Abstract
Background:
Collagen and chitosan are potential biomaterials for medical applications; chitosan-collagen membranes are used as a barrier membrane in guided tissue regeneration and guided bone regeneration.
Aims:
The purpose of this study was to analyze the effect of the chitosan-collagen membrane on wound healing in rat mandibular defect by counting the number of fibroblasts and new blood vessels.
Materials and Methods:
As much as 24 male Wistar rats were divided into two groups, the treatment and control group. Bone defects were made In the rat mandible with diamond bur with a diameter of 2 mm, then the defect was covered with a chitosan-collagen membrane, and the control group was covered without application of chitosan-collagen membrane. After the 3rd, 7th, 14th, and the 21st day, the defect site was analyzed histologically. The number of fibroblasts and blood vessels was counted under a light microscope, at five fields with ×1000 and ×400 microscope magnification.
Statistical Analysis Used:
This study was done by using analysis of variance and unpaired t-test.
Results:
The average number of fibroblasts and blood vessels in the treatment group was higher than the control group. There was a significant difference in the number of fibroblasts on the 3rd and 7th day (P = 0.001; P = 0.001) and the number of blood vessels on the 3rd day (P = 0.04).
Conclusion:
The chitosan-collagen membrane was able to increase the number of fibroblasts and new blood vessels in the wound healing process.
Keywords: Chitosan-collagen membrane, mandibular defect, wound healing
INTRODUCTION
Wound healing consists of three phases include inflammation, proliferation, and maturation. It is a complex process, involving the interaction of various cells, cytokines, and growth factors.[1,2] The proliferation phase is characterized by the formation of the extracellular matrix (ECM) and the beginning of angiogenesis.[1,2] Angiogenesis is a fundamental step in the healing process, in which, the new blood vessels are formed from the preexisting vessel. The new vessels involved in the formation of the granulation tissue supplies the growing tissue with oxygen and nutrients.[1,3]
One of the uses of collagen in dentistry is a membrane barrier used in guided tissue regeneration (GTR) or guided bone regeneration (GBR) procedures due to its excellent biocompatibility and capability to accelerate wound healing.[4,5] Collagen also has biological activities that play an important role in the formation of coagulum, chemotaxis, and activates neutrophils and fibroblast cells in the periodontal ligament and gingiva, with low immunogenicity. Therefore, collagen was chosen as one ingredient in the resorbable membranes manufacture.[4,6] The use of resorbable membrane does not require any surgery to remove the membrane due to its resorbability. There are two types of resorbable membrane, synthetic membrane, and natural membrane.[7,8] The synthetic membrane is derived from copolymers, such as polylactide and polyglycolide, while the natural membrane is derived from collagen.[9]
Chitosan as a deacetylated derivatives of chitin is applied in various fields including industries, agriculture and food, and also medicine.[10] Chitosan is widely used in medical applications because it can deliverer medication toward the tissue surface better. It coagulates blood quickly, hypoallergenic, antibacterial, biocompatible, and biodegradable.[11,12] Chitosan can be prepared in gel, film, membrane, fiber, and powder, as additional preparations for periodontal treatment, and implant. It has proven to be able to accelerate the alveolar bone healing.[13] The previous researches have suggested that chitosan has antibacterial properties due to its ability to bind on the bacterial cell surface, causing shrinkage of the cell membrane that leads to the cell death.[14] Research conducted by Ueno et al. implicated that chitosan was observed as an indicator of inflammatory cells infiltration acceleration activate fibroblast migration activation, and the stimulation of these cells’ proliferation in the wound area.[15] This study was aimed to evaluate the role of the chitosan-collagen membrane in the wound healing by analyzing the number of fibroblasts and blood vessels around the rat mandibular defect.
MATERIALS AND METHODS
This study was an in vivo study conducted toward male Wistar rats, with the age of 2–3 months old, weight of 250–300 g. The collagen source was extracted from the white snapper fish added with chitosan, manufactured at the National Nuclear Energy Agency of Indonesia (BATAN). The total of 24 rats was divided into two groups; the treatment group with the application of collagen-chitosan membrane, and the control group without the application of chitosan-collagen membrane. The study was conducted at the Animal Hospital of Bogor Agricultural University. The protocol and treatment of animals have been approved by the animal care and use committee at the Faculty of Veterinary Medicine, Bogor Agricultural University (No. 11-2016/ACUC/RSHP/FKH-IPB).
Chitosan-collagen membrane preparation
Collagen was extracted from the white snapper scales using 0.5 M acetic acid at 4°C for 3 days with periodic stirring. Wet collagen was dried using a freeze drier to produce dry collagen. Chitosan an amino polysaccharide (poly-1, 4-D-glucosamine) derived from chitin by deacetylation. The chitosan used was fabricated from the crab shells produced by Biotech Surindo Company, Cirebon, with 90% deacetylation degree. The chitosan solution was prepared with 2% chitosan in 1% acetic acid or, in other words, as much as 2 g of chitosan in 100 mL acetic acid to get a gel-like appearance. Collagen addition was performed by adding as much as 1 g collagen into the chitosan solution, then mixed using a homogenesis tool until homogenous. The collagen and chitosan mixture was then poured into a petri dish until the desired thickness was gained. The mixture was then frozen in a freeze drier. The membrane sterilization process included the gamma-ray irradiation with a 25 kGy doses for 1–3 h, depending on the number of materials being sterilized. The thickness of the chitosan-collagen membrane obtained was 0.6 mm, as shown in Figure 1.
Figure 1.

Chitosan-collagen membrane; size 1 cm × 1 cm
Surgical procedure
Twenty-four Wistar rats were obtained from local vendor farms in the city of Bogor. The anesthetic action was performed using Ketamine 75 mg/kg bw and Xylazine 10 mg/kg bw, injected intraperitoneum. The incision was performed extra orally with a #15 blade in the buccal region, followed by blunt dissection up to the mandibular angle. The mandibular bone defect was created in the mandibular angulus region with a diameter of 2 mm using low-speed bur followed by 0.9% NaCl irrigation. Choice of bone defects in mandibular because periodontal disease is a progressive condition that eventually leads to the destruction of the connective tissue and jawbone. The bone defect was then covered with the chitosan-collagen membrane in the treatment group, as shown in Figure 2. The closure of the injured incision was performed with suture using nylon 5.0. Each group was performed necropsies on every 3 rats on the 3rd, 7th, 14th, and 21st day, with exsanguination under the influence of anesthesia. The histology slide was prepared to calculate the number of fibroblasts and blood vessels in the area around the wound or bone defect.
Figure 2.
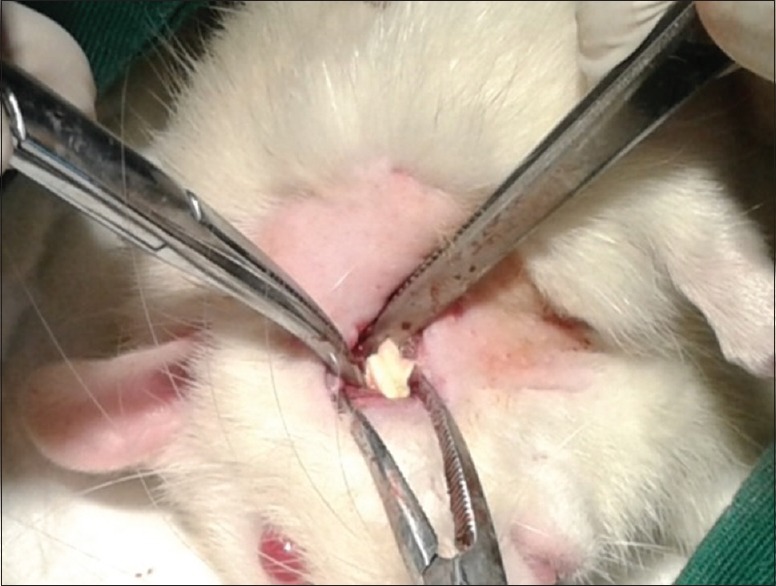
Chitosan-collagen membrane application covering the mandibular defect created
Histological examination
The mandibular slides, following the preparation methodology, were immersed in 10% formalin solution for 24 h. Specimens were embedded in the paraffin blocks and cut using a microtome with the thickness of 4–5 μm then stained with hematoxylin and eosin (H and E). Histological examination was performed by blind observation to determine the number of fibroblasts around bone defects under five view-fields with ×1000 microscope magnification. The number of blood vessels was observed under five view-fields with ×400 microscope magnification (Carl-Zeiss® Axio Imager Z.2).
Statistical analysis
Data were presented in the mean standard deviation, analysis of variance test was used to compare at four-time intervals in each group. Differences in the number of fibroblasts and blood vessels between the treatment and control groups and were statistically analyzed using the unpaired t-test, with the level of significance P < 0.05.
RESULTS
This study was analyzing the effect of the chitosan-collagen membrane on the wound healing by counting the number of fibroblasts and new blood vessels. The average number of fibroblasts and blood vessels in the group with chitosan-collagen membrane application was higher than the control group [Figures 3 and 4].
Figure 3.
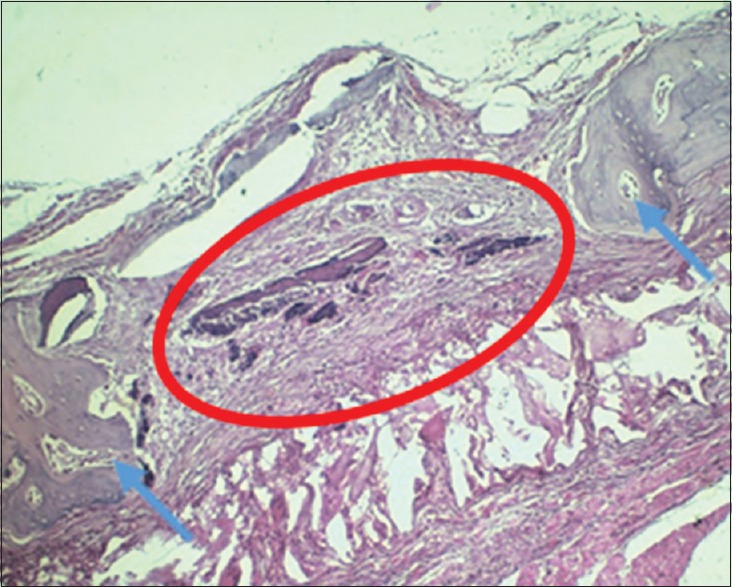
Histology H and E image; Bone defect was observed in the rat's mandibular with ×200 microscope; Blue arrows pointed to the rat's mandibular bone; Red circle marked the bone defect
Figure 4.
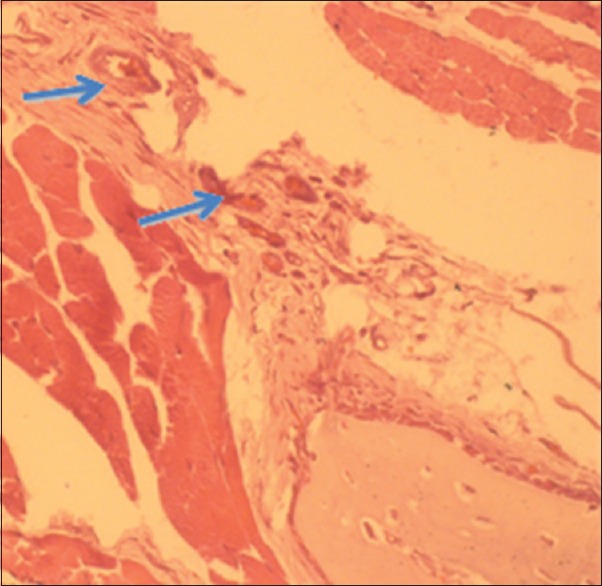
Histology H and E image with ×400; Blue arrows pointed to the blood vessels
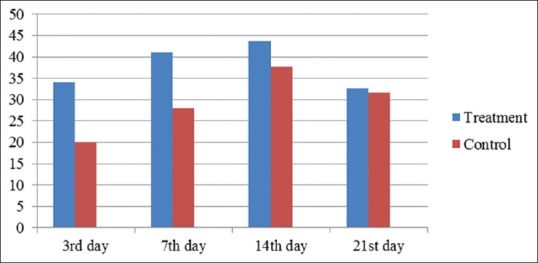
Table 1 showed the difference between the average number of fibroblasts between the treatment and control group. Based on the statistical analysis using the t-test showed that there were differences in the number of fibroblasts on the 3rd and 7th days (P = 0.001 and P = 0.001). This result showed that the application of chitosan-collagen membrane affected the number of fibroblasts in the wound healing process.
Table 1.
The difference in the number of fibroblasts between the treatment and control group
| Fibroblasts | Group Mean (SD) | P | Significance | |
|---|---|---|---|---|
| Treatment | Control | |||
| 3rd day | 34 (2.00) | 20 (2.00) | 0.001* | S |
| 7th day | 41 (1.73) | 28 (2.00) | 0.001* | S |
| 14th day | 43.7 (3.51) | 37.7 (2.52) | 0.07 | NS |
| 21st day | 32.7 (2.52) | 31.7 (1.53) | 0.58 | NS |
| P | 0.002* | 0.001* | ||
*P<0.05. SD – Standard deviation; S – Significant; NS – Nonsignificant; P – P value
Table 2 showed the difference between the average number of blood vessels between the treatment and control group. Based on the statistical analysis using the unpaired t-test showed that there were differences in the number of blood vessels on the 3rd day (P = 0.04; P < 0.05).
Table 2.
The difference in the number of blood vessels between the treatment and control group
| Blood vessels | Group Mean (SD) | P | Significance | |
|---|---|---|---|---|
| Treatment | Control | |||
| 3rd day | 4.7 (0.58) | 3.3 (0.58) | 0.04* | S |
| 7th day | 7.7 (2.52) | 6.3 (0.58) | 0.23 | NS |
| 14th day | 5.7 (1.15) | 5.0 (1.00) | 0.37 | NS |
| 21st day | 4.0 (1.00) | 4.3 (1.53) | 0.64 | NS |
| P | 0.01* | 0.005* | ||
*P<0.05. SD – Standard deviation; S – Significant; NS – Nonsignificant; P – P value
Histology H and E image of the bone defect observed in the rat's mandibular bone with ×200 microscope magnification was showed in Figure 5. In Figure 6, the histology image was magnified ×400 and showed the blood vessels formed as the rat's mandibular wound healing marker.
Figure 5.
The average number of fibroblasts in the treatment and control group
Figure 6.
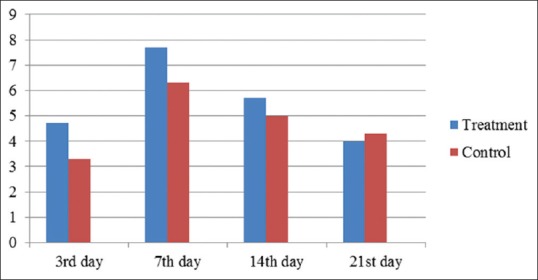
The average number of blood vessels in the treatment and control group
DISCUSSION
The results showed that the collagen membrane was able to increase the number of fibroblast cells and blood vessels. These results were consistent with previous research conducted by Wang et al. and Kasaj et al. The study conducted by Wang et al. was an in vitro study suggested that the collagen membrane was able to facilitate the cell attachment that leads to the formation of osteoblast cell layers thus increases the bone regeneration.[16] The study conducted by Kasaj et al. suggested that collagen membrane increased the proliferation of fibroblasts cells as well as osteoblasts cells of the gingival and periodontal ligaments compared to the synthetic membrane (polytetrafluoroethylene membrane).[17] Locci et al. demonstrated that matrix membrane composed of collagen and chondroitin glycosaminoglycan enhanced cellular proliferation and macromolecule accumulation.[18]
The major goal of periodontal therapy is a regeneration of the lost attachment apparatus and preventing disease progression.[19] Regenerative outcomes depend on the recruitment of the progenitor cell population resides in the remaining periodontium in the wound area. Ideally, a barrier membrane should facilitate this process by providing a substrate that can act as a matrix and provide signals necessary for the regenerating cells to proceed with the wound-healing cascade of proliferation, differentiation, and tissue maturation.[20]
This attachment process consists of adsorption of glycoproteins on to substrate surface, cell contacts, attachments, and spreading. The membranes used should not have any destructive effect toward the cell and having the capacity to promotes the cell growth and proliferation for the processes to occurred.[16,20]
GTR utilizes a physical barrier to exclude unwanted cells from the wound space to promote periodontal regeneration, also to providing cell occlusivity, space maintenance, tissue integration, and biocompatibility. This barrier membrane ideally must possess the ability to induce the cellular proliferation and differentiation.[20] Mesenchymal cell adhesion toward the barrier membrane depends on the type and nature of the barrier used and is thought to be positively influencing the regenerative outcomes.[20,21] The barrier is placed between the periodontal flap and the osseous defect to maintain a space for blood clot retention, as well as allowing the repopulation of the defect with cells having a regenerative potential.[20,22]
Collagen is a good natural protein used for GTR applications because this material is chemotactic toward the periodontal ligament fibroblasts, able to act as a migratory barrier of epithelial cells, contributes to the hemostasis process, and serves as the scaffold for early vascular reactions and tissue regeneration.[17] Collagen consists of basic molecules such as lysine and arginine, as the specific cell-adhesion site arginine-glycine-aspartic acid (RGD). RGD actively stimulates cellular attachment by binding the integrin receptors, and these bonds play an important role in the cell growth and differentiation as well as the overall regulation of cell function.[23] One problem encountered with collagen membrane is a rather fast degradation by collagenase, which able to affects the regenerating tissue.[17]
Chitosan may increase the formation of a well-organized ECM in the collagen Scaffold, which can be functioned as the cell modulator.[13] The previous study on chitosan-collagen suggested the ability to increase the cell growth in vitro and also affected the cells morphological picture. As a cross-linking agent, chitosan was able to not only increasing the scaffold resistance and prolonging degradation time but also showing an improved cell-adhesion and better and faster proliferation in vivo.[13,24] A study conducted by Song et al. concluded that the chitosan/fibroin-hydroxyapatite membrane had a similar bone regeneration capacity with the collagen membrane, and furthermore, the absorbable chitosan membrane offers the possibility to be used as a barrier membrane in the GBR.[25] Research, Wang et al., reports that a chitosan-collagen composite film accelerated osteoblast proliferation, differentiation, and matrix mineralization in vitro, its promising biomaterials for bone-tissue engineering in bone defect-related disease.[26] In this study, the membrane was made from the mixture of chitosan and collagen. When chitosan was combined with collagen, the chitosan-collagen blended membranes could improve its tensile strength or its elongation at break. The improving value was probably related to the formation of an attractive interaction between chitosan and collagen in the blended membranes.[27]
Fibroblast cells are used as indicators of wound healing because these cells are the dominant cells in the wound healing process. These cells are derived from the mesenchymal cells responsible for producing the new matrix necessary for restoring the structure and function of the injured tissue.[1] In this study, the number of fibroblast cells and blood vessels in the area around the rat's mandibular bone defect were observed with the H and E histologic preparations. The results showed that there were significant differences in the number of fibroblast cells and the blood vessels between the treatment and control group. The fibroblast cells begin proliferating within 24 h after injury, and the quantity begins to increase by the 3rd day.[1,2] This increase occurred because the 3rd day was the final phase of the inflammatory phase toward the beginning of the fibroblastic phase when the active macrophages produced the growth factors. The migration and proliferation of fibroblast cells together with the growth of new blood vessels responsible for the supply of oxygen-rich blood and nutrients, as the marker of the fibroplastic phase.[1,28]
The importance of early angiogenesis in the defected area has been increasingly stressed in connection with the tissue augmentation. Osteogenic cells developed from undifferentiated mesenchymal progenitor cells that are present either in the connective tissue of the bone marrow or derived from the pericytes of the connective tissue adjacent to the minor capillaries.[29] Hence, if the defect space is quickly colonized by blood vessels, this will also stimulate bone regeneration.[29] Some native membranes have been observed to exhibit selective permeability regarding the penetration of the membrane body by blood vessels within a few weeks after implantation.[29,30] In some membranes, small pores of fine-fibrillar collagen are serving as a guiding structure for invading blood capillaries.[30]
Bone defects in this study differ from periodontal bone defects. The presence of saliva, gingival crevicular fluid, type of bone damage, and bacteria in the mouth affect the chitosan-collagen membrane and wound healing. This research was observing the bone defects in the treatment and control group in different rat, so the possibility of differences in body response factors affect the results. Limitations found in this study were the small bone defects and the wound healing time evaluation. Further research is needed on the effect of the chitosan-collagen membrane on other healing parameters such as the formation of collagen and new bone with longer observation time and also comparing with the collagen membrane or chitosan application.
CONCLUSION
The chitosan-collagen membrane was able to increase the number of fibroblasts and new blood vessels in the wound healing process and also offered the possibility for use as a barrier membrane.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Leong M, Phillips LG. Wound healing. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, editors. The Biological Basic of Modern Surgical Practice. 19th ed. Philadelphia: Saunders-Elsevier; 2012. pp. 151–64. [Google Scholar]
- 2.Traversa B, Sussman G. The role of growth factor, cytokines and proteases in wound management. Prim Intention Aust J Wound Manag. 2001;9:161–7. [Google Scholar]
- 3.de Mendonça RJ. London: InTech Open; [Last accessed on 2018 Jun 20]. Angiogenesis in Wound Healing. Available from: http://www.cdn.intechopen. com/pdfs/34634/InTech-Angiogenesis_in_wound_healing.pdf . [Google Scholar]
- 4.Patino MG, Neiders ME, Andreana S, Noble B, Cohen RE. Cellular inflammatory response to porcine collagen membranes. J Periodontal Res. 2003;38:458–64. doi: 10.1034/j.1600-0765.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 5.Khan R, Khan MH, Bey A. Use of collagen as an implantable material in the reconstructive procedure – An overview. Biol Med. 2011;3:25–32. [Google Scholar]
- 6.Rothamel D, Benner M, Fienitz T, Happe A, Kreppel M, Nickenig HJ, et al. Biodegradation pattern and tissue integration of native and cross-linked porcine collagen soft tissue augmentation matrices – An experimental study in the rat. Head Face Med. 2014;10:10. doi: 10.1186/1746-160X-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hägi TT, Laugisch O, Ivanovic A, Sculean A. Regenerative periodontal therapy. Quintessence Int. 2014;45:185–92. doi: 10.3290/j.qi.a31203. [DOI] [PubMed] [Google Scholar]
- 8.Tal H, Moses O, Kozlovsky A, Nemcovsky C. Bioabsorbable collagen membranes for guided bone regeneration. In: Tal H, editor. Bone Regeneration. Rijeka: InTech; 2012. pp. 111–38. [Google Scholar]
- 9.Aurer A, Jorgic-Srdjak K. Membranes for periodontal regeneration. Acta Stomatol Croat. 2005;39:107–12. [Google Scholar]
- 10.Suzuki Y, Nishimura Y, Tanihara M, Suzuki K, Nakamura T, Shimizu Y, et al. Evaluation of a novel alginate gel dressing: Cytotoxicity to fibroblasts in vitro and foreign-body reaction in pig skin in vivo . J Biomed Mater Res. 1998;39:317–22. doi: 10.1002/(sici)1097-4636(199802)39:2<317::aid-jbm20>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Duarte AR, Mano JF, Reis RL. Preparation of chitosan scaffolds for tissue engineering using supercritical fluid technology. Eur Polym J. 2009;45:141–8. [Google Scholar]
- 12.Jia Y, Hu Y, Zhu Y, Che L, Shen Q, Zhang J, et al. Oligoamines conjugated chitosan derivatives: Synthesis, characterization, in vitro and in vivo biocompatibility evaluation. Carbohydr Polym. 2011;83:1153–61. [Google Scholar]
- 13.Archana D, Upadhyay L, Tewari RP, Dutta J, Huang YB, Dutta PK. Chitosan-pectin alginate as a novel scaffold for tissue engineering applications. Indian J Biotechnol. 2013;12:475–82. [Google Scholar]
- 14.Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2011;9:857–79. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno H, Yamada H, Tanaka I, Kaba N, Matsuura M, Okumura M, et al. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 1999;20:1407–14. doi: 10.1016/s0142-9612(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang HL, Miyauchi M, Takata T. Initial attachment of osteoblasts to various guided bone regeneration membranes: An in vitro study. J Periodontal Res. 2002;37:340–4. doi: 10.1034/j.1600-0765.2002.01625.x. [DOI] [PubMed] [Google Scholar]
- 17.Kasaj A, Reichert C, Götz H, Röhrig B, Smeets R, Willershausen B, et al. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008;4:22. doi: 10.1186/1746-160X-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locci P, Calvitti M, Belcastro S, Pugliese M, Guerra M, Marinucci L, et al. Phenotype expression of gingival fibroblasts cultured on membranes used in guided tissue regeneration. J Periodontol. 1997;68:857–63. doi: 10.1902/jop.1997.68.9.857. [DOI] [PubMed] [Google Scholar]
- 19.Garrett S. Periodontal regeneration around natural teeth. Ann Periodontol. 1996;1:621–66. doi: 10.1902/annals.1996.1.1.621. [DOI] [PubMed] [Google Scholar]
- 20.Thangakumaran S, Sudarsan S, Arun KV, Talwar A, James JR. Osteoblast response initial adhesion and alkaline phosphatase activity following exposure to a barrier membrane/enamel matrix derivative combination. Indian J Dent Res. 2009;20:7–12. doi: 10.4103/0970-9290.49048. [DOI] [PubMed] [Google Scholar]
- 21.Takata T, Wang HL, Miyauchi M. Attachment, proliferation and differentiation of periodontal ligament cells on various guided tissue regeneration membranes. J Periodontal Res. 2001;36:322–7. doi: 10.1034/j.1600-0765.2001.360508.x. [DOI] [PubMed] [Google Scholar]
- 22.Behring J, Junker R, Walboomers XF, Chessnut B, Jansen JA. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology. 2008;96:1–11. doi: 10.1007/s10266-008-0087-y. [DOI] [PubMed] [Google Scholar]
- 23.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tangsadthakun C, Kanokpanont S, Sanchavanakit N, Banaprasert T, Damrongsakkul S. Properties of collagen-chitosan scaffold for skin tissue engineering. J Metal Mater Miner. 2007;16:37–44. [Google Scholar]
- 25.Song JM, Shin SH, Kim YD, Lee JY, Baek YJ, Yoon SY, et al. Comparative study of chitosan/fibroin-hydroxyapatite and collagen membranes for guided bone regeneration in rat calvarial defects: Micro-computed tomography analysis. Int J Oral Sci. 2014;6:87–93. doi: 10.1038/ijos.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Wang G, Liu L, Zhang D. The mechanism of a chitosan-collagen composite film used as biomaterial support for MC3T3-E1 cell differentiation. Sci Rep. 2016;6:39322. doi: 10.1038/srep39322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Indrani DJ, Lukitowati F, Yulizar Y. Preparation of Chitosan/Collagen Blend Membranes for Wound Dressing: A Study on FTIR Spectroscopy and Mechanical Properties. Proceedings. 4th International Conference on Advanced Materials Science and Technology. Malang: IOP Conference Series: Materials Science and Engineering. 2017 27-28 September, 2016. [Google Scholar]
- 28.Putri SA, Ismardianita E. The effect of ethanol extract of Hypnophytum formicarum plant towards angiogenesis for wound healing after tooth extraction: Experimental research on Cavia cobaya. Padjadjaran J Dent. 2016;28:171–6. [Google Scholar]
- 29.Schwarz F, Rothamel D, Herten M, Wüstefeld M, Sager M, Ferrari D, et al. Immunohistochemical characterization of guided bone regeneration at a dehiscence-type defect using different barrier membranes: An experimental study in dogs. Clin Oral Implants Res. 2008;19:402–15. doi: 10.1111/j.1600-0501.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- 30.Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C, et al. Arole for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One. 2010;5:e9808. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]


