Abstract
Background:
In RET-rearranged lung cancers, data on the frequency of brain metastases and, in particular, the outcomes of multikinase inhibitor therapy in patients with intracranial disease are not well characterized.
Methods:
A global, multi-institutional registry (cohort A, n=114) and a bi-institutional data set (cohort B, n=71) of RET-rearranged lung cancer patients were analyzed. Patients were eligible if they had stage IV lung cancers harboring a RET rearrangement by local testing. The incidence of brain metastases and outcomes with multikinase inhibitor therapy were determined.
Results:
The frequency of brain metastases at the time of diagnosis of stage IV disease was 25% (95%CI 18%−32%) in all patients from both cohorts. The lifetime prevalence of brain metastasis in stage IV disease was 46% (95%CI 34%−58%) in patients for whom longitudinal data was available. The cumulative incidence of brain metastases was significantly different (p=0.0039) between RET-, ROS1-, and ALK- rearranged lung cancers, with RET intermediate between the other two groups. While intracranial response data was not available in cohort A, the median progression-free survival (PFS) of multikinase inhibitor therapy (cabozantinib, vandetanib, or sunitinib) in patients with brain metastases was 2.1 months (95%CI 1.3–2.9 months, n=10). In cohort B, an intracranial response was observed in 2 of 11 patients (18%) treated with cabozantinib, vandetanib (±everolimus), ponatinib, or alectinib; the median overall PFS (intracranial and extracranial) was 3.9 months (95%CI 2.0–4.9 months).
Conclusions:
Brain metastases occur frequently in RET-rearranged lung cancers, and outcomes with multikinase inhibitor therapy in general are suboptimal. Novel RET-directed targeted therapy strategies are needed.
INTRODUCTION
RET rearrangements are found in 1–2% of unselected patients with non-small cell lung cancers (NSCLCs).1 Like ALK and ROS1 rearrangements, these are often identified in younger patients with lung adenocarcinomas and a minimal to no history of prior cigarette smoking. RET-directed multikinase inhibitor therapy with drugs such as cabozantinib, vandetanib, and levantinib is active in a subset of patients with these tumors.2–5
While lung cancers are known to frequently metastasize to the brain, the incidence of brain metastases in patients with RET-rearranged lung cancers, particularly in comparison to other driverpositive lung cancers, has not been well-defined. Furthermore, the clinical outcomes with multikinase inhibitor therapy in patients with brain metastases are poorly characterized. We set out to establish this data using a global, multicenter registry and a complementary bi-institutional dataset of RET-rearranged lung cancer patients.
MATERIALS AND METHODS
Patients.
The global RET registry6 was used as a platform to identify cases; a multicenter network of thoracic oncologists from 29 centers and 12 countries identified eligible patients (cohort A). A separate, bi-institutional data set (Memorial Sloan Kettering Cancer Center and the Massachusetts General Hospital) was used as a complementary cohort (cohort B), given that data on the lifetime prevalence of brain metastases and intracranial response to therapy were not available in the former. Patients were identified under Institutional Review Board-approved waivers/protocols. For both cohorts, patients were eligible for inclusion if they had stage IV NSCLCs and a RET rearrangement identified by local molecular profiling (RT-PCR, FISH, DNA-based next-generation sequencing, or targeted RNA sequencing). A retrospective review of clinicopathologic and molecular features was performed. Response to therapy (overall and intracranial) was determined (RECIST v1.1).
Treatment.
Investigators administered multikinase inhibitor therapy with cabozantinib, vandetanib, vandetanib and everolimus, alectinib, ponatinib, and sunitinib off-label (treatment history as previously described6). Patients who received cabozantinib included four subjects on a phase II trial (NCT01 6395082: patients were eligible if they had asymptomatic, untreated brain metastases; 4 patients met these criteria while the other 22 did not have untreated brain metastases at baseline). A review of serial standard-of-care intracranial imaging was performed by a blinded radiologist.
Statistics.
The cumulative incidence of brain metastasis was compared to a published dataset of patients with ALK- and ROS1-rearranged lung cancers7 using the Gray’s test. Progression-free survival (PFS) and overall survival (OS) in patients with and without brain metastasis treated with multikinase inhibitors were determined using Kaplan-Meier estimates. Survival was measured from the date of treatment with the first multikinase inhibitor.
RESULTS
Patients.
A total of 114 patients with stage IV, RET-rearranged lung cancers were identified in cohort A (global RET registry). Information on the presence of brain metastases at the diagnosis of stage IV disease was available in 75 patients. In these 75 patients, the median age was 60 years (range 28–86 years) and 45% of patients were female. Most patients were never smokers (68%) with lung adenocarcinomas (96%). KIF5B was the most common upstream fusion partner (89%, Supplementary Table 1). Given that data on the lifetime prevalence of brain metastases and intracranial response to therapy were not available in cohort A, a separate bi-institutional cohort (cohort B, including 13 patients from cohort A for whom this data was available) was analyzed. Cohort B was composed of 71 patients. The median age was 58 years (range 33–79) and 47% of patients were female. Like cohort A, most patients were never smokers (69%) with lung adenocarcinomas (96%). KIF5B was the most common upstream partner (84%, Supplementary Table 2).
Frequency and cumulative incidence of brain metastases.
The frequency of brain metastases at diagnosis in stage IV RET-rearranged lung cancers was 25% (95%CI 18%−32%, n=33/133) in all patients from both cohorts. The lifetime prevalence of brain metastasis, including brain metastases at diagnosis, in patients with stage IV RET-rearranged lung cancers for whom data was available (cohort B) was 46% (95%CI 34%−58%, n=33/71, Figure 1A). The cumulative incidence of brain metastasis in patients from cohort B with stage IV RET-rearranged lung cancers and no history of brain metastases at diagnosis (n=54) was compared to a previously published cohort of patients with ROS1-rearranged (n=29) and ALK-rearranged (n=98) lung cancers. We focused on ROS1- and ALK-rearranged lung cancers as comparators as these are the most common, targetable oncogenic fusions in NSCLC. The cumulative incidence of brain metastases was significantly different between groups (Gray’s test, p=0.0039, Figure 1B), with RET-rearranged lung cancers intermediate between ALK- and ROS1-rearranged lung cancers. Pairwise comparisons revealed, however, that the cumulative incidence of brain metastases in RET- rearranged lung cancers was not different from ROS1-rearranged (p=0.051) or ALK-rearranged (p=0.150) lung cancers; the cumulative incidence of brain metastases was higher in ALK- compared to ROS1- rearranged lung cancers (p=0.004).
Figure 1. Brain metastases in RET-rearranged lung cancers.
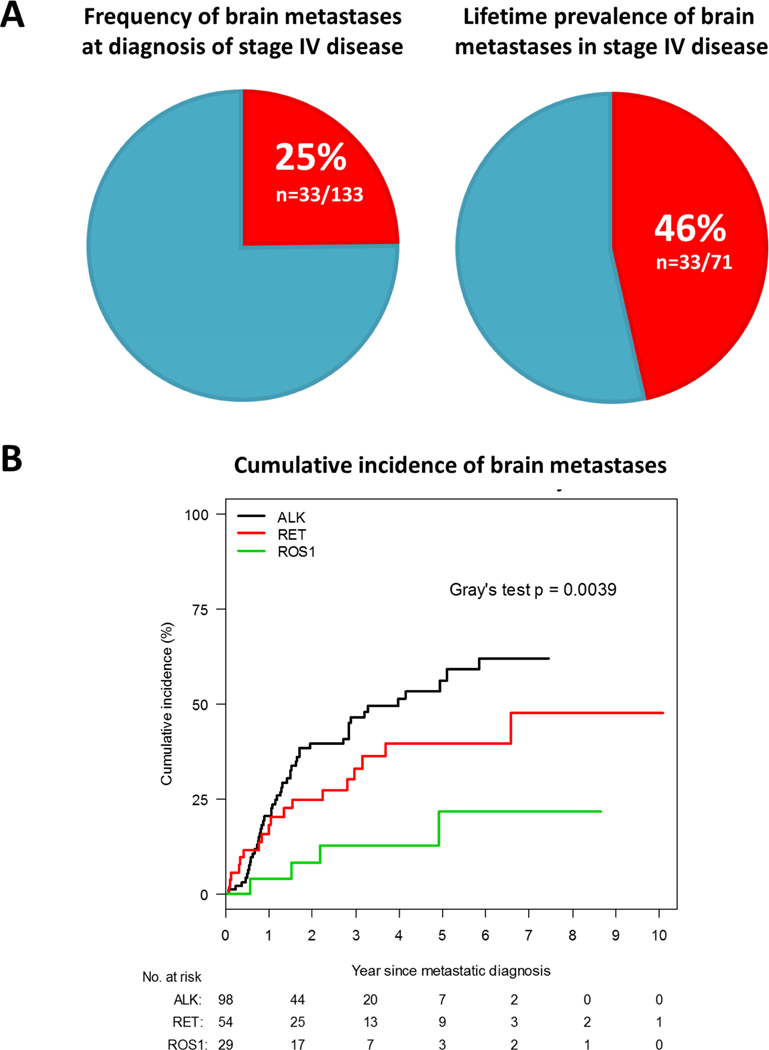
In Figure 1A, the frequency of brain metastases at the time of diagnosis of stage IV disease is shown on the left. The lifetime prevalence of brain metastases in stage IV disease, including brain metastases at diagnosis, is shown on the right. In Figure 1B, the cumulative incidence of brain metastases in RET-rearranged lung cancers is compared to ALK- and ROS1-rearranged lung cancers for patients without known brain metastases at diagnosis.
Outcomes of multikinase inhibitor therapy.
In cohort A, 37 patients received a multikinase inhibitor with activity against RET (cabozantinib n=16, vandetanib n=9, sunitinib n=7, ponatinib n=1, nintedanib n=1, other n=3). Active untreated brain metastases were present prior to multikinase inhibitor therapy in 10 of these patients. While data on objective response to therapy are summarized in Supplementary Table 1, data on intracranial response were not available. The median PFS in patients with brain metastases was 2.1 months (95% CI 1.3–2.9 months). The median OS was 3.9 months (95% CI 1.9–5.4). The median PFS and OS were not significantly different when compared to the remaining 27 patients without brain metastases (Figure 2).
Figure 2. Survival with multikinase inhibition in RET-rearranged lung cancers with intracranial disease.
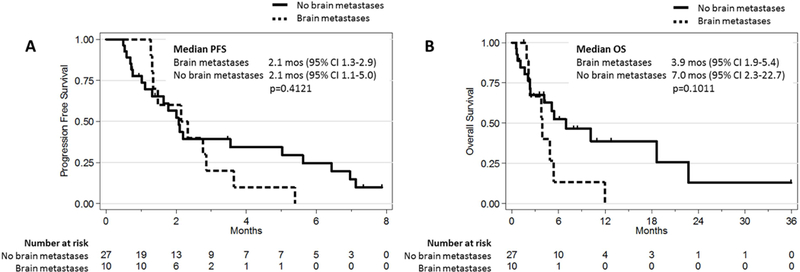
Kaplan-Meier progression-free survival (PFS) and overall survival (OS) curves of patients with stage IV, RFT-rearranged lung cancers treated with multikinase inhibitors are shown. The PFS and OS of patients with brain metastases prior to tyrosine kinase inhibitor (TKI) therapy were compared to patients without baseline brain metastases.
In cohort B, data on intracranial response was available in 13 patients with brain metastases prior to the initiation of multikinase inhibitor therapy. These data, including information on fusion type and line of therapy, are summarized in Table 1. Of note, two patients (cases 1 and 9) received one or more multikinase inhibitors in sequence. Patients had either measurable and/or evaluable intracranial disease prior to therapy. All patients had active untreated disease at baseline except for case 9 where prior RT was administered to all sites of disease, however, progression of disease was noted as the best intracranial response to therapy with sunitinib. No other patients received brain radiation or underwent intracranial surgery immediately prior to multkinase inhibitor therapy initiation.
Table 1. Response to multikinase inhibition in RET-rearranged lung cancers.
The best objective response and the best intracranial response to multikinase inhibitor therapy (single agent or combination therapy) with activity against RET were determined by RECIST v1.1.
| Drug | Fusion | Line of TKI Therapy |
Measurable Intracranial Disease at Baseline |
Evaluable Intracranial Disease at Baseline |
Active Untreated Intracranial Disease Pre- TKI |
Best Intracranial Response |
Best Overall Response* |
Duration of Therapy (months) |
TKI Response Rate1 |
|---|---|---|---|---|---|---|---|---|---|
| Alectinib | 1/2 (50%) | ||||||||
| Case 1 | CCDC6-RET | 2nd | Yes | Yes | Yes | PR | PR | 9.0 | |
| Case 2 | KIF5B-RET | 1st | Yes | Yes | Yes | SD | PD | 1.8 | |
| Cabozantinib | 0/3 (0%) | ||||||||
| Case 3 | KIF5B-RET | 1st | Yes | Yes | Yes | SD | SD | 7.4 | |
| Case 4 | FISH+ | 1st | Yes | Yes | Yes | SD | SD | 1.6 | |
| Case 5 | RUFY2-RET | 1st | Yes | Yes | Yes | PD | PD | 0.9 | |
| Case 6 | KIF5B-RET | 1st | No | Yes | Yes | SD2 | SD | 23.6 | |
| Case 7 | FISH+ | 1st | No | Yes | Yes | SD2 | SD | 40.8 | |
| Case 8 | FISH+ | 1st | No | Yes | Yes | PD2 | PD | 1.6 | |
| Ponatinib | 0/3 (0%) | ||||||||
| Case 1 | CCDC6-RET | 1st | Yes | Yes | Yes | PD | SD | 5.0 | |
| Case 9 | KIF5B-RET | 2nd | Yes | Yes | Yes | SD | PD | 3.0 | |
| Case 10 | KIF5B-RET | 1st | Yes | Yes | Yes | PD | PD | 0.9 | |
| Case 11 | KIF5B-RET | 1st | No | Yes | Yes | SD2 | SD | 4.0 | |
| Sunitinib | N/A | ||||||||
| Case 9 | KIF5B-RET | 1st | No | Yes | No (prior RT) | PD3 | SD | 4.4 | |
| Vandetanib | 0/1 (0%) | ||||||||
| Case 9 | KIF5B-RET | 3rd | Yes | Yes | Yes | SD | SD | 4.7+ | |
| Vandetanib + Everolimus | 1/2 (50%) | ||||||||
| Case 12 | CCDC6-RET | 3rd | Yes | Yes | Yes | PR | SD | 6.8 | |
| Case 1 | CCDC6-RET | 3rd | Yes | Yes | Yes | SD | SD | 3.7 | |
| Case 13 | KIF5B-RET | 2nd | No | Yes | Yes | SD3 | SD | 2.5 | |
overall response rate only in patients with measurable disease at baseline,
intracranial response in non-target lesions,
brain radiation (RT) administered prior to sunitinib, but first follow-up brain scan revealed progression of disease on sunitinib; TKI – tyrosine kinase inhibitor, FISH+ – positive RET fluorescence in situ break apart test;
(under duration of therapy) – indicates that therapy is ongoing; PR – partial response. SD – stable disease. PD – progression of disease. N/A – not applicable.
refers to the best overall response (extracranial and intracranial)
In patients with measurable disease at baseline, a confirmed intracranial response was only observed in 2/11 cases (18%): 0/3 (0%) with cabozantinib, 0/3 (0%) with ponatinib, 1/2 (50%) with vandetanib and everolimus, 1/2 (50%) with alectinib, and 0/1 (0%) with vandetanib. Radiation therapy was eventually administered in 5/11 patients who were fit to receive treatment (45%; whole brain radiation in 2 cases, or stereotactic radiosurgery in 3 cases). Both responses were patients whose lung cancers harbored a CCDC6-RET fusion. No intracranial responses were observed in KIF5B-RET fusion-positive tumors, consistent with select series showing lower rates of overall systemic response with KIF5B-RET- compared to non-KIF5B-RET-rearranged lung cancers.1 While hypothesis-generating, this requires further study in a larger cohort. The outcomes of cases with evaluable but non-measurable intracranial disease are detailed in Table 1. The median overall PFS, reflecting both intracranial and extracranial disease control, was 3.9 months (95% CI 2.0–4.9 months).
DISCUSSION
In this series, we demonstrate that brain metastases frequently occur in RET-rearranged lung cancers. A quarter of patients have brain metastases at diagnosis of stage IV disease, and close to half of patients will develop brain metastases during their lifetime. The cumulative incidence of brain metastases in RET-rearranged lung cancer patients was intermediate between that of ALK- and ROS1- rearranged lung cancers when all three groups were compared.
Whereas durable intracranial responses to multikinase inhibitor therapy have been reported in RET-rearranged lung cancers,8–10 we showed that overall clinical outcomes are poor, especially when compared to data on the activity of targeted therapies in ALK- and ROS1-rearranged lung cancers with metastatic disease to the brain.11 The intracranial response rate of multikinase RET inhibition was low, and the median PFS and OS were short in patients with brain metastases. This relative difference echoes the decreased overall efficacy noted with multikinase RET-directed versus ALK- and ROS1-directed targeted therapy in RET-, ALK-, and ROS1-rearranged lung cancers, respectively, reflecting how the currently available multikinase inhibitors are suboptimal at targeting RET.1 Prior to this report, data on the central nervous system activity of many single-agent multikinase inhibitors with anti-RET activity was not clearly defined; one exception to this is alectinib, however, data on intracranial outcomes was largely limited to ALK-rearranged lung cancers. Of note, many RET inhibitors have substantial anti- angiogenic activity that some have hypothesized may contribute, in part, to response.1 This series was limited by its retrospective nature and the inability to consistently assess all outcomes on both cohorts.
Novel RET-directed targeted therapy strategies are needed. The combination of vandetanib with everolimus has been postulated to increase brain penetrance;12 beyond the limited number of cases presented in this series, results of an ongoing trial of this combination (NCT01582191) will be informative. Furthermore, more selective RET inhibitors such as BLU-667 (NCT03037385) and LOXO-292 (NCT03157128) are currently in phase 1 testing.13,14 LOXO-292 demonstrated preclinical activity in a mouse model harboring an orthotopically implanted intracranial RET-rearranged tumor; treatment with LOXO-292 resulted in intracranial disease control in 12 patients with baseline brain metastases on a phase I trial, including 3 of 3 confirmed intracranial responses in patients with measurable intracranial target lesions (Drilon et al, ASCO Annual Meeting 2018), in addition to a near complete intracranial response in a RET-rearranged lung cancer patient with multiple brain metastases on a compassionate use protocol.15 Similarly, BLU-667 has also demonstrated preliminary intra-cranial anti-tumor activity in a RET-rearranged NSCLC patient enrolled in an ongoing phase I trial (Subbiah et al, AACR Annual Meeting 2018).
We await the results of ongoing trials of these and future approaches. In addition, we encourage that trials of RET-directed therapy be designed to include baseline and serial brain imaging even in patients without a history of brain metastases. This will help further characterize not only response to therapy, but baseline intracranial disease, and patterns of disease progression in the central nervous system. Should novel strategies not be available to patients with RET-rearranged lung cancers that metastasize to the central nervous system, best clinical judgement should be used to determine if single-agent multikinase inhibitor therapy could be complemented by local therapy such as radiation, either concurrently or in sequence.
Supplementary Material
Footnotes
DISCLOSURES
No Conflicts of interest were reported for: Jessica J. Lin, Thomas Filleron, Ai Ni, Julie Milia, Isabella Bergagnini, Vaios Hatzoglou, Vamsidhar Velcheti, Michael Offin, Julien Mazieres, David P. Carbone, Benjamin Besse, Tony Mok, Mark M. Awad, Dwight Owen, Ross Camidge, Nir Peled and Oliver Gautschi.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Drilon A, Hu ZI, Lai GGY, et al. : Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drilon A, Rekhtman N, Arcila M, et al. : Cabozantinib in patients with advanced RET- rearranged non-small-cell lung cancer: an open-label, single-centre, phase 2, single-arm trial. Lancet Oncol, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoh K, Seto T, Satouchi M, et al. : Vandetanib in patients with previously treated RET- rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Lee SH, Lee JK, Ahn MJ, et al. : Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann Oncol 28:292–297, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Velcheti VTH KL Reckamp JC. Yang H. Nokihara P Sachdev K. Feit T. Kubota T. Nakada CE. Dutcus M Ren T. Tamura Phase 2 study of lenvatinib (LN) in patients (Pts) with RET fusion-positive adenocarcinoma of the lung. ESMO Congress:1204PD, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Gautschi O, Milia J, Filleron T, et al. : Targeting RET in Patients With RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. J Clin Oncol 35:1403–1410, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gainor JF, Tseng D, Yoda S, et al. : Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol 2017, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JJ, Kennedy E, Sequist LV, et al. : Clinical Activity of Alectinib in Advanced RET- Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 11:2027–2032, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Velcheti V, Ahluwalia M: Intracranial and Systemic Response to Alectinib in a Patient with RET-KIF5B Oncogenic Fusion. J Thorac Oncol 12:e98–e99, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Li GG, Somwar R, Joseph J, et al. : Antitumor Activity of RXDX-105 in Multiple Cancer Types with RET Rearrangements or Mutations. Clin Cancer Res 23:2981–2990, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schram AM, Chang MT, Jonsson P, et al. : Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 14:735–748, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbiah V, Berry J, Roxas M, et al. : Systemic and CNS activity of the RET inhibitor vandetanib combined with the mTOR inhibitor everolimus in KIF5B-RET re-arranged non-small cell lung cancer with brain metastases. Lung Cancer 89:76–9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahal R, Evans EK, Hu W, et al. : The development of potent, selective RET inhibitors that target both wild-type RET and prospectively identified resistance mutations to multi-kinase inhibitors. Cancer Research 76, 2016 [Google Scholar]
- 14.Brandhuber BB, Haas J, Tuch BB, et al. : ENA-0490 The development of LOXO-292, a potent, KDR/VEGFR2-sparing RET kinase inhibitor for treating patients with RET-dependent cancers. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics Poster No. 441, 2016 [Google Scholar]
- 15.Velcheti V, Bauer T, Subbiah V, et al. : LOXO-292, a Potent, Highly Selective RET Inhibitor, in MKI-Resistant RET Fusion-Positive Lung Cancer Patients with and without Brain Metastases. Journal of Thoracic Oncology 12:S1778, 2017 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


