Abstract
Pericytes and other perivascular stem cells are of growing interest in orthopedics and tissue engineering. Long regarded as simple regulators of angiogenesis and blood pressure, pericytes are now recognized to have MSC (mesenchymal stem cell) characteristics, including multipotentiality, self-renewal, immunoregulatory functions, and diverse roles in tissue repair. Pericytes are typified by characteristic cell surface marker expression (including αSMA, CD146, PDGFRβ, NG2, RGS5, among others). Although alone no marker is absolutely specific for pericytes, collectively these markers appear to selectively identify an MSC-like pericyte. The purification of pericytes is most well described as a CD146+CD34−CD45− cell population. Pericytes and other perivascular stem cell populations have been applied in diverse orthopedic applications, including both ectopic and orthotopic models. Application of purified cells has sped calvarial repair, induced spine fusion, and prevented fibrous non-union in rodent models. Pericytes induce these effects via both direct and indirect mechanisms. In terms of their paracrine effects, pericytes are known to produce and secrete high levels of a number of growth and differentiation factors both in vitro and after transplantation. The following review will cover existing studies to date regarding pericyte application for bone and cartilage engineering. In addition, further questions in the field will be pondered, including the phenotypic and functional overlap between pericytes and culture-derived MSC, and the concept of pericytes as efficient producers of differentiation factors to speed tissue repair.
Keywords: Perivascular stem cell, Mesenchymal stem cell, PSC, MSC, Bone, Cartilage
1. Introduction
The interest in pericytes and related perivascular cell populations for tissue engineering has significantly increased over recent years. Just eight years ago, the MSC (mesenchymal stem cell) identity of pericytes was reported (Crisan et al., 2008). The second perivascular population with MSC characteristics was reported in 2012 (Corselli et al., 2012). The umbrella term “perivascular stem cells (PSC)” has been proposed to broadly encompass these bipartite cell population (James et al., 2012b; Murray et al., 2014). Since their initial identification, multiple research groups have confirmed the MSC characteristics of pericytes, and sought to leverage this cell type for tissue regeneration – both within and outside of the orthopedic field. In the present review, we will summarize what is known regarding the identity and characteristics of pericytes, as well as the studies to date in bone and cartilage tissue engineering and regeneration.
1.1. Review criteria
For the current review, PubMed search terms were used to identify articles of interest, including “pericyte, adventitial cell, perivascular stem cell” and “bone, cartilage.” Articles were then filtered by relevance by the corresponding author. An effort was made to include work published within the last 10 years, and include earlier works only when directly relevant.
1.2. Conventional culture-derived mesenchymal stem cell
Historically MSC have been isolated in laboratory culture, being selected from total cell suspensions based on their ability to adhere and proliferate for several weeks from primary culture (Murray & Peault, 2015). Bone marrow represents the gold standard source of culture-derived MSC, with the iliac crest being the most common site of harvest (Cox et al., 2011). The aspirated marrow is typically centrifuged to separate mononuclear cells from blood and fat. The mononu-clear cell layer is subsequently plated onto tissue culture plastic and allowed to proliferate, during which time the MSC predominate and become the most abundant cell type. Although these preparations are enriched for MSC, some residual heterogeneity is inevitable and they can therefore not be considered purified populations. Surface epitope characterization has demonstrated that a high number of culture-derived MSC isolated from both adipose tissue and bone marrow express pericyte markers (Khan et al., 2008, 2010).
Although, promising results have been achieved using MSC derived through culture in the treatment of bone (Muschler et al., 2005; Kawate et al., 2006; Marcacci et al., 2007) and cartilage lesions (Wakitani et al., 2002; Haleem et al., 2010), there are a number of clear challenges to widespread clinical application of cells isolated in this way. The selection and expansion of cells through cell culture is time consuming, precluding use in emergency scenarios, and often involves exposure to animal-derived culture products. Culture introduces risks including infection (Murray & Peault, 2015), immunogenicity (Gad, 2008), genetic instability (Dahl et al., 2008; Tonti & Mannello, 2008) and tumorogenicity (Rosland et al., 2009; Ren et al., 2011) although the latter has been contested (Rosland et al., 2009). Furthermore, increasing time in culture has been shown to reduce multipotentiality and increase replicative senescence that may impair their clinical efficacy (Muraglia et al., 2000; Baxter et al., 2004).
Strategies that utilize serum-free media supplemented with growth factors have been used to minimize potential immunologic responses and the risk of contamination although these approaches require further investigation. Production of clinically utilized MSC requires facilities that comply with good manufacturing practices and culturing of cells has considerable regulatory implications. Regulatory agencies in the United States qualify laboratory culture as beyond “minimal-manipulation”, requiring that potential therapies proceed through the arduous pathway 351 of the regulation covering “Human Cells, Tissues, and Cellular or Tissue-based Products” (HCT/Ps) (Anz et al., 2014).
2. Introduction to pericytes and perivascular stem cells
2.1. In situ identification of pericytes
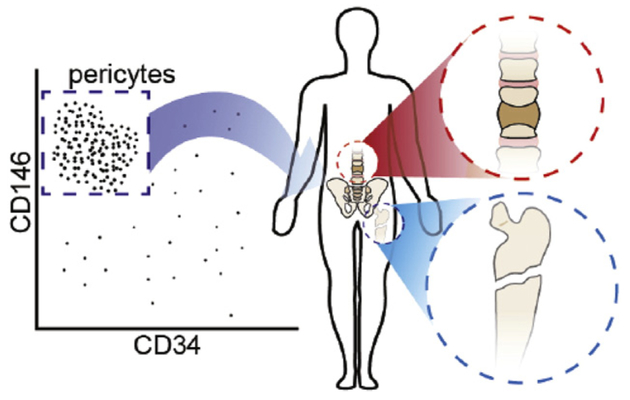
Pericytes had long been considered to have a limited role in simple blood pressure control. However it is now known that pericytes play diverse physiologic and pathologic roles throughout the human body (summarized in Table 1). The in situ identification of pericytes as a tissue resident MSC population was first reported by Crisan et al. (2008). Prior to this, scattered reports had described the possible multipotentiality of pericytes, including an ability to undergo osteogenic, chondrogenic, fibrogenic, and adipogenic differentiation (Doherty & Canfield, 1999; Farrington-Rock et al., 2004; Collett & Canfield, 2005) and with high expression of growth and differentiation factors (Howson et al., 2005; Invernici et al., 2007). Covas et al. (2005) had identified MSC-like cells within human veins, but did not show in situ localization. It was not until Crisan et al. (2008) utilized a combination of immunohistochemical and flow cytometry analysis that the mesenchymal stem cell (MSC) identity of pericytes was fully appreciated. Using a combination of immunohistochemical markers (including NG2, CD146, PDGFRB, and αSMA), the localization of pericytes was performed. Additional pericytes markers are listed in Table 2. Across diverse human organs, pericytes were ubiquitously identified around arterioles, capillaries and venules. Next, Crisan et al. found that isolated pericytes express MSC markers, including CD73, CD90 and CD105. They further identified the multilineage differentiation potential of FACS (fluorescence activated cell sorting) purified pericytes, including an ability to differentiate down muscle, bone, fat and cartilage lineages. This study was the first of its kind to identify and confirm the MSC attributes of pericytes. Since this time, multiple independent investigators have confirmed the pericytic/perivascular origins of MSC (see Murray et al., 2014 for a review). In addition, several research groups have identified pericytes as native progenitor cells that are involved in endogenous tissue development and repair (Dellavalle et al., 2007; Tang et al., 2008; Feng et al., 2011).
Table 1.
Brief description of physiologic and pathologic roles of pericytes.
| Function | Comments/reference |
|---|---|
| Physiological | |
| Blood brain barrier | The blood brain barrier serves to prevent the passive transport of cells, proteins and bioactive compounds from the systemic blood to the vasculature of the CNS (Abbott, Patabendige, Dolman, Yusof, & Begley, 2010). Studies have demonstrated that the brain has the highest coverage of pericytes and that they play a critical role in the maturation and maintenance of the barrier. Deficiency of pericytes results in increased permeability to water and low and high molecular weight compounds (Armulik et al., 2010). |
| Vascular permeability | Increased vascular permeability is seen in response to a number of pathological states including sepsis, trauma, tumors and microangiopathy where pericytes have been observed to migrate from the vessels wall resulting in increased permeability (Nag, Kapadia, & Stewart, 2011). |
| Vasoconstriction | The role that pericytes play in capillary constriction was one of the first described functions of pericytes and has been observed in vitro (Díaz-Flores et al., 2009), and in vivo (Fernández-Klett, Offenhauser, Dirnagl, Priller, & Lindauer, 2010). This contraction facilitates variations in regional blood flow and contributes to the control of blood pressure. |
| Stem cell | Many studies have demonstrated that pericytes represent a population of MSC progenitor cells that express MSC markers in vivo, and are capable of multilineage differentiation in vitro (Crisan et al., 2008). Subsequent in vivo studies have demonstrated the role that pericytes play in the regeneration of white adipose tissue (Tang et al., 2008), odontoblasts (Feng et al., 2011) and skeletal muscle (Dellavalle et al., 2011) among other tissues. |
| Pathological | |
| Fibrosis | The myofibroblast is a critical cell in fibrosis and is responsible for the deposition of pathological extracellular matrix. The pericyte has been demonstrated to be the progenitor of the myofibroblast in many solid organs including liver, lung, heart (Kramann et al., 2015) and skin (Dulauroy, Di Carlo, Langa, Eberl, & Peduto, 2012). |
| Neurodegeneration | Loss of pericytes from blood vessels in the brain has been shown to lead to increased vascular permeability and promotion of neurodegeneration in a murine model of aging. |
| Cancer | A significant proportion of a tumor’s bulk is the stromal component of which pericytes form a large part. The stroma has key roles in tumor growth and progression and therefore pericytes represent a potential target for anti-cancer therapies (Pietras & Ostman, 2010). The exact origin and role of pericytes in tumor biology remains relatively poorly understood. |
| Pericyte derived tumors | A group of related tumors demonstrates pericytes marker expression and may be derived from modified pericytes (Shen et al., 2015a; Shen et al., 2015b). |
| Diabetic retinopathy | Diabetic retinopathy is a common complication experienced by up to a third of adult diabetics. Pericyte death is the first in a chain of events that leads to basement membrane thickening, capillary leakage, vessel occlusion and the subsequent proliferative retinopathy (Barber, Gardner, & Abcouwer, 2011). |
Table 2.
Summary of pericytes markers.
2.2. Purification of pericytes
Advances in multicolor flow cytometry have enabled the prospective identification and purification of pericytes from heterogeneous cell preparations using fluorescence activated cell sorting (FACS). Isolating homogenous populations of MSC in this way overcomes many of the drawbacks of conventionally isolated MSC and unsorted populations, including issues relating to purity, potency and availability. Given the relative abundance of progenitors within adipose tissue, perivascular MSC can be isolated in sufficient quantities for direct clinical application without requirement for culture expansion. Analyzing the frequency of viable MSC purified by FACS from 60 consecutive donors of lipoaspirate, James et al. (2012a) reported a mean yield of over 15 million purified PSC per 100 ml of lipoaspirate. Importantly, from a clinical standpoint, the high number of autologous cells available means that MSC can be purified and returned for clinical use in sufficient quantities to avoid culture expansion and the necessity for a second surgical procedure. Purifying cells in this way has its own practical challenges including the expenses and availability of GMP facilities and reagents.
2.3. Adventitial cells: a second perivascular stem cell population
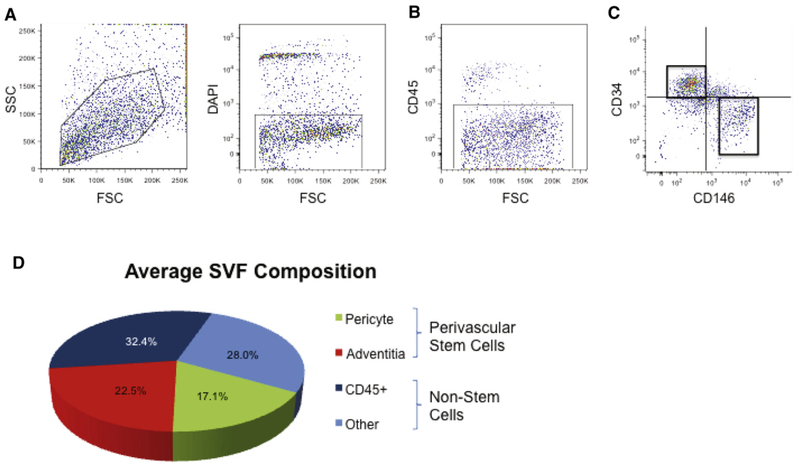
Prior studies have clearly shown that cells within the adventitia of larger vessels have MSC characteristics. Hu et al. (2004) identified Sca-1 (Stem cell antigen-1) expression and other MSC markers within the adventitia of the mouse aorta. Since then, investigators have isolated CD34+ MSC progenitors from human arteries and veins (Pasquinelli et al., 2007; Campagnolo et al., 2010). For example, Campagnolo et al. (2010) had previously characterized a similar CD34+ cell multipotent cell population from the saphenous vein (termed “saphenous vein-derived progenitor cells”). Likewise, Pasquinelli et al. (2007) described a similar CD34+ MSC-like cell from the thoracic aorta. After isolation of adipose tissue-derived pericytes, it became clear that within the blood vessels of adipose tissue there was a non-pericyte fraction with MSC characteristics. This led to the identification of a second (nonpericyte) perivascular cell with MSC characteristics (Corselli et al., 2012). These cells (identified by expression of CD34 and negativity for CD146 and CD31) were identified in the tunica adventitia of larger arteries and veins – and were thus named adventitial cells. When combined, pericytes and adventitial cells represent all known cells with MSC characteristics within human white adipose tissue (Corselli et al., 2012). By flow cytometry and immunohistochemistry analysis, adventitial cells have been demonstrated to express typical MSC markers including CD44, CD90, CD105, and CD73. By clonal analysis, adventitial cells evidence a capacity for multilineage differentiation, including osteogenesis, chondrogenesis, and adipogenesis (Corselli et al., 2012). Taken together, pericytes and adventitial cells (collectively termed PSC) comprise ~40% of lipoaspirate stromal tissues (James et al., 2012b). Unlike other MSC sources, PSC are prospectively identified using fluorescence activated cell sorting (rather than retrospective isolation via cell culture) (James et al., 2012b; Murray et al., 2014). These results were the first study to demonstrate that PSC could be derived in clinically meaningful numbers for orthopedic applications. The combined use of pericytes and adventitial cells has been pursued for use in bone and cartilage tissue engineering (Fig. 1).
Fig. 1.
Schematic overview of past studies involving orthopedic applications of pericyte and perivascular stem cells (PSC). From left to right: Pericytes and perivascular stem cells are most commonly derived from liposuction aspirate of human white adipose tissue. After enzymatic digestion, an unpurified stromal cell population is obtained, termed ‘stromal vascular fraction’ or SVF. Fluorescence activated cell sorting is performed to obtain CD146+CD34−CD45− pericytes and CD146−CD34+CD45− adventitial cells. Collectively, these perivascular cell populations are termed ‘perivascular stem cell’ or PSC. Pericytes and/or PSC have been applied to diverse orthopedic models, including mouse calvarial defects, mouse intramuscular implantation experiments, rat femoral fibrous non-union defects, and rat lumbar transverse spinal fusion models.
3. Pericytes in bone tissue engineering
3.1. Pericytes in ectopic bone formation
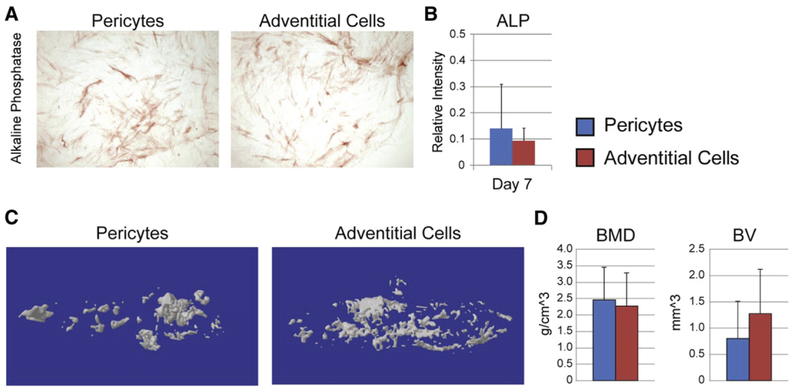
Animal models of ectopic bone formation have been used to confirm the capacity for in vivo osteogenic differentiation of implanted pericytes. First, a comparative study was performed examining pericytes and adventitial cells derived from the same patient sample of white adipose tissue. These studies were performed so as to determine the relative osteogenic potential of perivascular stem cell subpopulations (pericytes and adventitial cells), and to better justify the combining of these cell sources for bone tissue engineering application. Results showed that both perivascular populations had a similar baseline osteogenic potential (Fig. 2, unpublished data). These results were true of in vitro osteogenic differentiation (Fig. 2A,B), as shown by alkaline phosphatase staining and quantification. Moreover, when these same populations were tested in vivo in an intramuscular environment, similar amounts of bone matrix were produced (Fig. 2C,D). This pilot study demonstrated that pericytes and adventitial cells have a similar bone-forming potential, and laid the framework for later studies in which these two cell populations were combined (termed perivascular stem cells or PSC).
Fig. 2.
Human pericytes and adventitial cells undergo roughly similar osteogenic differentiation. (A,B) Human pericytes and adventitial cells from the same patient sample were cultured under osteogenic conditions (10% FBS, 100 μg/ml ascorbic acid, 10 mM β-glycerophosphate). (A) Representative image of alkaline phosphatase (ALP) staining at 5 d differentiation. (B) Semi-quantification of ALP staining. (C,D) Human pericytes or adventitial cells were implanted in the thigh complex of a SCID mouse using a collagen sponge carrier (2.5 × 105 cells, sponge size 2.0 × 1.0 × 0.5 cm). (C) 3-Dimensional MicroCT reconstructions. (D) MicroCT based quantification of bone mineral density (BMD) and bone volume (BV).
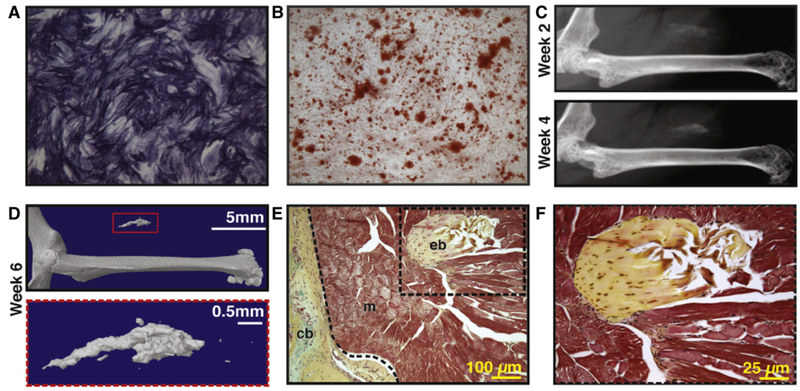
Next, the bipartite PSC population was again implanted in an intramuscular environment and bone formation assessed overtime (Fig. 3, reproduced with permission from James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, Pang S, Stoker D, Ting K, Peault B, Soo C. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. Jun 2012; 1(6):510–9. PMCID: PMC3659717). By both radiographic and histologic outcomes, PSC were observed to spontaneously ossify overtime without additional osteogenic stimuli. These studies demonstrated PSC to be an ‘osteocompetent’ cell population for further study.
Fig. 3.
Human perivascular stem cells (hPSC) are an ‘osteocompetent’ cell population. (A,B) hPSC were cultured under osteogenic conditions (10% FBS, 100 μg/ml ascorbic acid, 10 mM β-glycerophosphate). (A) Representative image of alkaline phosphatase staining at 5 d differentiation. (B) Representative image of Alizarin red staining, 10 d differentiation. (C–F) hPSC were implanted in the thigh complex of a SCID mouse using a collagen sponge carrier (2.5 × 105 cells, sponge size 2.0 × 1.0 × 0.5 cm). (C) High resolution radiography at 2 and 4 weeks post-implantation. (D) 3-Dimensional MicroCT reconstructions at Th90. (E,F) Pentachrome staining of histological sections. eb: ectopic bone, cb: cortical bone, m: muscle. N = 5 implants, from N = 1 patient specimens. Reproduced with permission from James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, Pang S, Stoker D, Ting K, Peault B, Soo C. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. Jun 2012; 1(6):510–9. PMCID: PMC3659717.
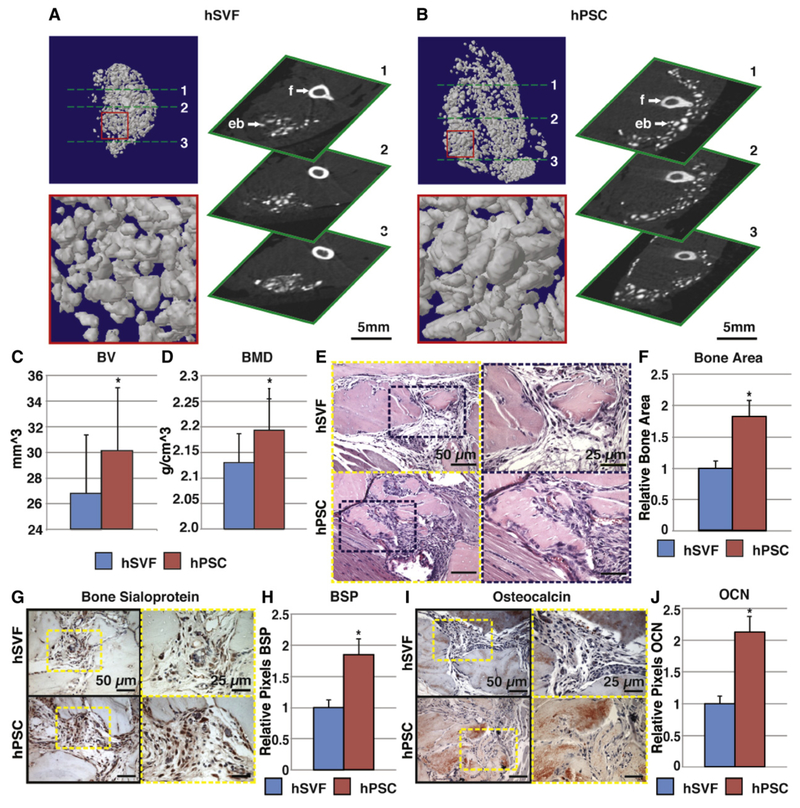
Next, the relative bone-forming efficacy of PSC was compared to the unpurified stromal vascular fraction (SVF) from the same patient’s adi-pose sample (Fig. 4, reproduced with permission from James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, Pang S, Stoker D, Ting K, Peault B, Soo C. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. Jun 2012; 1(6):510–9. PMCID: PMC3659717). Here, a demineralized bone matrix scaffold was used for an additional osteoinductive stimulus. Results showed that PSC intramuscular implantation led to larger particles of bone by microCT imaging (Fig. 4A,B), increased quantitative metrics of bone formation by microCT (Fig. 4C,D), and increased bone matrix accumulation by routine histology (Fig. 4E,F), which was confirmed by immunohistochemical staining of bone-specific markers (Fig. 4G–J). Increased bone formation among PSC treated groups was accompanied by a quantitative increase in vascularity of the implant site, accompanied by increased VEGF (vascular endothelial growth factor) elaboration (Askarinam et al., 2013). Overall, these studies showed that pericytes and adventitial cells either alone or combined result in significant ectopic bone formation. Moreover and for the first time it was observed that these FACS purified cell populations outcompete unpurified stroma from the same patient sample in terms of bone-forming efficacy.
Fig. 4.
hPSC undergo more robust differentiation in comparison to human stromal vascular fraction (hSVF). Equal numbers of viable hSVF cells or hPSC (2.5 × 105) from the same patient samples were implanted intramuscularly in the thigh of a SCID mouse. An osteoinductive demineralized bone matrix (DBX®) putty was used as scaffold. Assessments were performed at 4 weeks post-implantation. (A,B) MicroCT images of hSVF and hPSC-treated samples. Representative 3-dimensional microCT reconstructions at Th90 (left) and corresponding 2-dimensional axial slices (right). (C,D) Analysis of bone volume (BV) and bone mineral density (BMD) among hSVF- and hPSC-treated samples. Th50–120. (E) Representative H&E staining. (F) Representative histomorphometric quantification of bone area per 100× field. (G) Representative bone sialoprotein (BSP) immunohistochemistry and (H) quantification of relative staining per 400× field. (I) Representative osteocalcin (OCN) immunohistochemistry and (J) quantification of relative staining per 400× field. Histomorphometric quantification calculated from N = 6 random microscopical fields. N = 12 implants per cell type, N = 3 patient specimens. *P < 0.05 in comparison to control as assessed by a Student’s t-test. eb: ectopic bone; f: femur. Reproduced with permission from James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, Yuan W, Chang L, Corselli M, Shen J, Pang S, Stoker D, Ting K, Peault B, Soo C. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med. Jun 2012; 1(6):510–9. PMCID: PMC3659717.
3.2. Pericytes in bone defect healing
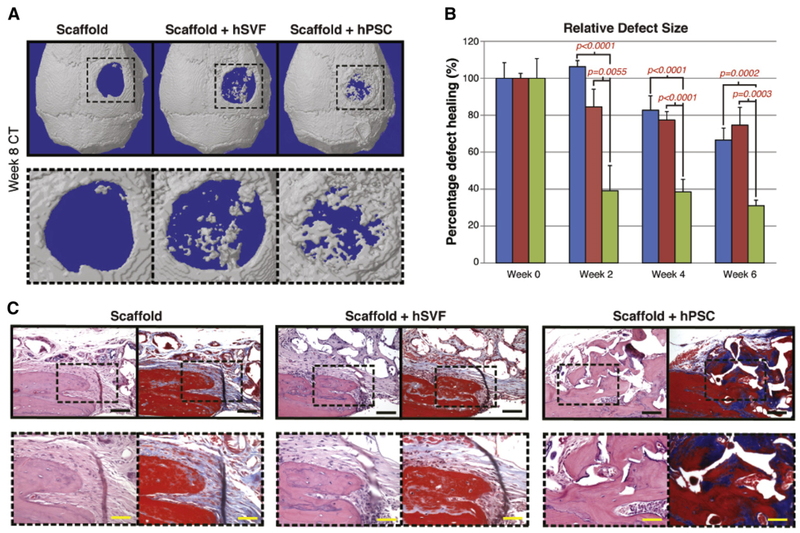
The bone-forming advantage of purified PSC was next extended to a more clinically relevant model of bone repair – a murine calvarial defect (Fig. 5, reproduced with permission from James AW, Zara JN, Corselli M, Askarinam A, Zhou AM, Hourfar A, Nguyen A, Megerdichian S, Asatrian G, Pang S, Stoker D, Zhang X, Wu B, Ting K, Peault B, Soo C. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med. Sep 2012; 1(9):673–84. PMCID: PMC3659737). Here, equal numbers of unpurified SVF or PSC from the same patient were implanted in a 3 mm non-healing calvarial defect centered in the parietal bone. A further acellular control was also used. Radiographic and histo-logic analysis showed that (similar to the intramuscular implantation model) PSC led to a significant increase in bony regenerate at the defect site with significant bone defect healing overtime. In comparison, unpurified SVF from the same patient had no statistically significant benefit in comparison to an acellular scaffold. PSC induced bone defect healing in this model was observed to be a result of both direct ossification of PSC and indirect paracrine effects exerted by PSC. Thus, across both ectopic and orthotopic bone models, PSC showed significantly greater potential for bone formation in comparison to unpurified stroma. Whether these findings correlated with the enrichment of osteogenic PSC, or conversely the elimination of an inhibitory cell type within the heterogeneous stroma, is still a matter of conjecture.
Fig. 5.
Calvarial healing by microCT and histology. hSVF or hPSC were used to treat a 3 mm diameter parietal bone defect in a SCID mouse. A custom-made hydroxyapatite coated PLGA scaffold was used as a carrier. Defects were either treated with an empty scaffold, or scaffold seeded with cells (scaffold + hSVF, or scaffold + hPSC). See Table S2 for details of treatment groups. (A) 3 dimensional reconstructions of control, hSVF, or hPSC treated calvarial defects shown at 8 weeks postoperative. CT threshold of 40 was used. (B) Relative defect healing as assessed by 0, 2, 4 and 6 weeks post-operative by serial live microCT scans. Relative defect area was calculated using a top-down view of the calvaria using AMIDE software images, followed by Adobe Photoshop quantification of relative defect size. (C) Representative H&E and Masson’s Trichrome images for the defect site. Images are taken from the lateral defect edge to delineate old from new bone. N = 16–18 mice per treatment group split equally between N = 4 separate patient samples. Black scale bar: 50 μm. Yellow scale bar: 25 μm. Reproduced with permission from James AW, Zara JN, Corselli M, Askarinam A, Zhou AM, Hourfar A, Nguyen A, Megerdichian S, Asatrian G, Pang S, Stoker D, Zhang X, Wu B, Ting K, Peault B, Soo C. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med. Sep 2012; 1(9):673–84. PMCID: PMC3659737.
3.3. Other orthopedic applications
Perivascular stem cell populations have been examined in other orthopedic models, including spinal fusion and atrophic non-union. Each will discussed sequentially below.
Spinal fusion models represent a tremendous biomedical burden. Spinal fusion procedures are performed at an approximate frequently of 350,000 surgeries performed per annum in the United States alone (Deyo et al., 2005; Gray et al., 2006; Katz, 1995; Lee & Langrana, 2004; Taylor et al., 1994). This represents an astounding 200% increase over the past decade (Deyo et al., 2005; Gray et al., 2006; Katz, 1995; Lee & Langrana, 2004; Taylor et al., 1994). Spinal fusion models represent a more functionally demanding environment for a bone graft substitute that must withstand significant biomechanical stress. Chung et al. (2015) examined the effects of PSC application in a rat posterolateral lumbar spinal fusion model. Briefly, human PSC implantation was performed across three cellular densities in the rat model, using a demineralized bone matrix scaffold as a carrier (Chung et al., 2015). Results showed that PSC showed a dose-dependent increase in endochondral ossification, increase in bone deposition, increase in theoretical measurements of bone strength, and 100% fusion between lumbar bone segments (Chung et al., 2015). Like other studies, new bone regenerate was observed to be a product of both direct PSC osteodifferentiation as well as host bone osteoblastogenesis (Chung et al., 2015). By species-specific immunohistochemistry, the indirect effects of host bone seemed to predominate (Chung et al., 2015). Later, Lee et al. (2015) extended these observations to rats rendered osteoporotic by gonadectomy. Here, greater numbers of implanted PSC were observed to surmount the effects of systemic osteoporosis (Lee et al., 2015).
Atrophic non-union is associated with biological failure of fracture healing. A prospective observational study performed in 41 trauma centers reported that the risk of delayed or non-union in open fractures with a wound either less than 5 cm or, greater than 5 cm was increased by 3.6 and 5.7 times respectively, when compared to fractures with no skin injuries (Audige et al., 2005). Other factors predisposing to atrophic non-union have been described, including smoking and vitamin D deficiency, among others. It has been thought that atrophic non-unions occurs in the setting of impaired biological activity, especially blood supply. Recent evidence does not, however, support this hypothesis. Reed et al. (2002) demonstrated no difference in the number of blood vessels in human atrophic non-union tissue when compare to hypertrophic non-union tissue. Animal studies have shown the pattern of change in vascularity at different time points in normal fracture healing and in atrophic non-union. The vascularity of atrophic non-unions was less in the first three weeks following injury but reached the same level as that in normal healing bone at later time-points (Reed et al., 2003). Bajada et al. (2009) successfully isolated MSC from atrophic non-union tissues; however, the growth kinetic and the osteogenic ability of these cells were diminished. These findings suggest that risk of atrophic non-union might be related to the number and functionality of progenitor cells at the site of atrophic non-union.
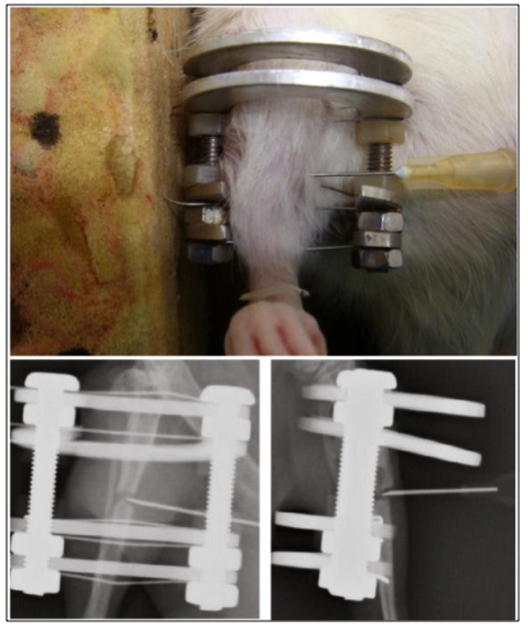
To test the applicability of pericyte therapies for non-union, we examined a well-established model of rat tibial atrophic non-union (Figs. 6,7) (Reed et al., 2003; Tawonsawatruk et al., 2014). Pericytes were percutaneously injected 3 weeks after the procedure for establishing fibrous non-union (Tawonsawatruk et al., 2016). Results showed that pericyte injection increased fracture callus size, increased mineralization, eventually resulting in increased osseous union (Tawonsawatruk et al., 2016). In contrast to the previously mentioned models, pericyte injection in non-union did not show significant persistence of implanted cells when defect sites were interrogated by species specific immunohistochemistry (Tawonsawatruk et al., 2016). These data suggest that at least in the inhospitable microenvironment of atrophic non-union, the benefit of pericytes primarily resides in their trophic abilities.
Fig. 6.
Atrophic non-union of the rat tibia. Atrophic non-unions are characterized by an absence of callus and atrophic bone ends.
Fig. 7.
Local percutaneous injection technique for pericyte delivery in an atrophic non-union model.
3.4. Mechanistic considerations for pericyte mediated bone formation
As previously mentioned, pericyte implantation likely results in improved bone healing by both direct and indirect mechanisms. Clearly, evidence for direct ossification of implanted perivascular cells has been found in vivo. For example, co-localization of labels of mineralization (Calcein or Alizarin complexone) with human-specific antigens identified foci of direct perivascular cell ossification (Lee et al., 2015). Nevertheless, immunohistochemical detection of species-specific antigens shows that osteoblasts and osteocytes within new bone are far more commonly of host rather than donor origin (Chung et al., 2015). For example, in a human perivascular cell implantation into rat model, rat derived osteoblasts outnumber human derived osteoblasts by a factor of 3.8 to 1 or more (Chung et al., 2015). Thus, the majority of new-formed bone is not of perivascular cell derivation, and an underlying paracrine mechanism was hypothesized. Supporting this hypothesis, original descriptions showed that pericytes are robust cytokine sources (Chen et al., 2009). For example, human pericytes secrete 5–20 times more heparin-binding epidermal growth factor (HB-EGF), fibroblast growth factor-2 (FGF-2), VEGF, and keratinocyte growth factor than classically derived adipose tissue or cord blood MSC (Chen et al., 2009). These initial observation have been confirmed by next generation sequencing studies examining pericytes to unpurified stroma from the same patient sample. By RNA-Seq, pericytes express 42 to 532 times higher expression of Bone Morphogenetic Protein transcripts (including BMP2, BMP4, and BMP7), as well as high expression of transcript within the fibroblast growth factor and platelet derived growth factor families (unpublished data). From this, it is clear that pericytes are both a rich source of a growth and differentiation factors, as well as a cell type directly responsible for the in vivo process of bone defect healing.
4. Pericytes in cartilage tissue engineering
4.1. Need for cell-based cartilage repair
Damage and degeneration of articular, meniscal and intervertebral cartilage is a significant problem with a large socio-economic burden. The end-stage treatment of deficient cartilage in di-arthroidal joints is prosthetic replacement, most commonly for the hip and knee but increasingly for the shoulder, ankle, elbow, intervertebral disks as well as for the small joints of the hand and foot. Fusion can also be undertaken, typically in the spine and ankle, but also in the small joints of the foot and hand. The economic burden of osteoarthritis has been estimated to cost ~1–2.5% of GDP in western countries (March & Bachmeier, 1997). In the US, this equates to more than $40 billion per year. Joint replacement and fusion both have significant drawbacks and an ability to regenerate cartilage would reduce their use.
Current biological therapies include [1] microfracture for marrow stimulation, [2] osteochondral autograft/allograft for replacement, and [3] cultured chondrocytes to generate new matrix. None of these approaches has so far demonstrated long-term efficacy (Vanlauwe, et al., 2011). Autologous chondrocytes lose their chondrogenic phenotype in vitro (Tuan, 2007; Zeifang et al., 2010; Oldershaw, 2012), which represents a significant barrier to use. Research has concentrated on the use of MSC for cartilage repair and regeneration (Sekiya et al., 2015; Yu et al., 2015). Filardo et al. (2013) reviewed animal and clinical studies into MSC and articular cartilage repair, and found that there was a very heterogeneous mix of studies and outcome data. In some studies, MSC implantation results in inappropriate ossification rather than cartilage formation (Hwang & Elisseeff, 2009; Oldershaw, 2012). The lack of uniformity in outcome measures and control groups results in substantial difficulty in drawing overarching conclusions.
Despite pericytes and other perivascular stem cells demonstrating increased bone-forming potential as compared to other conventional MSC, little is known at this time regarding their relative promise for cartilage formation.
4.2. Pericytes in cartilage development and pathology
The long bones and their synovial joints are formed from condensations of the apical ectodermal ridge. Pericytes have been identified within the vascular channels of the developing fetal epiphyseal cartilage (Anderson et al., 2000; Su et al., 2015). The development of the meniscal cartilages also has an association with pericytes. Osawa et al. isolated cells from fetal menisci and demonstrated that the cells found in the vascular region contained a greater proportion of perivascular cells compared to the relatively avascular part of the menisci suggesting they may be involved in the development of meniscal cartilage (Giurea et al., 2006; Osawa et al., 2013). Pericytes have also been found to be present in the callus formation between fractured long bones and are associated with the formation of chondroblasts and repair of the fracture (Brighton & Hunt, 1997). Strikingly, Matthews et al. (2014) used an inducible pericyte reporter animal to show that SMA+ pericytes give rise to a large portion of cells within a fracture callus.
As well as their association with the normal development and regeneration of cartilage, pericytes have been implicated in pathological processes involving cartilage formation. Angiogenesis and associated pericytes have been implicated in ectopic calcification of blood vessels, heart valves and skeletal muscle (Collett & Canfield, 2005). In the musculo-skeletal system they have been studied in osteoarthritis, but their functional role remains unclear. Su et al. (2015) analyzed cells from osteoarthritic cartilage and demonstrated a CD146+ population of chondroprogenitor cells with increased chondrogenic potential compared to chondrocytes or adipose derived MSC. This has been mirrored by an increase in Stro-1+ cells in the synovial tissue of patients with osteoarthritis (Giurea et al., 2006). In addition, pericytes have also been found in the increased microvascular network within cartilage tumors (Kalinski et al., 2009).
It is clear from the data above that pericytes have a functional role in normal development of cartilage, repair of osteocartilaginous tissues, as well as pathological processes involving cartilage formation.
4.3. Pericytes and cartilage regeneration
The majority of research has used chondrogenesis as a marker of the mesenchymal multi-lineage potential of pericytes but have not quanti-fied or compared this potential to other cell types (Brighton & Hunt, 1997; Anderson et al., 2000; Farrington-Rock et al., 2004). Microvascular pericytes have been shown to express Aggrecan, a key constituent of cartilage extra-cellular matrix (Diefenderfer & Brighton, 2000), as well as other proteins associated with chondrogenesis (Canfield & Schor, 1991). It has also been demonstrated that Wnt/β-catenin signaling enhances the chondrogenic differentiation of pericytes (Kirton et al., 2007; Zhang et al., 2015).
Recently, Li et al. (2016) endeavored to optimize perivascular stem cell chondrogenesis in vitro using a combination of growth and differentiation factors (GDFs). Here, a combination of NELL-1 (NEL-like molecule-1), TGF-β3 (transforming growth factor beta 3), and BMP-6 (bone morphogenetic protein 6) was observed to accelerate PSC chondrogenic differentiation in pellet culture in comparison to other growth and differentiation factor combinations, as shown by gene and protein markers of chondrogenesis. Interestingly, markers of chondrocyte hypertrophy, fibrosis, or osteogenic differentiation were not upregulated by this growth and differentiation factor combination, suggesting the potential clinical utility in this approach.
Zhang et al. investigated the ability of pericytes and adventitial cells to support chondrocytes in pellet cultures. Their results pointed to differential effects of pericytes versus adventitial cells in their support of chondrocytic differentiation. Specifically, adventitial cells were observed to promote chondrocyte proliferation, while pericytes predominantly supported the production of glycosaminoglycans and collagen II (Hagmann et al., 2014; Zhang et al., 2015). This enhancement of glycosaminoglycan production by pericytes was supported by Hagmann et al. (2014), in comparison to unsorted MSC.
In a somewhat related set of studies, Wu et al. separated cultured MSC on their expression of CD146 and found in vitro that the CD146+ MSC demonstrated increased chondrogenic potential compared to CD146− MSC (Collett & Canfield, 2005; Hagmann et al., 2014). They also found that the CD146+ MSC had a positive impact in their murine arthritis model compared with CD146− cells. At this point, it is not clear the exact phenotypic overlap between culture-derived CD146+ MSC and FACS derived pericytes.
5. Discussion
In summary, the application of pericytes for orthopedic indications is a significant and growing field of study. Among orthopedic indications, the two most promising clinical applications are use in a bone graft substitute for spinal fusion and stimulation of fracture repair (Fig. 8). The use of PSC to stimulate spinal fusion (as the cellular component of a bone graft substitute) has previously documented preclinical efficacy (Chung et al., 2015). Likewise, pericyte based stimulation of fracture healing (as an injectable cell suspension) has recently shown proof of principle efficacy in a murine model (Tawonsawatruk et al., 2016). There exist both pros and cons to an approach using a purified pericyte population for orthopedic applications. Indeed, there are numerous broadly applicable advantages to using a cell sorting rather than culture-based approach to cell purification. A cell sorting approach bypasses the time, costs, and risks of cell culture, and confers a greater degree of homogeneity to the cell population. Risks of cell culture range from actual to theoretical, and include issues of immunogenicity, infection, and genetic instability (Dahl et al., 2008; Rosland et al., 2009). Precise cell identity may also improve regulatory approval of a cellular constituent for tissue engineering. As well, high expression of growth and differentiation among purified perivascular cells may, from a pharmacologic perspective, exert greater paracrine effects to speed tissue regeneration. For an ease of use standpoint, a cell sorting approach allows for a one-time operative procedure, with the significant potential to reduce operating room and hospitalization costs.
Fig. 8.
Brief graphical illustration of potential clinical applications of pericytes. Among orthopedic applications, the use to stimulate spinal fusion (as the cellular component of a bone graft substitute), or fracture healing (as an injectable cell suspension) are the most promising. Both indications have preclinical studies demonstrating efficacy of a pericyte/perivascular cell based product.
Conversely, there are several drawbacks for the use of a purified cellular constituent for tissue engineering. First, the isolation of PSC is admittedly a complex procedure. For example, PSC isolation requires identification of multiple cell surface antigens, each requiring specific FACS antibodies. Each antibody used adds complexity to the regulatory approval for eventual clinical translation of a PSC-based bone graft substitute. In contrast, multiple companies already have SVF-based tissue engineering products in development (e.g. IntelliCell Biosciences, Osiris Therapeutics), and so regulatory approval of an SVF-based technology for orthopedic repair would have significant predicates.
Pericytes and other perivascular stem cells have significant overlap with culture-based MSC. In the field of bone tissue engineering, both PSC and other MSC sources have significant potential for local bone repair. It is clear that PSC exert significant paracrine effects on osseous regeneration, although a mixture of direct and indirect effects underlie their bone healing capacity. All these findings are broadly applicable comparisons to culture-derived MSC. In the case of adipose tissue, it is clear that PSC outperform unpurified stroma (SVF) in bone forming capacity. Nevertheless, direct head-to-head comparisons of PSC to other MSC sources has not yet been performed (e.g. BMSC or ASC). Our unpublished observation show that PSC undergo more robust osteogenic differentiation in vitro in comparison to other MSC cell types. Pericyte and adventitial PSC may compare favorably to classically derived MSC for future bone repair and regeneration applications.
The field of pericyte application for cartilage regeneration and repair is in its relative beginnings as compared to bone applications. Fascinatingly, pericytes and other perivascular stem cells may play differential roles when it comes to supporting the process of chondrogenic differentiation. Divergent effects of pericytes and other PSC have not been observed in the context of bone tissue engineering. All in all, pericytes may have a role to play in cell-based cartilage regeneration – where most cell-based therapies have yielded disappointing results.
Acknowledgments
The present work was supported by the NIH/NIAMS (grants R01 AR061399, R01 AR066782, K08 AR068316), and Orthopaedic Research and Education Foundation with funding provided by the Musculoskeletal Transplant Foundation (Grant no. 20151006). IRM is supported by a Wellcome Trust funded Edinburgh Clinical Academic Track (ECAT) Lectureship (ref. 097483).
Abbreviations:
- BMD
bone mineral density
- BMSC
bone marrow mesenchymal stem cells
- BV
bone volume
- FACS
fluorescence activated cell sorting
- MSC
mesenchymal stem cells
- SVF
stromal vascular fraction
- PSC
perivascular stem cells
Footnotes
Conflict of interest statement
K.T. and C.S. are inventors of perivascular stem cell-related patents filed from UCLA. K.T. and C.S. are founders of Scarless Laboratories, Inc. which sublicenses perivascular stem cell-related patents from the UC Regents, and who also hold equity in the company. C.S. is also an officer of Scarless Laboratories, Inc.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, & Begley DJ (2010). Structure and function of the blood-brain barrier. Neurobiol Dis. 37, 13–25. [DOI] [PubMed] [Google Scholar]
- Anderson HC, Hodges PT, Aguilera XM, Missana L, & Moylan PE (2000). Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J Histochem Cytochem 48, 1493–1502. [DOI] [PubMed] [Google Scholar]
- Anz AW, Hackel JG, Nilssen EC, & Andrews JR (2014). Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg 22, 68–79. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, … Betsholtz C (2010). Pericytes regulate the blood-brain barrier. Nature. 468, 557–561. [DOI] [PubMed] [Google Scholar]
- Askarinam A, James AW, Zara JN, Goyal R, Corselli M, Pan A, … Soo C (2013). Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue Eng Part A 19, 1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audige L, Griffin D, Bhandari M, Kellam J, & Ruedi TP (2005). Path analysis of factors for delayed healing and nonunion in 416 operatively treated tibial shaft fractures. Clin Orthop Relat Res 438, 221–232. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Gardner TW, & Abcouwer SF (2011). The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci 52, 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajada S, Marshall MJ, Wright KT, Richardson JB, & Johnson WE (2009). Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone 45, 726–735. [DOI] [PubMed] [Google Scholar]
- Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, & Bellantuono I (2004). Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 22, 675–682. [DOI] [PubMed] [Google Scholar]
- Brighton CT, & Hunt RM (1997). Early histologic and ultrastructural changes in microvessels of periosteal callus. J Orthop Trauma 11, 244–253. [DOI] [PubMed] [Google Scholar]
- Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R, … Madeddu P (2010). Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation 121, 1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield AE, & Schor AM (1991). Identification and partial characterisation of a low Mr collagen synthesised by bovine retinal pericytes. Apparent relationship to type X collagen. FEBS Lett 286, 171–175. [DOI] [PubMed] [Google Scholar]
- Chen CW, Montelatici E, Crisan M, Corselli M, Huard J, Lazzari L, & Peault B (2009). Perivascular multi-lineage progenitor cells in human organs: regenerative units, cytokine sources or both? Cytokine Growth Factor Rev 20, 429–434. [DOI] [PubMed] [Google Scholar]
- Chung CG, James AW, Asatrian G, Chang L, Nguyen A, Le K, … Soo C (2015). Human perivascular stem cell-based bone graft substitute induces rat spinal fusion. Stem Cells Transl Med 4, 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett GD, & Canfield AE (2005). Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res 96, 930–938. [DOI] [PubMed] [Google Scholar]
- Corselli M, Chen CW, Sun B, Yap S, Rubin JP, & Peault B (2012). The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21, 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covas DT, Piccinato CE, Orellana MD, Siufi JL, Silva WA Jr., Proto-Siqueira R, … Zago MA (2005). Mesenchymal stem cells can be obtained from the human saphena vein. Exp Cell Res 309, 340–344. [DOI] [PubMed] [Google Scholar]
- Cox G, McGonagle D, Boxall SA, Buckley CT, Jones E, & Giannoudis PV (2011). The use of the reamer–irrigator–aspirator to harvest mesenchymal stem cells. J Bone Joint Surg Br 93, 517–524. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, … Peault B (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313. [DOI] [PubMed] [Google Scholar]
- Dahl JA, Duggal S, Coulston N, Millar D, Melki J, Shahdadfar A, … Collas P (2008). Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol 52, 1033–1042. [DOI] [PubMed] [Google Scholar]
- Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, … Itskovitz-Eldor J (2012). Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 125, 87–99. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, … Cossu G (2007). Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9, 255–267. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, … Cossu G (2011). Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2, 499. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Gray DT, Kreuter W, Mirza S, & Martin BI (2005). United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 30, 1441–1445 (discussion 1446–1447). [DOI] [PubMed] [Google Scholar]
- Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, … Díaz-Flores L Jr. (2009). Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 24, 909–969. [DOI] [PubMed] [Google Scholar]
- Diefenderfer DL, & Brighton CT (2000). Microvascular pericytes express aggrecan message which is regulated by BMP-2. Biochem Biophys Res Commun 269, 172–178. [DOI] [PubMed] [Google Scholar]
- Doherty MJ, & Canfield AE (1999). Gene expression during vascular pericyte differentiation. Crit Rev Eukaryot Gene Expr 9, 1–17. [PubMed] [Google Scholar]
- Dulauroy S, Di Carlo SE, Langa F, Eberl G, & Peduto L (2012). Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 18, 1262–1270. [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, & Canfield AE (2004). Chondrogenic and adipogenic potential of microvascular pericytes. Circulation 110, 2226–2232. [DOI] [PubMed] [Google Scholar]
- Feng J, Mantesso A, De Bari C, Nishiyama A, & Sharpe PT (2011). Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A 108, 6503–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, & Lindauer U (2010). Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A 107, 22290–22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, & Kon E (2013). Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc 21, 1717–1729. [DOI] [PubMed] [Google Scholar]
- Gad S (2008). Pharmaceutical Manufacturing Handbook: Regulations and Quality. Hoboken, NJ: John Wiley and Sons. [Google Scholar]
- Giurea A, Ruger BM, Hollemann D, Yanagida G, Kotz R, & Fischer MB (2006). STRO-1+ mesenchymal precursor cells located in synovial surface projections of patients with osteoarthritis. Osteoarthritis Cartilage 14, 938–943. [DOI] [PubMed] [Google Scholar]
- Gray DT, Deyo RA, Kreuter W, Mirza SK, Heagerty PJ, Comstock BA, & Chan L (2006). Population-based trends in volumes and rates of ambulatory lumbar spine surgery. Spine (Phila Pa 1976) 31, 1957–1963 (discussion 1964). [DOI] [PubMed] [Google Scholar]
- Hagmann S, Frank S, Gotterbarm T, Dreher T, Eckstein V, & Moradi B (2014). Fluorescence activated enrichment of CD146+ cells during expansion of human bone-marrow derived mesenchymal stromal cells augments proliferation and GAG/DNA content in chondrogenic media. BMC Musculoskelet Disord 15, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, Chu CR, … Abdel Aziz MT (2010). The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage 1, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, & Nicosia RF (2005). The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol 289, C1396–C1407. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, & Xu Q (2004). Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest 113, 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang NS, & Elisseeff J (2009). Application of stem cells for articular cartilage regeneration. J Knee Surg 22, 60–71. [DOI] [PubMed] [Google Scholar]
- Invernici G, Emanueli C, Madeddu P, Cristini S, Gadau S, Benetti A, … Alessandri G (2007). Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol 170, 1879–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Zara JN, Corselli M, Askarinam A, Zhou AM, Hourfar A, … Soo C (2012a). An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med 1, 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Zara JN, Zhang X, Askarinam A, Goyal R, Chiang M, … Soo C (2012b). Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl Med 1, 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski T, Sel S, Kouznetsova I, Ropke M, & Roessner A (2009). Heterogeneity of angiogenesis and blood vessel maturation in cartilage tumors. Pathol Res Pract 205, 339–345. [DOI] [PubMed] [Google Scholar]
- Katz JN (1995). Lumbar spinal fusion. Surgical rates, costs, and complications. Spine (Phila Pa 1976) 20, 78S–83S. [PubMed] [Google Scholar]
- Kawate K, Yajima H, Ohgushi H, Kotobuki N, Sugimoto K, Ohmura T, … Takakura Y (2006). Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs 30, 960–962. [DOI] [PubMed] [Google Scholar]
- Khan WS, Adesida AB, Tew SR, Lowe ET, & Hardingham TE (2010). Bone marrow-derived mesenchymal stem cells express the pericyte marker 3G5 in culture and show enhanced chondrogenesis in hypoxic conditions. J Orthop Res 28, 834–840. [DOI] [PubMed] [Google Scholar]
- Khan WS, Tew SR, Adesida AB, & Hardingham TE (2008). Human infrapatellar fat pad-derived stem cells express the pericyte marker 3G5 and show enhanced chondrogenesis after expansion in fibroblast growth factor-2. Arthritis Res Ther 10, R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirton JP, Crofts NJ, George SJ, Brennan K, & Canfield AE (2007). Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res 101, 581–589. [DOI] [PubMed] [Google Scholar]
- Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, … Humphreys BD (2015). Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, & Langrana NA (2004). A review of spinal fusion for degenerative disc disease: need for alternative treatment approach of disc arthroplasty? Spine J 4, 173S–176S. [DOI] [PubMed] [Google Scholar]
- Lee S, Zhang X, Shen J, James AW, Chung CG, Hardy R, … Soo C (2015). Brief report: human perivascular stem cells and Nel-like protein-1 synergistically enhance spinal fusion in osteoporotic rats. Stem Cells 33, 3158–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Zhang X, Peault B, Jiang J, Ting K, Soo C, & Zhou YH (2016). Accelerated chondrogenic differentiation of human perivascular stem cells with NELL-1. Tissue Eng Part A 22, 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, … Cancedda R (2007). Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng 13, 947–955. [DOI] [PubMed] [Google Scholar]
- March LM, & Bachmeier CJ (1997). Economics of osteoarthritis: a global perspective. Baillieres Clin Rheumatol 11, 817–834. [DOI] [PubMed] [Google Scholar]
- Matthews BG, Grcevic D, Wang L, Hagiwara Y, Roguljic H, Joshi P, … Kalajzic I (2014). Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. J Bone Miner Res 29, 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraglia A, Cancedda R, & Quarto R (2000). Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 113(Pt 7), 1161–1166. [DOI] [PubMed] [Google Scholar]
- Murray IR, & Peault B (2015). Q&A: mesenchymal stem cells — where do they come from and is it important? BMC Biol 13, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, … Peault B (2014). Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci 71, 1353–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschler GF, Matsukura Y, Nitto H, Boehm CA, Valdevit AD, Kambic HE, … Powell KA (2005). Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Kapadia A, & Stewart DJ (2011). Review: molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol 37, 3–23. [DOI] [PubMed] [Google Scholar]
- Oldershaw RA (2012). Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol 93, 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa A, Harner CD, Gharaibeh B, Matsumoto T, Mifune Y, Kopf S, … Huard J (2013). The use of blood vessel-derived stem cells for meniscal regeneration and repair. Med Sci Sports Exerc 45, 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli G, Tazzari PL, Vaselli C, Foroni L, Buzzi M, Storci G, … Conte R (2007). Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells 25, 1627–1634. [DOI] [PubMed] [Google Scholar]
- Pietras K, & Ostman A (2010). Hallmarks of cancer: interactions with the tumor stroma.Exp Cell Res 316, 1324–1331. [DOI] [PubMed] [Google Scholar]
- Psaltis PJ, Harbuzariu A, Delacroix S, Holroyd EW, & Simari RD (2011). Resident vascular progenitor cells–diverse origins, phenotype, and function. J Cardiovasc Transl Res. 4, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AA, Joyner CJ, Brownlow HC, & Simpson AH (2002). Human atrophic fracture non-unions are not avascular. J Orthop Res 20, 593–599. [DOI] [PubMed] [Google Scholar]
- Reed AA, Joyner CJ, Isefuku S, Brownlow HC, & Simpson AH (2003). Vascularity in a new model of atrophic nonunion. J Bone Joint Surg Br 85, 604–610. [DOI] [PubMed] [Google Scholar]
- Ren Z, Wang J, Zhu W, Guan Y, Zou C, Chen Z, & Zhang YA (2011). Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro. Exp Cell Res 317, 2950–2957. [DOI] [PubMed] [Google Scholar]
- Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, … Schichor C (2009). Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 69, 5331–5339. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Muneta T, Horie M, & Koga H (2015). Arthroscopic transplantation of syno-vial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res 473, 2316–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Shrestha S, Yen YH, Asatrian G, Mravic M, Soo C, … James AW (2015b).Pericyte antigens in perivascular soft tissue tumors. Int J Surg Pathol 23, 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Shrestha S, Yen YH, Scott MA, Asatrian G, Barnhill R, … James AW(2015a). Pericyte antigens in angiomyolipoma and PEComa family tumours. Med Oncol 32, 210. [DOI] [PubMed] [Google Scholar]
- Su X, Zuo W, Wu Z, Chen J, Wu N, Ma P, … Qiu G (2015). CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J Orthop Res 33, 84–91. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, … Graff JM (2008). White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawonsawatruk T, Kelly M, & Simpson H (2014). Evaluation of native mesenchymal stem cells from bone marrow and local tissue in an atrophic nonunion model. Tissue Eng Part C Methods 20, 524–532. [DOI] [PubMed] [Google Scholar]
- Tawonsawatruk T, West CC, Murray IR, Soo C, Péault B, & Simpson AH (2016). Adipose derived pericytes rescue fractures from a failure of healing–non-union. Sci Rep 6, 22779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VM, Deyo RA, Cherkin DC, & Kreuter W (1994). Low back pain hospitalization. Recent United States trends and regional variations. Spine (Phila Pa 1976) 19, 1207–1212 (discussion 1213). [DOI] [PubMed] [Google Scholar]
- Tallone T, Realini C, Böhmler A, Kornfeld C, Vassalli G, Moccetti T, … Soldati G (2011). Adult human adipose tissue contains several types of multipotent cells. J Cardiovasc Transl Res. 4, 200–210. [DOI] [PubMed] [Google Scholar]
- Tonti GA, & Mannello F (2008). From bone marrow to therapeutic applications: different behaviour and genetic/epigenetic stability during mesenchymal stem cell expansion in autologous and foetal bovine sera? Int J Dev Biol 52, 1023–1032. [DOI] [PubMed] [Google Scholar]
- Tuan RS (2007). A second-generation autologous chondrocyte implantation approach to the treatment of focal articular cartilage defects. Arthritis Res Ther 9, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP, & Tig/Act, & Group, E. X. T. S. (2011). Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med 39, 2566–2574. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, & Yoneda M (2002). Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage 10, 199–206. [DOI] [PubMed] [Google Scholar]
- Yu H, Adesida AB, & Jomha NM (2015). Meniscus repair using mesenchymal stem cells — a comprehensive review. Stem Cell Res Ther 6, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannettino AC, Paton S, Kortesidis A, Khor F, Itescu S, & Gronthos S (2007). Human mulipotential mesenchymal/stromal stem cells are derived from a discrete subpopulation of STRO-1bright/CD34/CD45(−)/glycophorin-A-bone marrow cells. Haematologica 92, 1707–1708. [DOI] [PubMed] [Google Scholar]
- Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, & Schmitt H (2010). Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med 38, 924–933. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ba K, Wu L, Lee S, Peault B, Petrigliano FA, … Lin Y (2015). Adventitial cells and pericytes support chondrogenesis through different mechanisms in 3-dimensional cultures with or without nanoscaffolds. J Biomed Nanotechnol 11, 1799–1807. [DOI] [PubMed] [Google Scholar]
- Zimmerlin L, Donnenberg VS, Rubin JP, & Donnenberg AD (2013). Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 83, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]