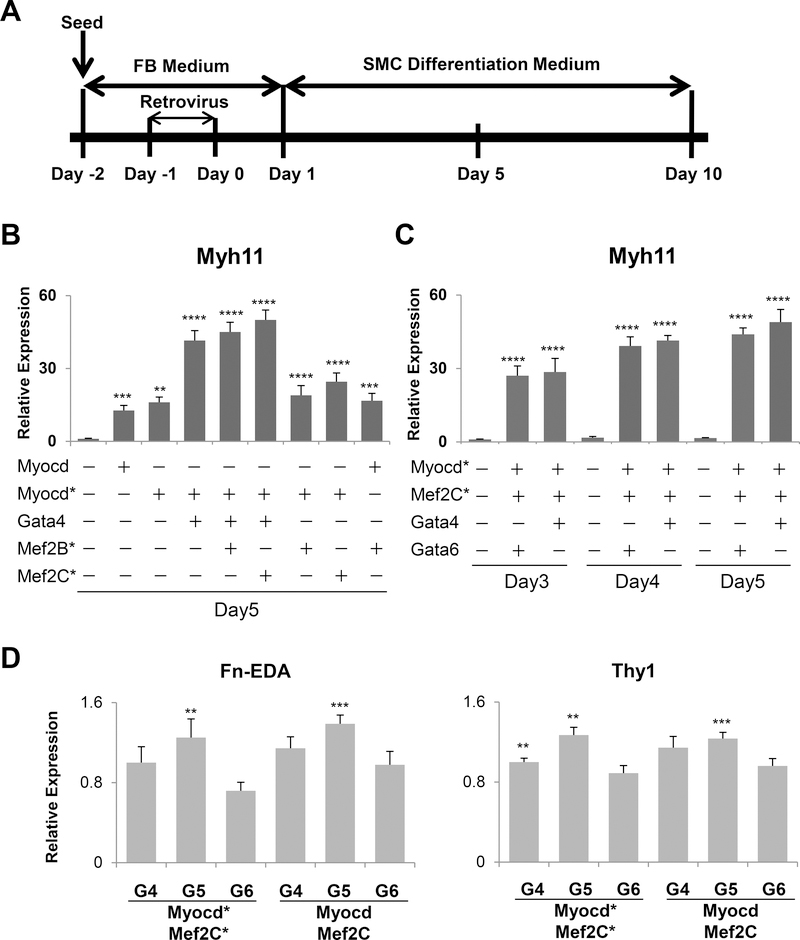
Figure 1.
Generation of smooth muscle-like cells (iSMCs) from MEFs. (A) Schematic drawing of the differentiation protocol used to generate iSMCs. (B) Induction of expression of the smooth muscle cell marker Myh11 in MEFs treated with a combination of Myocd*, Gata4, Mef2B*, and Mef2C* genes on day 5 post-transduction. (C) Induction of Myh11gene expression in MEFs treated with combination of Myocd*, Mef2C*, Gata4, and Gata6 genes and cultured for the 3, 4 and 5 days post-transduction. (D) Downregulation of expression of fibroblast marker genes Fn-EDA, Thy1 in MEFs at 5 days post-transduction with the retrovirus encoding the reprogramming factors indicated in the figure. Normalized with empty vector virus control group = 1.0 (B and C) or Myocd*, Mef2C*, and Gata6 group = 1.0 (D). The data are shown as mean ± SEM. N=3. ****p < 0.001, ***p < 0.01, **p < 0.05 compared with the control group. G4: Gata4, G5: Gata5, G6: Gata6, Md: Myocd, Md*: Myocd*
Human iSMC
The human MyoD M3 domain (amino acids 1–62) was fused to full-length cDNAs encoding human MEF2C at the carboxy-terminus to create the fusion gene MEF2C*. pLenti CMV Puro (kind gift from Drs. Campeau & Kaufman, Addgene plasmid # 17448) vector encoding the fusion gene MEF2*, and pReceiver-Lv105 (GeneCopoeia) vector encoding human wild-type genes MYOCD, and GATA6, as well as the “empty vector” virus control, were separately co-transfected with packaging plasmids, psPAX2 (kind gift of Dr. Trono, Addgene plasmid # 12260) and envelope plasmid, pCMV-VSV-G (kind gift of Dr. Weinberg, Addgene plasmid # 8454) into 293T cells using Lipofectamine 2000 (Thermo Fisher Scientific). Human adult dermal fibroblasts (hFBs, Lonza) were seeded at a density of 2.5× 103 cells/cm2 in each well of 48-well, or 24-well plates in human fibroblast medium containing DMEM/F-12, 10% fetal bovine serum (FBS), and Penicillin-Streptomycin (Thermo Fisher Scientific) for the virus transduction. Various combinations of fresh 0.3mL of virus supernatant were added to hFB on day −1 with 10 μg/mL polybrene. The virus supernatant was changed to fibroblast medium on day 0. On day 1, the human fibroblast medium was changed to SMC medium containing DMEM/F-12, 10% KSR, 2 ng/mL Recombinant Human TGF-β1, 10 ng/mL Human PDGF-BB, and 1% Penicillin-Streptomycin, which was replaced every other day thereafter (Figure 1 A).
Immunofluorescence staining and time-lapse image acquisition
Cells were fixed with 4% formaldehyde in PBS for 10 min and permeabilized with 0.5% Triton X-100 in PBS. Cells were stained with the primary and secondary antibodies for 1 hour each at room temperature. The following primary antibodies against Myosin-11 (Abcam), Calponin1 (Sigma-Aldrich), α-SMA (Sigma-Aldrich) and secondary antibodies either anti-Rabbit IgG (H+L), or anti-Mouse IgG (H+L) conjugated to Alexa Fluor 488 (Thermo Fisher Scientific) were used. Nuclei were counterstained with DAPI. Percentage of cells positive for of of Myosin-11, Calponin 1 and α-SMA was obtained from three independent experiments. Two hundred of cells were counted in each experiment. Fluorescence and time-lapse images were obtained with an OLYMPUS microscope as described below and processed with Adobe Photoshop CS6 and Adobe Illustrator CS6 (Adobe Systems). Carbachol-induced contractility assay was carried out as described previously19, 20. Briefly, Phase-contrast time lapse of carbachol (100 μM, Alfa Aesar) induced contraction of iSMC was recorded with a DP70 microscope digital camera with an IX71 phase-contrast fluorescent motorized inverted microscope using DP Controller and edited with DP Manager (Olympus) in a time-lapse manner at 1-minute intervals for 30min. Contraction was determined by tracking individual cells in the videos.
Real-Time Quantitative Reverse Transcription PCR (qRT-PCR)
Total RNA was purified from cells and from 5-week-old mouse aorta media and heart with a Direct-zol RNA MicroPrep (Zymo Research) and treated with DNase I as directed by the manufacturer. cDNA was synthesized using a SuperScript IV First-Strand Synthesis System (Thermo Fisher Scientific) and qRT-PCR was performed with iTaq universal SYBR Green supermix (Bio-Rad Laboratories) on a Realplex 2S system (Eppendorf). The mRNA level was normalized with that of 18S ribosomal RNA (18S rRNA). The standard error of the mean (SEM) was calculated from the values obtained from three independent experiments. Aorta media was used as a positive control. Please, see the “Major Resources Table” in the Supplemental Material for the list of primers used.
Statistical Analysis
Data are expressed as mean ± SEM. Analyses were performed using SPSS 24.0 (SPSS, Chicago, IL). All data were tested for normality and equal variance. If the data passed the normality and equal variance tests, Student t test was used to compare 2 groups or 1-way ANOVA followed by Tukey post hoc for comparisons among >2 groups. If the data did not pass the normality or equal variance test, non-parametric tests (Mann-Whitney U and Kruskall-Wallis tests) were used. A P value <0.05 was considered statistically significant.
Results
Since Myocd controls SMC gene expression and smooth muscle development2–6, we first tested the ability of Myocd to activate endogenous SMC specific gene expression by itself or in combination with other factors following the protocol outlined in Figure 1A. To determine the effects of Myocd on SMC reprogramming, we transduced MEF cells with retrovirus carrying Myocd in SMC culture medium. Myh11, Acta2, Cnn1, SmthB, and Tagln gene expression were used to assess the quality of induced iSMC. Consistent with previous reports2–6, Myocd induced endogenous SMC specific markers such as Myh11, Acta2, Cnn1, Smtn-B, and Tagln in MEFs (Supplemental figure I). Except for Myh11and endogenous Myocd, the expression level of other SMC genes tested was similar to those of aorta media (Supplemental figure I). We noticed that Myh11 expression level is more than three-fold lower compared to aorta media, even when Myocd expression levels were three-fold higher than in aorta media (Supplemental figure I). Interestingly, similar level of Myocd expression to that of aorta media induces less than 20% Myh11 expression compared to that in aorta media and even 4-times higher Myocd still fails to induce Myh11 to aorta media levels (Supplemental figure I). Aorta media was used as a positive control, since the expression level of Myh11, the most stringent smooth muscle marker, is expected to represent that in vivo, while primary vascular smooth muscle cells in culture consistently showed over 200-fold lower levels than aorta media for this gene (Supplemental figure II).
To search for an optimal combination of transcription factors capable of inducing smooth muscle lineage-reprogramming, we generated retroviruses to express each of the seven core smooth muscle and cardiac transcription factors Myocd*, Mef2B, Mef2B*, Mkl1, Mkl1*, Gata4 and Ets1 in MEFs (Supplemental figure III). In the first round of screening, the cocktail of four genes, Myocd*, Mkl1, Mef2B, Gata4 was the most effective. The results suggest that, in the absence of Gata4, Mef2B* is better than Mef2B for Cnn1 induction. The positive effects of Gata4, such as activation of Tagln gene expression were unexpected because former studies showed that GATA4 can repress the myocardin-induced Tagln promoter activation in A10 smooth muscle cells21. Yet, our results indicate that the combination of transcription factors tested here could activate smooth muscle genes including Tagln. When the MyoD activation domain was fused with Mkl1, that is in Mlk1*, the fusion gene was inhibitory for induction of smooth muscle genes. We then compared the combination of four genes, and found that, indeed, Mkl1 was not required for the induction of smooth muscle genes (Supplemental figure IV-A). Myocd*, Mef2B*, and Gata4 was the most effective combination of three genes in this first round. The three genes induced Myh11 expression in MEFs efficiently, and over 3-fold higher than the Myocd* alone as of day 10 (Supplemental figure IV-B). We considered the possibility that other isoform of Mef2, Mef2C, might promote SMC reprogramming. To test this, we compared Mef2B* and Mef2C* (Figure 1B and Supplemental figure IV-B). Myh11 reached a peak of expression on day 5 post-transduction. To our surprise, Mef2B* and Mef2C* had similar effect on Myh11 expression. There was no significant difference beyond a slightly but reproducible increase in Myh11 induction in the combination of Myocd* with Mef2C* and Gata4 compared to Myocd* with Mef2B* and Gata4 on days 5 and 10. Next, we used the combination of Myocd* with Mef2C* and Gata4 for further validation. Myh11 expression was consistently more efficient on day 5 than day 3 or 4 with Myocd* with Mef2C* and Gata4 (Figure 1C). Smooth muscle specific genes were efficiently induced when cultured with SMC culture medium, but not fibroblast medium (Supplemental figure V). We also addressed if other members of the Gata family could improve MEF differentiation into iSMCs by qRT-PCR analysis of gene expression patterns in iSMCs to ascertain increased expression of smooth muscle specific genes, and concomitant suppression of fibroblast-specific markers. Indeed, former studies showed that Gata6 and Myocd synergistically activate the promoter of smooth muscle myosin heavy chain in A10 smooth muscle cells21, while in 10T1/2 fibroblast cells overexpression of smooth muscle differentiation factor Gata622 was not able to induce expression of endogenous smooth muscle genes, suggesting that Gata6 alone is not sufficient to drive fibroblast cells to differentiate into smooth muscle cells21. Based on these data, we verified that the gene expression pattern of smooth muscle cell markers was optimized compared to Myocd alone through our protocol of reprogramming from MEFs. We see similar trends in the combination of Myocd*/Myocd with Mef2C*/C and Gata4/5/6 genes (Figure 1D, Supplemental figure IV-B, VI-A and VI-B). Myocd induces smooth muscle target genes, but does not repress fibroblast markers in MEFs efficiently in this context (Supplemental figure VI-C). Thus, compared to the other combinations tested, such as Myocd* with Mef2C* and Gata5/6, or Myocd with Mef2C and Gata4/5/6, the combination of the three factors Myocd*, Mef2C* and Gata6 was the most effective in overall suppression of the fibroblast specific genes (Figure 1D and Supplemental figure VI-B) while inducing Myh11 to levels comparable to Gata4 (Figure 1C and Supplemental figure VI-A). Furthermore, iSMCs derived from transduction of Myocd* in combination with Mef2C* and Gata6 showed smooth muscle-specific genes significantly up-regulated when compared to empty vector virus control, with expression patterns similar to those in the aorta media control (Figure 2A). In addition to the four maturation-related markers, we verified up-regulation of endogenous Myocd and Col3a1 and Smtn-B and confirmed the downregulation of fibroblast-enriched genes, specifically Fn-EDA, Thy1 and Snai1 and 2, in those iSMCs (Figure 2 A-B and Supplemental figure VII-A). KLF4 and KLF5 are negative regulators of Myocd transcription in mice and rats.23, 24, 25 To address if they might be responsible for a potential feedback repression of endogenous Myocd, we determined their expression levels in our experimental conditions and found that they were unresponsive to our differentiation protocol (Supplemental figure VII-A). Additionally, there was no induction of cardiac genes at day 10 post-transduction (Supplemental figure VII-B), indicating that in these conditions iSMCs acquire maturity similar to that of the aorta media. Immunofluorescent staining of iSMCs showed expression of smooth muscle cell markers Calponin 1 and α-SMA (Supplemental figure VIII-A-C) and further demonstrated that 93% of iSMCs were positively stained for the most specific smooth muscle cell marker, Myosin-11 (Figure 2C-D, left panels) and the contractile function was confirmed by treatment with carbachol26 (Fig. 2E, top panel, and Supplemental figure IX and on line movies). Taken together, these results indicate that the combination of Myocd*, Mef2C* and Gata6 can induce smooth muscle gene expression in MEFs better than the other combinations tested in this approach. We additionally compared the combination of Myocd*, Gata6 and Mef2C* with Myocd, Gata6 and Mef2C* for the induction of smooth muscle marker genes and fibroblast marker genes (Supplemental figure X-A-B). There was no significant difference in induction of smooth muscle and repression of fibroblast marker genes or in Klf4 and Klf5 expression between the combination of Myocd*, Gata6 and Mef2C* vs Myocd, Gata6 and Mef2C*, indicating that both Myocd* and Myocd are equally effective.
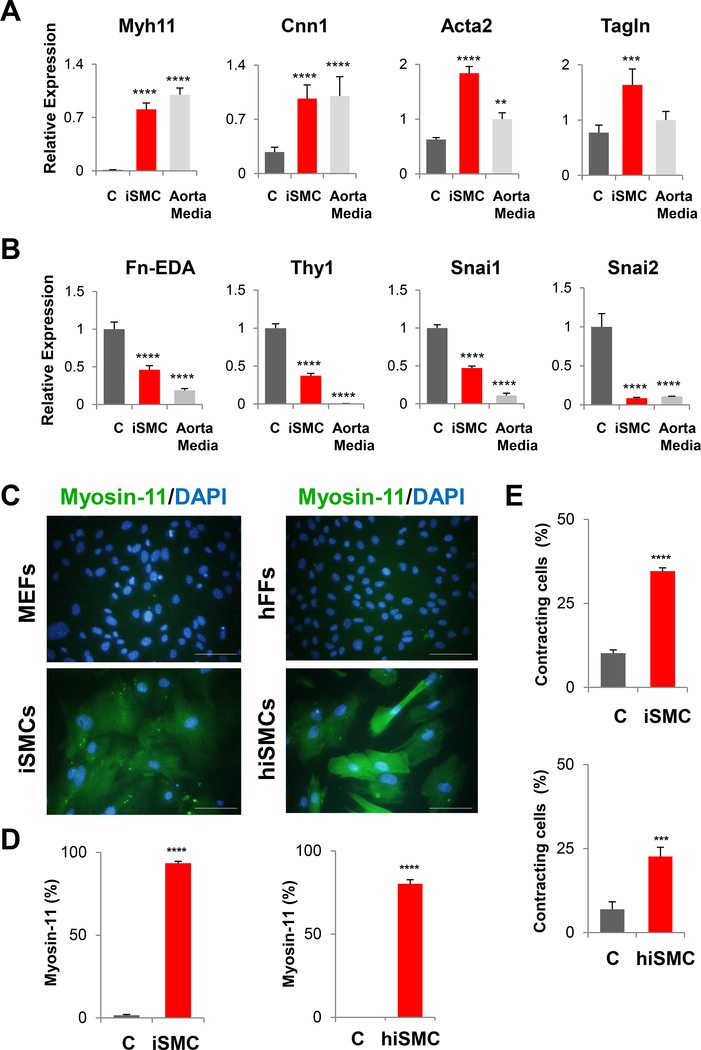
Figure 2.
Characterization of iSMCs induced from MEFs and hFBs with the combination of the three factors Myocd*, Mef2C* and Gata6. (A) Induction of smooth muscle marker genes, including Myh11, Cnn1, Acta2, and Tagln from MEFs at 5 days post-transduction. (B) Decrease of fibroblast marker genes Fn-EDA, Thy1, Snai1, and Snai2 from MEFs at 5 days post-transduction. Normalized relative to Aorta Media (A) or empty vector virus control (B). The data are shown as mean ± SEM. N=5. (C) Immunofluorescence staining of Myosin-11 (Green), on day 5 post-transduction transduction with Myocd*, Mef2C* and Gata6 and counterstained with DAPI (Blue). The scale bars represent 100 μm. (D) Percentage of cells positive for Myosin-11 on day 5 post-transduction. The data are shown as mean ± SEM. N=5. (E) Percentage of carbachol-induced contracting cells on day 5 post-transduction. The data are shown as mean ± SEM. N=5. ****p < 0.001, ***p < 0.01, **p < 0.05, compared with control group. * indicates potentiated genes with fusion of the M3 domain of human MyoD.
Finally, we applied MYOCD with GATA6 and MEF2C* for the reprogramming of human iSMCs from human adult dermal fibroblasts. MG2* induced MYH11 to aorta media levels and the other SMC markers above aorta media levels (Supplemental figure XI-A). Interestingly, in contrast to MEFs, in human fibroblasts, the three factors could not induce endogenous MYOCD (Supplemental figureXI-A). Indeed, in human dermal fibroblasts, MYOCD basal expression was the same level as in aorta media, while MYH11 was 1000-fold lower. Regulation of MYOCD is not well characterized in human compared to animal models27. KLF4 and KLF5 bind to the MYOCD promoter regions and mediate transcriptional repression in humans as well27, 28. KLF4 and KLF5 were highly expressed in aorta media compared to fibroblasts (Supplemental figure XI-A). On the other hand, in hiSMCs, KLF5 was expressed at higher level than in fibroblasts and aorta media (Supplemental figure XI-A), suggesting, that KLF5 might be responsible for the apparent repression of endogenous MYOCD in hiSMCs. More importantly, it is apparent that in human adult dermal fibroblasts, our MG2* was able to effectively and remarkably repress fibroblast genes, including SNAI1, SNAI2, and THY1 to aorta media levels, unlike in MEFs (Supplemental figure XI-B). Immunofluorescent staining of hiSMCs demonstrated expression of Smooth Muscle Cell Markers Calponin 1 and α-SMA (Supplemental figure XII-A-C) and 80% of cells were positively stained for the most stringent marker of smooth muscle cells, Myosin-11 (Figure 2C-D). Smooth muscle contractility to carbachol was confirmed (Fig. 2E, Supplemental figure XIII and on line movies).
Discussion
Elucidation of the regulatory mechanisms driving and controlling developmental programs of fate decisions towards smooth muscle cell lineage and differentiation is fundamental and clinically important in the study of vascular diseases. In this study, we report a novel combination of transcription factors coupled with forward reprogramming to significantly improve the expression of Myh11 from fibroblasts. We found a combination of three transcription factors, Myocd*, Mef2C* and Gata6, that facilitate direct reprogramming of mouse embryonic fibroblasts to smooth muscle-like cells, iSMCs. We demonstrated that in addition to enhancing the overall expression of Myh11 (Fig. 1C), the combination of those specific transcription factors, namely Myocd* (or Myocd), Mef2C* and Gata6, could enhance the maturation of induced smooth muscle cells by reducing fibroblast specific gene expression (Fig. 2B and Supplemental figure X). Indeed, Myh11 levels in iSMC were comparable to those of aorta media (Figure 2A) with over 90% of the cells expressing Myosin-11, a marker for terminally differentiated smooth muscle cells (Figure 2C and D). It is noted that reduction of fibroblast specific genes is still insufficient. Furthermore, we were able to reprogram human adult dermal fibroblasts using the Myocd, Mef2C* and Gata6 combination which rendered 80% of the cells expressing Myosin-11 (Figure 2C and D), with remarkable reduction in fibroblast markers in this case (Supplemental figure XI-B). Induction of endogenous Myocd does not achieve the same levels as the corresponding tissue aorta media (Supplemental figure VII and XI). Our findings point to a complex regulation of MYOCD in human aorta which is beyond the scope of this brief report but merits further investigation. Our results suggest the existence of MYOCD independent MYH11 induction in human aorta media. Conversely, it is also possible that in human dermal fibroblasts there is active repression of MYH11, overcoming any effects of basal levels of MYOCD in those cells. Understanding the complexity of such interplay will require systematic further dissection of MYOCD expression and function in human aorta as well as any potential differences between embryonic and adult fibroblasts and potential epigenetic modification 29, 30. Therefore, it should be assumed that these iSMCs represent partially reprogrammed smooth muscle-like cells. Furthermore, the direct reprogramming requires resetting of the epigenetic memory of the parent cells. Combining small-molecule epigenetic modulators and modified transcription factors within effective time windows31 could improve the generation of mature iSMCs by facilitating epigenetic reprogramming.
Although further improvements to the protocol will still be necessary to increase the population of mature smooth muscle cells through direct reprogramming of fibroblasts, forward reprogramming towards of the smooth muscle lineage holds great promise for the field of aorta vascular regeneration by providing the tools to generate new smooth muscle cells from differentiated adult cells, as for instance, resident myofibroblasts. Induced cells could be considered as a potential cell source for regeneration therapy for cardiovascular diseases. Furthermore, it is hoped that induced patient-specific smooth muscle cells will become experimental platforms to investigate cardiovascular disease in the context of personalized medicine. Except in Alzheimer’s disease in which increases in SMC contractile proteins are associated with the disorder16, most vascular diseases correlate with a decrease in SMC contractile proteins. Based on these findings, the identification of functional differences caused by various vascular diseases as well as differences in gene expression between diseased SMC and normal SMC would be important for preclinical applications of this approach.
Supplementary Material
Highlights.
The combination of transcription factors Myocd* with Mef2C* and Gata4/5/6 could reprogram mouse embryonic fibroblasts to smooth muscle-like cells.
The combination of transcription factors Myocd with Mef2C and Gata4/5/6 also could reprogram mouse embryonic fibroblasts to smooth muscle-like cells.
The combination of transcription factors Myocd* with Gata 6 and Mef2C* could sufficiently activate smooth muscle specific myosin heavy chain (Myh11) with partial induction of endogenous Myocd expression while most efficiently downregulate fibroblast markers in mouse embryonic fibroblasts.
The combination of transcription factors Myocd with Gata6 and Mef2C* could sufficiently reprogram adult human dermal fibroblasts to smooth muscle-like cells with remarkably high efficiency for downregulation of fibroblast markers.
Acknowledgements
We are grateful to Dr. Olson, Dr. Kitamura, Drs. Campeau & Kaufman, Dr. Trono and Dr. Weinberg for their selfless sharing of their reagents, as indicated in the Materials and Methods section.
Sources of Funding
This work was supported, in whole or in part, by National Institutes of Health Grants HL136231 (PXM and YEC), HL141891 (BY) and HL138139 (JZ).
Abbreviations
- MEFs
mouse embryonic fibroblasts
- hFBs
human adult dermal fibroblasts
- Myh11
myosin, heavy polypeptide 11, smooth muscle
- Myocd
myocardin
- Gata4
GATA binding protein 4
- Gata6
GATA binding protein 6
- Mef2B
myocyte enhancer factor 2B
- Mef2C
myocyte enhancer factor 2C
- Cnn1
calponin 1
- Acta2
actin, alpha 2, smooth muscle, aorta
- Tagln
transgelin (SM22-alpha)
- Fn-EDA
Fibronectin extra domain A
- Thy1
thymus cell antigen 1, theta
- Snai1
snail family zinc finger 1
- Snai2
snail family zinc finger 2
- Smtn-B
smoothelin b
- Mkl1
MKL (megakaryoblastic leukemia)/myocardin-like 1
- Est1
E26 avian leukemia oncogene 1, 5’ domain
- Myh6
Myh6 myosin, heavy polypeptide 6, cardiac muscle, alpha
- Myh7
myosin, heavy polypeptide 7, cardiac muscle, beta
- iSMC
induced Smooth Muscle-like Cells
- α-SMA
α-smooth muscle actin
- MG2 (or M*G2*)
Myocd (or Myocd*), Gata6 and Mef2C (or Mef2C*, a.k.a. MM31) combination
- (MG2*)
Myocd, Gata6 and Mef2C* combination
Footnotes
Disclosures
None.
References
- 1.Hirai H, Katoku-Kikyo N, Keirstead SA and Kikyo N. Accelerated direct reprogramming of fibroblasts into cardiomyocyte-like cells with the MyoD transactivation domain. Cardiovasc Res. 2013;100:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung KK, Bogunovic N, Keekstra N, Beunders AA, Pals J, van der Kuij K, Overwater E, Wisselink W, Blankensteijn JD, van Hinsbergh VW, Musters RJ, Pals G, Micha D and Zandieh-Doulabi B. Transdifferentiation of Human Dermal Fibroblasts to Smooth Muscle-Like Cells to Study the Effect of MYH11 and ACTA2 Mutations in Aortic Aneurysms. Hum Mutat. 2017;38:439–450. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Kitchen CM, Streb JW and Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol. 2002;34:1345–56. [DOI] [PubMed] [Google Scholar]
- 4.Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK and Parmacek MS. Myocardin Is a Critical Serum Response Factor Cofactor in the Transcriptional Program Regulating Smooth Muscle Cell Differentiation. Molecular and Cellular Biology. 2003;23:2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN and Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res. 2003;92:856–64. [DOI] [PubMed] [Google Scholar]
- 6.Pipes GC, Sinha S, Qi X, Zhu CH, Gallardo TD, Shelton J, Creemers EE, Sutherland L, Richardson JA, Garry DJ, Wright WE, Owens GK and Olson EN. Stem cells and their derivatives can bypass the requirement of myocardin for smooth muscle gene expression. Dev Biol. 2005;288:502–13. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida T, Kawai-Kowase K and Owens GK. Forced expression of myocardin is not sufficient for induction of smooth muscle differentiation in multipotential embryonic cells. Arterioscler Thromb Vasc Biol. 2004;24:1596–601. [DOI] [PubMed] [Google Scholar]
- 8.Long X, Bell RD, Gerthoffer WT, Zlokovic BV and Miano JM. Myocardin is sufficient for a smooth muscle-like contractile phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creemers EE, Sutherland LB, Oh J, Barbosa AC and Olson EN. Coactivation of MEF2 by the SAP domain proteins myocardin and MASTR. Mol Cell. 2006;23:83–96. [DOI] [PubMed] [Google Scholar]
- 10.Ji H, Atchison L, Chen Z, Chakraborty S, Jung Y, Truskey GA, Christoforou N and Leong KW. Transdifferentiation of human endothelial progenitors into smooth muscle cells. Biomaterials. 2016;85:180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML and Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollong MJ, Yang B, Vergani N, Beyer BA, Chin EN, Zambaldo C, Wang D, Chatterjee AK, Lairson LL and Schultz PG. Small molecule-mediated inhibition of myofibroblast transdifferentiation for the treatment of fibrosis. Proc Natl Acad Sci U S A. 2017;114:4679–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirai H, Tani T, Katoku-Kikyo N, Kellner S, Karian P, Firpo M and Kikyo N. Radical acceleration of nuclear reprogramming by chromatin remodeling with the transactivation domain of MyoD. Stem Cells. 2011;29:1349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhikari N, Shekar KC, Staggs R, Win Z, Steucke K, Lin YW, Wei LN, Alford P, Hall JL and International Society of Cardiovascular Translational R. Guidelines for the isolation and characterization of murine vascular smooth muscle cells. A report from the International Society of Cardiovascular Translational Research. J Cardiovasc Transl Res. 2015;8:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T and Kumagai H. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–14. [PubMed] [Google Scholar]
- 16.Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM and Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A. 2007;104:823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita S, Kojima T and Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–6. [DOI] [PubMed] [Google Scholar]
- 18.Hirai H, Katoku-Kikyo N, Karian P, Firpo M and Kikyo N. Efficient iPS cell production with the MyoD transactivation domain in serum-free culture. PLoS One. 2012;7:e34149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao J, Xiong W, Wang L, Yang J, Qiu P, Hirai H, Shao L, Milewicz D, Chen YE and Yang B. Differentiation defect in neural crest-derived smooth muscle cells in patients with aortopathy associated with bicuspid aortic valves. EBioMedicine. 2016;10:282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Qiu P, Jiao J, Hirai H, Xiong W, Zhang J, Zhu T, Ma PX, Chen YE and Yang B. Yes-Associated Protein Inhibits Transcription of Myocardin and Attenuates Differentiation of Vascular Smooth Muscle Cell from Cardiovascular Progenitor Cell Lineage. Stem Cells. 2017;35:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin F and Herring BP. GATA-6 can act as a positive or negative regulator of smooth muscle-specific gene expression. J Biol Chem. 2005;280:4745–52. [DOI] [PubMed] [Google Scholar]
- 22.Mano T, Luo Z, Malendowicz SL, Evans T and Walsh K. Reversal of GATA-6 downregulation promotes smooth muscle differentiation and inhibits intimal hyperplasia in balloon-injured rat carotid artery Circulation research. 1999;84:647–654. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH and Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–27. [DOI] [PubMed] [Google Scholar]
- 24.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R and Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES and Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolton TB, Lang RJ and Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984;351:549–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner EC, Huang CL, Govindarajan K and Caplice NM. Identification of a Klf4-dependent upstream repressor region mediating transcriptional regulation of the myocardin gene in human smooth muscle cells. Biochim Biophys Acta. 2013;1829:1191–201. [DOI] [PubMed] [Google Scholar]
- 28.Miano JM. Myocardin in biology and disease. J Biomed Res. 2015;29:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imamura M, Long X, Nanda V and Miano JM. Expression and functional activity of four myocardin isoforms. Gene. 2010;464:1–10. [DOI] [PubMed] [Google Scholar]
- 30.Grauss RW, van Tuyn J, Steendijk P, Winter EM, Pijnappels DA, Hogers B, Gittenberger-De Groot AC, van der Geest R, van der Laarse A, de Vries AA, Schalij MJ and Atsma DE. Forced myocardin expression enhances the therapeutic effect of human mesenchymal stem cells after transplantation in ischemic mouse hearts. Stem Cells. 2008;26:1083–93. [DOI] [PubMed] [Google Scholar]
- 31.Hirai H and Kikyo N. Inhibitors of suppressive histone modification promote direct reprogramming of fibroblasts to cardiomyocyte-like cells. Cardiovasc Res. 2014;102:188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.