Abstract
Background
Brain death initiates hemodynamic, immunological, and hormonal changes that potentially compromise organ quality for transplantation. Therefore, it is generally believed that organs should be procured as soon as possible after the declaration of brain death. However, conflicting data exist regarding the impact of brain death duration on long-term graft function and survival.
Material/Methods
The effect of duration of brain death on graft survival and function of 1869 adult transplant recipients receiving kidneys from deceased donors after brain death was analyzed, using relevant donor and recipient characteristics and allograft related factors.
Results
Duration of brain death was a significant predictor for long-term graft survival, whilst there was no significant effect of duration of brain death on the incidence of delayed graft function or acute graft rejection after kidney transplantation. After dividing the study population into a “short durBD” (<10.6 hours) group and a “long durBD” (>10.6 hours) group, the 15-year graft survival estimates were significantly higher and the serum creatinine at 3 months after transplantation was significantly lower in the “long durBD” group.
Conclusions
Duration of brain death does not affect the incidence of delayed graft function or acute rejection after kidney transplantation. However, longer duration of brain death is associated with better kidney allograft function and survival.
MeSH Keywords: Brain Death, Delayed Graft Function, Graft Rejection, Kidney Transplantation, Survival Analysis
Background
The immunological changes and hormonal dysregulation in brain-dead donors may contribute to the inferior outcomes of transplantation with a kidney from a brain-dead donor as compared to a kidney from a living donor [1–3]. This can be explained by the hemodynamic instability in the donor: when cerebral ischemia reaches the medulla oblongata, the sympathetic nerves are excited, causing the explosive release of endogenous catecholamines. This leading to an abrupt elevation of blood pressure and tachycardia, also called the “sympathetic or catecholamine storm”. The release of catecholamines increases the heart load and oxygen consumption. High levels of catecholamines can also lead to overload of intracellular calcium, depletion of adenosine triphosphate, and therefore overproduction of oxygen free radicals and cell injury. Thereafter, a hypotensive phase commences, due to gradual depletion of catecholamines, causing further reduction of the oxygen supply to the internal organs [4,5]. Therefore, it is generally believed that organs should be procured as soon as possible once brain death is confirmed. This is supported by evidence from animal studies indicating that prolonged duration of brain death (durBD) is deleterious [6–8]. However, evidence from clinical cohort studies indicates that longer durBD is not detrimental, but beneficial by reducing the incidence of delayed graft function [9–12]. Conflicting evidence exists regarding the influence of brain death duration on long-term kidney graft function and survival [10,12].
We aimed to study the impact of brain death duration on long-term outcome in deceased donor kidney transplantation. Therefore, we analyzed data from the Netherlands Organ Transplant Registry (NOTR).
Material and Methods
Patients
Data was obtained from a prospectively maintained electronic database called the Netherlands Organ Transplant Registry (NOTR, Dutch Transplant Foundation, Leiden, the Netherlands). The dataset that we used included a consecutive series of donation after brain death kidney transplantations from May 1, 2002 to December 31, 2015 in the Netherlands. Inclusion criteria were: initial kidney transplantation in adult recipients aged ≥18 years with recorded data of durBD, immediate or delayed graft function, graft rejection within the first year after kidney transplantation, and graft survival.
The following characteristics were available and extracted from the database: donor age, gender, body mass index, hypotensive period(s) and lowest creatinine during the brain death period, history of systemic diseases (e.g., hypertension, diabetes mellitus), date and time of brain death declaration and start of cold perfusion; recipient gender, age, body mass index and duration of dialysis prior to kidney transplantation; anastomosis time, cold ischemia time and human leukocyte antigen mismatches. DurBD was calculated by subtracting the time of start of cold perfusion and the time of declaration of brain death.
Endpoints
The long-term outcome measure was graft survival. Graft failure was defined as the return to dialysis or re-transplantation and was censored upon death with a functioning graft. The short-term outcome measures were delayed graft function, defined as the need for dialysis after transplantation; acute rejection, defined as the need for treatment for graft rejection within the first year after kidney transplantation; serum creatinine 3 and 12 months after kidney transplantation.
Risk of bias
To address potential sources of bias, baseline donor and recipient characteristics and allograft related factors of exposed and unexposed participants were compared, and follow-up outcome data on immediate or delayed graft function, graft rejection within the first year after kidney transplantation, and graft survival were considered for selection bias; multivariable analysis with relevant donor and recipient demographics and allograft related were performed for confounding factors [13–18].
Statistical analysis
To evaluate the effect of durBD on graft survival after kidney transplantation, uni- and multivariable Cox proportional hazards models were performed with relevant donor and recipient characteristics and allograft related factors. Binary logistic regression, with relevant donor and recipient characteristics and allograft related factors, was used to identify whether durBD was associated with the incidence of delayed graft function or acute rejection. After measuring the effect of durBD as a continuous variable, we divided our study population equally by the median in the “short durBD” (<10.6 hours) group and the “long durBD” (>10.6 hours) group [10]. The effect of “short durBD” versus “long durBD” on graft survival was expressed graphically using the Kaplan-Meier method for illustrational purposes; the statistical difference between groups was assessed by the log-rank test. The effect on serum creatinine 3 and 12 months post-operatively was evaluated using the Mann-Whitney U test. All statistical analyses were performed using SPSS software, version 25 (SPSS Inx., Chicago, IL, USA). P-values <0.05 were considered statistically significant.
Results
Demographics
Between May 1, 2002 and December 31, 2015 there were 2460 initial transplantations performed with kidneys donated after brain death, in adults in the Netherlands, including 1869 transplants with recorded data of durBD. Data on time of start of cold perfusion or time of declaration of brain death was not recorded for 591 transplants (24.0%), and these transplants where therefore excluded. Data on graft function and/or graft survival was not recorded for 286 transplant recipients (15.3%) who were lost to follow-up. Therefore, 1583 donor-recipient pairs were included in this analysis. Demographics of donors and recipients, and allograft-related factors are shown in Table 1. The median durBD was 10.6 hours; the distribution of durBD is shown in Figure 1. For 99.0% of all donors the durBD was shorter than 24 hours.
Table 1.
Baseline donor and recipient demographics and allograft related factors (n=2460).
| Baseline characteristics | Included pairs (n=1,869) | n | Excluded pairs (n=591) | n | P-value |
|---|---|---|---|---|---|
| Duration of brain death period (hours) | 10.6 (8.9–12.5) | 1,869 | N/A | 591 | N/A |
| Donor gender (Male) | 833 (44.6%) | 1,869 | 313 (53.0%) | 591 | .000 |
| Donor age (years) | 54 (45–63) | 1,869 | 51 (42–58) | 591 | .000 |
| Donor body mass index (kg/m2) | 24.5 (22.5–26.9) | 1,869 | 25.1 (23.3–27.8) | 591 | .000 |
| Expanded criteria donor | 696 (37.2%) | 1,869 | 157 (26.6%) | 591 | .000 |
| Donor history of hypertension | 505 (28.0%) | 1,806 | 179 (42.3%) | 423 | .000 |
| Donor hypotensive period(s) | 658 (37.3%) | 1,762 | 122 (34.2%) | 357 | .252 |
| Donor history of diabetes mellitus | 24 (1.3%) | 1,859 | 0 (0.0%) | 19 | .618 |
| Donor history of cardiac arrest | 449 (24.4%) | 1,843 | 76 (20.1%) | 378 | .064 |
| Donor use of inotropic medication | 1,616 (86.5%) | 1,869 | 22 (95.7%) | 23 | .049 |
| Donor cause of death: stroke | 587 (31.4%) | 1,869 | 163 (27.6%) | 591 | .073 |
| Donor cause of death: trauma | 290 (15.5%) | 1,869 | 89 (15.1%) | 591 | .789 |
| Donor lowest creatinine (μmol/L) | 64 (50–100) | 1,868 | 69 (53–104) | 591 | .707 |
| Recipient gender (Male) | 1,151 (61.6%) | 1,869 | 300 (50.8%) | 591 | .000 |
| Recipient age (years) | 57 (45–65) | 1,869 | 54 (44–61) | 591 | .000 |
| Recipient body mass index (kg/m2) | 25.4 (22.8–28.4) | 1,725 | 24.8 (22.2–28.1) | 564 | .181 |
| Recipient dialysis duration (years) | 3.67 (2.42–4.99) | 1,702 | 2.98 (1.90–4.58) | 542 | .017 |
| Cold ischemia time (hours) | 14.6 (11.5–19.0) | 1,665 | 18.0 (14.5–22.7) | 551 | .000 |
| Anastomosis time (minutes) | 33 (26–40) | 1,662 | 32 (25–40) | 534 | .376 |
| Number of HLA mismatches | 3 (2–4) | 1,859 | 2 (0–3) | 589 | .000 |
| Delayed graft function | 277 (17.5%) | 1,583 | 82 (16.0%) | 513 | .429 |
| Acute graft rejection within 1 year | 110 (5.9%) | 1,869 | 38 (6.4%) | 591 | .628 |
| Graft failure | 293 (15.7%) | 1,869 | 100 (16.9%) | 591 | .472 |
Values are expressed as the median (25th–75th percentile), unless stated otherwise. HLA – human leukocyte antigen.
Figure 1.
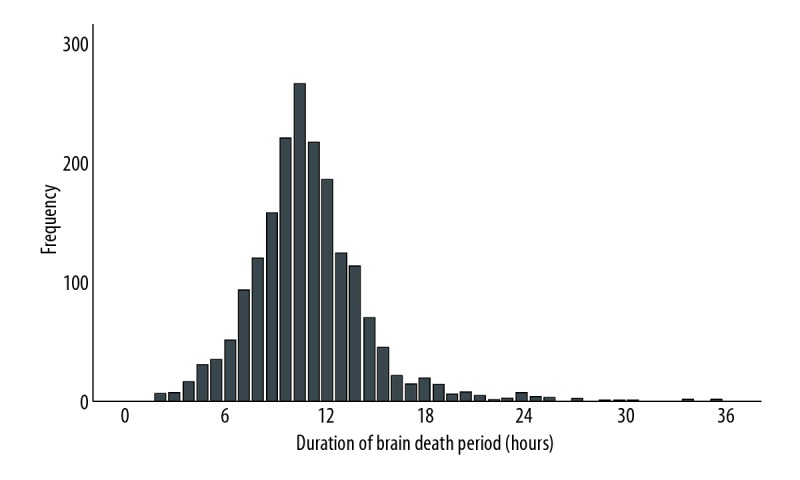
Distribution of duration of brain death period in hours.
Duration of brain death and graft function
Data on direct or delayed graft function after kidney transplantation were available for 1583 recipients (84.7%). Donors of these recipients who had immediate graft function (n=1306) had a median durBD of 10.7 hours (range, 9.0 to 12.5 hours), while donors of recipients who suffered from delayed graft function (n=277) had a median durBD of 10.5 hours (range, 8.9 to 12.8 hours). In recipients who needed treatment for rejection within 1 year after transplantation (n=110), the donors had a median durBD of 10.2 hours (range, 9.0 to 12.5 hours), while durBD was 10.7 hours (range, 8.9 to 12.5 hours) in donors of rejection-free recipients (n=1759).
To evaluate the effect of durBD on the incidence of delayed graft function and acute rejection, binary logistic analyses with relevant donor and recipient characteristics and allograft related factors were performed. In a univariable logistic regression analysis, the effect of durBD was not significant for the incidence of delayed graft function with an odds ratio (OR) of 1.012 (P=0.503) or the incidence of graft rejection in the first year after transplantation with an OR of 1.012 (P=0.641). In a multivariable logistic regression analysis, the effect of durBD remained nonsignificant for the incidence of delayed graft function with an OR of 1.042 (P=0.058) or acute rejection with an OR of 1.054 (P=0.055). Table 2 shows the variables used in the multivariable analysis.
Table 2.
Multivariable binary logistic regression analysis for delayed graft function and acute graft rejection.
| Graft function | Delayed graft function | Acute graft rejection | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Duration of brain death period (hours) | 1.042 (0.999–1.087) | .058 | 1.054 (0.999–1.112) | .055 |
| Donor gender (Female/Male) | 1.569 (1.128–2.183) | .008 | 0.942 (0.588–1.508) | .804 |
| Donor age (years) | 1.036 (1.015–1.057) | .001 | 1.034 (1.003–1.067) | .033 |
| Donor body mass index (kg/m2) | 1.044 (1.004–1.086) | .031 | 1.006 (0.952–1.062) | .841 |
| Expanded criteria donor | 0.955 (0.564–1.617) | .863 | 0.975 (0.459–2.069) | .948 |
| Donor history of hypertension | 1.365 (0.946–1.970) | .096 | 0.663 (0.380–1.158) | .149 |
| Donor hypotensive period(s) | 0.774 (0.547–1.095) | .148 | 1.027 (0.633–1.667) | .915 |
| Donor history of diabetes mellitus | 0.671 (0.078–5.791) | .717 | 1.595 (0.195–13.039) | .663 |
| Donor history of cardiac arrest | 1.461 (0.992–2.152) | .055 | 0.895 (0.501–1.598) | .707 |
| Donor use of inotropic medication | 1.238 (0.744–2.061) | .411 | 1.240 (0.595–2.582) | .566 |
| Donor cause of death: stroke | 0.709 (0.482–1.043) | .081 | 0.832 (0.492–1.407) | .493 |
| Donor cause of death: trauma | 1.069 (0.662–1.727) | .784 | 0.502 (0.223–1.131) | .096 |
| Donor lowest creatinine (μmol/L) | 1.001 (1.000–1.001) | .079 | 1.001 (1.000–1.001) | .029 |
| Recipient gender (Female/Male) | 1.307 (0.930–1.839) | .123 | 1.496 (0.910–2.461) | .112 |
| Recipient age (years) | 0.996 (0.982–1.010) | .595 | 0.992 (0.973–1.012) | .433 |
| Recipient body mass index (kg/m2) | 1.083 (1.043–1.124) | .000 | 1.038 (0.984–1.095) | .175 |
| Recipient dialysis duration (years) | 1.196 (1.100–1.300) | .000 | 0.970 (0.857–1.098) | .633 |
| Cold ischemia time (hours) | 1.050 (1.022–1.078) | .000 | 1.023 (0.984–1.095) | .245 |
| Anastomosis time (minutes) | 1.002 (0.989–1.015) | .761 | 0.987 (0.968–1.007) | .195 |
| Number of HLA mismatches | 1.038 (0.915–1.177) | .562 | 0.988 (0.829–1.176) | .888 |
HLA – human leukocyte antigen.
To identify factors influencing durBD, we entered each variable separately into the regression model. Donor age was the only factor that significantly influenced durBD. Subsequently, we tested the correlation between durBD and donor age. These variables were correlated with a Pearson’s coefficient of −0.144 (P=0.000), indicating donors with a prolonged durBD were younger of age.
We divided our study population equally by the median in a “short durBD” (<10.6 hours) group and “long durBD” (>10.6 hours) group. At 3 months after transplantation, serum creatinine was significantly lower in the “long durBD” group: “short durBD” group 138 μmol/L (range, 110 to 175 μmol/L) versus “long durBD” group 132 μmol/L (range, 106 to 162 μmol/L), P=0.003. At twelve months after transplantation, there was no significant difference: “short durBD” 133 μmol/L (range 107 to 168 μmol/L) versus “long durBD” 129 μmol/L (range, 107 to 159 μmol/L), P=0.085.
Duration of brain death and graft survival
Recipients with functioning grafts after transplantation (84.2%) had kidneys from donors with a median durBD of 10.7 hours (range, 9.0 to 12.6 hours), while recipients with graft failure had donors with a median durBD of 10.1 hours (range, 8.2 to 12.0 hours).
Using the Cox proportional hazards model, the effect of durBD on the graft survival after kidney transplantation was analyzed with relevant donor and recipient characteristics and allograft related factors. In a univariable Cox analysis, durBD influenced the graft survival significantly with a hazard ratio (HR) of 0.941 (P=0.001). In a multivariable Cox analysis, durBD remained a significant independent predictor for graft survival after kidney transplantation with a HR of 0.933 (P=0.015). Table 3 shows the variables used in the multivariable analysis.
Table 3.
Multivariable Cox proportional hazards analysis for graft survival.
| Graft survival | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Duration of brain death period (hours) | 0.933 (0.882–0.987) | .015 |
| Donor gender (Female/Male) | 1.247 (0.870–1.789) | .230 |
| Donor age (years) | 1.035 (1.012–1.058) | .003 |
| Donor body mass index (kg/m2) | 0.965 (0.923–1.009) | .120 |
| Expanded criteria donor | 1.203 (0.699–2.073) | .505 |
| Donor history of hypertension | 1.164 (0.781–1.733) | .456 |
| Donor hypotensive period(s) | 0.921 (0.640–1.325) | .657 |
| Donor history of diabetes mellitus | 5.415 (1.608–18.237) | .006 |
| Donor history of cardiac arrest | 1.213 (0.802–1.835) | .360 |
| Donor use of inotropic medication | 1.886 (0.994–3.578) | .088 |
| Donor cause of death: stroke | 0.611 (0.398–0.937) | .024 |
| Donor cause of death: trauma | 0.812 (0.481–1.370) | .435 |
| Donor lowest creatinine (μmol/L) | 1.001 (1.000–1.001) | .013 |
| Recipient gender (Female/Male) | 1.088 (0.755–1.567) | .651 |
| Recipient age (years) | 0.973 (0.959–0.987) | .000 |
| Recipient body mass index (kg/m2) | 1.008 (0.966–1.051) | .726 |
| Recipient dialysis duration (years) | 0.996 (0.903–1.099) | .939 |
| Cold ischemia time (hours) | 1.015 (0.987–1.045) | .286 |
| Anastomosis time (minutes) | 0.994 (0.980–1.007) | .336 |
| Number of HLA mismatches | 0.927 (0.808–1.064) | .283 |
| Delayed graft function | 2.279 (1.541–3.369) | .000 |
| Acute graft rejection within 1 year | 4.122 (2.541–6.686) | .000 |
HLA – human leukocyte antigen.
After dividing our study population equally by the median in a “short durBD” (<10.6 hours) group and a “long durBD” (>10.6 hours) group, Kaplan-Meier analysis showed that the 15-year graft survival (Figure 2) was significantly higher for recipients whom received a kidney from a donor with “long durBD” compared to a kidney received from a donor with a “short durBD” with a log rank of 6.094 (P=0.014). The estimated 15-year death-censored graft survival was 73.8%, for kidneys from donors with “short durBD” and 79.5% for kidneys from donors with “long durBD”.
Figure 2.
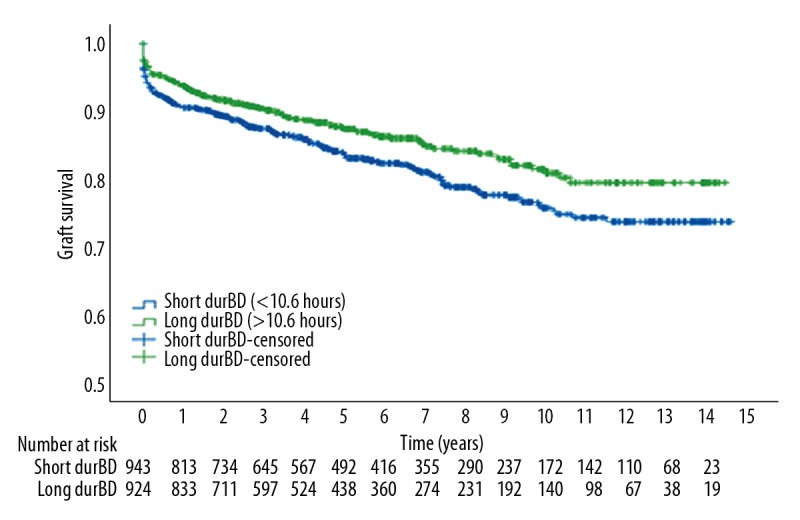
Kaplan-Meier survival curve and number at risk table for 15-years graft survival.
Discussion
We show that prolonged durBD was a significant independent predictor for better graft survival after kidney transplantation. Since brain death has well known deleterious hemodynamic effects, it seems logical to remove donor organs as soon as possible after brain death has been established. However, our data suggest that longer duration of brain death in the donor was not detrimental, but was correlated with better graft survival after kidney transplantation. At 3 months after transplantation, “long durBD” was associated with lower creatinine indicating better graft function. There was no association between duration of brain death and incidence of delayed graft function or acute rejection.
In 2001, Muruve et al. performed the first study comparing the effect of durBD on graft survival [9]. The authors retrospectively analyzed the effect of durBD on graft survival of 627 donor-recipient pairs with “short durBD (<24.7 hours), “medium durBD” (24.7 to 59.2 hours), and “long durBD” (>59.2 hours). Using the univariate analyses, durBD had no significant effect on graft survival 1 year and 10 years after kidney transplantation. In the same year, Kunzendorf et al. performed a similar study and concluded that kidney allografts procured from donors with “long durBD” (>7.8 hours) in comparison to “short durBD” (<7.8 hours), exhibited a significantly better graft survival 10 year after kidney transplantation and lower incidence of delayed graft function [10]. In our view, it is important to note that Kunzendorf et al. only performed a Kaplan-Meier survival analysis. Therefore, their data did not show that “long durBD” was an independent predictor of long-term graft survival. In 2007, Guner et al. performed a retrospective analysis of 24 donor-recipient pairs with “short durBD” (<12 hours) and “long durBD” (>12 hours), according to their observation that 12 hours was the period usually required to stabilize the condition of the brain-dead organ donor [11]. Serum creatinine at 3 months after kidney transplantation was significantly lower in the “long durBD” group. There was no difference in serum creatinine at 12 months post kidney transplantation. In 2010, a retrospective analysis of the Organ Procurement and Transplant Network was performed by Nijboer et al. for 20 773 donor-recipient pairs [12]. A multivariate Cox regression hazards model and multivariate binary logistics regression indicated that the effect of durBD was not significant for graft survival at 1 year and 3 years after kidney transplantation or for the incidence of delayed graft function, respectively.
A beneficial effect of long durBD is contra-intuitive, since brain death causes hemodynamic changes, hormone dysregulation, a pro-inflammatory environment, and apoptosis of liver and kidney cells [2,18–20]. There are several possible explanations for this paradox. First, longer durBD implies longer stay at the intensive care unit and therefore more opportunity to counterbalance the effects of a hypotensive period. However, in our series, we did not find that hypotensive period(s) during brain death modulated the effects of durBD on graft function and long-term graft survival.
Second, a longer durBD could provide opportunity for the donor organs to recover from the catecholamine storm. A study in rats showed that there is a continuous deterioration of liver and kidney function from 1 to 6 hours after brain death, but, provided that the donor is hemodynamically stable, no further deterioration occurs in the subsequent 5 hours [21].
Moreover, there is increasing evidence that the application of brief, non-lethal periods of ischemia and reperfusion, activates an innate immune response that confers protection against later prolonged periods of ischemia. These effects are also present in remote areas, called “remote ischemic preconditioning” [22]. Kunzendorf et al. suggested that the sublethal ischemia of organs due to brain death can lead to ischemic preconditioning, and therefore have protective effects on the kidney allograft [10].
Strengths and limitations
An important strength of this study was the use of a large cohort and the fact that the data were collected in a prospective manner. However, data were obtained from multiple centers, which implies multiple protocols with respect to the transplantation procedure. This could have introduced some heterogeneity. Also, durBD was defined as the interval between declaration of donor brain death and start of cold perfusion. This could have caused some underestimation of the durBD since brain death can already be present before it is formally established. It should also be noted that the durBD in the United States, as shown by Muruve et al. (median durBD 24.7 to 59.2 hours) and Nijboer et al. (median durBD 23.8 hours), are generally longer when compared to Europe, as shown by Kunzendorf et al. (median durBD 7.8 hours). This is probably due to the fact that in the United States more time is spent to obtain informed consent for donation and because multi-organ procurement is usually planned during office hours. Whereas in Europe the surgery of the donor is commonly performed as soon as possible, often during the night [12]. Since the 2 largest studies comparing the impact of durBD on graft survival were performed with data from the United States, it is difficult to compare these outcomes with our data [9,12].
Conclusions
In conclusion, we showed that prolonged duration of brain death is a significant independent predictor for better long-term kidney graft survival. There was no significant effect of duration of brain death period on the incidence of delayed graft function or acute rejection within 1 year after kidney transplantation, but kidney function at 3 months after transplantation was better in the “long durBD” group. Therefore, our recommendation is to optimize donor management, in contrast to procuring the organs as fast as possible. Further research is necessary to unravel the mechanism of this effects and to define the optimal duration of the brain death period.
Acknowledgements
The authors are indebted to all colleagues in the various Dutch transplant centers for submitting their data to the Netherlands Organ Transplant Registry and to Cynthia Konijn of the Dutch Transplant Foundation for assistance with the data management.
Abbreviations
- durBD
duration of brain death
- HR
hazard ratio
- OR
odds ratio
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Lee S, Kim J, Shin M, Kim E, et al. Comparison of outcomes of living and deceased donor kidney grafts surviving longer than 5 years in Korea. Transplant Proc. 2010;42(3):775–77. doi: 10.1016/j.transproceed.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Stiegler P, Sereinigg M, Puntschart A, et al. Oxidative stress and apoptosis in a pig model of brain death (BD) and living donation (LD) J Transl Med. 2013;11:244. doi: 10.1186/1479-5876-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Maset R, Perich LG, Vallespin EV, et al. Living donor renal transplantation in Catalonia: overall results and comparison of survival with cadaveric donor renal transplantation. Transplant Proc. 2005;37(9):3682–83. doi: 10.1016/j.transproceed.2005.09.100. [DOI] [PubMed] [Google Scholar]
- 4.Pratschke J, Wilhelm MJ, Kusaka M, et al. Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation. 1999;67(3):343–48. doi: 10.1097/00007890-199902150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Zhang SJ, Wang T. The influence of brain death on donor liver and the potential mechanisms of protective intervention. Front Med. 2011;5(1):8–14. doi: 10.1007/s11684-011-0109-y. [DOI] [PubMed] [Google Scholar]
- 6.van der Hoeven JA, Ploeg RJ, Postema F, et al. Induction of organ dysfunction and up-regulation of inflammatory markers in the liver and kidneys of hypotensive brain-dead rats: A model to study marginal organ donors. Transplantation. 1999;68(12):1884–90. doi: 10.1097/00007890-199912270-00012. [DOI] [PubMed] [Google Scholar]
- 7.van der Hoeven JA, Molema G, Ter Horst GJ, et al. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int. 2003;64(5):1874–82. doi: 10.1046/j.1523-1755.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 8.Tang Y, Zhao J, Liu D, et al. Evaluation of early kidney damage caused by brain death using real-time ultrasound elastography in a Bama pig model. Ultrasound Med Biol. 2017;43(10):2395–401. doi: 10.1016/j.ultrasmedbio.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Muruve NA, Helling TS, Luger AM, et al. Effect of donor brain-death duration on graft outcome. Transplant Proc. 2001;33(6):2980–81. doi: 10.1016/s0041-1345(01)02279-5. [DOI] [PubMed] [Google Scholar]
- 10.Kunzendorf U, Hohenstein B, Oberbarnscheid M, et al. Duration of donor brain death and its influence on kidney graft function. Am J Transplant. 2002;2(3):292–94. doi: 10.1034/j.1600-6143.2002.20316.x. [DOI] [PubMed] [Google Scholar]
- 11.Guner M, Pirat A, Zeyneloglu P, et al. Effect of the interval between organ donor brain death and organ harvesting on kidney graft function after transplantation. Transplant Proc. 2007;39(4):837–41. doi: 10.1016/j.transproceed.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Nijboer WN, Moers C, Leuvenink HG, Ploeg RJ. How important is the duration of the brain death period for the outcome in kidney transplantation? Transpl Int. 2011;24(1):14–20. doi: 10.1111/j.1432-2277.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldfarb-Rumyantzev AS, Scandling JD, Pappas L, et al. Prediction of 3-year cadaveric graft survival based on pre-transplant variables in a large national dataset. Clin Transplant. 2003;17(6):485–97. doi: 10.1046/j.0902-0063.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 14.Issa N, Stephany B, Fatica R, et al. Donor factors influencing graft outcomes in live donor kidney transplantation. Transplantation. 2007;83(5):593–99. doi: 10.1097/01.tp.0000256284.78721.ba. [DOI] [PubMed] [Google Scholar]
- 15.Salahudeen AK, Haider N, May W. Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int. 2004;65(2):713–18. doi: 10.1111/j.1523-1755.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 16.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294(21):2726–33. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 17.Ojo AO, Leichtman AB, Punch JD, et al. Impact of pre-existing donor hypertension and diabetes mellitus on cadaveric renal transplant outcomes. Am J Kidney Dis. 2000;36(1):153–59. doi: 10.1053/ajkd.2000.8288. [DOI] [PubMed] [Google Scholar]
- 18.Nijboer WN, Schuurs TA, van der Hoeven JA, et al. Effects of brain death on stress and inflammatory response in the human donor kidney. Transplant Proc. 2005;37(1):367–69. doi: 10.1016/j.transproceed.2004.12.262. [DOI] [PubMed] [Google Scholar]
- 19.Saat TC, Susa D, Roest HP, et al. A comparison of inflammatory, cytoprotective and injury gene expression profiles in kidneys from brain death and cardiac death donors. Transplantation. 2014;98(1):15–21. doi: 10.1097/TP.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 20.Ranasinghe AM, Bonser RS. Endocrine changes in brain death and transplantation. Best Pract Res Clin Endocrinol Metab. 2011;25(5):799–812. doi: 10.1016/j.beem.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 21.van Der Hoeven JA, Ter Horst GJ, Molema G, et al. Effects of brain death and hemodynamic status on function and immunologic activation of the potential donor liver in the rat. Ann Surg. 2000;232(6):804–13. doi: 10.1097/00000658-200012000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gassanov N, Nia AM, Caglayan E, Er F. Remote ischemic preconditioning and renoprotection: From myth to a novel therapeutic option? J Am Soc Nephrol. 2014;25(2):216–24. doi: 10.1681/ASN.2013070708. [DOI] [PMC free article] [PubMed] [Google Scholar]


