Abstract
Therapeutic hypothermia following neonatal encephalopathy is neuroprotective. However, approximately one in two cooled infants still die or develop permanent neurological impairments. Further understanding of variables associated with the effectiveness of cooling is important to improve the therapeutic regimen. To identify clinical factors associated with short-term outcomes of cooled infants, clinical data of 509 cooled infants registered to the Baby Cooling Registry of Japan between 2012 and 2014 were evaluated. Independent variables of death during the initial hospitalization and survival discharge from the cooling hospital at ≤28 days of life were assessed. Death was associated with higher Thompson scores at admission (p < 0.001); higher heart rates after 3–72 hours of cooling (p < 0.001); and higher body temperature after 24 hours of cooling (p = 0.002). Survival discharge was associated with higher 10 minutes Apgar scores (p < 0.001); higher blood pH and base excess (both p < 0.001); lower Thompson scores (at admission and after 24 hours of cooling; both p < 0.001); lower heart rates at initiating cooling (p = 0.003) and after 24 hours of cooling (p < 0.001) and lower average values after 3–72 hours of cooling (p < 0.001); higher body temperature at admission (p < 0.001); and lower body temperature after 24 hours and lower mean values after 3–72 hours of cooling (both p < 0.001). Survival discharge was best explained by higher blood pH (p < 0.05), higher body temperature at admission (p < 0.01), and lower body temperature and heart rate after 24 hours of cooling (p < 0.01 and <0.001, respectively). Lower heart rate, higher body temperature at admission, and lower body temperature during cooling were associated with favorable short-term outcomes.
Keywords: : body temperature, heart rate, selective-head cooling, therapeutic hypothermia, whole-body cooling
Introduction
Accumulated evidence supports that therapeutic hypothermia following perinatal asphyxia is associated with reduced death and disability up to 18 to 22 months of age (Jacobs et al., 2013). The neuroprotective effect of therapeutic hypothermia is persistently observed up to school age (Guillet et al., 2012; Shankaran et al., 2012; Azzopardi et al., 2014). Subsequently, therapeutic hypothermia has become a routine part of clinical practice for infants with moderate to severe neonatal encephalopathy (Perlman et al., 2015). However, previous large-scale randomized controlled trials demonstrated that 44% to 55% of infants do not respond to therapeutic hypothermia and die or develop permanent disability (Edwards et al., 2010). In addition, recent studies suggest that even infants with mild neonatal encephalopathy who currently have no indication of cooling develop cerebral lesions on MRI and neurodevelopmental impairments thereafter (Rollins et al., 2014; Gagne-Loranger et al., 2016; Walsh et al., 2017).
To improve the therapeutic regimen for neonatal encephalopathy, detailed understanding of clinical variables associated with the effectiveness of therapeutic hypothermia is essential. Recent studies reassessed whether traditional prognostic markers of neonatal encephalopathy are still valid even when cooling is applied. Clinical variables representing the severity of hypoxia-ischemia, such as Apgar scores, blood gas pH and base excess, and encephalopathy scores, showed consistent associations with outcomes in cooled infants (Wyatt et al., 2007; Pappas et al., 2011; Azzopardi et al., 2012), whereas cooling altered the predictive value of several established markers, such as amplitude-integrated encephalogram and cerebral Doppler velocimetry (Takenouchi et al., 2011; Skranes et al., 2014; Del Rio et al., 2016; Chandrasekaran et al., 2017). Apart from traditional markers, several novel independent variables of outcomes have been identified, including body weight and blood glucose and carbon dioxide levels (Wyatt et al., 2007; Pappas et al., 2011; Chouthai et al., 2015; Basu et al., 2017). In contrast, the role of experimentally established independent variables of outcomes, such as delay in cooling and target cooling temperature, has not been confirmed in clinical settings (Gunn et al., 2015; Thoresen, 2015).
This study aimed to identify early clinical factors that may influence short-term outcomes after therapeutic hypothermia in newborn infants with neonatal encephalopathy.
Materials and Methods
Ethics approval and consent
This study was conducted in compliance with the Declaration of Helsinki. The protocols of the registry were approved by the Ethics Committees of Kurume University School of Medicine and Saitama Medical University, Japan. Since no patient identifiers were or are collected, the Ethics Committees advised that there is no statutory requirement for parental consent for data collection, and consent was not sought for the current registry.
Population and data collection
The Baby Cooling Registry of Japan is an online case registry that was established in January 2012 by inviting all registered Japanese level II/III neonatal intensive care centers. The detail of this registry has been reported previously (Tsuda et al., 2017). In brief, participating centers were requested to register all neonates who were referred to the unit for consideration of cooling. Clinical information was provided via the official website, including patient characteristics, severity of encephalopathy, body temperature, cardiovascular/respiratory parameters, supportive treatments, and short-term outcomes. Discharge from the cooling hospital was followed up at least up to 12 months of life. For this observational study, registered data of 509 cooled infants compiled between January 1, 2012 and December 31, 2014 were analyzed.
Statistical analysis
The Baby Cooling Registry of Japan is currently collecting the follow-up data at 2 years of age, findings of which will be reported elsewhere. For the current study, independent variables of death during the initial hospitalization and survival discharge from the cooling hospital at ≤28 days of life were assessed. Requirements for respiratory and feeding support at discharge (i.e., tube feeding, oxygen supplementation, and other invasive/noninvasive respiratory support) were also assessed. For the analysis, the following 10 clinical background variables were selected: gestational age; birth weight; birth location; 10 minutes Apgar score; cord or first blood pH and base excess; Thompson encephalopathy score at admission and after 24 hours of cooling; elapsed time from birth to the commencement of cooling; and the mode of cooling. Additional 10 physiological variables during cooling were also chosen as potential independent variables of the outcome: body temperature, heart rate, and mean blood pressure at admission (at admission only body temperature was taken) and after 0 and 24 hours and mean values after 3–72 hours of cooling (see Supplementary Table S1 for the analysis involving additional variables; Supplementary Data are available online at www.liebertpub.com/ther). Cases with unexplained missing data for >10% of the aforementioned variables and whose cooling mode and discharge status were not specified were not considered further.
To reduce attrition biases due to missing data, multiple imputation of variables was performed (n = 5 imputations) based on the correlation between variables with missing values and other subject characteristics (SPSS ver. 21.0; IBM, Armonk, NY). Univariate logistic regression analysis was performed to evaluate the crude effects of the potential independent variables on the outcome, where statistical significance was assumed for p-values <0.005 after correcting for multiple comparisons over 10 variables within the category. Final logistic models to explain the favorable outcome were developed using the independent variables available after 0 and 24 hours of cooling by forward selection.
Subsequently, several novel independent variables of the outcome were identified, including body temperature and heart rate. Independent variables of heart rate (after 0 and 24 hours of cooling) and body temperature (at admission and after 24 hours of cooling) were further investigated using general linear models. Finally, for outborn infants, the association between the target body temperature during transportation and subsequent body temperature at admission was assessed using simple linear regression model.
Results
Final study cohort
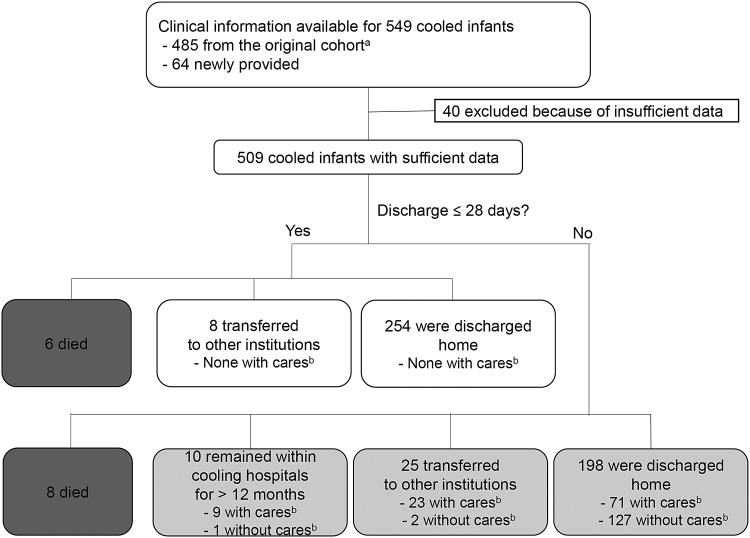
In addition to the data set of 485 cooled infants used in our previous analysis (Tsuda et al., 2017), data were newly obtained for 64 cooled infants, whose data submission was suspended at the time of the previous analysis. Of the 549 infants, 40 did not have sufficient data for the current analysis, and, thus, were excluded. Consequently, the final cohort of the current study comprised 509 cooled newborn infants (Fig. 1). The differences in the study population from that of the original cohort resulted in only subtle changes in the clinical backgrounds, physiological variables, and outcomes of infants (Table 1; see Tsuda et al., 2017 for the outline of the data from the original cohort).
FIG. 1.
Profile of the study population. aStudy cohort used in the previous analysis (see Tsuda et al., 2017 for details). bContinuous medical care, including tube feeding and/or respiratory support (invasive/noninvasive ventilation and oxygen supplementation).
Table 1.
Independent Variables of Short-term Outcomes
| Death during initial hospitalization | Survival discharge ≤28 days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||||||
| Variables | Yes (n = 14) | No (n = 495) | Odds ratio | Lower | Upper | p | Yes (n = 262) | No (n = 247) | Odds ratio | Lower | Upper | p |
| Background variables | ||||||||||||
| Gestational age (weeks) | 38.6 ± 1.8 | 38.9 ± 1.7 | 0.876 | 0.655 | 1.173 | 0.374 | 39.1 ± 1.6 | 38.7 ± 1.8 | 1.152 | 1.040 | 1.278 | 0.007 |
| Birth weight (kg) | 2.8 ± 0.5 | 2.9 ± 0.5 | 0.831 | 0.274 | 2.521 | 0.743 | 2.9 ± 0.4 | 2.9 ± 0.5 | 1.094 | 0.760 | 1.576 | 0.628 |
| Birth location | ||||||||||||
| Outborn | 11 (3.0) | 350 (97.0) | 1 | Reference | 174 (48.2) | 187 (51.8) | 1 | Reference | ||||
| Inborn | 3 (2.0) | 145 (98.0) | 0.657 | 0.172 | 2.502 | 0.536 | 88 (59.5) | 60 (40.5) | 1.576 | 1.070 | 2.323 | 0.021 |
| 10 minutes Apgar | 2 (1–5) | 5 (3–7) | 0.705 | 0.527 | 0.942 | 0.020 | 6 (4–7) | 4 (2–5) | 1.500 | 1.362 | 1.652 | <0.001 |
| Cord or first blood gas ≤1 hour of birth | ||||||||||||
| pH | 6.85 ± 0.29 | 6.95 ± 0.21 | 0.814a | 0.602 | 1.101 | 0.177 | 6.98 ± 0.18 | 6.90 ± 0.23 | 1.212a | 1.109 | 1.325 | <0.001 |
| BE (mmol/L) | −19.8 ± 12.0 | −14.5 ± 10.6 | 0.796b | 0.442 | 1.432 | 0.437 | −12.1 ± 9.6 | −17.4 ± 11.1 | 1.575b | 1.315 | 1.887 | <0.001 |
| Thompson encephalopathy score | ||||||||||||
| At admission | 18 (16–19) | 10 (7–14) | 1.261 | 1.108 | 1.434 | <0.001 | 9 (6–11) | 13 (9–17) | 0.862 | 0.831 | 0.895 | <0.001 |
| 24 hoursc | 16 (13–18) | 10 (5–13) | 1.169 | 1.047 | 1.304 | 0.006 | 7 (3–11) | 12 (9–16) | 0.865 | 0.835 | 0.896 | <0.001 |
| Cooling modality | ||||||||||||
| Selective-head | 9 (5.1) | 167 (94.9) | 1 | Reference | 79 (44.9) | 97 (55.1) | 1 | Reference | ||||
| Whole-body | 5 (1.5) | 327 (98.5) | 0.343 | 0.108 | 1.089 | 0.069 | 182 (54.8) | 150 (45.2) | 1.498 | 1.038 | 2.163 | 0.031 |
| Initiating cooling after birth (minutes) | 233 ± 104 | 212 ± 96 | 1.034d | 0.979 | 1.093 | 0.229 | 214 ± 95 | 211 ± 97 | 1.003d | 0.985 | 1.022 | 0.730 |
| Physiological variables during cooling | ||||||||||||
| Heart rate (beat/min) | ||||||||||||
| 0 hourc | 142 ± 21 | 132 ± 20 | 1.226e | 0.957 | 1.570 | 0.107 | 129 ± 20 | 135 ± 19 | 0.857e | 0.777 | 0.946 | 0.003 |
| 24 hoursc | 133 ± 14 | 114 ± 18 | 1.777e | 1.206 | 2.618 | 0.005 | 108 ± 17 | 121 ± 17 | 0.644e | 0.563 | 0.737 | <0.001 |
| Mean (3–72 hoursc) | 133 ± 14 | 113 ± 14 | 2.595e | 1.749 | 3.850 | <0.001 | 106 ± 12 | 120 ± 14 | 0.429e | 0.354 | 0.519 | <0.001 |
| Mean blood pressure (mmHg) | ||||||||||||
| 0 hourc | 41 ± 12 | 46 ± 10 | 0.588f | 0.347 | 0.995 | 0.048 | 47 ± 9 | 46 ± 11 | 1.103f | 0.924 | 1.317 | 0.276 |
| 24 hoursc | 41 ± 10 | 47 ± 8 | 0.306f | 0.118 | 0.792 | 0.017 | 47 ± 7 | 47 ± 8 | 1.012f | 0.807 | 1.268 | 0.921 |
| Mean (3–72 hoursc) | 42 ± 10 | 49 ± 6 | 0.295f | 0.083 | 1.051 | 0.059 | 48 ± 5 | 48 ± 7 | 0.854f | 0.618 | 1.180 | 0.339 |
| Body temperature (°C) | ||||||||||||
| At admission | 35.2 ± 1.0 | 36.0 ± 1.3 | 0.653 | 0.463 | 0.922 | 0.016 | 36.2 ± 1.1 | 35.7 ± 1.4 | 1.372 | 1.157 | 1.627 | <0.001 |
| 0 hourc | 34.5 ± 1.3 | 35.3 ± 1.3 | 0.639 | 0.425 | 0.960 | 0.031 | 35.4 ± 1.2 | 35.1 ± 1.3 | 1.176 | 1.010 | 1.369 | 0.037 |
| 24 hoursc | 34.2 ± 1.2 | 33.8 ± 0.5 | 3.558 | 1.631 | 7.761 | 0.002 | 33.7 ± 0.5 | 33.9 ± 0.5 | 0.459 | 0.316 | 0.666 | <0.001 |
| Mean (3–72 hoursc) | 34.1 ± 0.7 | 33.8 ± 0.5 | 2.268 | 1.063 | 4.842 | 0.034 | 33.7 ± 0.5 | 33.9 ± 0.5 | 0.404 | 0.269 | 0.606 | <0.001 |
Values are shown as number (%), mean ± standard deviation, or median (interquartile range).
Statistical significance was assumed for p < 0.005 (indicated in bold, Bonferroni correction).
Per 0.1 change.
Per 10 mmol/L.
After initiating cooling.
Per 10 minutes.
Per 10 beat/min.
Per 10 mmHg.
BE, base excess; CI, confidence interval.
Outcome of infants
By the 28th day of life, 6 infants died during the initial hospitalization, 8 infants were transferred to a birth hospital or neighboring institutions, and 254 infants were discharged home (none required respiratory/feeding support; Fig. 1). Of the remaining 241 infants who required hospital care beyond 28 days of life, 8 infants died during the initial hospital stay, 10 infants remained hospitalized within the cooling unit at 12 months of life, 25 infants were transferred to other institutions, and 198 infants were discharged home.
Independent variables of death during initial hospitalization
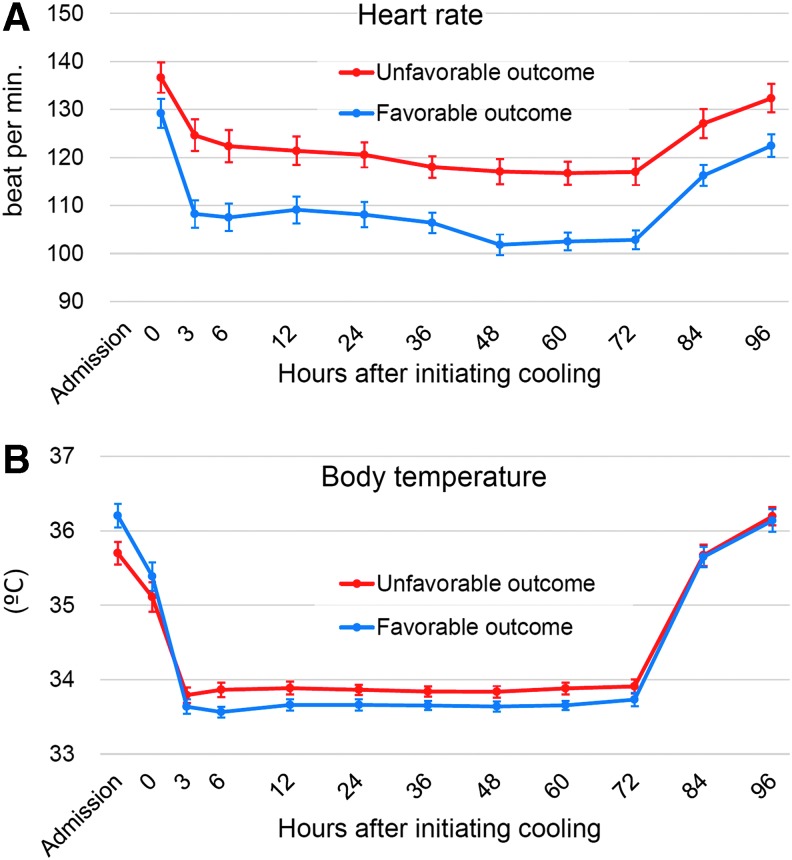
Death during initial hospitalization was associated with higher Thompson encephalopathy scores at admission (p <0.001); higher heart rates after 3–72 hours of cooling (p < 0.001); and higher body temperature after 24 hours of cooling (p = 0.002) (Table 1, Fig. 2, and Supplementary Table S1). Multivariate analysis was not performed because of the small number of mortality events. Potential dependence of the outcome on 10 minutes Apgar scores; Thompson encephalopathy scores after 24 hours of cooling; heart rates after 24 hours of cooling; mean blood pressure at initiating cooling and after 24 hours of cooling; and body temperature at admission, at initiating cooling; and mean values after 3–72 hours of cooling was lost after correction for multiple comparisons.
FIG. 2.
Temporal changes of heart rate (A) and body temperature (B) in infants with favorable and unfavorable outcomes. Values are shown as mean (95% confidence interval). Data at admission were not collected for the heart rate.
Independent variables of survival discharge within 28 days of life
Survival discharge ≤28 days was associated with higher 10 minutes Apgar scores (p < 0.001); higher blood pH and base excess (both p < 0.001); lower Thompson encephalopathy scores (at admission and after 24 hours of cooling; both p < 0.001); lower heart rates at initiating cooling (p = 0.003) and after 24 hours of cooling (p < 0.001); and lower mean values after 3–72 hours of cooling (p < 0.001); higher body temperature at admission (p < 0.001); and lower body temperature after 24 hours and lower mean values after 3–72 hours of cooling (both p < 0.001) (Table 1, Fig. 2, and Supplementary Table S1). Potential dependence of the outcome on greater gestational age, inborn, whole-body cooling, and higher body temperature at initiating cooling was lost after correction for multiple comparisons.
The multivariate logistic regression model to estimate survival discharge ≤28 days at the time of cooling initiation comprised inborn (p < 0.05), greater gestational age (p < 0.05), higher blood gas pH (p < 0.005), lower heart rate at initiation of cooling (p < 0.001), and higher body temperature at admission (p < 0.001) (Table 2). When clinical variables obtained up to 24 hours after initiation of cooling were incorporated, the final model to predict survival discharge ≤28 days consisted of higher blood gas pH (p < 0.05), higher body temperature at admission (p < 0.01), lower body temperature after 24 hours of cooling (p < 0.01), and lower heart rate after 24 hours of cooling (p < 0.001) (Table 3).
Table 2.
Multivariate Model Using Variables Available at the Commencement of Cooling
| 95% CI | ||||
|---|---|---|---|---|
| Odds ratio | Lower | Upper | p | |
| Birth location (inborn) | 1.604 | 1.049 | 2.453 | 0.029 |
| Gestational age (per week) | 1.140 | 1.019 | 1.276 | 0.022 |
| Cord or first blood gas pH (per 0.1 change) | 1.148 | 1.046 | 1.258 | 0.003 |
| Heart rate at 0 houra (per 10 beat/min) | 0.824 | 0.741 | 0.917 | <0.001 |
| Body temperature at admission (per degree) | 1.364 | 1.149 | 1.620 | <0.001 |
Statistical significance was assumed for p < 0.05 (indicated in bold).
After initiating cooling.
Table 3.
Multivariate Model Using Variables Available After 24 Hours of Cooling
| 95% CI | ||||
|---|---|---|---|---|
| Odds ratio | Lower | Upper | p | |
| Cord or first blood gas pH (per 0.1 change) | 1.112 | 1.006 | 1.228 | 0.037 |
| Body temperature at admission (per degree) | 1.271 | 1.076 | 1.503 | 0.005 |
| Heart rate at 24 hoursa (per 10 beat/min) | 0.689 | 0.594 | 0.799 | <0.001 |
| Body temperature at 24 hoursa (per degree) | 0.558 | 0.372 | 0.838 | 0.005 |
After initiating cooling. Statistical significance was assumed for p < 0.05 (indicated in bold).
Control variables of heart rate and body temperature
Higher heart rate at initiation of cooling was associated with lower blood base excess (p < 0.01), higher Thompson encephalopathy scores at admission (p < 0.01), and higher body temperature at admission (p < 0.001) (Supplementary Table S2). The higher heart rate after 24 hours of cooling could be best explained by the lower first blood base excess, higher Thompson encephalopathy scores at admission, and higher body temperature after 24 hours of cooling (all p < 0.001) (Supplementary Table S3). Higher body temperature at admission was associated with inborn (p < 0.01), greater birth weight (p < 0.001), higher first blood base excess (p < 0.005), and smaller Thompson encephalopathy scores (p < 0.05) (Supplementary Table S4). Higher body temperature after 24 hours of cooling was associated with lower 10 minutes Apgar score (p < 0.01) and selective-head cooling (p < 0.001). In a cohort of outborn infants, the body temperature at admission was dependent on the target body temperature during transportation (p < 0.001, r = 0.394) (Supplementary Table S5).
Discussion
Using a large-scale data set from a national registry, we identified a range of potentially important clinical variables associated with outcomes of cooled infants. Outborn, younger gestational age, and higher heart rate before, during, and after cooling were associated with adverse outcomes. Higher body temperature at admission and lower body temperature during cooling were paradoxically associated with survival discharge ≤28 days.
Heart rate and outcome
Bradycardia is a major physiological response to hypothermia (Thoresen et al., 2000; Erecinska et al., 2003). Studies of out-of-hospital cardiac arrest suggested that sinus bradycardia of <50–60 beat/min under cooling is associated with favorable outcomes (Staer-Jensen et al., 2014; Thomsen et al., 2015). Elstad et al. (2016) found in 60 cooled newborn infants that poor outcome is associated with higher heart rate after 12 hours of birth. In a large cohort of newborn infants, we confirmed that higher heart rates before, during, and after cooling are consistently associated with adverse outcome. The precise mechanism remains unclear, however, following severe hypoxia–ischemia, sympathetic stimulation due to excessive release of excitatory neurotransmitters may impair autoregulation of the cardiac system (Drury et al., 2014; Govindan et al., 2016). Restricted cardiac output and relatively higher body temperature may also be a common causative of tachycardia and adverse outcomes. However, in our data, tachycardia was not associated with hypotension; the relationship between tachycardia and adverse outcomes was observed even when corrected for the influence of body temperature. Although we did not obtain information on the use of inotropic and sedative drugs, it is possible that these therapeutic options were predominantly used for relatively more severely asphyxiated infants, resulting in a spurious correlation between tachycardia and adverse outcomes. Further studies are required to investigate the interactions between body temperature, heart rate, and outcome by incorporating various clinical variables, including cardiac support.
Body temperature and outcome
Preclinical studies consistently demonstrated the dependence of the outcome on the timing of cooling initiation (Davidson et al., 2015; Thoresen, 2015). However, this has not been confirmed in clinical studies. In addition, unlike induced hypothermia, spontaneous temperature reduction before initiating cooling might have a different influence on the outcome. Spontaneous hypothermia following severe hypoxia–ischemia or behavioral hypothermia has been observed in numerous vertebrates (Wood et al., 1996). Using a postnatal day 7 rat pup model of hypoxia–ischemia, Wood et al. (2017) demonstrated that spontaneous body temperature reduction of <32.2°C 1 hour after hypoxia–ischemia is associated with more severe brain injury, whereas active cooling to 32°C improved histopathological brain injury compared with normothermic temperature management. Consistent with these findings, in our study population, lower body temperature at admission and higher body temperature during cooling were paradoxically associated with adverse outcomes. Several explanations are possible. Severe hypoxia–ischemia may trigger self-protection programs to downregulate intrinsic thermogenesis (Wood et al., 1996). Profound brain stem injury may affect the thermoregulatory response to heat loss (George et al., 2004). Our data suggested that lower body temperature at admission was associated with outborn, whereas the body temperature in outborn infants was dependent on the target body temperature during transportation. It is possible that the initiation of cooling before receiving intensive care is deleterious under certain circumstances.
Regarding the cooling temperature, experimental studies suggested that even subtle temperature differences may alter the neuroprotective effect of hypothermia (Leonov et al., 1990; Iwata et al., 2005; Wood et al., 2017); however, this trend has not been confirmed in the clinical setting. Although a recent large-scale trial did not find any additional benefits of cooling newborn infants to 32°C (Shankaran et al., 2017), our data suggested the potential advantage of using relatively lower cooling temperatures within the currently recommended range. In our study, slightly higher temperature levels during cooling were primarily associated with selective-head cooling, which uses 1°C-higher body temperature than whole-body cooling. Hoque et al. (2010) also reported that the fluctuation of the rectal temperature was greater for selective-head cooling compared with whole-body cooling. Potential advantages in using whole-body cooling need to be investigated.
Other independent variables of outcome
Our data confirmed the dependence of outcomes of cooled infants on established markers for the severity of hypoxia–ischemia and encephalopathy (Sarnat et al., 1976; Thompson et al., 1997; van de Riet et al., 1999; Malin et al., 2010). In addition, outborn and younger gestational age were identified as independent variables of adverse outcomes. Rao et al. (2017) assessed the safety of cooling preterm infants (34–35 weeks' gestation), compared the results with those of term infants, and found consistent trends toward increased adverse events, such as hypo/hyperglycemia, MRI brain lesions, and death. In our study cohort, the dependence of outcomes on gestational age was consistently observed even when the analysis was repeated after excluding 18 infants <36 weeks gestation (data not shown). A special consideration would be necessary in cooling infants with a relatively younger gestational age.
Limitations
Because of the revision of the national guideline for the handling of the clinical data in 2017, the Baby Cooling Registry of Japan is currently suspending the data collection. Subsequently, the outcome was assessed using short-term endpoints. Furthermore, multivariate models were developed only for survival discharge ≤28 days due to the low mortality rate. The timing of discharge is affected by subjective decisions. However, we rarely encounter near-term and term infants requiring prolonged hospitalization >28 days unless they have serious respiratory/feeding problems. Indeed, of 198 infants who were discharged home after 28 days, 35.9% were dependent on continuous respiratory support and/or enteral feeding, as opposed to none requiring medical care for ones discharged ≤28 days. We previously speculated that a Japanese cultural background, where withdrawal from life support is relatively uncommon, is at least, in part, responsible for the low mortality rate of cooled infants in our registry (Tsuda et al., 2017). Therefore, careful consideration is required when interpreting our current findings into clinical practice in other part of the world.
Because of these limitations, the precise relationships between the clinical variables and outcomes largely remain unknown. Nonetheless, the use of short-term measures would be justified when the safety of specific cooling procedures is concerned (Gunn et al., 1998; Thoresen et al., 2000; Shankaran et al., 2014; Rao et al., 2017). We believe that clinicians and researchers, who wish to improve the outcome of infants with neonatal encephalopathy, should be aware of our preliminary findings, especially of the possible risk of relatively lower body temperature at admission and relatively higher body temperature during cooling in some specific conditions.
Conclusions
In addition to the established outcome markers of cooled infants, greater gestational age, inborn, higher body temperature at admission, lower body temperature during cooling, and bradycardia before, during, and after cooling were identified as potential independent variables of favorable short-term outcomes. With further investigations, these novel variables may help improve the therapeutic regimen for neonatal encephalopathy by (1) uncovering new mechanisms (and therapeutic target) of brain injury, (2) improving the algorithm of outcome prediction, and (3) renewing the criteria for patient selection. Prospective studies need to investigate the cause-consequence relationships between body temperature, heart rate, and outcomes. Meanwhile, secondary analyses of pooled data from previous large-scale trials should be conducted.
Supplementary Material
Acknowledgments
The authors are grateful to the staff of participating centers for their contribution to the data collection, and the infants and their parents for sharing the clinical information. This work was supported by the Japan Society of Perinatal and Neonatal Medicine, and the Ministry of Health, Labour and Welfare, Japan (H27-001, Special research in perinatal medicine). Dr. Tsuda is funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research B26860856). Dr. S. Iwata was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C15K09733). Dr. Takeuchi was funded by the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research C15K09737). Dr. Takenouchi was funded by Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research C16K09974 and B17H04232). Dr. O. Iwata was funded by the Japan Science and Technology Agency and the Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research C16K09005). The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Baby Cooling Registry of Japan Collaboration Team: Hiroyuki Adachi, Department of Pediatrics, Akita University Hospital, Akita, Japan.; Satoru Aiba, Department of Pediatrics, Yamagata Prefectural Central Hospital, Yamagata, Japan.; Shinnosuke Akiyoshi, Division of Neonatology, Ehime Prefectural Central Hospital, Ehime, Japan.; Takasuke Amizuka, Division of Neonatology, Aomori Prefectural Central Hospital, Aomori, Japan.; Mikihiro Aoki, Department of Pediatrics, National Hospital Organization Nagasaki Medical Center, Nagasaki, Japan.; Hirokazu Arai, Division of Neonatology, Japanese Red Cross Akita Hospital, Akita, Japan.; Junichi Arai, Division of Neonatology, Ibaraki Children's Hospital, Ibaraki, Japan.; Hideomi Asanuma, Division of Neonatology, Hokkaido Medical Center for Child Health and Rehabilitation, Hokkaido, Japan.; Atsushi Baba, Department of Pediatrics, Shinshu University Hospital, Nagano, Japan.; Motoki Bonno, Division of Neonatology, National Hospital Organization Mie Chuo Medical Center, Mie, Japan.; Yusuke Daimon, Department of Pediatrics, Tomakomai City Hospital, Hokkaido, Japan.; Tomoko Egashira, Department of Pediatrics, National Hospital Organization Saga Hospital, Saga, Japan.; Rie Fukuhara, Division of Neonatology, Hiroshima Prefectural Hospital, Hiroshima, Japan.; Naoki Fukushima, Division of Neonatology, Almeida Memorial Hospital, Oita, Japan.; Yasumasa Yamada, Department of Pediatrics, Aichi Medical University Hospital, Aichi, Japan.; Sayaka Harada, Department of Neonatology, Osaka City General Hospital, Osaka, Japan.; Masahiro Hayakawa, Division of Neonatology, Center for Maternal-Neonatal Care, Nagoya University Hospital, Aichi, Japan.; Nobuhide Henmi, Division of Neonatal Intensive Care Unit, Tokyo Women's Medical University Medical Center East, Tokyo, Japan.; Takehiko Hiroma, Division of Neonatology, Nagano Children's Hospital, Nagano, Japan.; Tadashi Hisano, Division of Neonatology, St. Mary's Hospital, Fukuoka, Japan.; Kuniko Ieda, Division of Neonatology, Tosei General Hospital, Aichi, Japan.; Koichi Iida, Division of Neonatology, Oita Prefectural Hospital, Oita, Japan.; Shigeo Iijima, Perinatal Center, Hamamatsu University School of Medicine, Shizuoka, Japan.; Ken Imai, Department of Neonatology, Tokyo Women's Medical University, Tokyo, Japan.; Takashi Imamura, Department of Pediatrics, Takeda General Hospital, Fukushima, Japan.; Shinkai Inoue, Department of Pediatrics, Fukuoka University Hospital, Fukuoka, Japan.; Akio Ishiguro, Division of Neonatology, Center for Maternal, Fetal and Neonatal Medicine, Saitama Medical Center, Saitama Medical University, Saitama, Japan.; Tsutomu Ishii, Department of Pediatrics, Fukushima National Hospital, Fukushima, Japan.; Manabu Kenmochi, Department of Pediatrics, Kitasato University Hospital, Kanagawa, Japan.; Masanori Iwai, Department of Pediatrics, Kumamoto University Hospital, Kumamoto, Japan.; Shinnichiro Iwataki, Department of Pediatrics, Onomichi Hospital, Hiroshima, Japan.; Wataru Jinnai, Division of Neonatology, Shikoku Medical Center for Children and Adults, Kagawa, Japan.; Akihiko Kai, Department of Pediatrics, Aizenbashi Hospital, Osaka, Japan.; Taro Kanbe, Department of Pediatrics, Sasebo City General Hospital, Nagasaki, Japan.; Hiroshi Kanda, Division of Neonatology, Iizuka Hospital, Fukuoka, Japan.; Masatoshi Kaneko, Department of Pediatrics, Fukushima Medical University, Fukushima, Japan.; Takenori Kato, Department of Neonatology and Pediatrics, Nagoya City University Graduate School of Medical Sciences, Aichi, Japan.; Akihiko Kawase, Division of Neonatology, Kumamoto City Hospital, Kumamoto, Japan.; Hitoshi Kawato, Division of Neonatology, Narita Red Cross Hospital, Chiba, Japan.; Yoshikazu Kida, Division of Neonatology, Matsudo City Hospital, Chiba, Japan.; Masahiro Kinoshita, Department of Paediatrics and Child Health, Kurume University School of Medicine, Fukuoka, Japan.; Makoto Kishigami, Department of Neonatology, Kanagawa Children's Medical Center, Kanagawa, Japan.; Hiroyuki Kitano, Division of Neonatology, Ishikawa Prefectural Central Hospital, Ishikawa, Japan.; Osamu Kito, Division of Neonatology, Japanese Red Cross Nagoya First Hospital, Aichi, Japan.; Akira Kobayashi, Department of Pediatrics, Nagaoka Red Cross Hospital, Niigata, Japan.; Yoshinori Kohno, Division of Neonatology, Gifu Prefectural General Medical Center, Gifu, Japan.; Minoru Kokubo, Department of Pediatrics, Kainan Hospital, Aichi, Japan.; Masatoshi Kondo, Department of Neonatology, Tokyo Metropolitan Children's Medical Center, Tokyo, Japan.; Eri Konishi, Department of Pediatrics, Matsue Red Cross Hospital, Shimane, Japan.; Takahiro Sugiura, Division of Neonatology, Toyohashi Municipal Hospital, Aichi, Japan.; Masaaki Kugo, Department of Pediatrics, Perinatal Medical Center, Himeji Red Cross Hospital, Hyogo, Japan.; Takeshi Kumagai, Neonatal Intensive Care Unit, Department of Pediatrics, Wakayama Medical University Hospital, Wakayama, Japan.; Takashi Kusaka, Division of Neonatology, Kagawa University Hospital, Kagawa, Japan.; Takeshi Kusuda, Department of Pediatrics, Yamaguchi University Hospital, Yamaguchi, Japan.; Tomoki Maeda, Department of Pediatrics, Oita University Hospital, Oita, Japan.; Yoshinobu Maede, Division of Neonatology, Perinatal Medical Center, Kagoshima City Hospital, Kagoshima, Japan.; Tomoaki Maji, Department of Pediatrics and Neonatology, Japan Red Cross Ise Hospital, Mie, Japan.; Tomoko Makiya, Division of Neonatology, Okinawa Prefectural Chubu Hospital, Okinawa, Japan.; Kenichi Maruyama, Department of Neonatology, Gunma Children's Medical Center, Gunma, Japan.; Ken Masunaga, Division of Neonatology, Tokyo Metropolitan Ohtsuka Hospital, Tokyo, Japan.; Atsushi Matsumoto, Department of Pediatrics, Iwate Medical University, Iwate, Japan.; Hiroshi Matsumoto, Division of Neonatology, Asahi General Hospital, Chiba, Japan.; Naoko Matsumoto, Department of Pediatrics, Kitakyushu Municipal Medical Center, Fukuoka, Japan.; Aya Mima, Department of Pediatrics, Yodogawa Christian Hospital, Osaka, Japan.; Kyoko Minagawa, Department of Pediatrics, Hyogo College of Medicine College Hospital, Hyogo, Japan.; Yoshihiro Minosaki, Neonatal Center, Kawaguchi Municipal Medical Center, Saitama, Japan.; Hideki Minowa, Division of Neonatology, Nara Prefecture General Medical Center, Nara, Japan.; Mazumi Miura, Division of Pediatrics and Perinatology, Tottori University Faculty of Medicine, Tottori, Japan.; Masafumi Miyata, Department of Pediatrics, Fujita Health University School of Medicine, Aichi, Japan.; Yayoi Miyazono, Department of Child Health, Faculty of Medicine, University of Tsukuba, Ibaraki, Japan.; Hiroshi Mizumoto, Department of Pediatrics, Kitano Hospital, Osaka, Japan.; Masato Mizushima, Division of Neonatology, Sapporo City General Hospital, Hokkaido, Japan.; Kazuhiro Mori, Department of Pediatrics, Tokushima Prefectural Central Hospital, Tokushima, Japan.; Ichiro Morioka, Department of Pediatrics, Kobe University Hospital, Hyogo, Japan.; Takeshi Morisawa, Department of Pediatrics, Kakogawa West City Hospital, Hyogo, Japan.; Ken Nagaya, Division of Neonatal Intensive Care Unit, Asahikawa Medical University Hospital, Hokkaido, Japan.; Yoshihisa Nagayama, Division of Neonatology, Niigata City General Hospital, Niigata, Japan.; Atsushi Naito, Division of Neonatology, Yamanashi Prefectural Central Hospital, Yamanashi, Japan.; Kenji Nakamura, Division of Neonatology, Japanese Red Cross Otsu Hospital, Shiga, Japan.; Makoto Nakamura, Division of Neonatology, National Hospital Organization Okayama Medical Center, Okayama, Japan; Masako Nakamura, Department of Pediatrics, Kurashiki Central Hospital, Okayama, Japan.; Atsushi Nakao, Division of Neonatology, Japanese Red Cross Medical Center, Tokyo, Japan.; Hideto Nakao, Division of Neonatology, Hyogo Prefectural Kobe Children's Hospital, Hyogo, Japan.; Yusuke Nakazawa, Division of Neonatology, Shizuoka Children's Hospital, Shizuoka, Japan; Yutaka Nishimura, Division of Neonatology, Hiroshima City Hiroshima Citizens Hospital, Hiroshima, Japan.; Naoto Nishizaki, Perinatal Medical Center, Juntendo University Urayasu Hospital, Chiba, Japan.; Kazuhiko Nosaka, Division of Neonatology, Fukui Prefectural Hospital, Fukui, Japan.; Masatoshi Nozaki, Department of Neonatal Medicine,Osaka Women's and Children's Hospital, Osaka, Japan.; Masayuki Ochiai, Department of Pediatrics, Kyushu University Hospital, Fukuoka, Japan.; Atsushi Ohashi, Department of Pediatrics, Kansai Medical University Hospital, Osaka, Japan.; Shigeru Ohki, Division of Neonatology, Seirei Hamamatsu General Hospital, Shizuoka, Japan.; Isaku Omori, Division of Neonatology, Tokyo Metropolitan Bokutoh Hospital, Tokyo, Japan.; Yoshiteru Osone, Division of Neonatology, Kimitsu Chuo Hospital, Chiba, Japan.; Junko Saito, Division of Neonatology, Miyagi Children's Hospital, Miyagi, Japan.; Yoshitake Sato, Department of Pediatrics, Ota Memorial Hospital, Gunma, Japan.; Kazuo Seki, Yokohama City University Medical Center, Perinatal Center, Kanagawa, Japan.; Naoaki Shibata, Department of Pediatrics, Shimane University School of Medicine, Shimane, Japan.; Yoshitsugu Shirakawa, Division of Neonatology, Fukuoka Shin Mizumaki Hospital, Fukuoka, Japan.; Hiroyuki Shiro, Division of Neonatology, Yokohama Rosai Hospital, Kanagawa, Japan.; Keiji Suzuki, Department of Pediatrics, Tokai University Hospital, Kanagawa, Japan.; Hiroshi Suzumura, Department of Pediatrics, Dokkyo Medical University Hospital, Tochigi, Japan.; Ritsuko Takahashi, Division of Neonatology, Japanese Red Cross Sendai Hospital, Miyagi, Japan.; Yasushi Takahata, Division of Neonatology, Fukuoka Children's Hospital, Fukuoka, Japan.; Atsuko Taki, Division of Neonatology, Tokyo Medical and Dental University, University Hospital of Medicine, Tokyo, Japan.; Taihei Tanaka, Division of Neonatology, Japanese Red Cross Nagoya Daini Hospital, Aichi, Japan.; Koichi Tanda, Department of Pediatrics, Japanese Red Cross Kyoto Daiichi Hospital, Kyoto, Japan.; Itaru Tateishi, Department of Pediatrics, Saiseikai Yokohamashi Tobu Hospital, Kanagawa, Japan.; Chihiro Tsuchiyama, Department of Paediatrics and Child Health, Kurume University School of Medicine, Fukuoka, Japan.; Mikio Tsunei, Department of Pediatrics, Tottori Prefectural Central Hospital, Tottori, Japan.; Touhei Usuda, Maternal and Perinatal Center, Niigata University Medical & Dental Hospital, Niigata, Japan.; Yukari Yada, Division of Neonatology, Jichi Medical University Hospital, Tochigi, Japan.; Junko Yamamoto, Division of Neonatology, Japan Community Healthcare Organization Kyushu Hospital, Fukuoka, Japan.; Masahito Yamamoto, Department of Pediatrics, Nagahama Red Cross Hospital, Shiga, Japan.; Hitoshi Yoda, Division of Neonatology, Toho University Omori Medical Center, Tokyo, Japan.; Akiko Yokoi, Department of Pediatrics, Nagoya City West Medical Center, Aichi, Japan.; Shinobu Yoshida, Department of Pediatrics, Omihachiman Community Medical Center, Shiga, Japan.; Taketoshi Yoshida, Department of Pediatrics, Toyama University Hospital, Toyama, Japan.; Tomohide Yoshida, Department of Pediatrics, University of the Ryukyus Hospital, Okinawa, Japan.; Kayo Yoshikawa, Division of Neonatology, Nihon University Itabashi Hospital, Tokyo, Japan.
Contributor Information
Collaborators: On behalf of The Baby Cooling Registry of Japan Collaboration Team, Hiroyuki Adachi, Satoru Aiba, Shinnosuke Akiyoshi, Takasuke Amizuka, Mikihiro Aoki, Hirokazu Arai, Junichi Arai, Hideomi Asanuma, Atsushi Baba, Motoki Bonno, Yusuke Daimon, Tomoko Egashira, Rie Fukuhara, Naoki Fukushima, Yasumasa Yamada, Sayaka Harada, Masahiro Hayakawa, Nobuhide Henmi, Takehiko Hiroma, Tadashi Hisano, Kuniko Ieda, Koichi Iida, Shigeo Iijima, Ken Imai, Takashi Imamura, Shinkai Inoue, Akio Ishiguro, Tsutomu Ishii, Manabu Kenmochi, Masanori Iwai, Shinnichiro Iwataki, Wataru Jinnai, Akihiko Kai, Taro Kanbe, Hiroshi Kanda, Masatoshi Kaneko, Takenori Kato, Akihiko Kawase, Hitoshi Kawato, Yoshikazu Kida, Masahiro Kinoshita, Makoto Kishigami, Hiroyuki Kitano, Osamu Kito, Akira Kobayashi, Yoshinori Kohno, Minoru Kokubo, Masatoshi Kondo, Eri Konishi, Takahiro Sugiura, Masaaki Kugo, Takeshi Kumagai, Takashi Kusaka, Takeshi Kusuda, Tomoki Maeda, Yoshinobu Maede, Tomoaki Maji, Tomoko Makiya, Kenichi Maruyama, Ken Masunaga, Atsushi Matsumoto, Hiroshi Matsumoto, Naoko Matsumoto, Aya Mima, Kyoko Minagawa, Yoshihiro Minosaki, Hideki Minowa, Mazumi Miura, Masafumi Miyata, Yayoi Miyazono, Hiroshi Mizumoto, Masato Mizushima, Kazuhiro Mori, Ichiro Morioka, Takeshi Morisawa, Ken Nagaya, Yoshihisa Nagayama, Atsushi Naito, Kenji Nakamura, Makoto Nakamura, Masako Nakamura, Atsushi Nakao, Hideto Nakao, Yusuke Nakazawa, Yutaka Nishimura, Naoto Nishizaki, Kazuhiko Nosaka, Masatoshi Nozaki, Masayuki Ochiai, Atsushi Ohashi, Shigeru Ohki, Isaku Omori, Yoshiteru Osone, Junko Saito, Yoshitake Sato, Kazuo Seki, Naoaki Shibata, Yoshitsugu Shirakawa, Hiroyuki Shiro, Keiji Suzuki, Hiroshi Suzumura, Ritsuko Takahashi, Yasushi Takahata, Atsuko Taki, Taihei Tanaka, Koichi Tanda, Itaru Tateishi, Chihiro Tsuchiyama, Mikio Tsunei, Touhei Usuda, Yukari Yada, Junko Yamamoto, Masahito Yamamoto, Hitoshi Yoda, Akiko Yokoi, Shinobu Yoshida, Taketoshi Yoshida, Tomohide Yoshida, and Kayo Yoshikawa
Author Disclosure Statement
No competing financial interests exist.
References
- Azzopardi D, Strohm B, Linsell L, Hobson A, Juszczak E, Kurinczuk JJ, Brocklehurst P, Edwards AD. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK—analysis of national data. PLoS One 2012;7:e38504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med 2014;371:140–149 [DOI] [PubMed] [Google Scholar]
- Basu SK, Salemi JL, Gunn AJ, Kaiser JR. Hyperglycaemia in infants with hypoxic-ischaemic encephalopathy is associated with improved outcomes after therapeutic hypothermia: a post hoc analysis of the CoolCap Study. Arch Dis Child Fetal Neonatal Ed 2017;102:F299–F306 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran M, Chaban B, Montaldo P, Thayyil S. Predictive value of amplitude-integrated EEG (aEEG) after rescue hypothermic neuroprotection for hypoxic ischemic encephalopathy: a meta-analysis. J Perinatol 2017;37:684–689 [DOI] [PubMed] [Google Scholar]
- Chouthai NS, Sobczak H, Khan R, Subramanian D, Raman S, Rao R. Hyperglycemia is associated with poor outcome in newborn infants undergoing therapeutic hypothermia for hypoxic ischemic encephalopathy. J Neonatal Perinatal Med 2015;8:125–131 [DOI] [PubMed] [Google Scholar]
- Davidson JO, Wassink G, Yuill CA, Zhang FG, Bennet L, Gunn AJ. How long is too long for cerebral cooling after ischemia in fetal sheep? J Cereb Blood Flow Metab 2015;35:751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio R, Ochoa C, Alarcon A, Arnaez J, Blanco D, Garcia-Alix A. Amplitude integrated electroencephalogram as a prognostic tool in neonates with hypoxic-ischemic encephalopathy: a systematic review. PLoS One 2016;11:e0165744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury PP, Gunn ER, Bennet L, Gunn AJ. Mechanisms of hypothermic neuroprotection. Clin Perinatol 2014;41:161–175 [DOI] [PubMed] [Google Scholar]
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010;340:c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstad M, Liu X, Thoresen M. Heart rate response to therapeutic hypothermia in infants with hypoxic-ischaemic encephalopathy. Resuscitation 2016;106:53–57 [DOI] [PubMed] [Google Scholar]
- Erecinska M, Thoresen M, Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab 2003;23:513–530 [DOI] [PubMed] [Google Scholar]
- Gagne-Loranger M, Sheppard M, Ali N, Saint-Martin C, Wintermark P. Newborns referred for therapeutic hypothermia: association between initial degree of encephalopathy and severity of brain injury (What About the Newborns with Mild Encephalopathy on Admission?). Am J Perinatol 2016;33:195–202 [DOI] [PubMed] [Google Scholar]
- George S, Gunn AJ, Westgate JA, Brabyn C, Guan J, Bennet L. Fetal heart rate variability and brain stem injury after asphyxia in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol 2004;287:R925–R933 [DOI] [PubMed] [Google Scholar]
- Govindan RB, Al-Shargabi T, Massaro AN, Metzler M, Andescavage NN, Joshi R, Dave R, du Plessis A. Baroreflex dysfunction in sick newborns makes heart rate an unreliable surrogate for blood pressure changes. Pediatr Res 2016;79:929–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet R, Edwards AD, Thoresen M, Ferriero DM, Gluckman PD, Whitelaw A, Gunn AJ. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr Res 2012;71:205–209 [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics 1998;102(4 Pt 1):885–892 [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Thoresen M. Animal studies of neonatal hypothermic neuroprotection have translated well in to practice. Resuscitation 2015;97:88–90 [DOI] [PubMed] [Google Scholar]
- Hoque N, Chakkarapani E, Liu X, Thoresen M. A comparison of cooling methods used in therapeutic hypothermia for perinatal asphyxia. Pediatrics 2010;126:e124–e130 [DOI] [PubMed] [Google Scholar]
- Iwata O, Thornton JS, Sellwood MW, Iwata S, Sakata Y, Noone MA, O'Brien FE, Bainbridge A, De Vita E, Raivich G, Peebles D, Scaravilli F, Cady EB, Ordidge R, Wyatt JS, Robertson NJ. Depth of delayed cooling alters neuroprotection pattern after hypoxia-ischemia. Ann Neurol 2005;58:75–87 [DOI] [PubMed] [Google Scholar]
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013;1:CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonov Y, Sterz F, Safar P, Radovsky A, Oku K, Tisherman S, Stezoski SW. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab 1990;10:57–70 [DOI] [PubMed] [Google Scholar]
- Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ 2010;340:c1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas A, Shankaran S, Laptook AR, Langer JC, Bara R, Ehrenkranz RA, Goldberg RN, Das A, Higgins RD, Tyson JE, Walsh MC. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J Pediatr 2011;158:752–758.e751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman JM, Wyllie J, Kattwinkel J, Wyckoff MH, Aziz K, Guinsburg R, Kim HS, Liley HG, Mildenhall L, Simon WM, Szyld E, Tamura M, Velaphi S. Part 7: neonatal resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2015;132(16 Suppl 1):S204–S241 [DOI] [PubMed] [Google Scholar]
- Rao R, Trivedi S, Vesoulis Z, Liao SM, Smyser CD, Mathur AM. Safety and short-term outcomes of therapeutic hypothermia in preterm neonates 34–35 weeks gestational age with hypoxic-ischemic encephalopathy. J Pediatr 2017;183:37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins N, Booth T, Morriss MC, Sanchez P, Heyne R, Chalak L. Predictive value of neonatal MRI showing no or minor degrees of brain injury after hypothermia. Pediatr Neurol 2014;50:447–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696–705 [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, Poindexter BB, Schibler K, Bell EF, Heyne RJ, Pedroza C, Bara R, Van Meurs KP, Grisby C, Huitema CM, Garg M, Ehrenkranz RA, Shepherd EG, Chalak LF, Hamrick SE, Khan AM, Reynolds AM, Laughon MM, Truog WE, Dysart KC, Carlo WA, Walsh MC, Watterberg KL, Higgins RD. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA 2014;312:2629–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, Poindexter BB, Schibler K, Bell EF, Heyne RJ, Pedroza C, Bara R, Van Meurs KP, Huitema CMP, Grisby C, Devaskar U, Ehrenkranz RA, Harmon HM, Chalak LF, DeMauro SB, Garg M, Hartley-McAndrew ME, Khan AM, Walsh MC, Ambalavanan N, Brumbaugh JE, Watterberg KL, Shepherd EG, Hamrick SEG, Barks J, Cotten CM, Kilbride HW, Higgins RD. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy: a randomized clinical trial. JAMA 2017;318:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 2012;366:2085–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes JH, Elstad M, Thoresen M, Cowan FM, Stiris T, Fugelseth D. Hypothermia makes cerebral resistance index a poor prognostic tool in encephalopathic newborns. Neonatology 2014;106:17–23 [DOI] [PubMed] [Google Scholar]
- Staer-Jensen H, Sunde K, Olasveengen TM, Jacobsen D, Draegni T, Nakstad ER, Eritsland J, Andersen GO. Bradycardia during therapeutic hypothermia is associated with good neurologic outcome in comatose survivors of out-of-hospital cardiac arrest. Crit Care Med 2014;42:2401–2408 [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Rubens EO, Yap VL, Ross G, Engel M, Perlman JM. Delayed onset of sleep-wake cycling with favorable outcome in hypothermic-treated neonates with encephalopathy. J Pediatr 2011;159:232–237 [DOI] [PubMed] [Google Scholar]
- Thompson CM, Puterman AS, Linley LL, Hann FM, van der Elst CW, Molteno CD, Malan AF. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr 1997;86:757–761 [DOI] [PubMed] [Google Scholar]
- Thomsen JH, Hassager C, Bro-Jeppesen J, Soholm H, Nielsen N, Wanscher M, Kober L, Pehrson S, Kjaergaard J. Sinus bradycardia during hypothermia in comatose survivors of out-of-hospital cardiac arrest—a new early marker of favorable outcome? Resuscitation 2015;89:36–42 [DOI] [PubMed] [Google Scholar]
- Thoresen M. Who should we cool after perinatal asphyxia? Semin Fetal Neonatal Med 2015;20:66–71 [DOI] [PubMed] [Google Scholar]
- Thoresen M, Whitelaw A. Cardiovascular changes during mild therapeutic hypothermia and rewarming in infants with hypoxic-ischemic encephalopathy. Pediatrics 2000;106(1 Pt 1):92–99 [DOI] [PubMed] [Google Scholar]
- Tsuda K, Mukai T, Iwata S, Shibasaki J, Tokuhisa T, Ioroi T, Sano H, Yutaka N, Takahashi A, Takeuchi A, Takenouchi T, Araki Y, Sobajima H, Tamura M, Hosono S, Nabetani M, Iwata O. Therapeutic hypothermia for neonatal encephalopathy: a report from the first 3 years of the Baby Cooling Registry of Japan. Sci Rep 2017;7:39508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Riet JE, Vandenbussche FP, Le Cessie S, Keirse MJ. Newborn assessment and long-term adverse outcome: a systematic review. Am J Obstet Gynecol 1999;180:1024–1029 [DOI] [PubMed] [Google Scholar]
- Walsh BH, Neil J, Morey J, Yang E, Silvera MV, Inder TE, Ortinau C. The frequency and severity of magnetic resonance imaging abnormalities in infants with mild neonatal encephalopathy. J Pediatr 2017;187:26–33.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SC, Gonzales R. Hypothermia in hypoxic animals: mechanisms, mediators, and functional significance. Comp Biochem Physiol B Biochem Mol Biol 1996;113:37–43 [DOI] [PubMed] [Google Scholar]
- Wood T, Hobbs C, Falck M, Brun AC, Loberg EM, Thoresen M. Rectal temperature in the first five hours after hypoxia-ischaemia critically affects neuropathological outcomes in neonatal rats. Pediatr Res 2017. DOI: 10.1038/pr.2017.51 [DOI] [PubMed] [Google Scholar]
- Wyatt JS, Gluckman PD, Liu PY, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 2007;119:912–921 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.