Abstract
Background
This study aimed to investigate the effects of abdominal aortic transplantation of bone marrow mesenchymal stem cells (BMMSCs) on the expression of inflammatory cytokines in a rat model of spinal cord ischemia-reperfusion injury.
Material/Methods
Adult female Sprague-Dawley rats (N=160) were divided into five groups: the sham operation group (N-32); the control group (N=32); the BMMSC transplanted group (N=32); the anti-ciliary neurotrophic factor (CNTF)-treated BMMSC transplanted group (N=32); and the CNTF small interfering RNA (siRNA)-treated BMMSC transplanted group (N=32). Motor behavior was assessed using the Basso, Beattie, and Bresnahan (BBB) locomotor scale. Motor evoked potentials (MEPs) and cortical somatosensory evoked potentials (CSEPs) were measured. Immunohistochemistry, quantitative real-time polymerase chain reaction (qRT-PCR), and Western blot analysis evaluated the expression of spinal inflammatory cytokines.
Results
Following surgery, compared with the control group the findings in the BMMSC transplant groups included significantly increased BBB scores; the latency and the amplitude of MEP and CSEP were reduced and increased, respectively; spinal neuronal necrosis was reduced; the number of normal neurons increased; CNTF mRNA and protein expression levels increased; expression levels of interleukin-6 (IL-6) were reduced and IL-10 levels were significantly increased (P<0.05). The effects of abdominal aortic BMMSC transplantation were at least partially reversed by both anti-CNTF and CNTF siRNA treatment.
Conclusions
In a rat model of spinal cord ischemia-reperfusion injury, abdominal aortic transplantation of BMMSCs increased the expression of CNTF, which improved hindlimb locomotor recovery by regulating the expression of IL-6 and IL-10 to reduce inflammation of the spinal cord.
MeSH Keywords: Cell Transplantation, Cytokines, Mesenchymal Stromal Cells, Reperfusion Injury
Background
Spinal cord ischemia-reperfusion injury results from spinal cord arterial disorders, spinal fractures, intraspinal surgery, surgery for spinal stenosis, and can be associated with an abdominal aortic aneurysm or thoracoabdominal aortic aneurysm [1]. Spinal cord ischemia-reperfusion injury is characterized by high disability and mortality rates [1]. The mechanisms underlying the pathogenesis of spinal cord ischemia-reperfusion injury are complex and involve intracellular calcium overload, oxygen free radical-mediated lipid peroxidation, inflammation, leukocyte activation, and neuronal apoptosis [2,3]. An inflammatory response represents an initiating step in the pathological changes of spinal cord ischemia-reperfusion injury. Pro-inflammatory genes can be activated by changes in cellular calcium levels, activation of free radicals, and ischemia, which further mobilizes neutrophils and glial cells. Activated pro-inflammatory genes induce the synthesis of pro-inflammatory cytokines to initiate the inflammation cascade, and increases vascular permeability, leading to vascular endothelial cell edema, which aggravates spinal neural ischemia [4,5].
Currently, the role of stem cell treatment for spinal cord injury has become widely recognized. Bone marrow mesenchymal stem cells (BMMSCs) have the advantages of ease of availability, a reduced risk of immune rejection, multipotential cell differentiation, and multiple transplantation pathways, which make them ideal tissue engineering and transplant stem cells, which can also be used in arterial and aortic transplantation [6]. Transplanted BMMSCs have been shown to secrete a variety of neurotrophic factors, which exert a synergistic neuroprotective effect with exogenous neurotrophic factors [7–9]. In a previous study, BMMSCs have been shown to express and secrete ciliary neurotrophic factor (CNTF) in vitro [10]. BMMSC transplantation through the infrarenal abdominal aorta has been shown to increase the local expression of CNTF in the spinal cord in ischemia-reperfusion injury [10].
CNTF and inflammatory factors belong to the same family [11], and their receptors consist of three components, the ciliary neurotrophic factor receptor (CNTFR), leukemia inhibitory factor receptor (LIFR) or CD118, and interleukin-6 (IL-6). When CNTF binds to LIFR and glycoprotein 130 (gp130) [12], it can directly affect the level of inflammatory factors and regulate cell fate. Therefore, BMMSCs may affect the expression of inflammatory cytokines in the injured spinal cord of rats with spinal cord ischemia-reperfusion injury by inducing increased expression of CNTF, promoting neuronal survival, improving spinal cord repair and improving hind limb function.
Therefore, this study aimed to investigate the effects of abdominal aortic transplantation of BMMSC transplantation on the expression of inflammatory cytokines in a rat model of spinal cord ischemia-reperfusion injury. The role of CNTF and the behavioral performance of the rats in the model before and after CNTF inhibition were studied. The morphology and number of cells that showed positive expression of neuronal nuclei (NeuN) neuron-specific nuclear in the cells in the ischemic segments of the spinal cord, and the expression levels of CNTF, IL-6, and IL-10 were investigated to determine the relationship between CNTF and inflammation in the rat model of spinal cord ischemia-reperfusion injury.
Material and Methods
Animals
All animal experiments were conducted according to the ethical guidelines of the First Peoples’ Hospital of Yunnan Province and with an animal license (License No. SCXK; certificate No. 43004700045114, Hunan, China). Specific pathogen-free (SPF) Sprague-Dawley rats (N=180) were purchased from Slac Jingda Laboratory Animal Co. Ltd. (Hunan, China). There were 160 adult rats (female) (mean weight, 200±20 g) and 3-week-old juvenile rats (male and female) (N=20) (mean weight, 30±10 g). Juvenile rats were chosen because the proliferative properties of cultured stem cells decreased with age, and compared with newborn mice, the tissues were easier to obtain.
Primary culture of bone marrow mesenchymal stem cells (BMMSCs)
Twenty juvenile Sprague-Dawley rats were sacrificed by cervical dislocation, and washed in 75% ethanol for 2–3 min. After rinsing with 0.1 M phosphate-buffered saline (PBS), both femurs were dissected out under sterile conditions, and the muscle fascia was removed. After washing with 0.1 M PBS, each femur was placed in a culture dish containing Dulbecco’s modified Eagle’s medium (DMEM) with F12 basic medium (Hyclone, Logan, UT, USA), supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA), 2 mmol/L glutamine, 50,000 U/L of penicillin, and 50 mg/L of streptomycin. The epiphysis tissue at the two ends of the femur was cut, and the bone marrow cavity was exposed. The bone marrow was retained, and the cell suspension was obtained by aspiration. After filtering in a mesh sieve, the cell suspension was isolated using Percoll gradient for blood cell separation, followed by centrifugation at 1,500 rpm for 20 min. After washing twice with 10% FBS, the cells were placed into lysine-coated 6-well culture plates, at the density of 1×106 cells/mL, and cultured in and incubator at 37°C in 5% CO2. After 48 h, the non-adherent cells were aspirated, and one-half of the medium was changed every other day until the cells covered reached 80% confluence.
Establishment of the rat model of spinal cord ischemia-reperfusion injury and the five study groups
Adult female Sprague-Dawley rats (N=160) were divided into five groups: the sham operation group (N-32); the control group (N=32); the BMMSC transplanted group (N=32); the anti-ciliary neurotrophic factor (CNTF)-treated BMMSC transplanted group (N=32); and the CNTF small interfering RNA (siRNA)-treated BMMSC transplanted group (N=32). In each group, 8 rats were investigated at 1, 2, 3, and 7 days, respectively.
The rats were anesthetized with an intraperitoneal injection of 3.6% chloral hydrate (1 mL/100 g). The left hind limb was sterilized, and following the skin preparation, the skin was opened to expose the femoral artery. A 24-gauge trocar was inserted into the femoral artery, which was connected to a pressure sensor to monitor the average pressure of the femoral artery. The left abdominal skin was cut longitudinally, and the posterior peritoneum was blunt dissected. The left renal artery and the abdominal aorta (1.5 cm each side) were exposed and freed. The vertebral artery from the superior abdominal aorta was occluded using bipolar electrocautery. The blood supply of the infrarenal abdominal aorta was occluded at the left renal artery origin for 120 min. On occlusion of the blood supply, the femoral artery pressure waveform disappeared, resulting in a mean arterial pressure (MAP) ≤10 mmHg (1.33 kPa). When the occlusion ceased, the femoral artery pressure waveform was restored. After 5 min of spinal cord reperfusion, a trocar was placed through the femoral cannula into the infrarenal abdominal aorta. For the sham operation group, the surgery and cannula implantation were performed, with no arterial occlusion.
For the sham operation group and the control group, 1 mL 10% FBS was injected through the cannula within 5 min, and the right femoral artery was temporarily occluded from before injection until 5 min after injection. For the other study groups, 1 mL of BMMSC suspension (1×106 cell/mL) was injected. For the anti-CNTF-treated BMMSC transplanted group and the CNTF siRNA-treated BMMSC transplanted group, on the day after transplantation, 1 mL of 10 μg/mL anti-CNTF (Chemicon International Inc., Temecula, CA, USA) and 1 mL of 2 μg/mL CNTF siRNA (Santa Cruz Biotechnology Inc., Dallas, TX, USA) were injected, respectively, once daily, for 7 consecutive days, while sterile water was injected for the other groups. After the operation, the abdominal layers were carefully sutured.
Neurobehavioral evaluation using the Basso, Beattie, and Bresnahan (BBB) hindlimb locomotor scale
The behavior of the rats was evaluated at 1, 2, 3, and 7 days after surgery using the BBB scoring system proposed by Basso et al. [13]. Normal locomotor function in rats had a BBB score of 21, while the rats with complete lack of locomotor function had a BBB score of 0. The evaluation criteria included hind limb movement, trunk position and stability, gait, coordination, claw placement, the gap between the toes, and tail position. Average scores were obtained. Scoring was performed by three independent assessors who were familiar with the scoring criteria.
Determination of motor evoked potentials (MEPs)
The behavior and electrophysiological tests were performed at an early period after the intervention, with the electrophysiological testing performed after the last BBB scoring was undertaken. Therefore, evaluation of motor evoked potentials (MEPs) were performed at day 7 d after surgery, according to the method previously described [14]. Briefly, each experimental animal was fixed in a prone position, awake, on the examination platform. Transcranial magnetic stimulation (TMS) (Dantec Medical, Skovlunde, Denmark) was used, with a circular stimulation coil, consisting of an inner diameter of the wire mesh of 10 mm, and an outer diameter of 50 mm (2×10 circles). The recording needle and reference electrodes were placed on the gastrocnemius muscle on the study side, and the surface electrode was connected to the ground wire, which was attached to the rat ear. The positive and negative electrodes were placed with 1 cm of each other. The center of the magnetic plate was placed to stimulate the cerebral cortex, with 40% of the stimulation in the motor zone. The waveform was recorded using the Keypoint signal acquisition system (Dantec Medical, Skovlunde, Denmark). The stimuli were repeated between 3–5 times and the latency and amplitude were recorded.
Determination of cortical somatosensory evoked potentials (CSEPs)
At day 7 after surgery, the cortical somatosensory inducing device was used to detect the cortical somatosensory evoked potentials (CSEPs). Briefly, the stimulating electrode was placed in the posterior tibial nerve, and the reference electrode was placed subcutaneously in the middle of the nose. The recording electrode was placed under the skin overlying the right cerebral cortex, and the rat ear was connected to the grounding electrode. The space between the positive and negative electrodes was 1 cm. The current was of the square-wave pulse type, with the following parameters: scanning speed, 10 ms/D; sensitivity, 10 uv/D; low frequency of filtering, 10 Hz; high frequency of filtering, 2 KHz. In total, 300 waveforms were recorded and averaged to obtain the final CSEP detection result.
Specimen collection and processing
The rats were euthanized by intraperitoneal injection with 3.6% chloral hydrate (1 mL/100 g), and the femoral artery was opened. After disinfection with 75% alcohol, the spinal canal was opened to expose the spinal cord, the L1 and L2 spinal cord was isolated and washed with pre-cooled 0.1% diethyl pyrocarbonate (DEPC) water, and stored at −80°C. The spinal cord below L2 was freed and fixed in 4% paraformaldehyde for 15 days. The L3 segment of the spinal cord was isolated and fixed in 4% paraformaldehyde for several days, followed by dehydration with 15% and 30% sucrose solution. The tissues were embedded in paraffin wax and serially sectioned at 5-μm onto glass slides.
Immunohistochemical staining
The primary cultured BMMSCs were identified by CD44 immunostaining. Third-generation BMMSCs were fixed in 4% paraformaldehyde at 4°C for 30 min. Immunohistochemical staining was performed by the PV-9000 two-step method (ZSGB-BIO, Beijing, China). The mouse anti-rat primary antibody to CD44 (1: 200) (Chemicon, Temecula, CA, USA) and the rabbit anti-rat primary antibody to neuronal nuclei (NeuN) protein (1: 200) (Chemicon, Temecula, CA, USA) were used. The negative control was the substitution of the primary antibody with 2% normal sheep serum. The localization of immunostaining was determined by incubation with the brown chromogen, 3,3′-diaminobenzidine (DAB) (ZSGB-BIO, Beijing, China), and the images were captured and analyzed.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the spinal cord tissue with TRIzol (Invitrogen, Carlsbad, CA, USA). The cDNA template was obtained with the Revert Aid™ First Strand cDNA Synthesis Kit (Fermentas International Inc., Burlington, Ontario, Canada). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using a PCR kit (Thermofisher Scientific, Waltham, MA, USA) using the Gene Cycle™ PCR instrument (Bio-Rad, Hercules, CA, USA). The qRT-PCR reaction was used to determine the expression of anti-ciliary neurotrophic factor (CNTF), interleukin (IL)-6, and IL-10.
The primer sequences were as follows:
CNTF, forward: 5′-CTTTCGCAGAGCAAACACCT-3′;
CNTF, reverse: 5′-CATCCCATCAGCCTCATTTT-3′;
IL-6 forward: 5′-GCCTTCTTGGGACTGATGT-3′;
IL-6, reverse: 5′-CTGGCTTTGTCTTTCTTGTTA-3′;
IL-10, forward: 5′-ACTGCTATGTTGCCTGCTCTT-3′;
IL-10, reverse: 5′-TCATTCTTCACCTGCTCCACT-3′;
GAPDH, forward: 5′-ATGGGGAAGGTGAAGGTCGGAG-3′; and
GAPDH, reverse 5′-GATGACAAGCTTCCCGTTCTCA-3′.
The PCR conditions were set as follows: 95°C for 2 min; 95°C for 10 s, 60°C for 30 s, 72°C for 15 s, for 40 cycles. GAPDH was used internal reference.
Western blot
The L2 segment spinal cord was lysed with the lysis buffer. After homogenizing, standing, and centrifugation, the protein concentration was determined. The protein samples underwent analysis with 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred onto the SDS-PAGE membrane. After blocking with 10% dried skimmed milk powder at 4°C overnight, the membrane was incubated for 1 h with the primary antibodies, including mouse anti-rat CNTF primary antibody (1: 500) (Chemicon International Inc., Temecula, CA, USA), goat anti-rat IL-6 (1: 200) (R&D Systems, Minneapolis, MN, USA), goat anti-rat IL-10 (1: 2000 dilution; R&D Systems), and mouse anti-rat β-actin (1: 2000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA). The membrane was incubated with secondary antibody conjugated with horseradish peroxidase (HRP), goat anti-mouse IgG (1: 5,000) (Santa Cruz Biotechnology Inc., Dallas, TX, USA) at room temperature for 70 min. Color development was performed with the enhanced chemiluminescence (ECL) kit, and protein band images were obtained and analyzed with Quantity One software (Bio-Rad, Hercules, CA, USA). β-actin was used as the internal reference.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). SPSS version 17.0 software (IBM, Chicago, IL, USA) was used for statistical analysis. One-way analysis of variance (ANOVA) and the Student-Newman-Keuls (SNK) q-test were performed for the group comparison. A P-value <0.05 was considered to be statistically significant.
Results
Identification and characterization of cultured bone marrow mesenchymal stem cells (BMMSCs)
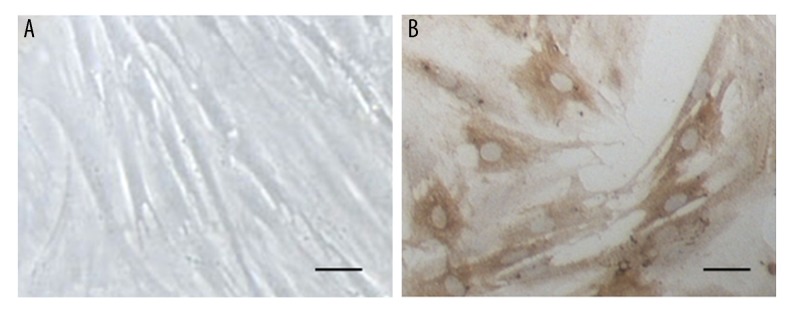
After two days of primary culture, bone marrow mesenchymal stem cells (BMMSCs) were observed to form spherules with reduced adherence, and some cells with elongated ends were present. On days 4 and 5, most of the cultured cells had short processes at both ends, with the cord-like cell groups. On day 7 of culture, the cultured cells had a swirling pattern of growth and cellular polarity was observed for most cells, with more flattened cell bodies and short processes (Figure 1A). The third-generation BMMSCs had flattened or triangular cell bodies with short processes. Immunohistochemical staining showed that most of these cultured BMMSCs were positive for CD44, which mainly stained the cytoplasm and cellular processes, while the cell nuclei were not stained (Figure 1B). These results showed that the cultured cells showed morphological and immunohistochemical features of BMMSCs.
Figure 1.
Identification and characterization of primary cultures of bone marrow mesenchymal stem cells (BMMSCs). (A) Primary cultures of bone marrow stem cells (BMMSCs) observed under light microscopy. (B) Immunohistochemical staining of BMMSCs with a primary antibody to CD44. Scale bar, 20 μm.
Basso, Beattie, and Bresnahan (BBB) neurobehavioral scores
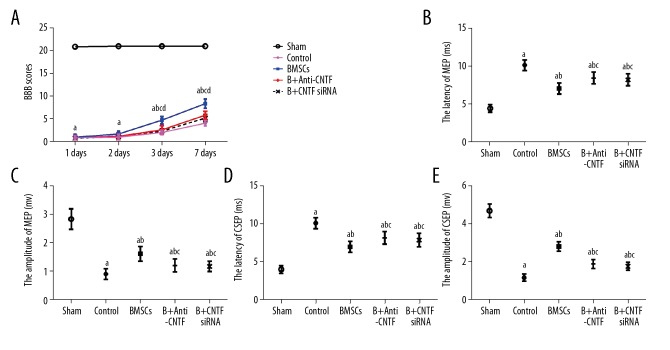
Adult female Sprague-Dawley rats (N=160) were divided into five groups: the sham operation group (N-32); the control group (N=32); the BMMSC transplanted group (N=32); the anti-ciliary neurotrophic factor (CNTF)-treated BMMSC transplanted group (N=32); and the CNTF small interfering RNA (siRNA)-treated BMMSC transplanted group (N=32). Following surgery, the rats from each group underwent BBB scoring. The results showed that when compared with the sham operation group, significantly reduced BBB scores were found in the control group, the BMMSC transplanted group, the anti-CNTF-treated BMMSC transplanted group, and the CNTF siRNA-treated BMMSC transplanted group, at each time point (P<0.05).
Following surgery, the BBB scores of all the groups were gradually restored, but to different degrees. For the BMMSC transplanted group, the BBB scores on days 3 and 7 after surgery were significantly increased when compared with the control group, the anti-CNTF-treated BMMSC transplanted group, and the CNTF siRNA-treated BMMSC transplanted group (P<0.05). The BBB scores in the anti-CNTF-treated BMMSC transplanted group, and the CNTF siRNA-treated BMMSC transplanted group on day 7 after surgery were significantly increased compared with the control group (P<0.05). No significant differences were found between the treated groups (P>0.05) (Table 1; Figure 2). These results showed that BMMSC transplantation significantly improved the hindlimb motor function in the rat model of spinal cord ischemia-reperfusion injury. Anti-CNTF treatment and CNTF siRNA inhibition could delay the recovery of hindlimb motor function in rats after BMMSC transplantation.
Table 1.
The Basso, Beattie, and Bresnahan (BBB) locomotor scoring scale for animal behavior following surgery.
| Groups in the rat model of spinal cord ischemia-reperfusion injury | Days post-surgery | |||
|---|---|---|---|---|
| 1 day | 2 days | 3 days | 7 days | |
| Sham group | 20.88±0.09 | 21±0 | 21±0 | 21±0 |
| Control group | 0.88±0.19# | 1.00±0.24# | 2.04±0.18#,** | 4.08±0.21#,** |
| BMMSC transplanted group | 1.00±0.24# | 1.71±0.24# | 4.75±0.29#,##,** | 8.38±0.36#,##,** |
| Anti-CNTF-treated BMMSC transplanted group | 0.88±0.20# | 1.21±0.27# | 2.63±0.33#,*,** | 5.84±0.32#,##,*,** |
| CNTF small interfering RNA (siRNA)-treated BMMSC transplanted group | 0.75±0.22# | 1.13±0.20# | 2.33±0.19#,*,** | 5.21±0.27#,##,*,** |
BMMSC – bone marrow mesenchymal stem cell; CNTF – ciliary neurotrophic factor. Compared with sham group,
P<0.05; compared with control group,
P<0.05; compared with transplanted group,
P<0.05; compared with the last time-point within the same group,
P<0.05.
Figure 2.
Behavioral performance of the rat model of spinal cord ischemia-reperfusion injury at different time points following surgery. (A) Basso, Beattie, and Bresnahan (BBB) locomotor scale scores. (B) Motor evoked potential (MEP) latency. (C) MEP amplitude. (D) Cortical somatosensory evoked potential (CSEP) latency. (E) CSEP amplitude. Compared with the sham operation group, a P<0.05; compared with the control group, b P<0.05; compared with the bone marrow mesenchymal stem cell (BMMSC) transplanted group, c P<0.05; and compared with the former time point within the same group, d P<0.05.
Electrophysiological findings
Electrophysiological studies were performed in the rat model of spinal cord ischemia-reperfusion injury following surgery. On day 7 after surgery, when compared with the sham operation group, the other groups had significantly prolonged motor evoked potential (MEP) and cortical somatosensory evoked potential (CSEP) latencies (P<0.05), with significantly reduced amplitudes (P<0.05). Compared with the control group, the latencies were shortened while the amplitudes were increased in the anti-CNTF-treated BMMSC transplanted group and the CNTF siRNA-treated BMMSC transplanted group (all P<0.05). Compared with the anti-CNTF-treated BMMSC transplanted group and the CNTF siRNA-treated BMMSC transplanted group, the latencies were further shortened while the amplitudes were further increased (all, P<0.05). However, no significant differences were observed between the inhibition or treatment groups (P>0.05) (Table 2; Figure 2). These results showed that BMMSC transplantation significantly promoted the recovery of spinal nerve conduction function in the rat model of spinal cord ischemia-reperfusion injury. Inhibition of anti-CNTF and CNTF siRNA delayed the recovery of spinal nerve conduction function after BMMSC transplantation.
Table 2.
Motor evoked potentials (MEPs) and cortical somatosensory evoked potentials (CSEPs) at day 7 following surgery.
| Groups in the rat model of spinal cord ischemia-reperfusion injury | Motor evoked potentials (MEPs) | Cortical somatosensory evoked potentials (CSEPs) | ||
|---|---|---|---|---|
| Latency (ms) | Amplitude (mv) | Latency (ms) | Amplitude (mv) | |
| Sham group | 4.42±0.18 | 2.84±0.13 | 4.07±0.18 | 4.69±0.13 |
| Control group | 10.19±0.26* | 0.91±0.07* | 10.16±0.25* | 1.17±0.06* |
| BMMSC transplanted group | 7.08±0.26*,** | 1.62±0.09*,** | 7.04±0.25*,** | 2.82±0.10*,** |
| Anti-CNTF-treated BMMSC transplanted group | 8.48±0.28*,**,# | 1.21±0.08*,**,# | 8.23±0.30*,**,# | 1.89±0.09*,**,# |
| CNTF small interfering RNA (siRNA)-treated BMMSC transplanted group | 8.25±0.28*,**,# | 1.17±0.06*,**,# | 7.92±0.31*,**,# | 1.77±0.07*,**,# |
BMMSC – bone marrow mesenchymal stem cell; CNTF – ciliary neurotrophic factor. Compared with sham group,
P<0.05; compared with control group,
P<0.05; compared with transplantation group,
P<0.05.
Expression of neuronal nuclei (NeuN) neuron-specific nuclear protein in the spinal cord
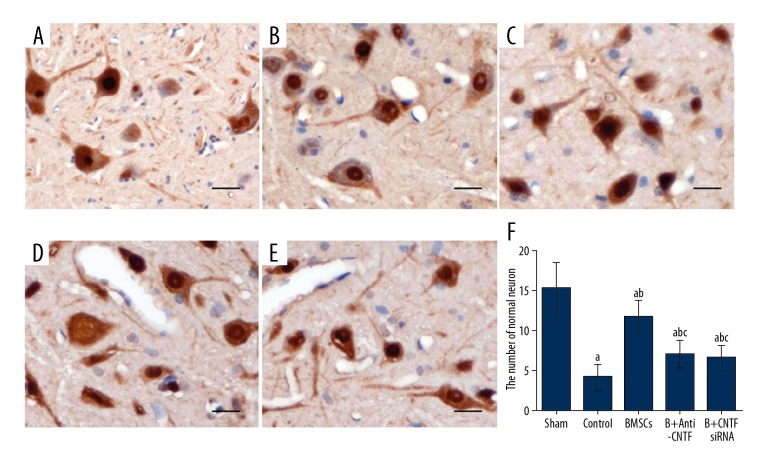
The expression of neuronal nuclei (NeuN) neuron-specific nuclear protein in the spinal cord was detected by immunohistochemical staining. On day 7 following surgery, NeuN-positive immunostaining was observed in the gray matter of the spinal cord of the rats in the sham operation group, and the cells showed polygonal morphology, intensely stained cellular nuclei (with prominent nucleoli), relatively lightly stained cytoplasm and cell processes. Also, the cell bodies of the neurons in lamina VIII and IX in the anterior horn were significantly larger than the surrounding cells (Figure 3A). In the control group, the cell bodies of a large number of neurons were atrophied, with vacuolar degeneration, ruptured nuclei, and disrupted chromatin (Figure 3B). The numbers of normal neurons in lamina VIII and IX were significantly lower in the sham operation group, compared with the BMMSC transplantation, and treatment groups (P<0.05). In the transplantation groups, only some of the neurons exhibited nuclear disruption (Figure 3C). The number of normal neurons was significantly reduced compared with the sham operation group (P<0.05), which was higher than the control group, the anti-CNTF-treated BMMSC transplanted group, and the CNTF siRNA-treated BMMSC transplanted group (P<0.05). In the anti-CNTF-treated BMMSC transplanted group and the CNTF siRNA-treated BMMSC transplanted group, the numbers of normal neurons were significantly increased compared with the control group (both, P<0.05) (Figures 3D, 3E). No significant differences were observed between the treatment inhibition groups (P>0.05) (Figure 3). These results showed that BMMSC transplantation significantly reduced neuronal necrosis and promoted neuronal survival in the ischemic segment of the rat model of spinal cord ischemia-reperfusion injury. CNTF inhibition reduced the therapeutic effects of BMMSC transplantation.
Figure 3.
Expression of neuronal nuclei (NeuN) neuron-specific nuclear protein in the L3 segment of the spinal cord on day 7 after surgery in the five study groups. The expression levels of neuronal nuclei (NeuN) neuron-specific nuclear protein were detected with the immunohistochemical staining. Scale bar, 20 μm. (A) The sham operation group. (B) The control group. (C) The bone marrow mesenchymal stem cell (BMMSC) transplanted group. (D) The anti-ciliary neurotrophic factor (CNTF)-treated BMMSC transplanted group. (E) The CNTF small interfering RNA (siRNA)-treated BMMSC transplanted group. (F) Statistical analysis. Compared with the sham operation group, a P<0.05; compared with the control group, b P<0.05; and compared with the transplanted groups, c P<0.05.
Expression levels of CNTF, IL-6, and IL-10
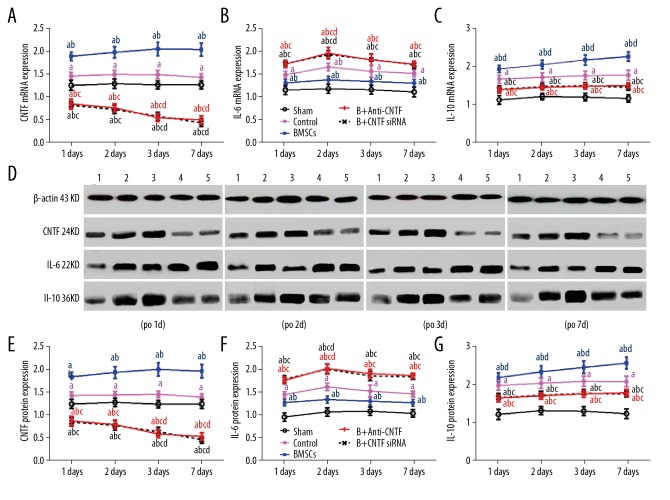
The mRNA and protein expression levels of CNTF, IL-6, and IL-10 were detected with quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot analysis, respectively. The results showed that on days 1, 2, 3, and 7 after surgery, when compared with the sham operation group, the mRNA and protein expression levels of CNTF in the spinal cord were significantly increased in the control and BMMSC transplanted groups (P<0.05). Also, the expression levels of CNTF were significantly decreased in the anti-CNTF-treated BMMSC transplanted group and the CNTF siRNA-treated BMMSC transplanted group (P<0.05) by day 7 following surgery (P<0.05).
Also, compared with the sham operation group, the mRNA and protein expression levels of IL-6 and IL-10 in the spinal cord were significantly increased after surgery (all, P<0.05). However, the expression of IL-10 was consistently increased after surgery. Compared with the control group, the expression of IL-6 was reduced and the expression of IL-10 was significantly increased in the BMMSC transplantation group (both, P<0.05). For the anti-CNTF-treated BMMSC transplanted group and the CNTF siRNA-treated BMMSC transplanted group, the expression of IL-6 was significantly increased and the expression of IL-10 was significantly reduced (P<0.05) (Figure 4). These results indicated that BMMSC transplantation significantly promoted the expression of CNTF and the anti-inflammatory cytokine, IL-10, in the spinal cord of the rat model of spinal cord ischemia-reperfusion injury, but inhibited the expression of the pro-inflammatory cytokine, IL-6, which could be altered by CNTF inhibition.
Figure 4.
(A–G) Expression levels of ciliary neurotrophic factor (CNTF), interleukin (IL)-6, and IL-10 in the lumbar spinal cord in the five study groups of rats. The mRNA and protein expression levels of CNTF, IL-6, and IL-10 in the lumbar spinal cord were detected by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot analysis, respectively. (A) The mRNA expression levels of ciliary neurotrophic factor (CNTF) in the lumbar spinal cord on days 1, 2, 3, and 7 after surgery. (B) The mRNA expression levels of interleukin (IL)-6 in the lumbar spinal cord on days 1, 2, 3, and 7 after surgery. (C) The mRNA expression levels of IL-10 in the lumbar spinal cord on days 1, 2, 3, and 7 after surgery. (D) The protein expression levels of CNTF, IL-6, and IL-10 in the lumbar spinal cord on days 1, 2, 3, and 7 after surgery. Adult female Sprague-Dawley rats (N=160) were divided into five groups: 1, the sham operation group; 2, the control group; 3, the bone marrow mesenchymal stem cell (BMMSC) transplanted group; 4, the anti-CNTF-treated BMMSC transplanted group; 5, the CNTF small interfering RNA (siRNA)-treated BMMSC transplanted group. Compared with the sham operation group, a P<0.05; compared with the control group, b P<0.05; compared with the transplantation group, c P<0.05; and compared with the different time points within the same group, d P<0.05.
Discussion
In this study, homologous bone marrow mesenchymal stem cells (BMMSCs), derived from 20 juvenile Sprague-Dawley rats, were used to treat rats in a model of spinal cord ischemia-reperfusion injury through subrenal abdominal aorta transplantation [15], with anti-ciliary neurotrophic factor (CNTF) and treatment with CNTF small interfering RNA (siRNA) via a subrenal abdominal aortic approach. Adult female Sprague-Dawley rats (N=160) were divided into five groups: the sham operation group (N-32); the control group (N=32); the BMMSC transplanted group (N=32); the anti-ciliary neurotrophic factor (CNTF)-treated BMMSC transplanted group (N=32); and the CNTF small interfering RNA (siRNA)-treated BMMSC transplanted group (N=32).
Severe spinal cord dysfunction was observed in the rat model following surgery. However, with time, the motor function of the rat hind limbs was restored but with varying degrees. BMMSC transplantation significantly accelerated the recovery of hind limb function, as shown by the shortened latencies of motor evoked potentials (MEPs) and increased amplitude of the cortical somatosensory evoked potentials (CSEPs), and increased Basso, Beattie, and Bresnahan (BBB) locomotor scores. However, following treatment with anti-CNTF and CNTF siRNA, the changes in the amplitude of MEP and CSEP, and BBB scores were significantly reduced compared with the transplant group, indicating that the recovery of hind limb function was delayed in rats following inhibition of CNTF. These results suggest that BMMSC transplantation into the infrarenal abdominal aorta contributed to the spinal cord repair process in the rats with spinal cord ischemia-reperfusion injury, resulting in improved hind limb function in rats after injury. However, CNTF inhibition significantly reduced the spinal cord reparative effects, indicating that the transplanted BMMSCs may secrete CNTF.
Previous studies have shown that BMMSCs could induce neural cell repair by secreting neurotrophic factors, promoting axonal regeneration leading to functional improvement following spinal cord injury [7–9]. In this study, immunohistochemistry showed that on day 7 after surgery, the transplanted BMMSCs could significantly reduce neuronal necrosis in the spinal cord of the ischemic segments, and increase the number of normal neurons. Also, the degree of neuronal necrosis was significantly increased after CNTF inhibition, with significantly reduced the numbers of normal neurons. These results suggest that BMMSC transplantation into the infrarenal abdominal aorta improved hind limb functional recovery of rats with spinal cord ischemia-reperfusion injury by secreting CNTF. The inhibition of CNTF could significantly reduce the efficacy of neuronal survival, indicating its possible role in promoting the survival of spinal cord neurons in the rat model of spinal cord ischemia-reperfusion injury.
CNTF was first discovered by Helfand et al. in 1976 [16]. In 1984, Barbin et al. successfully extracted CNTF and showed that it could protect the chicken ciliary neurons and maintain the survival of parasympathetic ganglia [17]. CNTF belongs to the regulatory cytokine family, which participate in the regulation of inflammatory responses by regulating inflammatory cytokine levels [11,12]. The inflammatory cascade is one of the important mechanisms of spinal cord ischemia-reperfusion injury, which involves the complex effects of inflammatory factors including the pro-inflammatory cytokines IL-2, IL-6, and IL-8, as well as anti-inflammatory factors that include IL-4 and IL-10 [5,18]. Ryu et al. [19] showed that, in a dog model of spinal cord injury, autologous and allogeneic BMMSC transplantation could promote neural regeneration after spinal cord ischemia and improved neurological function, which was associated with IL-6 and cyclooxygenase-2 (COX-2) expression.
The findings of the present study showed that BMMSC transplantation significantly upregulated the levels of CNTF and IL-10, and down-regulated IL-6 levels, in the spinal cord of rats in the model of spinal cord ischemia-reperfusion injury. However, following CNTF inhibition, IL-10 expression levels were significantly reduced, while IL-6 expression levels were significantly increased. These results suggest that CNTF participates in the regulation of the balance between the pro-inflammatory cytokine IL-6, and anti-inflammatory cytokine IL-10. Also, BMMSC transplantation may indirectly regulate the balance of inflammatory factors through the elevated expression of CNTF, reducing the local inflammatory responses in the injured spinal cord, promoting the neuron survival, and promoting the improvement of hind limb functions in the rat model of spinal cord ischemia-reperfusion injury
Conclusions
In a rat model of spinal cord ischemia-reperfusion injury, abdominal aortic transplantation of bone marrow mesenchymal stem cells (BMMSCs) increased the expression of ciliary neurotrophic factor (CNTF), which improved hindlimb locomotor recovery by regulating the expression of interleukin (IL)-6 and IL-10 to reduce inflammation of the spinal cord. These therapeutic effects were reduced by inhibition of CNTF. These preliminary findings may provide the basis for further studies on the clinical mechanism of BMMSC transplantation in spinal cord ischemia-reperfusion injury.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 31360246 and No. 81300973), the Science Research Fund Project from the Yunnan Department of Education (No. 2016ZZX279), the Yunnan Academic Science and Technology Reserve Talent Project (No. 2017HB042), and the Yunnan Academic Leader Project (No. D-201631)
References
- 1.Panthee N, Ono M. Spinal cord injury following thoracic and thoracoabdominal aortic repairs. Asian Cardiovasc Thorac Ann. 2015;23:235–46. doi: 10.1177/0218492314548901. [DOI] [PubMed] [Google Scholar]
- 2.Gokce EC, Kahveci R, Gokce A, et al. Curcumin attenuates inflammation, oxidative stress, and ultrastructural damage induced by spinal cord ischemia-reperfusion injury in rats. J Stroke Cerebrovasc Dis. 2016;25:1196–207. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Gokhan L, Selcuk GH, Kemal K, et al. Efficacy of iloprost and montelukast combination on spinal cord ischemia/reperfusion injury in a rat model. J Cardiothorac Surg. 2013;8:64. doi: 10.1186/1749-8090-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang JY, Min SW, Jeon YT, et al. Effect of coenzyme Q10 on spinal cord ischemia-reperfusion injury. J Neurosurg Spine. 2015;22:432–38. doi: 10.3171/2014.12.SPINE14487. [DOI] [PubMed] [Google Scholar]
- 5.Palencia G, Medrano JAN, Ortiz-Plata A, et al. Anti-apoptotic, anti-oxidant, and anti-inflammatory effects of thalidomide on cerebral ischemia/reperfusion injury in rats. J Neurol Sci. 2015;351:78–87. doi: 10.1016/j.jns.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg J, Södersten E, Sundström E, et al. Targeted intra-arterial transplantation of stem cells to the injured CNS is more effective than intravenous administration: Engraftment is dependent on cell type and adhesion molecule expression. Cell Transplant. 2012;21:333–43. doi: 10.3727/096368911X576036. [DOI] [PubMed] [Google Scholar]
- 7.Abbaszadeh HA, Tiraihi T, Noori-Zadeh A, et al. Human ciliary neurotrophic factor-overexpressing stable bone marrow stromal cells in the treatment of a rat model of traumatic spinal cord injury. Cytotherapy. 2015;17:912–21. doi: 10.1016/j.jcyt.2015.03.689. [DOI] [PubMed] [Google Scholar]
- 8.Mendez-Ferrer S, Scadden DT, Sanchez-Aguilera A. Bone marrow stem cells: Current and emerging concepts. Ann NY Acad Sci. 2015;1335:32–44. doi: 10.1111/nyas.12641. [DOI] [PubMed] [Google Scholar]
- 9.Xiong LL, Liu F, Lu BT, et al. Bone marrow mesenchymal stem-cell transplantation promotes functional improvement associated with CNTF-STAT3 activation after hemi-sectioned spinal cord injury in tree shrews. Front Cell Neurosci. 2017;11:172. doi: 10.3389/fncel.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen SS, Guo XB, Jin H, Surgery DO. [Effects of the bone marrow mesenchymal stem cells transplantated by Abdominal aortic on CNTF and STAT3 in spinal cord ischemic reperfusion injured rats]. J Apoplexy Nerv Dis. 2014 [in Chinese] [Google Scholar]
- 11.Davis S, Yancopoulos GD. The molecular biology of the CNTF receptor. Curr Opin Neurobiol. 1993;3:20–24. doi: 10.1016/0959-4388(93)90030-3. [DOI] [PubMed] [Google Scholar]
- 12.Davis S, Aldrich TH, Stahl N, et al. LIFRβ and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993;260:1805–8. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- 13.Basso DM, Beattie MS, Bresnahan JC. Descending systems contributing to locomotor recovery after mild or moderate spinal cord injury in rats: Experimental evidence and a review of literature. Restor Neurol Neurosci. 2002;20(5):189–218. [PubMed] [Google Scholar]
- 14.Copelman CA, Cuzner ML, Groome N, Diemel LT. Temporal analysis of growth factor mRNA expression in myelinating rat brain aggregate cultures: Increments in CNTF, FGF-2, IGF-I, and PDGF-AA mRNA are induced by antibody-mediated demyelination. Glia. 2000;30:342–51. doi: 10.1002/(sici)1098-1136(200006)30:4<342::aid-glia30>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Chen SS, Ning J, Zhu LX, et al. [Model of bone marrow mesenchymal stem cells transplanted to ischemic reperfusion injured spinal cord through abdomen aorta in rats]. JKM UST. 2014 [in Chinese] [Google Scholar]
- 16.Helfand SL, Smith GA, Wessells NK. Survival and development in culture of dissociated parasympathetic neurons from ciliary ganglia. Dev Biol. 1976;50:541–47. doi: 10.1016/0012-1606(76)90174-3. [DOI] [PubMed] [Google Scholar]
- 17.Barbin G, Manthorpe M, Varon S. Purification of the chick eye ciliary neuronotrophic factor. J Neurochem. 1984;43:1468–78. doi: 10.1111/j.1471-4159.1984.tb05410.x. [DOI] [PubMed] [Google Scholar]
- 18.Segal JL, Gonzales E, Yousefi S, et al. Circulating levels of IL-2R, ICAM-1, and IL-6 in spinal cord injuries. Arch Phys Med Rehabil. 1997;78:44–47. doi: 10.1016/s0003-9993(97)90008-3. [DOI] [PubMed] [Google Scholar]
- 19.Ryu HH, Kang BJ, Park SS, et al. Comparison of mesenchymal stem cells derived from fat, bone marrow, Wharton’s jelly, and umbilical cord blood for treating spinal cord injuries in dogs. J Vet Med Sci. 2012;74:1617–30. doi: 10.1292/jvms.12-0065. [DOI] [PubMed] [Google Scholar]