Abstract
Prescription opioids (POs) such as oxycodone and hydrocodone are highly effective medications for pain management, yet they also present a substantial risk for abuse and addiction. The consumption of POs has been escalating worldwide, resulting in tens of thousands of deaths due to overdose each year. Pharmacokinetic strategies based upon vaccination present an attractive avenue to suppress PO abuse. Herein, the preparation of two active PO vaccines is described that were found to elicit high-affinity anti-opioid antibodies through a structurally-congruent drug-hapten design. Administration of these vaccines resulted in a significant blockade of opioid analgesic activity, along with an unprecedented increase in drug serum half-life and protection against lethal overdose.
Graphical Abstract
Prescription opioid (PO) pain relievers form the foundation of modern pain management therapy, yet they are also among the most commonly misused and abused medications (Figure 1). Indeed, the illicit use of these medicines has reached epidemic levels in the U. S., with 18,893 PO overdose deaths reported in 2014.1 Notably, these drugs now cause more fatalities than heroin each year.2 This trend is not isolated to North America—the consumption of POs and PO overdoses have also increased across Europe, especially in the United Kingdom, Germany and Spain.3 While the misuse of nearly all POs has increased in recent years, oxycodone and hydrocodone products are by far the most widely and commonly abused opioids.1–2, 4
Figure 1.
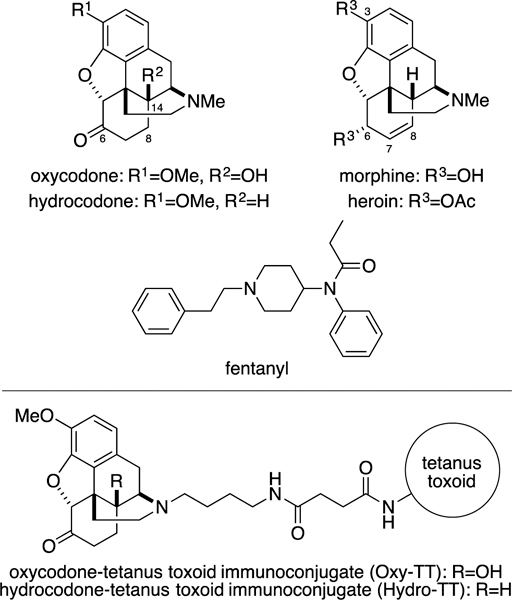
Structure of opioids and conjugate vaccines
Oxycodone and hydrocodone are effective semisynthetic opioid analgesics that predominantly activate mu opioid receptors (MORs) in the central nervous system (CNS). However, the euphoric effects of oxycodone and hydrocodone make them a common source for substance dependence and iatrogenic addiction. Both acute and chronic abuse of these medicines can lead to several serious complications, such as respiratory depression and cerebral hypoxia. The Drug Abuse Warning Network (DAWN) has reported that oxycodone is by far the most common PO associated with emergency department visits (151,218), followed immediately by hydrocodone (82,480).5 Devastatingly, PO-related fatalities involving oxycodone and hydrocodone overdose increased by nearly 4-fold between 2000 and 2014 in the U.S.4
To combat the deadly and addictive effects of oxycodone and hydrocodone, we pursued a vaccine-mediated pharmacokinetic (PK) strategy. In this approach, a small molecule-immunogenic protein conjugate is used to elicit drug-specific IgG antibodies that can bind to freely-circulating opioid molecules and prevent them from entering the CNS to induce MOR activation. When compared to the traditional pharmacologic strategy for treating PO addiction (MOR agonists or antagonists; e.g. methadone, buprenorphine, naltrexone), a PK strategy has several advantages including: (1) an ability to block drug activity without directly interacting with MORs, (2) fewer adverse or dysphoric side-effects, and (3) persistence of circulating antibody for up to a year, using strategically-spaced booster injections.[6] On the whole, such a vaccine could effectively suppress the addiction liability and overdose potential of the target drug over an extended time period without placing excessive compliance demands on the patient.
Previous disclosures of oxycodone and hydrocodone-targeted vaccines have focused on haptens that were linked to an immunogenic protein through the C-6 or C-8 position of the morphinan skeleton.[7] However, none of the studies with prescription opioid vaccines to date have addressed the vital challenge of protection against overdose. Because it has previously been reported that haptens which faithfully preserve the original structure of the target drug often generate higher-affinity antibodies than those which do not, we decided to initiate our studies with an alternative hapten design, replacing the N-methyl group of both oxycodone and hydrocodone with a succinic anhydride-derived linker.[8] Furthermore, molecular modeling exercises based upon this hapten design show the C-14 position to be spatially sequestered, allowing for the hydroxyl group of oxycodone to be immunochemically silenced while retaining a faithful presentation of the essential opioid pharmacophore.
Following this enabled logic, syntheses of Oxycodone-TT (Oxy-TT) and Hydrocodone-TT (Hydro-TT) were accomplished (Figure 1 and Supplemental Information). Importantly, replacement of the N-methyl group of oxycodone and hydrocodone with an N-butyl group possessing a succinamic acid moiety allowed the haptens to be conjugated to tetanus toxoid (TT) under standard amide-coupling conditions (Supplemental Figure 1 and 2).[9] We selected TT as our carrier protein due to its demonstrated efficacy in vaccines against drugs of abuse.[10] After conjugation, both Oxy-TT and Hydro-TT were formulated with alum (Alhydrogel®, Invivogen) and CpG ODN 1826 as adjuvants, which have likewise demonstrated good efficacy in the course of our previous studies.[10–11]
The resulting vaccines were administered to mice over the course of four intraperitoneal (IP) injections (Supplemental Figure 3). This sequence of events led to both Oxy-TT and Hydro-TT inducing high antibody mid-point titers when tested against their cognate coating antigen (ca. 400,000 for Oxy -TT and 200,000 for Hydro-TT) (Supplemental Figure 4). Notably, although these titers dropped somewhat by the end of the study, there was still a substantial antibody presence in both cohorts of animals on day 70.
To assess the functional efficacy of our vaccines, we conducted both hot plate and tail flick antinociception assays, which are often used for measuring the supraspinal and spinal analgesic potency of opioid drugs in rodent models.[12] In these assays, oxycodone and hydrocodone raise pain thresholds in a dose-responsive manner, and their efficacy can be quantified by measuring a response latency to the heat stimulus across several doses. Since antibodies from vaccinated mice bind to the target drugs and blunt their pharmacologic effect, an effective vaccine will present a rightward shift in these antinociception assays. In the current study, vaccination resulted in large increases for both oxycodone and hydrocodone ED50s (9.06 ± 0.61 mg by Oxy-TT, 15.17 ± 0.50 mg by Hydro-TT). Quantification of these ED50 shifts as dose ratios, (ca. 10-fold shift in oxycodone ED50, and ca. 6-fold shift in hydrocodone ED50) revealed the significant pharmacodynamic buffering capacity that vaccination was able to achieve (Figure 2). When Oxy-TT and Hydro-TT were delivered via subcutaneous injection, a translationally relevant route, they retained their ability to blunt opioid antinociceptive efficacy. The overall efficacy reduction at 2.25 mg/kg of oxycodone was comparable to that seen with previous hapten designs, although Oxy-TT and Hydro-TT also exhibited protection at higher doses of oxycodone (Supplemental Figure 5).
Figure 2.
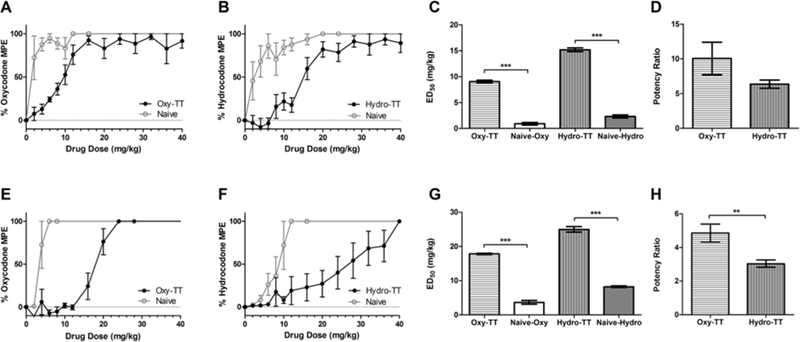
Behavioral results for intraperitoneally vaccinated and unvaccinated mice (n=6) in the hot plate (A-D) and tail flick (E-H) antinociception assays. Vaccinated and unvaccinated mice were cumulatively dosed with the specified drug and latency to nociception was measured by hot plate and tail flick tests. Bars denote means ± SEM. **=p < 0.01; ***=p < 0.001 two-tailed t-test
In order to further characterize the functional utility of this strategy, we next explored the ability of the resulting antibodies to bind clinically relevant concentrations of the two POs in vitro. A recent clinical study in oxycodone and hydrocodone abusers indicated that a steady state serum concentration of ca. 300 ng/mL was correlated with the subjective perception of feeling high.[13] Thus, in order to judge whether our vaccines could generate a robust antibody response to effectively block this concentration, serum from mice vaccinated with Oxy-TT and Hydro-TT were challenged with 300 ng/mL of oxycodone and hydrocodone. The amount of drug able to diffuse across a dialysis membrane was then quantified by LCMS.[14} Gratifyingly, we found that serum from vaccinated mice could bind all free drug at this concentration, while unvaccinated serum exhibited no specific binding for these species (Supplemental Table 1). As anticipated, the antibodies generated by each vaccine did not discriminate between oxycodone and hydrocodone.
We were next led to consider the vaccines’ effect on long-term drug disposition and metabolism, because even though active vaccination is a PK strategy for decreasing abuse all of the PO vaccine studies undertaken to date have measured drug distribution at only a single time point. To measure the rate of metabolic clearance of oxycodone and hydrocodone, a cassette preparation containing both drugs was administered to vaccinated and unvaccinated mice, followed by serial blood sampling over the course of 24 h.[14] The amount of drug remaining in the blood at each time point was then quantified using LCMS (Figure 3 A and B). In unvaccinated mice, oxycodone and hydrocodone were found to have a serum half-life between 10–20 minutes.
Figure 3.
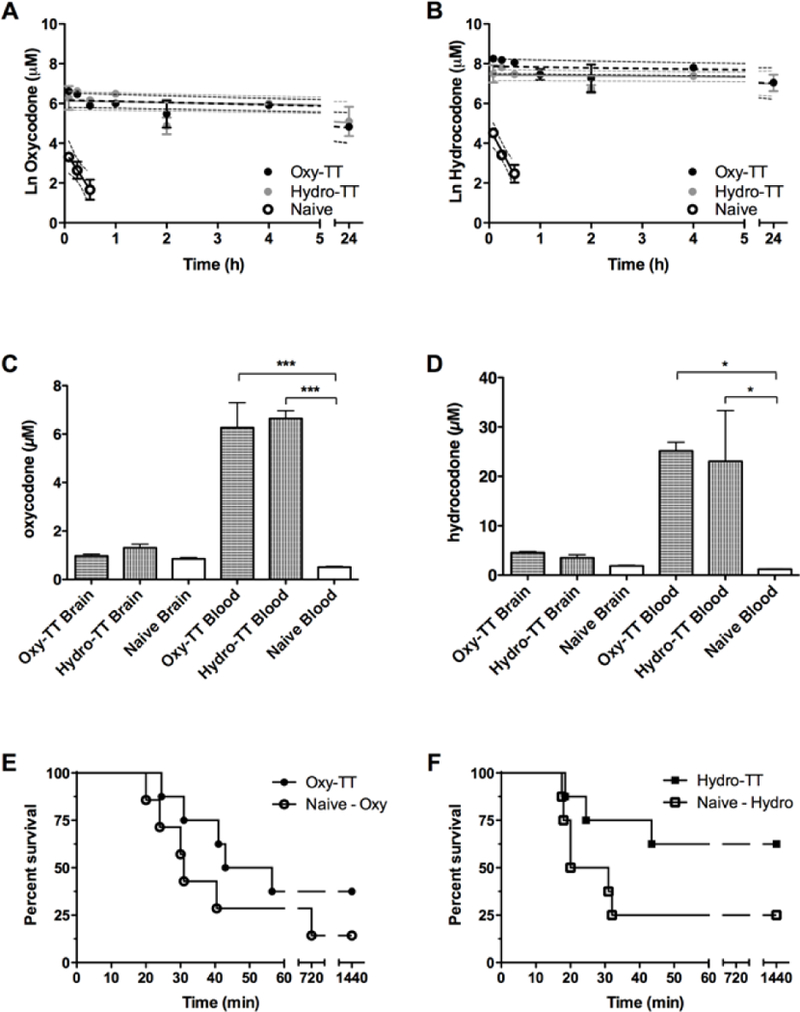
Serum pharmacokinetic results for vaccinated and unvaccinated mice (n=6 overall, n=2 per time point) following intraperitoneal cassette injection of 4 mg/kg oxycodone (A) and hydrocodone (B). Dotted lines represent the 95% CI for each regression. Biodistribution of oxycodone and hydrocodone in blood and brain samples. Vaccinated and naive mice (n=3) were intraperitoneally dosed with oxycodone at 4 mg/kg (C) or hydrocodone at 8 mg/kg (D), then trunk blood and brain were harvested 15 min post-injection. Survival rates following administration of lethal doses of prescription opioids. Vaccinated and unvaccinated animals (n=7–8) were dosed SC with 426 mg/kg of oxycodone (E) or 86 mg/kg of hydrocodone (F), and survival was measured continuously for 120 min, then again at 12 and 24 hours post injection. Bars denote means ± SEM. *=p < 0.01; ***=p < 0.0001 one way ANOVA.
To our surprise, vaccination was able to prolong drug half-life to between 12–38.5 hours—a 68 to 268-fold increase over unvaccinated animals (Supplemental Table 2). We contrast this to a report by Pfizer, where vaccination against nicotine only generated a 7.4-fold in half-life.[14] Our analysis also indicated that the vaccines were increasing the peak concentration of the drugs in the serum, which is consistent with the expectation that vaccination will sequester free drug in the periphery. As half-life (t1/2) is governed by the relationship t1/2 = (0.693*Vd)/CL, these findings indicate that the vaccines’ effect on half-life is likely to be due to a decreased rate of clearance (CL), rather than an increase in the volume of distribution (Vd) of the drugs.
To assess the link between these observed pharmacodynamic and pharmacokinetic effects, we next investigated how the vaccines could alter the biodistribution of oxycodone and hydrocodone. After treating vaccinated and unvaccinated mice with oxycodone or hydrocodone, the mice were sacrificed at 15 min via rapid decapitation and the drug concentration in blood and brain was quantified by LCMS (Supplemental Figure 6). As with the PK data discussed vide supra, our results from this experiment clearly show that vaccine-induced serum antibodies can effectively trap large amounts of free drug in the blood (Figure 3 C and D). Interestingly, although all mice received the same amount of the drug, the total concentration of drug found in vaccinated mice appeared higher than in unvaccinated animals. When considered together with the in vitro serum binding results vide supra, it is plausible that the significant decrease in the fraction of unbound drug in the bloodstream of vaccinated animals is ultimately limiting the rate of hepatic clearance for the test compounds.
Having established the remarkable pharmacokinetic effects of these two vaccines, we decided to seek a deeper understanding of the vaccines’ polyclonal response, investigating their kinetics as a means to further tease out efficacy. We therefore looked to surface plasmon resonance (SPR) (Table 1), where we were able to directly measure the antibody binding kinetics for the POs (Supplemental Figure 7).[10b] Using this design strategy affinity purified polyclonal immunoglobulin G (pIgG) was loaded on an SPR chip and challenged with free POs. As shown in Table 1, both anti-oxycodone and anti-hydrocodone pIgGs exhibited extremely high affinity to both cognate drugs (sub-nanomolar KD). As we have stated vide supra, this cross-reactivity is due to the C-14 positon being an immunosilent chemical epitope. On the other hand, the pIgGs possessed lower affinity for morphine, presumably due to its vast structural differences at C-3 (hydroxyl), C-6 (hydroxyl) and C-7,8 (alkene). This interpretation is also supported by the relatively weak KDs for heroin and fentanyl. Additional drug-hapten structural comparisons demonstrate why, as heroin contains two bulky acetyls and a C-7,8-alkene functionality, while fentanyl lacks the morphinan skeleton entirely, resulting in poor antibody affinity for these drugs.
Table 1.
The kinetic constants of anti-oxycodone or anti-hydroxycodone pIgG with different compounds. Kinetic analysis was performed on a BIAcore 3000 instrument (GE Healthcare) equipped with a research-grade CM7 sensor chip. Concentration series: a=0, 1, 2, 4, 8, 16, and 32 nM, b=0, 1.25, 2.5, 5, 10, 20, and 40 μM, c: No significant binding was observed at concentration of 50 μM.
| purified plgG |
compound | ka (M−1s−1) | kd (s−1) | KD (M) |
|---|---|---|---|---|
| anti-oxycodone | oxycodonea | 3.54 × 106 | 1.39 × 10−3 | 3.94 × 10−10 |
| hydrocodonea | 3.78 × 106 | 1.59 × 10−3 | 4.19 × 10−10 | |
| morphineb | 4.28 × 102 | 4.70 × 10−3 | 1.10 × 10−5 | |
| heroinc | N.D. | N.D. | N.D. | |
| fentanylc | N.D. | N.D. | N.D. | |
| anti-hydrocodone | oxycodonea | 1.47 × 106 | 1.22 × 10−3 | 8.30 × 10−10 |
| hydrocodonea | 5.06 × 106 | 1.21 × 10−3 | 2.40 × 10−10 | |
| morphineb | 1.05 × 104 | 3.96 × 10−3 | 3.78 × 10−7 | |
| heroinc | N.D. | N.D. | N.D. | |
| fentanylc | N.D. | N.D. | N.D. |
Because induced antibodies exhibited both a prolonged duration of binding and extremely high affinity for free oxycodone and hydrocodone, we assessed the ability of Oxy-TT and Hydro-TT to protect against acute overdose. When unvaccinated mice were dosed subcutaneously (SC) with 426 mg/kg of oxycodone, a 14.2% survival rate was seen at 24 hours, while overall survival was increased to 37.5% in Oxy-TT vaccinated animals. Likewise, in unvaccinated mice given 86 mg/kg of hydrocodone SC, a 25% survival rate was observed at 24 hours, whereas 62.5% of the animals vaccinated with Hydro-TT survived (Figure 3 E and F). This increased protection from prescription opioid overdose is very encouraging for further development of active vaccination against opioids, considering the enormous and growing number of fatalities and emergency department visits caused by these drugs annually.
In summary, these opioid conjugate vaccines demonstrated robust production of high-affinity antibodies with ample in vivo neutralization of their cognate opioids’ centrally-mediated pharmacodynamic effects, even while dramatically prolonging the half-life of these compounds in the bloodstream.
While the functional implications of prolonging drug half-life so profoundly are likely to vary depending on the intended clinical application and the persistence of drug challenge, the current study firmly established that Oxy-TT and Hydro-TT vaccines can limit mortality from lethal doses of hydrocodone and oxycodone. This finding indicates the potential of active vaccination as a route to halt the ongoing PO overdose epidemic.
Finally, the hapten methodologies designed herein are expected to inform future vaccination studies for other classes of frequently abused drugs, with a particular emphasis on understanding the links between antibody affinity, long-term PK alterations of target compounds, and persistence of vaccine efficacy.
METHODS
Details of experimental procedures are provided in the Supporting Information.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank Beverly Ellis for her assistance with mouse tissue collection. The authors thank the National Institute on Drug Abuse for funding under grant 1UH2DA041146–02. This is manuscript #29414 from TSRI.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
REFERENCES
- [1].CDC, National Vital Statistics System Mortality File (2015) 2015. [Google Scholar]
- [2].NIDA, Overdose Death Rates (Revised December 2015) 2015. [Google Scholar]
- [3].a) Giraudon I, Lowitz K, Dargan PI, Wood DM, Dart, Br J Clin Pharmacol 2013, 76, 823–824; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) van Amsterdam J, van den Brink W, Curr Drug Abuse Rev 2015, 8, 3–14; [DOI] [PubMed] [Google Scholar]; c) del Pozo JG, Carvajal A, Viloria JM, Velasco A, del Pozo VG, Eur J Clin Pharmacol 2008, 64, 411–415. [DOI] [PubMed] [Google Scholar]
- [4].CDC, Morbidity and Mortality Weekly Report 2016, 50. [Google Scholar]
- [5].NAHDA, HHS Publication No. (SMA) 13–4760, DAWN Series D-39; Rockville, MD, 2013. 2011. [Google Scholar]
- [6].Martell BA, Mitchell E, Poling J, K Gonsai, Kosten TR , Biol Psychiatry 2005, 58, 158–164. [DOI] [PubMed] [Google Scholar]
- [7].a) Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR, J Pharmacol Exp Ther 2012, 341, 225–232; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pravetoni M, Le Naour M, Tucker AM, Harmon TM, Hawley TM, Portoghese PS, Pentel PR, J Med Chem 2013, 56, 915–923; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, Birnbaum AK, Portoghese PS, Pentel PR, Vaccine 2012, 30, 4617–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD, J Med Chem 2011, 54, 5195–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Kunz H, von dem Bruch K, Methods Enzymol 1994, 247, 3–30; [DOI] [PubMed] [Google Scholar]; b) Kunz H, Birnbach S, Angewandte Chemie-International Edition in English 1986, 25, 360–362; [Google Scholar]; c) Kunz H, Birnbach S, Wernig P, Carbohyd Res 1990, 202, 207–223. [DOI] [PubMed] [Google Scholar]
- [10].a) Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD, Mol. Pharmaceutics 2010, 7 (0), 431–441.; [DOI] [PubMed] [Google Scholar]; b) Jalah R.; Torres OB.; Mayorov AV.; Li F.; Antoline JF.; Jacobson AE.; Rice KC.; Deschamps JR.; Beck Z.; Alving CR.; Matyas GR., Bioconjug Chem 2015. 26 (6), 1041–53.; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jacob NT, Lockner JW, Schlosburg JE, Ellis BA, Eubanks LM, Janda KD, J Med Chem 2016, 59, 2523–2529;d) Bremer PT, Kimishima A, Schlosburg JE, Zhou B, Collins KC, Janda KD, Angew Chem Int Ed Engl 2016, 55, 3772–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bremer PT, Schlosburg JE, Lively JM, Janda KD, Mol Pharm 2014, 11, 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bannon AW, Malmberg AB, Curr Protoc Neurosci 2007, Chapter 8, Unit 8 9. [DOI] [PubMed] [Google Scholar]
- [13].Morton TL, Devarakonda K, Kostenbader K, Montgomery J, Barrett T, Webster L, Pain Med 2015. [DOI] [PubMed] [Google Scholar]
- [14].McCluskie MJ, Evans DM, Zhang N, Benoit M, McElhiney SP, Unnithan M, DeMarco SC, Clay B, Huber C, Deora A, Thorn JM, Stead DR, Merson JR, Davis HL, Immunopharmacol Immunotoxicol 2016, 38, 184–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


