Abstract
The majority of human immunodeficiency virus (HIV) type 1 infections in infants are acquired orally through breastfeeding. Toward development of a pediatric HIV vaccine to prevent breastmilk transmission, we tested the efficacy of a simultaneous oral and intramuscular (IM) vaccination regimen for preventing oral simian immunodeficiency virus (SIV) transmission in infant rhesus macaques. Two groups of neonatal macaques were immunized with DNA encoding SIV virus-like particles (DNA-SIV) on weeks 0 and 3, then boosted with modified vaccinia Ankara (MVA) virus expressing SIV antigens (MVA-SIV) on weeks 6 and 9. One group was prime/boosted by the IM route only. Another group was immunized with DNA by both the IM and topical oral (O) buccal routes, and boosted with MVA-SIV by both the IM and sublingual (SL) routes. A third group of control animals received saline by O + IM routes on weeks 0 and 3, and empty MVA by SL + IM routes on weeks 6 and 9. On week 12, infants were orally challenged once weekly with SIVmac251 until infected. The vaccine regimen that included oral routes resulted in reduced peak viremia. The rate of infection acquisition in vaccinated infants was found to be associated with prechallenge intestinal immunoglobulin G (IgG) responses to SIV gp120 and V1V2. Peak viremia was inversely correlated with postinfection intestinal IgG responses to gp120, gp41, and V1V2. These results suggest that codelivery of a pediatric HIV vaccine by an oral route may be superior to IM-only regimens for generating mucosal antibodies and preventing HIV breastmilk transmission in neonates.
Keywords: HIV, SIV, pediatrics, oral, vaccine, buccal, sublingual
Introduction
Breastfeeding is critical for nutrient provision and passive immunity to infants in nonindustrialized nations, but it poses a considerable risk for postpartum mother-to-child transmission (MTCT) of HIV.1–4 Indeed, of the estimated 150,000 new cases of infant HIV infections in 2015, >50% have been acquired through breastfeeding.5 HIV breastmilk transmission has been dramatically reduced by initiating antiretroviral therapy (ART) early in pregnant women and maintaining treatment throughout lactation.6 However, 30% of infected women do not adequately comply with ART.7 In addition, 30%–40% of HIV-infected pregnant or breastfeeding women still do not have access to ART.5 Thus, MTCT breastmilk transmission of HIV remains unacceptably high, and development of additional interventions, such as a pediatric vaccine, is an immediate need.
A pediatric HIV vaccine will need to be administered immediately after birth and will likely require expedited booster vaccinations to rapidly generate sufficient antiviral immune responses. The immaturity of the neonatal innate and adaptive immune systems8–11 poses a challenge in generating high-quality HIV-specific immune responses, and likely explains in part why there has been limited success preventing oral simian immunodeficiency virus (SIV) or SHIV transmission in neonatal macaques immunized with vaccines by the conventional intramuscular (IM) route.12–14
However, the efficacy of these vaccines could potentially be improved if they were also administered by an oral route, which should induce greater immune responses at sites of oral viral entry, such as the tonsils and intestine.15–19 Using adult macaques, others have demonstrated the feasibility of delivering DNA, viral vectors, and proteins in the small intestine for induction of mucosal antibodies or T cells,20–22 and prevention or control of rectal or vaginal SIV infection.22,23 Vaccines topically applied to the buccal mucosa or tonsils in the oral cavity of adult macaques have also prevented infection or reduced viremia after challenge by oral, rectal, or vaginal routes.23–25 Recently, sublingual (SL) application of HIV envelope (Env) and SIV gag,pol expressing vaccinia virus vectors followed by IM gp120 boosting in adult macaques was shown to provide protection against rectal SHIV challenge.26 Thus, the easily accessible SL mucosa may be another efficacious oral delivery site for pediatric vaccines.
In a recent pilot study, we tested whether a DNA-SIV prime/modified vaccinia Ankara (MVA)-SIV boost regimen administered at distinct sites in the oral cavity could induce mucosal T and B cell responses in juvenile macaques.27 SIV-specific intestinal T cells but no mucosal or systemic antibody responses were generated when DNA-SIV was topically applied to the oral buccal mucosa (O), and the MVA-SIV was placed on top of either the palatine tonsils or the SL mucosa. However, simultaneous O + IM DNA priming followed by SL MVA boosting induced SIV-specific plasma immunoglobulin G (IgG), intestinal IgA, and T cells.27
Based on these findings, this study evaluated the ability of an O + IM DNA-SIV prime/SL + IM MVA-SIV boost regimen to protect infant macaques against SIVmac251 infection utilizing a repeated oral exposure model to simulate breastmilk transmission of HIV in human infants. Although the O + IM/SL + IM vaccine regimen did not prevent infection, it resulted in lower viremia. These beneficial effects were not observed in neonates, given the same vaccine components by the IM route alone. Thus, the results support the inclusion of oral immunization routes in addition to the traditional IM route for administration of pediatric vaccines intended to prevent HIV breastmilk transmission.
Materials and Methods
Animals
Rhesus macaques were housed in pairs according to the “Guide for Care and Use of Laboratory Animals” as outlined by the American Association for Assessment and Accreditation of Laboratory Animal Care at the California National Primate Research Center (Davis, CA). All animal procedures were approved by the UC Davis Institutional Animal Care and Use Committee before study implementation. The study included 18 nursery-reared rhesus macaques (Macaca mulatta) that were between 3 and 10 days old at the time of the first immunization.
All procedures were performed under ketamine anesthesia (10 mg/kg body weight) administered IM.
Vaccines
Neonates were primed with two previously described SIV/adjuvant-coexpressing DNA plasmids, DG and D40L.28–30 The amounts of granulocyte-macrophage colony-stimulating factor (GM-CSF) secreted in vivo after immunization of macaques with DG are not known, but transient transfection of 293T cells has been found to result in production of ∼200 ng GM-CSF per 106 cells.28 The D40L plasmid coexpresses SIVmac239 virus-like particles (VLP) and a membrane-bound form of macaque CD40L, resulting in secretion of VLP, which express CD40L on their surface.29 For oral immunizations, the two DNA plasmids were formulated in cationic 1,2-dioleoyl-3-trimethylammonium liposomes by Encapsula Nanosciences (Brentwood, TN). The recombinant DR2 MVA-SIVmac239 gag, pol, env, and empty MVA vectors used for boosting were provided by Dr. Bernie Moss (National Institute of Allergy and Infectious Diseases). Viral vectors were expanded and titrated for plaque forming units (PFUs) using chick embryo fibroblasts as previously described.31
Immunizations
The vaccination groups, vaccine dosages, and schedule are outlined in Table 1. Neonatal macaques in Groups B and C were immunized on weeks 0 and 3 with a 1:1 mixture of DG + D40L (hereafter referred to as DNA-SIV), and boosted on weeks 9 and 12 with MVA-SIV. Group B was IM immunized with a total of 3 mg DNA-SIV in 1 mL, and Group C received 1.5 mg DNA-SIV IM in 0.5 mL. The IM immunizations with DNA were done by injecting 250 μL (Group B) or 125 μL (Group C) into both the left and right quadriceps and biceps. For O immunizations (Group C), 0.7 mL of liposomal DNA-SIV was topically applied in each cheek pouch (1.4 mL total containing 1.5 mg DNA-SIV). All IM immunizations with MVA were done by injecting 0.5 × 107 PFUs in 0.25 mL into both the left and right quadriceps (108 PFU total). For SL administration of MVA-SIV, 108 PFU in 30 μL was placed under the tongue. Group A mock control animals were administered saline both orally (1 mL) and IM (0.25 mL) on weeks 0 and 3, then boosted SL + IM on weeks 9 and 12 with empty MVA by applying 108 PFU topically onto the SL mucosa and injecting 108 PFU into the quadriceps. It should be noted that neonates in Groups A and B were immunized (and challenged) in parallel. Age-matched Group C neonates started the immunization regimen 1 month later.
Table 1.
Vaccination Groups and Schedule
| Group | Prime (weeks 0 and 3) | Dose and route | Boost (weeks 6 and 9) | Dose and route | First oral SIVmac251 exposure |
|---|---|---|---|---|---|
| A (n = 6) | Saline | 1 mL O + 0.25 mL IM | Empty MVA | 108 PFU SL + 108 PFU IM | Week 12 |
| B (n = 6) | DNA-SIVa | 3.0 mg IMb | MVA-SIV | 108 PFU IM | Week 12 |
| C (n = 6) | DNA-SIV | 1.5 mg O + 1.5 mg IMc | MVA-SIV | 108 PFU SL + 108 PFU IM | Week 12 |
DNA-SIV: 1:1 mixture of DG + D40L.
1.5 mg DG + 1.5 mg D40L.
0.75 mg DG + 0.75 mg D40L by each route.
SIV, simian immunodeficiency virus; O, oral; IM, intramuscular; SL, sublingual; MVA, modified vaccinia Ankara; PFU, plaque forming units.
SIV challenge
Beginning on week 12, infant macaques were orally exposed once weekly to SIVmac251 (CNPRC Stock 08/12). Each dose consisted of 5 × 102 50% tissue culture infective doses in sucrose-containing RPMI1640 medium and was administered under ketamine anesthesia using a blunted syringe.14 Although the oral SIV challenge was initiated 4 weeks later in Group C infants, the weekly exposure regimen resulted in overlap of SIV challenge time points between Groups A and C, and SIV inocula were prepared from the same virus stock for infants in all groups. Animals were monitored for plasma viremia weekly using reverse transcription-polymerase chain reaction (RT-PCR) and considered to be infected after two consecutive positive results. Upon confirmation of systemic infection, the first positive time point was considered week 1 postinfection (pi). Approximately 12 weeks pi, infants were euthanized.
Plasma SIV RNA analysis
After the first challenge, virological analysis of plasma samples was performed weekly using RT-PCR for SIV RNA as described, but with manual RNA extraction due to limited plasma volumes.32,33 The limit of detection was 15 RNA copies/mL. Samples showing transient low viremia followed by SIV RNA-negative time points were retested to confirm the initial PCR results. Animals were considered to be persistently infected when plasma tested SIV RNA positive on two consecutive time points. Data are reported as the number of SIV RNA copy equivalents per milliliter of plasma.
Specimens and sample processing
Plasma and lymphocytes were isolated from ethylenediaminetetraaceticacid anticoagulated whole blood using established protocols.12,34 Isolated peripheral blood mononuclear cells (PBMCs) were used fresh or stored in liquid nitrogen for future use. On weeks 9 and 12, saliva and stool samples were collected and processed as described.35–37 Stool, but not saliva, was collected also during the challenge phase and early after infection. Lymph nodes (submandibular, retropharyngeal, submental, and mesenteric), spleen, tonsil, ileum, and colon were collected at necropsy and processed as previously reported.4,12
Plasma, saliva, and stool antibody analysis
SIV-specific IgA and IgG antibodies were measured in plasma using enzyme-linked immunosorbent assay (ELISA), and in saliva and fecal extracts using a binding antibody multiplex assay (BAMA) with magnetic Luminex beads as previously described.35,37,38 Antibodies were measured against recombinant SIVmac239 gp140, SIVmac239 gp120 (both Immune Technology, NY), SIV gp41 (#5009; ImmunoDx, Woburn, MS), and a murine leukemia virus gp70 scaffolded SIVmac239 gp120 V1V2 protein (Dr. Abraham Pinter, Rutgers Medical School, NJ). Total IgA and IgG were measured by ELISA as described36 using goat antimonkey IgA and IgG antibodies (Rockland Immunochemicals, Pottstown, PA). Concentrations of SIV-specific IgG or IgA in saliva and fecal extracts were divided by the total IgG or IgA to obtain the specific activity (sp act) and considered positive if ≥mean sp act +3 SD in mock control secretions. The avidity of anti-gp140 or -gp120 plasma IgG antibodies was measured using a chaotropic displacement ELISA with 1.5 M NaSCN as described.39 The avidity index was calculated by dividing the concentration of antibody in NaSCN-treated wells by that in untreated wells.
Antibody-dependent phagocytosis (ADP)
Plasma was tested for antibodies able to enhance phagocytosis of SIV gp140-labeled beads by THP-1 monocytes as previously described.38 In brief, 1 μm avidin-coated fluorospheres (Invitrogen, Carlsbad, CA) were labeled with anti-HIS tag antibody followed by HIS-tagged recombinant SIV gp140 (Immune Technology). THP-1 were incubated with beads and diluted plasma samples for 6 h, then treated with trypsin and fixed with paraformaldehyde. After flow cytometric analysis, an initial phagocytic score was determined by multiplying the percentage of bead-positive cells by their median fluorescence intensity. The final phagocytic score was calculated by dividing the initial score for test samples by the average score obtained with mock control plasma at the same dilution. Final scores >2 were significant in this assay.
Antibody-dependent cellular cytotoxicity
The Granzyme B GranToxiLux assay (OncoImmunin, Gaithersburg, MD) was used to detect plasma antibody-dependent cellular cytotoxicity (ADCC) activity directed against CCR5+ CEM.NKR T cells (AIDS Reagent Program; contributed by Alexandra Trkola) coated with SIVmac239 or SIVmac251 gp120 protein as described previously.37,40 ADCC activity was measured in fourfold serial plasma dilutions starting at 1:100. Cryopreserved human PBMCs from an HIV-seronegative donor with the 158F/V FcRIIIa genotype were used as the source of effector cells.41 ADCC endpoint titers were determined by interpolating the dilutions of plasma that intercept the positive cutoff.
SIV-specific T cell responses
SIV Gag-specific T cell responses were determined as described previously.14,37,39 In brief, PBMCs were stimulated with 5 μg of SIV Gag peptide pools for 6 h, with Brefeldin A (eBioscience, San Diego, CA) present during the last 5 h. At the end of the culture period, PBMCs were stained for cell surface markers and for the cytokines interferon-gamma (IFN-γ), interleukin (IL)-2, IL-17, and tumor necrosis factor-alpha (TNF-α). Negative and positive controls included media only and Cell Stimulation Cocktail (eBioscience), respectively.14,37,39 Postchallenge, we also assessed the frequencies of activated T cells (Ki-67, CCR5, and CXCR3) and intestinal homing potential as described14,37,39 using the A4B7 antimacaque α4β7 antibody (obtained from the NIH Nonhuman Primate Reagent Resource Program). A live/dead cell discriminator dye was included in all assays, and gates were based on fluorescence minus one control. A total of 300,000 cells were acquired on an LSRFortessa using FACSDiva v8.0 (BD) and analyzed using FlowJo Software v10.2 (TreeStar).
Immunofluorescence assay to assess mucosal barrier function in intestinal tissues
Colonic sections (5 μm) were analyzed for IL-17-producing T cells and zonula occludens-1 (ZO-1) expression by immunofluorescence assay as described.18 In brief, formalin-fixed, paraffin-embedded tissue sections were deparaffinized, blocked, and incubated with the primary antibody for 1 h in the dark at room temperature. CD3 and ZO-1 were detected by polyclonal rabbit IgG antibodies (Life Technologies) used at a 1:200 or 1:100 dilution, respectively. The IL-17 antibody (clone: eBio64CAP17) was applied at a 1:50 dilution. After two washes with phosphate-buffered saline (PBS)-Fish Skin Gelatin containing Triton X-100 (Sigma) and a third wash with PBS-Fish Skin Gelatin lacking Triton X-100, sections were treated with secondary antibody (goat antirabbit IgG Alexa Fluor 488; goat antimouse IgG Alexa Fluor 594 or goat antirabbit IgG Alexa Fluor 594) protected from light for 1 h at room temperature. Slides were washed, counterstained with 4′,6-diamidino-2-phenylindole, mounted, and coverslipped with DPX mounting medium (Sigma-Aldrich, St. Louis, MO).
Negative controls included tissue sections without the primary antibody and tissues from healthy SIV-naive infants. Each fluorescent signal for a specific marker was analyzed individually to exclude false-positive signals due to color overlap. Five randomly chosen (10 × ) microscope fields/sections were analyzed. Images were captured digitally on a Zeiss Axio Observer microscope with an AxioCam MRm monochromatic camera and analyzed with Zen 2.3 Lite imaging software (Zeiss). Quantitative measurements were obtained using ImageJ Software (https://imagej.net/).42 ZO-1, CD3, and IL-17 single channel images were analyzed for circular signals (circularity = 0.80–1.00) larger than 12.5 μm2. CD3+IL17+ double positive cells were enumerated by hand using the Cell Counter Plugin for ImageJ (De Vos, https://imagej.nih.gov/ij/plugins/cell-counter.html). Cell numbers were reported as the number of cells per square millimeter. Total image area was determined using the acquisition-defined scale bar.
Statistical analysis
Per-exposure risk of infection was analyzed using the Mantel–Cox logrank test. Comparisons between groups were performed using the nonparametric two-tailed Mann–Whitney rank sum test. Spearman's rank correlation tests were used to evaluate associations between variables. Calculations were performed with GraphPad Prism v6 (GraphPad Software, La Jolla, CA). The p values ≤.05 were considered significant.
Results
Plasma and mucosal antibody responses before oral SIV challenge
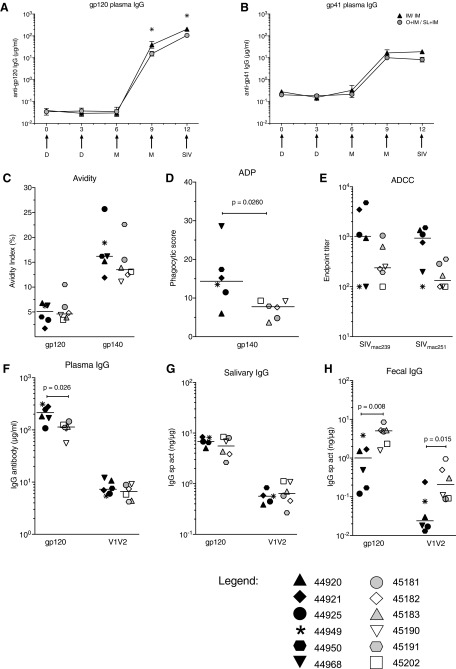
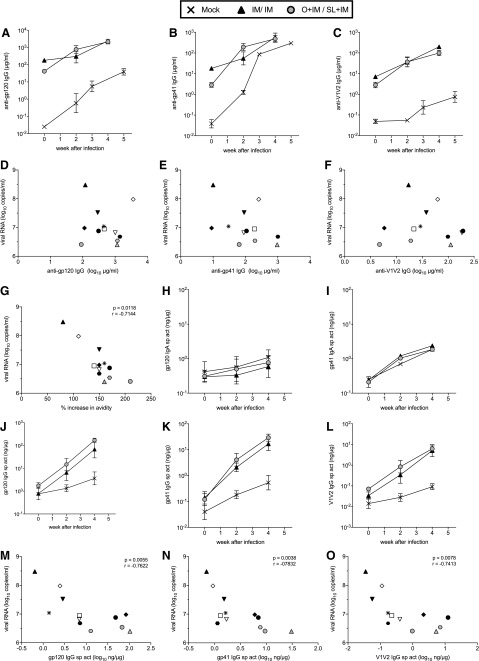
As found in studies with adult macaques,43,44 DNA-SIV priming did not induce significant antibodies in plasma of infants (Fig. 1A, B). However, high levels of SIV Env-specific plasma IgG antibodies were detected after the first MVA boost, and these were further increased after the second MVA immunization (Fig. 1A, B). Infant macaques in the IM/IM group had twofold higher concentrations of anti-gp120 plasma IgG antibodies compared with the O + IM/SL + IM group, and although modest, these differences were statistically significant on week 9 (p = .03) and week 12 (p = .03) (Fig. 1A). The greater systemic IgG response in IM/IM animals was most likely due to priming with twice as much DNA by the IM route. The avidity of plasma IgG antibodies to gp120 and gp140 was similar in both groups, although very low (Fig. 1C). The median antibody-dependent phagocytosis (ADP) activity in plasma of the IM/IM group was roughly twofold higher than that of the O + IM/SL + IM group (Fig. 1D), consistent with the higher level of binding antibodies in IM/IM animals. ADCC antibody titers against SIVmac239 gp120 (representative of the immunogen) and SIVmac251 gp120 (representative of the challenge virus) also tended to be higher in IM/IM animals, but they did not differ significantly between the groups (Fig. 1E).
FIG. 1.
Vaccine-induced IgG antibody responses. Geometric mean concentrations of SIV (A) gp120- and (B) gp41-specific IgG measured by enzyme-linked immunosorbent assay in plasma of vaccinated infants up to week 12, when the first oral SIV challenge was performed. Error bars represent the standard error of the mean. Arrows below the x-axis indicate the times of DNA (D) and MVA (M) immunization and the time of the first oral SIV challenge. (C) Avidity to Env proteins, (D) ADP activity at 1/20 dilutions and (E) titers of antibody-dependent cellular cytotoxicity antibody were measured in week 12 plasma as described in the section “Materials and Methods”. Bars denote medians. SIV gp120 and gp70-V1V2mac239 IgG antibodies in week 12 (F) plasma, (G) saliva, and (H) fecal extracts. IgG antibodies in mucosal fluids were measured by BAMA and are expressed as specific activity (sp act) in nanogram antibody per microgram total IgG. In graphs (A) and (B), white triangles and gray circles represent animals in the IM/IM and O + IM/SL + IM groups, respectively. In all other graphs, individual animals in the IM/IM group are represented by distinct black symbols, and animals in the O + IM/SL + IM group can be distinguished by distinct white or gray symbols. Individual animal symbols are maintained consistent for all figures. Between-group comparisons were performed using the two-tailed Mann–Whitney rank sum test; statistically significant differences are denoted by the asterisk. ADP, antibody-dependent phagocytosis; BAMA, binding antibody multiplex assay; IgG, immunoglobulin G; IM, intramuscular; MVA, modified vaccinia Ankara; O, oral; SIV, simian immunodeficiency virus; SL, sublingual.
Both vaccine regimens also elicited Env-specific IgA antibodies in plasma by week12, but concentrations were 1,000-fold lower than IgG (data not shown). Env-reactive IgA in saliva and fecal extracts of vaccinated infants was not greater than that in mock controls (not shown). However, significant levels of IgG antibodies to gp120, gp41, and V1V2 were found in these samples. In saliva, Env-specific IgG did not differ between the two groups (Fig. 1G). The gp41-specific IgG in fecal extracts also did not differ (not shown). However, O + IM/SL + IM-vaccinated animals had greater levels of gp120- and V1V2-specific IgG in stool samples when compared with the IM/IM-vaccinated animals (Fig. 1H). This markedly contrasted with plasma (Fig. 1F), and suggests that coadministration of vaccine by both IM and oral routes may have generated IgG plasma cells in the intestine.
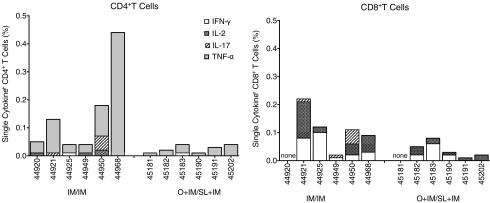
SIV Gag-specific T cells in blood
We also evaluated SIV Gag-specific CD4+ and CD8+ T cells in blood on the day of the first challenge (week 12). Only low frequencies of gag-specific T cells, mostly producing IFN-γ and TNF-α, were observed (Fig. 2), and there were no differences between the groups. Due to the small size of neonatal macaques, we did not collect lymph node or intestinal biopsies until necropsy. Therefore, it is possible that frequencies of vaccine-specific T cells in tissues or lymph nodes may have differed between the groups at the time of challenge. Furthermore, because the SIV-specific T cell responses were measured 3 weeks after the last boost (week 12), we likely missed the peak T cell response.
FIG. 2.
Vaccine-induced T cells in blood on the day of challenge. SIV Gag-specific (left graph) CD4+ and (right graph) CD8+ T cells measured by flow cytometry after stimulating peripheral blood mononuclear cells with overlapping Gag p27 peptides and staining for CD3, CD4, CD8, and intracellular cytokines. The sum of the percentage of single cytokine-producing cells within each T population is shown for animals in each group. The total frequency of SIV Gag-specific CD4+ T cells in the IM/IM group was greater than the O + IM/SL + IM group (p = .013 by Mann–Whitney).
Oral SIVmac251 challenge outcome
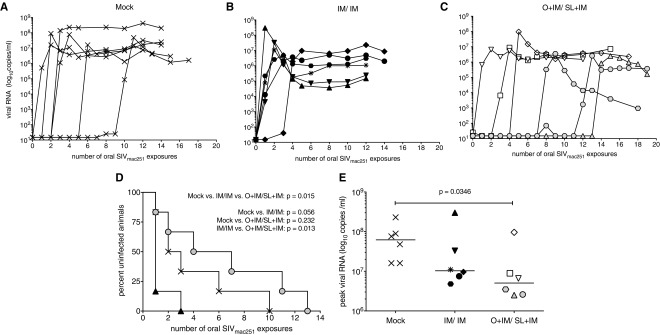
All mock-vaccinated controls became infected after oral challenge with SIVmac251, although the number of oral exposures required for infection of these animals differed. Consistent with our previous studies,14,39 the per-exposure risk of infection was 0.25 in the control group (Fig. 3A). In the IM/IM group, five of six infants became infected after the first challenge, and the remaining animal after three exposures (Fig. 3B), translating into a threefold higher risk of infection per exposure (0.75). In contrast, O + IM/SL + IM vaccination resulted in a reduced risk (0.15), with animals requiring 1, 2, 4, 7, 11, or 13 challenges to become infected (Fig. 3C). The animal requiring 13 challenges (#45181) did exhibit low viremia after seven exposures (70 copies/mL), but upon retesting another plasma aliquot of the same time point tested negative. Furthermore, viremia remained below the limit of detection at the following five consecutive time points before it was again detected after the 13th challenge (1,700 copies/mL) and each week thereafter. Differences in the infection risk per oral SIVmac251 exposure between the three groups were statistically significant by Mantel–Cox test. Among the two vaccine groups, only the IM/IM group exhibited a trend toward an increased infection risk compared with controls (p = .066), and although the O + IM/SL + IM-vaccinated infants had a lower infection risk per exposure compared with IM/IM group (p = .013), their risk did not differ from controls (p = .232) (Fig. 3D). However, orally immunized infants had significantly lower peak viremia when compared with mock controls (Fig. 3E).
FIG. 3.
Outcome of oral challenge. Plasma viral loads measured by reverse transcription-polymerase chain reaction in (A) mock controls, (B) IM/IM-vaccinated, and (C) vaccinated infants after initiating the weekly oral challenge regimen with SIVmac251. (D) Rate of infection acquisition for all groups. A significant difference was found among the three groups using the Mantel–Cox logrank test. (E) Peak viral loads for each group. Differences in peak viremia were assessed by the Mann–Whitney rank sum test.
Improved control of viremia in the O + IM/SL + IM group was unlikely due to the expression of major histocompatibility complex (MHC) Class I alleles that have been associated with better control of viremia. Due to the age of the animals at study initiation, we could not evenly distribute infant macaques to specific groups based on their MHC Class I genotype. However, retrospective genotyping of the Mamu A*01, Mamu B*08, and Mamu B*17 alleles did not reveal any correlation between control of viremia and protective MHC Class I genotypes (Table 2). Similarly, there was no correlation between the TRIM5α genotype and challenge outcome (Table 2).
Table 2.
MHC Class I Genotype
| MHC Class I genotypea(Mamu) | TRIM5α | Plasma viremia (copies/mL) | |||||
|---|---|---|---|---|---|---|---|
| Group | Animal no. | A*01 | B*08 | B*17 | Genotypeb | Peak | Necropsy |
| A | 44900 | − | − | − | TFP/TFP | 8.9 × 107 | 1.6 × 107 |
| 44905 | + | − | − | TFP/Q | 4.8 × 107 | 1.6 × 107 | |
| 44919 | − | − | − | TFP/CypA | 2.3 × 108 | 2.0 × 108 | |
| 44936 | + | − | − | TFP/TFP | 1.6 × 107 | 2.9 × 10 | |
| 44937 | − | − | − | TFP/Q | 7.6 × 107 | 2.5 × 107 | |
| 44939 | + | − | − | Q/CypA | 1.6 × 107 | 7.4 × 106 | |
| B | 44920 | − | − | − | TFP/TFP | 3.0 × 108 | 1.5 × 105 |
| 44921 | + | − | − | TFP/Q | 9.6 × 106 | 8.7 × 106 | |
| 44925 | + | − | − | TFP/Q | 7.5 × 106 | 2.0 × 106 | |
| 44949 | − | − | + | TFP/TFP | 1.1 × 107 | 1.1 × 106 | |
| 44950 | − | − | − | TFP/CypA | 4.8 × 106 | 3.0 × 106 | |
| 44968 | + | − | − | TFP/Q | 3.3 × 107 | 2.4 × 105 | |
| C | 45181 | − | − | + | TFP/Q | 2.6 × 106 | 3.7 × 105 |
| 45182 | − | − | + | TFP/Q | 9.6 × 107 | 2.3 × 106 | |
| 45183 | + | − | − | TFP/CypA | 2.5 × 106 | 1.6 × 105 | |
| 45190 | − | + | − | Q/Q | 6.6 × 106 | 1.5 × 106 | |
| 45191 | − | − | + | TFP/TFP | 3.5 × 106 | 9.5 × 102 | |
| 45202 | − | − | − | TFP/TFP | 8.9 × 106 | 7.3 × 106 | |
The Mamu A*01, Mamu B*08, and Mamu B*17 alleles have been associated with better control of viremia.
The TRIM5α genotype TFP/TFP confers resistance to SIV, while the genotype Q/Q is associated with increased susceptibility to infection. The heterozygous genotypes TFP/CypA and Q/CypA support intermediate replication.
MHC, major histocompatibility complex.
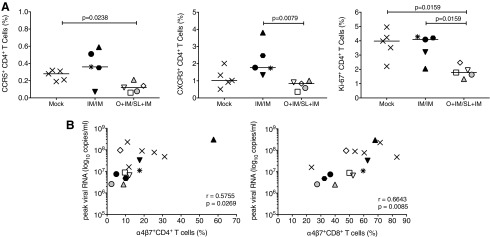
Reduced virus replication in orally immunized infants was supported by lower frequencies of activated CD4+ T cells expressing CCR5, CXCR3, or Ki-67 (Fig. 4A); a similar trend was observed for CD8+ T cells (data not shown). Enhanced frequencies of α4β7+ CD4+ and CD8+ T cells in blood at week 4 pi positively correlated with peak viremia, suggesting that higher plasma SIV RNA levels likely reflected increased virus replication in intestinal tissues and caused an expansion of T cells with mucosal homing potential (Fig. 4B). If this conclusion is correct, mock animals and IM/IM-immunized animals with higher viremia should also present with more signs of mucosal barrier damage.
FIG. 4.
T cell activation and intestinal barrier function post SIV infection. Activated peripheral blood CD4+ T cells expressing CCR5, CXCR3, or Ki-67 at week 4 pi (A). The nonparametric Mann–Whitney test was applied to determine statistically significant differences between groups. Both CD4+ and CD8+ T cells positive for the intestinal homing marker α4β7 correlated with peak viremia (B). Correlation analysis was performed using the Spearman rank test.
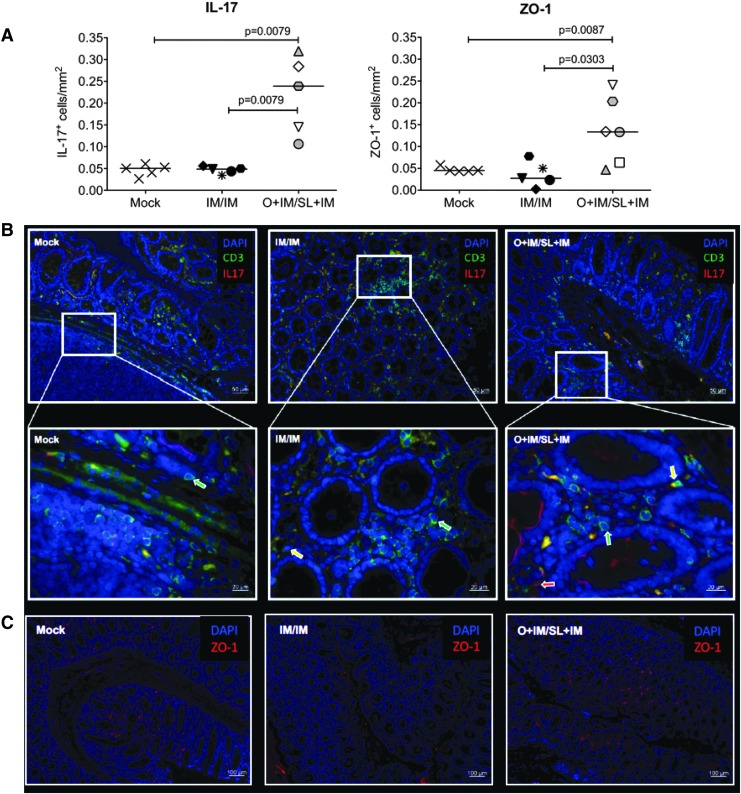
To confirm better maintenance of mucosal barrier function in orally compared with IM/IM- and mock-immunized animals, we analyzed colonic tissue sections at the time of euthanasia for the presence of IL-17-producing cells and the tight junction protein ZO-1, both of which are considered paramount for mucosal integrity.45–47 Indeed, median colon frequencies of IL-17- and ZO-1-positive cells were higher in infants of the O + IM/SL + IM group compared with the mock and the IM/IM groups (Fig. 5). It should be noted that IL-17 can be produced both by CD3 T cells and by innate lymphoid cells (Fig. 5B), and therefore we reported total frequencies of IL-17-positive cells.
FIG. 5.
Mucosal integrity and barrier function. Immunofluorescence staining of colon sections for CD3, IL-17, and ZO-1 at the time of euthanasia. For each marker, five randomly chosen fields were analyzed per animal. (A) Average counts of IL-17 (left graph)- and ZO-1 (right graph)-positive cells per square millimeter colon. Differences in the frequencies of IL-17+ or ZO-1+ cells between the groups were assessed by the nonparametric Mann–Whitney test. (B) Images of individual animals representative of infants in the mock (left), IM-immunized (middle), or O + IM-immunized infants (right). The top row shows images taken with a 20 × objective. The lower row images show a higher magnification (63 × ) of the white box area indicated in the respective top images. Green and red arrows indicate CD3+ T cells or IL-17+ cells, respectively; yellow arrows point to CD3+IL-17+ T cells. (C) ZO-1 staining in colon sections (10 × ). The scale bar for each image is provided in the lower right-hand corner. IL, interleukin; ZO-1, zonula occludens-1.
Impact of prechallenge immune responses on challenge outcome
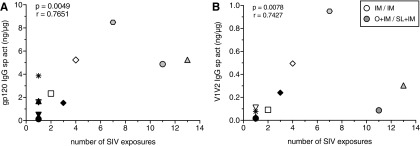
To determine if vaccine-induced immune responses contributed to the rate of infection acquisition, we compared week 12 immune responses in all vaccinated animals to the number of challenges required for infection. Neither the frequencies of Gag-specific T cells in blood nor the levels of antibodies in plasma or saliva correlated with the number of challenges required for infection (not shown). There was also no association between ADP or ADCC function of plasma IgG antibodies and challenge outcome. However, there was a modest positive correlation between levels of gp120- and V1V2-specific IgG in stool and the number of exposures required for infection (Fig. 6A, B).
FIG. 6.
Prechallenge intestinal IgG responses and rate of infection acquisition. Association of (A) SIV gp120 or (B) gp70-V1V2mac239 in week 12 fecal extracts with the number of oral SIV challenges required for infection of vaccinated infants. p and r values above each graph are obtained using the Spearman rank correlation test.
Antibody responses and viremia post SIV infection
To determine if antibodies may have contributed to control of viremia, early pi responses in vaccinated infants were compared with peak viral loads, the majority of which were observed at week 2 pi. During the first 2 weeks of infection, concentrations of anti-Env plasma IgG antibodies increased more rapidly in O + IM/SL + IM animals (Fig. 7A–C), but no associations with viremia were observed (Fig. 7D–F). The avidity indices for gp120-specific IgG in plasma of IM/IM and O + IM/SL + IM groups were still very low on week 2 pi (geometric mean: 6.2 and 12.3, respectively), and they did not correlate with viremia (not shown). However, we did find an inverse association between peak viremia and the magnitude of the increase in gp120 avidity during the first 2 weeks of infection (Fig. 7G).
FIG. 7.
Antibody responses after SIV infection. Geometric mean concentrations of plasma IgG antibodies to (A) gp120, (B) gp41, and (C) gp70-V1V2 measured early after infection. (D–F) Comparison of peak viral loads and week 2 pi concentrations of anti-Env plasma antibodies. (G) inverse association between peak viral loads and the percentage increase in anti-gp120 IgG plasma antibody avidity from week 0 to 2 pi. IgA antibodies to (H) gp120 and (I) gp41 in fecal extracts of vaccinated and control infants. Fecal IgG antibodies to (J) gp120, (K) gp41, and (L) gp70-V1V2. (M–O) Inverse associations between peak viremia and anti-Env fecal IgG antibodies. All comparisons were done using the Spearman rank correlation test, and were only significant where p and r values are indicated.
Levels of IgA antibodies to gp120 and gp41 in fecal extracts of vaccinated and control infants did not differ after infection (Fig. 7H, I), confirming a lack of vaccine-induced mucosal IgA responses. In contrast, anamnestic anti-Env intestinal IgG responses were observed in both groups of vaccinated infants (Fig. 7J–L). In addition, the levels of gp120-, gp41-, and V1V2-specific IgG in fecal extracts on week 2 pi were inversely correlated with peak viral load (Fig. 7M–O). These correlations remained statistically significant for gp120 and gp41 antibodies when we corrected for multiple comparisons of the seven correlation analyses and apply p = .007; the correlation between V1V2 antibodies and peak viremia trended toward significance (p = .0078). These results demonstrate that the higher the postchallenge fecal IgG responses, the lower was peak viremia, supporting the importance of mucosal antibody responses. In contrast, the correlation between plasma IgG avidity increase and peak viremia was no longer significant.
Discussion
The goal of this study was to test whether vaccine delivery by an oral route, in addition to the IM route, would enhance the induction of mucosal immune responses that could protect infants against immunodeficiency virus transmission through breastmilk. We chose a DNA/MVA vaccine strategy because DNA prime/poxviral vector boost regimens have proven safe and immunogenic in infants, and they have demonstrated efficacy for delaying or controlling SIV or SHIV infection in adult macaques.4,12,13,28,29,43,48,49 A primary objective of the current approach in infants was to elicit mucosal IgA at the site of virus exposure.18 This was based on previous findings that induction of Env-specific salivary and fecal IgA responses was associated with better control of viremia in infant macaques12 and in adult macaques.50,51 We selected GM-CSF and CD40L as molecular adjuvants in the DNA prime due to their ability to enhance dendritic cell priming and increase the induction of mucosal IgA responses in adult macaques.28,29 As found for most vaccines tested in adult macaques, the DNA/MVA vaccine did not prevent mucosal SIVmac251 transmission to infant macaques. Nonetheless, the differences in vaccine outcome between the IM-only immunized infants and the infants that received the vaccine by both the IM and oral routes raise some important questions that will inform future vaccine studies.
Infant macaques vaccinated by the IM route alone had a threefold higher risk of SIV infection per exposure compared with mock controls and a fivefold higher risk compared with orally immunized infants. We considered that the higher risk of infection in IM-immunized animals might have been related to vaccine-induced immune activation. We previously found that IM vaccination with mycobacterial-based SIV vectors induced monocyte and CD4 T cell activation and enhanced oral SIV acquisition in infant macaques.39 In that study, increased frequencies of SIV target cells were readily detected in blood before challenge.39 However, in this study, we did not observe any evidence of vaccine-induced CD4+ T cell activation in prechallenge blood samples (data not shown). However, we cannot exclude local activation in specific tissues (e.g., orogastrointestinal tract) that served as virus entry sites after oral SIV exposure. Furthermore, the group size of six infants per group is relatively small, and therefore the data should not be overinterpreted.
Instead, we want to highlight some of the potential benefits that were observed in the group of infants that received the vaccine by both the IM and the oral routes. The finding here that greater gp120- and V1V2-specific intestinal IgG responses at the time of the first oral SIV challenge were significantly associated with the number of exposures required for infection supports the potential importance of mucosal antibodies. In addition, there was an inverse relationship between peak viremia and pi Env-specific intestinal IgG antibodies that was not observed for plasma IgG. These data suggest that IgG antibodies in the intestine of vaccinated infants may have played a role in early clearance of free virus or infected cells. This conclusion is indirectly supported by fewer α4β7-positive T cells in orally immunized infants during the acute phase of infection, whereas mock and IM-immunized infants with higher peak plasma viremia, and presumably also higher virus replication in intestinal tissues, had higher T cell numbers with intestinal homing potential. To determine if mucosal IgG plasma cells were truly generated in this tissue, future studies will need to analyze the isotype of antibody-producing cells in the intestine.
In this study, Env-specific plasma antibodies were insufficient to protect against oral SIV infection. In fact, IM-only vaccinated infants had higher gp120-specific plasma IgG antibodies than orally immunized infants, and these antibodies trended toward higher avidity and better ADP and ADCC function. In several nonhuman primate SIV vaccine studies, the avidity of Env-specific IgG antibodies has been correlated with protection.52,53 Here, vaccine-induced gp120-specific plasma IgG antibodies at the time of the first oral SIV exposure (week 12) were of very low avidity in both vaccine groups. Neonates do characteristically develop lower affinity antibodies than adults,10 but the poor avidity of antibodies generated here is also likely due to the short intervals between immunizations.54–57 We recently demonstrated that the extension of vaccine intervals from 3 to 6 weeks improved both avidity and Fc-mediated function of Env-specific plasma IgG antibodies in infant macaques.37 In the study here, the percentage increase in antibody avidity in the first 2 weeks pi was inversely correlated with peak viremia.
The more rapid affinity maturation of plasma IgG antibodies in animals with lower viremia may reflect better preservation of T helper cells, especially follicular T helper (TFH) cells.58 Due to the small size of infants, we did not collect intestinal or lymph node biopsies before challenge to test TFH frequencies or function. However, since the completion of this study, assays to detect antigen-specific TFH in peripheral blood have been developed59–61 and can be applied to future studies. Furthermore, in infant mice, the ability of TFH to induce antigen-specific germinal center B cells is severely limited compared with adults but can be significantly enhanced by vaccine adjuvantation.62 Similarly, the induction of antibodies to the human pneumococcal vaccine, which is poorly immunogenic in infants, was dramatically accelerated in neonatal macaques when vaccine was administered with 3M-052 adjuvant.63 The 3M-052 adjuvant belongs to the group of low molecular weight imidazoquinolines that can activate toll-like receptors (TLRs) 7 and 8, and these TLR7/8 agonists are especially potent in activating infant dendritic cells.64–66 We recently tested different adjuvants for their ability to enhance antibody responses in infant macaques, and found that both the magnitude and function of Env-specific IgG antibodies were highest in infants IM immunized with HIV Env protein and 3M-052 in stable emulsion compared with animals given protein in alum or glucopyranosyl lipid adjuvant in stable emulsion (GLA-SE).67
In contrast, GM-CSF, one of the adjuvants used in this study, can cause immune suppression at high doses,68 and thereby interfere with vaccine efficacy.69 Infants immunized only by the IM route received twice as much GM-CSF encoding DNA by the IM route than did the O + IM-immunized infants. After we had immunized neonates with the GM-CSF DNA, it was discovered that GM-CSF can inhibit both systemic and mucosal IgA responses in adult macaques.70 In our pilot study,27 IgA antibodies were generated in stool of animals primed with GM-CSF DNA by the O + IM route, but we only immunized once with the DNA, and we used juvenile macaques instead of neonates. Thus, the dose of GM-CSF in these larger animals was probably not sufficient to interfere with mucosal IgA induction. Interestingly, in this study, oral and IM coadministration of vaccine generated intestinal IgG antibodies instead of IgA. It is possible that this was also related to vaccine priming in the presence of more GM-CSF. Although we did not test for any causative relationship between GM-CSF and mucosal antibodies, our results emphasize the importance of testing adjuvants at different doses and routes for their efficacy in enhancing specific immune responses, and these adjuvants need to be tested in the age group that is targeted by the vaccine. Further studies are warranted to understand the mechanistic interplay between innate and adaptive responses, and how it can be influenced by vaccine immunogens, adjuvants, and intervals.71,72
Despite the inability of this O + IM/SL + IM vaccine to prevent oral SIV transmission, it may have provided some benefit on disease outcome. At the end of the study, all mock control animals had high plasma viremia, ranging from 106 to 108 copies/mL. In IM-immunized animals, plasma viremia had dropped to 105 copies/mL in two of six infants but remained >106 copies/mL in the other four infants. In contrast, infants immunized by the oral and IM routes experienced the lowest average chronic viremia, with one infant having <103 copies/mL and two having <4 × 105 copies of SIV RNA/mL. Even the animal with the highest plasma viremia in the O + IM group (#45202) appeared to have better preservation of mucosal barrier function at the time of euthanasia, as evidenced by greater frequencies of IL-17-producing T cells and higher expression of the ZO-1 tight junction protein in colonic tissue. It should be noted that not all IL-17-producing cells in the colon coexpressed CD3. These cells likely represented type 3 innate lymphoid cells (ILC3) that are frequently found at mucosal sites, and are rapidly depleted in HIV and SIV infection.73–76 Importantly, the preservation of ILCs at various mucosal sites has been correlated with better disease outcome in SIV-infected monkeys77,78 and HIV-infected humans.79
Several SL vaccines against infectious diseases have proved successful in mouse models,80–83 and the first immunogenicity studies of a SL papillomavirus vaccine have been conducted in humans.84 While the current O + IM DNA/SL + IM MVA vaccine strategy was not able to prevent oral SIV transmission in infant macaques, it did result in better control of viremia, and it generated mucosal immune responses that were associated with both a delay in the rate of infection acquisition and improved viral control. A recent study on adult cynomolgous macaques further supports the feasibility of a combined SL/IM vaccine for the induction of mucosal antibodies to protect against mucosal SIV infection.85 The protective efficacy of such a vaccine will depend on a better understanding of the interactions of innate and adaptive immunity at both mucosal and systemic sites and how to exploit these by age-relevant adjuvants, immunogen choice, and vaccine administration at different routes and intervals.
Acknowledgments
This work was supported by National Institutes of Health grants R01 DE022287 (K.D.P.), T32 5108303 (A.D.C.), U19 AI109633 (to R.R.A.), the Office of Research Infrastructure Programs/OD P51OD011107 (to CNPRC), and the Center for AIDS Research award P30AI050410 (to UNC). The UNC Flow Cytometry Core Facility is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The SIV Gag peptides were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, and the α4β7 antibody was provided by the NIH-funded Nonhuman Primate Reagent Resource. We thank Drs. Bernie Moss and Patricia Earl (NIAID) for the MVA vectors, Olga Nichols (LSUHSC Vector Core Facility) for production of MVA stocks, Robert L. Wilson (LSUHSC) for technical assistance in antibody measurements, and Tori Huffmann for help with the ADCC assays. We also thank Dr. J. Lifson and colleagues in the Quantitative Molecular Diagnostics Core of the AIDS and Cancer Virus Program of the Frederick National Laboratory for expert assistance with viral load measurements and for MHC Class I genotyping. Our special thanks to Dr. B. Keele at the Cancer Virus Program of the Frederick National Laboratory for performing the TRIM5α genotyping. The work was supported in part with federal funds from the National Cancer Institute, National Institutes of Health (contract no. HHSN261200800001E, J.L.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ananworanich J, Abrams EJ: Time to prioritise the UNAIDS 90-90-90 targets for infants. Lancet HIV 2016;3:e241–e243 [DOI] [PubMed] [Google Scholar]
- 2. Hennet T, Borsig L: Breastfed at Tiffany's. Trends Biochem Sci 2016;41:508–518 [DOI] [PubMed] [Google Scholar]
- 3. Pedersen SH, Wilkinson AL, Andreasen A, et al. : Longitudinal analysis of mature breastmilk and serum immune composition among mixed HIV-status mothers and their infants. Clin Nutr 2016;35:871–879 [DOI] [PubMed] [Google Scholar]
- 4. Van Rompay KK, Abel K, Earl P, et al. : Immunogenicity of viral vector, prime-boost SIV vaccine regimens in infant rhesus macaques: Attenuated vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) recombinant SIV vaccines compared to live-attenuated SIV. Vaccine 2010;28:1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. UNAIDS: New HIV infections in children. Available at http://aidsinfo.unaids.org/ (2016), accessed June4, 2018
- 6. Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J: FCGR2A and FCGR3A genotypes in human immunodeficiency virus mother-to-child transmission. Open Forum Infect Dis 2015;2:ofv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haas AD, Msukwa MT, Egger M, et al. : Adherence to antiretroviral therapy during and after pregnancy: Cohort study on women receiving care in Malawi's option B+ program Malawi. Clin Infect Dis 2016;63:1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O: Protecting the newborn and young infant from infectious diseases: Lessons from immune ontogeny. Immunity 2017;46:350–363 [DOI] [PubMed] [Google Scholar]
- 9. Philbin VJ, Levy O: Developmental biology of the innate immune response: Implications for neonatal and infant vaccine development. Pediatr Res 2009;65:98R–105R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adkins B, Leclerc C, Marshall-Clarke S: Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004;4:553–564 [DOI] [PubMed] [Google Scholar]
- 11. Levy O: Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat Rev Immunol 2007;7:379–390 [DOI] [PubMed] [Google Scholar]
- 12. Marthas ML, Van Rompay KK, Abbott Z, et al. : Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques. Vaccine 2011;29:3124–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Rompay KK, Abel K, Lawson JR, et al. : Attenuated poxvirus-based simian immunodeficiency virus (SIV) vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J Acquir Immune Defic Syndr 2005;38:124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen K, Nabi R, Van Rompay KKA, et al. : Vaccine-elicited mucosal and systemic antibody responses are associated with reduced simian immunodeficiency viremia in infant rhesus macaques. J Virol 2016;90:7285–7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abel K, Pahar B, Van Rompay KK, et al. : Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J Virol 2006;80:6357–6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Rasmussen T, Pahar B, et al. : Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood 2007;109:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Xu H, Pahar B, et al. : Simian immunodeficiency virus selectively infects proliferating CD4+ T cells in neonatal rhesus macaques. Blood 2010;116:4168–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amedee AM, Phillips B, Jensen K, et al. : Early sites of virus replication after oral SIVmac251 infection of infant macaques: Implications for pathogenesis. AIDS Res Hum Retroviruses 2018;34:286–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neutra MR, Kozlowski PA: Mucosal vaccines: The promise and the challenge. Nat Rev Immunol 2006;6:148–158 [DOI] [PubMed] [Google Scholar]
- 20. Kubota M, Miller CJ, Imaoka K, et al. : Oral immunization with simian immunodeficiency virus p55gag and cholera toxin elicits both mucosal IgA and systemic IgG immune responses in nonhuman primates. J Immunol 1997;158:5321–5329 [PubMed] [Google Scholar]
- 21. Manrique M, Kozlowski PA, Cobo-Molinos A, et al. : Immunogenicity of a vaccine regimen composed of simian immunodeficiency virus DNA, rMVA, and viral particles administered to female rhesus macaques via four different mucosal routes. J Virol 2013;87:4738–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou Q, Hidajat R, Peng B, et al. : Comparative evaluation of oral and intranasal priming with replication-competent adenovirus 5 host range mutant (Ad5hr)-simian immunodeficiency virus (SIV) recombinant vaccines on immunogenicity and protective efficacy against SIV(mac251). Vaccine 2007;25:8021–8035 [DOI] [PubMed] [Google Scholar]
- 23. Manrique M, Kozlowski PA, Cobo-Molinos A, et al. : Resistance to infection, early and persistent suppression of simian immunodeficiency virus SIVmac251 viremia, and significant reduction of tissue viral burden after mucosal vaccination in female rhesus macaques. J Virol 2014;88:212–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vagenas P, Williams VG, Piatak M, Jr., et al. : Tonsillar application of AT-2 SIV affords partial protection against rectal challenge with SIVmac239. J Acquir Immune Defic Syndr 2009;52:433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stahl-Hennig C, Eisenblatter M, Franz M, et al. : A single vaccination with attenuated SIVmac 239 via the tonsillar route confers partial protection against challenge with SIVmac 251 at a distant mucosal site, the rectum. Front Biosci 2007;12:2107–2123 [DOI] [PubMed] [Google Scholar]
- 26. Thippeshappa R, Tian B, Cleveland B, Guo W, Polacino P, Hu SL: Oral immunization with recombinant vaccinia virus prime and intramuscular protein boost provides protection against intrarectal simian-human immunodeficiency virus challenge in macaques. Clin Vaccine Immunol 2015;23:204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Curtis AD, 2nd, Jensen K. Van Rompay KKA. Amara RR. Kozlowski PA. De Paris K: A simultaneous oral and intramuscular prime/sublingual boost with a DNA/modified vaccinia Ankara viral vector-based vaccine induces simian immunodeficiency virus-specific systemic and mucosal immune responses in juvenile rhesus macaques. J Med Primatol 2018;47:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai L, Kwa S, Kozlowski PA, et al. : Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis 2011;204:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwa S, Lai L, Gangadhara S, et al. : CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge. J Virol 2014;88:9579–9589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwa S, Sadagopal S, Shen X, et al. : CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus (SIV) vaccine enhances protection against neutralization-resistant mucosal SIV infection. J Virol 2015;89:4690–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cotter CA, Earl PL, Wyatt LS, Moss B: Preparation of cell cultures and vaccinia virus stocks. Curr Protoc Microbiol 2015;39:14A.3.1–14A.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cline AN, Bess JW, Piatak M, Jr., Lifson JD: Highly sensitive SIV plasma viral load assay: Practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol 2005;34:303–312 [DOI] [PubMed] [Google Scholar]
- 33. Li H, Wang S, Kong R, et al. : Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc Natl Acad Sci U S A 2016;113:E3413–E3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jensen K, Ranganathan UD, Van Rompay KK, et al. : A recombinant attenuated Mycobacterium tuberculosis vaccine strain is safe in immunosuppressed simian immunodeficiency virus-infected infant macaques. Clin Vaccine Immunol 2012;19:1170–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jensen K, Pena MG, Wilson RL, et al. : A neonatal oral Mycobacterium tuberculosis-SIV prime/intramuscular MVA-SIV boost combination vaccine induces both SIV and Mtb-specific immune responses in infant macaques. Trials Vaccinol 2013;2:53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR: Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr 2000;24:297–309 [DOI] [PubMed] [Google Scholar]
- 37. Phillips B, Fouda GG, Eudailey J, et al. : Impact of poxvirus vector priming, protein coadministration, and vaccine intervals on HIV gp120 vaccine-elicited antibody magnitude and function in infant macaques. Clin Vaccine Immunol 2017;24: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iyer SS, Gangadhara S, Victor B, et al. : Virus-like particles displaying trimeric simian immunodeficiency virus (SIV) envelope gp160 enhance the breadth of DNA/modified vaccinia virus Ankara SIV vaccine-induced antibody responses in rhesus macaques. J Virol 2016;90:8842–8854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jensen K, Dela Pena-Ponce MG, Piatak M, Jr., et al. : Balancing trained immunity with persistent immune activation and the risk of SIV infection in infant macaques vaccinated with attenuated Mycobacterium tuberculosis or BCG vaccines. Clin Vaccine Immunol 2017;24: pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pollara J, Hart L, Brewer F, et al. : High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 2011;79:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M: Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 1997;90:1109–1114 [PubMed] [Google Scholar]
- 42. Schneider CA, Rasband WS, Eliceiri KW: NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lai L, Kwa SF, Kozlowski PA, et al. : SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine 2012;30:1737–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amara RR, Villinger F, Altman JD, et al. : Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Vaccine 2002;20:1949–1955 [DOI] [PubMed] [Google Scholar]
- 45. Pan D, Kenway-Lynch CS, Lala W, et al. : Lack of interleukin-10-mediated anti-inflammatory signals and upregulated interferon gamma production are linked to increased intestinal epithelial cell apoptosis in pathogenic simian immunodeficiency virus infection. J Virol 2014;88:13015–13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Estes JD, Harris LD, Klatt NR, et al. : Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 2010;6:e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klatt NR, Harris LD, Vinton CL, et al. : Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol 2010;3:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chea LS, Amara RR: Immunogenicity and efficacy of DNA/MVA HIV vaccines in rhesus macaque models. Expert Rev Vaccines 2017;16:973–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Iyer SS, Amara RR: DNA/MVA vaccines for HIV/AIDS. Vaccines (Basel) 2014;2:160–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brocca-Cofano E, McKinnon K, Demberg T, et al. : Vaccine-elicited SIV and HIV envelope-specific IgA and IgG memory B cells in rhesus macaque peripheral blood correlate with functional antibody responses and reduced viremia. Vaccine 2011;29:3310–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vargas-Inchaustegui DA, Tuero I, Mohanram V, et al. : Humoral immunity induced by mucosal and/or systemic SIV-specific vaccine platforms suggests novel combinatorial approaches for enhancing responses. Clin Immunol 2014;153:308–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lai L, Vödrös D, Kozlowski PA, et al. : GM-CSF DNA: An adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 2007;369:153–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pegu P, Vaccari M, Gordon S, et al. : Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol 2013;87:1708–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pichichero ME: Booster vaccinations: Can immunologic memory outpace disease pathogenesis? Pediatrics 2009;124:1633–1641 [DOI] [PubMed] [Google Scholar]
- 55. Castiglione F, Mantile F, De Berardinis P, Prisco A: How the interval between prime and boost injection affects the immune response in a computational model of the immune system. Comput Math Methods Med 2012;2012:842329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brice GT, Dobaño C, Sedegah M, et al. : Extended immunization intervals enhance the immunogenicity and protective efficacy of plasmid DNA vaccines. Microbes Infect 2007;9:1439–1446 [DOI] [PubMed] [Google Scholar]
- 57. Masopust D, Ha SJ, Vezys V, Ahmed R: Stimulation history dictates memory CD8 T cell phenotype: Implications for prime-boost vaccination. J Immunol 2006;177:831–839 [DOI] [PubMed] [Google Scholar]
- 58. Crotty S: A brief history of T cell help to B cells. Nat Rev Immunol 2015;15:185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Havenar-Daughton C, Reiss SM, Carnathan DG, et al. : Cytokine-independent detection of antigen-specific germinal center T follicular helper cells in immunized nonhuman primates using a live cell activation-induced marker technique. J Immunol 2016;197:994–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dan JM, Arlehamn CS, Weiskopf D, da Silva Antunes R, et al. : A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J Immunol 2016;197:983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reiss S, Baxter AE, Cirelli KM, et al. : Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS One 2017;12:e0186998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mastelic B, Kamath AT, Fontannaz P, et al. : Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol 2012;189:5764–5772 [DOI] [PubMed] [Google Scholar]
- 63. Dowling DJ, van HAren SD, Scheid A, et al. : TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight 2017;2:e91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pettengill MA, van Haren SD, Li N, et al. : Distinct TLR-mediated cytokine production and immunoglobulin secretion in human newborn naive B cells. Innate Immun 2016;22:433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Philbin VJ, Dowling DJ, Gallington LC, et al. : Imidazoquinoline toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol 2012;130:195–204.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR: Selective impairment of TLR-mediated innate immunity in human newborns: Neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol 2004;173:4627–4634 [DOI] [PubMed] [Google Scholar]
- 67. Phillips B, Van Rompay KKA, Rodriguez-Nieves J, et al. : Adjuvant-dependent enhancement of HIV Env-specific antibody responses in infant rhesus macaques. J Virol 2018;92 [Epub ahead of print]; DOI: 10.1128/JVI.01051-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Qin L, Greenland JR, Moriya C, Cayabyab MJ, Letvin NL: Effects of type I interferons on the adjuvant properties of plasmid granulocyte-macrophage colony-stimulating factor in vivo. J Virol 2007;81:10606–10613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Barouch DH, Santra S, Tenner-Racz K, et al. : Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J Immunol 2002;168:562–568 [DOI] [PubMed] [Google Scholar]
- 70. Kannanganat S, Wyatt LS, Gangadhara S, et al. : High doses of GM-CSF inhibit antibody responses in rectal secretions and diminish modified vaccinia Ankara/simian immunodeficiency virus vaccine protection in TRIM5alpha-restrictive macaques. J Immunol 2016;197:3586–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Walker BD, Ahmed R, Plotkin S: Moving ahead an HIV vaccine: Use both arms to beat HIV. Nat Med 2011;17:1194–1195 [DOI] [PubMed] [Google Scholar]
- 72. Pulendran B, Ahmed R: Immunological mechanisms of vaccination. Nat Immunol 2011;12:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shah SV, Manickam C, Ram DR, Reeves RK: Innate lymphoid cells in HIV/SIV Infections. Front Immunol 2017;8:1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aparicio-Domingo P, Romera-Hernandez M, Karrich JJ, et al. : Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J Exp Med 2015;212:1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mortha A, Chudnovskiy A, Hashimoto D, et al. : Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014;343:1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kløverpris HN, Kazer SW, Mjösberg J, et al. : Innate lymphoid cells are depleted irreversibly during acute HIV-1 infection in the absence of viral suppression. Immunity 2016;44:391–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li H, Richert-Spuhler LE, Evans TI, et al. : Hypercytotoxicity and rapid loss of NKp44+ innate lymphoid cells during acute SIV infection. PLoS Pathog 2014;10:e1004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Reeves RK, Kang G, Li H, Li Q: Depletion of lamina propria innate lymphoid cells in simian immunodeficiency virus infection. AIDS Res Hum Retroviruses 2014;30:1160–1161 [DOI] [PubMed] [Google Scholar]
- 79. Krämer B, Goeser F, Lutz P, et al. : Compartment-specific distribution of human intestinal innate lymphoid cells is altered in HIV patients under effective therapy. PLoS Pathog 2017;13:e1006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huang CF, Wang CC, Wu TC, Wu KG, Lee CC, Peng HJ: Neonatal sublingual vaccination with Salmonella proteins and adjuvant cholera toxin or CpG oligodeoxynucleotides induces mucosal and systemic immunity in mice. J Pediatr Gastroenterol Nutr 2008;46:262–271 [DOI] [PubMed] [Google Scholar]
- 81. Song JH, Nguyen HH, Cuburu N, et al. : Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A 2008;105:1644–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lee HJ, Cho H, Kim MG, et al. : Sublingual immunization of trivalent human papillomavirus DNA vaccine in baculovirus nanovector for protection against vaginal challenge. PLoS One 2015;10:e0119408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP: Buccal and sublingual vaccine delivery. J Control Release 2014;190:580–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huo Z, Bissett SL, Giemza R, Beddows S, Oeser C, Lewis DJ: Systemic and mucosal immune responses to sublingual or intramuscular human papilloma virus antigens in healthy female volunteers. PLoS One 2012;7:e33736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bekri S, Bourdely P, Luci C, et al. : Sublingual priming with a HIV gp41-based subunit vaccine elicits mucosal antibodies and persistent B memory responses in non-Human primates. Front Immunol 2017;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]