Abstract
Background
Confidence in any diagnostic and antimicrobial susceptibility testing data is provided by appropriate and regular quality assurance (QA) procedures. In Europe, the European Gonococcal Antimicrobial Susceptibility Programme (Euro-GASP) has been monitoring the antimicrobial susceptibility in Neisseria gonorrhoeae since 2004. Euro-GASP includes an external quality assessment (EQA) scheme as an essential component for a quality-assured laboratory-based surveillance programme. Participation in the EQA scheme enables any problems with the performed antimicrobial susceptibility testing to be identified and addressed, feeds into the curricula of laboratory training organised by the Euro-GASP network, and assesses the capacity of individual laboratories to detect emerging new, rare and increasing antimicrobial resistance phenotypes. Participant performance in the Euro-GASP EQA scheme over a 10 year period (2007 to 2016, no EQA in 2013) was evaluated.
Methods
Antimicrobial susceptibility category and MIC results from the first 5 years (2007–2011) of the Euro-GASP EQA were compared with the latter 5 years (2012–2016). These time periods were selected to assess the impact of the 2012 European Union case definitions for the reporting of antimicrobial susceptibility.
Results
Antimicrobial susceptibility category agreement in each year was ≥91%. Discrepancies in susceptibility categories were generally because the MICs for EQA panel isolates were on or very close to the susceptibility or resistance breakpoints. A high proportion of isolates tested over the 10 years were within one (≥90%) or two (≥97%) MIC log2 dilutions of the modal MIC, respectively. The most common method used was Etest on GC agar base. There was a shift to using breakpoints published by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) in the latter 5 years, however overall impact on the validity of results was limited, as the percentage categorical agreement and MIC concordance changed very little between the two five-year periods.
Conclusions
The high level of comparability of results in this EQA scheme indicates that high quality data are produced by the Euro-GASP participants and gives confidence in susceptibility and resistance data generated by laboratories performing decentralised testing.
Keywords: Gonorrhoea, EUCAST, Euro-GASP, European Union (EU), European economic area (EEA)
Background
Gonorrhoea is the second most common bacterial sexually transmitted infection (STI) worldwide, with a global estimate of 78 million new cases among adults in 2012 [1]. If untreated, gonorrhoea can result in complications and sequelae such as pelvic inflammatory disease, ectopic pregnancy and infertility [2]. In the absence of an effective vaccine, antimicrobial treatment along with appropriate prevention, diagnostics and surveillance, is the mainstay in the clinical and public health management of gonorrhoea and prevention of these complications. However, due to the emergence and spread of antimicrobial resistance in the causative agent, Neisseria gonorrhoeae, most previously used therapeutic agents can no longer be recommended for first-line treatment [3]. Dual antimicrobial therapy, mainly ceftriaxone 250–500 mg plus azithromycin 1–2 g, is the current recommended empirical first-line treatment for gonorrhoea in many countries [4]. As strongly emphasized in the WHO global action plan [5] and the European response plan [6] to control multidrug-resistant N. gonorrhoeae, enhanced worldwide, quality-assured surveillance of gonococcal antimicrobial susceptibility is crucial in order to ensure the effectiveness of the recommended empiric treatment, to monitor antimicrobial resistance trends, and to identify new emerging resistance.
In general, confidence in any diagnostic and antimicrobial susceptibility testing data is provided by appropriate and regular quality assurance (QA) procedures. These include validations of testing methods used, internal quality controls, and quality assessments such as internal quality assessment and, importantly, external quality assessment (EQA). In Europe, the European Gonococcal Antimicrobial Susceptibility Programme (Euro-GASP) has been monitoring the antimicrobial susceptibility in N. gonorrhoeae since 2004 [7–11]. Isolates are tested either centrally or via a decentralised testing model where antimicrobial susceptibility testing is performed in laboratories in participating countries after fulfilling set quality criteria. Criteria include acceptable performance in the EQA and good comparability between the laboratories own national susceptibility testing data and susceptibility data generated by centralised susceptibility testing [11]. Euro-GASP includes an EQA scheme as an essential component for a quality-assured laboratory-based surveillance programme [12]. This EQA scheme aims to ensure high-quality, accurate and comparable susceptibility data between and within testing laboratories. Furthermore, successful performance in the EQA is one of the quality criteria (introduced in 2010) required for Euro-GASP to include susceptibility data generated by laboratories performing decentralised testing [11]. Participation in the EQA scheme enables any problems with the performed antimicrobial susceptibility testing to be identified and addressed, feeds into the curricula of laboratory training organised by the Euro-GASP network, and assesses the capacity of individual laboratories to detect emerging new, rare and increasing antimicrobial resistance phenotypes.
The first Euro-GASP EQA gonococcal strain panel was distributed to the newly created Euro-GASP network in 2003 [13], before the first sentinel susceptibility study in 2004 [9]. The results showed an overall susceptibility category agreement of only 70% and a crucial need for enhanced standardisation of the susceptibility testing methods used in Europe. In 2007, the Euro-GASP EQA scheme was re-established and subsequently run until 2009 as part of the European Surveillance of Sexually Transmitted Infections (ESSTI) programme [14]. Since 2010, the EQA has been co-ordinated by the European Centre for Disease Prevention and Control (ECDC).
The aims of the present study were to evaluate the performance of the Euro-GASP EQA over a 10-year period (2007 to 2016) and to compare the results of the first 5 years (2007–2011) with the latter 5 years (2012–2016; no EQA in 2013) in order to assess whether Euro-GASP data provide a high-quality and valid picture of gonococcal antimicrobial resistance in the European Union/European Economic Area (EU/EEA), i.e. on which treatment recommendations can be based. These time periods were selected to additionally assess the impact of the 2012 European Union case definitions for the reporting of antimicrobial susceptibility (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:262:0001:0057:EN:PDF).
Methods
EQA panel and antimicrobial susceptibility testing methods
The EQA gonococcal strain panels were selected by Public Health England (PHE) and Örebro University Hospital, Sweden. From 2007 to 2009, the EQA was run annually, consisted of 30 cultures of N. gonorrhoeae (10 strains in triplicate to measure intra-laboratory reproducibility), and was distributed by PHE to 19 participating laboratories in 2007 and 2008, and 16 laboratories in 2009. Since 2010, the United Kingdom National External Quality Assessment Service (UK NEQAS) has distributed the EQA panels. The EQA panels from 2010 to 2016 (no EQA was performed in 2013) consisted of 65 gonococcal isolates, some in duplicate or triplicate. In 2010, 15 isolates were distributed in two EQAs; ten isolates in March (19 laboratories) and five in October (20 laboratories). In 2011, two panels of five isolates each were distributed in February (20 laboratories) and October (21 laboratories). Ten isolates were distributed in one panel in 2012 (22 laboratories), 2014 (21 laboratories), 2015 (26 laboratories), and 2016 (27 laboratories). The gonococcal strains in the EQA panels demonstrated a range of antimicrobial susceptibility profiles to therapeutic agents. The gonococci were selected from clinical isolates and a panel of well characterised strains, including current WHO reference strains [15]. Over the 10 years, 46 different strains were included in the EQAs; ten of these strains were included in more than one distribution (four strains in three distributions, two strains in two and four distributions (four strains in total), one strain in five and another in seven distributions). Of the ten different strains, eight (WHO F, G, K, L, M, N, O, P) are incorporated in the WHO N. gonorrhoeae control panel [15] and were included a total of 29 times.
The laboratories participating in the EQA scheme were requested to test the isolates using their own routine antimicrobial susceptibility testing methodology and standard operating procedures, against a panel of therapeutically relevant antimicrobial agents, ideally ceftriaxone, cefixime (included since 2010), azithromycin, ciprofloxacin, spectinomycin, and gentamicin (included since 2010). Penicillinase production (β-lactamase testing) was also monitored where performed. The antimicrobial susceptibility testing methodologies, including media used, and the guidelines/breakpoints used for each antimicrobial agent were requested. Data on the clinical breakpoints (interpretative criteria) used by each laboratory for each individual agent was available until 2012. Post 2012, data were collected on adherence to published breakpoints. For each isolate tested, minimum inhibitory concentrations (MICs) (mg/L) or zones of inhibition (mm), as well as the susceptibility category (susceptible (S), intermediate susceptible (I), or resistant (R)) were reported. Results were reported directly to PHE until 2012 and to UK NEQAS from 2014.
Analysis and interpretation of the results
For the analysis, Etest (or more rarely other MIC gradient strip tests such as the Oxoid M.I.C.Evaluator strips or the Liofilchem MIC Test Strips) whole MIC log2 dilutions were used. The minimum, maximum and modal MIC of each strain was established. To avoid relying upon one set of MIC results from the laboratory that selected the isolates, the modal MIC was used as the ‘expected’ MIC. The number of MICs within one MIC log2 dilution of the modal MIC (essential agreement), as well as number of MICs within two and > 2 MIC log2 dilutions of the modal MIC for each strain was established for all years and for each five-year period (2007–2011 and 2012–2016). The MICs for the eight WHO strains (total of 29 appearances in the EQA) were analysed to assess comparability, consistency and performance of participating laboratories over the 10 years.
The consensus antimicrobial susceptibility category (S, I or R) was assigned for each strain and antimicrobial agent tested and from all isolates in the triplicate or duplicate sets, irrespective of breakpoint criteria used. The overall percentage susceptibility category agreement for each antimicrobial agent was established by calculating the average of each strain percentage concordance. The susceptibility category agreement for each year was the average of each of the concordances for each agent. Due to the confidential nature of the EQA, all results were aggregated by year and antimicrobial.
Troubleshooting
Any laboratory that reported more than 5% of strains with MICs > 2 MIC log2 dilutions from the modal MIC was contacted to identify problems with contamination, reagents, testing and interpretation.
Results
Antimicrobial susceptibility testing methods
The Etest (or more rarely other MIC gradient strip tests) was the most common antimicrobial susceptibility testing method, used in 55.0% of laboratories in 2007–2011 and 76.8% in 2012–2016 (Table 1). The use of disk diffusion as the sole method decreased from 21.4% in 2007–2011 to 3.2% of laboratories in 2012–2016, when most laboratories had replaced their disk diffusion methods with Etest due to its better performance in the EQAs in general, and the recommendations by the Euro-GASP. The most frequently used agar media or agar base for the antimicrobial susceptibility testing was GC agar base (63.4% of laboratories in 2007–2011 and 53.7% in 2012–2016). An increased use of non-selective Thayer-Martin and non-specified agar/s accounted for the reduction in the use of GC agar over the two time periods. Guidelines/breakpoints from the Clinical and Laboratory Standards Institute (CLSI) [16] were adhered to most frequently in the first five-year time period (69.8%), whereas breakpoints from the European Committee on Antimicrobial Susceptibility testing (EUCAST) [17] were the most common in the second five-year time period (increased from 6.5 to 65.3%) (Table 1). Most variation in the applied SIR breakpoints was observed for azithromycin (Table 2) with some harmonisation to EUCAST breakpoints observed from 2007 (0%) to 2012 (42.9%). However, many different breakpoints were used also for cefixime, ceftriaxone, ciprofloxacin and spectinomycin (Table 3).
Table 1.
Details of antimicrobial susceptibility testing methods used in the European gonococcal External Quality Assessments (EQAs)
| 2007–2011 (n=131a) | 2012–2016 (n=95a; no EQA in 2013) | ||||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Antimicrobial susceptibility test | Etestb | 72 | 55.0 | 73 | 76.8 |
| Agar dilution | 26 | 19.8 | 13 | 13.7 | |
| Disk diffusion | 28 | 21.4 | 3 | 3.2 | |
| Disk diffusion and Etests | 5 | 3.8 | 6 | 6.3 | |
| Culture media/base | GC agar base | 83 | 63.4 | 51 | 53.7 |
| Chocolatised blood agar | 33 | 25.2 | 23 | 24.2 | |
| Diagnostic sensitivity (DST) agar | 7 | 5.3 | 5 | 5.3 | |
| Thayer-Martin agar (non-selective) | 5 | 3.8 | 10 | 10.5 | |
| Blood agar base | 2 | 1.5 | 1 | 1.1 | |
| None specified | 1 | 0.8 | 5 | 5.3 | |
| Guidelines/breakpoints c | CLSI | 97 | 69.8 | 23 | 24.2 |
| None specified | 17 | 12.2 | 1 | 1.1 | |
| GRASP – United Kingdom | 7 | 5.0 | 4 | 4.2 | |
| CACFM – France | 3 | 2.2 | 4 | 4.2 | |
| SRGA – Sweden | 5 | 3.6 | 0 | 0.0 | |
| EUCAST | 9 | 6.5 | 62d | 65.3 | |
| WHO | 1 | 0.7 | 1 | 1.1 | |
CLSI Clinical and Laboratory Standards Institute [16], GRASP Gonococcal Resistance to Antimicrobials Surveillance Programme [34], CA-SFM Committee of the French Society for Microbiology (http://www.sfm-microbiologie.org), SRGA Swedish Reference Group for Antibiotics (no longer operational), EUCAST European Committee on Antimicrobial Susceptibility testing [17], WHO World Health Organization
aSome methods and guidelines changed throughout the time periods for some laboratories. Thus, analysis of the comparison of methods was performed using each laboratory for each year to give a total of 131 comparisons for 2007–2012 and 95 for 2012–2016
bDuring recent years, some countries have also used other MIC gradient strip tests
cn = 139 for guidelines/breakpoints as some laboratories used more than one guidance on methodology/breakpoints over the time period
dIncludes one laboratory that also used BSAC disk diffusion breakpoints for azithromycin
Table 2.
Different MIC breakpoints for azithromycin used in the European gonococcal External Quality Assessments (EQAs) from 2007 to 2012
| No. of laboratories* (n = 104) | No. of laboratories 2007 (n = 12) | No. of laboratories 2012 (n = 14) | Azithromycin MIC breakpoints (mg/L) | ||
|---|---|---|---|---|---|
| S ≤ | I | R > | |||
| 31 (29.8%) | 4 (33.3%) | 4 (28.6%) | 0.5 | – | 0.5 |
| 29 (27.9%) | 0 | 6 (42.9%) | 0.25a | 0.5a | 0.5a |
| 11 (10.6%) | 2 (16.7%) | 1 (7.1%) | 1 | – | 1b |
| 7 (6.7%) | 2 (16.7%) | 0 | 0.5 | 1 | 1 |
| 6 (5.8%) | 1 (8.3%) | 0 | 2 | – | – |
| 6 (5.8%) | 0 | 2 (14.3%) | 0.25 | – | 0.25 |
| 1 (1.0%) | 0 | 0 | 0.125 | 0.25–0.5 | 0.5 |
| 1 (1.0%) | 0 | 0 | 0.125 | 0.25–1 | 1 |
| 1 (1.0%) | 1 (8.3%) | 0 | 0.25 | 0.5–1 | 1 |
| 1 (1.0%) | 1 (8.3%) | 0 | 4 | ||
| 10 (9.6%) | 1 (8.3%) | 1 (7.1%) | None given | ||
Note: Detailed interpretative MIC data was only available until 2012. Adherence to published breakpoints collected post-2012
*Includes total number of participants at each distribution, i.e. the same laboratory will be counted at each distribution
aCurrent EUCAST breakpoints
bUS GISP alert value MIC (https://www.cdc.gov/std/gisp/GISP-Protocol-May-2016.pdf)
Table 3.
Different MIC and zone diameter breakpoints for cefixime, ceftriaxone, spectinomycin and ciprofloxacin used in the European gonococcal External Quality Assessments (EQAs) from 2007 to 2012
| Antimicrobial agent | MIC (mg/L) and zone diameter (mm) breakpoints | ||
|---|---|---|---|
| S ≤ | I | R > | |
| Cefixime | 0.06 | ≥0.12 | – |
| 0.12 | ≥0.25 | – | |
| 0.12a | – | 0.12a | |
| 0.25b | – | – | |
| ≥31c | – | – | |
| Ceftriaxone | 0.06 | ≥0.12 | – |
| 0.12 | ≥0.25 | – | |
| 0.12a | – | 0.12a | |
| 0.25b | – | – | |
| ≥35c | – | – | |
| Spectinomycin | 64a | – | 64a |
| 32 | – | 32 | |
| 32b | 64b | 64b | |
| ≥18c | 15 – 17c | ≤14c | |
| Ciprofloxacin | 0.03 | 0.06 - 0.5 | 0.5 |
| 0.03 | – | 0.03 | |
| 0.03a | – | 0.06a | |
| 0.12 | – | 0.25 | |
| 0.06b | 0.12 - 0.5a | 0.5b | |
| ≥41c | 28 – 40c | ≤27c | |
All centres using the disk diffusion method referred to the antibiotic contents recommended by the CLSI [16], with exception of the azithromycin (15 μg) and gentamicin (10 μg) disks for which CLSI does not have any recommendations. The majority of the centres using disk diffusion method also adhered to the recommended CLSI zone diameter breakpoints [16], again with the exception of the azithromycin resistance zone diameter breakpoints of ≤25 mm, ≤27 mm (BSAC) and ≤ 30 mm and gentamicin which has no defined SIR breakpoints.
Antimicrobial susceptibility category agreement
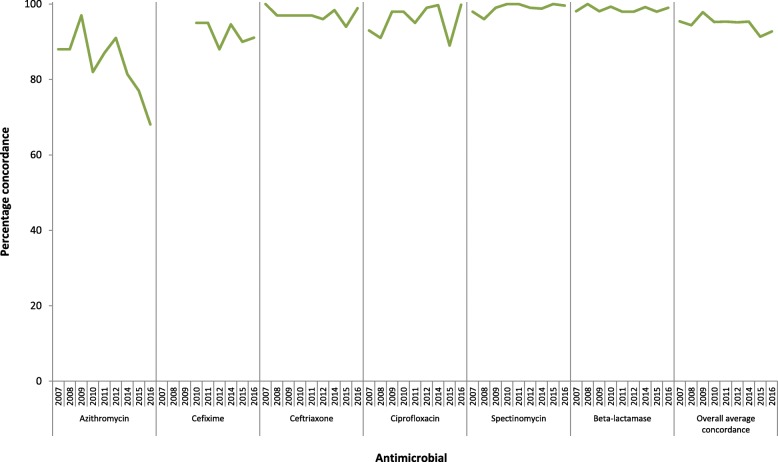
The overall antimicrobial susceptibility category agreement was consistently very high for spectinomycin (mean: 99.0%; range over the years: 96–100%), β-lactamase testing (98.6%; 98–100%) and ceftriaxone (97.2%; 94–100%). The concordance was also high for ciprofloxacin (95.9%; 89–100%) and cefixime (92.3%; 88–95%). However, for azithromycin the concordance was lower and fluctuated substantially over the years (84.3%; 68–97%) (Fig. 1). The lowest concordance for azithromycin (68%) was noted in 2016. Consensus antimicrobial susceptibility categories were not assigned for gentamicin as no international organisation has stated any SIR breakpoints for interpretation of results.
Fig. 1.
Overall antimicrobial susceptibility category agreement in the European gonococcal External Quality Assessments (EQAs), 2007–2016 (no EQA in 2013). Note: Cefixime was not tested before 2010
The susceptibility category agreement was either the same or higher for agar dilution compared with Etest, with ceftriaxone (2012–2015) being the exception. In general, the susceptibility category agreement over the two time periods of 2007–2011 and 2012–2015 was lowest for azithromycin (88 and 83%, respectively) and highest for spectinomycin (99%) (Table 4).
Table 4.
Overall concordance (%) of susceptibility category agreement for all EQA distributions and methods 2007–2011 and 2012–2015 (no EQA in 2013)
| 2007–2011 | 2012–2015 (no EQA in 2013)a | |||||
|---|---|---|---|---|---|---|
| All methods (n = 131)b | Etest (n = 72)c | Agar dilution (n = 26) | All methods (n = 66)b | Etest (n = 52)c | Agar dilution (n = 11) | |
| Azithromycin | 88 | 85 | 97 | 83 | 80 | 92 |
| Cefiximed | 95 | 93 | 99 | 91 | 91 | 91 |
| Ceftriaxone | 98 | 97 | 98 | 96 | 97 | 96 |
| Ciprofloxacin | 95 | 96 | 97 | 96 | 96 | 96 |
| Spectinomycin | 99 | 99 | 99 | 99 | 99 | 100 |
a2016 results not included as only two laboratories performed agar dilution in 2016 so concordance was not calculated
bIncludes disk diffusion results
cSome countries have also used other MIC gradient strip tests
dNot tested prior to 2010
Concordance of MIC
Overall, high proportions of the reported MICs of each antimicrobial agent were within one MIC log2 dilution (+/− two-fold variation) of the modal MIC, that is, gentamicin (95%), cefixime (93%), ciprofloxacin (92%), spectinomycin (91%), azithromycin (89%), and ceftriaxone (85%) (Table 5). Ninety percent of the total MICs were also within one MIC log2 dilution of the modal MIC during the two five-year periods (2007–2011 and 2012–2016), with exception of azithromycin (88% in 2012–2016) and ceftriaxone (84% in 2007–2011 and 88% in 2012–2016). In both time periods and for all antimicrobial agents combined, ≥97% of isolates were within two MIC log2 dilutions (+/− four-fold variation) of the modal MIC. The overall concordance did not change over the two five-year time periods (Table 5).
Table 5.
MIC concordance and variation from the modal MIC in the European gonococcal External Quality Assessments (EQAs), 2007–2016 (no EQA in 2013)
| Azithromycin | Cefiximea | Ceftriaxone | Ciprofloxacin | Gentamicina | Spectinomycin | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
| 2007–2011 | Within 1 MIC log2 dilutionb | 1284 | 90 | 301 | 94 | 1331 | 84 | 1502 | 94 | 233 | 99 | 1038 | 91 | 5690 | 90 |
| Within 2 MIC log2 dilutions | 123 | 99 | 13 | 98 | 172 | 95 | 80 | 99 | 2 | 100 | 76 | 98 | 466 | 98 | |
| > 2 MIC log2 dilutions | 19 | 1 | 6 | 2 | 76 | 5 | 24 | 1 | 0 | 0 | 26 | 2 | 151 | 2 | |
| Total no. of isolates with MIC data | 1426 | 320 | 1579 | 1606 | 235 | 1140 | 6307 | ||||||||
| 2012–2016 | Within 1 MIC log2 dilutionb | 701 | 88 | 757 | 93 | 728 | 88 | 728 | 90 | 482 | 93 | 660 | 92 | 4056 | 91 |
| Within 2 MIC log2 dilutions | 65 | 96 | 33 | 97 | 77 | 97 | 45 | 96 | 30 | 99 | 45 | 98 | 295 | 97 | |
| > 2 MIC log2 dilutions | 28 | 4 | 22 | 3 | 27 | 3 | 36 | 4 | 4 | 1 | 11 | 2 | 128 | 3 | |
| Total no. of isolates with MIC data | 794 | 812 | 832 | 809 | 516 | 716 | 4479 | ||||||||
| All years | Within 1 MIC log2 dilutionb | 1985 | 89 | 1058 | 93 | 2059 | 85 | 2230 | 92 | 715 | 95 | 1698 | 91 | 9745 | 90 |
| Within 2 MIC log2 dilutions | 188 | 98 | 46 | 98 | 249 | 96 | 125 | 98 | 32 | 99 | 121 | 98 | 761 | 97 | |
| > 2 MIC log2 dilutions | 47 | 2 | 28 | 2 | 103 | 4 | 60 | 2 | 4 | 1 | 37 | 2 | 279 | 3 | |
| Total no. of isolates with MIC data | 2220 | 1132 | 2411 | 2415 | 751 | 1856 | 10,785 | ||||||||
aCefixime and gentamicin were not included in the 2007–2009 External Quality Assessment distributions
bEssential agreement
The overall MIC concordances for each EQA distribution were ≥ 85% (mean: 90.7%; range: 85–94%) and ≥ 95% (97.6%; 95–99%) within one and two MIC log2 dilutions of the modal MIC, respectively (Fig. 2). Modal MICs for the eight WHO strains [15], used 29 times throughout the years, varied by one MIC log2 dilution, except for WHO M and ciprofloxacin which varied from 1 to 4 mg/L between two distributions, but the category remained the same, resistant. All susceptibility categories were identical except for WHO K and cefixime between two distributions (S and R), however the modal MIC was the same at 0.25 mg/L.
Fig. 2.
MIC concordance within one and two MIC log2 dilutions of the modal MIC in the European gonococcal External Quality Assessments (EQAs), 2007–2016 (no EQA in 2013). *contains isolates from 2010 (11–15) and 2011 (1–15)
Troubleshooting
More than 5% variation from the modal MIC was mainly identified in laboratories that did not yet participate in Euro-GASP decentralised testing. Issues identified and mostly corrected included use of media suboptimal for N. gonorrhoeae antimicrobial susceptibility testing, the use of MIC gradient strip tests other than Etests (BioMerieux), suboptimal reading of the MIC gradient strips, mis-interpretation of the colour change with nitrocefin, contamination and transcription errors.
Discussion
An increasing number of laboratories participated in the ECDC Euro-GASP EQA; 27 in 2016 compared with 16 in 2009. Etest was the most common methodology and GC agar base the most frequently used agar. In the last 5 years, there was a marked shift among participants to the use of EUCAST [17] breakpoints from the CLSI [16] breakpoints, most likely influenced by Euro-GASP and the publication of the EU case definitions in August 2012 (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:262:0001:0057:EN:PDF), which include definitions of antimicrobial resistance and state that EUCAST clinical breakpoints [17, 18] should be adhered to. However, the lack of a recommended methodology for N. gonorrhoeae susceptibility testing by EUCAST might result in some laboratories continuing to use the CLSI breakpoints [16], which are inherently linked to the CLSI methodology, which may impact the interpretation. The harmonisation of antimicrobial susceptibility testing methods in the latter five-year period (2012–2016) in Europe is a substantial improvement compared with when the first EQA was performed and generally no common methods were used [13]. However, even though methods and SIR breakpoints have increasingly been harmonised, the overall impact on the validity of results has been very limited since 2007, as the percentage susceptibility category agreement and MIC concordances changed very little between the two five-year periods. Nevertheless, Euro-GASP will work together with EUCAST to establish best practice so laboratories across Europe can use standardised antimicrobial susceptibility testing methods for N. gonorrhoeae.
The United States Food and Drug Administration (FDA) recommends that essential agreement (within one MIC log2 dilution of the modal MIC) and antimicrobial susceptibility category agreement should be at a minimum 90% for each antimicrobial agent [19]. In the present study, the overall concordance of antimicrobial susceptibility categories in each year reached this target (≥91%), which is a major improvement compared with the first EQA performed in 2003 (> 70%). This demonstrates an improved level of comparability of antimicrobial susceptibility results, despite the diversity of methods. In future Euro-GASP EQAs, categorical agreement using a known number of non-susceptible strains and the number of very major, major and minor errors will be established, as recommended by CLSI [20]. The comparability of Etest and agar dilution observed in this study, has also been observed elsewhere, particularly for cephalosporins [21–23]. MIC differences in our study could be due to agar media and inoculum size differences as established previously [24, 25], along with varying levels of comparability between different disk diffusion methods and agar dilution and/or Etests [26–30]. Identified agar media differences in this study (data not shown) in general agree with data presented from other studies, including that cephalosporin MICs were generally slightly higher from agar dilution with GC agar compared with Etests [21–23], MICs from Etests with chocolate agar were higher [31], as well as differing MIC variation depending upon which media was used for agar dilution [32, 33]. It was suggested by Liao et al. [31] that laboratories should adhere to CLSI media recommendations (GC agar base with 1% growth supplement) [16], however the lack of commercial, off-the-shelf options of this medium makes this challenging for laboratories who do not have in-house agar-plate pouring facilities.
Discrepancies in susceptibility categories were generally because the MICs for EQA panel isolates were on or very close to the breakpoints, particularly for azithromycin in 2016 (68%), as well as for ciprofloxacin in 2014 (89%) when a triplicate was composed of isolates with MICs exactly on the resistance breakpoint. The use of triplicates/duplicates allows laboratories to investigate their own intra-laboratory concordance. However, if strains with MICs exactly on or very close to a breakpoint are selected, the overall category agreement for that antimicrobial agent can be highly affected. For this reason comparisons over time are difficult, so the inclusion of the same strains over the years, as achieved in this EQA and with comparable results, is an important aspect to consider when analysing EQA performance. Even though strains with MICs close to a breakpoint can impact on susceptibility category agreement, they are clinically relevant, effectively challenge the antimicrobial susceptibility testing in participating laboratories, and should be included in EQAs. However, when interpreting susceptibility category results it is important to consider the actual MIC for individual strains in respect to patient management and the MIC distribution for isolates contributing to susceptibility surveillance data, so isolates near or on breakpoints can be identified and appropriate caution issued. Differences in breakpoints will also impact on susceptibility category agreement, for example ten different breakpoint schemes for azithromycin were used by EQA participants from 2007 to 2012, and the breakpoints for cefixime were less harmonised during earlier years, which may have contributed to the lower concordance in 2012 (88%).
High comparability of results was also demonstrated by the high proportion of isolates tested over the 10 years that were within one (≥90%) MIC log2 dilution and two MIC log2 dilutions (≥97%) of the modal MIC. The overall lower MIC concordance for ceftriaxone (85%) may be due to the smaller dilution scales due to the mostly lower ceftriaxone MICs and requiring more precision, e.g. at 0.004, 0.008 and 0.016 mg/L, as compared with other antimicrobial agents with higher MICs in the dilution scales such as 4, 8, 16 mg/L. The lower MIC concordance for azithromycin (89%) was likely affected by the fact that MIC testing for azithromycin is very sensitive to minor differences in methodologies, in particular the medium used and pH (which is affected by the concentration of CO2), as has been demonstrated previously [33, 34]. Full concordance in antimicrobial susceptibility categories and MICs will likely never be possible, due to the inherent inter-assay variation of any testing method, and particularly not before there is a complete harmonisation of antimicrobial susceptibility testing methods.
The use of the same WHO strains over the years allowed the measurement of variability over time, which was shown to be very low in this EQA. The present study has shown that the inter-laboratory reproducibility was high amongst participating laboratories, comparable in different distributions and years, and the use of standardised quality control strains [15] allows improved comparison of results over time and between as well as within laboratories.
The Euro-GASP EQA revealed high levels of competence and capability in recovering and testing strains of unknown phenotype. The high level of comparability over the 10 years of the EQA indicates that high quality data are produced by the Euro-GASP participants and gives confidence in decentralised testing and comparison of antimicrobial susceptibility surveillance data in the EU/EEA. The results from this EQA compared well with similar national schemes in Canada (> 90% for MIC and interpretation concordance) [35], India (82% interpretation concordance) [36] and Australia (3.1% error rate in respect to penicillin MICs) [32], even though the Euro-GASP EQA is regional with many different participating countries, which by default means more variability in methodologies. A quality control comparison programme for the Latin America and the Caribbean GASP region recently reported that most participants had acceptable results and the impact of the different methods on the results was also highlighted [37].
It should be noted that the Euro-GASP laboratories are frequently experienced national reference laboratories with a high level of expertise and access to training and advice from the Euro-GASP coordinators. In contrast, the global GASP coordinated by the WHO includes antimicrobial susceptibility data from both experienced as well as less experienced laboratories. It would be exceedingly valuable to implement a global EQA scheme, particularly in regions not participating in existing schemes, to monitor and support comparability of antimicrobial susceptibility surveillance data from different countries and laboratories globally. In addition, the use of a global EQA could support primary diagnostic laboratories that perform antimicrobial susceptibility testing for patient management and local surveillance studies to ensure adequate quality. The crucial need for this was illustrated in a national survey in the United Kingdom [38], where low levels of QA in gonococcal antimicrobial susceptibility procedures were identified. Confidence in the reporting of patient related antimicrobial susceptibility results is essential to avoid administering inappropriate treatment. A global EQA would additionally allow the global dissemination of important reference strains for QA and clinical strains with interesting/emerging resistance profiles or diagnostically challenging characteristics, and provide a further opportunity for laboratories to achieve accreditation standards.
Conclusions
Gonorrhoea remains a public health concern because of the increasing incidence and the threat of multidrug-resistant N. gonorrhoeae. Strengthening surveillance of gonococcal antimicrobial susceptibility is imperative worldwide and, in Europe, Euro-GASP has been expanding annually. The high level of QA of the data from Euro-GASP and other similar surveillance programmes is essential in order to identify novel emerging resistance, appropriately monitor antimicrobial resistance trends and to ensure national and international gonorrhoea treatment guidelines are updated based on high quality and valid antimicrobial susceptibility data.
Acknowledgements
Many thanks to Stephanie Chisholm (formerly of PHE, London, United Kingdom), Gaute Syversen, (Oslo University Hospital, Norway), Paul Reichert and Monique Perrin (E.P Laboratoire national de santé, Luxembourg), and Agathe Goubard (Institut Alfred Fournier, Paris, France) for their contributions to the work.
Funding
The study was funded by the European Centre for Disease Prevention and Control (Framework Contract No. ECDC/2013/015). The funding body contributed to the design of the study, the interpretation of the data and to the writing of the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the Public Health England and the European Centre for Disease Prevention and Control, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data may however be available from the authors upon reasonable request and with permission of the European Centre for Disease Prevention and Control.
Abbreviations
- CLSI
Clinical and Laboratory Standards Institute
- ECDC
European Centre for Disease Prevention and Control
- EEA
European Economic Area
- EQA
External quality assessment
- ESSTI
European Surveillance of Sexually Transmitted Infections
- EU
European Union
- EUCAST
European Committee on Antimicrobial Susceptibility testing
- Euro-GASP
European Gonococcal Antimicrobial Surveillance Programme
- FDA
Food and Drug Administration
- MIC
Minimum inhibitory concentration
- PHE
Public Health England
- QA
Quality assurance
- S, I, R
Susceptible, intermediate susceptible, resistant
- STI
Sexually transmitted infection
- UK NEQAS
United Kingdom National External Quality Assessment Service
- US
United States
- WHO
World Health Organization
Authors’ contributions
MC and CI initiated and designed the study. MC, NQ, CI, NW, GS and MU analysed and interpreted the combined EQA data, and wrote a first draft of the paper. MC, NQ, SJ, MD, EF, SS and MU were responsible for the selection and distribution of the EQAs. AS, SP, TC, BH, PM, SH, JV1, SB, PK, ET, ES, EB, GSH, LR, PS, AC, GP, FM, RZC, IL, TB, MS, BMB, MJB, JS, PP, SJ, JV2 and RA tested the EQA isolates, submitted results and contributed to the development and design of the EQA schemes and the present study. MC, NQ, SJ, MD, RP, SW and MU performed the analysis and write-up of the individual EQA distributions. All authors read, commented and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. However, PHE’s AMRHAI Reference Unit has received financial support for conference attendance, lectures, research projects or contracted evaluations from numerous sources, including: Accelerate Diagnostics, Achaogen Inc., Allecra Therapeutics, Amplex, AstraZeneca UK Ltd., AusDiagnostics, Basilea Pharmaceutica, Becton Dickinson Diagnostics, bioMérieux, Bio-Rad Laboratories, BSAC, Cepheid, Check-Points B.V., Cubist Pharmaceuticals, Department of Health, Enigma Diagnostics, Food Standards Agency, GlaxoSmithKline Services Ltd., Helperby Therapeutics, Henry Stewart Talks, IHMA Ltd., Innovate UK, Kalidex Pharmaceuticals, Melinta Therapeutics, Merck Sharpe & Dohme Corp., Meiji Seika Pharma Co. Ltd., Mobidiag, Momentum Biosciences Ltd., Neem Biotech, Nordic Pharma Ltd., Norgine Pharmaceuticals, Rempex Pharmaceuticals Ltd., Roche, Rokitan Ltd., Smith & Nephew UK Ltd., Shionogi & Co. Ltd., Speedx, Trius Therapeutics, VenatoRx Pharmaceuticals and Wockhardt Ltd.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michelle J. Cole, Email: michelle.cole@phe.gov.uk
Nerteley Quaye, Email: Nerteley.Quaye@phe.gov.uk.
Susanne Jacobsson, Email: susanne.jacobsson@regionorebrolan.se.
Michaela Day, Email: Michaela.Day@phe.gov.uk.
Elizabeth Fagan, Email: Elizabeth.Fagan@phe.gov.uk.
Catherine Ison, Email: ison.catherine@gmail.com.
Rachel Pitt, Email: Rachel.Pitt@phe.gov.uk.
Shila Seaton, Email: Shila.Seaton@phe.gov.uk.
Neil Woodford, Email: Neil.Woodford@phe.gov.uk.
Angelika Stary, Email: angelika.stary@meduniwien.ac.at.
Sonja Pleininger, Email: sonja.pleininger@ages.at.
Tania Crucitti, Email: tcrucitti@itg.be.
Blaženka Hunjak, Email: blazenka.hunjak@hzjz.hr.
Panayiota Maikanti, Email: pmaikanti@mphs.moh.gov.cy.
Steen Hoffmann, Email: HOF@ssi.dk.
Jelena Viktorova, Email: Jelena.Viktorova@terviseamet.ee.
Susanne Buder, Email: dr.susanne.buder@web.de.
Peter Kohl, Email: Peter.Kohl@vivantes.de.
Eva Tzelepi, Email: tzelepi@pasteur.gr.
Eirini Siatravani, Email: siatravani@pasteur.gr.
Eszter Balla, Email: drballa.eszter@gmail.com.
Guðrún Svanborg Hauksdóttir, Email: gusvhauk@landspitali.is.
Lisa Rose, Email: LRose@stjames.ie.
Paola Stefanelli, Email: paola.stefanelli@iss.it.
Anna Carannante, Email: anna.carannante@iss.it.
Gatis Pakarna, Email: gatis.pakarna@inbox.lv.
Francesca Mifsud, Email: francesca.vella@gov.mt.
Rosann Zammit Cassar, Email: rosann.zammit@gov.mt.
Ineke Linde, Email: ilinde@ggd.amsterdam.nl.
Thea Bergheim, Email: UXTHBE@ous-hf.no.
Martin Steinbakk, Email: Martin.Steinbakk@so-hf.no.
Beata Mlynarczyk-Bonikowska, Email: beata.mlynarczyk@wum.edu.pl.
Maria-José Borrego, Email: M.Jose.Borrego@insa.min-saude.pt.
Jill Shepherd, Email: Jill.Shepherd@nhslothian.scot.nhs.uk.
Peter Pavlik, Email: peter.pavlik@medirex.sk.
Samo Jeverica, Email: Samo.Jeverica@mf.uni-lj.si.
Julio Vazquez, Email: jvazquez@isciii.es.
Raquel Abad, Email: rabad@isciii.es.
Sabrina Weiss, Email: sabrina.weiss@charite.de.
Gianfranco Spiteri, Email: Gianfranco.Spiteri@ecdc.europa.eu.
Magnus Unemo, Email: magnus.unemo@regionorebrolan.se.
References
- 1.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ison C, Lewis D: Gonorrhea. In: Atlas of sexually transmitted diseases and AIDS. 4th edition. Edited by Morse SA, Ballard RC, Holmes KK, Moreland AA: Saunders Elsevier; 2010:24–39.
- 3.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson C, Arnason T, Friedman DS, Metz G, Grimshaw JM. A systematic review and appraisal of the quality of practice guidelines for the management of Neisseria gonorrhoeae infections. Sex Transm Infect. 2017;93:487–492. doi: 10.1136/sextrans-2016-052939. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organisation. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. 2012. http://whqlibdoc.who.int/publications/2012/9789241503501_eng.pdf. Accessed 04 Jan 2019.
- 6.European Centre for Disease Prevention and Control. Response plan to control and manage the threat of multidrug-resistant gonorrhoea in Europe. 2012. http://www.ecdc.europa.eu/en/publications/Publications/1206-ECDC-MDR-gonorrhoea-response-plan.pdf. Accessed 04 Jan 2019.
- 7.Cole MJ, Spiteri G, Jacobsson S, Pitt R, Grigorjev V, Unemo M. Is the tide turning again for cephalosporin resistance in Neisseria gonorrhoeae in Europe? Results from the 2013 European surveillance. BMC Infect Dis. 2015;15:321. doi: 10.1186/s12879-015-1013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole MJ, Spiteri G, Jacobsson S, Woodford N, Tripodo F, Amato-Gauci AJ, et al. Overall low extended-spectrum cephalosporin resistance but high azithromycin resistance in Neisseria gonorrhoeae in 24 European countries, 2015. BMC Infect Dis. 2017;17:617. doi: 10.1186/s12879-017-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin IM, Hoffmann S, Ison CA. European surveillance of sexually transmitted infections (ESSTI): the first combined antimicrobial susceptibility data for Neisseria gonorrhoeae in Western Europe. J Antimicrob Chemother. 2006;58:587–593. doi: 10.1093/jac/dkl265. [DOI] [PubMed] [Google Scholar]
- 10.Cole MJ, Spiteri G, Town K, Unemo M, Hoffmann S, Chisholm SA, et al. Risk factors for antimicrobial-resistant Neisseria gonorrhoeae in Europe. Sex Transm Dis. 2014;41:723–729. doi: 10.1097/OLQ.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control. Gonococcal antimicrobial susceptibility surveillance in Europe 2015. 2017. https://ecdc.europa.eu/sites/portal/files/documents/gonococcal-antimicrobial-susceptibility-surveillance-Europe-2015.pdf. Accessed 04 Jan 2019.
- 12.European Centre for Disease Prevention and Control. Euro-GASP 2016 external quality assessment (EQA) scheme for Neisseria gonorrhoeae antimicrobial susceptibility testing 2017. https://ecdc.europa.eu/sites/portal/files/documents/EQA%20Report%202016%20final.pdf. Accessed 04 Jan 2019.
- 13.Ison CA, Martin IM, Lowndes CM, Fenton KA. Comparability of laboratory diagnosis and antimicrobial susceptibility testing of Neisseria gonorrhoeae from reference laboratories in Western Europe. J Antimicrob Chemother. 2006;58:580–586. doi: 10.1093/jac/dkl264. [DOI] [PubMed] [Google Scholar]
- 14.Cole MJ, Chisholm SA, Hoffmann S, Stary A, Lowndes CM, Ison CA. European surveillance of antimicrobial resistance in Neisseria gonorrhoeae. Sex Transm Infect. 2010;86:427–432. doi: 10.1136/sti.2010.044164. [DOI] [PubMed] [Google Scholar]
- 15.Unemo M, Golparian D, Sanchez-Buso L, Grad Y, Jacobsson S, Ohnishi M, et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: phenotypic, genetic and reference genome characterization. J Antimicrob Chemother. 2016;71:3096–3108. doi: 10.1093/jac/dkw288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CLSI: Performance standards for antimicrobial susceptibility testing. Twenty-first informational supplement: Clinical and Laboratory Standards Institute 2011; 2011.
- 17.European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.1. 2018. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf. Accessed 04 Jan 2019.
- 18.Kahlmeter G. The 2014 Garrod lecture: EUCAST - are we heading towards international agreement? J Antimicrob Chemother. 2015;70:2427–2439. doi: 10.1093/jac/dkv145. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services. Food and Drug Administration. Class II Special Controls Guidance Document: Antimicrobial Susceptibility Test (AST) Systems. 2009. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071462.pdf. Accessed 04 Jan 2019.
- 20.CLSI. Development of In Vitro Susceptibility Testing Criteria and Quality Control Parameters. Clinical and Laboratory Standards Institute. 2018. https://clsi.org/standards/products/microbiology/documents/m23/#. Accessed 04 Jan 2019.
- 21.Enriquez RP, Goire N, Kundu R, Gatus BJ, Lahra MM. A comparison of agar dilution with the calibrated dichotomous sensitivity (CDS) and Etest methods for determining the minimum inhibitory concentration of ceftriaxone against Neisseria gonorrhoeae. Diagn Microbiol Infect Dis. 2016;86:40–43. doi: 10.1016/j.diagmicrobio.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Taylor TH, Jr, Pettus K, Trees D. Assessment of Etest as an alternative to agar dilution for antimicrobial susceptibility testing of Neisseria gonorrhoeae. J Clin Microbiol. 2014;52:1435–1440. doi: 10.1128/JCM.02131-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shende T, Sood S, Singh R, Kapil A, Kar HK, Sharma VK. Comparison of E test and agar dilution for testing activity of ceftriaxone against Neisseria gonorrhoeae. J Med Microbiol. 2016;65:701–702. doi: 10.1099/jmm.0.000283. [DOI] [PubMed] [Google Scholar]
- 24.Hall WH, Opfer BJ. Influence of inoculum size on comparative susceptibilities of penicillinase-positive and -negative Neisseria gonorrhoeae to 31 antimicrobial agents. Antimicrob Agents Chemother. 1984;26:192–195. doi: 10.1128/AAC.26.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dillon JA, Tostowaryk W, Pauze M. Effects of different media and methods of inoculum preparation on results of antimicrobial susceptibility testing of Neisseria gonorrhoeae by agar dilution. Antimicrob Agents Chemother. 1987;31:1744–1749. doi: 10.1128/AAC.31.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Taylor TH, Jr, Pettus K, Johnson S, Papp JR, Trees D. Comparing the disk-diffusion and agar dilution tests for Neisseria gonorrhoeae antimicrobial susceptibility testing. Antimicrob Resist Infect Control. 2016;5:46. doi: 10.1186/s13756-016-0148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mal PB, Jabeen K, Farooqi J, Unemo M, Khan E. Antimicrobial susceptibility testing of Neisseria gonorrhoeae isolates in Pakistan by Etest compared to calibrated dichotomous sensitivity and clinical laboratory standards institute disc diffusion techniques. BMC Microbiol. 2016;16:236. doi: 10.1186/s12866-016-0707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khaki P, Sharma A, Bhalla P. Comparison of two disc diffusion methods with minimum inhibitory concentration for antimicrobial susceptibility testing of Neisseria gonorrhoeae isolates. Ann Med Health Sci Res. 2014;4:453–456. doi: 10.4103/2141-9248.133477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh V, Bala M, Kakran M, Ramesh V. Comparative assessment of CDS, CLSI disc diffusion and Etest techniques for antimicrobial susceptibility testing of Neisseria gonorrhoeae: a 6-year study. BMJ Open. 2012;2. [DOI] [PMC free article] [PubMed]
- 30.Bala M, Ray K, Gupta SM. Comparison of disc diffusion results with minimum inhibitory concentration (MIC) values for antimicrobial susceptibility testing of Neisseria gonorrhoeae. Indian J Med Res. 2005;122:48–51. [PubMed] [Google Scholar]
- 31.Liao CH, Lai CC, Hsu MS, Chu FY, Wu MY, Huang YT, et al. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates determined by the agar dilution, disk diffusion and Etest methods: comparison of results using GC agar and chocolate agar. Int J Antimicrob Agents. 2010;35:457–460. doi: 10.1016/j.ijantimicag.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Tapsall JW. Use of a quality assurance scheme in a long-term multicentric study of antibiotic susceptibility of Neisseria gonorrhoeae. Genitourin Med. 1990;66:8–13. doi: 10.1136/sti.66.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodford N, Ison CA. The effect of media on antimicrobial susceptibility testing of Neisseria gonorrhoeae. J Antimicrob Chemother. 1988;22:463–471. doi: 10.1093/jac/22.4.463. [DOI] [PubMed] [Google Scholar]
- 34.Public Health England. Surveillance of antimicrobial resistance in Neisseria gonorrhoeae. 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/567602/GRASP_Report_2016.pdf. Accessed 04 Jan 2019.
- 35.Sawatzky P, Liu G, Dillon JA, Allen V, Lefebvre B, Hoang L, et al. Quality assurance for antimicrobial susceptibility testing of Neisseria gonorrhoeae in Canada, 2003 to 2012. J Clin Microbiol. 2015;53:3646–3649. doi: 10.1128/JCM.02303-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bala M, Tapsall JW, Limnios A, Sood S, Ray K. Experience with an external quality assurance scheme for antimicrobial susceptibility testing of Neisseria gonorrhoeae in India, 2001-2007. Epidemiol Infect. 2010;138:69–75. doi: 10.1017/S0950268809990148. [DOI] [PubMed] [Google Scholar]
- 37.Sawatzky P, Martin I, Galarza P, Carvallo MET, Araya Rodriguez P, Cruz OMS, et al. Quality assurance for antimicrobial susceptibility testing of Neisseria gonorrhoeae in Latin American and Caribbean countries, 2013-2015. Sex Transm Infect. 2018;94:479–482. doi: 10.1136/sextrans-2017-053502. [DOI] [PubMed] [Google Scholar]
- 38.Jain A, Cole MJ, Planche T, Ison CA. An evaluation of Neisseria gonorrhoeae antimicrobial susceptibility testing in the UK. J Clin Pathol. 2014;67:1013–1016. doi: 10.1136/jclinpath-2014-202392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Public Health England and the European Centre for Disease Prevention and Control, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data may however be available from the authors upon reasonable request and with permission of the European Centre for Disease Prevention and Control.