Abstract
Objectives:
To investigate the impact on metal artifacts and dose efficiency of using a tin filter in combination with high-energy-threshold (TH) images of a photon-counting-detector (PCD)-CT system.
Materials and Methods:
A 3D-printed spine with pedicle screws was scanned on a PCD-CT system with and without tin filtration. Image noise and severity of artifacts were measured for low-energy threshold (TL) and TH images. In a prospective, IRB-approved, HIPAA compliant study, 20 patients having a clinical energy-integrating-detector (EID)-CT were scanned on a PCD-CT system using tin filtration. Images were reviewed by 3 radiologists to evaluate visualization of anatomic structures, diagnostic confidence and image preference. Artifact severity and image noise were measured. Wilcoxon signed rank was used to test differences between PCD-CT TH and EID-CT images.
Results:
TH phantom images with tin filtration reduced metal artifacts and had comparable noise (32 HU) to TL images (29 HU) acquired without filtration. Visualization scores for the cortex, trabeculae, and implant-trabecular interface from PCD-CT TH images (4.4 ± 0.9, 4.4 ± 1.0 and 4.4 ± 1.0) were significantly higher (P<0.0001) than EID-CT images (3.3 ± 1.3, 3.3 ± 1.2 and 3.3 ± 1.6). A strong preference was shown for PCD-CT TH images due to improved diagnostic confidence and decreased artifact severity. Noise in PCD-CT TH images (93 ± 41 HU) was significantly lower than in EID-CT images (133 ± 92 HU, P < 0.05).
Conclusions:
TH images acquired with tin filtration on PCD-CT demonstrated a substantial decrease in metal artifacts and an increase in dose efficiency compared to EID-CT.
Keywords: CT, Photon counting detector, Metal artifact reduction, tin filtration
Introduction
Computed tomography (CT) serves as an essential imaging modality for a wide range of diagnostic and treatment planning applications, including evaluation of postoperative orthopedic surgery patients 1, 2. However, metal implants typically cause severe artifacts in CT images, which appear as streaks and dark/bright regions. These metal artifacts are caused by multiple mechanisms, including x-ray scatter, photon starvation and beam hardening 3. Depending on the atomic number, density and shape of the metal, as well as the surrounding anatomy and scan acquisition/reconstruction parameters, the resulting metal artifacts can dramatically obscure critical structures. This can result in a significant reduction in diagnostic confidence for the task of distinguishing pathologic findings from normal structures 4.
Metal artifacts have been a serious problem for CT since its invention and multiple types of metal artifact reduction (MAR) techniques have been proposed 5–9. Scanning techniques that use a high tube potential and tube current can reduce metal artifacts somewhat by lessening photon starvation 4. However, this approach increases radiation dose to the patient and is effective primarily for implants made of less dense or lower atomic number metals. Reconstruction 7, 10–16 and post processing algorithms 9, 17–19 have also been developed to reduce metal artifacts. With respect to spinal hardware, iterative metal artifact reduction demonstrated promising artifact reduction for spinal fusion patients 20. However, algorithmic methods are computationally expensive and their effectiveness depends on the assumptions made or prior knowledge about the implants.
In 2014, a whole-body photon counting detector (PCD) CT system was installed in a research setting at our institution. To date, it has demonstrated several advantages compared to commercial energy-integrating-detector (EID) CT systems 21–27. In PCDs, X-ray photons are grouped into multiple data sets according to their energies, with each data set corresponding to photons with energy above a predefined threshold 28. By excluding lower energies photons, PCD-CT high-energy threshold (TH) images suffer from less beam hardening and photon starvation artifacts from metal implants. Use of additional x-ray spectra filtration, such as a thin layer of tin, can further increase the mean energy of the TH images.
The purpose of this study was to investigate the impact on metal artifacts and dose efficiency of using a tin filter in combination with TH images of a PCD-CT system.
Materials and Methods
Phantom Studies
A 3D-printed spine model with metallic pedicle screws was placed in a 35-cm wide water phantom, which emulated the attenuation of a typical healthy adult, and scanned on a PCD-CT system (CounT, Siemens Healthcare, Forchheim, Germany) using abdomen protocols with and without 0.4 mm tin filtration. The tube potential (140 kV) and energy thresholds (25 and 75 keV) were held constant for all acquisitions. For both scans, the volume CT dose index (CTDIvol) was 12.1 mGy. This value was determined using an initial scan of the phantom at 120 kV, 200 quality reference mAs on a 128-slice clinical CT (SOMATOM Flash, Siemens Healthcare, Forchheim, Germany) with automatic exposure control on, which is the routine abdomen protocol in our practice. This dose level is somewhat lower than the 50th (17 mGy) and 75th (21 mGy) percentile CTDIvol values for abdominal/pelvic CT reported in a recent U.S.-based survey of CT doses 29. The CT number difference between water regions with and without metal artifacts was measured to quantify artifact severity for both the low-energy threshold images (TL) and TH images for scans acquired without and with the tin filter. Image noise was measured as the standard deviation of CT numbers inside uniform water regions that were unaffected by metal artifacts.
Patient Data Collection
This HIPPA compliant, prospective study was approved by our Institutional Review Board, and written informed consent was obtained from each participant. From May 30, 2017 to October 24, 2017, patients aged 25 to 80 years of both sexes with known metal implants in the spine, shoulders, or extremities undergoing a CT scan for clinical indications were recruited to the study, which entailed receiving a research PCD-CT scan. Patients were excluded from the study if they were pregnant or unable to provide written informed consent.
CT Image Acquisition and Reconstruction
All clinical EID-CT exams were performed on 64- or 128-slice CT scanners in our practice (SOMATOM Sensation 64, Definition Edge/Flash; Siemens Healthcare, Forchheim, Germany). For the clinically-indicated scan, the supervising radiologist selected the appropriate clinical protocol for each patient; participation in this study in no way impacted the protocol choice for the clinical EID-CT exam. Immediately following their clinical EID-CT exams, each patient was scanned over the identical body region using the PCD-CT system with use of a 0.4 mm tin filter 30. For each patient, tube potential was set to 140 kV and energy thresholds were set to 25 and 75 keV. The tube current on the PCD-CT system was adjusted to match the radiation dose, in terms of CTDIvol, to the patient’s clinical EID-CT exam. Image reconstructions of both EID and PCD acquisitions were performed according to our routine clinical standards for the corresponding anatomic regions. A summary of EID-CT and PCD-CT acquisition and reconstruction parameters is given in Table 1.
Table 1.
Acquisition and reconstruction parameters for EID- and PCD-CT scans performed with metal implants within the image field of view. * indicates the tube current of PCD-CT was adjusted to match the CTDIvol values of the clinical EID-CT scan for each patient. ** indicates the PCD reconstruction kernel was matched to the corresponding kernel for the EID system on which a specific patient was scanned. Thus, if the EID CT used iterative reconstruction (IR) in our clinical protocol (e.g., Flash scanner), we used IR for the PCD-CT images; if the EID CT used filtered back projection (FPB) in our clinical protocol (e.g., Sens64 scanner), we used FBP for the PCD-CT images.
| Body Part |
Scanner Type |
kV | Quality Reference |
Collimation | Kernels | Slice Thickness |
|---|---|---|---|---|---|---|
| Cervical Spine | Flash | 140 | 280 mAs | 128 × 0.6 mm | I31(2), I70(2) | 2.0 mm |
| Sens64 | 140 | 350 mAs | 64 × 0.6 mm | B31, B70 | 2.0 mm | |
| PCD-CT | 140Sn | * | 48 × 0.25 mm | ** | 2.0 mm | |
| Lumbar Spine | Flash | 120 | 260 mAs | 128 × 0.6 mm | I31(2), I70(2) | 2.0 mm |
| Sens64 | 120 | 350 mAs | 64 × 0.6 mm | B31, B70 | 2.0 mm | |
| PCD-CT | 140Sn | * | 48 × 0.25 mm | ** | 2.0 mm | |
| Shoulder | Edge | 140 | 250 mAs | 128 × 0.6 mm | I40(2), I70(2) | 1.0 mm |
| PCD-CT | 140Sn | * | 48 × 0.25 mm | I40(2), I70(2) | 1.0 mm | |
| Wrist | Edge | 140 | 200 mAs | 128 × 0.6 mm | I40(1), I70 (1) | 0.6 mm |
| Edge | 140 | 250 mAs | 16 × 0.3 mm | U40, U70 | 0.6 mm | |
| PCD-CT | 140Sn | * | 48 × 0.25 mm | ** | 0.6 mm | |
| Elbow | Edge | 140 | 250 mAs | 16 × 0.6 mm | U40, U70 | 1.0 mm |
| PCD-CT | 140Sn | * | 48 × 0.25 mm | U40, U70 | 1.0 mm | |
| Ankle | Edge | 140 | 250 mAs | 16 × 0.6 mm | U40, U70 | 1.0 mm |
| PCD-CT | 140Sn | * | 48 × 0.25 mm | U40, U70 | 1.0 mm | |
Image Analysis
Qualitative Measurements:
Images were loaded onto on a clinical viewing workstation that was calibrated in compliance with the ACR Technical Standard for Electronic Practice 31. The images from each patient case were independently assessed by three radiologists, each with > 8 years of experience. For each case, the EID-CT and PCD-CT TH images were randomly displayed side-by-side and reviewed in a blinded fashion. The type of metal implant and its location was recorded for each case.
For each review session, reviewers were asked to assess pre-defined structures that were specified for each anatomic region (see Table, Supplemental Digital Content 1, which lists critical structures identified for reader assessment). Osseous structures were evaluated around the region of the metal hardware using a 6-point scale, particularly focusing on the ability to identify periprothestic fractures, implant loosening, cortex, trabeculae and implant-bone interface (0 = critical structures totally obscured, no structures identifiable; 1 = marked artifacts, questionable anatomic recognition of critical structures; 2 = faint anatomic recognition, no confidence in the ability to identify pathology; 3 = anatomic recognition with low confidence in diagnosis; 4 = anatomic recognition with medium confidence in diagnosis; 5 = anatomic recognition with high confidence in diagnosis) 1.
In addition, readers were asked to rate their preference for the left image to the right image for each pair using a 5-point preference scale (−2 = significant decline in diagnostic confidence, −1 = less preference but no decline in diagnostic confidence, 0 = same, no difference or preference in diagnostic confidence; +1 = better, preference but no improvement in diagnostic confidence; +2 = substantial improvement in diagnostic confidence). For both sets of images, the readers also evaluated the overall effect of the artifacts on diagnosis using a 3 point scale (1 = artifact has no/minimal effect on images, 2 = artifact degrades images without affecting diagnosis, 3 = artifact impedes diagnosis).
Quantitative Measurements:
Artifact size was measured as the width of the most prominent artifact in the axial plane closest to the metal implant (Figure 3). The severity of metallic artifacts were measured as the absolute value of the difference in mean CT numbers between a pair of circular region of interests (ROIs), one in the most prominent hypodense artifact region next to the metal implant and the other in adjacent fat outside of the artifact region. The standard deviation of the CT numbers in the ROI of the fat region was calculated to represent image noise.
Figure 3.

59-year-old female with posterior rod and screw fixation of L2-L5 and a posterolateral bone graft demonstrating improved visualization of critical anatomic structures of the spine with threshold high (TH) Sn140 kV PCD-CT. EID-CT 120 kV (A-B) and TH Sn 140 kV PCD-CT (C-D) axial images from the same patient. The TH Sn 140 kV PCD-CT images (C-D) have reduced metal artifact compared with the EID-CT images (A-B) showing improved visualization of the central canal, neural foramina, and nerve roots which are the overall most improved critical anatomic structures in this study. The metal artifact reduction is predominantly evident with the soft tissue algorithm (A) vs. (C) than with the bone algorithm (B) vs. (D). In this particular example, the readers mean evaluation scores for central canal, neural foramina, and nerve roots for TH Sn 140 kV PCD-CT (C-D) were 3.7, 4.0, and 4.0 vs. 0.3, 3.0, and 2.3 for EID-CT (A-B), respectively. A, C: W/L = 400/40 HU. B, D: W/L = 1500/450 HU.
Statistics
Overall image preference and diagnostic confidence, artifact size, and image noise were compared between PCD-CT TH and EID-CT images using the Wilcoxon signed rank accounting for the paired data structure between the two CT technologies. A sub-analysis was performed for spine cases, where the diagnostic image quality scores of soft tissue and osseous structures from PCD-CT TH images were compared to that from EID-CT acquisitions using the Wilcoxon signed rank test. For other body parts (e.g. extremities), sub-analysis was not performed due to a limited number of cases. Statistical significance was defined as a two-sided p < 0.05. Statistical analysis was conducted using JMP version 12 (SAS Institute Inc., Cary, North Carolina).
Results
Phantom results
Phantom studies demonstrated substantial metal artifact reduction in PCD-CT TH images compared with the TL images (Figure 1, A-B). Further artifact reduction was observed using the tin filter (Figure 1, C-D). These observations were also reflected in quantitative measurements (Figure 2A), where PCD TH images (w/o tin: 30 HU; w/ tin: 21 HU) substantially reduced the median difference in water CT numbers between artifact-free and artifact-contaminated regions compared to those of PCD TL images (w/o tin: 89 HU; w/ tin: 43 HU). Importantly, the added tin filtration reduced the noise level of TH images by 26% (w/o tin: 43 HU; w/ tin: 32 HU), resulting in noise level close to that of TL images before adding the tin filtration (29 HU) (Figure 2B).
Figure 1.

Representative images of a 3D-printed vertebrae containing metallic pedicle screws acquired without (A,B) and with (C,D) use of a 0.4 mm tin filter. (A,C) = Low-energy threshold images that use the full energy spectrum. (B,D) High-energy threshold images that use only higher-energy photons. W/L = 400/40 HU
Figure 2.
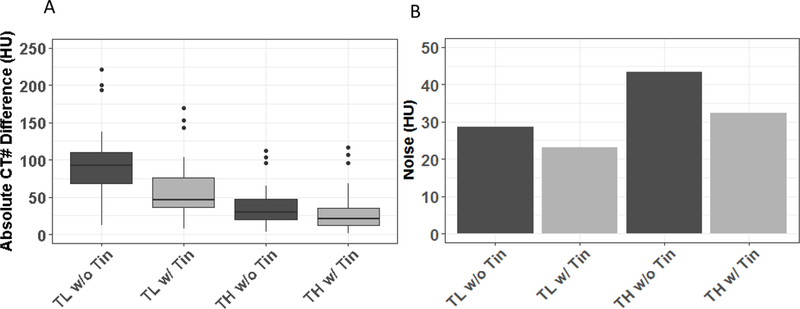
(A) Absolute values of the CT number difference between artifact-free and artifact-contaminated regions of interest. (B) Image noise in uniform regions of interest without metal artifacts. Data are provided for the low-energy threshold [25–140 keV] (TL) and high-energy threshold [75–140 keV] (TH) images, both without (dark grey) and with (light grey) use of the tin filter.
Patient image examples
Twenty patients with orthopedic metal implants (see Table, Supplemental Digital Content 2, which lists patients’ demographic data and information) were included in the study (13 females [65%]; age range, 25–80 years), including twelve spine (60%) [3 (15%) cervical spine and 9 (45%) lumbar spine], 3 (15%) shoulder, 3 (15%) wrist, 1 (5%) elbow, and 1 (5%) ankle cases.
Qualitative image rating
For evaluation of critical anatomic structures in the spine, there was a significant improvement (P<0.001) in the diagnostic image quality for every structure evaluated (Table 2). The central canal had the worst image quality scores using EID-CT (0.9 ±1.3), and showed the greatest improvement with the PCD-CT (3.3 ± 1.5, p<.0001). There was also improvement in scores for the neural foramina, nerve roots, prevertebral soft tissues, psoas muscle, cortex, trabeculae, and implant-trabecular interface on the PCD-CT TH images compared to the EID-CT (Figure 3). A similar trend was observed for extremity cases (shoulder, wrist, elbow, and ankle) when comparing critical anatomic structures, effects of artifacts and overall preference between PCD-CT TH and EID-CT images (Table 2; Figure 4). Statistical analysis was not performed for extremity cases due to the small sample size. For all anatomic regions combined, the overall visualization scores of the cortex, trabeculae, and implant-trabecular interface from PCD-CT TH images (4.4 ± 0.9, 4.4 ± 1.0 and 4.4 ± 1.0) were significantly higher (P<0.0001) than EID-CT images (3.3 ± 1.3, 3.3 ± 1.2 and 3.3 ± 1.6). This difference corresponds to an increase from low to medium confidence in diagnosis for the anatomic structures evaluated.
Table 2.
Comparison of the mean (standard deviation) score for comparing PCD and EID images. 0 = critical structures totally obscured, no structures identifiable; 1 = marked artifacts, questionable anatomic recognition of critical structures; 2 = faint anatomic recognition, no confidence in the ability to identify pathology; 3 = anatomic recognition with low confidence in diagnosis; 4 = anatomic recognition with medium confidence in diagnosis; 5 = anatomic recognition with high confidence in diagnosis). An average score of each task from all readers was calculated for each patient case and pairwise Wilcoxon signed rank was applied to calculate the P value for spine cases.
| Body Part | Structures | PCD-CT | EID-CT | P value |
|---|---|---|---|---|
|
Spine (N = 12) |
Central Canal | 3.3 (0.8) | 0.9 (1.1) | <.0001 |
| Neural Foramina | 4.3 (0.5) | 2.8 (1.1) | <.0001 | |
| Nerve Roots | 4.3 (0.6) | 2.8 (1.0) | <.0001 | |
| Prevertebral Soft Tissues | 3.9 (0.5) | 2.4 (0.5) | <.0001 | |
| Psoas Muscle (N = 9) | 4.7 (0.6) | 3.6 (1.0) | 0.0002 | |
| Cortex | 4.5 (0.4) | 3.2 (0.7) | <.0001 | |
| Trabeculae | 4.6 (0.5) | 3.1 (0.7) | <.0001 | |
| Implant Trabecular Interface | 4.5 (0.5) | 3.2 (0.7) | 0.0003 | |
| Shoulder (N = 3) | Glenohumeral Joint Space | 3.7 (1.5) | 4 (1.0) | NA |
| Humeral Head (N = 1) | 5 (0) | 5 (0) | NA | |
| Greater/Lesser Tuberosity | 4.1 (0.8) | 4 (0.9) | NA | |
| Coracoid Process | 4.0 (1.2) | 3.7 (0.6) | NA | |
| Acromion/Clavicle | 5 (0) | 5 (0) | NA | |
| Rotator Cuff Musculature | 3.4 (1.2) | 3.2 (1.6) | NA | |
| Scapula | 4.5 (0) | 4.0 (0.3) | NA | |
| Axilla (for nodes) | 3.4 (2.0) | 3.2 (1.8) | NA | |
| Cortex | 3.8 (0.6) | 3.1 (0.3) | NA | |
| Trabeculae | 3.6 (0.9) | 3.1 (0.2) | NA | |
| Implant Trabecular Interface | 4.1 (0.7) | 3.1 (0.5) | NA | |
|
Wrist (N = 3) |
Scaphoid | 5 (0) | 5 (0) | NA |
| Lunate | 5 (0) | 5 (0) | NA | |
| Other Carpal Bones | 5 (0) | 5 (0) | NA | |
| 1st CMC Joint | 5 (0) | 5 (0) | NA | |
| Distal Radioulnar Joint | 5 (0) | 4.8 (0.3) | NA | |
| Styloid Processes | 5 (0) | 5 (0) | NA | |
| Ulnar Head | 5 (0) | 5 (0) | NA | |
| Flexor Tendons | 4.4 (0.5) | 3.9 (1.5) | NA | |
| Cortex | 4.4 (0.3) | 3.9 (0.5) | NA | |
| Trabeculae | 4.4 (0.3) | 3.8 (0.5) | NA | |
| Implant Trabecular Interface | 4.4 (0.4) | 3.7 (0.8) | NA | |
|
Elbow (N = 1) |
Olecranon & Fossa | 5 | 5 | NA |
| Coronoid Process | 4.7 | 4 | NA | |
| Trochlear Notch | 5 | 5 | NA | |
| Trochlea & Capitellum | 4 | 3.3 | NA | |
| Medial/Lateral Epicondyles | 5 | 4.7 | NA | |
| Joint Spaces | 2.3 | 3.0 | NA | |
| Triceps Tendon | 5 | 5 | NA | |
| Cortex | 4.7 | 4.0 | NA | |
| Trabeculae | 4.7 | 4.3 | NA | |
| Implant Trabecular Interface | 4.3 | 3.3 | NA | |
|
Ankle (N = 1) |
Calcaneus/Achilles Tendon | 5 | 5 | NA |
| Medial/Lateral Malleolus | 4.7 | 4.3 | NA | |
| Tibiofibular/Tibiotalar/ Subtalar Joint | 4.7 | 4.3 | NA | |
| Talus | 4.7 | 4.3 | NA | |
| Navicular | 5 | 5 | NA | |
| Talocalcaneal/ Talonavicular Joint | 4.7 | 4.3 | NA | |
| Tarsal Bones | NA | 5 | NA | |
| Cortex | 4.3 | 3.0 | NA | |
| Trabeculae | 4 | 2.3 | NA | |
| Implant Trabecular Interface | 4 | 2.7 | NA | |
Figure 4.

69 -year-old male with left tibiotalar arthrodesis with lateral plate and screw fixation showing improved metal artifact reduction of the ankle with threshold high (TH) Sn140 kV PCD-CT. EID-CT 120 kV (A-B) and TH Sn 140 kV PCD-CT (C-D) are shown. The TH Sn 140 kV PCD-CT images (C-D) have reduced metal artifact compared to the EID-CT images (A-B) demonstrating improved visualization of the implant trabecular interface, cortex, trabecula, and soft tissue in the distal tibia near the tibiofibular joint. In this particular example, the readers mean evaluation scores for implant trabecular interface, cortex, and trabecula for TH Sn 140 kV PCD-CT (C-D) were 4.0, 4.3 and 4.0 vs. 2.7, 3.0, and 2.3 for EID-CT (A-B), respectively. A, C: soft tissue algorithm kernel; W/L = 400/40 HU. B, D: bone algorithm kernel; W/L = 1500/450 HU.
In side-by-side comparisons, the PCD-CT TH images were preferred by the majority of readers (≥ 2), with increased diagnostic confidence in 13 of all 20 patient cases (65%), preferred with no increase of diagnostic confidence in 6 cases (30%), and no preference/equivalent in 1 case (5%) when compared to the EID-CT images. More specifically, PCD-CT TH images were preferred with increased diagnostic confidence in 39 of 60 comparisons (65%, 20 cases x 3 reviewers), preferred with no increase of diagnostic confidence in 23% of comparisons (14/60), and no preference/equivalent in 8% of comparisons (5/60) (Figure 5). For only 3% (2/60) of all comparisons, the readers preferred the EID-CT images without increased diagnostic confidence. None of the EID-CT images was scored as preferred with increased diagnostic confidence.
Figure 5.
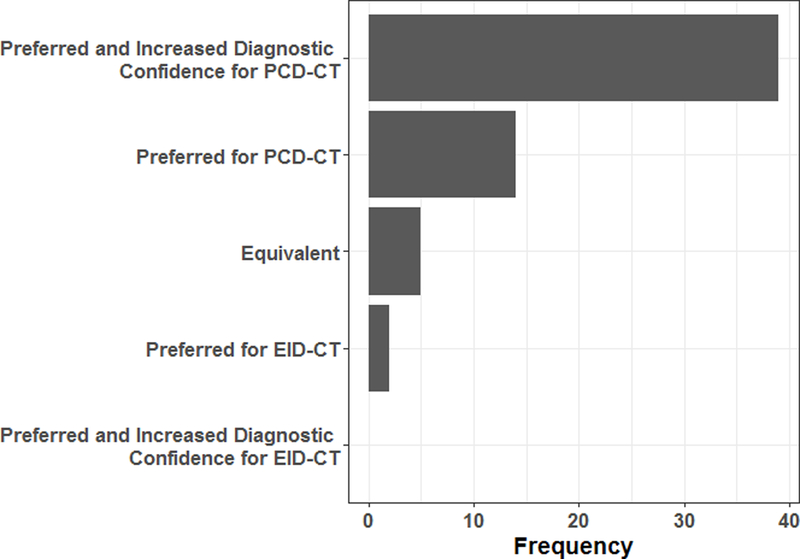
Summary of the preference and diagnostic confidence rating for the comparison of photon-counting-detector (PCD) CT high-energy threshold images and conventional energy integrating detector (EID) images.
Using PCD-CT TH images, there was less of an effect of metal artifacts on diagnosis compared to using EID-CT images (Table 3; significantly for all cases and for spine cases, P<0.0001). PCD-CT significantly reduced the ranking of the effect of artifacts on diagnosis from a mean of 2.6 for PCD to1.9 for EID for all anatomic locations (P<0.0001), and from 2.8 to 1.9 for spine, respectively (P<0.001). These results imply that artifacts appeared in both systems but with a statistically different impact on diagnosis. Artifacts in general impeded diagnosis (score 3) in EID images, while they had minimal effect on images (score 1) or degraded images without affecting diagnosis (score 2). A similar trend was observed when comparing artifacts effects for upper/lower extremity cases.
Table 3.
Comparison of overall effects of the metal artifacts on diagnosis between PCD and EID images. 1 = artifact has no/minimal effect on images, 2 = artifact degrades images without affecting diagnosis, 3 = artifact impedes diagnosis. An average score of each task from all readers was calculated for each patient case and pairwise Wilcoxon signed rank was applied to calculate the P value for all anatomical regions and spine cases.
| Body Part | PCD-CT | EID-CT | P Value |
|---|---|---|---|
| All Anatomic Locations | 1.9 (0.7) | 2.6 (0.6) | <.0001 |
| Spine | 1.9 (0.4) | 2.8 (0.3) | <.0001 |
| Shoulder | 2.1 (0.2) | 2.6 (0.2) | NA |
| Wrist | 1.6 (0.3) | 1.8 (0.3) | NA |
| Elbow | 2.0 | 2.0 | NA |
| Ankle | 1.7 | 2.3 | NA |
Quantitative image analysis
PCD-CT TH images demonstrated a smaller difference in the mean CT number (440 ± 182 HU) between artifact and non-artifact regions (Figure 6A) compared to EID-CT (539 ± 376 HU, P < 0.01). A significant decrease of the size of artifacts was also observed for PCD-CT TH compared to EID-CT images (0.45 ± 0.47 mm vs. 1.11 ± 1.42 mm, p < 0.0001) (Figure 6B). In addition, the noise level (Figure 7) in PCD-CT TH images (93 ± 41 HU) was significantly lower than EID-CT images (133 ± 92 HU, P < 0.05).
Figure 6.
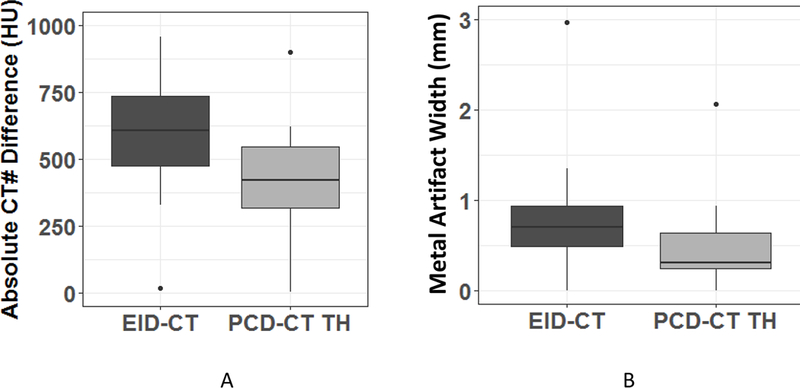
Quantitative comparison of the severity of metal artifacts between photon-counting-detector (PCD)-CT high-energy threshold images (TH) and energy-integrating-detector (EID)-CT images. (A) Distribution of the absolute values of the differences in mean CT numbers between region of interests in the artifact-containing region and fat adjacent to but outside of the artifact-containing region. (B) Distribution of the size of the metal artifacts across patients for each imaging protocol.
Figure 7.
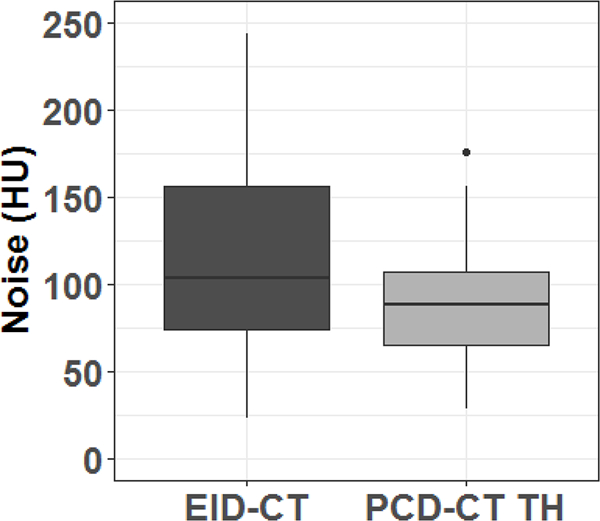
Comparison of noise (standard deviation of CT numbers in a uniform region of interest in fat) between photon-counting-detector (PCD)-CT high-energy threshold images (TH) and energy-integrating-detector (EID)-CT images.
Discussion
In this study, we proposed a new concept for metal artifact reduction using PCD-CT technology. Both subjective and objective assessments of image quality demonstrated that TH images obtained using a whole-body PCD-CT system and 0.4 mm of tin filtration substantially improved the diagnostic confidence and had higher radiation dose efficiency (lower noise for the same dose) when directly compared to clinical EID-CT systems. To the best of our knowledge, this is the first study exploring the impact of the multi-energy capability of PCD-CT technology on metal artifacts.
TH images acquired on a PCD-CT are intrinsically more immune to metal artifacts because they exclude low-energy photons, which are primarily responsible for beam hardening and photon starvation effects 3; however, these TH images have an increased noise level because they do not use all delivered photons 21, 24. Our study mitigated this noise increase by using tin filtration on the PCD-CT to move the majority of photons into the TH energy bin. Thus by applying tin filtration, the effective energy in TH images was increased, resulting in a substantial decrease of artifact severity. Additionally, after tin filtration, the photons in the beam occupied a higher percentage of the TH energy bin, leading to improved dose efficiency for the TH images. Our phantom data demonstrated that the filtration reduced noise by 26% for TH images, achieving a level that was comparable to that of the TL “full spectrum” images without tin filtration. Moreover, our patient comparison results showed that the noise level of the proposed tin-filtered TH images on PCD-CT was ~12% lower than the clinical EID images, yielding a 23% improvement in dose efficiency. Another possible contribution to lower noise level in the tin-filtered TH images compared to clinical EID images could be the immunity of PCD-CT to electronic noise 23, 25.
The proposed approach using tin filtration and the TH images to reduce metal artifacts is compatible with projection- or image-based MAR strategies, which could lead to further improvements in image quality. For instance, iMAR 10, 15 could be adapted and applied to the TH projection data acquired with the tin filtration. In addition, PCD technology generates multi-energy CT data, allowing for the creation of virtual monoenergetic images (VMIs) for a single acquisition 32. VMIs created using dual-energy CT have been reported to reduce metal-related artifacts 33. For exams with extreme dense metal implants, the use of tin filtration could be combined with iMAR and VMI techniques on PCD-CT to achieve the best possible metal artifact reduction 34.
This study was performed on a research scanner from a specific manufacturer. However, the proposed method is not manufacturer specific; it could be applied to any PCD-CT system with energy resolving capabilities. Use of a filter to harden the beam may offer some level of metal artifact reduction in EID scanners as well, although the final image would still retain a substantial number of low-energy photons, and hence metal artifacts, due to the inability of EID CT to generate an image from only higher-energy photons. In addition, although a 0.4 mm tin filter was used in this study, other filtration materials, thicknesses and material types could also be used. Future investigations to determine the optimal configuration of filter material and thickness for metal artifact reduction would be of interest.
There are some limitations in this study. First, the sample size for upper/lower extremities is relatively small. Future studies should include more patients and with a wider variety of implant materials and designs. Second, the tube potential used for the EID lumbar spine exams was 120 kV, per clinical protocol. For PCD-CT, all patients were scanned at 140 kV, as, based on the phantom studies, it provided the best image quality. For the lumbar spine exams, this created a difference between the EID- and PCD-CT exams that potentially could be a factor contributing to the observed differences in image quality assessment. However, as shown in the other exam types, where 140 kV was used for both EID- and PCD-CT systems, the proposed method still provided better image quality. Third, the soft tissue contrast was slightly changed with the filtration. Although this small alteration did not affect the diagnosis for the spine and extremity cases, the impact on other clinical applications needs to be evaluated. Finally, we did not include an evaluation of currently-available post-processing metal artifact reduction methods (e.g., iMAR, Siemens Healthcare) in this study because iMAR is not currently available on either the Sensation 64 or research PCD-CT scanners used in this study. However, the PCD-CT metal artifact reduction method described in this study is fully compatible with currently-available post-processing methods to reduce metal artifacts; the software is simply not available at this time. Rather, the primary point of our work was to demonstrate the inherently better data that can be acquired with our technique – prior to any post-processing. This is because improved source data, in this case by the described method, will produce better results for any subsequent image processing. Future studies should investigate the improvement in image quality obtained by combining the improved data obtained using tin filtration on the PCD-CT with the iMAR post-processing (once available) and then compare the composite performance to EID-CT using iMAR.
This report showed the first clinical results using PCD-CT technology for patients with metal implants. The high-energy threshold PCD-CT images acquired with tin filtration demonstrated a significant decrease in artifact size and severity, an increase in diagnostic confidence, and an increase in dose efficiency compared to conventional EID-CT systems.
Supplementary Material
Acknowledgments
Research reported in this article was supported by the National Institutes of Health under award number BRP 016966 and C06 RR018898. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Notification of Conflicts of Interest: Drs. McCollough and Fletcher receive industry funding from Siemens Healthcare. For the remaining authors none were declared.
References
- 1.Wood KB, Fritzell P, Dettori JR, et al. Effectiveness of spinal fusion versus structured rehabilitation in chronic low back pain patients with and without isthmic spondylolisthesis: a systematic review. Spine (Phila Pa 1976). 2011;36(21 Suppl):S110–9. [DOI] [PubMed] [Google Scholar]
- 2.Cheng JS, Lee MJ, Massicotte E, et al. Clinical guidelines and payer policies on fusion for the treatment of chronic low back pain. Spine (Phila Pa 1976). 2011;36(21 Suppl):S144–63. [DOI] [PubMed] [Google Scholar]
- 3.Barrett JF, Keat N. Artifacts in CT: Recognition and avoidance. Radiographics. 2004;24(6):1679–91. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter KA, Parr JA, Choplin RH, Capello WN. Multichannel CT Imaging of Orthopedic Hardware and Implants. Semin Musculoskelet Radiol. 2006;10(1):86–97. [DOI] [PubMed] [Google Scholar]
- 5.Glover GH, Pelc NJ. An algorithm for the reduction of metal clip artifacts in CT reconstructions. Med Phys. 1981;8(6):799–807. [DOI] [PubMed] [Google Scholar]
- 6.Hinderling T, Ruegsegger P, Anliker M, Dietschi C. Computed tomography reconstruction from hollow projections: an application to in vivo evaluation of artificial hip joints. J Comput Assist Tomogr. 1979;3(1):52–7. [PubMed] [Google Scholar]
- 7.Morsbach F, Bickelhaupt S, Wanner GA, et al. Reduction of metal artifacts from hip prostheses on CT images of the pelvis: value of iterative reconstructions. Radiology. 2013;268(1):237–44. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Snyder DL, O’Sullivan JA, Vannier MW. Iterative deblurring for CT metal artifact reduction. IEEE Trans Med Imaging. 1996;15(5):657–64. [DOI] [PubMed] [Google Scholar]
- 9.Zhao SY, Roberston DD, Wang G, et al. X-ray CT metal artifact reduction using wavelets: Arm application for imaging total hip prostheses. Ieee Transactions on Medical Imaging. 2000;19(12):1238–47. [DOI] [PubMed] [Google Scholar]
- 10.Subhas N, Primak AN, Obuchowski NA, et al. Iterative metal artifact reduction: evaluation and optimization of technique. Skeletal Radiol. 2014;43(12):1729–35. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Li H, Mueller J, et al. Metal artifact reduction from reformatted projections for hip prostheses in multislice helical computed tomography: techniques and initial clinical results. Invest Radiol. 2009;44(11):691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasirudin RA, Mei K, Penchev P, et al. Reduction of metal artifact in single photon-counting computed tomography by spectral-driven iterative reconstruction technique. PLoS One. 2015;10(5):e0124831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Noel C, Chen H, et al. Clinical evaluation of a commercial orthopedic metal artifact reduction tool for CT simulations in radiation therapy. Med Phys. 2012;39(12):7507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagenaar D, van der Graaf ER, van der Schaaf A, Greuter MJ. Quantitative comparison of commercial and non-commercial metal artifact reduction techniques in computed tomography. PLoS One. 2015;10(6):e0127932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aissa J, Boos J, Schleich C, et al. Metal Artifact Reduction in Computed Tomography After Deep Brain Stimulation Electrode Placement Using Iterative Reconstructions. Invest Radiol. 2017;52(1):18–22. [DOI] [PubMed] [Google Scholar]
- 16.Lell MM, Meyer E, Kuefner MA, et al. Normalized metal artifact reduction in head and neck computed tomography. Invest Radiol. 2012;47(7):415–21. [DOI] [PubMed] [Google Scholar]
- 17.De Man B, Nuyts J, Dupont P, et al. An iterative maximum-likelihood polychromatic algorithm for CT. IEEE Trans Med Imaging. 2001;20(10):999–1008. [DOI] [PubMed] [Google Scholar]
- 18.Lee YH, Park KK, Song HT, et al. Metal artefact reduction in gemstone spectral imaging dual-energy CT with and without metal artefact reduction software. Eur Radiol. 2012;22(6):1331–40. [DOI] [PubMed] [Google Scholar]
- 19.Mangold S, Gatidis S, Luz O, et al. Single-source dual-energy computed tomography: use of monoenergetic extrapolation for a reduction of metal artifacts. Invest Radiol. 2014;49(12):788–93. [DOI] [PubMed] [Google Scholar]
- 20.Kotsenas AL, Michalak GJ, DeLone DR, et al. CT Metal Artifact Reduction in the Spine: Can an Iterative Reconstruction Technique Improve Visualization? AJNR Am J Neuroradiol. 2015;36(11):2184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Z, Leng S, Jorgensen SM, et al. Evaluation of conventional imaging performance in a research whole-body CT system with a photon-counting detector array. Phys Med Biol. 2016;61(4):1572–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pourmorteza A, Symons R, Sandfort V, et al. Abdominal Imaging with Contrast-enhanced Photon-counting CT: First Human Experience. Radiology. 2016;279(1):239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutjahr R, Halaweish AF, Yu Z, et al. Human Imaging With Photon Counting-Based Computed Tomography at Clinical Dose Levels: Contrast-to-Noise Ratio and Cadaver Studies. Invest Radiol. 2016;51(7):421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pourmorteza A, Symons R, Reich DS, et al. Photon-Counting CT of the Brain: In Vivo Human Results and Image-Quality Assessment. AJNR Am J Neuroradiol. 2017;38(12):2257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannil M, Hickethier T, von Spiczak J, et al. Photon-Counting CT: High-Resolution Imaging of Coronary Stents. Invest Radiol. 2018;53(3):143–9. [DOI] [PubMed] [Google Scholar]
- 26.Symons R, Reich DS, Bagheri M, et al. Photon-Counting Computed Tomography for Vascular Imaging of the Head and Neck: First In Vivo Human Results. Invest Radiol. 2018;53(3):135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leng S, Rajendran K, Gong H, et al. 150-mum Spatial Resolution Using Photon-Counting Detector Computed Tomography Technology: Technical Performance and First Patient Images. Invest Radiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Spiczak J, Mannil M, Peters B, et al. Photon Counting Computed Tomography With Dedicated Sharp Convolution Kernels: Tapping the Potential of a New Technology for Stent Imaging. Invest Radiol. 2018;53(8):486–94. [DOI] [PubMed] [Google Scholar]
- 29.Kanal KM, Butler PF, Sengupta D, et al. U.S. Diagnostic Reference Levels and Achievable Doses for 10 Adult CT Examinations. Radiology. 2017;284(1):120–33. [DOI] [PubMed] [Google Scholar]
- 30.Primak AN, Giraldo JC, Eusemann CD, et al. Dual-source dual-energy CT with additional tin filtration: Dose and image quality evaluation in phantoms and in vivo. AJR Am J Roentgenol. 2010;195(5):1164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norweck JT, Seibert JA, Andriole KP, et al. ACR-AAPM-SIIM technical standard for electronic practice of medical imaging. J Digit Imaging. 2013;26(1):38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leng S, Zhou W, Yu Z, et al. Spectral performance of a whole-body research photon counting detector CT: quantitative accuracy in derived image sets. Phys Med Biol. 2017;62(17):7216–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou C, Zhao YE, Luo S, et al. Monoenergetic imaging of dual-energy CT reduces artifacts from implanted metal orthopedic devices in patients with factures. Acad Radiol. 2011;18(10):1252–7. [DOI] [PubMed] [Google Scholar]
- 34.Grosse Hokamp N, Hellerbach A, Gierich A, et al. Reduction of Artifacts Caused by Deep Brain Stimulating Electrodes in Cranial Computed Tomography Imaging by Means of Virtual Monoenergetic Images, Metal Artifact Reduction Algorithms, and Their Combination. Invest Radiol. 2018;53(7):424–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


