Abstract
Objective: We have shown that “euglycemic DKA” in patients with type 1 diabetes receiving a sodium–glucose cotransporter 2-inhibitor (SGLT2i) is due to normal increases in rates of ketogenesis but blunted increases in plasma glucose levels. In this analysis, we assessed whether rescue treatment of early ketoacidosis with insulin is altered by SGLT2i use.
Research Design and Methods: Participants received 0.2 U/kg of aspart insulin after two 6-h interruptions of basal insulin that increased beta-hydroxybutyrate (BHB) by 1.2 ± 0.7 mmol/L before and by 1.5 ± 0.2 mmol/L during canagliflozin treatment. BHB and free fatty acid (FFA) were monitored every 30 min for 120 min after receiving a 0.2 U/kg subcutaneous injection of aspart insulin.
Results: Ten adults (23 ± 5 years) were studied. During the 120 min after rescue therapy with insulin, the reductions in BHB and FFA were nearly identical between the pre- and during canagliflozin treatment studies, respectively (−1.27 ± 0.76 and −1.13 ± 0.69, P = 0.671 for BHB and −0.50 ± 0.35 vs. −0.41 ± 0.41, P = 0.603 for FFA).
Conclusion: These data indicate that turning ketogenesis off, as well as on, does not appear to be affected by SGLT2i use.
Keywords: Type 1 diabetes, SGLT2i, Ketogenesis, Insulin pump.
Introduction
By increasing urinary glucose excretion, sodium–glucose cotransporter 2 inhibitors (SGLT2i) have shown promising results in reducing plasma glucose (PG), HbA1c, and body weight in patients with type 1 diabetes (T1D) and in lowering the risk of cardiovascular disease in type 2 diabetes.1–10 Despite these benefits, enthusiasm for therapy with SGLT2i in T1D has been tempered by reports of “euglycemic diabetic ketoacidosis” (euDKA), which has been suggested to be due, in part, to the need to reduce total daily insulin doses due to the insulin-independent lowering of PG levels by these agents.11
Pump-treated T1D patients are at risk of euDKA when receiving SGLT2i therapy, since infusion set failures are common.12 During paired 6-h insulin interruption studies in insulin-pump-treated adults before and after ∼3 weeks of treatment with canagliflozin, we were surprised to find that SGLT2i treatment did not significantly increase the rise in beta-hydroxybutyrate (BHB) or free fatty acid (FFA) levels, even in the face of a 16% decrease in total daily insulin doses.13 Instead, the major difference between the studies was a blunted rise in PG during canagliflozin treatment. In this study, we report results of the second phase of that study, which evaluated whether SGLT2i treatment altered the ability of a rescue dose of aspart insulin to lower elevated BHB levels.
Research Design and Methods
Participants
Participants were recruited from the Yale Diabetes Program; inclusion criteria included age 18–45 years, T1D >1 year duration, on insulin-pump therapy for >3 months, and HbA1c ≤9%. The study was approved by Yale University Human Investigations Committee and registered at clinicaltrials.gov (NCT02673138).
Study protocol
Participants were admitted to the Research Unit of Yale-New Haven Hospital for two insulin interruption studies: one before and one after ∼3 weeks of canagliflozin therapy (dose 300 mg/day). The insulin interruption study was previously described in detail.13 In brief, the participant's pump was shut off at ∼3 am and remained off for up to 6-h unless bedside BHB rose >2.5 mmol/L or PG reached >350 mg/dL (19.4 mmol/L). At the end of the suspension period, each participant during each study received a subcutaneous dose of insulin aspart (0.2 U/kg). At the bedside, PG was measured by YSI 2300 Glucose Analyzer every 30 min for 120 min. Blood was also obtained for laboratory-based BHB and FFA measurements. Laboratory BHB and FFA levels were measured by UV/Vis colorimetric spectrophotometer (Wako Autokit3-HB and NEFA-HR2, respectively).
Statistical analysis
BHB and FFA percentage change from baseline to 120 min after insulin injection was calculated; thus, within subject comparisons between the pre- and post-treatment admission was performed using paired Student's t-test. The distribution of continuous variables was assessed for skewness and kurtosis. Data are expressed as mean ± standard deviation.
Analyses were conducted using Prism 7 software (GraphPad Software, La Jolla, CA).
Results
Ten participants (age 23 ± 5 years, HbA1c 7.4% ± 0.8% [57 ± 8.7 mmol/mol]) completed the insulin interruption studies before and after 3 weeks of treatment with canagliflozin.
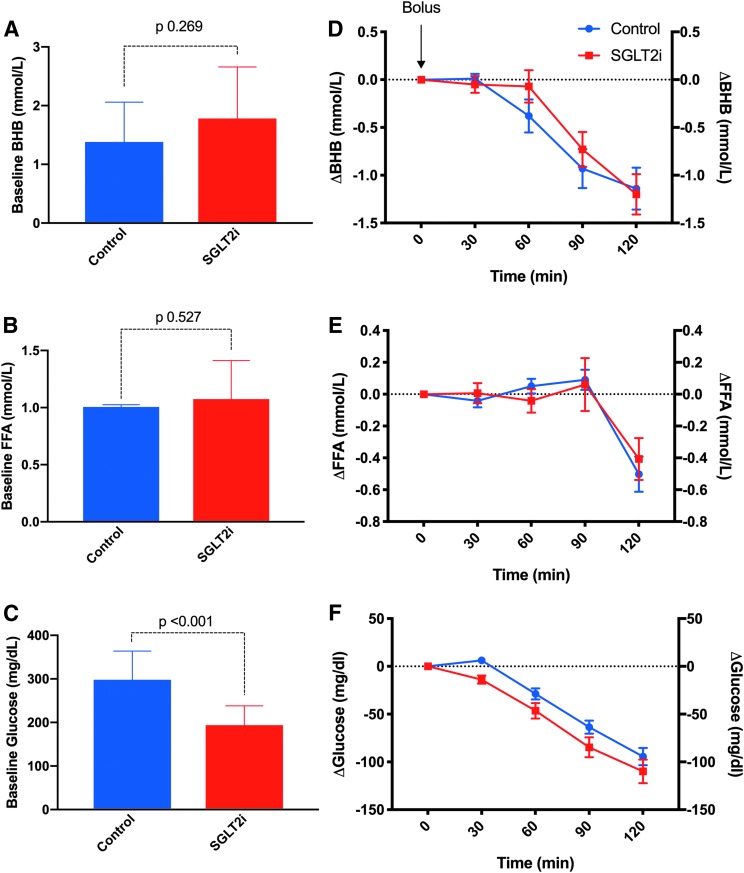
At the end of the two insulin suspension periods, BHB levels did not differ between the pre-canagliflozin (1.4 ± 0.7 mmol/L) and during canagliflozin treatment (1.8 ± 0.9 mmol/L, P = 0.269) (Fig. 1A), as did FFA levels (pretreatment 1.01 ± 0.02 mmol/L vs. 1.07 ± 0.34 mmol/L, during treatment, P = 0.527 (Fig. 1B), whereas glucose was higher at the pretreatment admission at baseline (Fig. 1C). As shown in Figure 1D and E, during the 120 min after rescue therapy with a 0.2 U/kg subcutaneous dose of aspart insulin, the reductions in BHB and FFA were nearly identical between the pre- and during canagliflozin treatment studies (−1.27 ± 0.76 and −1.13 ± 0.69, respectively, P = 0.671 for BHB and −0.50 ± 0.35 vs. −0.41 ± 0.41, P = 0.603 for FFA). The fall in PG after the rescue bolus of insulin did not differ between the two admissions, with a reduction of ∼100 mg/dL on both the study days (Fig. 1F).
FIG. 1.
BHB (A), FFA (B), and PG (C) at baseline. Incremental change (Δ) in BHB (D), FFA (E), and glucose (F) from baseline after administration of a 0.2 IU/kg bolus of insulin aspart at the end of the suspension period. BHB, beta-hydroxybutyrate; FFA, free fatty acid; PG, plasma glucose.
Discussion
Having demonstrated that SGLT2i do not exaggerate increases in BHB and FFA levels that are observed after interruption of insulin infusion in insulin pump-treated patients with T1D,13 this study showed that SGLT2i treatment does not impede the recovery from ketosis after rescue doses of rapid-acting insulin analogs. Rather, the potential pitfall of using SGLT2i as an adjunct to insulin in T1D lies in the lack of marked hyperglycemia that usually follows a suspension of basal insulin delivery. In the absence of hyperglycemia, patients may not recognize an infusion site failure early enough to prevent severe metabolic decompensation.
Importantly, studies of SGLT2i use in patients with T1D highlight the magnitude of the potential risk of DKA with this adjunctive therapy. As given in Supplementary Table S1 (Supplementary Data available online at https://www.libertpub.com/suppl/doi/10.1089/dia.2018.0356), rates of DKA varied from 2% to 3% for dapagliflozin, 0.8%–4% for empagliflozin, 5%–9.4% for canagliflozin, and 3% for the dual SGLT1/2 inhibitor sotagliflozin.3,4,14–16 This is in comparison with the 0%–1.2% rate of DKA detected in the placebo-treated participants. Factors contributing to the development of DKA were varied and included insulin pump failure, missed insulin doses, severe illness, and categories combined as other/not identified in our review. Yet, interruption of basal insulin delivery associated with insulin pump therapy accounted for 15%–30% of events in the aggregated studies. Although pump users accounted for 30%–40% of the participant cohort in most of the trials, data from the U.S.-based T1D Exchange Registry shows that roughly 60% of registry participants use this modality of insulin delivery. Thus, the impact of infusion set failures and issues with insulin interruption in clinical practice may be underestimated. Furthermore, a recent analysis conducted on the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS)17 for empagliflozin, dapagliflozin, and canagliflozin confirmed that 12% of users experienced an episode of DKA, with the highest number of event occurring in patients with T1D.
Although the mechanisms of ketosis during SGLT2i treatment remain controversial,18 the risk of ketosis is likely propagated by a number of other factors, including mechanisms of action associated with SGLT2i use such as the reduced insulin requirements these agents afford those living with diabetes. Therefore, rates of metabolic decompensation and subsequent reversal of ketosis associated with insulin omission/interruption, which was investigated in this trial, should not be extrapolated to identify other precipitators of DKA identified both in case reports and clinical trials. These would include stressful events, such as intercurrent illness or excessive alcohol intake.
In this study we demonstrated that SGLT2i themselves do not impair recovery from ketosis and considering the positive effect on glycemic control and on body weight of this class of drugs, a convincing argument can be made for using them as adjuvant therapy in T1D. Although our study shows a slight delay in the initial drop of BHB levels after the insulin bolus as a consequence of the SGLT2i treatment (Fig. 1D), this finding cannot be confirmed with this study sampling time of 30 min.
Nevertheless, our findings also indicate the need to develop more effective strategies for early detection of interruption of insulin delivery in pump users. In view of the technological revolution in diabetes care, one future direction should be the development of algorithms to alert pump-treated T1D patients of infusion set failures. Such algorithms would reduce the burden placed on patients to be ever vigilant, especially those on adjunctive SGLT2i therapy.19
Limitations of this study include its small sample size and the short duration, fixed dose of one SGLT2i therapy due to the large commitment of time, and effort required of participants to do two separate insulin interruption studies. In addition, we have not examined whether SGLT2i therapy impairs the ability of insulin to reverse ketogenesis due to intercurrent infections or other stresses. Data regarding oral intake and urine output were not recorded during the study; increases in fluid intake may have assisted in the recovery from ketosis and ketoacidosis in study participants.
As approval of SGLT2i agents for use in T1D is on the horizon, our findings that use of canagliflozin, a selective SGLT2-inhibitor, does not affect the recovery phase after suspension of insulin delivery allows clinicians to focus on educating patients about risk and the need to adopt new strategies to detect a potential episode of ketosis. Patients with diabetes who are receiving SGLT2i need to monitor blood and/or urine ketone levels regularly and more often if they have any signs or symptoms suggestive of DKA. Once recognized, Garg et al. have proposed the STICH protocol for treatment of ketosis in patients with T1D receiving SGLT2i: (1) STop SGLT2i, (2) take insulin bolus, (3) consume 30 g carbohydrates, and (4) hydrate.18 Our findings support the efficacy of the second step in this regimen; namely, turning ketogenesis off, as well as on, does not appear to be affected by SGLT2i use, at least in the instances related to insulin interruption.
Supplementary Material
Acknowledgments
The authors thank the participants and their families, the health care professionals, and staff of the Yale Children's Diabetes Clinic, the Yale Center for Clinical Investigation, and the dedicated nursing staff of the Hospital Research Unit, whose support and participation made this project possible.
Authors' Contributions
S.S. and A.G. researched and analyzed the data and drafted the article; N.S.P. researched data and conducted the clinical study; L.R.C. researched the data; W.V.T. critically revised the article; J.L.S. designed and conducted the study and revised the article. J.L.S. is the guarantor of this work, and as such had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Author Disclosure Statement
J.L.S. serves as a consultant to Medtronic Diabetes and is on advisory boards for Bigfoot Biomedical, Eli Lilly, and Insulet. J.L.S. has received research support from Medtronic Diabetes and Insulet. WVT serves as a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Medtronic Diabetes, and NovoNordisk. No other potential conflicts of interest relevant to this article were reported.
This work was supported by Endocrine Fellows Foundation, Juvenile Diabetes Research Foundation (5-ECR-2014-112-A-N), the National Institutes of Health (K12-DK-094714, UL1TR000142, P30 DK45735), the ISPAD Research Fellowship Program (2016), and Robert E. Leet and Clara Guthrie Patterson Trust Mentored Research Award (2017).
References
- 1. Pieber TR, Famulla S, Eilbracht J, et al. : Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab 2015;17:928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perkins BA, Cherney DZ, Partridge H, et al. : Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 2014;37:1480–1483 [DOI] [PubMed] [Google Scholar]
- 3. Rosenstock J, Marquard J, Laffel LM, et al. : Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 2018;41:2560–2569 [DOI] [PubMed] [Google Scholar]
- 4. Mathieu C, Dandona P, Gillard P, et al. : Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care 2018;41:1938–1946 [DOI] [PubMed] [Google Scholar]
- 5. Rodbard HW, Peters AL, Slee A, et al. : The effect of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care 2017;40:171–180 [DOI] [PubMed] [Google Scholar]
- 6. Sha S, Devineni D, Ghosh A, et al. : Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011;13:669–672 [DOI] [PubMed] [Google Scholar]
- 7. Ang KH, Sherr JL: Moving beyond subcutaneous insulin: the application of adjunctive therapies to the treatment of type 1 diabetes. Expert Opin Drug Deliv 2017;14:1113–1131 [DOI] [PubMed] [Google Scholar]
- 8. Rådholm K, Figtree G, Perkovic V, et al. : Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018;138:458–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zinman B, Wanner C, Lachin JM, et al. : Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 10. Inzucchi SE, Zinman B, Fitchett D, et al. : How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018;41:356–363 [DOI] [PubMed] [Google Scholar]
- 11. Peters AL, Buschur EO, Buse JB, et al. : Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wheeler BJ, Heels K, Donaghue KC, et al. : Insulin pump-associated adverse events in children and adolescents—a prospective study. Diabetes Technol Ther 2014;16:558–562 [DOI] [PubMed] [Google Scholar]
- 13. Patel NS, Van Name MA, Cengiz E, et al. : Altered patterns of early metabolic decompensation in type 1 diabetes during treatment with a SGLT2 inhibitor: an insulin pump suspension study. Diabetes Technol Ther 2017;19:618–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dandona P, Mathieu C, Phillip M, et al. : Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care 2018;41:2552–2559 [DOI] [PubMed] [Google Scholar]
- 15. Garg SK, Henry RR, Banks P, et al. : Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017;377:2337–2348 [DOI] [PubMed] [Google Scholar]
- 16. Henry RR, Thakkar P, Tong C, et al. : Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 2015;38:2258–2265 [DOI] [PubMed] [Google Scholar]
- 17. Fadini GP, Bonora BM, Avogaro A: SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA adverse event reporting system. Diabetologia 2017;60:1385–1389 [DOI] [PubMed] [Google Scholar]
- 18. Garg SK, Peters AL, Buse JB, Danne T: Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther 2018;20:571–575 [DOI] [PubMed] [Google Scholar]
- 19. Herrero P, Calm R, Vehi J, et al. : Robust fault detection system for insulin pump therapy using continuous glucose monitoring. J Diabetes Sci Technol 2012;6:1131–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.