Abstract
Background:
Vitamin D, an important hormone required by the body, exerts its biological effects through Vitamin D receptors (VDRs) present on target cells. Vitamin D is ineffective in tissues which lack VDR. Various tissues show the presence of VDRs. However, evidence for the presence of VDRs in human periodontal ligament tissue in fully erupted teeth in adults is lacking. The present study intends to evaluate the presence of VDRs in periodontal ligament (PDL) tissue and assess their response to serum Vitamin D3 levels in chronic periodontic patients.
Materials and Methods:
A total of 19 chronic periodontitis patients were enrolled in the study and tested for serum 25(OH)D3 levels. Deficient patients were supplemented with Vitamin D3. PDL tissue of these patients was isolated after tooth extraction before and after supplementation of Vitamin D3 and analyzed for the presence of VDR in PDL tissue by using enzyme-linked immunosorbent assay.
Results:
All the chronic periodontitis patients were found to be deficient in Vitamin D3. The mean serum 25(OH)D3 level before supplementation was 13.96 ng/mL which significantly increased to 35.12 ng/mL after supplementation of Vitamin D3 for 6 weeks. VDR analysis determined mean VDR conc. in PDL tissue to be -1.443 ng/mL, which increased to 2.38 ng/mL after supplementation. A concentration dependent correlation was seen between serum 25(OH)D3 levels and VDR conc. in PDL tissue after supplementation.
Conclusions:
The study determined Vitamin D Receptors (VDR) in PDL tissue after supplementation of Vitamin D. Thus in addition to the standard treatment modalities, Vitamin D3 supplementation would be an important factor for generation of adequate immune response.
Keywords: Chronic periodontitis, enzyme-linked immunosorbent assay, periodontal ligament tissue, Vitamin D deficiency, Vitamin D receptor
INTRODUCTION
Vitamin D is a fat-soluble secosteroid required for intestinal absorption of calcium, magnesium, and phosphate. It has direct as well as indirect effects on bone. It is also essential for optimal functioning of various organs and tissues. It exerts its effect by binding to Vitamin D receptors (VDRs) on target cells. Vitamin D is derived from food and ultraviolet B photons. It undergoes hydroxylation in liver to form 25(OH)D3 and further in kidneys by enzyme 1α hydroxylase (1αOHase) to form 1, 25,(OH)2D3 (calcitriol), an active form. This active form is also produced at extrarenal sites. Enzyme 1αOHase (CYP27B1) required for this conversion is expressed by several target cells. In cases of infection, the pathogen-associated molecular patterns such as lipopolysaccharides, interferon gamma, and nitric oxide get attracted to toll-like receptors of macrophages. This regulates cytochrome P27B1 (CYP27B1) genes of the target cells. The expressed enzyme locally hydroxylates the inactive form (25[OH] D3) resulting in the production of calcitriol (1,25[OH] 2D3).[1] This calcitriol exerts its effect on target cells which express VDR. Calcitriol enters the target cell through caveolae, containing VDR or by endocytic uptake of 25(OH)D3-D-binding protein, with the help of glycoproteins such as cubilin and megalin.[2] After entering the cell, 1,25(OH)2D3 ligates with VDR in nucleus and generates vital biological responses. The 1,25(OH)2D3-VDR complex heterodimerizes with retinoid X receptor and shows DNA binding at Vitamin D response element, leading to RNA transcription and biological actions which include modulation of inflammatory response via promoting monocyte differentiation to macrophages; preventing release of inflammatory cytokines; preventing cell surface expression of major histocompatibility complex-II;[3] downregulating T-cells and monocytes; downregulating the expression and production of several pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8; and decreasing the nuclear factor-κB activity which is a transcription factor having a key role in immunomodulation.[4] Vitamin D also regulates the expression of specific endogenous antimicrobial peptides.[5]
Since the discovery of VDR, three decades ago, more than fifty target cells have been identified, involving the receptor-mediated Vitamin D functions.[6] To the best of our knowledge, very few studies have mentioned the presence of VDR in periodontal ligament (PDL) tissue. Investigations by Andrukhov et al.[7] Tang and Meng,[8] McMahon et al.,[9] and Liu et al.[10] have shown VDR-mediated activities in PDL tissue cells. These studies were however investigated on cell cultures and according to Bhalla et al.[11] VDRs actually may not be present in tissues but may be acquired in cultures. They also stated that receptors may appear in vivo when cells are exposed to vascular or inflammatory cell products. Study by Onishi et al. in 2003[12] and Chen in 2012[13] elucidated immunolocalization of VDR during and at completion of root formation. VDR was detected in periodontium. However, these investigations do not confirm the presence of VDR in human PDL (hPDL) tissue of completely erupted teeth in adults. The present study was intended to evaluate the presence of VDRs in PDL tissue and study its response to serum 25(OH)D3 levels. Detection of VDR in hPDL tissue would implicate the involvement of Vitamin D-mediated response in periodontal tissue.
MATERIALS AND METHODS
Study design
A total of 19 chronic periodontitis patients of both sexes with age ranging from 35 to 65 years, visiting the outpatient department of periodontology (from February 2015 to March 2017), were recruited for the present study. Their medical history was taken followed by a detailed periodontal examination. Individuals with a history of any systemic disease, medications (such as bisphosphonates, barbiturates, cardiac glycosides, cholestyramine, glucocorticoids, and laxatives), hypersensitivity reactions, Vitamin D or calcium supplements, smokers, pregnant women, and those with a history of antibiotic therapy within the last 3 months were excluded from the study.
Periodontal examination
The selected patients were examined clinically. Participants diagnosed with moderate-to-severe chronic periodontitis requiring extractions of at least two teeth (e.g., teeth with compound or complex pockets having 70%–75% bone loss on the involved surfaces and 50%–60% bone loss on the remaining surfaces; teeth with Grade III furcation involvement with severe vertical bone loss in interradicular area with 50%–60% bone loss on mesial and distal surfaces; teeth with severe recession on one side and 50%–60% bone loss on other sides along with mobility; patients with partially edentulous arches with 50%–60% bone loss on few remaining teeth and indicated for extraction for prosthodontic purposes) were considered for the study. PDL tissue was scraped from the remaining surfaces for evaluation of VDRs in the study.
The study was approved by the Institutional Ethics Committee (reference number: Perio-II-1/2015–2016). Detailed informed written consent was obtained from all the selected participants, indicating voluntary participation in the study.
Venous blood sample of approximately 2–3 ml was withdrawn in a vacutainer (with clot activator and gel for serum separation) from all the 19 chronic periodontitis patients for assessment of serum 25(OH)D3 levels. ADVIA Centaur Vitamin D Total (Vitamin D) assay (antibody competitive immunoassay) was used for the determination of total 25(OH)D3 in human serum. The reference range[14] used for the study was as follows: deficiency (<20 ng/mL), insufficiency (20–30 ng/mL), sufficiency (30–100 ng/mL), and toxicity (above 100 ng/mL). The sensitivity range of ADVIA Centaur Vitamin D assay is 4.2–150 ng/mL.
Periodontal Phase I therapy (nonsupportive periodontal therapy) and extraction of tooth were carried out. The participants were then referred to physician for supplementation of Vitamin D3. The dose of cholecalciferol (Torrent Pharmaceuticals Ltd, Ahmedabad, India) was 60,000 IU once a week for 6 weeks. After supplementation, the participants were tested for serum 25(OH)D3 levels. This was followed by extraction of the second tooth.
PDL tissue of the extracted tooth was used for the evaluation of VDR concentration. First, the extracted tooth was collected in a vial containing solution of 0.9%W/V sodium chloride, 50 U/mL of penicillin, and 50 μg/mL of streptomycin. The tooth was then washed with the same solution to remove blood completely. PDL tissue was scraped from the root surfaces with sterile curettes and placed in Eppendorf tube containing 1 ml of phosphate-buffered saline. The tubes were centrifuged at 4°C at 5000 gravity for 10 min. The supernatant was removed. Pellet was resuspended in 0.3ml of phosphate buffered saline. The tube was then subjected to three freeze-thaw cycles to facilitate cell lysis.
Enzyme-linked immunosorbent assay procedure
PDL tissue samples were analyzed by using Human VDR ELISA Kit (Elabscience Catalog no E-EL-H2043) for in vitro quantitative determination of human VDR concentrations. The minimum detectable dose of human VDR by this kit was 0.38 ng/ml. The detection range was 0.63–40 ng/ml. The enzyme-linked immunosorbent assay (ELISA) procedure was performed as per the manufacturer's instructions. After preparing standard stock solution of 40 ng/ml and its serial dilutions, optical density (OD) values were obtained. Standard concentrations and OD values were plotted to get a standard curve. Actual ELISA for samples was then assayed using stock solution of 40 ng/ml as control. 100 μl of PDL tissue samples was added in respective wells of ELISA microplate in duplicate. They were incubated for 90 min at 37°C. 100 μl of diluted biotinylated detection antibody was added to all the wells. After incubation for 60 min at 37°C, the wells were washed with wash buffer for 3 times. 100 μl of horseradish peroxide conjugate was added and incubated for 30 min at 37°C. The wash process was repeated 5 times followed by addition of 90 μl of substrate reagent and incubation for 15 min at 37°C. 50 μl of stop solution was then added to it. ELISA plate was read on microplate reader at 450 nm. The OD readings of the samples were plotted on standard graph to get its VDR concentration.
Statistical analysis
Data were statistically analyzed using SPSS version 16.0, IBM Corporation, Armonk, NY, USA. The intragroup comparison was done by paired t- test. To study the predictive reliability (goodness of fit) of the regression models, percentage R2 as a coefficient of determination was used. Correlation analysis was done using Pearson's method (Pearson's r value). P <0.05 indicated statistically significant results (P < 0.05, **P < 0.001, ***P < 0.001), and NS indicated statistically nonsignificant results. Power of the study was 80%.
RESULTS
A total of 19 chronic periodontitis patients were evaluated for 25(OH)D3 levels in serum and VDR concentration in human PDL tissue in the present study.
The mean serum level before supplementation was 13.96 ng/mL with standard deviation of 3.36. The participants then underwent Vitamin D3 supplementation as prescribed by the physician. The mean serum 25(OH)D3 levels 6 weeks after supplementation of 60,000 IU Vitamin D3 increased to 35.12 ng/mL with a standard deviation of 5.27. The results are presented in Table 1 and Figure 1 which depict significant increment in serum 25(OH)D3 levels after supplementation of Vitamin D3 (P < 0.001).
Table 1.
Comparison of mean serum 25(OH)D3 levels before and after supplementation of Vitamin D3
| Before supplementation (n=19) | After supplementation (n=19) | P | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Serum 25(OH)D3 (ng/ml) | 13.96 | 3.36 | 35.12 | 5.27 | 0.001*** |
*Is a standard notation used to determine the level of significance. P value by independent sample “t-” test. P<0.05 is considered to be statistically significant. *P<0.05, **P<0.01, ***P<0.001. SD – Standard deviation; n – Sample size; NS – Statistically nonsignificant
Figure 1.
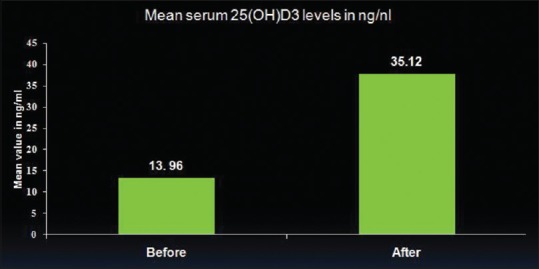
Comparison of mean serum 25(OH)D3 levels before and after supplementation
The present study also involved the investigation of VDR in PDL tissue isolated from 19 participants of the above study group at both time intervals. VDR analysis was done by using Human Vitamin D Receptor ELISA kit. The mean VDR concentration in PDL tissue was calculated from OD values obtained from ELISA readings.
The mean VDR concentration in periodontal ligament tissue of Vitamin D deficient, chronic periodontitis subjects was -1.443/ml with standard deviation of 0.604. The values increased to 2.38 /ml with standard deviation of 2.64 after supplementation, indicating highly significant increment (P-value < 0.001) [Table 2 and Figure 2].
Table 2.
Comparison of mean Vitamin D receptor concentrations before and after supplementation of Vitamin D3
| Before supplementation (n=19) | After supplementation (n=19) | P | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| VDR concentration (ng/ml) | 3.31 | 1.73 | 8.78 | 3.24 | 0.001*** |
*Is a standard notation used to determine the level of significance. P value by independent sample t-test. P<0.05 is considered to be statistically significant. *P<0.05, **P<0.01, ***P<0.001. VDR – Vitamin D receptor; SD – Standard deviation; n – Sample size; NS – Statistically nonsignificant
Figure 2.
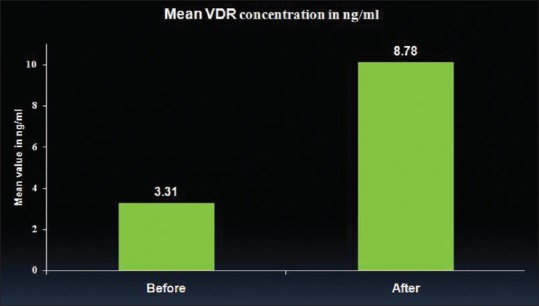
Comparison of mean Vitamin D receptor concentration before and after supplementation
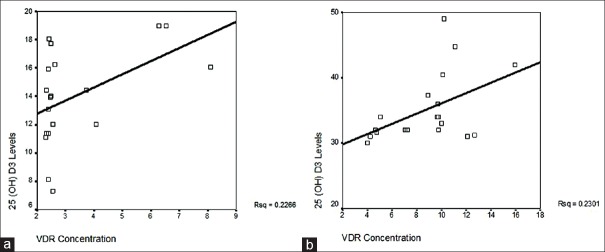
The study also points out the correlation between VDR concentration and serum 25(OH)D3. When correlation was studied between VDR concentration and serum 25(OH)D3. The correlation coefficient “r” value before supplementation was 0.433. The p value obtained was 0.063 which indicated no significant correlation before supplementation. After supplementation the correlation coefficient “r” value was 0.480. The P value obtained was 0.048 which indicated significant positive correlation [Table 3]. The Linear relationship, before as well as after supplementation is presented in Figure 3a and 3b.
Table 3.
Correlation analysis of 25(OH)D3 levels and Vitamin D receptor concentrations before and after supplementation
| Before supplementation (n=19) | After supplementation (n=19) | |||
|---|---|---|---|---|
| Correlation between | ||||
| r | P | r | P | |
| 25(OH)D3 with VDR concentration | 0.476 | 0.039* | 0.480 | 0.038* |
**Is a standard notation used to determine the level of significance. Pearson’s r value, P<0.05 indicated statistically significant correlation. *P<0.05, **P<0.01, ***P<0.001. There was a statistically significant positive correlation between 25(OH)D3 levels and VDR concentrations before supplementation (P<0.05) as well as after supplementation (P<0.05). NS – Statistically nonsignificant; VDR – Vitamin D receptors; n – Sample size
Figure 3.
(a) Linear relationship between serum 25(OH)D3 and VDR concentration in PDL tissue in chronic periodontitis subjects during deficiency state. On pearson's correlation analysis the “r” value before supplementation was 0.433 and the P value obtained was 0.063 indicating no significant positive correlation (P > 0.05) between 25(OH)D3 and VDR concentration in PDL tissue when subjects were deficient; (b) Linear relationship between serum 25(OH)D3 and VDR concentration in periodontitis cases after attaining sufficient range. The correlation coefficient “r” value on supplementation was 0.480 with P value of 0.048. A significant positive correlation was noted (P< 0.05) between serum 25(OH)D3 levels and VDR concentration as subjects became sufficient in D3
The results of present study clearly demonstrated that VDR concentration in periodontal ligament tissue is fully dependent on serum 25(OH)D3 levels. Lower serum concentration of 25(OH)D3 was associated with decreased expression of VDRs and increase in 25(OH)D3 levels in serum was associated with increased expression of VDRs in periodontal ligament tissue.
DISCUSSION
The present study evaluated serum concentrations of 25(OH)D3 and VDR concentration in PDL tissue in chronic periodontitis patients, before as well as after supplementation of Vitamin D3. The study also attempted to correlate serum 25(OH)D3 levels with VDR concentration in PDL tissue.
The results of the study revealed that all the chronic periodontic patients were found to be deficient in Vitamin D3. According to Ritu,[15] Vitamin D3 deficiency is common in the Indian population due to dark skin (increased melanin pigmentation), social and religious norms to cover maximum part of the body, tendency to protect self from sun exposure, vegetarian diet (which lacks Vitamin D), less Vitamin D fortification of food products, and because people live in highly dense areas. Marwaha,[16] Sahu,[17] and Khadilkar[18] have reported high prevalence of Vitamin D deficiency in various groups of the Indian population including healthy School children, adolescents, rural girls, pregnant women, and health-care professionals. The study by Patil[19] also confirmed Vitamin D deficiency in Indian population. They carried out a study on 48 chronic periodontitis and 37 periodontally healthy controls. In their study, 97.9% of chronic periodonttis patients demonstrated 25(OH)D3 deficiency or insufficiency, whereas only 2.1% of the participants had sufficient levels of 25(OH)D3. Studies by Boggess et al.,[20] Chang et al.,[21] Antonoglou et al.,[22] and Abreu et al.[23] have shown association of low Vitamin D3 levels and periodontitis. Thus, Vitamin D deficiency may be considered a risk factor for periodontitis and therefore its evaluation needs to be considered in periodontic patients.
Comparison of 25(OH)D3 levels in the study group determined significant increase after supplementation of Vitamin D3 in all the study participants [Table 1 and Figure 1]. The results corroborated with earlier findings. Harinarayan[24] showed that Vitamin D of 9572 IU/day for 8 weeks significantly raised the serum levels of 25(OH)D and that supplementation up to upper tolerable intake levels is safe and sufficient. The present study suggests that loading dose of cholecalciferol (60,000 IU weekly)[25] for 6 weeks helps in increasing the levels of Vitamin D3 to sufficient range (≥30 ng/ml).
In the present study, Vitamin D3 supplementation was given instead of Vitamin D2. Evidence suggests that cholecalciferol (D3) is superior to ergocalciferol (D2) in terms of potency, elevating and sustaining 25(OH)D concentrations, and maintaining the storage form of Vitamin D.[26,27] Studies by Trang et al.,[28] Armas et al.,[29] and Heaney[30] also found that the D3 supplement was more effective in increasing serum 25(OH)D3 than D2. The dosage used in our study was 60,000 IU/week for 6 weeks as recommended by Vieth et al.[31] and Hathcock et al.[32] Similarly, Gupta et al.[33] and Goswami et al.[25] also used comparable dose and found serum 25(OH)D3 levels to increase.
ELISA kit with D6 antibody was used to determine detectable levels of VDR in the PDL tissue. VDR concentration was found to be very less. Lower VDR concentration has also been demonstrated earlier in infectious condition. Marshall in 2008[34] determined that sulphono-lipid capnine substance produced by some gliding bacteria disabled the VDR. Capnine binds to and inactivates the VDR leading to exaggerated inflammatory responses. Waterhouse et al.[35] found that intracellular bacteria in chronic infectious condition dysregulated Vitamin D metabolism by causing VDR dysfunction within phagocytes. These data suggest the mechanism for reduced VDR levels in chronic infectious condition. In inflammatory conditions, the active macrophages through CYP27B1 vigorously convert 25(OH)D3 to its active form (1,25[OH] 2D3), further increasing the expression of VDR.[36] Conversion of 25(OH)D3 into 1,25(OH)2D3 up to toxic levels has been demonstrated by Marshall in 2008[34] and Waterhouse et al.[36]
In contrast to the above findings, the VDR concentration in the present study was found to be very low. The lower concentration of VDR was probably because of severe deficiency of Vitamin D in chronic periodontitis patients; hence, less 25(OH)D3 was available for conversion to active form, further leading to less expression of VDRs. Moreover, as Phase I therapy was initiated prior to the supplementation of Vitamin D3, there was considerable reduction of plaque-induced inflammation. As inflammation reduced, the response to inflammation was low. The results of the present study thus indicate that in cases of Vitamin D deficiency and infection or inflammation, the concentration of VDR decreases as seen in chronic periodontitis. The decreased levels of VDR would in turn be responsible for reduced effect of Vitamin D-mediated generation of adequate immune and inflammatory response.
The VDR concentration in PDL tissue in chronic periodontic patients in the present study showed direct correlation with serum 25(OH)D3 levels after supplementation with Vitamin D3. After supplementation, D3 ligand binds to VDR and brings about conformational changes in VDR, making it less vulnerable to proteolytic attack. The following studies determined the increased stability of VDR with Vitamin D3. Li et al.[37] found that VDR is degraded by ubiquitin/proteosome pathway. However, after supplementation, D3 bound to VDR and inhibited ubiquitination and retarded degradation, resulting in elevated VDR levels. Kongsbak et al.,[38] Costa et al.,[39] Zineb et al.,[40] and Wiese et al.[41] also found that 1,25(OH)2D3 stabilized and upregulated VDR protein expression by approximately 2–2.3 folds, by protecting it from proteasomal degradation, thus increasing half-life and mean lifetime of the VDR protein. In our study also, supplementation of Vitamin D3 after periodontal Phase I therapy may have helped in decreased degradation/increased stability of VDR and increased availability of 25(OH)D3 for conversion to 1,25(OH)2D3, increased binding of 1,25(OH)2D3 to VDR, thus leading to increased expression of VDRs.
The results of the present study showed that 25(OH)D3 levels in serum and VDR concentration in periodontal ligament tissue was very less in chronic periodontitis patients before supplementation, which increased significantly after supplementation of Vitamin D3. In addition, a significant positive correlation was seen between serum 25(OH)D3 and VDR concentration in Periodontal Ligament tissue, after supplementation of Vitamin D3.
Limitations
The limitation of the study was patient compliance regarding supplementation of Vitamin D3.
CONCLUSION
The present article determined the presence of VDRs in PDL tissue which responded to levels of Vitamin D3 in serum. The VDR levels were very low in subjects patients with Vitamin D3 deficiency, which increased significantly after supplementation of Vitamin D3 for 6 weeks. Vitamin D3 through VDR-mediated effect plays a crucial role in generating immune and anti-inflammatory responses (decreased production of pro-inflammatory cytokines such as interferon-γ, IL-17, IL-21, decreased levels of IL-6, IL-1β, and TNF-α, and increased apoptotic mechanisms in pro-inflammatory cells). Low levels of VDR are indicative of low immune response in these patients. Vitamin D supplementation could enhance VDR levels which in turn may contribute to enhanced immune response. The innovative finding of expression of VDRs in PDL tissue in fully erupted teeth has opened a new gate for further research. Mechanisms to understand its effect on periodontal regeneration (PDL fibroblast uniqueness and bone formation) need to be studied.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bikle DD. Extrarenal synthesis of 1,25 dihydroxyvitamin D and its health implications. Clin Rev Bone Min Metab. 2009;7:114–25. [Google Scholar]
- 2.Rowling MJ, Kemmis CM, Taffany DA, Welsh J. Megalin-mediated endocytosis of Vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr. 2006;136:2754–9. doi: 10.1093/jn/136.11.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine. 2010;77:552–7. doi: 10.1016/j.jbspin.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Harant H, Andrew PJ, Reddy GS, Foglar E, Lindley IJ. 1alpha, 25-dihydroxyvitamin D3 and a variety of its natural metabolites transcriptionally repress nuclear-factor-kappaB-mediated interleukin-8 gene expression. Eur J Biochem. 1997;250:63–71. doi: 10.1111/j.1432-1033.1997.00063.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartley J. Vitamin D: Emerging roles in infection and immunity. Expert Rev Anti Infect Ther. 2010;8:1359–69. doi: 10.1586/eri.10.102. [DOI] [PubMed] [Google Scholar]
- 6.Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206:1188–90. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 7.Andrukhov O, Andrukhova O, Hulan U, Tang Y, Bantleon HP, Rausch-Fan X, et al. Both 25-hydroxyvitamin-D3 and 1,25-dihydroxyvitamin-D3 reduces inflammatory response in human periodontal ligament cells. PLoS One. 2014;9:e90301. doi: 10.1371/journal.pone.0090301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang X, Meng H. Osteogenic induction and 1,25-dihydroxyvitamin D3 oppositely regulate the proliferation and expression of RANKL and the Vitamin D receptor of human periodontal ligament cells. Arch Oral Biol. 2009;54:625–33. doi: 10.1016/j.archoralbio.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 9.McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, Diamond G, et al. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect Immun. 2011;79:2250–6. doi: 10.1128/IAI.00099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K, Meng H, Hou J. Characterization of the autocrine/paracrine function of Vitamin D in human gingival fibroblasts and periodontal ligament cells. PLoS One. 2012;7:e39878. doi: 10.1371/journal.pone.0039878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhalla AK, Wojno WC, Goldring MB. Human articular chondrocytes acquire 1,25-(OH)2 vitamin D-3 receptors in culture. Biochim Biophys Acta. 1987;931:26–32. doi: 10.1016/0167-4889(87)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Onishi T, Okawa R, Murakami H, Ogawa T, Ooshima T, Wakisaka S, et al. Immunolocalization of calbindin D28k and Vitamin D receptor during root formation of murine molar teeth. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:700–4. doi: 10.1002/ar.a.10084. [DOI] [PubMed] [Google Scholar]
- 13.Chen YC, Ninomiya T, Hosoya A, Hiraga T, Miyazawa H, Nakamura H, et al. 1α,25-dihydroxyvitamin D3 inhibits osteoblastic differentiation of mouse periodontal fibroblasts. Arch Oral Biol. 2012;57:453–9. doi: 10.1016/j.archoralbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 15.Ritu G, Gupta A. Vitamin D deficiency in India: Prevalence, causalities and interventions. Nutrients. 2014;6:729–75. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marwaha RK, Sripathy G. Vitamin D & bone mineral density of healthy school children in Northern India. Indian J Med Res. 2008;127:239–44. [PubMed] [Google Scholar]
- 17.Sahu M, Bhatia V, Aggarwal A, Rawat V, Saxena P, Pandey A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in Northern India. Clin Endocrinol (Oxf) 2009;70:680–4. doi: 10.1111/j.1365-2265.2008.03360.x. [DOI] [PubMed] [Google Scholar]
- 18.Khadilkar AV. Vitamin D deficiency in Indian adolescents. Indian Pediatr. 2010;47:755–6. doi: 10.1007/s13312-010-0110-6. [DOI] [PubMed] [Google Scholar]
- 19.Patil VS, Mali RS, Moghe AS. Evaluation of 25(OH)D3 levels in chronic periodontitis and periodontally healthy subjects. Int J Periodontol Implantol. 2017;2:55–60. [Google Scholar]
- 20.Boggess KA, Espinola JA, Moss K, Beck J, Offenbacher S, Camargo CA., Jr Vitamin D status and periodontal disease among pregnant women. J Periodontol. 2011;82:195–200. doi: 10.1902/jop.2010.100384. [DOI] [PubMed] [Google Scholar]
- 21.Chang WP, Chang WC, Wu MS, Pai JT, Guo YC, Chen KC, et al. Population-based 5-year follow-up study in Taiwan of osteoporosis and risk of periodontitis. J Periodontol. 2014;85:e24–30. doi: 10.1902/jop.2013.130256. [DOI] [PubMed] [Google Scholar]
- 22.Antonoglou GN, Knuuttila M, Niemelä O, Raunio T, Karttunen R, Vainio O, et al. Low serum level of 1,25(OH)2 D is associated with chronic periodontitis. J Periodontal Res. 2015;50:274–80. doi: 10.1111/jre.12207. [DOI] [PubMed] [Google Scholar]
- 23.Abreu OJ, Tatakis DN, Elias-Boneta AR, López Del Valle L, Hernandez R, Pousa MS, et al. Low Vitamin D status strongly associated with periodontitis in Puerto Rican adults. BMC Oral Health. 2016;16:89. doi: 10.1186/s12903-016-0288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harinarayan CV, Appicatlaa L, Nalini BA, Joshi S. Efficacy and safety of cholecalciferol supplementation in Vitamin D deficient subjects based on endocrine society clinical practice guidelines. Endocrinol Metabol Syndr. 2012;S4:4. [Google Scholar]
- 25.Goswami R, Vatsa M, Sreenivas V, Singh U, Gupta N, Lakshmy R, et al. Skeletal muscle strength in young Asian Indian females after Vitamin D and calcium supplementation: A double-blind randomized controlled clinical trial. J Clin Endocrinol Metab. 2012;97:4709–16. doi: 10.1210/jc.2012-2340. [DOI] [PubMed] [Google Scholar]
- 26.Houghton LA, Vieth R. The case against ergocalciferol (Vitamin D2) as a Vitamin supplement. Am J Clin Nutr. 2006;84:694–7. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]
- 27.Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96:981–8. doi: 10.1210/jc.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R, et al. Evidence that Vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does Vitamin D2. Am J Clin Nutr. 1998;68:854–8. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 29.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than Vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–91. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 30.Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D (3) is more potent than Vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96:E447–52. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 31.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, et al. The urgent need to recommend an intake of Vitamin D that is effective. Am J Clin Nutr. 2007;85:649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 32.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for Vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 33.Gupta R, Sharma U, Gupta N, Kalaivani M, Singh U, Guleria R, et al. Effect of cholecalciferol and calcium supplementation on muscle strength and energy metabolism in vitamin D-deficient Asian Indians: A randomized, controlled trial. Clin Endocrinol (Oxf) 2010;73:445–51. doi: 10.1111/j.1365-2265.2010.03816.x. [DOI] [PubMed] [Google Scholar]
- 34.Marshall TG. Vitamin D discovery outpaces FDA decision making. Bioessays. 2008;30:173–82. doi: 10.1002/bies.20708. [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse JC, Perez TH, Albert PJ. Reversing bacteria-induced Vitamin D receptor dysfunction is key to autoimmune disease. Ann N Y Acad Sci. 2009;1173:757–65. doi: 10.1111/j.1749-6632.2009.04637.x. [DOI] [PubMed] [Google Scholar]
- 36.Waterhouse JC, Marshall TG, Fenter B, Mangin M, Blaney G. High levels of active 1,25-dihydroxyvitamin D despite low levels of the 25-hydroxyvitamin D precursor-implications of dysregulate Vitamin D for diagnosis and treatment of chronic disease. In: Veronica D, editor. Vitamin D: New Research. Vol. 1. New York: Nova Science Publishers; 2006. pp. 1–23. [Google Scholar]
- 37.Li XY, Boudjelal M, Xiao JH, Peng ZH, Asuru A, Kang S, et al. 1,25-dihydroxyvitamin D3 increases nuclear Vitamin D3 receptors by blocking ubiquitin/proteasome-mediated degradation in human skin. Mol Endocrinol. 1999;13:1686–94. doi: 10.1210/mend.13.10.0362. [DOI] [PubMed] [Google Scholar]
- 38.Kongsbak M, von Essen MR, Boding L, Levring TB, Schjerling P, Lauritsen JP, et al. Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+T cells. PLoS One. 2014;9:e96695. doi: 10.1371/journal.pone.0096695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa EM, Hirst MA, Feldman D. Regulation of 1,25-dihydroxyvitamin D3 receptors by Vitamin D analogs in cultured mammalian cells. Endocrinology. 1985;117:2203–10. doi: 10.1210/endo-117-5-2203. [DOI] [PubMed] [Google Scholar]
- 40.Zineb R, Zhor B, Odile W, Marthe RR. Distinct, tissue-specific regulation of Vitamin D receptor in the intestine, kidney, and skin by dietary calcium and Vitamin D. Endocrinology. 1998;139:1844–52. doi: 10.1210/endo.139.4.5903. [DOI] [PubMed] [Google Scholar]
- 41.Wiese RJ, Uhland-Smith A, Ross TK, Prahl JM, DeLuca HF. Up-regulation of the Vitamin D receptor in response to 1,25-dihydroxyvitamin D3 results from ligand-induced stabilization. J Biol Chem. 1992;267:20082–6. [PubMed] [Google Scholar]