Abstract
Background
Campylobacteriosis is a zoonotic infectious disease that can be mostly undiagnosed or unreported due to fastidious Campylobacter species. The aim of this study was to develop a simple, sensitive, and quick assay for the detection of Campylobacter spp. and taking advantage of the great sensitivity of gold nanorods (GNRs) to trace changes in the local environment and interparticle distance.
Methods
Characterized GNRs were modified by specific ssDNA probes of cadF gene. First, the biosensor was evaluated using recombinant plasmid (pTG19-T/cadF) and synthetic single-stranded 95 bp gene, followed by a collection of the extracted DNAs of the stool samples. The sensing strategy was compared by culture, PCR, and real-time PCR.
Results and discussion
Analysis of 283 specimens showed successful detection of Campylobacter spp. in 44 cases (16%), which was comparable to culture (7%), PCR (15%), and real-time PCR (18%). In comparison with real-time PCR, the sensitivity of the biosensor was reported 88%, while the specificity test for all assays was the same (100%). However, it was not able to detect Campylobacter in 6 positive clinical samples, as compared to real-time PCR. The limit of detection was calculated to be the same for the biosensor and real-time PCR (102 copy number/mL).
Conclusions
Taking high speed and simplicity of this assay into consideration, the plasmonic nanobiosensor could pave the way in designing a new generation of diagnostic kits for detection of C. jejuni and C. coli species in clinical laboratories.
Electronic supplementary material
The online version of this article (10.1186/s12951-019-0476-0) contains supplementary material, which is available to authorized users.
Keywords: Campylobacter spp., Gold nanorods, Surface plasmon resonance, Nanobiosensor
Introduction
Campylobacter spp. are fastidious Gram-negative bacteria which are known as a common cause of human acute gastroenteritis (campylobacteriosis) worldwide [1]. Among various species, Campylobacter (C.) jejuni is a species with more clinical prevalence (about 90%), followed by C. coli (about 10%) [2, 3]. The reservoir of the bacteria is mainly poultry, but it can also be transmitted to humans through consumption of animal products, contaminated water, and untreated milk, [4–6]. Although many cases of campylobacteriosis are undiagnosed the disease incidence has been estimated to affect over 1.3 million cases annually [7].
There are several methods (e.g. culture, molecular, and serological tests) for the detection of campylobacteriosis [8–10].
Stool culture is considered as the gold standard for the detection of the disease but often requires several days to complete and sometimes presents unreliable results because of (i) fastidious conditions of the bacteria, (ii) low sensitivity, (iii) and conversion to viable but nonculturable cells (VBNC) [5, 11, 12]. Molecular techniques are other assays for the identification of the Campylobacters with more specificity and sensitivity than culture [13, 14]. Nevertheless, these methods are sensitive to stool, blood, and urine inhibitors (such as bile, heme, and urea, etc.), expensive, and requiring trained staffs [15, 16]. Serological techniques are other platforms which have been reported for the detection of the Campylobacters. Unfortunately, cross-reaction between bacterial antigens have been reported [17, 18]. Hence, it is important to develop a simple, sensitive, and quick assay for the identification of Campylobacter species.
In recent years, researchers have been considering to improve new biosensors because of their sensitivity, simplicity, and low cost by different nanostructures, including quantum dots, gold nanoparticles, carbon nanotubes, etc. [19, 20]. Gold nanoparticle-based methods have recently attracted significant attention in biotechnological fields for biosensing applications due to outstanding properties. Amongst them, Gold nanorods with two strong longitudinal surface plasmon resonance (SPR) [21] bands, tunable aspect ratio and extreme sensitivity to trace changes in the dielectric properties of the surrounding have been regarded as promising candidates as diagnostic nanobiosensor to analyze different biomarkers [22–26]. Recently, a few studies have reported the design of GNRs-DNA biosensors for the identification of the microorganisms. For example, Hepatitis B virus, Chlamydia trachomatis, and Ochratoxin A were detected by GNRs. [22, 24, 27, 28]. DNA-biosensors are able to form a double-stranded hybrid with their complementary sequences, resulting in the aggregation of the GNRs and change of SPR absorption peak [24]. Although several studies have been conducted on detecting Campylobacters by the SPR properties [29, 30], this is the first study based on ssDNA-GNRs biosensing system for the identification of both C. jejuni and C. coli species using specific cadF gene, giving a comparison with bacterial culture, PCR, and real-time PCR assays.
Methods
Bioinformatics and design of probes and primers
The cadF gene of Campylobacter was considered as the target gene in all molecular techniques. Deposited partial and complete sequences of the cadF gene from C. jejuni and C. coli (accession numbers NC_022660.1, CP006702.1, GL405235.1, CP017025.1, KC575115.1, AAFL01000010.1, CP007183.1, CP007179.1, CP004066.1, CP007181.1, AEER01000022.1, HE978252.1, CP017029.1, CP006707.2, and CP006709.2) were downloaded from the NCBI GenBank (http://www.ncbi.nlm.nih.gov/), and multiple alignments of sequences were done using CLC Sequence Viewer 7.6 software (CLC bio, Aarhus, Denmark) to determine conserved regions among members of both species. GeneRunner, AllelID, and CLC software were used for designing the probe and primers of real-time PCR assay and probes of the biosensor (Table 1).
Table 1.
Primers and probes used in this study
| Primer name | Sequence (5′ → 3′) | Target | Size | Reference |
|---|---|---|---|---|
| PCR | F: TTGAAGGTAATTTAGATATG R: CTAATACCTAAAGTTGAAAC |
C. jejuni/C. coli | 400 bp | [49] |
| Duplex-PCR | FU: TTGAAGGTAATTTAGATATG R1: TTTATTAACTACTTCTTTTG R2: ATATTTTTCAAGTTCATTAG |
C. coli C. jejuni |
461 bp 737 bp |
[10, 49] |
| Real-time PCR | F: AACCCAAATTCTAATTGATC R: GAAGGTAATTTAGATATGGATAA P: FAM-AAATGATAACCAAGTCTAATCCCTGG |
C. jejuni/C. coli | 95 bp | This study |
| Biosensor | Probe 1: TTATCCATATCTAAATTACC-SH Probe 2: SH-AGTCTAATCCCTGGTGCATA |
C. jejuni/C. coli | 44 bp | This study |
| Cloning | M13F: AGGGTTTTCCCAGTCACGA M13R: GAGCGGATAACAATTTCACAC |
Recombinant plasmid (for sequencing) | 193 bp | [50] |
Reagents for nanobiosensor
Chloroauric acid (HAuCl4·3H2O), cetyltrimethylammonium bromide (CTAB), sodium borohydride (NaBH4), sodium acetate, and phosphate buffered saline tablet (PBS; 10 mM, pH 7.4) were procured from Sigma Aldrich. Ascorbic acid, silver nitrate (AgNO3), sodium chloride (NaCl), and Dithiothreitol (DTT) were purchased from Merck. Probes, primers, and synthetic single-stranded 95 bp cadF gene were synthesized by Eurofins MWG Operon (Ebensburg, Germany) company.
Preparation and characterization of gold nanorods
GNRs were synthesized using the previously described seed-mediated growth method reported by Tohidi Moghadam and Nikoobakht [31, 32]. Briefly, the seed solution was prepared by mixing CTAB (7.5 mL, 0.095 M) and HAuCl4·3H2O (250 μL, 0.01 M) followed by mixing with fresh ice-cold NaBH4 (600 μL, 0.01 M) and rapid inversion for 2 min to form a yellow–brown solution. Keeping the reaction mixture undisturbed at ambient temperature for 2 h, the growth solution was prepared by addition of CTAB (9.5 mL, 0.095 M), HAuCl4·3H2O (400 μL, 0.01 M), AgNO3 (60 μL, 0.01 M), and ascorbic acid (64 μL 0.10 M) to a test tube sequentially. Then, 40 μL of the seed particles were added to the mixture to initiate the growth of GNRs.
Then, excess CTAB and unreacted gold ions were removed from the solution by two rounds of centrifugation (13,000 rpm, 7 min). The supernatant was carefully decanted, and the pellet containing GNRs was re-dispersed in a PBS buffer (pH 7.4, 10 mM). Prior to bioconjugation with probes, optical density (OD) of the stock GNRs was adjusted to 1. The SPRs characteristic of the GNRs was evaluated by UV–vis spectrophotometer (Perkin-Elmer, Thermo Scientific, USA) in the wavelength region of 400–900 nm. Size and morphology of the nanostructures were analyzed by Zeiss EM900 transmission electron microscopy at an accelerating voltage of 50 kV (ZEISS, Germany).
Preparation of ssDNA-GNRs nanobioconjugates (Nanoprobe)
The thiol group (–SH) of each probe was reduced by Dithiothreitol (DTT). Briefly, 10 μL of 1.0 N DTT (in 0.01 M Sodium acetate, pH 5.2) was added to the thiolated probes. The mixture was vortexed and incubated at room temperature (RT) for 15 min. To remove DTT, thiolated-oligonucleotide mixture was washed 3 times with 50 µL ethyl acetate. Each time, the upper layer was discarded after vortexing.
A total of 300, 200, 150, 100, 50, and 25 nM of freshly cleaved probes were then added immediately to 250 μL of GNRs buffered to 0.1 M NaCl and incubated for 30 min at RT. After the bioconjugation process, the GNRs were centrifuged and the concentration of the unbound ss-DNAs were measured by spectrophotometer (Eppendorf, Hamburg, Germany). Using ICP-AAS (Inductively coupled plasma-atomic absorption spectroscopy), the concentration of the GNRs was evaluated. Both results were used for estimating the ratio of GNRs:ssDNA. Immobilization of the ssDNA probe on the GNRs was analyzed by Fourier Transform Infrared Spectrometer (FTIR, Thermo Nicolet-Nexus 870, USA), UV–vis spectra, Dynamic Light Scattering (DLS), and zeta potential (Zetasizer Nano ZS, England, Malvern).
Preparation of recombinant pTG19-T/cadF plasmids as a standard control
To provide a standard control for gold nanobiosensor and real-time PCR analyses, a recombinant pTG19-T/cadF plasmid was prepared and used according to a protocol described by Soleimani et al. [33]. Briefly, PCR amplification of the cadF gene was performed using primers designed for real-time PCR assay (forward primer: AACCCAAATTCTAATTGATC and reverse primer: GAAGGTAATTTAGATATGGATAA). After purification of PCR products by PCR purification Kit (Bioneer, Daejeon, Korea), a 95 bp fragment of cadF gene was ligated into a pTG19-T vector according to manufacturer’s instructions (TA Cloning kit, CinnaClon Co., Iran). Extracted plasmids of the cloned E. coli TOP10F´ were evaluated by PCR and sequencing using specific primers (M13F and M13R) described in Table 1.
Initial evaluation of nanobiosensor efficacy by spiking pTG19-T/cadF plasmid and synthetic single-stranded 95 bp cadF gene in stool samples
The nanobiosensor was initially evaluated by spiking recombinant pTG19-T/cadF plasmid and a synthetic single-stranded 95 bp cadF gene in Campylobacter-free stools. Accordingly, 250 µL of each ssDNA-GNR Probe 1 and 2 solutions (Probes 1 and 2 conjugated to GNRs in 10 mM PBS buffer) were mixed together at RT. Then, the plasmid and the single-stranded 95 bp gene were extracted from spiked samples and the targets (~ 4 ng) were denatured by heating at 100 °C for 15 min in a thermoblock and immediately chilled in ice for another 5 min to obtain denatured single-stranded DNA. After adding DNA targets to the biosensors, the changes in morphology of the GNRs and absorbance peaks of the biosensor were evaluated using transmission electron microscopy at 50 kV and UV–vis spectrophotometry, respectively.
The resultant SPR spectra from the recombinant plasmid and the single-stranded 95 bp gene were compared with extracted DNA from standard bacteria.
Collection and culture of human stool specimens
In a 1-year period (2016–2017), 283 children under 5-year of age with intestinal signs and suspected to campylobacteriosis, referring to two pediatric hospitals in Tehran, Iran, were enrolled in the present study. Stool specimens were transferred to the bacteriological laboratory into Carry-Blair transport media (Micro Media, Hungry) and immediately cultured on Brucella agar with 5% sheep blood and modified charcoal–cefoperazone–deoxycholate agar (mCCDA) (Merck, Germany) supplemented with specific antibiotics (ibresco, Iran). Incubation was performed at 42 °C for 48 h under microaerobic conditions. Suspicious colonies were evaluated by Gram staining, standard biochemical testing, and PCR using primers reported by Konkel et al. (Table 1).
DNA extraction of the stool
DNA extraction was performed from all fecal samples using QIAamp DNA Stool Mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instruction. Usually, the kit removes all inhibitors of the stool and a pure DNA can be obtained. This can avoid the interferences in the molecular techniques, especially in the biosensor. The extracted DNAs were stored at − 20 °C until further procedures About 4 ng of each extracted DNAs from the patients were uniformly used in all assays.
Direct evaluation of stool by PCR
A PCR assay was performed on all extracted DNA from stools using primers designed for real-time PCR assay. The PCR was carried out in a 25-μL reaction mixture containing 4 ng DNA template, 2.5 μL PCR buffer 10×, 200 μM dNTP, 5 mM MgCl2, 0.1 μM cadF primers, 1 unit of Taq DNA polymerase, and sterile deionized water. The cycling conditions were as follows: 95 °C for 3 min (1 cycle), followed by 32 cycles of denaturation at 94 °C for 30 s, annealing at 43 °C for 30 s, and extension at 72 °C for 30 s in a thermocycler (Eppendorf, Hamburg, Germany) with an additional extension step (5 min, 72 °C). To evaluate whether the designed nanobiosensor and real-time PCR can simultaneously detect both C. jejuni and C. coli species in stool specimens, an alternative duplex-PCR assay was also done on the specimens as previously described [10]. C. jejuni ATCC 29428 and C. coli ATCC 43478 strains were used as reference strains in the method.
Direct evaluation of stool by real-time PCR
Evaluation of DNA by real-time PCR assay was carried out using a StepOne instrument (Applied Biosystems, Foster, CA, USA). The real-time PCR reaction was performed in a final volume of 25 µL using the TaqMan Universal PCR Master Mix (Ampliqon, Denmark), containing 250 nM fluorogenic probe, 900 nM of each primer, and 4 ng of template DNA. Thermal cycling conditions were as follows: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. In each round of the PCR, the recombinant plasmid was used as positive control.
Direct evaluation of stool by nanobiosensor
The biosensor efficacy for the detection of the C. jejuni or C. coli DNA directly in the stool samples was evaluated. The SPR absorbance changes of the assay were immediately evaluated in the presence of the target gene by UV–vis spectrophotometry. The recombinant plasmid was used as positive control. Visual evaluation was also performed by naked eye.
Limits of Detection (LODs), specificity, and sensitivity of designed nanobiosensor, real-time PCR, and PCR
The LODs of the gold biosensor, real-time PCR, and PCR assays were evaluated using serial 10-fold dilution of the pTG19-T/cadF plasmid stock (e.g., 100 µL of plasmid: 900 µL of DNase-free water). The concentration of the diluted DNAs was determined from 4 to 4 × 10−10 ng (~ 109 to 1 copy number/mL). Similar initial amounts of ~ 4 ng of plasmid were spiked in the stool and serial dilutions were prepared. After extraction step, LODs of the biosensor, real-time PCR, and PCR methods were evaluated. In all assays, the above-mentioned calculation was performed with different concentrations of the plasmid. The lowest concentration which can be detected by each technique was defined as LODs. To quantify the LOD of biosensor, a standard curve was generated by plotting the Tt (time threshold) values against log copy number, and linear regression was considered using the Microsoft Excel program. Each test was performed in independent triplicates.
A collection of the genomic DNA of enteropathogenic bacterial strains, e.g. E. coli ATCC 25922, Vibrio cholerae ATCC 14035, Aeromonas hydrophila ATCC 7966, Enterobacter cloacae PTCC 1798, Shigella sonnei ATCC 9290, Campylobacter jejuni ATCC 29428, Campylobacter coli ATCC 43478, and clinical isolates of Enteropathogenic E. coli, Enterohemorrhagic E. coli, and Salmonella enterica were used for specificity test. The DNAs of the strains were extracted using an EZ-10 Spin Column Genomic DNA Mini-Preps Kit (Bio Basic Inc., Canada) according to the manufacturer’s instruction. Used concentrations of the DNAs for all assays were adjusted to ~ 4 ng and the protocol was done with the above- mentioned identical conditions. The sensitivity and specificity of three assays for direct detection of the Campylobacters in fecal specimens was calculated according to presented formula by Parikh et al. [34]. In sensitivity tests, the real-time PCR method was used as gold standard.
Stability of biosensor
In order to investigate the stability, the GNR-ssDNA biosensor was kept at 4 °C for a period of 6 months and was monitored by UV–vis spectrophotometer. The specificity and the LOD of nanobiosensor were also evaluated during the stability period by the recombinant plasmid and non-target DNAs. The done conditions for the stability assays were similar to the above-mentioned conditions. All experiments on the clinical samples were carried out with fresh nanobiosensor.
Results and discussion
TA cloning and positive control
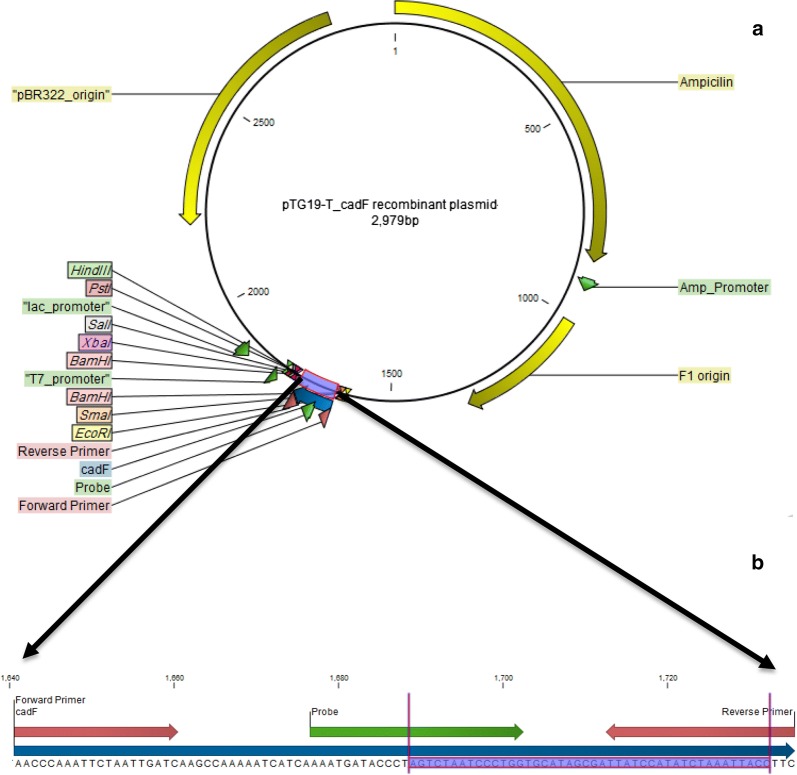
TA cloning of cadF gene following recombinant plasmids sequencing confirmed that target fragment was correctly assembled into the vector and can efficiently hybridize with target gene from both Campylobacters. The position of the cloned cadF fragment and biosensor target is presented in Fig. 1.
Fig. 1.
Schematic map of the positive control. A; pTG19-T/cadF recombinant plasmid + 95 bp fragment of cadF gene inserted into vector (blue box). B: The attachment position of probe/primers used in real-time PCR (red and green arrows) and target sequence of biosensor (blue scheme)
Synthesis and characterization of nanorods
Compared with spherical gold nanoparticles, gold nanorods show greater absorption cross sections and stronger light scattering properties, giving two absorption peaks in the visible and near-infrared (NIR) regions that correspond to the transverse and longitudinal surface plasmon bands, respectively. In this study, the characteristic transverse absorption band appeared at ~ 500 nm while the longitudinal absorption band emerged around 730 nm, representing the formation of rod-shaped nanostructures. Analysis of transmission microscopy also confirmed the rod morphology and size of the GNRs with the average length and the diameter of ~ 32.43 ± 5.5 nm and ~ 11.53 ± 1.03 nm, respectively. Size of the nanostructures was estimated by ImageJ Software (Fig. 2b).
Fig. 2.
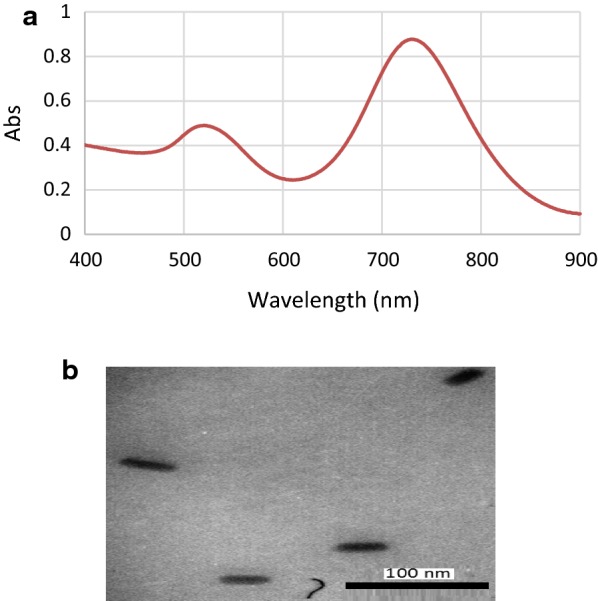
Synthetized nanorods. a Characteristic surface plasmon resonance bands of GNRs; b the inset shows TEM of the nanostructures
Analysis and optimization of GNRs-ssDNA nano-bioconjugates
Surface functionalization of gold with ssDNA has been reported previously [35]. Thiol activated ssDNA is usually utilized for adsorption onto the gold surface with high affinity. Formation of the ssDNA-GNRs complex was first monitored by UV–vis spectroscopy. Although it is very important to maximize DNA loading onto GNRs, due to the high sensitivity of these nanostructures care must be taken to maintain the rod morphology during the bioconjugation process. Upon the bioconjugation process with different concentrations of the probe (25–300 nM), the characteristic SPR bands of GNRs experienced a decrease in intensity. The results showed that addition of higher concentrations of the probe (above 25 nM) has led to the disappearance of typical SPR bands, representing a nonspecific aggregation of the nanostructures. Therefore, the specified concentration was used for further experiments to have a proper control on interparticle distance before target addition without losing the characteristic morphology as well as showing stability over a period of time. Such an optimization is of great significance in the process of design and development of SPR based nanobiosensors [32]. The GNRs were centrifuged after the bioconjugation process (with 25 nM DNA) and concentration of the unbound ss-DNAs was measured to be ~ 8 nM. Using ICP-AAS, the concentration of the GNRs can also be calculated. Based on the average length and diameter of the GNRs (to obtain the number of atoms in each GNR) and ICP results, molarity of GNRs in this study was calculated to be 1200 pM. Therefore, the ratio of GNRs:ss-DNAs in which the nanostructures can maintain their rod morphology was calculated to be 1.2: 17.
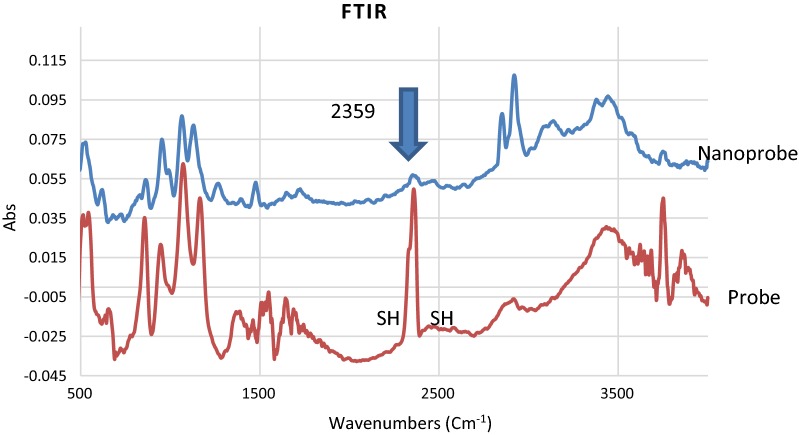
Figure 3 shows that the absorption peak of the FTIR around 2359 cm−1 can be correlated to –SH group of the probes. Upon immobilization of the probes onto the matrix of GNRs, the peak disappeared, resulting the attachment of –SH to Au [36]. It is worth mentioning that the strong absorption bands around 2920 and 2850 cm−1 in the nanoprobe sample appeared due to C–H stretching vibrations of methyl and methylene groups of CTAB (the cationic surfactant) on the matrix of GNRs. The small band around 1470 cm−1 can also be attributed to C-H bending vibrations of the CTAB layer around GNRs [37].
Fig. 3.
FTIR spectra of the nanoprobe before and after immobilization onto GNRs
Moreover, to monitor the interaction of the probes with GNRs, zeta potential of the samples before and after the bioconjugation process was analyzed. The zeta value for the GNRs decreased from + 30.1 to + 15.8 mV after interaction with ssDNA, indicating changes on the surface of GNRs (Additional file 1). Polydispersity index (PdI) for both nanostructures were ~ 0.4 (Additional file 2).
Analysis of nanobiosensor in the presence of recombinant plasmid and synthetic 95 bp cadF gene
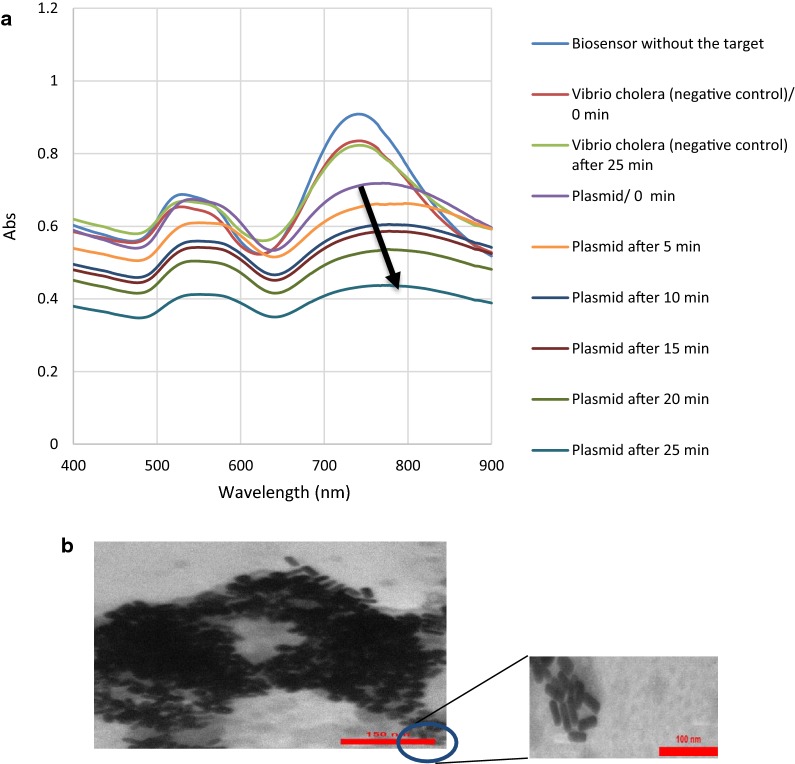
Figure 4 shows the SPR bands and TEM image of the nanobiosensor after hybridization with recombinant plasmid of cadF gene of Campylobacter. Upon addition of the extracted genomic DNA, the shape of the SPR bands of the nanoprobes apparently changed. This phenomenon is thought to have occurred due to the hybridization of cadF specific sequence with capture probes that are complementary to each other, leading to the notable decrease of interparticle distance. A glance at the SPR response of the nanobiosensor shows the significant decrease in the intensity as well as red-shifts in the position of the longitudinal surface plasmon absorption bands upon aging (up to 25 min) and final loss of the SPR bands. This could be attributed to the change in the refractive index at the surface layer of the GNRs, induced by binding to the target [38]. The very first change can be observed after immediate addition of the target DNA to the nanoprobe. A decrease in the intensity of the transverse plasmon band was also detected around 500 nm. The SPR bands of the Vibrio cholerae DNA, as the negative control, showed no changes upon interaction. Transmission electron microscopy image (Fig. 4b) revealed the remarkable decrease of interparticle distance and aggregation of the nanoprobe after hybridization with the target.
Fig. 4.
Nanobiosensor upon addition of recombinant plasmid as the target gene. a surface plasmon resonance bands; b transmission electron microscopy image
The same procedure was performed to assess the efficacy of the nanobiosensor against the stool samples spiked with synthetic single-stranded 95 bp cadF gene. Upon addition of synthetic single-stranded 95 bp cadF target, the SPR response of the nanobiosensor was changed immediately; however, the reaction became stronger within 25 min. Accordingly, the results were similar regarding recombinant plasmid as the target and indicated the biosensor efficacy for the detection of cadF gene of Campylobacter in stool samples. In addition, SPRs spectra were compared for the three positive controls including the DNAs from standard strains, the recombinant plasmid, and the single-stranded 95 bp cadF gene. Our results showed that plasmonic changes were identical for three targets (Fig. 5). Therefore, it seems that the current biosensor can be directly applied for the detection of the Campylobacter in the fecal specimen of patients suspected to campylobacteriosis.
Fig. 5.
SPRs spectra of the DNAs from standard strains, the recombinant plasmid, and the single-stranded 95 bp cadF gene
Comparison of LOD, sensitivity, and specificity of the nanobiosensor with PCR and real-time PCR and evaluation of stability tests
Ten-fold dilutions of pTG19-T/cadF recombinant plasmid were used to determine the detection limit of the molecular methods. According to the complete sequence of cloned plasmid (2884 + 95 = 2979 bp) and copy number formula, LODs of PCR, real-time PCR, and biosensor were calculated. Although the biosensor was stable when the recombinant plasmid concentrations were 40 × 10−9 and 40 × 10−10 ng, the aggregation of gold nanoprobes took place after adding 40 × 10−1 to 40 × 10−8 ng of DNA. Consequently, the 10−8 dilution (102 copy number/mL) was determined as LOD of the biosensor. The LOD values for biosensors are different in published studies. Singh et al. reported 50 copy number. mL−1 for the detection of E. coli O157:H7 by gold nanorods while Wei et al. described 103 copy number. mL−1 of Campylobacter using a SPR biosensor [30, 39].
Figure 6 indicates the standard linear regression curve of log copy number/reaction against SPR position with a significant difference in the intensity of the SPR between 109 and 102 copy number of target DNA. The changes in the SPR position was linearly observed when the plasmid increased with a correlation coefficient of ~ 0.95. Considering the concentration of C. jejuni during human infection which can reach as high as 108 copy number/g in fecal material (3), the biosensor can efficiently detect the target with a remarkable change in the SPR absorption peak. In this study, the broadening of the longitudinal surface plasmon resonance band and disappearance of characteristic LSPR band can be correlated to the presence of the target (Fig. 6).
Fig. 6.
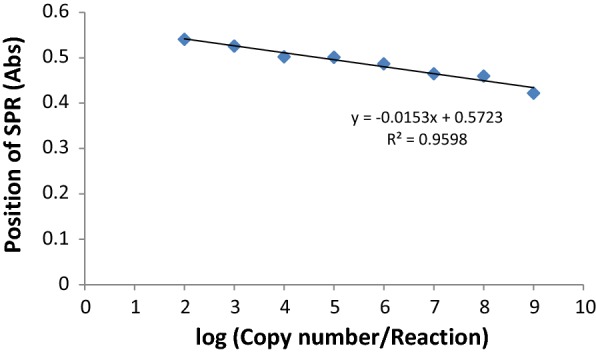
Standard linear regression curve of log (copy number/reaction) against SPR position
The detection limit of the PCR and real-time PCR was also detected as 10−7 and 10−8 dilutions; equal to ~ 103 and ~ 102 copy number/mL, respectively. It was found that LODs of the nanobiosensor and real-time PCR were the same, and it seems that this system can be applied as a potential tool in the genomic hybridization assay for diagnostic purposes. Considering the technical basis of the mentioned methods which are DNA targeted, analysis of their higher sensitivity should be interpreted with cautious as DNA targeted diagnostic tools cannot discriminate between viable and dead cells. Although, considering the pathogenic nature of C. jejuni and C. coli which are not categorized within normal flora population, makes its DNA detection confirmative of the disease. The detection limit of the methods on spiked stool was 10−6, 10−7 and 10−7 for PCR, real-time PCR, and nanobiosensor, respectively, each of which is one-fold higher than what was obtained for spiked DNase-free water. The highest sensitivity for the detection of Campylobacter spp. in clinical samples was related to real-time PCR (50 of 50, 100%). This technique was used as the standard to assess the sensitivity of other assays. Higher sensitivity (100%) of real-time PCR was also reported in Zhang study [40]. When compared with real-time PCR, the sensitivity of the biosensor and PCR were reported as 88% (44 of 50) and 84% (42 of 50), respectively. Our nanobiosensor showed an acceptable sensitivity range between PCR and real-time PCR which proposes its application for development of a promising diagnostic tool in the upcoming future.
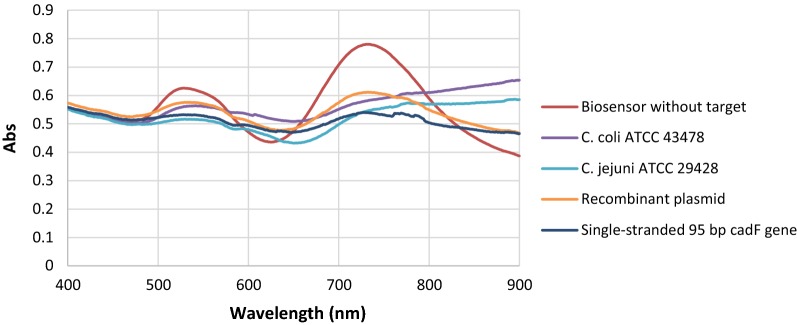
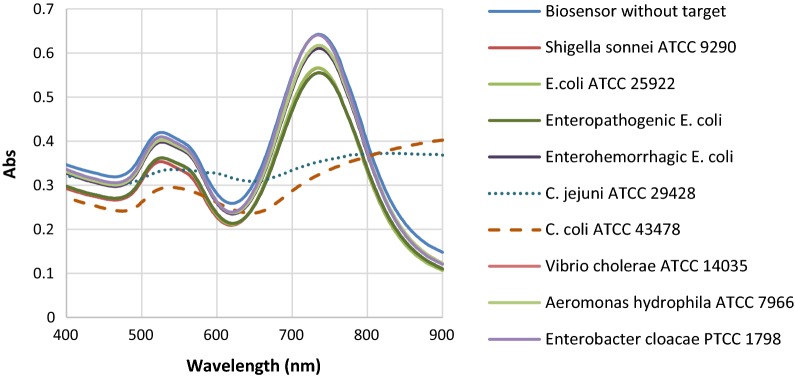
For specificity evaluation, a list of intestinal bacteria was used for PCR, real-time PCR, and biosensor. The specificity results for all 3 tests were 100% with exclusive detection of both C. jejuni and C. coli species and no non-Campylobacter detection in the 3 test (data not shown). Changes in the SPR absorption peak of the nanobiosensor was also specific for standard strains of Campylobacter while no remarkable variation was found for the other non-Campylobacter enteric bacteria (Fig. 7).
Fig. 7.
Specificity results of the nanobiosensor
Based on stability findings, there was no aggregation of the nanostructures, and the sensor could maintain its characteristic rod morphology. A small shift in the longitudinal surface plasmon resonance of the GNRs represents some changes in their microenvironment which is mainly size (Fig. 8). Therefore, the nanbiosensor reported in this study was stable over this period of time.
Fig. 8.
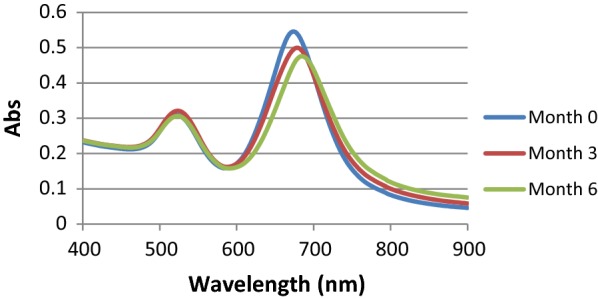
Stability of the gold nanostructure under a period of time
During stability time, LOD of the nanobiosensor were also evaluated by recombinant plasmid and was calculated as ~ 102 copy number/mL which was similar to freshly prepared nanobiosensor. In the specificity test on the control biosensors, no measurable change of the SPR was observed when non-target DNAs were used. The aggregation was only identified in the samples with recombinant plasmid and synthetic single-stranded 95 bp gene. All these data supported that the designed sensor is highly stable, specific, reliable, and sensitive for the detection of Campylobacter.
Culture of clinical specimens
According to morphological and phenotypical characterizations, total numbers of Campylobacter spp. positive clinical specimens were 19 out of 283 cases (7%), including 17 C. jejuni (89%) and 2 C. coli species (11%). Ghorbanalizadgan et al. [41] isolated Campylobacter spp. in 12 out of 200 evaluated children (6%) in Iran. Other studies also published data on the isolation rate of Campylobacter spp. between 0 to 16% from clinical stool sample by culture in Iran and some other countries [9, 42].
Detection of Campylobacter spp. in stool by direct PCR
Using direct PCR, cadF gene was detected in 42 (15%) patients. This technique identified bacterial DNA in 19 out of 19 (100% sensitivity) culture-positive specimens and in 23 culture-negative samples. In bacteriology, the culture is the gold standard of the detection of the bacteria, but some those such as Campylobacters aren’t able to grow properly on the media because of fastidious conditions, VBNC, etc. Therefore, despite the disease, the bacteria cannot be isolated in culture method. However, the culture only can detect the live bacteria, while molecular methods can detect DNA from both dead and live bacteria. So it is normal that higher rates of the positive cases can be detected by the molecular tests [43].
O’Leary et al. [44] reported that Campylobacter detection by PCR is more sensitive than culture. Singh et al. [45] also indicated that PCR could detect C. jejuni more efficiently than culture method.
In addition, all specimens were examined to test the presence of target gene using duplex-PCR described in our previous study [10], which could simultaneously detect and differentiate C. jejuni and C. coli species. Its result was similar to the common PCR (42 positive cases, including 40 C. jejuni, 95%, and 2 C. coli species, 5%).
Evaluation of campylobacteriosis in fecal samples by direct real-time PCR
In this study, a real-time PCR was developed using new TaqMan probe and primer sets, which were able to amplify a 95 bp fragment of the cadF gene with accurate specificity confirmed by direct sequencing of the real-time PCR products and analysis of results in NCBI, PubMed (http://www.ncbi.nlm.nih.gov/). With real-time PCR, 50 (18%) out of 283 clinical samples were positive for Campylobacter spp. The assay was also able to identify 19 of 19 and 42 of 42 culture and PCR-positive specimens, respectively. Moreover, the designed real-time PCR showed 100% sensitivity compared with two other methods. However, another potential benefit of real-time PCR test over the standard PCR and culture was a short time (about 40 min) needed for the detection whilst the minimum times for PCR and culture, with confirmatory tests to identify a Campylobacter isolate at the species level, were about 3 h and 5 days, respectively. This finding is in agreement with De Boer et al. [47] and Zhang et al. [46] studies, which showed the sensitivity of real-time PCR for the identification of Campylobacters was both higher and faster than culture method [40, 47].
Direct detection of Campylobacter spp. in stool samples by the nanobiosensor
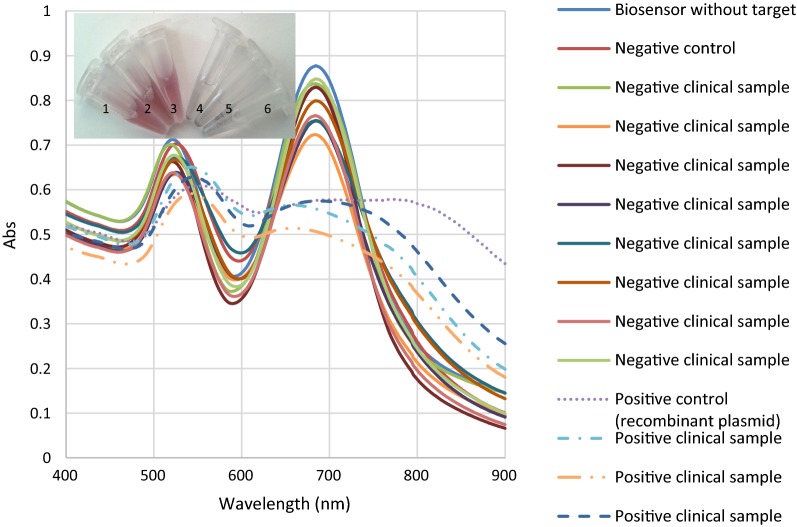
Of 283 evaluated specimens by the biosensor, C. jejuni and C. coli species were detected in 44 cases (16%). This method showed higher sensitivity (100%) than PCR (95%) and culture (43%), when it was considered as gold standard. In comparison with real-time PCR, our nanobiosensor was not able to detect Campylobacter in 6 positive clinical samples which were also negative in culture method. As shown in Fig. 9, no change in curve shapes of the SPR was observed in the negative clinical specimens, while significant changes representing a specific aggregation of the nanoprobes were observed in the presence of both the target Campylobacter DNA and recombinant plasmid. Overall, in DNA genosensing system, there was 12% false negative. The system colour changes were readily observable in the presence of target DNA even by the naked eye (the inset of Fig. 9). This result is consistent with the report by Su et al. [48], developing a colorimetric biosensor for the detection of E. coli O157:H7.
Fig. 9.
SPR bands of the nanobiosensor upon interaction with different clinical enteropathogenic bacterial strains. The inset shows naked eye detection of Campylobacter DNA. 1: negative control, 2 and 3: negative clinical samples, 4 and 5: positive clinical samples, 6: positive control
Conclusion
In summary, the present study focused on the design and development of the biosensor for the detection of both target species in human. Similar to real-time PCR, as a standard method, the detection level of the biosensor was 102 copy number/mL, being lower than that of the PCR assay. The false negatives of the nanobiosensor were an unexpected problem, but the system with an acceptable sensitivity and specificity could be useful for the identification of the Campylobacter spp. in the clinical setting due to its simplicity, high speed, cost-effectiveness, and portability. It also seems that these advantages can convert the biosensor to a commercial production in the future.
Additional files
Additional file 1. Zeta potential analysis of nanostructures: A) GNRs before conjugation with probe; B) ssDNA-GNRs nanobioconjugates (nanoprobe).
Additional file 2. DLS analysis of nanostructures. A) bare GNRs; B) nanoprobes.
Authors’ contributions
SS performed the experiments and drafted the manuscript. BB and TTM developed and supervised the work. SS, BB, TTM and MB contributed to data interpretation. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We wish to thank the Research Council of Tarbiat Modares University for developing the project.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current work are available from the corresponding authors on reasonable request.
Ethics approval and consent to participate
The study was reviewed and approved by Medical Ethics Committee of Tarbiat Modares University (Code: IR.MODARES.REC). The participants provided their written informed consent in order to participate in this study.
Funding
The study was supported by Research Council of Tarbiat Modares University and National Institute for Medical Research Development (NIMAD) (Grant Number: 942608).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- GNRs
gold nanorods
- C
Campylobacter
- VBNC
viable but nonculturable cells
- SPR
surface plasmon resonance
- PCR
polymerase chain reaction
- CTAB
cetyltrimethylammonium bromide
- NaBH4
sodium borohydride
- PBS
phosphate buffered saline
- AgNO3
silver nitrate
- NaCl
sodium chloride
- DTT
dithiothreitol
- UV–vis
ultraviolet–visible
- ss-DNA
single-stranded DNA
- FTIR
Fourier transform infrared spectrometer
- LOD
limit of detection
- ATCC
American type culture collection
- PTCC
Persian type culture collection
- NIR
near-infrared
- mV
millivolt
- DNA
deoxyribonucleic acid
- TEM
transmission electron microscopy
Contributor Information
Saeed Shams, Email: sshams@muq.ac.ir, Email: sshamsmed@gmail.com.
Bita Bakhshi, Phone: +982182884558, Email: b.bakhshi@modares.ac.ir.
Tahereh Tohidi Moghadam, Phone: +982182883914, Email: t.tohidi@modares.ac.ir.
Mehrdad Behmanesh, Email: behmanesh@modares.ac.ir.
References
- 1.Platts-Mills JA, Liu J, Gratz J, Mduma E, Amour C, Swai N, Taniuchi M, Begum S, Penataro Yori P, Tilley DH, et al. Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries. J Clin Microbiol. 2014;52:1074–1080. doi: 10.1128/JCM.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonjoch X, Calvó L, Soler M, Ruiz-Rueda O, Garcia-Gil LJ. A new multiplexed real-time PCR assay to detect Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. Food Anal Methods. 2010;3:40–46. doi: 10.1007/s12161-009-9110-3. [DOI] [Google Scholar]
- 3.Janssen R, Krogfelt KA, Cawthraw SA, van Pelt W, Wagenaar JA, Owen RJ. Host-pathogen interactions in Campylobacter infections: the host perspective. Clin Microbiol Rev. 2008;21:505–518. doi: 10.1128/CMR.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. Global epidemiology of campylobacter infection. Clin Microbiol Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sails AD, Fox AJ, Bolton FJ, Wareing DR, Greenway DL. A real-time PCR assay for the detection of Campylobacter jejuni in foods after enrichment culture. Appl Environ Microbiol. 2003;69:1383–1390. doi: 10.1128/AEM.69.3.1383-1390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gölz G, Kittler S, Malakauskas M, Alter T. Survival of campylobacter in the food chain and the environment. Curr Clin Microbiol Rep. 2018;5:1–9. doi: 10.1007/s40588-018-0085-y. [DOI] [Google Scholar]
- 7.https://www.cdc.gov/foodsafety/diseases/campylobacter/.
- 8.Taylor B, Williamson J, Luck J, Coleman D, Jones D, McGregor A. Sensitivity and specificity of serology in determining recent acute Campylobacter infection. Intern Med J. 2004;34:250–258. doi: 10.1111/j.1444-0903.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 9.Shams S, Bakhshi B, Nikmanesh B. Designing a rapid and accurate method for transportation and culture of the Campylobacter jejuni and Campylobacter coli-fastidious bacteria in the children with bacterial gastrointestinal symptoms. Koomesh J. 2016;18:71–78. [Google Scholar]
- 10.Shams S, Bakhshi B, Tohidi Moghadam T. In silico analysis of the cadF gene and development of a duplex polymerase chain reaction for species-specific identification of Campylobacter jejuni and Campylobacter coli. Jundishapur J Microbiol. 2016;9:1–7. doi: 10.5812/jjm.29645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva J, Leite D, Fernandes M, Mena C, Gibbs PA, Teixeira P. Campylobacter spp. as a foodborne pathogen: a review. Front Microbiol. 2011;2:200. doi: 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bessede E, Delcamp A, Sifre E, Buissonniere A, Megraud F. New methods for detection of campylobacters in stool samples in comparison to culture. J Clin Microbiol. 2011;49:941–944. doi: 10.1128/JCM.01489-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persson S, Olsen KE. Multiplex PCR for identification of Campylobacter coli and Campylobacter jejuni from pure cultures and directly on stool samples. J Med Microbiol. 2005;54:1043–1047. doi: 10.1099/jmm.0.46203-0. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni SP, Lever S, Logan JM, Lawson AJ, Stanley J, Shafi MS. Detection of campylobacter species: a comparison of culture and polymerase chain reaction based methods. J Clin Pathol. 2002;55:749–753. doi: 10.1136/jcp.55.10.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors—occurrence, properties and removal. J Appl Microbiol. 2012;113:1014–1026. doi: 10.1111/j.1365-2672.2012.05384.x. [DOI] [PubMed] [Google Scholar]
- 16.Buckwalter S, Sloan L, Cunningham S, Espy M, Uhl J, Jones M, Vetter E, Mandrekar J, Cockerill F, Pritt B. Inhibition controls for qualitative real-time PCR assays: are they necessary for all specimen matrices? J Clin Microbiol. 2014;52:2139–2143. doi: 10.1128/JCM.03389-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boswell T, Kudesia G. Serological cross-reaction between Legionella pneumophila and campylobacter in the indirect fluorescent antibody test. Epidemiol Infect. 1992;109:291–295. doi: 10.1017/S095026880005024X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mæland JA, Bevanger L, Enge J. Serological testing for campylobacteriosis with sera forwarded for Salmonella and Yersinia serology. APMIS. 1993;101:647–650. doi: 10.1111/j.1699-0463.1993.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 19.Luo C, Tang H, Cheng W, Yan L, Zhang D, Ju H, Ding S. A sensitive electrochemical DNA biosensor for specific detection of enterobacteriaceae bacteria by exonuclease III-assisted signal amplification. Biosens Bioelectron. 2013;48:132–137. doi: 10.1016/j.bios.2013.03.084. [DOI] [PubMed] [Google Scholar]
- 20.Inbaraj BS, Chen B. Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J Food Drug Anal. 2016;24:15–28. doi: 10.1016/j.jfda.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desar IM, van Deuren M, Sprong T, Jansen JB, Namavar F, Vandenbroucke-Grauls CM, van der Meer JW. Serum bactericidal activity against Helicobacter pylori in patients with hypogammaglobulinaemia. Clin Exp Immunol. 2009;156:434–439. doi: 10.1111/j.1365-2249.2009.03909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu X, Dong X, Zhang K, Han X, Fang X, Zhang Y. A gold nanorods-based fluorescent biosensor for the detection of hepatitis B virus DNA based on fluorescence resonance energy transfer. Analyst. 2013;138:642–650. doi: 10.1039/C2AN36099C. [DOI] [PubMed] [Google Scholar]
- 23.Ma Z, Tian L, Wang T, Wang C. Optical DNA detection based on gold nanorods aggregation. Anal Chim Acta. 2010;673:179–184. doi: 10.1016/j.aca.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 24.Parab HJ, Jung C, Lee J-H, Park HG. A gold nanorod-based optical DNA biosensor for the diagnosis of pathogens. Biosens Bioelectron. 2010;26:667–673. doi: 10.1016/j.bios.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 25.Cao J, Galbraith EK, Sun T, Grattan KT. Effective surface modification of gold nanorods for localized surface plasmon resonance-based biosensors. Sens Actuat B Chem. 2012;169:360–367. doi: 10.1016/j.snb.2012.05.019. [DOI] [Google Scholar]
- 26.Yasun E, Gulbakan B, Ocsoy I, Yuan Q, Shukoor MI, Li C, Tan W. Enrichment and detection of rare proteins with aptamer-conjugated gold nanorods. Anal Chem. 2012;84:6008–6015. doi: 10.1021/ac300806s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JH, Byun JY, Mun H, Shim WB, Shin YB, Li T, Kim MG. A regeneratable, label-free, localized surface plasmon resonance (LSPR) aptasensor for the detection of ochratoxin A. Biosens Bioelectron. 2014;59:321–327. doi: 10.1016/j.bios.2014.03.059. [DOI] [PubMed] [Google Scholar]
- 28.Dash SK, Sharma M, Khare S, Kumar A. rmpM genosensor for detection of human brain bacterial meningitis in cerebrospinal fluid. Appl Biochem Biotechnol. 2013;171:198–208. doi: 10.1007/s12010-013-0339-3. [DOI] [PubMed] [Google Scholar]
- 29.Gnanaprakasa TJ, Oyarzabal OA, Olsen EV, Pedrosa VA, Simonian AL. Tethered DNA scaffolds on optical sensor platforms for detection of hipO gene from Campylobacter jejuni. Sensors Actuators B Chem. 2011;156:304–311. doi: 10.1016/j.snb.2011.04.037. [DOI] [Google Scholar]
- 30.Wei D, Oyarzabal OA, Huang TS, Balasubramanian S, Sista S, Simonian AL. Development of a surface plasmon resonance biosensor for the identification of Campylobacter jejuni. J Microbiol Methods. 2007;69:78–85. doi: 10.1016/j.mimet.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Nikoobakht B, El-Sayed MA. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem Mater. 2003;15:1957–1962. doi: 10.1021/cm020732l. [DOI] [Google Scholar]
- 32.Tohidi Moghadam T, Ranjbar B. Heat induced aggregation of gold nanorods for rapid visual detection of lysozyme. Talanta. 2015;144:778–787. doi: 10.1016/j.talanta.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Soleimani M, Shams S, Majidzadeh AK. Developing a real-time quantitative loop-mediated isothermal amplification assay as a rapid and accurate method for detection of Brucellosis. J Appl Microbiol. 2013;115:828–834. doi: 10.1111/jam.12290. [DOI] [PubMed] [Google Scholar]
- 34.Parikh R, Mathai A, Parikh S, Sekhar GC, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol. 2008;56:45. doi: 10.4103/0301-4738.37595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HJ, Yook JG. Recent research trends of radio-frequency biosensors for biomolecular detection. Biosens Bioelectron. 2014;61:448–459. doi: 10.1016/j.bios.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Jazayeri MH, Amani H, Pourfatollah AA, Pazoki-Toroudi H, Sedighimoghaddam B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sensing Bio Sens Res. 2016;9:17–22. doi: 10.1016/j.sbsr.2016.04.002. [DOI] [Google Scholar]
- 37.Su G, Yang C, Zhu JJ. Fabrication of gold nanorods with tunable longitudinal surface plasmon resonance peaks by reductive dopamine. Langmuir. 2015;31(2):817–823. doi: 10.1021/la504041f. [DOI] [PubMed] [Google Scholar]
- 38.Unser S, Bruzas I, He J, Sagle L. Localized surface plasmon resonance biosensing: current challenges and approaches. Sensors (Basel) 2015;15:15684–15716. doi: 10.3390/s150715684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh AK, Senapati D, Wang S, Griffin J, Neely A, Candice P, Naylor KM, Varisli B, Kalluri JR, Ray PC. Gold nanorod based selective identification of Escherichia coli bacteria using two-photon Rayleigh scattering spectroscopy. ACS Nano. 2009;3:1906–1912. doi: 10.1021/nn9005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang MJ, Qiao B, Xu XB, Zhang JZ. Development and application of a real-time polymerase chain reaction method for Campylobacter jejuni detection. World J Gastroenterol. 2013;19:3090–3095. doi: 10.3748/wjg.v19.i20.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghorbanalizadgan M, Bakhshi B, Kazemnejad Lili A, Najar-Peerayeh S, Nikmanesh B. A molecular survey of Campylobacter jejuni and Campylobacter coli virulence and diversity. Iran Biomed J. 2014;18:158–164. doi: 10.6091/ibj.1359.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osbjer K, Tano E, Chhayheng L, Mac-Kwashie AO, Fernstrom LL, Ellstrom P, Sokerya S, Sokheng C, Mom V, Chheng K, et al. Detection of Campylobacter in human and animal field samples in Cambodia. APMIS. 2016;124:508–515. doi: 10.1111/apm.12531. [DOI] [PubMed] [Google Scholar]
- 43.Lleo MM, Ghidini V, Tafi MC, Castellani F, Trento I, Boaretti M. Detecting the presence of bacterial DNA by PCR can be useful in diagnosing culture-negative cases of infection, especially in patients with suspected infection and antibiotic therapy. FEMS Microbiol Lett. 2014;354:153–160. doi: 10.1111/1574-6968.12422. [DOI] [PubMed] [Google Scholar]
- 44.O’Leary J, Corcoran D, Lucey B. Comparison of the EntericBio multiplex PCR system with routine culture for detection of bacterial enteric pathogens. J Clin Microbiol. 2009;47:3449–3453. doi: 10.1128/JCM.01026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh H, Rathore RS, Singh S, Cheema PS. Comparative analysis of cultural isolation and PCR based assay for detection of Campylobacter jejuni in food and faecal samples. Braz J Microbiol. 2011;42:181–186. doi: 10.1590/S1517-83822011000100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.https://www.hindawi.com/journals/bmri/2013/852195/fig1/.
- 47.Boer P, Rahaoui H, Leer R, Montijn R, Vossen J. Real-time PCR detection of Campylobacter spp.: a comparison to classic culturing and enrichment. Food Microbiol. 2015;51:96–100. doi: 10.1016/j.fm.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Su H, Ma Q, Shang K, Liu T, Yin H, Ai S. Gold nanoparticles as colorimetric sensor: a case study on E. coli O157: H7 as a model for Gram-negative bacteria. Sens Actuat B Chem. 2012;161:298–303. doi: 10.1016/j.snb.2011.10.035. [DOI] [Google Scholar]
- 49.Konkel ME, Gray SA, Kim BJ, Garvis SG, Yoon J. Identification of the enteropathogens Campylobacter jejuni and Campylobacter coli based on the cadF virulence gene and its product. J Clin Microbiol. 1999;37:510–517. doi: 10.1128/jcm.37.3.510-517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fotouhi-Ardakani R, Dabiri S, Ajdari S, Alimohammadian MH, AlaeeNovin E, Taleshi N, Parvizi P. Assessment of nuclear and mitochondrial genes in precise identification and analysis of genetic polymorphisms for the evaluation of Leishmania parasites. Infect Genet Evol. 2016;46:33–41. doi: 10.1016/j.meegid.2016.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Zeta potential analysis of nanostructures: A) GNRs before conjugation with probe; B) ssDNA-GNRs nanobioconjugates (nanoprobe).
Additional file 2. DLS analysis of nanostructures. A) bare GNRs; B) nanoprobes.
Data Availability Statement
The datasets used and/or analyzed during the current work are available from the corresponding authors on reasonable request.