Abstract
HIV-positive individuals are at increased risk for precancerous anal squamous intraepithelial lesions (SILs). Anal cytology and digital rectal examination are performed as screening tools, but extensive training and appropriate instruments are required to follow up on an abnormal anal cytology. Thus, novel approaches to SIL evaluation could improve better health care follow-up by efficient and timely diagnosis to offer treatment options. Recently, Raman-enhanced spectroscopy (RESpect) has emerged as a potential new tool for early identification of SIL. RESpect is a noninvasive, label-free, laser-based technique that identifies molecular composition of tissues and cells. HIV-serodiscordant couples had anal biopsies obtained during high-resolution anoscopy. RESpect was performed on the specimens. Principal component analysis of the data identified differences between normal and abnormal tissue as well as HIV-positive and HIV-negative individuals of each couple even with similar pathologies. RESpect has the potential to change the paradigm of anal pathology diagnosis and could provide insight into different pathways leading to SIL in HIV-serodiscordant couples.
Keywords: Raman spectroscopy, anal neoplasia, HIV serodiscordant
Introduction
The incidence of anal neoplasia is increased in people living with HIV and is particularly high among HIV-positive men who have sex with men (MSM) as well as transgender women.1–4 However, even in HIV-negative MSM, anal cancer incidence is high.5 Anal cancer incidence has been increasing 2.2% per year over the past decade with more than 7,200 cases reported in the United States in 2015, with anal squamous cell carcinomas comprising 80% of the cases.6 While ∼80% of anal cancers in persons with HIV infection occur in MSM, limited data suggest similar trends for transgender women.2,7 Progress in understanding the pathology and diagnostic approaches to anal neoplasia was paved by advances that were made in cervical cancer.8 However, anal cancer is not an AIDS-defining cancer even though the incidence is higher in HIV-positive individuals and is linked to human papillomavirus (HPV).5,9 While early detection of invasive anal carcinoma may be associated with improved prognosis, universal acceptance of screening and follow-up remains challenging in the absence of randomized controlled clinical studies and long-term surveillance studies.10–12
Although HPV is implicated in anal cancer, the actual role that HIV plays remains relatively unknown.13 Immunosuppression associated with HIV likely plays a part in anal cancer pathogenesis along with HPV, but delineating specific HIV effects could further improve our understanding of anal cancer and other cancers.14 Thus, in addition to understanding HPV/HIV mechanisms leading to anal cancer, better screening and follow-up of at-risk patients remain challenging particularly since there are no universally accepted screening guidelines.3,9,14,15 Therefore, better tools could assist health care providers through more efficient screening paradigms to assist with anal cancer diagnosis.12
As a relatively new technique to assess biological specimens, Raman-enhanced spectroscopy (RESpect) has been applied to analyze malignant cells and tissues.16–18 It is a laser-based tool that characterizes molecular and chemical composition through inelastic scattering of photons.19 As light passes through chemical bonds, photons interacting with chemical bonds can pass on energy to photons and create a shift in energy. This resulting energy shift of scattered photons corresponds to specific molecular bonds of cells and tissues.20 The RESpect data provide details of proteins, carbohydrates, lipids, and DNA content, which could potentially be used to enhance diagnosis.20
Regarding RESpect as a potential clinical tool, it is nondestructive and label-free, and thus could provide a unique opportunity to identify cellular composition without requiring chemical manipulation of tissue for diagnosis. Using cell lines, previous data demonstrated unique RESpect chemical characteristics of B cell lymphoid cells, which were different from normal B cells.21 Others analyzed cervical cancer tissues that identified distinct RESpect profiles.22–24 RESpect could be used to characterize unique fingerprints of normal and abnormal anal pathology to aid in diagnosis and treatment responses.
The present study reports on a unique opportunity to identify RESpect fingerprint differences and/or similarities of anal tissue pathology between individuals of HIV-serodiscordant couples. The study also reports on the RESpect fingerprint of HIV itself in the context of the HIV-serodiscordant dyad couples. The RESpect profiles of normal anal tissue and anal tissue with high-grade squamous intraepithelial lesion (HSIL) and low-grade squamous intraepithelial lesion (LSIL), intraepithelial lesions from HIV-positive and HIV-negative individuals, contribute novel information about differences due to HIV as well as new information about chemical compositions across the spectrum of anal pathology. To what extent HIV affects the pathogenesis of anal neoplasia is currently unknown, and thus, the data from this study supplement present knowledge about LSIL and HSIL.
Materials and Methods
Participants and specimens
The study was approved in accordance with the University of Hawaii Institutional Review Board. Participants were enrolled in a clinical research study as HIV-serodiscordant couples focusing on identifying differences/similarities in anal pathology. Inclusion criteria were MSM and/or transgender women, self-identified couple relationship, and self-identified HIV-serodiscordant status. Exclusion criteria were current treatment for any type of malignancy or any condition preventing full participation in the study. Anal cytology and high-resolution anoscopy (HRA) were performed on each participant and at least two biopsies were obtained from areas that appeared abnormal under HRA: the first specimen was flash frozen in liquid nitrogen for RESpect analysis and the second biopsy was obtained from the same site for pathology assessment. Obtaining biopsies from the same abnormal area by HRA allowed for comparing pathology with RESpect analysis. Any other abnormal areas observed by HRA were also assessed clinically.
The frozen tissue was cut and placed in 0.9% saline solution for RESpect analysis. All participants were followed up clinically after the clinical pathology reports were available.
Laboratory HIV-1 strains were grown and also assessed by RESpect to characterize unique peaks attributable to the virus.25,26 U937 cells (NIH AIDS Reagents Program, MD) were added to a 15 mL conical tube and pelleted by spinning at 2,000 rpm for 20 min at 4°C. Cells (2 × 106 cells) were resuspended with HIV-1 strains of IIIB, JR-CSF, or P89.6 (NIH AIDS Reagents Program, MD) at a concentration of 15 ng/mL. Inoculated cells were incubated at 37°C for 60 min with light shaking every 10 min. Cells were washed with phosphate-buffered saline, vortexed, and spun down at 2,000 rpm for 20 min at 4°C. Cells were resuspended in complete Roswell Park Memorial Institute Medium and transferred to a T-25 culture flask. Cells were incubated upright at 37°C and 5% CO2. Cultures were examined every other day for the presence of giant cells. Virus culture supernatants were cryopreserved at 4–5-day intervals, 6–8 days after initial viral exposure. Virus cultures were diluted with 0.9% saline solution and mounted onto reflective aluminum slides and dried before RESpect analysis.
Raman spectroscopy data acquisition
RESpect data were acquired using a Raman microscope as previously described.21 Briefly, the tissue (0.5 mm thickness) was mounted on reflective aluminum slides (Anomet, Inc., ON, Canada) cleaned with ethanol. The RESpect data were captured using a 40-point scan with 15-s exposures with 15 accumulations, using a micro-Raman RXN system (KOSI, Inc., Ann Arbor, MI) with a 785 nm laser and automated xyz-microscope stage and 50 μm slit width. Each data point was collected with 10 mW laser power at the sample.
Data analysis
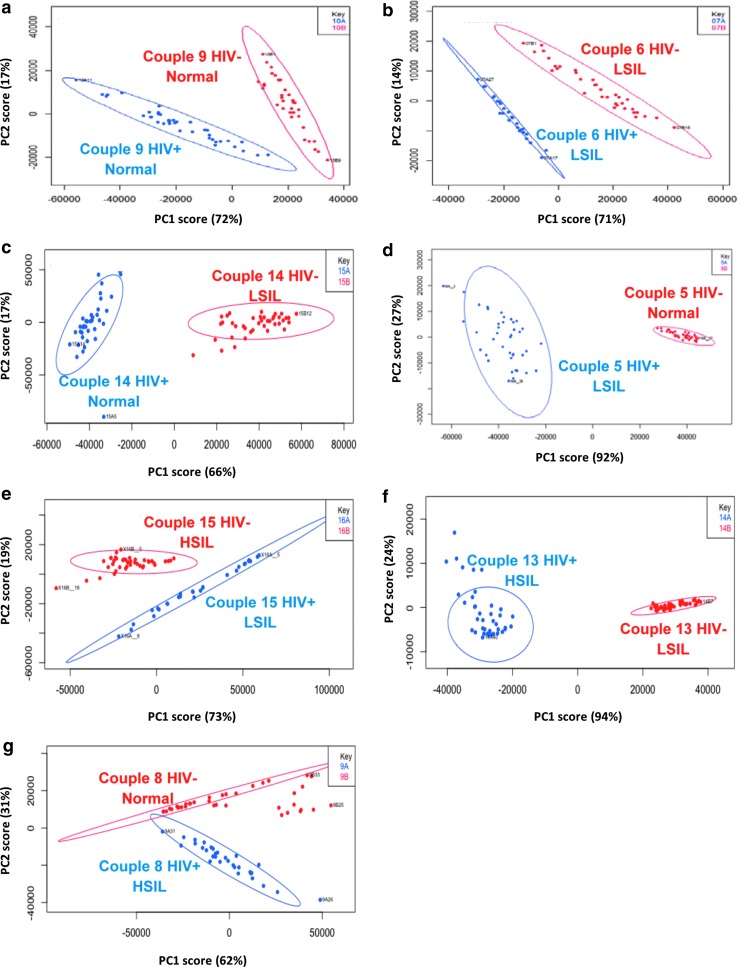
Asymmetric least square (AsLS) was applied to each spectrum for baseline correction data (n = 40 scans per sample).27 AsLS simultaneously corrects baselines of the acquired spectra by estimating the baselines by penalizing differences in corrected signals and smoothes the resultant peaks in the region of 600–1,800 cm−1. Spectra from the processed data were averaged to produce the RESpect profile of each sample (Fig. 1). To compare RESpect data between individuals of each HIV-serodiscordant couple, principal component analysis (PCA) was conducted and a scree plot was generated to represent the total coverage of variance in the data.
FIG. 1.
Principal component analysis graphs for representative HIV-serodiscordant couples. Couples 9 and 6 had similar anal pathologies between serodiscordant individuals (a, b). Couples 14, 5, 15, 13, and 8 had different anal pathologies between the serodiscordant individuals (c–g). Ninety-nine percent robust prediction ellipses were created, which group the points not affected by the outliers (points outside the circle). LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
Spectra were graphed using the first two principal component (PC) scores. The first two PC scores were used for clustering analysis since >90% of the total variance scores were covered. Data analyses were conducted using custom scripts written in MATLAB and R. Mahalanobis distances (MDs) were computed to assess distance between the centers of each PCA cluster.28 Hotelling's two-sample T2 statistics was then calculated and an F-value was obtained for each couple. F-values were converted to a p-value based on degrees of freedom.
Results
Thirty individuals representing 15 HIV-serodiscordant couples had adequate specimens available for this study. A summary of the demographics of the participants is provided in Table 1, including age, ethnicity, and mean/nadir CD4 cell count (HIV positive). Even though HIV status was self-reported, the HIV-positive individuals were referred by clinics from which they were receiving HIV care. The HIV-seronegative status of the partner was not confirmed. All participants had anal cytology performed and underwent HRA with anal biopsies for the study.
Table 1.
Participant Demographics
| HRA survey variable | HIV negative (n = 15) | HIV positive (n = 15) | p-Value |
|---|---|---|---|
| Ethnicity, non-Hispanic | 13 (87%) | 12 (80%) | .564 |
| Race | .892 | ||
| Black | 1 (7%) | 0 (0%) | |
| Asian | 1 (7%) | 2 (13%) | |
| White | 7 (47%) | 6 (40%) | |
| NHOPI | 1 (7%) | 3 (20%) | |
| More than one | 5 (33%) | 4 (27%) | |
| HIV status, positive | 0 (0%) | 15 (100%) | <.001 |
| Gender | .480 | ||
| Male | 15 (100%) | 13 (87%) | |
| Transgender | 0 (0%) | 2 (13%) | |
| Smoking status | .262 | ||
| Current smoker | 3 (20%) | 3 (20%) | |
| Past smoker | 6 (40%) | 4 (27%) | |
| Never smoked | 5 (33%) | 6 (40%) | |
| Missing | 1 (7%) | 2 (13%) | |
| History of anal dysplasia | .041 | ||
| No | 13 (87%) | 9 (60%) | |
| Yes | 0 (0%) | 6 (40%) | |
| Missing | 2 (13%) | 0 (0%) | |
| Anal cytology result | .892 | ||
| Negative | 8 (53%) | 6 (40%) | |
| LSIL | 1 (7%) | 2 (13%) | |
| HSIL | 0 (0%) | 1 (7%) | |
| ASCUS | 6 (40%) | 6 (33%) | |
| ASC-H | 0 (0%) | 1 (7%) | |
| High-resolution anoscopy | |||
| Negative | 11 (73%) | 10 (67%) | .655 |
| LSIL | 7 (47%) | 7 (47%) | 1.000 |
| HSIL | 2 (13%) | 3 (20%) | .655 |
| Other (insufficient) | 1 (7%) | 1 (7%) | 1.000 |
| Age (years), median (IQR) | 49 (33–63) | 50 (36–59) | .838 |
| Most recent viral load, median (IQR) | 0 (0–0) | NA | |
| Nadir CD4 count, median (IQR) | 100 (40–300) | NA |
McNemar's test or Bowker's test for categorical variables and paired t-test for continuous variables were used to compare how well the pairs match on each variable.
HRA, high-resolution anoscopy; NHOPI, native Hawaiian, other Pacific Islander; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; ASCUS, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells, cannot exclude HSIL; IQR, interquartile range (25–75 percentile); NA, not applicable.
The focus of the analyses was to assess PCA comparisons between the two individuals of each of the 15 dyads. The PCA graphs demonstrated unique differences between the two individuals of each couple separated by anal pathology and RESpect data as demonstrated in representative PCA graphs in serodiscordant couples (Fig. 1). Within each couple, distinct clusters were formed that demonstrated statistically significant separations between HIV-positive and HIV-negative individuals. Within each HIV-serodiscordant couple who had different pathology, PCA was able to discriminate between low-grade and high-grade pathologies in HIV-positive (blue) and HIV-negative (red) individuals (Fig. 1). In couples from whom the anal biopsies had the same pathologies (Fig. 1a, b) and those with different pathologies (Fig. 1c–g), the PCA plots continued to demonstrate statistically significant (p < .05) separation of RESpect data.
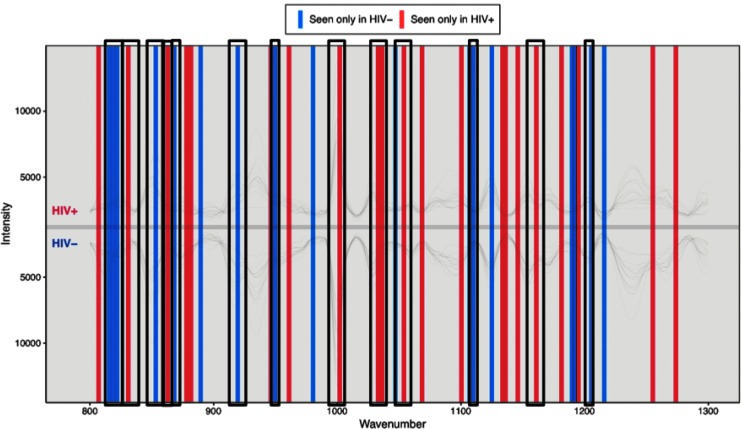
Within all individuals, analysis was conducted to obtain peaks that contributed to PCA separation. PCA loadings were graphed against each other to identify unique peaks, accounting for the maximum PCA separation in HIV-negative and HIV-positive individuals (Fig. 2). For HIV-negative individuals, the peaks corresponded to proline (815, 853, 868, 918, and 950 cm−1), tyrosine (822, 853, and 1,205 cm−1), collagen (1,204 cm−1), and phenylalanine (1,107 and 1,208 cm−1). For HIV-positive individuals, the peaks corresponded to proline (830 and 1,066 cm−1), tyrosine (831, 1,032, and 1,066 cm−1), collagen (859 and 1,034 cm−1), and phenylalanine (1,002 and 1,031 cm−1).29 In addition, analysis of HIV-1 strains IIIB, JR-CSF, and P89.6 showed prominent peaks at Raman shifts of 855, 1,000, 1,454, and 1,659 cm−1 after baseline correction by AsLS using the PROcess package in R. These peaks correspond to tyrosine in collagen, phenylalanine, collagen CH3 bending and CH2 scissoring, and amide I in collagen-like proteins.29
FIG. 2.
RESpect peaks. RESpect plot depicts unique peaks in HIV-negative and HIV-positive individuals at specific wave numbers after asymmetric least squares baseline correction. Peaks correspond to various chemical compositions such as DNA bases, lipids, proteins, and bond stretching. Highlighted peaks correspond to proline (815, 830, 853, 868, 918, 950, 1,066 cm−1), tyrosine (822, 831, 853, 1,032, 1,066, 1,205 cm−1), collagen (859, 1,034, 1,204 cm−1), and phenylalanine (1,002, 1,031, 1,107, 1,208 cm−1). RESpect, Raman-enhanced spectroscopy.
Discussion
RESpect is a tool that can be used in a variety of settings as it is both nondestructive and label free, making it particularly attractive to analyze biological specimens.30–32 In contrast to preparing tissue samples for clinical diagnostic tests, RESpect analysis requires minimal tissue preparation with no staining, fixing, or other tissue manipulation. For clinical pathology assessment, paraffin-embedded tissue is typically prepared for the diagnostic workup.33 It is possible to analyze paraffin-embedded tissue by RESpect, however, the paraffin manipulation potentially induces background peaks.34 In addition to the background paraffin RESpect signals, clean RESpect signals are difficult to obtain because of the use of xylene to remove paraffin from the tissue before RESpect analysis.35,36
Thus, the protocol used in this study consisted of flash freezing the tissue specimen; tissue preservation and integrity were maintained, providing cleaner and more representative PCA data. The use of 0.9% saline solution in preparing the tissue for RESpect capture did not alter the spectra of the samples as saline does not present any additional spectral peaks and could actually enhance the signal of Raman scattering.37
RESpect data from all 15 couples showed clustering and significant separation of PCA data based on MD calculation (Table 2). From the 10 couples whose anal pathologies differed, the RESpect data showed significant separation of the PCA plots. These differences may reflect not only differences in anal pathologies but could also represent an overlapping HIV effect since the couples are HIV serodiscordant. These data are consistent with studies of cervical neoplasia that demonstrated RESpect differences across the spectrum of cervical cancers.22,38,39
Table 2.
Summary of Principal Component Analysis Results
| Couple participants | Anal biopsy pathology | RESpect result Mahalanobis distance significance (p < .0001) |
|---|---|---|
| Couple 1 HIV+ | Normal | Yes |
| Couple 1 HIV− | Normal | |
| Couple 2 HIV+ | LSIL | Yes |
| Couple 2 HIV− | Normal | |
| Couple 3 HIV+ | LSIL | Yes |
| Couple 3 HIV− | HSIL | |
| Couple 4 HIV+ | LSIL | Yes |
| Couple 4 HIV− | LSIL | |
| Couple 5 HIV+ | LSIL | Yes |
| Couple 5 HIV− | Normal | |
| Couple 6 HIV+ | LSIL | Yes |
| Couple 6 HIV− | LSIL | |
| Couple 7 HIV+ | Normal | Yes |
| Couple 7 HIV− | Normal | |
| Couple 8 HIV+ | HSIL | Yes |
| Couple 8 HIV− | Normal | |
| Couple 9 HIV+ | Normal | Yes |
| Couple 9 HIV− | Normal | |
| Couple 10 HIV+ | Normal | Yes |
| Couple 10 HIV− | LSIL | |
| Couple 11 HIV+ | Normal | Yes |
| Couple 11 HIV− | LSIL | |
| Couple 12 HIV+ | HSIL | Yes |
| Couple 12 HIV− | LSIL | |
| Couple 13 HIV+ | HSIL | Yes |
| Couple 13 HIV− | LSIL | |
| Couple 14 HIV+ | Normal | Yes |
| Couple 14 HIV− | LSIL | |
| Couple 15 HIV+ | LSIL | Yes |
| Couple 15 HIV− | HSIL |
RESpect, Raman-enhanced spectroscopy.
To support a hypothesis that HIV per se may account for RESpect differences, the five couples whose anal pathologies between the HIV-serodiscordant individuals were the same, the separation of their respective PCA data was statistically significant. Analysis of HIV strains IIIB, JR-CSF, and P89.6 produced peaks that were consistent with peaks previously described for HIV antigens and HIV antibodies.40 Furthermore, a loading plot generated peaks that contributed the most to the separation of PCA clustering. The protein/chemical peaks identified from the tissue RESpect data could potentially be used to differentiate differences in pathology and possibly infectious agents.40–42
If validated in the future, use of RESpect technology in this setting could change the paradigm of diagnostic algorithms across the spectrum of diseases. Peaks corresponding to tyrosine, collagen, and phenylalanine were found using RESpect that also differentiated peaks in HIV itself. The potential importance and application of tyrosine/phenylalanine peaks related to HIV lie in both use of RESpect to detect HIV and also understanding the potential mechanistic role of tyrosine/phenylalanine in inflammatory pathways leading to diseases.43–45 If these findings are confirmed in future studies and analyses, the HIV RESpect characteristics could be leveraged to understand cancer pathogenesis and possibly novel intervention strategies, as well as a new tool to assess viral reservoirs in HIV cure agenda.46
Because of the importance of differentiating LSIL from HSIL from a clinical intervention perspective, identifying chemical characteristics through RESpect could also potentially lead to a more improved and efficient approach to diagnosis and treatment strategies. Currently, during HRA, biopsies are obtained from areas of concern after anal cytology and/or digital rectal examination, suggesting further evaluation.11,15,47 The poor sensitivity and specificity of anal cytology screening advocate for improving screening tools for anal cancer.47 When concurrent anal cytology screens and high-resolution anal biopsies were compared, concordance with specificity and sensitivity ranged from poor to moderate.47 Abnormal anal cytologies overall had an 83.8% rate of biopsy-proven disease, however, sensitivity was higher (92%) for high-grade anal intraepithelial neoplasia or worse (AIN2+).47 Overall detection of AIN2+ by cytology showed specificity of 26% with negative predictive value of 92% and positive predictive value of 26%.47 Therefore, if HSIL is diagnosed, treatment may be recommended, but for LSIL diagnoses, treatment intervention may not be necessary and screening capabilities could be improved.48,49
If RESpect technology could be adapted to differentiate LSIL from HSIL during HRA, an improved paradigm for screening, treatment, and follow-up could potentially transform health care for this disease by decreasing the time interval from diagnosis to therapeutic decisions. Recently, clinical applications of Raman systems have been theorized and explored.50 The development of probes coupled with Raman systems has been proposed for a variety of epithelial cell cancers.51–54 These systems described in the literature are all custom built using commercially available Raman lasers, probes, and charge-coupled devices. These probes open the possibility of cancer detection but are limited to only spectral analysis. Currently, high-speed, label-free, optical Raman systems are being explored to create images of cancerous tissues and cells.55,56 Future work into coupling a visual system into a probe could prove to be cost-effective as it would provide instant pathological identification of cancerous tissue without the need for additional pathology work or sample manipulation.
While there are limitations of the study design and interpretation of the results, the data provide a compelling alternative or supplemental tool for screening and studying anal neoplasia. A cost analysis of incorporating the probe into clinical practice is beyond the scope of this study, however, given that, three to four anal biopsies associated with clinical pathology-related costs versus a real-time assessment of tissue during HRA could surmount to potential cost savings. The number of HIV-serodiscordant couples is relatively small, but the study is ongoing and the resulting data could provide additional validation for testing a real-time RESpect probe in a clinical trial. Another limitation is that even though HIV status was known for the HIV-positive individual, the HIV status of the seronegative partner was not confirmed due to limited resources. However, the ability to test for HIV DNA in the biopsy specimens is possible as one of the next steps for the future as well as assessing HPV subtypes and copy numbers. Our group and others have used RESpect to evaluate cervical pathology as well as differentiating HPV itself.22,57,58
The present study provides the foundation that RESpect technology has the potential to differentiate anal pathology along with insight into HIV. Additional work and studies are needed to determine the specificity and sensitivity of RESpect in differentiating anal pathologies. Future work in this field could pave the way toward new diagnosis paradigms and less invasive techniques in the management of anal SIL.
Acknowledgments
The authors acknowledge all participants in the ongoing study at the Hawaii Center for AIDS and the Histology and Imaging core of John A. Burns School of Medicine for guidance on handling of tissue samples. Supported, in part, by R21CA216830, U54MD007584, and U54MD007601.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Roberts JR, Siekas LL, Kaz AM: Anal intraepithelial neoplasia: A review of diagnosis and management. World J Gastrointest Oncol 2017;9:50–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kobayashi T, Sigel K, Gaisa M: Prevalence of anal dysplasia in human immunodeficiency virus-infected transgender women. Sex Transm Dis 2017;44:714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palefsky JM: Anal squamous intraepithelial lesions in human immunodeficiency virus-positive men and women. Semin Oncol 2000;27:471–479 [PubMed] [Google Scholar]
- 4. Buzard CL, Rizzolo D: An overview of anal intraepithelial neoplasia. JAAPA 2018;31:1–5 [DOI] [PubMed] [Google Scholar]
- 5. Salati SA, Al Kadi A: Anal cancer—a review. Int J Health Sci 2012;6:206–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Cancer Institute Surveillance E and End Results Program: SEER stat fact sheets. Available at http://seer.cancer.gov/statfacts/html/anus.html accessed October24, 2018
- 7. Thompson AB, Gillespie SE, Mosunjac MB, Hussen SA, Flowers LC, Camacho-Gonzalez AF: Prevalence of anal squamous intraepithelial lesions in HIV-1-infected young men who have sex with men and transwomen. J Low Genit Tract Dis 2018;22:340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wasserman P, Rubin DS, Turett G: Review: Anal intraepithelial neoplasia in HIV-Infected men who have sex with men: Is screening and treatment justified? AIDS Patient Care STDS 2017;31:245–253 [DOI] [PubMed] [Google Scholar]
- 9. Palefsky JM, Rubin M: The epidemiology of anal human papillomavirus and related neoplasia. Obstet Gynecol Clin North Am 2009;36:187–200 [DOI] [PubMed] [Google Scholar]
- 10. Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM: The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA 1999;281:1822–1829 [DOI] [PubMed] [Google Scholar]
- 11. Mallari AO, Schwartz TM, Luque AE, Polashenski PS, Rauh SM, Corales RB: Anal cancer screening in HIV-infected patients: Is it time to screen them all? Dis Colon Rectum 2012;55:1244–1250 [DOI] [PubMed] [Google Scholar]
- 12. Ong JJ, Temple-Smith M, Chen M, et al. : Why are we not screening for anal cancer routinely-HIV physicians' perspectives on anal cancer and its screening in HIV-positive men who have sex with men: A qualitative study. BMC Public Health 2015;15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shiels MS, Engels EA: Increased risk of histologically defined cancer subtypes in human immunodeficiency virus-infected individuals: Clues for possible immunosuppression-related or infectious etiology. Cancer 2012;118:4869–4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grulich AE, Vajdic CM: The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin Oncol 2015;42:247–257 [DOI] [PubMed] [Google Scholar]
- 15. Leeds IL, Fang SH: Anal cancer and intraepithelial neoplasia screening: A review. World J Gastrointest Surg 2016;8:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teixeira RAR, Lataliza AAB, Raposo NRB, Costa LAS, Sant'Ana AC: Insights on the transport of tamoxifen by gold nanoparticles for MCF-7 breast cancer cells based on SERS spectroscopy. Colloids Surf B Biointerfaces 2018;170:712–717 [DOI] [PubMed] [Google Scholar]
- 17. Wu Q, Qiu S, Yu Y, et al. : Assessment of the radiotherapy effect for nasopharyngeal cancer using plasma surface-enhanced Raman spectroscopy technology. Biomed Opt Express 2018;9:3413–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui S, Zhang S, Yue S: Raman spectroscopy and imaging for cancer diagnosis. J Healthc Eng 2018;2018:8619342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butler HJ, Ashton L, Bird B, et al. : Using Raman spectroscopy to characterize biological materials. Nat Protoc 2016;11:664–687 [DOI] [PubMed] [Google Scholar]
- 20. Eberhardt K, Stiebing C, Matthäus C, Schmitt M, Popp J: Advantages and limitations of Raman spectroscopy for molecular diagnostics: An update. Expert Rev Mol Diagn 2015;15:773–787 [DOI] [PubMed] [Google Scholar]
- 21. Shiramizu B, Oda R, Kamada N, et al. : Unique Raman spectroscopic fingerprints of B-cell non-Hodgkin lymphoma: Implications for diagnosis, prognosis and new therapies. J Biol Med Sci 2018;2:pii: [PMC free article] [PubMed] [Google Scholar]
- 22. Kamemoto LE, Misra AK, Sharma SK, et al. : Near-infrared micro-Raman spectroscopy for in vitro detection of cervical cancer. Appl Spectrosc 2010;64:255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyng FM, Traynor D, Ramos IR, Bonnier F, Byrne HJ: Raman spectroscopy for screening and diagnosis of cervical cancer. Anal Bioanal Chem 2015;407:8279–8289 [DOI] [PubMed] [Google Scholar]
- 24. Rubina S, Krishna CM: Raman spectroscopy in cervical cancers: An update. J Cancer Res Ther 2015;11:10–17 [DOI] [PubMed] [Google Scholar]
- 25. Munsaka SM, Agsalda M, Troelstrup D, Hu N, Yu Q, Shiramizu B: Characteristics of activated monocyte phenotype support R5-tropic human immunodeficiency virus. Immunol Immunogenet Insights 2009;1:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gartner S, Popovic M: Virus isolation and production. Techniques in HIV research. Springer, Berlin, Germany, 1990, pp. 53–70 [Google Scholar]
- 27. Boelens HF, Dijkstra RJ, Eilers PH, Fitzpatrick F, Westerhuis JA: New background correction method for liquid chromatography with diode array detection, infrared spectroscopic detection and Raman spectroscopic detection. J Chromatogr A 2004;1057:21–30 [DOI] [PubMed] [Google Scholar]
- 28. Goodpaster AM, Kennedy MA: Quantification and statistical significance analysis of group separation in NMR-based metabonomics studies. Chemometr Intell Lab Syst 2011;109:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Movasaghi Z, Rehman S, Rehman IU: Raman spectroscopy of biological tissues. Appl Spectrosc Rev 2007;42:493–541 [Google Scholar]
- 30. Niedieker D, GrosserÜschkamp F, Schreiner A, et al. : Label-free identification of myopathological features with coherent anti-Stokes Raman scattering. Muscle Nerve 2018;58:456–459 [DOI] [PubMed] [Google Scholar]
- 31. Satoh S, Otsuka Y, Ozeki Y, et al. : Label-free visualization of acetaminophen-induced liver injury by high-speed stimulated Raman scattering spectral microscopy and multivariate image analysis. Pathol Int 2014;64:518–526 [DOI] [PubMed] [Google Scholar]
- 32. Zhang C, Winnard PT, Jr., Dasari S, et al. : Label-free Raman spectroscopy provides early determination and precise localization of breast cancer-colonized bone alterations. Chem Sci 2018;9:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mies C: Molecular pathology of paraffin-embedded tissue. Current clinical applications. Diagn Mol Pathol 1992;1:206–211 [PubMed] [Google Scholar]
- 34. Gaifulina R, Maher AT, Kendall C, et al. : Label-free Raman spectroscopic imaging to extract morphological and chemical information from a formalin-fixed, paraffin-embedded rat colon tissue section. Int J Exp Pathol 2016;97:337–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aydin I, Yörükoglu K, Cingöz S, Agilkaya S: The effect of the alternative solutions to formaldehyde and xylene on tissue processing. Indian J Pathol Microbiol 2013;56:221–230 [DOI] [PubMed] [Google Scholar]
- 36. Meksiarun P, Ishigaki M, Huck-Pezzei VA, et al. : Comparison of multivariate analysis methods for extracting the paraffin component from the paraffin-embedded cancer tissue spectra for Raman imaging. Sci Rep 2017;7:44890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu B, Li P, Zhou B, Tang X, Li S, Yang L: Sodium chloride crystal-induced SERS platform for controlled highly sensitive detection of illicit drugs. Chemistry 2018;24:4800–4804 [DOI] [PubMed] [Google Scholar]
- 38. Jess PR, Smith DD, Mazilu M, Dholakia K, Riches AC, Herrington CS: Early detection of cervical neoplasia by Raman spectroscopy. Int J Cancer 2007;121:2723–2728 [DOI] [PubMed] [Google Scholar]
- 39. Winnard PT, Jr., Zhang C, Vesuna F, et al. : Organ-specific isogenic metastatic breast cancer cell lines exhibit distinct Raman spectral signatures and metabolomes. Oncotarget 2017;8:20266–20287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zinin PV, Hu N, Kamemoto LE, Yu Q, Misra AK, Sharma SK: Raman spectroscopy of HIV-1 antigen and antibody. Paper presented at Smart Biomedical and Physiological Sensor Technology VIII 2011. Orlando, FL [Google Scholar]
- 41. Jermyn M, Desroches J, Aubertin K, et al. : A review of Raman spectroscopy advances with an emphasis on clinical translation challenges in oncology. Phys Med Biol 2016;61:R370–R400 [DOI] [PubMed] [Google Scholar]
- 42. Moor K, Terada Y, Taketani A, Matsuyoshi H, Ohtani K, Sato H: Early detection of virus infection in live human cells using Raman spectroscopy. J Biomed Opt 2018;23:1–7 [DOI] [PubMed] [Google Scholar]
- 43. Gostner JM, Becker K, Kurz K, Fuchs D: Disturbed amino acid metabolism in HIV: Association with neuropsychiatric symptoms. Front Psychiatry 2015;6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Y, Lu C: Raman spectroscopic study on structure of human immunodeficiency virus (HIV) and hypericin-induced photosensitive damage of HIV. Sci China C Life Sci 2005;48:117–132 [DOI] [PubMed] [Google Scholar]
- 45. Zangerle R, Kurz K, Neurauter G, Kitchen M, Sarcletti M, Fuchs D: Increased blood phenylalanine to tyrosine ratio in HIV-1 infection and correction following effective antiretroviral therapy. Brain Behav Immun 2010;24:403–408 [DOI] [PubMed] [Google Scholar]
- 46. Margolis DM, Garcia JV, Hazuda DJ, Haynes BF: Latency reversal and viral clearance to cure HIV-1. Science 2016;353:aaf6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morency EG, Harbert T, Fatima N, Samolcyzk J, Maniar KP, Nayar R: Anal cytology: Institutional statistics, correlation with histology, and development of multidisciplinary screening program with review of the current literature. Arch Pathol Lab Med 2018; [Epub ahead of print]; DOI: 10.5858/arpa.2017-0242-RA [DOI] [PubMed] [Google Scholar]
- 48. Stier EA, Goldstone SE, Berry JM, et al. : Infrared coagulator treatment of high-grade anal dysplasia in HIV-infected individuals: An AIDS malignancy consortium pilot study. J Acquir Immune Defic Syndr 2008;47:56–61 [DOI] [PubMed] [Google Scholar]
- 49. Weis SE: Current treatment options for management of anal intraepithelial neoplasia. OncoTargets Ther 2013;6:651–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pence I, Mahadevan-Jansen A: Clinical instrumentation and applications of Raman spectroscopy. Chem Soc Rev 2016;45:1958–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ming LC, Gangodu NR, Loh T, et al. : Real time near-infrared Raman spectroscopy for the diagnosis of nasopharyngeal cancer. Oncotarget 2017;8:49443–49450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schleusener J, Gluszczynska P, Reble C, et al. : Perturbation factors in the clinical handling of a fiber-coupled Raman probe for cutaneous in vivo diagnostic Raman spectroscopy. Appl Spectrosc 2015;69:243–256 [DOI] [PubMed] [Google Scholar]
- 53. Pramanik A, Chavva SR, Viraka Nellore BP, et al. : Development of a SERS probe for selective detection of healthy prostate and malignant prostate cancer cells using ZnII. Chem Asian J 2017;12:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sharma M, Marple E, Reichenberg J, Tunnell JW: Design and characterization of a novel multimodal fiber-optic probe and spectroscopy system for skin cancer applications. Rev Sci Instrum 2014;85:083101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang D, Wang P, Slipchenko MN, Cheng JX: Fast vibrational imaging of single cells and tissues by stimulated Raman scattering microscopy. Acc Chem Res 2014;47:2282–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Egawa M, Tokunaga K, Hosoi J, Iwanaga S, Ozeki Y: In situ visualization of intracellular morphology of epidermal cells using stimulated Raman scattering microscopy. J Biomed Opt 2016;21:086017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Choi S, Park HK, Min GE, Kim YH: Biochemical investigations of human papillomavirus-infected cervical fluids. Microsc Res Tech 2015;78:200–206 [DOI] [PubMed] [Google Scholar]
- 58. Kim YH, Chang B, Choi JH, Park HK, Choi S: Biochemical fingerprints of human papillomavirus infection and cervical dysplasia using cervical fluids: Spectral pattern investigation. Microsc Res Tech 2016;79:966–972 [DOI] [PubMed] [Google Scholar]