Abstract
A Negishi cross-coupling of alkyl pyridinium salts and alkylzinc halides has been developed. This is the first example of alkyl–alkyl bond formation via cross-coupling of an alkyl amine derivative with an unactivated alkyl group, and allows both primary and secondary alkyl pyridinium salts to react with primary alkylzinc halides with high functional group tolerance. When combined with formation of the pyridinium salts from primary amines, this method enables the non-canonical transformation of NH2 groups into a wide range of alkyl substituents with broad functional group tolerance.
Graphical Abstract

Alkyl amines are abundant molecules ranging from simple starting materials to complex natural products and drug candidates.1 As an example of their prevalence, a search of Pfizer’s internal chemical store revealed over 47,000 alkyl primary amines vs. about 28,000 primary and secondary alkyl halides.2 In addition, the use of alkyl amines as intermediates is advantageous, because they can be prepared directly and convergently with simultaneous formation of the amine and C–C bonds via classic reactions, such as Mannich, Petasis, Strecker, and others.3 Due to their ubiquity, methods that allow alkyl amines to be converted into electrophiles for cross-coupling reactions present an exciting opportunity for development. However, the utilization of amine-derived alkyl electrophiles remains limited with cross-couplings largely restricted to alkyl amine derivatives with electronic or strain activation of the C(sp3)–N bond (Scheme 1A).4,5,6 We recently overcame this limitation by developing a Suzuki–Miyaura arylation of Katritzky pyridinium salts, which are easily and selectively prepared from primary alkyl amines (Scheme 1B).7,6m, 6n,5b, 8 This reaction was the first cross-coupling of an alkyl amine derivative with an unactivated alkyl group. Recognizing that our proposed alkyl radical intermediate could also be formed via photoredox catalysis, Glorius, Gryko, Aggarwal, Li and Shi then developed Minisci arylation, alkynylation, and borylations.9 These exciting reports demonstrate the potential of alkyl pyridinium salts in synthesis, and offer avenues to convert ubiquitous NH2 groups into C(sp2) and C(sp) substituents. However, no current methods enable C(sp3)–C(sp3) formation via cleavage of these unactivated alkyl pyridinium salts.10
Scheme 1.

Alkyl amine derivatives in cross-coupling reactions.
The prevalence of C(sp3)–C(sp3) bonds in bioactive molecules has led to intense efforts to develop methods to incorporate fully saturated C–C bonds in complex products.11 In particular, Negishi cross-couplings have emerged as a reliable and functional group tolerant method.6a, 6d, 12 Motivated by the myriad advantages of alkyl amine derivatives as alkyl electrophiles, we envisioned a Negishi cross-coupling for the generation of C(sp3)–C(sp3) bonds from alkyl pyridinium salts (Scheme 1C). However, despite the precedent with other alkyl electrophiles, we were concerned that competitive nucleophilic addition of the organozinc to the pyridinium ring and/or deprotonation of the acidic α-proton of the alkyl pyridinium salts would prevent the desired cross-coupling. Overcoming these challenges, we have developed the first example of alkyl–alkyl bond formation via cross-coupling of an alkyl amine derivative with an unactivated alkyl group. This method allows both primary and secondary alkyl pyridinium salts to react with primary alkylzinc halides with high functional group tolerance. In combination with efficient pyridinium formation, this reaction enables the non-canonical transformation of amino groups into alkyl substituents, providing unprecedented entry to complex, saturated organic frameworks via versatile and attractive amine intermediates.
We selected the cross-coupling of phenethyl pyridinium salt 1a and commercially available (2-(1,3-dioxolan-2-yl)ethyl)zinc bromide for our initial studies. We prioritized the use of alkyl zinc halides, as opposed to dialkyl zinc reagents, due to their greater commercial availability, higher functional group tolerance, and ease of handling.13 Using bathophenanthroline (BPhen), the optimal ligand for our Suzuki–Miyaura arylation, resulted in <20% yield. However, with NiCl2·DME/4,4’,4’’-tri-tert-butyl terpyridine (ttbtpy) as catalyst and DMA as a polar co-solvent, 68% yield was observed (Table 1, entry 1).14 Increasing the amount of organozinc halide further improved the yield (entry 2); however, more than 1.6 equivalents led to decreased product formation (see Supporting Information). The use of other redox non-innocent ligands also resulted in lower yields (entries 3–6). To maximize the practicality, less expensive NiII sources and lower catalyst loadings were examined. Although NiCl2·6H2O was ineffective (entry 7), Ni(acac)2·xH2O gave slightly higher yields than NiCl2·DME (entry 8). Lowering the Ni(acac)2·xH2O and ttbtpy loading to 5 and 6 mol %, furnished a similar yield (entry 9). Control experiments confirmed that both nickel and ligand are required (entries 10 and 11).
Table 1.
Reaction Optimizationa
 | |||
|---|---|---|---|
| entry | [Ni] | ligand | yield (%)b |
| 1c | NiCl2·DME | ttbtpy | 68 |
| 2 | NiCl2·DME | ttbtpy | 75 |
| 3 | NiCl2·DME | tpy | 47 |
| 4 | NiCl2·DME | phen | 14 |
| 5 | NiCl2·DME | dtbbpy | 15 |
| 6 | NiCl2·DME | 1-bpp | 49 |
| 7 | NiCl2·6H2O | ttbtpy | 20 |
| 8 | Ni(acac)2·xH2O | ttbtpy | 81 |
| 9d | Ni(acac)2·xH2O | ttbtpy | 80 |
| 10 | none | ttbtpy | 3 |
| 11 | Ni(acac)2·xH2O | none | 9 |
 | |||
| 12 | NiCl2·DME | ttbtpy | 18 |
| 13 | NiCl2·DME | tpy | 68 |
| 14 | NiCl2·DME | 1-bpp | 83 |
Conditions: Pyridinium salt 1a (0.1 mmol), [Ni] (10 mol %), ligand (12 mol %), AlkZnBr (1.6 equiv), THF/DMA (2:1, 0.2 M), 60 °C, 16–22 h, unless noted otherwise.
Determined by 1H NMR spectroscopic analysis using 1,3,5-trimethoxybenzene as internal standard.
AlkZnBr (1.4 equiv).
5 mol % [Ni], 6 mol % ligand.
Although ttbtpy proved effective for primary alkyl pyridinium salts, only 18% yield was observed with secondary alkyl pyridinium salt 1b (Table 1, entry 12). However, 2,6-bis(N-pyrazolyl)pyridine (1-bpp) promoted the cross-coupling with high efficiency (entry 14). This ligand has been used previously in Negishi cross-couplings, including those of bulky alkylzinc iodides.12f, 12g Notably, tpy also bested the more hindered ttbtpy (entries 12 vs. 13), suggesting that steric hindrance of the ligand may be primarily responsible for the success of 1-bpp.12g
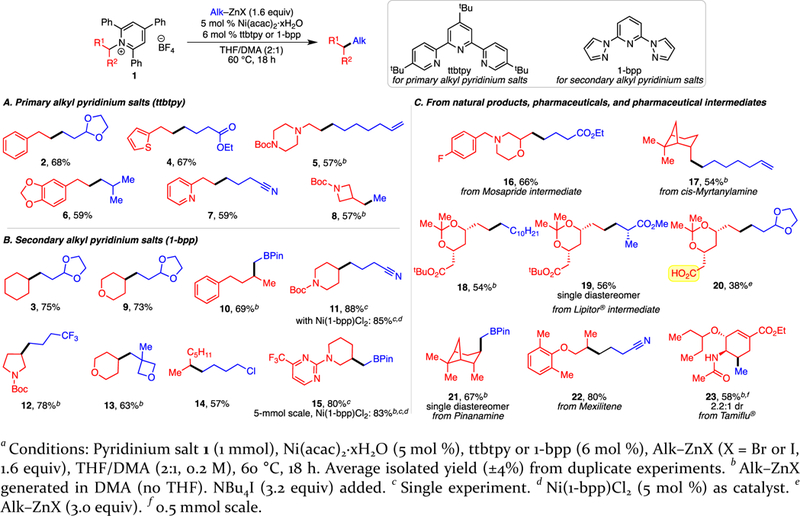
Wide scope of primary and secondary alkyl pyridinium salts was observed (Scheme 2). Aryl and heteroaryl groups were tolerated, including thiophene (4), benzodioxole (6), pyridine (7), and pyrimidine (15). Products with saturated heterocycles, such as piperazine 5, morpholine 16, pyrans 9 and 13, piperidines 11 and 15, pyrrolidine 12, and strained azetidine 8, also formed in good yields. Highlighting the potential for late-stage functionalization, pyridinium salts of several drugs, pharmaceutical intermediates, and natural products underwent alkylation. Alkylations of the pyridinium salts of amine intermediates in the synthesis of the gastrointestinal drug Mosapride (16) and Lipitor (18–20) were effective.15 A single diastereomer of 19 was observed, indicating that stereocenters bearing acidic protons are not epimerized. The unprotected carboxylic acid of 20 is tolerated in both the pyridinium formation and cross-coupling, demonstrating orthogonality of the amine and carboxylate groups, either of which can be used to generate alkyl radicals.12h, 16 The pyridinium salts of natural cis-myrtanylamine (17), pinanamine (21), the anti-arrhythmic drug Mexiletine (22) and antiviral Tamiflu (23) also coupled well.17 The cross-coupling of the Tamiflu pyridinium salt demonstrates that a high degree of steric hindrance and chemical complexity is tolerated. However, this method is limited to primary and secondary alkyl 2,4,6-triphenyl pyridinium salts, because we cannot yet form them from unconstrained tertiary alkyl amines.9e To simplify set-up, a single-component catalyst, Ni(1-bpp)Cl2, can be used (see 11, 15).
Scheme 2.
Reaction Scopea
Both commercial and prepared primary alkyl zinc halides proved effective (Scheme 2). We used Hou’s method to generate organozinc reagents due to its high functional group tolerance.18 In line with findings by Organ,19 a salt additive was crucial for maintaining high reactivity of non-commercial organozinc reagents, with NBu4I as the optimal additive.20 Homemade organozinc halides were generated in DMA (no THF present). In terms of steric hindrance, β-branching is tolerated (6, 19), including quaternary carbons (13). However, secondary and tertiary alkylzinc halides do not undergo the desired cross-coupling. Instead, a non-nickel-catalyzed addition to the 4-position of the pyridinium ring occurs.21 Regarding functional group tolerance, various groups can be incorporated in the alkylzinc halide, including acetal (2, 3, 9, 20), ester (4, 16, 19), alkene (5, 17), nitrile (7, 11, 22), trifluoromethyl (12), and oxetane (13). Notably, the pyridinium group couples preferentially in the presence of an alkyl chloride (14). Methylzinc iodide is also effective (8, 23). When coupled with formation of the pyridinium salt, this strategy allows NH2 to be replaced with a steric isostere CH3, offering opportunities for efficient structure-activity relationship studies.22 Finally, synthetically versatile α-methylpinacolboronate (CH2Bpin) can also be installed (10, 15, 21). To our knowledge, this is the first example utilizing α-methylpinacolboronate zinc bromide in a Negishi cross-coupling.23 Product 15 is formed in 83% yield on a 5-mmol scale with the single-component catalyst. Further, we demonstrated the versatility of the newly installed Bpin by converting it to an alcohol (22),6g bromide (23),24 and thiophene (24, Scheme 3).25
Scheme 3.

Elaboration of Boronic Ester
To facilitate use of this chemistry in parallel medicinal chemistry and other synthesis efforts, we developed conditions for the one-pot transformation of amines directly to the alkylated products. By increasing the equivalents of alkylzinc halide, similar yields were obtained in this one-pot procedure as the two-step method (Scheme 4). Notably, these conditions are not sequential addition; primary amine and pyrylium tetrafluoroborate are simply added in place of the pyridinium salt under the previous conditions.
Scheme 4.

One-pot activation and cross-coupling
In analogy to Negishi cross-couplings of other alkyl electrophiles, our arylation of alkyl pyridinium salts,7, 12f, 12g and other reactions of alkyl pyridinium salts,9a, 9b, 26 we hypothesize that this reaction proceeds through single-electron transfer (SET) to the alkyl pyridinium salt from a Ni(I) intermediate or a Ni(II) cation with a reduced ligand.12g Fragmentation of the resulting neutral pyridyl radical then gives an alkyl radical, which recombines with a Ni(II) intermediate to then deliver the product. Consistent with an alkyl radical intermediate, we observe ring-opened 30 in the reaction of cyclopropylmethyl pyridinium salt and TEMPO-adduct 31 when TEMPO is added (Scheme 5). No cross-coupled product 2 is observed upon addition of TEMPO.
Scheme 5.

Support for Alkyl Radical Intermediate
In summary, we have developed the first example of an alkyl–alkyl cross-coupling of alkyl amine derivatives with unactivated alkyl groups, specifically alkyl pyridinium salts. When combined with chemoselective pyridinium formation from primary amines, this Negishi reaction provides the ability to convert NH2 into a range of alkyl groups with broad functional group tolerance. The reaction appears to proceed via an alkyl radical intermediate, likely generated after SET from a Ni species to the pyridinium cation. Future efforts will expand the scope of alkyl groups and pursue detailed mechanistic understanding.
Supplementary Material
ACKNOWLEDGMENT
We thank NIH (R01 GM111820) and University of Delaware (UD) for a University Graduate Fellowship (C.H.B.). We thank Drs. Michelle Garnsey, Joe Tucker, and Brian Boscoe for helpful discussions. Data were acquired at UD on instruments obtained with assistance of NSF and NIH funding (NSF CHE0421224, CHE1229234, CHE0840401, and CHE1048367; NIH P20 GM104316, P20 GM103541, and S10 OD016267).
Footnotes
Notes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information.
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details and data (PDF)
REFERENCES
- 1.(a) Ruiz-Castillo P; Buchwald SL Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev 2016, 116, 12564–12649; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) McGrath NA; Brichacek M; Njardarson JT A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ 2010, 87, 1348–1349; [Google Scholar]; (c) Liu Y; Ge H Site-selective C–H arylation of primary aliphatic amines enabled by a catalytic transient directing group. Nat. Chem 2017, 9, 26–32. [Google Scholar]
- 2.Garnsey MR, Pfizer Worldwide Research and Development, 2018.
- 3.(a) Lawrence SA, Amines: Synthesis, Properties and Applications Cambridge University Press: New York, NY, 2004; [Google Scholar]; (b) Nugent TC, Chiral Amine Synthesis Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2010. [Google Scholar]
- 4.Ouyang K; Hao W; Zhang WX; Xi Z Transition-Metal-Catalyzed Cleavage of C-N Single Bonds. Chem. Rev 2015, 115, 12045–12090. [DOI] [PubMed] [Google Scholar]
- 5.(a) For C(sp2)–N bond activation, see:Blakey S; MacMillan D The first Suzuki cross-couplings of aryltrimethylammonium salts. J. Am. Chem. Soc 2003, 125, 6046–6047; [DOI] [PubMed] [Google Scholar]; (b) Buszek KR; Brown N N-Binylpyridinium and -ammonium Tetrafluoroborate Salts: New Electrophilic Coupling Partners for Pd(0)-Catalyzed Suzuki Cross-Coupling Reactions. Org. Lett 2007, 9, 707–710; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hie L; Baker EL; Anthony SM; Desrosiers J-N; Senanayake C; Garg NK Nickel-Catalyzed Esterification of Aliphatic Amides. Angew. Chem., Int. Ed 2016, 55, 15129–15132; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Meng G; Szostak M Rhodium-Catalyzed C–H Bond Functionalization with Amides by Double C–H/C–N Bond Activation. Org. Lett 2016, 18, 796–799; [DOI] [PubMed] [Google Scholar]; (e) Peng C; Wang Y; Wang J Palladium-Catalyzed Cross-Coupling of alpha-Diazocarbonyl Compounds with Arylboronic Acids. J. Am. Chem. Soc 2008, 130, 1566–1567; [DOI] [PubMed] [Google Scholar]; (f) Shi S; Meng G; Szostak M Synthesis of Biaryls through Nickel-Catalyzed Suzuki–Miyaura Coupling of Amides by Carbon–Nitrogen Bond Cleavage. Angew. Chem., Int. Ed 2016, 55, 6959–6963; [DOI] [PubMed] [Google Scholar]; (g) Simmons BJ; Weires NA; Dander JE; Garg NK Nickel-Catalyzed Alkylation of Amide Derivatives. ACS Catal 2016, 6, 3176–3179; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Tobisu M; Nakamura K; Chatani N Nickel-Catalyzed Reductive and Borylative Cleavage of Aromatic Carbon–Nitrogen Bonds in N-Aryl Amides and Carbamates. J. Am. Chem. Soc 2014, 136, 5587–5590; [DOI] [PubMed] [Google Scholar]; (i) Walker JA; Vickerman KL; Humke JN; Stanley LM Ni-Catalyzed Alkene Carboacylation via Amide C–N Bond Activation. J. Am. Chem. Soc 2017, 139, 10228–10231; [DOI] [PubMed] [Google Scholar]; (j) Weires NA; Baker EL; Garg NK Nickel-catalysed Suzuki–Miyaura coupling of amides. Nat. Chem 2016, 8, 75–79; [DOI] [PubMed] [Google Scholar]; (k) Wu G; Deng Y; Wu C; Zhang Y; Wang J Synthesis of alpha-aryl esters and nitriles: deaminative coupling of alpha-aminoesters and alpha-aminoacetonitriles with arylboronic acids. Angew. Chem., Int. Ed 2014, 53, 10510–10514. [DOI] [PubMed] [Google Scholar]
- 6.(a) For electronically or strain-activated alkyl groups, see:Huang C-Y; Doyle AG Nickel-Catalyzed Negishi Alkylations of Styrenyl Aziridines. J. Am. Chem. Soc 2012, 134, 9541–9544; [DOI] [PubMed] [Google Scholar]; (b) Li M-B; Wang Y; Tian S-K Regioselective and Stereospecific Cross-Coupling of Primary Allylic Amines with Boronic Acids and Boronates through Palladium-Catalyzed C–N Bond Cleavage. Angew. Chem., Int. Ed 2012, 51, 2968–2971; [DOI] [PubMed] [Google Scholar]; (c) Maity P; Shacklady-McAtee DM; Yap GPA; Sirianni ER; Watson MP Nickel-Catalyzed Cross Couplings of Benzylic Ammonium Salts and Boronic Acids: Stereospecific Formation of Diarylethanes via C–N Bond Activation. J. Am. Chem. Soc 2013, 135, 280–285; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jensen KL; Standley EA; Jamison TF Highly Regioselective Nickel-Catalyzed Cross-Coupling of N-Tosylaziridines and Alkylzinc Reagents. J. Am. Chem. Soc 2014, 136, 11145–11152; [DOI] [PubMed] [Google Scholar]; (e) Shacklady-McAtee DM; Roberts KM; Basch CH; Song Y-G; Watson MP A general, simple catalyst for enantiospecific cross couplings of benzylic ammonium triflates and boronic acids: no phosphine ligand required. Tetrahedron 2014, 70, 4257–4263; [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhang H; Hagihara S; Itami K Making Dimethylamino a Transformable Directing Group by Nickel-Catalyzed C–N Borylation. Chem. Eur. J 2015, 21, 16796–16800; [DOI] [PubMed] [Google Scholar]; (g) Basch CH; Cobb KM; Watson MP Nickel-Catalyzed Borylation of Benzylic Ammonium Salts: Stereospecific Synthesis of Enantioenriched Benzylic Boronates. Org. Lett 2016, 18, 136–139; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Hu J; Sun H; Cai W; Pu X; Zhang Y; Shi Z Nickel-Catalyzed Borylation of Aryl- and Benzyltrimethylammonium Salts via C-N Bond Cleavage. J. Org. Chem 2016, 81, 14–24; [DOI] [PubMed] [Google Scholar]; (i) Moragas T; Gaydou M; Martin R Nickel-Catalyzed Carboxylation of Benzylic C−N Bonds with CO2. Angew. Chem., Int. Ed 2016, 55, 5053–5057; [DOI] [PubMed] [Google Scholar]; (j) Yi Y-Q-Q; Yang W-C; Zhai D-D; Zhang X-Y; Li S-Q; Guan B-T Nickel-catalyzed C-N bond reduction of aromatic and benzylic quaternary ammonium triflates. Chem. Commun 2016, 52, 10894–10897; [DOI] [PubMed] [Google Scholar]; (k) Gui Y; Tian S-K Stereospecific Nucleophilic Substitution of Enantioenriched Tertiary Benzylic Amines via in Situ Activation with Benzyne. Org. Lett 2017, 19, 1554–1557; [DOI] [PubMed] [Google Scholar]; (l) Guisán-Ceinos M; Martín-Heras V; Tortosa M Regio- and Stereospecific Copper-Catalyzed Substitution Reaction of Propargylic Ammonium Salts with Aryl Grignard Reagents. J. Am. Chem. Soc 2017, 139, 8448–8451; [DOI] [PubMed] [Google Scholar]; (m) Liao J; Guan W; Boscoe BP; Tucker JW; Tomlin JW; Garnsey MR; Watson MP Transforming Benzylic Amines into Diarylmethanes: Cross-Couplings of Benzylic Pyridinium Salts via C–N Bond Activation. Org. Lett 2018, 20, 3030–3033; [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Guan W; Liao J; Watson MP Vinylation of Benzylic Amines via C–N Bond Functionalization of Benzylic Pyridinium Salts. Synthesis 2018, 50, 3231–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basch CH; Liao J; Xu JY; Piane JJ; Watson MP Harnessing Alkyl Amines as Electrophiles for Nickel-Catalyzed Cross Couplings via C-N Bond Activation. J. Am. Chem. Soc 2017, 139, 5313–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Bapat JB; Blade RJ; Boulton AJ; Epsztajn J; Katrizky AR; Lewis J; Molina-Buendia P; Nie P-L; Ramsden CA Pyridines as Leaving Groups in Synthetic Transformations: Nucleophilic Displacements of Amino Groups, and Novel Preparations of Nitriles and Isocyanates. Tetrahedron Lett 1976, 31, 2691–2694; [Google Scholar]; (b) Katrizky AR; Marson CM Pyrylium Mediated Transformations of Primary Amino Groups into Other Functional Groups. Angew. Chem., Int. Ed 1984, 23, 420–429; [Google Scholar]; (c) Moser D; Duan Y; Wang F; Ma Y; O’Neill Matthew J; Cornella J Selective Functionalization of Aminoheterocycles by a Pyrylium Salt. Angew. Chem., Int. Ed 2018, 57, 11035–11039; [DOI] [PubMed] [Google Scholar]; (d) Sowmiah S; Esperança JMSS; Rebelo LPN; Afonso CAM Pyridinium salts: from synthesis to reactivity and applications. Org. Chem. Front 2018, 5, 453–493. [Google Scholar]
- 9.(a) Klauck FJR; James MJ; Glorius F Deaminative Strategy for the Visible‐Light‐Mediated Generation of Alkyl Radicals. Angew. Chem., Int. Ed 2017, 56, 12336–12339; [DOI] [PubMed] [Google Scholar]; (b) Ociepa M; Turkowska J; Gryko D Redox-Activated Amines in C(sp3)–C(sp) and C(sp3)–C(sp2) Bond Formation Enabled by Metal-Free Photoredox Catalysis. ACS Catal 2018, 8, 11362–11367; [Google Scholar]; (c) Wu J; He L; Noble A; Aggarwal VK Photoinduced Deaminative Borylation of Alkylamines. J. Am. Chem. Soc 2018, 140, 10700–10704; [DOI] [PubMed] [Google Scholar]; (d) Sandfort F; Strieth-Kalthoff F; Klauck FJR; James MJ; Glorius F Deaminative Borylation of Aliphatic Amines Enabled by Visible Light Excitation of an Electron Donor–Acceptor Complex. Chem. Eur. J 2018, 24, 17210–17214; [DOI] [PubMed] [Google Scholar]; (e) Hu J; Wang G; Li S; Shi Z Selective C−N Borylation of Alkyl Amines Promoted by Lewis Base. Angew. Chem., Int. Ed 2018, 57, 15227–15231. [DOI] [PubMed] [Google Scholar]
- 10.For a recent three-component coupling of activated alkyl pyridinium salts, see: Klauck FJR; Yoon H; James MJ; Lautens M; Glorius F Visible-Light-Mediated Deaminative Three-Component Dicarbofunctionalization of Styrenes with Benzylic Radicals. ACS Catal 2019, 9, 236–241. [Google Scholar]
- 11.(a) Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem 2009, 52, 6752–6756; [DOI] [PubMed] [Google Scholar]; (b) Choi J; Fu GC Transition metal-catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 2017, 356, eaaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Jana R; Pathak TP; Sigman MS Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev 2011, 111, 1417–1492; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rudolph A; Lautens M Secondary Alkyl Halides in Transition-Metal-Catalyzed Cross-Coupling Reactions. Angew. Chem., Int. Ed 2009, 48, 2656–2670; [DOI] [PubMed] [Google Scholar]; (c) Giovannini R; Studemann T; Dussin G; Knochel P An efficient nickel-catalyzed cross-coupling between sp(3) carbon centers. Angew. Chem., Int. Ed 1998, 37, 2387–2390; [DOI] [PubMed] [Google Scholar]; (d) Devasagayaraj A; Stüdemann T; Knochel P A New Nickel-Catalyzed Cross-Coupling Reaction between sp3 Carbon Centers. Angew. Chem., Int. Ed. Engl 1996, 34, 2723–2725; [Google Scholar]; (e) Zhou JS; Fu GC Cross-Couplings of Unactivated Secondary Alkyl Halides: Room-Temperature Nickel-Catalyzed Negishi Reactions of Alkyl Bromides and Iodides. J. Am. Chem. Soc 2003, 125, 14726–14727; [DOI] [PubMed] [Google Scholar]; (f) Smith SW; Fu GC Nickel-Catalyzed Negishi Cross-Couplings of Secondary Nucleophiles with Secondary Propargylic Electrophiles at Room Temperature. Angew. Chem., Int. Ed 2008, 47, 9334–9336; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Jones GD; Martin JL; McFarland C; Allen OR; Hall RE; Haley AD; Brandon RJ; Konovalova T; Desrochers PJ; Pulay P; Vicic DA Ligand Redox Effects in the Synthesis, Electronic Structure, and Reactivity of an Alkyl−Alkyl Cross-Coupling Catalyst. J. Am. Chem. Soc 2006, 128, 13175–13183; [DOI] [PubMed] [Google Scholar]; (h) Cornella J; Edwards JT; Qin T; Kawamura S; Wang J; Pan CM; Gianatassio R; Schmidt M; Eastgate MD; Baran PS Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters. J. Am. Chem. Soc 2016, 138, 2174–2177; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Gong H; Sinisi R; Gagne MR A room temperature negishi cross-coupling approach to C-alkyl glycosides. J. Am. Chem. Soc 2007, 129, 1908–1909; [DOI] [PubMed] [Google Scholar]; (j) Phapale VB; Bunuel E; Garcia-Iglesias M; Cardenas DJ Ni-catalyzed cascade formation of C(sp(3))-C(sp(3)) bonds by cyclization and cross-coupling reactions of iodoalkanes with alkyl zinc halides. Angew. Chem., Int. Ed 2007, 46, 8790–8795; [DOI] [PubMed] [Google Scholar]; (k) Terao J; Todo H; Watanabe H; Ikumi A; Kambe N Nickel-catalyzed cross-coupling reaction of alkyl halides with organozinc and Grignard reagents with 1,3,8,10-tetraenes as additives. Angew. Chem., Int. Ed 2004, 43, 6180–6182. [DOI] [PubMed] [Google Scholar]
- 13.Knochel P; Singer RD Preparation and Reactions of Polyfunctional Organozinc Reagents in Organic Synthesis. Chem. Rev 1993, 93, 2117–2188. [Google Scholar]
- 14.Commercial samples of alkyl zinc halides are universally supplied in THF. The ratio of polar solvent present could thus not be increased further without reducing the overall concentration of the reaction mixture.
- 15.(a) Kato S; Morie T; Yoshida N Synthesis and Biological Activities of Metabolites of Mosapride, a New Gastroprokinetic Agent. Chem. Pharm. Bull 1995, 43, 699–702; [DOI] [PubMed] [Google Scholar]; (b) Roth BD Trans-6-[2-(3- or 4-carboxamido-substituted pyrrol-1-yl)alkyl]-4-hydroxypyran-2-one Inhibitors of Cholesterol Synthesis US 4,681,893, 1987.
- 16.(a) Johnston CP; Smith RT; Allmendinger S; MacMillan DW Metallaphotoredox-catalysed sp(3)-sp(3) cross-coupling of carboxylic acids with alkyl halides. Nature 2016, 536, 322–325; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ventre S; Petronijevic FR; MacMillan DW Decarboxylative Fluorination of Aliphatic Carboxylic Acids via Photoredox Catalysis. J. Am. Chem. Soc 2015, 137, 5654–5657; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Qin T; Cornella J; Li C; Malins LR; Edwards JT; Kawamura S; Maxwell BD; Eastgate MD; Baran PS A General Alkyl-Alkyl Cross-Coupling Enabled by Redox-Active Esters and Alkylzinc Reagents. Science 2016, 352, 801–805; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Huihui KM; Caputo JA; Melchor Z; Olivares AM; Spiewak AM; Johnson KA; DiBenedetto TA; Kim S; Ackerman LK; Weix DJ Decarboxylative Cross-Electrophile Coupling of N-Hydroxyphthalimide Esters with Aryl Iodides. J. Am. Chem. Soc 2016, 138, 5016–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Koppe H; Zeile K; Kummer W; Stahle H; Danneberg P 1-(2’,6’-Dimethyl-phenoxy)-2-amino-alkanes and salts thereof US3954872A, 1976;; (b) Davies BE Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemother 2010, 65, ii5–ii10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Rieke RD Preparation of Organometallic Compounds from Highly Reactive Metal Powders. Science 1989, 246, 1260–1264; [DOI] [PubMed] [Google Scholar]; (b) Rieke RD; Li PTJ; Burns TP; Uhm ST Preparation of Highly Reactive Metal Powders - A New Procedure for the Preparation of Highly Reactive Zinc and Magnesium Metal Powders. J. Org. Chem 1981, 46, 4323–4324. [Google Scholar]
- 19.(a) McCann LC; Organ MG On The Remarkably Different Role of Salt in the Cross-Coupling of Arylzincs From That Seen With Alkylzincs. Angew. Chem., Int. Ed 2014, 53, 4386–4389; [DOI] [PubMed] [Google Scholar]; (b) Achonduh GT; Hadei N; Valente C; Avola S; O’Brien CJ; Organ MG On the role of additives in alkyl-alkyl Negishi cross-couplings. Chem. Commun 2010, 46, 4109–4111. [DOI] [PubMed] [Google Scholar]
- 20.We assume commercial Rieke organozinc halides contain halide salts.
- 21.(a) Shibuya J; Nabeshima M; Nagano H; Maeda K Photochemical reaction of 2,4,4,6-tetrasubstituted 1,4-dihydropyridines in deaerated media: photocolouration and photorearrangement accompanying dehydrogenation. J. Chem. Soc. Perkin Trans. 2 1988, 1607–1612;; (b) Schwarz M; Kuthan J Reaction of organolithium compounds with 1-substituted 2,4,6-triphenylpyridinium perchlorates. Collect. Czech. Chem. Commun 1989, 54, 1880–1887; [Google Scholar]; (c) Karl D; Karlheinz W; Hermann K Über Derivate von 2H- und 4H-Pyran sowie von 2H- und 4H-Thiopyran. Justus Liebigs Ann. Chem 1964, 678, 183–201. [Google Scholar]
- 22.Patani GA; LaVoie EJ Bioisosterism: A Rational Approach in Drug Design. Chem. Rev 1996, 96, 3147–3176. [DOI] [PubMed] [Google Scholar]
- 23.Shang-Zheng S; Ruben M Nickel-Catalyzed Umpolung Arylation of Ambiphilic α-Bromoalkyl Boronic Esters. Angew. Chem., Int. Ed 2018, 57, 3622–3625. [DOI] [PubMed] [Google Scholar]
- 24.Larouche-Gauthier R; Elford TG; Aggarwal VK Ate Complexes of Secondary Boronic Esters as Chiral Organometallic-Type Nucleophiles for Asymmetric Synthesis. J. Am. Chem. Soc 2011, 133, 16794–16797. [DOI] [PubMed] [Google Scholar]
- 25.Bonet A; Odachowski M; Leonori D; Essafi S; Aggarwal VK Enantiospecific sp(2)-sp(3) coupling of secondary and tertiary boronic esters. Nat. Chem 2014, 6, 584–589. [DOI] [PubMed] [Google Scholar]
- 26.Katritzky AR; De Ville G; Patel RC Carbon-alkylation of simple nitronate anions by N-substituted pyridiniums. Tetrahedron 1981, 37, 25–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


