Abstract
Background and Aims
Glycoprotein acetylation [GlycA] is a novel nuclear magnetic resonance [NMR] biomarker, measured in serum or plasma, that summarizes the signals originating from glycan groups of certain acute-phase glycoproteins. This biomarker has been shown to be robustly associated with cardiovascular and short-term all-cause mortality, and with disease severity in several inflammatory conditions. We investigated GlycA levels in a cohort of healthy individuals [HCs], patients with Crohn’s disease [CD] and patients with ulcerative colitis [UC] prior to and after therapeutic control of inflammation.
Methods
Serum samples of 10 HCs, 37 CD patients and 21 UC patients before and after biologic therapy were subjected to high-throughput NMR analysis by Nightingale Health Ltd. Paired C-reactive protein [CRP] and fecal calprotectin [fCal] measurements were used to characterize baseline differences, treatment effects and post-treatment association with endoscopic response [50% SES-CD decrease at Week 24] and mucosal healing [SES-CD ≤ 2 for CD, Mayo endoscopic score ≤ 1 for UC].
Results
GlycA levels were significantly higher in patients with active inflammamtory bowel disease [IBD] compared with those in healthy controls, and accurately reflected the mucosal recovery to a ‘healthy’ state in both CD and UC patients achieving mucosal healing. In CD patients who experienced an endoscopic response without achieving full mucosal healing, GlycA levels also decreased but did not normalize to HC levels. Overall, GlycA correlated well with CRP and fCal, and accurately tracked disease activity in CRP-negative patients [<5 mg/dL].
Conclusion
GlycA holds promise as a viable serological biomarker for disease activity in IBD, even in patients without elevated CRP, and should therefore be tested in large prospective cohorts.
Keywords: Glycoprotein acetylation, GlycA, disease activity, biomarker, CRP, calprotectin, inflammatory bowel disease
1. Introduction
Glycans are complex oligosaccharides that play a fundamental role in human health and contribute to the development of many complex inflammatory diseases.1 Glycoprotein acetylation [GlycA] is a novel nuclear magnetic resonance [NMR] biomarker, measured in blood serum or plasma, that summarizes the NMR signal originating from the glycan groups of certain acute-phase glycoproteins [mainly α-1-acid glycoprotein, haptoglobin, α-1-antichymotrypsin, and transferrin].2,3 GlycA has been extensively investigated in cardiovascular disease [CVD], where it was found to be robustly associated with atherosclerosis and risk of future CVD.3,4 Large demographic studies have also shown an association with cardiovascular and short-term all-cause mortality.5 Robust associations with both CVD and diabetes in large cohorts have prompted the conclusion that GlycA summarizes the summative risk resulting from multiple inflammatory pathways.4,6,7 In several inflammatory conditions, such as rheumatoid arthritis,8,9 Kawasaki disease10 and psoriasis,11 GlycA has been shown to be associated with disease severity, even after adjustment for traditional acute inflammation metrics such as C-reactive protein [CRP]. Furthermore, GlycA levels decrease during successful treatment of psoriatic skin inflammation with anti-TNF therapy,11 suggesting it could be a clinically relevant biomarker for monitoring disease severity.
Despite growing evidence of the role of glycosylation in other immune-mediated entities, including inflammatory bowel disease [IBD],1,12,13 GlycA has not yet been studied in this context. Current IBD treatment algorithms are not solely symptom driven, but additionally guided by biomarkers, an approach that has been shown to improve endoscopic outcomes.14 However, CRP has little discriminatory power in IBD: many, but not all, patients with Crohn’s disease [CD] show a strong CRP response, whereas patients with ulcerative colitis [UC] generally only have a modest or absent CRP response.15 A better alternative biomarker has been found in fecal calprotectin [fCal], which correlates well with mucosal inflammation and can be used as a surrogate marker for mucosal healing [MH] in both UC and CD.16 However, in many countries, serial fCal measurements are not reimbursed, and patients generally do not prefer regular fecal sampling.17
We therefore investigated GlycA in a cohort of healthy individuals [HCs], patients with CD, and patients with UC. We not only studied differences between healthy and affected individuals, but also collected post-treatment samples, allowing the detection of associations with mucosal healing relative to established inflammatory biomarkers such as CRP and fCal.
2. Materials and Methods
2.1. Study design and patients
We conducted this prospective study at the tertiary IBD referral center of the University Hospitals Leuven [Leuven, Belgium]. All patients included in the analysis had given written consent to participate in the Institutional Review Board–approved IBD Biobank [B322201213950/S53684], allowing collection of serum data, clinical [baseline] features, patient-reported outcomes [PRO2], Harvey–Bradshaw Index [HBI] characteristics, among other items.
We randomly selected samples from patients with active endoscopic disease who were initiating biologic therapy. Patients with an ostomy were excluded. All patients were prospectively monitored, including clinical and endoscopic assessment, and CRP and fCal measurements at predefined outcomes [baseline and 6 months for CD patients, baseline and Week 8 [adalimumab, ADM] or Week 14 [infliximab, IFX, and vedolizumab, VDM] for UC patients]. In addition, serum samples of 10 gender- and age-matched healthy controls [HCs] were collected.
2.2. Outcomes
Endoscopic outcomes were assessed 6 months after treatment initiation in CD patients,18 whereas in UC patients timing depended on the national reimbursement criteria of the individual drug [8 weeks for ADM, 14 weeks for IFX and VDM]. In UC patients, mucosal healing was defined as a Mayo endoscopic subscore of ≤1, whereas in CD patients, mucosal healing was defined as a Simple Endoscopic Score for CD [SES-CD] of ≤2. Because of the low MH rates in ustekinumab [UST]-treated patients,19 only endoscopic response [minimal 50% decrease in SES-CD] was evaluated.
2.3. Samples
Serum samples were collected at baseline prior to the first administration of the drug, and during maintenance. Samples were centrifuged and stored aliquoted at –20°C. Fecal samples were collected at home, stored at 4°C in the home fridge and transported [cooled] within 24 h to the hospital. fCal measurements were performed for all patients with the fCAL ELISA kit [Bühlmann, Schönenbuch, Switzerland]. CRP was determined by the routine laboratory of the University Hospitals Leuven. GlycA concentration was quantified using the Nightingale Health Ltd high-throughput metabolomics platform [Helsinki, Finland].2,3
2.4. Statistical analysis
Continuous variables are expressed as median and interquartile range [IQR]. The Wilcoxon rank-sum test was used to compare GlycA levels of HC samples with those of baseline CD and UC samples, and to compare patients showing MH with those without signs of healing in these conditions. All statistical analyses were performed in R, version 3.4.3 [R Development Core Team, Vienna, Austria]20 using the base stats package. Graphics were generated using the ggplot2 R package.21
3. Results
3.1. Patient characteristics
Fifty-eight IBD patients [37 CD, 21 UC] were included in this prospective, observational study prior to the initiation of biologic therapy [9 ADM, 13 IFX, 24 VDM, and 12 UST]. Of the examined patients, 34.5% [n = 20] did not have an elevated CRP at baseline. In this cohort of patients with a median disease duration of 4.8 [2.4–14.9] years, mucosal healing was obtained in 39.7% [n = 23] after a median of 23.7 [22.4–24.7, CD] and 13.7 [11.0–14.3, UC] weeks, respectively [Table 1].
Table 1.
Baseline disease characteristics.
| Crohn’s disease [n = 37] |
Ulcerative colitis [n = 21] |
|
|---|---|---|
| Sex, women, n [%] | 15 [40.5] | 9 |
| Disease duration, y, median [IQR] | 4.6 [2.6–16.3] | 5.0 [1.8–9.6] |
| Age at inclusion, y, median [IQR] | 30.2 [22.9–41.6] | 37.5 [26.2–47.2] |
| Disease location, n [%] | L1 Ileal disease—10 [27] | E1 Proctitis—3 [14.3] |
| L2 Colonic disease—9 [24.3] | E2 Left sided—8 [38.1] | |
| L3 Ileocolonic disease—18 [48.6] | E3 Pancolitis—10 [47.6] | |
| Disease behavior, n [%] | ||
| Inflammatory [B1] | 22 [59.5] | |
| Stricturing [B2] | 5 [13.5] | NA |
| Penetrating [B3] | 10 [27.0] | |
| Perianal disease [p] | 12 [32.4] | |
| Smoking status, n [%] | ||
| Active smoking | 7 [18.9] | 0 [0.0] |
| Previously smoking | 10 [27.0] | 6 [28.6] |
| Never smoked | 20 [54.1] | 15 [71.4] |
| Body Mass Index, kg/m2, median [IQR] | 21.6 [19.8–25.3] | 22.9 [22.0–26.4] |
| C-reactive protein, mg/L, median [IQR] | 12.5 [3.5–22.1] | 5.9 [1.5–20.7] |
| Fecal calprotectin, μg/g, median [IQR] | 1800.0 [1554.2–1800.0] | 1800.0 [924.8–1800.0] |
| PRO2, median [IQR] | 15.0 [7.0–21.0] | 4.0 [4.0–5.0] |
| Initiated biological therapy, n [%] | ||
| Adalimumab | 5 [13.5] | 4 [19.0] |
| Infliximab | 8 [21.6] | 5 [23.8] |
| Vedolizumab | 12 [32.4] | 12 [57.1] |
| Ustekinumab | 12 [32.4] | NA |
| Timing of endoscopic assessment, weeks, median [IQR] | 23.7 [22.4–24.7] | 13.7 [11.0–14.3] |
IQR = interquartile range; n = number of patients; PRO2 CD = patient-reported outcome for Crohn’s disease = 5 × abdominal pain score + 2 × liquid stool frequency; PRO2 UC = patient-reported outcome for ulcerative colitis = stool frequency + rectal bleeding.
3.2. GlycA accurately reflects disease activity
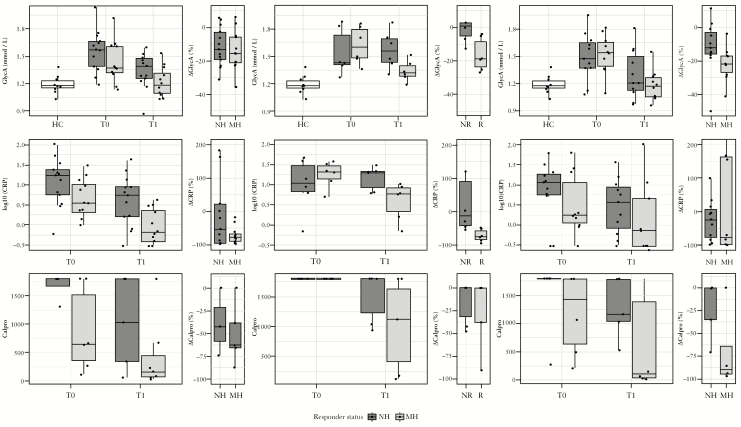
GlycA concentrations were significantly increased in both CD and UC patients compared to HCs [p < 10–4 and p < 10–3, respectively]. Variability of GlycA was likewise higher in CD and UC patients than what is observed in HCs [coefficient of variation of 14.8%, 15.5%, and 8.0%, respectively]. At baseline, no significant difference between GlycA levels of CD and UC patients [p = 0.92] was observed, and GlycA concentration in both CD and UC patients achieving mucosal healing dropped back to HC levels [p = 0.90, p = 0.91] [Figure 1A and C]. While CD patients responding well to UST treatment [without achieving mucosal healing] showed a significant decrease in GlycA concentration in comparison with their baseline measurements [p = 0.03], their post-treatment GlycA levels remained elevated in comparison with HC levels [one-sided wilcox p = 0.006] [Figure 1B]. The observed drop in GlycA levels during maintenance therapy was consistent regardless of treatment used [supplementary Figure S1]. Analysis of variance across the different treatments found no significant differences between the baseline GlycA, post-treatment GlycA, and the difference between these two timepoints of the different treatments.
Figure 1.
Glycoprotein acetylation [GlycA], CRP, and fCal measurements. The top row depicts GlycA concentrations in healthy controls [HCs], and IBD patients at baseline [T0] and during maintenance therapy [T1], as well as the observed percentage change after treatment. The middle row depicts CRP measurements; the bottom row depicts fCal levels. Columns, from left to right, show samples originating from [A] IFX-, ADM-, or VDM-treated CD, [B] UST-treated CD, and [C] IFX-, ADM- or VDM-treated UC. Patients are divided into non-responding [no mucosal healing, NH, or no endoscopic response, NR, depicted in dark grey] and responding [mucosal healing, MH, or endoscopic response, R, depicted in light grey] groups. CRP, C-reactive protein; fCal, fecal calprotectin; IBD, inflammatory bowel disease; IFX, infliximab; ADM, adalimumab; VDM, vedolizumab; UST, ustekinumab;UC, ulcerative colitis.
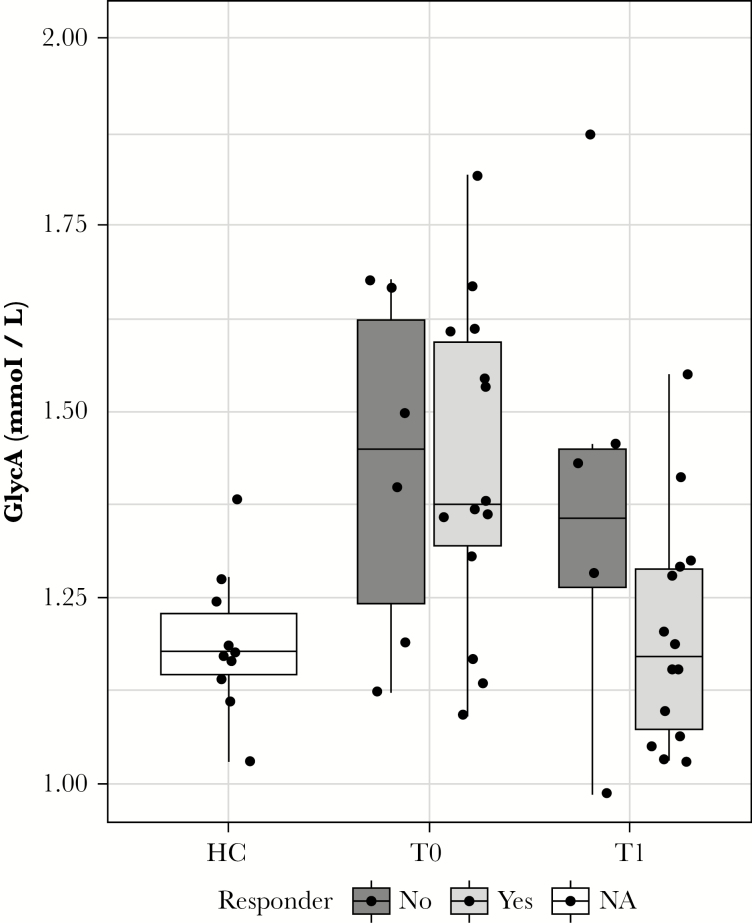
In patients without elevated CRP [<5 mg/dL] at baseline [n = 20, 11 CD, 9 UC], GlycA levels were significantly higher than those observed in HCs [p < 0.01] [Figure 2]. In healers, GlycA levels dropped to levels similar to those in HCs, while in non-healers GlycA levels remained elevated compared with those in HCs [p = 0.07].
Figure 2.
Glycoprotein acetylation [GlycA] levels of CRP-negative patients. Patients with healthy CRP levels [<5 mg/dL], [n = 20, 11 CD, 9 UC] have significantly increased GlycA levels compared with healthy controls [p < 0.01]. After treatment [T1], patients who responded to treatment returned to the GlycA levels observed in HCs, while non-responder GlycA levels remain elevated over HC levels [p = 0.07]. CRP, C-reactive protein; CD, Crohn’s disease; UC, ulcerative colitis; HC, healthy control.
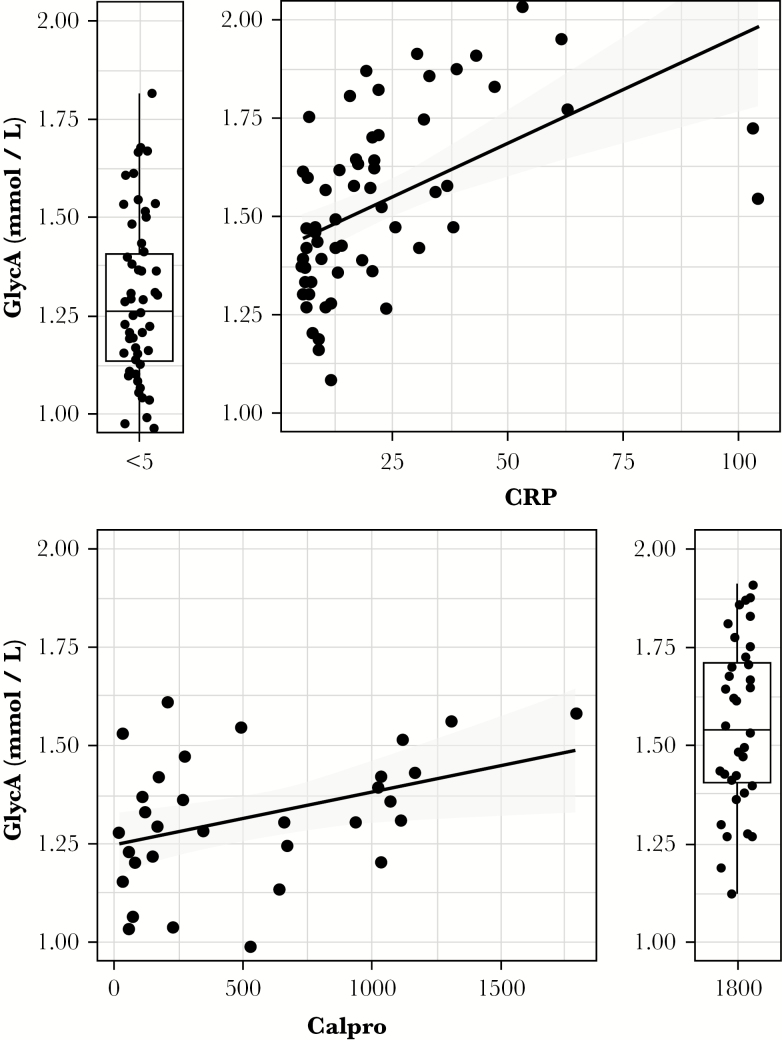
GlycA correlated well with both fCal and CRP [spearman ρ = 0.39, p = 0.02 and ρ = 0.65, p < 10–8, respectively] [Figure 3]. Across the three considered settings [IFX-, ADM-, or VDZ-treated CD, UST-treated CD and IFX, ADM- or VDZ-treated UC], of these three biomarkers, only GlycA post-treatment levels consistently showed a significant difference between responder and non-responder levels [Figure 1]. Endoscopic activity, quantified by the Mayo endoscopic subscore in UC, correlated well with GlycA [ρ = 0.51, p = 0.0016], but not with the SES-CD, though this latter measure was only available for 15 of 37 CD patients. Disease location was associated with GlycA levels, with a Wilcoxon test showing a borderline significant [p = 0.06] difference between GlycA levels of L1 and L3 CD patients, and UC patients with E1 disease showing significantly lower GlycA levels than those of E2/E3-afflicted patients [wilcoxon p = 0.04]. CD patients with prior enterectemy showed similar baseline GlycA levels to those of patients without occurrence of any resection of a part of the gut, or stricturoplasty for stenosing complications. The greater GlycA decrease observed in treatment responders compared with non-responders was observed in resectioned patients and non-resectioned patients alike, indicating prior enterectemy does not impact GlycA’s association with disease activity. Finally, the expected association of GlycA with body–mass index [BMI], robustly shown in large demographic studies,22,23 was absent in this IBD cohort, analogous to observations in rheumatoid arthritis.9
Figure 3.
Glycoprotein acetylation [GlycA], CRP, and fCal correlations. Correlation between GlycA and CRP [spearman ρ = 0.40, p = 0.02, top] and fCal [spearman ρ = 0.61, p < 10–8, bottom] is illustrated in all available data. Data at the lower detection limit for CRP [<5] or the upper detection limit for fCAL [>1800] are depicted as boxplots alongside the scatterplot. CRP, C-reactive protein; fCal, fecal calprotectin.
4. Discussion
In this prospective pilot project, we provide the first evidence that GlycA can be a relevant biomarker in the context of IBD. Glycoprotein acetylation levels were found to be significantly higher in patients with active IBD, compared with HCs. As elevated GlycA levels have previously been associated with atherosclerosis and cardiovascular disease,3,4 our observations support previous findings regarding increased cardiovascular risk reported in IBD due to the persistence of chronic inflammation.24 While pre-treatment GlycA levels showed no association with eventual treatment outcome, a treatment-induced decrease in GlycA was strongly associated with successful treatment response and mucosal healing, and associated with treatment response at least as well as traditional inflammatory biomarkers such as CRP or fCal. Glycoprotein acetylation accurately reflected the mucosal recovery to a healthy state, as evidenced by the return to healthy GlycA levels in healers, but not in non-healers or partial responders.
The discovery of GlycA as a relevant biomarker for IBD disease activity makes pathophysiological sense, as glycans are important in fine-tuning immune responses.13 Furthermore, recent mass spectrometry data demonstrated that plasma N-glycomes show distinct glycosylation patterns differentiating CD and UC patients, and that these patterns can be associated with disease progression and the need for more potent medication and surgery.25 The method used in our report summarizes NMR signal originating from acetyl groups of specific glycosylated proteins present in these patterns [specifically haptoglobin and α1-acid-glycoprotein].25
Our results suggest that GlycA is a viable serological biomarker for monitoring disease activity and treatment response in CRP-negative IBD patients. The advantage of GlycA over CRP likely stems from its greater stability, owing to its composite nature as a summary measure of glycan signal originating from several acute-phase proteins. It has been shown that calculating the true set point for biological homeostasis of CRP requires 33 concurrent measurements, whereas for GlycA only a single measurement is required.10 Contrary to CRP, GlycA has been shown to be a stable and sensitive marker for chronic inflammation in rheumatoid arthritis.9 Additionally, replacing CRP with another serological biomarker in patients who cannot be biochemically monitored using CRP might be a better alternative than a fecal biomarker, as patients prefer regular blood instead of regular stool sampling.17 The cost of GlycA determination is comparable with that of fCal measurement [20–50 euro, depending on sample size, and ~25 euros, respectively]. Though both biomarkers are considerably more expensive than a CRP measurement [~5 euro], the NMR spectrum used to quantify GlycA additionally quantifies several metabolic biomarkers such as amino acid levels, glycolysis and fatty acid metabolites, and an extensive lipoprotein profile.
We recognize that our study has both strengths and weaknesses. To our knowledge, this is the first study that assesses glycosylation levels of plasma proteins through NMR in IBD patients. The study had access to both baseline pre-treatment samples and post-treatment samples of CD and UC patients, which facilitated characterization of baseline differences, treatment effects, and post-treatment association with mucosal healing and treatment response. However, sample size was limited, necessitating the grouping of samples from several treatment regimens for meaningful statistical analysis. The sample size would need to be increased to assess the effects of, and biomarkers for successful treatment with, specific drug regimens. Likewise, our sample numbers necessitated the grouping of CD and UC samples to assess GlycA’s potential as a biomarker in pre-treatment CRP-negative patients. The limited availability of certain clinical metadata in this pilot study, specifically fCal and SES-CD, presents a strong incentive for a more extensive follow-up study. Finally, the GlycA biomarker summarizes the degree of acetylation of specific acute-phase glycoproteins, and to our knowledge it is currently not known whether the principal contribution underlying the associations with GlycA can be ascribed to the concentrations of these proteins, their glycosylation, or their acetylation profiles.
In conclusion, we identified GlycA as a promising candidate biomarker for monitoring disease activity in IBD patients, even in patients without elevated CRP. Additional experiments in larger cohorts are necessary to confirm our findings, and to elucidate whether measuring the GlycA component glycoproteins directly is a low-cost proxy that can track successful treatment response as accurately as the GlycA measurement by NMR.
Author Contributions
TD: study design, data interpretation, statistical analysis, and writing of the manuscript. BV: study design, data acquisition and interpretation, and writing of the manuscript. SV: study design, data interpretation, supervision, and critical revision of the manuscript. JvW: critical revision of the manuscript.
Funding
Research was funded by the Research Foundation – Flanders [FWO] [grant G0D6817N]. TD is funded by a grant from the Agency for Innovation and Entrepreneurship [VLAIO, #141614]. BV is a doctoral fellow and SV is a Senior Clinical Investigator of the FWO, Belgium. BV has also received research grants from the Belgium Week of Gastroenterology, the Belgian IBD Research and Development [BIRD], the European Crohn’s and Colitis Organization [ECCO], and the IBD Patient’s Association Flanders [CCV VZW].
Conflicts of Interest
BV received lecture fees from Ferring, R-biopharm, Takeda Pharmaceuticals, and Janssen; and a consultancy fee from Janssen.
SV received financial support for research from MSD, Abbvie, Janssen, and UCB Pharma; lecture fees from Abbott, Abbvie, Merck Sharpe & Dohme, Ferring Pharmaceuticals, and UCB Pharma; and consultancy fees from Pfizer, Ferring Pharmaceuticals, Shire Pharmaceuticals Group, Merck Sharpe & Dohme, and AstraZeneca Pharmaceuticals.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Supplementary Material
Acknowledgments
The authors would like to thank Vera Ballet and Eline Vandeput for an excellent job in maintaining the Leuven IBD patient database; and Nooshin Ardeshir Davani, Helene Blevi, Tamara Coopmans, Sophie Organe, and Willem-Jan Wollants for an excellent job in maintaining the Biobank database.
References
- 1. Theodoratou E, Campbell H, Ventham NT, et al. The role of glycosylation in IBD. Nat Rev Gastroenterol Hepatol 2014;11:588–600. [DOI] [PubMed] [Google Scholar]
- 2. Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: A primer on -omic technologies. Am J Epidemiol 2017;186:1084–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soininen P, Kangas AJ, Würtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet 2015;8:192–206. [DOI] [PubMed] [Google Scholar]
- 4. Akinkuolie AO, Glynn RJ, Padmanabhan L, Ridker PM, Mora S. Circulating N‐linked glycoprotein side‐chain biomarker, rosuvastatin therapy, and incident cardiovascular disease: An analysis from the JUPITER trial. J Am Heart Assoc 2016;5:e003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer K, Kettunen J, Würtz P, et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: An observational study of 17,345 persons. PLoS Med 2014;11:e1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawler PR, Akinkuolie AO, Chandler PD, et al. Circulating N-linked glycoprotein acetyls and longitudinal mortality risk. Circ Res 2016;118:1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ritchie SC, Würtz P, Nath AP, et al. The biomarker GlycA is associated with chronic inflammation and predicts long-term risk of severe infection. Cell Syst 2015;1:293–301. [DOI] [PubMed] [Google Scholar]
- 8. Bartlett DB, Connelly MA, AbouAssi H, et al. A novel inflammatory biomarker, GlycA, associates with disease activity in rheumatoid arthritis and cardio-metabolic risk in BMI-matched controls. Arthritis Res Ther 2016;18:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ormseth MJ, Chung CP, Oeser AM, et al. Utility of a novel inflammatory marker, GlycA, for assessment of rheumatoid arthritis disease activity and coronary atherosclerosis. Arthritis Res Ther 2015;17:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connelly MA, Shimizu C, Winegar DA, et al. Differences in GlycA and lipoprotein particle parameters may help distinguish acute kawasaki disease from other febrile illnesses in children. BMC Pediatr 2016;16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joshi AA, Lerman JB, Aberra TM, et al. GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circ Res 2016;119:1242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dias AM, Dourado J, Lago P, et al. Dysregulation of T cell receptor N-glycosylation: A molecular mechanism involved in ulcerative colitis. Hum Mol Genet 2014;23:2416–27. [DOI] [PubMed] [Google Scholar]
- 13. Dias AM, Correia A, Pereira MS, et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc Natl Acad Sci U S A 2018;115:E4651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease [CALM]: A multicentre, randomised, controlled phase 3 trial. Lancet 2018;390:2779–89. [DOI] [PubMed] [Google Scholar]
- 15. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys?Gut 2006;55:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn’s disease. Inflamm Bowel Dis 2016;22:623–30. [DOI] [PubMed] [Google Scholar]
- 17. Adler A, Geiger S, Keil A, et al. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol 2014;14:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR guideline for diagnostic assessment in inflammatory bowel disease. J Crohns Colitis 2019; 13:273–90. [DOI] [PubMed] [Google Scholar]
- 19. Verstockt B, Noman M, Aerden I, et al. Mo1880 – Ustekinumab induces limited mucosal healing after 6 months in a real-life, prospective cohort of patients with refractory Crohn’s disease. Gastroenterology 2018;154:S-836. [Google Scholar]
- 20. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 21. Wickam H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag New York; 2009. [Google Scholar]
- 22. Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR Jr. Comparison of the predictive value of GlycA and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clin Chem 2016;62:1020–31. [DOI] [PubMed] [Google Scholar]
- 23. Titan SM, Pecoits-Filho R, Barreto SM, Lopes AA, Bensenor IJ, Lotufo PA. GlycA, a marker of protein glycosylation, is related to albuminuria and estimated glomerular filtration rate: The ELSA-Brasil study. BMC Nephrol 2017;18:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: A nationwide Danish cohort study. Gut 2013;62:689–94. [DOI] [PubMed] [Google Scholar]
- 25. Clerc F, Novokmet M, Dotz V, et al. ; IBD-BIOM Consortium Plasma N-glycan signatures are associated with features of inflammatory bowel diseases. Gastroenterology 2018;155:829–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.